Determinants of hospital length of stay after transcatheter aortic valve implantation with self-expanding prostheses: a prospective, single centre observational study
DOI: https://doi.org/10.4414/smw.2019.20095
Angela
Freia, Dionysios
Adamopoulosa, Hajo
Müllera, Bernhard
Walderb, Nils
Perrina, Thomas
Reynaudb, Sylvain
Hoc, Marco
Roffia, François
Macha, Marc-Joseph
Lickerb, Stéphane
Noblea
aDivision of Cardiology, University Hospital of Geneva, Switzerland
bDivision of Anaesthesiology, University Hospital of Geneva, Switzerland
cDepartment of Nutrition and Dietetics, University Hospital of Geneva, Switzerland
Summary
INTRODUCTION
We sought to identify baseline and periprocedural variables affecting hospital length of stay (LoS) in patients undergoing transcatheter aortic valve implantation (TAVI).
METHODS
Data on 304 consecutive patients undergoing TAVI at a single centre between August 2008 and December 2017 were collected prospectively. All patients underwent a complete clinical, echocardiographic and laboratory evaluation including a comprehensive frailty assessment at baseline. LoS was defined as the number of in-hospital days after the TAVI procedure during the index hospitalisation until the time the patient left the hospital for home or a rehabilitation clinic.
RESULTS
The mean LoS was 10.4 ± 7.1 days (median 8, interquartile range 5–12) with a significant trend towards shorter LoS over time (p <0.001). Patients discharged directly home were more likely to have shorter LoS (p = 0.007). All periprocedural complications were significantly associated with prolonged LoS (p <0.05 for all). Multivariate analysis showed an independent association between LoS and emergency admission (beta 3.24 ± 1.56, p = 0.039), baseline gait speed (beta: 0.39 ± 0.16, p = 0.018), baseline serum C-reactive protein (CRP, beta 0.14 ± 0.04, p = 0.001) and subclavian access (beta 8.27 ± 2 .9, p = 0.005). Gait speed and serum CRP remained significant determinants of LoS even after adjustment for periprocedural complications and patients’ discharge destination.
CONCLUSION
Baseline gait speed and serum CRP are significant independent determinants of LoS after TAVI.
Introduction
Recent years have witnessed a worldwide expansion of transcatheter aortic valve implantation (TAVI) as an alternative to surgery for aortic valve stenosis. As the indication for TAVI extends to the intermediate risk population [1, 2], with the price of available devices remaining high, the cost of this procedure will become more and more of a concern. In order to limit costs, the use of hospital resources should be optimised. Previous studies have reported the positive results of a simplified approach (true percutaneous access with procedures performed in a catheterisation laboratory with fluoroscopic guidance only, under local anaesthesia) for the TAVI procedure [3, 4], the reduction or elimination of an intensive care unit (ICU) stay for elective cases, and the safety and feasibility of early discharge [5, 6] (≤72 hours after uncomplicated transfemoral TAVI). The advantages of this streamlining are multiple: not only could it improve the patient experience and quality of care in an elderly and/or frail population at risk for extended hospitalisation, but in a country such as Switzerland, where the cost of in-hospital days is high and the postprocedure length of hospital stay (LoS) remains long (9.7 ± 5.9 days in 2015), it could also help reduce the costs of this expensive treatment [7, 8]. [9]
At our centre, frailty is systematically assessed for all patients prior to TAVI. Over the last few years we noted that some patients experienced a prolonged hospital stay after TAVI despite the absence of any expected causes, such as periprocedural complications. We consequently hypothesised that frailty alone has an impact on LoS. Therefore, we sought to identify baseline features – including elements of the frailty assessment – and periprocedural variables affecting LoS to determine the causes of prolonged hospitalisation in patients undergoing TAVI.
Methods
Study design
This was a single centre prospective cohort study. The study was approved by the local ethics committee (Commission cantonale d'éthique de la recherche, Genève. Study number: 10-137) and conforms to the principles outlined in the Declaration of Helsinki. All patients gave informed written consent.
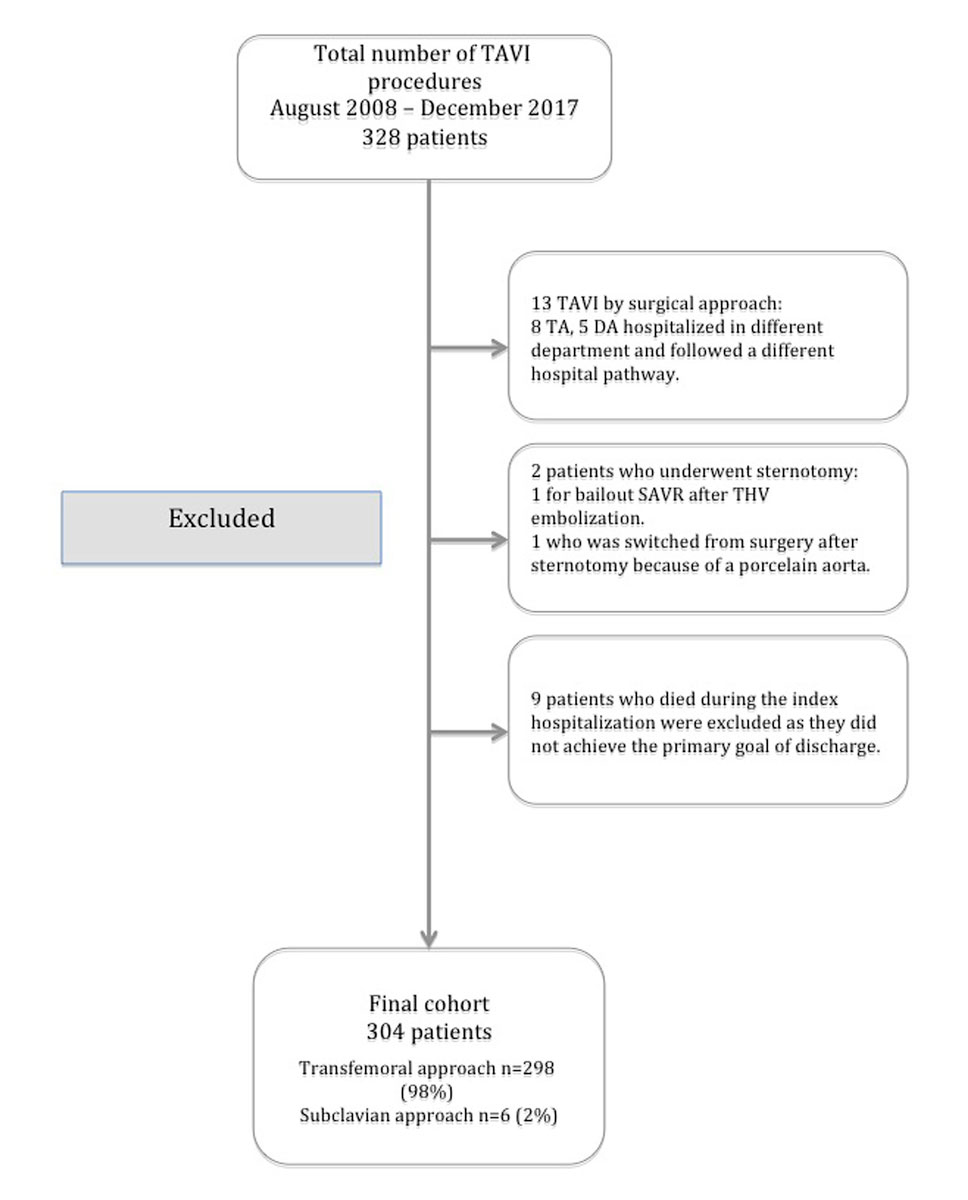
From August 2008 to December 2017, 328 consecutive patients with severe symptomatic aortic valve stenosis underwent implantation of a transcatheter heart valve (THV) at our institution. The study population flow chart showing patients excluded from the final cohort according to the predefined study criteria are presented in summary in figure 1.
Patient population
We consecutively included all patients who were admitted to and treated at our centre for severe symptomatic aortic stenosis by transfemoral or subclavian TAVI using exclusively a Medtronic self-expanding THV. Each case was discussed by our local heart team, which includes interventional cardiologists, cardiovascular anaesthetists, cardiac surgeons, radiologists, echocardiographers, intensivists and a nurse coordinator. The decision for TAVI was based on current guidelines [1, 2].
Baseline assessment
All patients underwent a standard pre-TAVI baseline assessment, including medical history, physical examination, electrocardiogram (ECG), transthoracic echocardiography (ETT), and laboratory tests, as well as multislice computed tomography. Risk scores estimating surgical mortality (Logistic EuroSCORE, Society of Thoracic Surgeons predicted risk of mortality [STS-PROM] score and more recently the EuroSCORE 2) were calculated for all patients. Additionally, the majority of patients (n = 254) underwent frailty assessment, encompassing evaluation of:
- Mobility: whether normal or poor (affecting the patient’s ability to perform daily activities), a history of recent falls, and the gait speed test [10].
- Strength: by measuring hand grip strength with a hand dynamometer (assessed according to the norms set out by Mathiowetz V. et al. [11]).
- Nutritional status: assessment of recent unintentional weight loss, pre-procedure serum albumin level, and body mass index.
- Mental capacity: assessed using the mini mental state evaluation (MMSE) and full psychogeriatric evaluation if the patient scored below 20 points on the MMSE.
Procedure and postprocedural management
As previously described, since the beginning of our experience, we have adopted a simplified approach [12, 13] as the default strategy for TAVI procedures. General anaesthesia was used in 18% of our cohort, in the following conditions:
- Elective cases requiring surgical access – subclavian approach or femoral cut-down
- Patients in a critical preoperative state, intubated in the ICU
- In emergency situations, such as flash pulmonary oedema, acute agitation or patient discomfort
Outcome
LoS was defined as the number of in-hospital days post-TAVI during the index hospitalisation. The day of the procedure was considered as day zero. Discharge was considered as the time the patient left hospital from the index hospitalisation, either to return to their own home (or that of a family member) or to go to a rehabilitation facility or other (sheltered accommodation, nursing home, or other hospital).
Patients were judged eligible for discharge once all of the following criteria were met, as assessed by the operator:
- TTE, puncture sites and blood results (haemoglobin, C-reactive protein [CRP] and renal function) satisfactory.
- Vital signs normal, New York Heart Association (NYHA) grade ≤2 and no oxygen therapy.
- Stable PR and QRS intervals on the ECG.
- Mobilisation adequate to return home.
Data collection
All data were collected prospectively and entered into our local database (from 2009) and the national registry Swiss TAVI (from 2012), with independent adjudication of all events. Complications were categorised as defined by the Valve Academic Research Consortium 2 criteria [15].
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) unless indicated otherwise. LoS was treated as a continuous variable (length of stay in days). In the first part of the analysis, univariate associations between the dependent variable (LoS) and selected clinical, laboratory and echocardiographic variables were identified by the use of the Pearson correlation and/or simple linear regression coefficients. In the case of categorical variables, group comparisons in terms of LoS were made with the use of the student t-test or after analysis of variance (ANOVA, in the case of ≥3 levels). The interaction between patients’ destination and living status was evaluated by the use of Chi-Square. Stepwise multiple linear regression analysis was performed to assess the independent effect of the potential baseline or periprocedural parameters on the LoS in days used as a continuous variable. All univariate variables identified by the correlation coefficients and/or group comparisons were included in the multivariate model. Finally, two models were constructed. In model A, independent variables tested: admission type, history of peripheral artery disease, gait speed test, serum albumin levels, forced expiratory volume in 1 second (FEV1), CRP, haemoglobin, mean aortic pressure gradient and type of TAVI access. Model B: Model A covariates, presence of any complication and patients’ destination at discharge. Significance was assumed at a level of P < 0.05. The cutoff value to define standard versus prolonged LoS was derived from the median LoS. All analyses were performed using the SPSS package version 13.0 (Chicago, Illinois, USA).
Results
Study population
From August 2008 to December 2017, 304 consecutive patients underwent a transfemoral (n = 298, 98%) or subclavian (n = 6, 2%) TAVI procedure at our institution with either a Medtronic Corevalve (n = 153, 50.3%), an Evolut R (n = 145, 47.3%) or an Evolut PRO THV (n = 6, 2%). Forty-five (14.8%) patients also underwent a concomitant procedure. The mean LoS was 10.4 ± 7.1 days (25th – 75th percentile: 5–12 days) with a minimum of 2 days and a maximum of 39 days (procedure in 2008). The median length of stay was 8 days; we used this value as the cutoff to define standard or prolonged LoS.
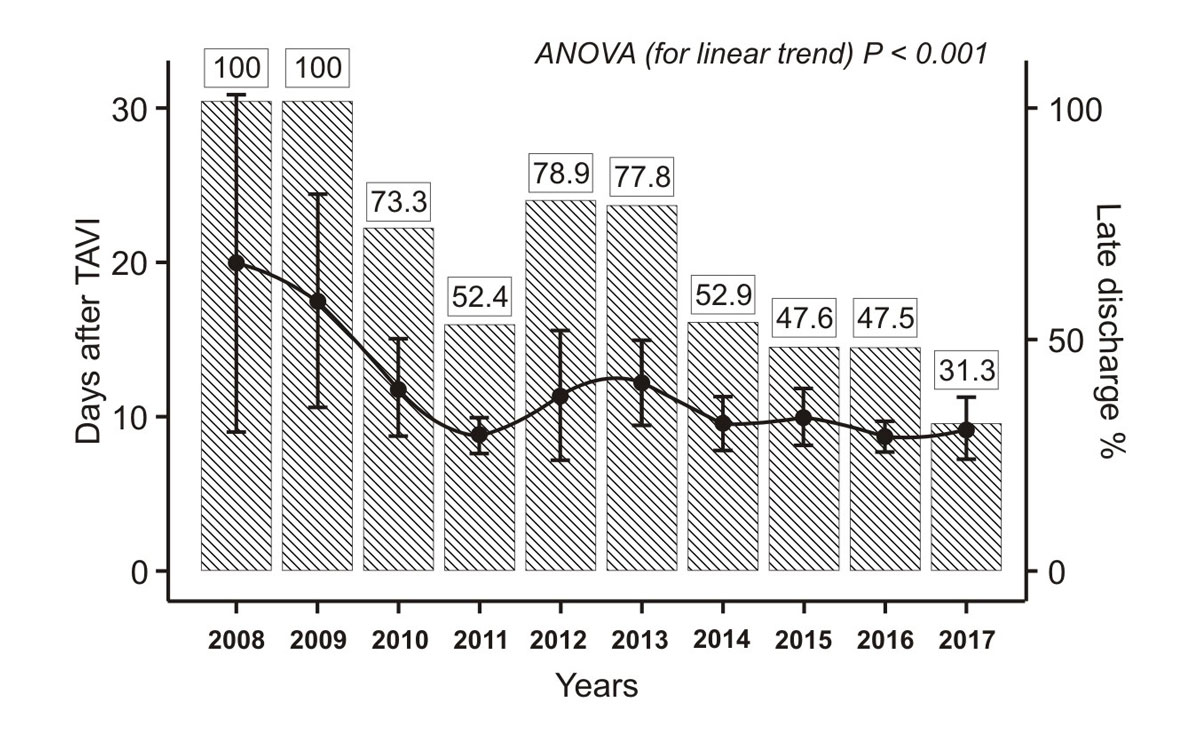
Figure 2 presents the mean LoS after TAVI by year, from 2008 to 2017. A significant time effect was noted (p for linear trend <0.001) with a progressive decrease in mean LoS from 20 ± 13.4 days in 2008 to 9.2 ± 7.3 days in 2017.
Discharge destination
A total of 139 (45.7%) patients were discharged directly home from hospital and 152 (50%) went to a rehabilitation facility. The remaining 13 (4.3%) returned to sheltered accommodation or the nursing home where they previously resided, or to the referring hospital. The vast majority of patients (93.7%) were living in their own home before the intervention (52.1% alone and 41.6% accompanied by a family member) and only a small minority were in another type of accommodation such as a nursing home (fig. 3). Patients living at home alone were more frequently referred to a rehabilitation clinic after the procedure (fig. 3 panel B, p <0.001). Referral to a rehabilitation clinic or other destination at discharge was also associated with prolonged hospitalisation (fig. 3 panel A, p = 0.007). The 30-day readmission rate was 12.5%. Cardiovascular causes (heart failure, new pacemaker implantation or atrial fibrillation) accounted for 47.3% of readmissions (fig. 3).

Figure 3 Length of hospital stay and patients’ destination after the intervention (panel A). Patient destination according to the baseline living status after the TAVI (panel B).
Los = length of stay; TAVI = transcatheter aortic valve replacement
* Other clinic/hospital
Baseline frailty and preoperative risk assessment
The baseline characteristics of the study population and the frailty parameters measured in the initial baseline assessment are presented in table 1.
Table 1 Baseline characteristics of the study population.
|
Characteristics
|
Mean ± SD
or %
|
| Age (years) |
83 ± 6.5 |
| Males (%) |
43 |
| BMI (Kg/m2) |
26.1 ± 4.9 |
| Active smokers (%) |
5.9 |
| Arterial hypertension (%) |
83.6 |
| Diabetes (%) |
29.6 |
| Dyslipidaemia (%) |
67.8 |
| History of atrial fibrillation (%) |
36.2 |
| History of PAD (%) |
12.2 |
| History of COPD (%) |
19.4 |
| History of stroke/TIA (%) |
10.9 |
| History of CAD (%) |
55.9 |
| FEV1 (L) |
1.6 ± 0.55 |
| Gait speed test (sec) |
6.9 ± 2.7 |
| Grip strength (kg) |
20.1 ± 8.2 |
| Poor mobility (%) |
10.2 |
| Recent falls (%) |
30.6 |
| Recent unintentional weight loss (%) |
24.3 |
| Emergency admission to hospital (%) |
16 |
| Logistic EuroSCORE I (%) |
20 ± 14.6 |
| Linear EuroSCORE (pts) |
9.9 ± 2.5 |
| Logistic EuroSCORE II (%) |
6.7 ± 6.6 |
| STS PROM score (%) |
6.4 ± 3.9 |
| MMSE score (pts) |
26.8 ± 3.2 |
| Peak aortic valve velocity (m/sec) |
4.1 ± 0.7 |
| Mean pressure gradient (mm Hg) |
40.8 ± 14.8 |
| Ejection fraction (%) |
55 ± 12.7 |
| Aortic valve area (cm2) |
0.7 ± 0.2 |
| CRP (mg/l) |
8.7 ± 14.1 |
| Haemoglobin (g/l) |
118 ± 15.9 |
| Serum albumin (g/l) |
37.6 ± 5.6 |
A significant association was noted between LoS, gait speed (Pearson r = +0.203, p = 0.004) and serum albumin levels (Pearson r = −0.195, p = 0.004, table 2). No associations between the preoperative risk assessment scores and LoS were noted with the exception of the STS PROM score (Pearson r = +0.129, p = 0.026, table 2).
Table 2 Univariate correlations and multiple linear regression analysis for hospital length of stay.
|
Univariate
|
Pearson r
|
Beta
|
SE
|
p-value
|
| CRP |
+0.214 |
+0.096 |
0.027 |
<0.001 |
| Gait speed test |
+0.203 |
+0.46 |
0.16 |
0.004 |
| Albumin |
−0.195 |
−0.22 |
0.08 |
0.004 |
| FEV1
|
−0.168 |
−2.207 |
0.81 |
0.007 |
| Haemoglobin |
−0.151 |
−0.067 |
0.025 |
0.009 |
| Mean aortic pressure gradient |
+0.167 |
+0.081 |
0.027 |
0.004 |
|
Multivariate
|
–
|
Beta
|
SE
|
p-value
|
| Emergency admission |
– |
3.24 |
1.56 |
0.039 |
| Subclavian access |
– |
8.27 |
2.9 |
0.005 |
| CRP |
– |
0.14*
|
0.04 |
0.001 |
| Gait speed test |
– |
0.39†
|
0.16 |
0.018 |
Comorbidities and baseline clinical presentation
No associations were noted between baseline clinical symptoms and LoS. As can be expected, emergency admission to hospital was found to be associated with prolonged hospitalisation after TAVI (14.6 ± 11 vs 9.5 ± 5.7 days, p <0.001). Moreover, history of peripheral artery disease (12.7 ± 5.9 vs 10 ± 7.2 days, p = 0.036) and history of chronic obstructive pulmonary disease (12.1 ± 8.5 vs 9.8 ± 6.7 days, p = 0.03) were found to be associated with longer hospitalisation. In accordance, baseline FEV1 was strongly related to LoS (Pearson r = -0.168, p = 0.007, table 2).
Preprocedural echocardiographic and laboratory evaluation
Baseline mean aortic pressure gradient (Pearson r = +0.167, p = 004), maximum aortic pressure gradient (Pearson r = +0.143, p = 0.014) and maximum aortic velocity (Pearson r = +0.130, p = 0.024) were associated with LoS. Moreover, baseline haemoglobin (Pearson r = −0.151, p = 0.009) and baseline CRP (Pearson r = +0.214, p <0.001) were strongly related to the LoS (table 2).
Periprocedural complications
Subclavian access was associated with a prolonged LoS (median 13.5, range 7–23 days vs median 8, range 2–54 days, p = 0.026) (table 3). All periprocedural complications and the need for blood transfusion were associated with prolonged LoS.
Table 3 Periprocedural complications and hospital length of stay.
|
LoS (days)
|
p-value
|
Beta*
|
SE
|
Complication (+)
mean ± SD
|
Complication (−)
mean ± SD
|
| Pacemaker implantation |
12.3 ± 8.7 |
9.8 ± 6.5 |
0.013 |
2.46 |
0.98 |
| Stroke |
17.9 ± 8.7 |
10 ± 6.9 |
<0.001 |
7.84 |
2.14 |
| Acute kidney injury |
19.4 ± 13 |
9.5 ± 5.7 |
<0.001 |
9.82 |
1.37 |
| Bleeding |
13.6 ± 10.1 |
9.7 ± 6.2 |
<0.001 |
3.94 |
1.08 |
| Vascular complication |
12.6 ± 8.8 |
9.9 ± 6.7 |
0.019 |
2.69 |
1.11 |
| Transfusion |
14.5 ± 11.3 |
9.4 ± 5.3 |
<0.001 |
5.02 |
1.14 |
| Any complication |
12.4 ± 8.9 |
8.6 ± 4.6 |
<0.001 |
3.7 |
0.79 |
Multiple regression model
In stepwise multiple regression analysis, emergency hospital admission, subclavian access, gait speed and CRP remained significant independent determinants of LoS after TAVI (table 2). The independent association between gait speed, CRP levels and LoS remained significant even after adjustment for the presence of any periprocedural complication and patients’ discharge destination (beta 0.41 ± 0.16 and 0.16 ± 0.04, respectively, p <0.05).
Discussion
Our single centre study evaluated the causes of prolonged hospitalisation after TAVI, the potential determinants of LoS from baseline parameters, and the impact of frailty and periprocedural variables on LoS. The major findings of our study can be summarised as follows:
- Patients with a slower baseline gait speed, lower serum albumin level, higher STS PROM score and those admitted as emergencies or with a history of chronic obstructive pulmonary disease were more likely to experience a prolonged LoS.
- Higher baseline aortic valve measurements of mean gradient, maximum gradient and maximum velocity were associated with a longer LoS.
- Subclavian access, all periprocedural complications and blood transfusion were associated with a longer LoS.
- Patients who were discharged to a rehabilitation facility or a destination other than home were more likely to experience a prolonged hospital stay.
- Independent determinants of prolonged LoS after TAVI were emergency hospital admission, subclavian approach, slow gait speed (1 second represented 0.4 days longer in hospital post TAVI), and higher serum CRP level. This remained significant even after statistical adjustment for periprocedural complications and patient’s discharge destination.
From the results of our study we can see that the factors influencing discharge timing are multiple, and in order to achieve earlier discharge, we need to address each of them. Since previous studies have already highlighted a link between frailty and mortality after TAVI [16–18] unsurprisingly our results suggest that the more frail a patient is the more likely they are to experience a prolonged hospitalisation. Slow gait speed at baseline was an independent determinant of LoS in our analysis. As this association was independent of periprocedural complications and the patients’ discharge destination, it would seem that frail patients do not stay hospitalised longer because they experience more complications or because they are more frequently referred to a rehabilitation facility, but because these patients simply require more time to achieve our discharge criteria.
Similarly, it seems that patients with a higher serum CRP level at baseline are more likely to remain hospitalised longer after TAVI, but that these patients do not experience a higher periprocedural complication rate. These results reflect those that have already been observed in surgical patients, where higher CRP levels have been associated with long-term mortality and extended hospital length of stay after coronary bypass surgery [19]. However, we cannot exclude the possibility that these patients have a pre-existing, subclinical infection, which could be responsible for a longer postintervention recuperation time and normalisation of blood analysis to achieve our discharge criteria.
In Switzerland, for TAVI performed between 2011 and 2015, the mean LoS was 10.1 ± 6.0 days (Swiss TAVI registry [9]), which is comparable to earlier European registries, such as the Italian CoreValve registry (June 2007 to December 2012), in which the median LoS for patients was 8 and 7 days for procedures performed under general and local anaesthesia, respectively [20]. The Pilot European Sentinel TAVI Registry, which included patients from nine European countries and Israel who were implanted with the Sapiens XT and CoreValve between January 2011 and May 2012, reported a wide variation between countries of LoS after TAVI, ranging from a mean of 5.8 days in Israel to 12.6 days in Poland [21]. However, recent North American studies reported greatly reduced LoS. In the Vancouver TAVI clinical pathway study from 2012 to 2014, early discharge (≤48 hours) was achieved in 38.2% of patients [22] and in 2019 the 3M TAVR study reported a rate of 80.1% for next day discharge [23]. Nevertheless, in the SOLVE trial, recently presented at the Transcatheter Cardiovascular Therapeutics (TCT) meeting 2018, the mean length of stay in this German study randomising the latest valve generation from Medtronic (Evolut R) and Edwards Lifesciences (SAPIEN 3) was 9 ± 7 days [24]. This demonstrates that fast-tracking is not routinely practiced in all centres.
At our centre, a short stay at a rehabilitation facility is organised for selected patients at discharge (e.g., patients living alone or without family support, patients who have complications or those more severely decompensated before the intervention). A negative repercussion of this practice is that patients often remain hospitalised while waiting for an available place in an appropriate facility. The discharge destination may influence the waiting time; however, as our registry was not specifically designed around this question, we do not have the detailed information on which facilities/destinations cause the greatest delay. Although some patients can benefit from an in-patient rehabilitation programme, a considerable proportion of patients are admitted to such facilities because of cultural expectations. Interestingly, in the multicentre European FAST-TAVI, the most common reason for prolonged hospitalisation (>72 h) was logistic reasons in 34.8% [25]. The other reasons were conduction disturbances (25.9%), bleeding (16.3%), mobilisation (14.8%), renal (10.4%), vascular (9.6%) and other (27.4%) [25]. Therefore an important message is the necessity to prepare the discharge as soon as possible involving the family and assessing the patient’s home environment to minimise prolonged LoS for logistical reasons.
The potentially detrimental effect of hospitalisation on the health of elderly and/or frail patients has already been highlighted in previous studies [26, 27]. Our study showed that patients who return home directly from hospital are discharged more rapidly than those who are referred to a rehabilitation facility.
In line with current literature, patients in our cohort who experienced periprocedural complications also experienced a prolonged LoS. TAVI complicated by acute kidney injury was seen to have the longest LoS in our cohort, followed by stroke and finally blood transfusion. Procedures performed via a subclavian approach also resulted in longer LoS, probably due to extra in-hospital days required for wound care of the surgical incision used for access.
Emergency admission was also a factor strongly related to prolonged LoS. This association is probably due to patients often arriving at hospital in cardiac failure and therefore generally sicker prior to the intervention than those who are admitted electively, therefore requiring a longer hospital stay for medical treatment.
Strengths and limitations
The first strength of this study is that the cohort was homogenous with consecutive patients, no exclusion criteria, no patients lost to follow-up at 30 days and prospective data collection at baseline and during follow-up. Secondly, for the whole of the study period, the same TAVI operators (SN and MR) performed all the TAVI procedures, and from 2012 the same trained TAVI nurse (AF) performed all frailty assessments.
The results of the present study need to be interpreted with the following limitations in mind: this is a single centre observational study, so structures and processes may be different in other settings. As LoS is not only determined by medical criteria, but also by local resources and the healthcare system, the generalisability of our study may be limited, and centre specific studies should be encouraged. Also, further studies would need to be performed to verify our findings and the statistical power of our analysis may be limited due to the number of factors analysed within a relatively small cohort.
Conclusion
Slow gait speed, emergency hospital admission and a raised serum CRP at baseline appear to be independent determinants of LoS after TAVI. A subclavian access seems to favour longer hospital stay. Discharge directly home after TAVI is strongly associated with a shorter LoS. In order to reduce LoS after TAVI it is essential to target the specific needs of this patient population. Considering there is often a time lapse of several weeks between a patient’s assessment for TAVI and the procedure itself, there exists a window to improve certain frailty parameters prior to the intervention, such as patients’ nutritional status. Therefore nutritionists should be involved in patients’ initial assessment. Additionally, specialised TAVI nurses can contribute to increase the rate of patients discharged directly home avoiding unnecessary delays caused by waiting lists for places in rehabilitation.
Author contributions
AF and DA contributed equally to authorship of the article.
References
1
Baumgartner
H
,
Falk
V
,
Bax
JJ
,
De Bonis
M
,
Hamm
C
,
Holm
PJ
, et al.; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739–91. doi:.https://doi.org/10.1093/eurheartj/ehx391
2
Nishimura
RA
,
Otto
CM
,
Bonow
RO
,
Carabello
BA
,
Erwin
JP, 3rd
,
Fleisher
LA
, et al.
2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159–95. doi:.https://doi.org/10.1161/CIR.0000000000000503
3
Durand
E
,
Borz
B
,
Godin
M
,
Tron
C
,
Litzler
PY
,
Bessou
JP
, et al.
Transfemoral aortic valve replacement with the Edwards SAPIEN and Edwards SAPIEN XT prosthesis using exclusively local anesthesia and fluoroscopic guidance: feasibility and 30-day outcomes. JACC Cardiovasc Interv. 2012;5(5):461–7. doi:.https://doi.org/10.1016/j.jcin.2012.01.018
4
Greif
M
,
Lange
P
,
Näbauer
M
,
Schwarz
F
,
Becker
C
,
Schmitz
C
, et al.
Transcutaneous aortic valve replacement with the Edwards SAPIEN XT and Medtronic CoreValve prosthesis under fluoroscopic guidance and local anaesthesia only. Heart. 2014;100(9):691–5. doi:.https://doi.org/10.1136/heartjnl-2013-304918
5
Durand
E
,
Eltchaninoff
H
,
Canville
A
,
Bouhzam
N
,
Godin
M
,
Tron
C
, et al.
Feasibility and safety of early discharge after transfemoral transcatheter aortic valve implantation with the Edwards SAPIEN-XT prosthesis. Am J Cardiol. 2015;115(8):1116–22. doi:.https://doi.org/10.1016/j.amjcard.2015.01.546
6
Barbanti
M
,
Capranzano
P
,
Ohno
Y
,
Attizzani
GF
,
Gulino
S
,
Immè
S
, et al.
Early discharge after transfemoral transcatheter aortic valve implantation. Heart. 2015;101(18):1485–90. doi:.https://doi.org/10.1136/heartjnl-2014-307351
7
Mallikethi-Reddy
S
,
Akintoye
E
,
Telila
T
,
Sudhakar
R
,
Jagadeesh
K
,
Briasoulis
A
, et al.
Transcatheter aortic valve implantation in the United States: Predictors of early hospital discharge. J Interv Cardiol. 2017;30(2):149–55. doi:.https://doi.org/10.1111/joic.12373
8
Reynolds
MR
,
Lei
Y
,
Wang
K
,
Chinnakondepalli
K
,
Vilain
KA
,
Magnuson
EA
, et al.; CoreValve US High Risk Pivotal Trial Investigators. Cost-Effectiveness of Transcatheter Aortic Valve Replacement With a Self-Expanding Prosthesis Versus Surgical Aortic Valve Replacement. J Am Coll Cardiol. 2016;67(1):29–38. doi:.https://doi.org/10.1016/j.jacc.2015.10.046
9
Stortecky
S
,
Franzone
A
,
Heg
D
,
Tueller
D
,
Noble
S
,
Pilgrim
T
, et al.
Temporal Trends in Adoption and Outcomes of Transcatheter Aortic Valve Implantation: A Swisstavi Registry Analysis. Eur Heart J Qual Care Clin Outcomes. 2018:qcy048. doi:.https://doi.org/10.1093/ehjqcco/qcy048
10
Afilalo
J
,
Eisenberg
MJ
,
Morin
JF
,
Bergman
H
,
Monette
J
,
Noiseux
N
, et al.
Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56(20):1668–76. doi:.https://doi.org/10.1016/j.jacc.2010.06.039
11
Mathiowetz
V
,
Kashman
N
,
Volland
G
,
Weber
K
,
Dowe
M
,
Rogers
S
. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66(2):69–74.
12
Perrin
N
,
Roffi
M
,
Frei
A
,
Hachulla
AL
,
Ellenberger
C
,
Müller
H
, et al.
Thirty-day Outcome Following CoreValve Evolut R Transcatheter Aortic Valve Implantation: An All-comers Prospective Study. Rev Esp Cardiol (Engl Ed). 2017;70(9):713–9. doi:.https://doi.org/10.1016/j.rec.2016.11.024
13
Perrin
N
,
Ellenberger
C
,
Licker
M
,
Hachulla
AL
,
Cikirikcioglu
M
,
Frei
A
, et al.
Management of vascular complications following transcatheter aortic valve implantation. Arch Cardiovasc Dis. 2015;108(10):491–501. doi:.https://doi.org/10.1016/j.acvd.2015.03.007
14
Noble
S
,
Roffi
M
,
Burri
H
. Use of an explanted pacemaker connected to a regular screw-in lead for temporary pacing. Rev Esp Cardiol (Engl Ed). 2011;64(12):1229–30. doi:.https://doi.org/10.1016/j.recesp.2011.01.004
15
Kappetein
AP
,
Head
SJ
,
Généreux
P
,
Piazza
N
,
van Mieghem
NM
,
Blackstone
EH
, et al.
Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33(19):2403–18. doi:.https://doi.org/10.1093/eurheartj/ehs255
16
Kleczynski
P
,
Dziewierz
A
,
Bagienski
M
,
Rzeszutko
L
,
Sorysz
D
,
Trebacz
J
, et al.
Impact of frailty on mortality after transcatheter aortic valve implantation. Am Heart J. 2017;185:52–8. doi:.https://doi.org/10.1016/j.ahj.2016.12.005
17
Green
P
,
Woglom
AE
,
Genereux
P
,
Daneault
B
,
Paradis
JM
,
Schnell
S
, et al.
The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5(9):974–81. doi:.https://doi.org/10.1016/j.jcin.2012.06.011
18
Afilalo
J
,
Lauck
S
,
Kim
DH
,
Lefèvre
T
,
Piazza
N
,
Lachapelle
K
, et al.
Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J Am Coll Cardiol. 2017;70(6):689–700. doi:.https://doi.org/10.1016/j.jacc.2017.06.024
19
Perry
TE
,
Muehlschlegel
JD
,
Liu
KY
,
Fox
AA
,
Collard
CD
,
Body
SC
, et al.; CABG Genomics Investigators. Preoperative C-reactive protein predicts long-term mortality and hospital length of stay after primary, nonemergent coronary artery bypass grafting. Anesthesiology. 2010;112(3):607–13. doi:.https://doi.org/10.1097/ALN.0b013e3181cea3b5
20
Petronio
AS
,
Giannini
C
,
De Carlo
M
,
Bedogni
F
,
Colombo
A
,
Tamburino
C
, et al.
Anaesthetic management of transcatheter aortic valve implantation: results from the Italian CoreValve registry. EuroIntervention. 2016;12(3):381–8. doi:.https://doi.org/10.4244/EIJY15M03_05
21
Di Mario
C
,
Eltchaninoff
H
,
Moat
N
,
Goicolea
J
,
Ussia
GP
,
Kala
P
, et al.; Transcatheter Valve Treatment Sentinel Registry (TCVT) Investigators of the EURObservational Research Programme (EORP) of the European Society of Cardiology. The 2011-12 pilot European Sentinel Registry of Transcatheter Aortic Valve Implantation: in-hospital results in 4,571 patients. EuroIntervention. 2013;8(12):1362–71. doi:.https://doi.org/10.4244/EIJV8I12A209
22
Lauck
SB
,
Wood
DA
,
Baumbusch
J
,
Kwon
JY
,
Stub
D
,
Achtem
L
, et al.
Vancouver Transcatheter Aortic Valve Replacement Clinical Pathway: Minimalist Approach, Standardized Care, and Discharge Criteria to Reduce Length of Stay. Circ Cardiovasc Qual Outcomes. 2016;9(3):312–21. doi:.https://doi.org/10.1161/CIRCOUTCOMES.115.002541
23
Wood
DA
,
Lauck
SB
,
Cairns
JA
,
Humphries
KH
,
Cook
R
,
Welsh
R
, et al.
The Vancouver 3M (Multidisciplinary, Multimodality, But Minimalist) Clinical Pathway Facilitates Safe Next-Day Discharge Home at Low-, Medium-, and High-Volume Transfemoral Transcatheter Aortic Valve Replacement Centers: The 3M TAVR Study. JACC Cardiovasc Interv. 2019;12(5):459–69. doi:.https://doi.org/10.1016/j.jcin.2018.12.020
24Thiele H. SOLVE-TAVI: A 2x2 Randomized Trial of Self-Expandable vs Balloon-Expandable Valves and General vs Local Anesthesia in Patients Undergoing Transcatheter Aortic Valve Implantation. Transcatheter Cardiovascular Therapeutics; 2018 September 21–25; San Diego, CA.
25
Barbanti
M
,
van Mourik
MS
,
Spence
MS
,
Icovelli
F
,
Martinelli
GL
,
Muir
DF
, et al.
Optimizing Patient Discharge Management after Transfemoral Transcatheter Aortic Valve Implantation: The Multicentre European FAST-TAVI Trial. EuroIntervention. 2019. [Epub ahead of print] doi:.https://doi.org/10.4244/EIJ-D-18-01197
26
Lefevre
F
,
Feinglass
J
,
Potts
S
,
Soglin
L
,
Yarnold
P
,
Martin
GJ
, et al.
Iatrogenic complications in high-risk, elderly patients. Arch Intern Med. 1992;152(10):2074–80. doi:.https://doi.org/10.1001/archinte.1992.00400220090016
27
George
AJ
,
Boehme
AK
,
Siegler
JE
,
Monlezun
D
,
Fowler
BD
,
Shaban
A
, et al.
Hospital-Acquired Infection Underlies Poor Functional Outcome in Patients with Prolonged Length of Stay. ISRN Stroke. 2013;2013:312348. doi:.https://doi.org/10.1155/2013/312348