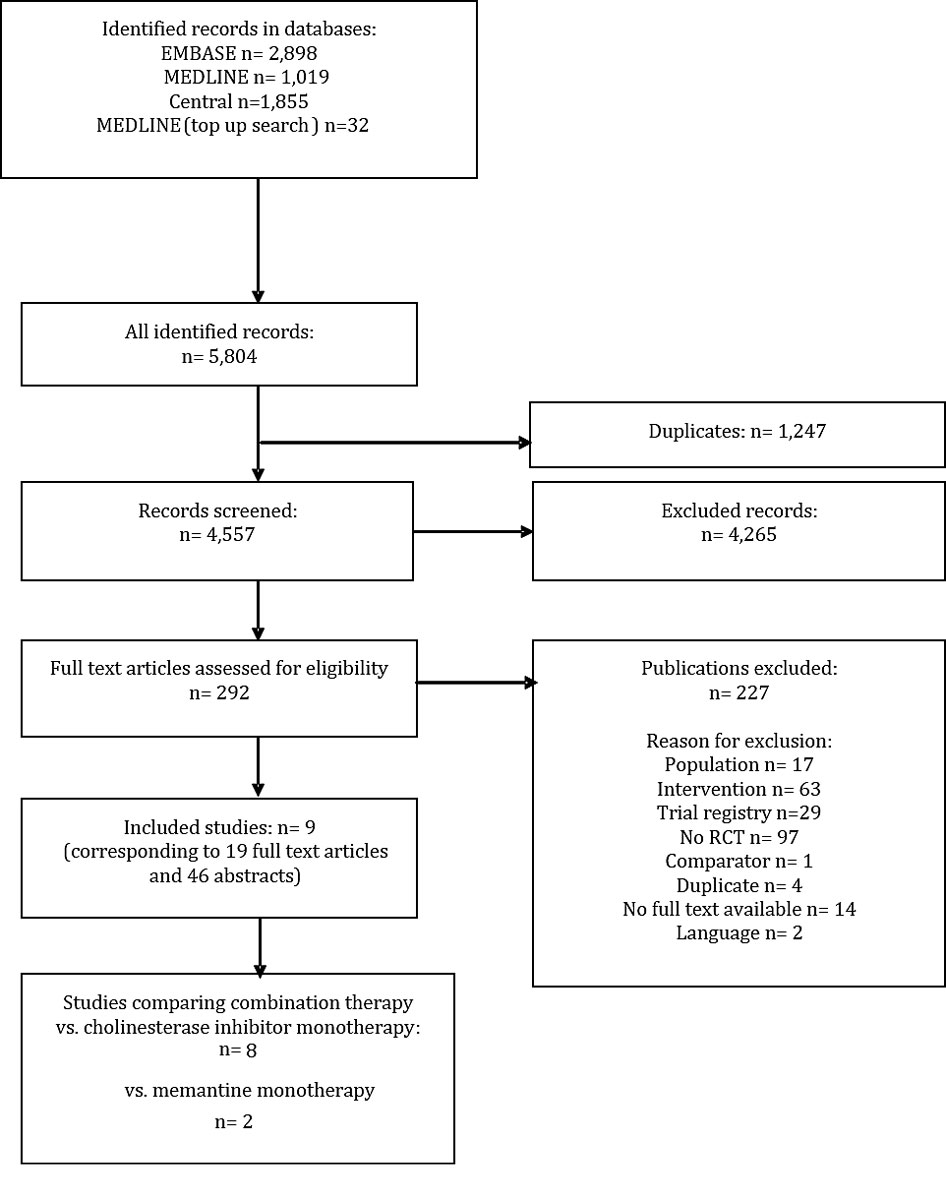
Figure 1 Article identification, inclusion and exclusion.
DOI: https://doi.org/10.4414/smw.2019.20093
Alzheimer's Association and the National Institute on Ageing
acetylcholinesterase inhibitor
confidence intervals
Alzheimer’s Disease Assessment Scale, cognitive subscale
Diagnostic and Statistical Manual of Mental Disorders
European Medicines Agency
Food and Drug administration
Grading of Recommendations Assessment, Development and Evaluation
International Classification of Diseases
individual patient data
last observation carried forward
mean difference
Mini-Mental State Examination
National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association
Neuropsychiatric Inventory
quality of evidence
randomised controlled trials
relative risk ratio
Swiss Medical Board
standardised mean difference
United States
World Health Organization
Alzheimer’s disease is a serious neurocognitive disorder characterised by a progressive decline of cognitive functions and memory (disturbances in attention, executive functions, learning and memory, speech, perceptual-motor ability and social cognition) [1, 2]. The resulting disabilities can limit independent daily living [2]. Alzheimer’s disease is the most common form of dementia, which affects millions of elderly people worldwide and is classified as a public health priority by the WHO [3].
Pharmaceutical therapy of Alzheimer’s disease typically includes acetylcholinesterase inhibitors (AChEIs), which inhibit chemical neurotransmitter breakdown. Cochrane reviews have reported that AChEIs may reduce disease progression, i.e. delay the decline in cognition and activities of daily living, but also cause adverse events, most frequently mild, such as diarrhoea, nausea or vomiting [4–6]. The N-methyl-D-aspartate receptor antagonist memantine is an alternative pharmaceutical treatment option and has been reported to delay Alzheimer’s disease progression with side effects similar to those of a placebo [7].
AChEIs are approved for dementia irrespective of the severity, but are commonly administered to patients with mild to moderate Alzheimer’s disease (Mini-Mental State Examination, MMSE score of around 26 to 10), while memantine is approved for moderate to severe dementia (MMSE score ≤19). Combination therapy is frequently used in Europe, the United States (US) and Switzerland in patients with moderate to severe Alzheimer's disease (MMSE score 19 to 10), especially if patient cognition deteriorates. In contrast to the practice in other countries, the Swiss mandatory basic health insurance does not cover the combination therapy [8]. This means that in Swiss clinical practice both substances can be prescribed in combination, but only one drug will be reimbursed and the other is paid for by the patient. Memantine is often added to an ongoing AChEI therapy with donepezil, galantamine or rivastigmine. Fixed combination products for combined therapy of AChEI and memantine have been approved by the US Food and Drug Administration (FDA), but not by the European Medicines Agency (EMA) [9, 10] and Switzerland. Previous systematic reviews comparing the efficacy of combination therapy with monotherapy overlooked a number of relevant studies [11], included studies of mildly severe dementia [12], for which memantine is not approved, or confined their assessment by using a selective comparator regimen, i.e. donepezil [13]. The objective of the present systematic review and meta-analysis was to comprehensively assess the clinical efficacy and safety of all combination therapy modalities compared to monotherapy with AChEI or memantine in patients with moderate to severe Alzheimer’s disease. The present systematic review stems from the clinical effectiveness section of a Health Technology Assessment report commissioned to us by the Swiss Medical Board (SMB) [14]. For the present report, we updated the literature search and aimed to add newly published studies.
We registered the protocol of this systematic review with PROSPERO (CRD42018079508). We searched MEDLINE and EMBASE (via OvidSP), and the Cochrane central register of controlled trials (Central) from inception until 5 February 2018. We conducted a top-up search via PubMed to identify not yet indexed publications in MEDLINE. The search strategy (see appendix, supplement 1) combined search terms for dementia and memantine, but did not contain terms for AChEI; these would have only complicated the search strategy without adding relevant articles, as combination therapy with memantine was the intervention of interest. We used the optimised randomised controlled trial (RCT) filter with regard to sensitivity and specificity in EMBASE [15], and the sensitivity- and precision-maximising filter in MEDLINE [16]. After removal of duplicate publications, two reviewers independently screened the titles and abstracts of the identified records for potentially eligible studies. Discrepant screening results were discussed and resolved by consensus. Subsequently, two reviewers independently screened the full text articles of the potentially relevant studies for eligible RCTs. Discrepant screening results were discussed and resolved by consensus or third-party arbitration.
Our inclusion criteria for relevant studies were:
Since we did not expect patients to be AChEI treatment naïve, we set no restriction regarding precedent or ongoing AChEI or memantine treatment.
We predefined outcomes with clinical experts (AUM and RWK) and categorised them as critical or important according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) [17]. Those outcomes we thought most relevant for decision-making we classified as “critical”, and the lowest quality of the evidence available for any of these outcomes is the basis for judging the overall quality of the evidence [18]. We pre-defined cognition, activities of daily living, clinical global impression, behavioural and psychological symptoms of dementia and delay of nursing home placement (only relevant to studies starting in an ambulatory setting) as critical outcomes. Withdrawal from the study, adverse events, caregiver burden or distress and quality of life were classified as important outcomes [17]. One reviewer extracted the data on study characteristics and outcomes into a standardised, pilot-tested form, a second reviewer checked it. Discrepancies were resolved by discussion or third-party arbitration. Continuous outcome data were extracted as means and standard deviations for each treatment group at follow-up. If means were not available at follow-up, mean changes were extracted from the baseline. Because outcomes were assessed by different instruments, we ranked them hierarchically and decided which data to extract (see appendix, supplement 2). In addition to the pre-specified protocol registered at PROSPERO (CRD42018079508), we also extracted information on responder analyses of the individual RCTs. Outcome data were summarised quantitatively in a meta-analysis by using inverse variance weighting models assuming random effects [19]. The analyses were performed using Review Manager (Version 5.3.5). Two follow-up time points were defined based on expert opinions (AUM and RWK); hence, meta-analyses were done for short-term (closest to 6 months) and long-term follow-up (at 9 months or the latest available follow-up). In case of continuous data, mean treatment group differences were pooled, either on the original scale or by using standardised mean differences (SMD) [20, 21].
One reviewer assessed the internal validity (risk of bias) [21] of each trial and the quality of evidence (QoE) [22] of every effect estimate for each outcome, which was then checked by a second reviewer. Discrepancies were resolved by discussion or third-party arbitration. The quality of evidence was judged according to GRADE [22].
We identified nine eligible RCTs (fig. 1, table 1). Seven RCTs assessed combination therapy versus AChEI monotherapy (Araki 2014 [23], EXPECT 2011 [26, 27], Grossberg 2013 [28], Herrmann 2013 [29], Porsteinsson 2008 [30], Tariot 2004 [31–35] and Wilkinson 2012 [37]), one RCT compared combination therapy versus memantine monotherapy (Shao 2015 [36]) and one four-armed RCT assessed combination therapy with both monotherapies with a two-by-two factorial study design (DOMINO-AD 2012 [24, 25]). Since DOMINO-AD 2012 unblinded patients and physicians after 52 weeks, we did not include later follow-up time-points.

Figure 1 Article identification, inclusion and exclusion.
Table 1 Overview of included studies.
| Study ID [ref.] | Country (setting) | MMSE range | Number randomised | Intervention vs comparator | Follow-up extracted (weeks) |
|---|---|---|---|---|---|
| Araki 2014 [23] | Japan (ambulatory, single centre) | Not reported | 37 | Memantine + donepezil vs donepezil | 24 |
| DOMINO-AD 2012 [24, 25] | UK (ambulatory, multicentre) | 5–13 | 146 | Memantine + donepezil vs donepezil + placebo | 30, 52 150 |
| EXPECT 2011 [26, 27] | South Korea (ambulatory, multicentre) | 10–20 | 172 | Memantine + transdermal rivastigmine vs transdermal rivastigmine | 16 |
| Grossberg 2013 (MEM-MD-50) [28] | Argentina, USA, Mexico, Chile (ambulatory, multicentre) | 3–14 | 677 | Memantine + AChEI vs AChEI | 24 |
| Herrmann 2013 [29] | Canada (ambulatory, multicentre) | 5-15 | 369 | Memantine + AChEI vs placebo + AChEI | 24 |
| Porsteinsson 2008 (MEM-MD-12) [30] | USA (ambulatory, multicentre) | 10–22 | 433 | Memantine + AChEI vs AChEI | 24 |
| Tariot 2004 (MEM-MD-02) [31–35] | USA (ambulatory, multicentre) | 5–14 | 404 | Memantine + donepezil vs donepezil | 24 |
| Shao 2015 [36] | China (not reported, single centre) | 10–24 | 88 | Memantine + donepezil vs memantine + rivastigmine vs memantine + galantamine vs memantine + placebo |
24 |
| Wilkinson 2012 [37] | France, Germany, Switzerland and UK (ambulatory, multicentre) | 12–20 | 278 | Memantine vs placebo (subgroups were included for the report: memantine + AChEI vs placebo + AChEI) | 52 |
AChEI = acetylcholinesterase inhibitor; MMSE = Mini-Mental State Examination
Most RCTs included a mix of patients with moderate to severe Alzheimer’s disease. Although the EXPECT 2011 and Porsteinsson 2008 trials included patients with mild to moderate Alzheimer’s disease, and the two trials Wilkinson 2012 and Shao 2015 did not report on disease severity, we estimated that at least 80% of patients in these trials had moderate Alzheimer’s disease based on reported baseline MMSE in these trials.
Araki 2014, DOMINO-AD 2012, Herrmann 2013, Grossberg 2013, Porsteinsson 2008 and Tariot 2004 reported that patients were on AChEI therapy for at least 3 months. The EXPECT 2011 trial recruited AChEI treatment-naïve patients whose dosage of the transdermal rivastigmine patch was titrated depending on tolerability until reaching a stable dose prior to randomisation. Wilkinson 2012 did not report on precedent AChEI therapy. The EXPECT 2011 study was the only study to apply a transdermal patch as route of administration. In the two trials comparing combination versus memantine monotherapy, one study (DOMINO-AD 2012) reported a 4-week period during which AChEI was decreased to 0 mg (placebo) and memantine therapy was initiated in parallel with 5-mg weekly increments of memantine. The other study (Shao 2015) reported a one week ‘wash-out phase’ (the authors did not specify of what) before the study treatment initiation.
At short-term follow-up, combination therapy had a significantly better effect than AChEI monotherapy on cognition (SMD 0.20, 95% CI 0.05 to 0.35, I2 = 62%, 7 RCTs, low QoE, fig. 2) and clinical global impression (SMD −0.15, 95% CI −0.28 to −0.01, I2 = 45%, 4 RCTs, moderate QoE, fig. 3). There was no significant difference for activities of daily living (SMD 0.09, 95% CI −0.01 to 0.18, I2 = 0%, 5 RCTs, moderate QoE, fig. 4) or behavioural and psychological symptoms (MD −3.07, 95% CI −6.53 to 0.38, I2 = 85%, 6 RCT, low QoE, fig. 5). Caregiver burden or distress was only reported by Araki 2014 and DOMINO-AD 2012. Araki 2014 reported reduced caregiver burden or distress with combination therapy (Japanese version of the Zarit Burden Interview scale mean scores ±SD: −0.33±10.87 versus 18.23±7.89) and DOMINO-AD 2012 reported a non-significant estimated average difference over all follow-up time points (−0.5, 99% CI −1.3 to 0.3, mixed regression model analysis, up to 52 weeks). Only DOMINO-AD 2012 reported on quality of life (Dementia Quality of Life), and the estimated difference over all follow-up time points was not significantly different (2.0, 99% CI –2.3 to 6.2, mixed regression model analysis, up to 52 weeks). Only Tariot 2004 reported ‘response rates’ for global impression based on CIBIC-plus, a 7-point scale of change. Every change on this scale can be considered to be clinically relevant [38]. At 24 weeks, Tariot 2004 reported no worsening of global impression in 109 out of 198 (55%) patients in the combination therapy and 89 out of 197 (45%) patients in the AChEI monotherapy group. Tariot 20014 did not report response rates for the other outcomes. The other studies did not report response rates, although EXPECT 2011 reported having assessed response rates for all outcomes, but provided no results, and only stated that no differences in response rates were found.
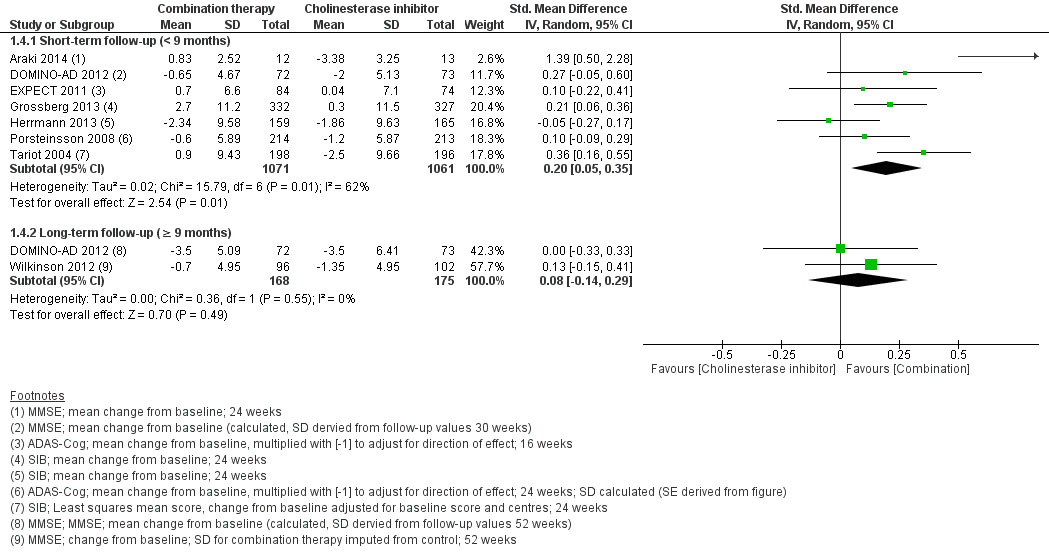
Figure 2 Cognition.
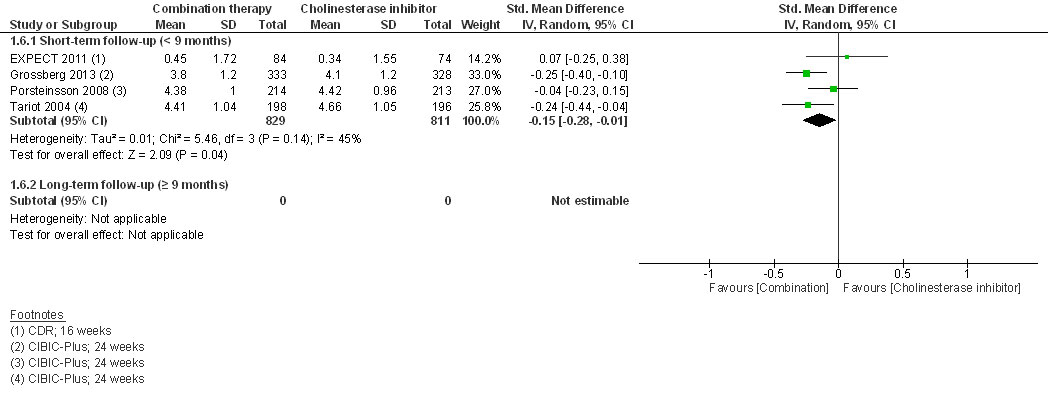
Figure 3 Clinical global impression.
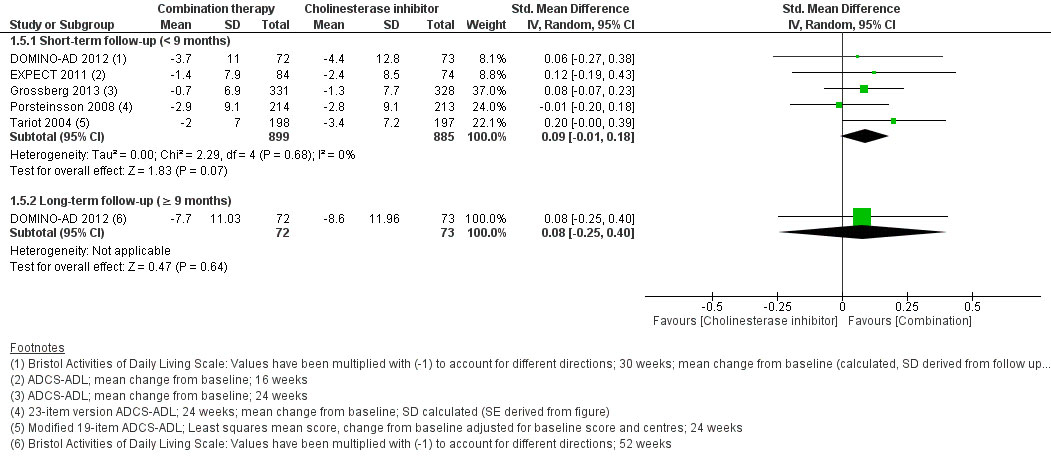
Figure 4 Activities of daily living.
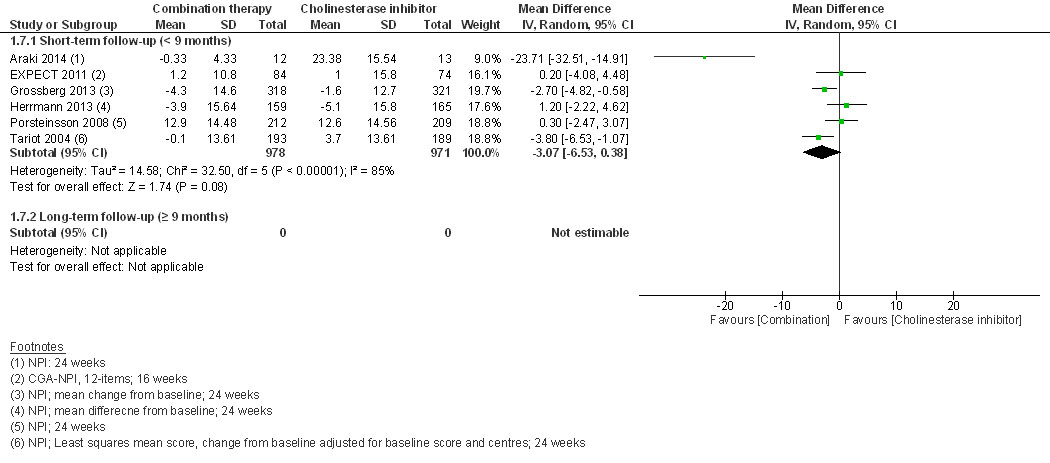
Figure 5 Behavioural and psychological symptoms of dementia.
At short-term follow-up, the RR for experiencing at least one adverse event (RR 1.05, 95% CI 0.98 to 1.12, I2 = 0%, 4 RCTs, low QoE, fig. 6) or withdrawal from the study (RR 0.89, 95% CI 0.72 to 1.11, I2 = 19%, low QoE, 6 RCTs, fig. 7) was not significantly different.
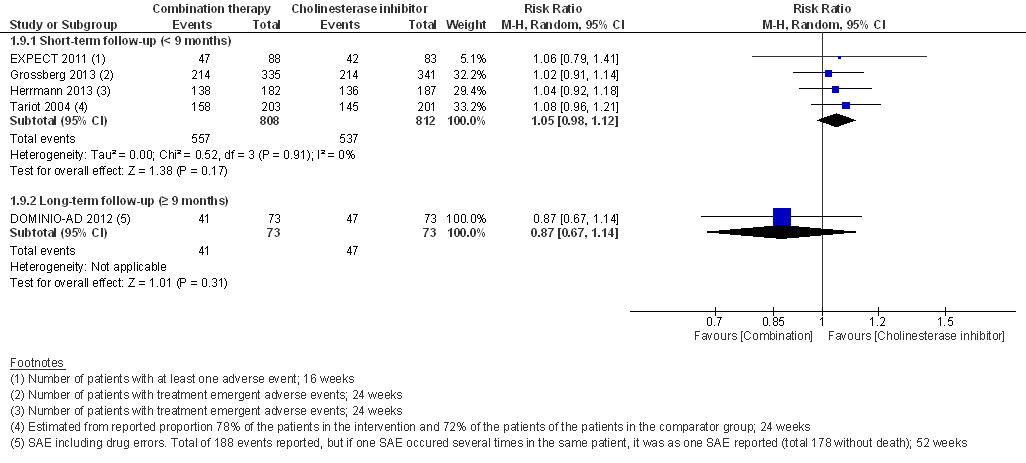
Figure 6 Adverse events.
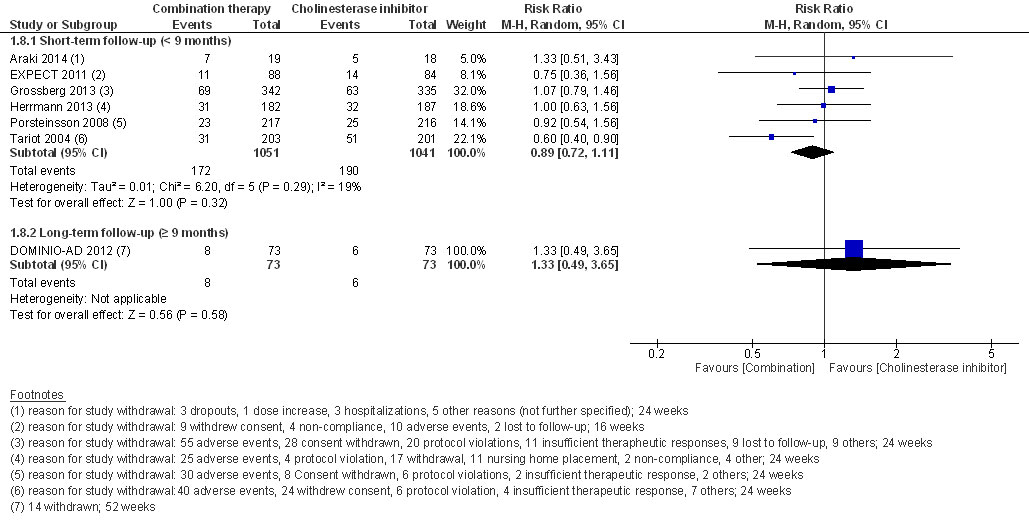
Figure 7 Withdrawals from study.
At long-term follow-up, there was no significant difference in cognition (SMD 0.08, 95% CI −0.14 to 0.29, I2 = 0%, 2 RCTs, low QoE, fig. 2), activities of daily living (SMD 0.08, 95% CI −0.25 to 0.40, 1 RCT, low QoE, fig. 3), adverse events (RR 0.87, 95% CI 0.67 to 1.14, 1 RCT, very low QoE, fig. 5) or the withdrawal rate (RR 0.87, 95% CI 0.67 to 1.14, 1 RCT, very low QoE, fig. 7). No other outcomes were reported at long-term follow-up.
Two RCTs reported on nursing home placement. Herrmann 2013 reported that fewer patients, who received combination therapy, i.e. 2 of 182 (1.1%) versus 9 of 187 (4.8%), and discontinued the trial due to nursing home placement at short-term follow-up (24 weeks). However, the authors did not report whether any patients who completed the trial were placed in a nursing home. For the outcome of delay in nursing home placement, DOMINO-AD 2012 reported that the confidence intervals of the probabilities after combination therapy overlapped with those after monotherapy with donepezil (Kaplan-Meier estimates: 0.20, 95% CI 0.12 to 0.32 versus 0.21, 95% CI 0.12 to 0.33) at 12 months of follow-up; hence, there was no difference. Outcomes on delay in nursing home placement were not pooled, hence, the QoE for the outcome delay in nursing home placement was judged to be very low.
At short- and long-term follow-up, there were no significant differences for cognition (short follow-up: MD 1.32, 95% CI −0.44 to 3.08, I2 = 68%, 2 RCTs, very low QoE; long follow-up: MD 0.80, 95% CI −1.01 to 2.61, 1 RCT, low QoE) or activities of daily living (short follow-up: SMD 0.06, 95% CI −0.55 to 0.68, I2 = 78%, 2 RCTs, very low QoE; long follow-up: SMD 0.22, 95% CI −0.10 to 0.55, 1 RCT, low QoE). Only DOMINO-AD 2012 assessed caregiver burden/distress and quality of life, and reported a non-significant average difference over all FU time-points from mixed regression model analysis (−0.6, 99% CI −1.3 to 0.2, up to 52 weeks and −0.9, 99% CI –5.2 to 3.4). Both RCTs did not report on clinical global impression, behavioural and psychological symptoms of dementia, or reported response rates for any of the outcomes.
At short- and long-term follow-up, the RR for the number of patients with at least one adverse event was not statistically significantly different (short follow-up: RR 1.40, 95% CI 0.60 to 3.27, 1 RCT, very low QoE; long follow-up: RR 1.07, 95% CI 0.80 to 1.43, 1 RCT, very low QoE). Withdrawals were only reported at long-term FU and RR was not statistically significantly different (RR 0.56, 95% CI 0.25 to 1.23, 1 RCT, very low QoE).
DOMINO-AD was the only study reporting on nursing home placement. At 12 months, the confidence intervals of the probabilities for nursing home placement after combination therapy overlapped with those after memantine monotherapy (Kaplan-Meier estimates: 0.21, 95% CI 0.12 to 0.33 versus 0.37, 95% CI 0.26 to 0.51).
The overall QoE for both comparisons, i.e. AChEI or memantine monotherapy, were judged to be very low for short- and long-term follow-up based on the QoE available for the critical outcomes, where the lowest judgment determines the overall QoE. At short-term follow-up with AChEI monotherapy as comparator, the judgment of very low quality of evidence of the outcome of delay in nursing home placement led to an overall very low quality of evidence. In all the other instances, the QoE was very low for other critical outcomes as well (see appendix, supplement 3).
We did not conduct the pre-specified subgroup analyses for any of the outcomes because too few trials were available. Sensitivity analyses addressing differences between trials in disease severity at baseline, type of instruments used for assessing dementia, drug adherence, industry versus non-industry sponsoring, AChEI route of administration or memantine target dose did not explain the high heterogeneity of the pooled results on cognition and behavioural and psychological symptoms.
In this meta-analysis of eight trials comparing combination therapy with AChEI monotherapy in patients with moderate to severe Alzheimer’s disease, combination therapy had significantly greater effects on cognition and clinical global impression at short-term follow-up, but not on behavioural and psychological symptoms. There was no evidence that combination therapy in comparison to AChEI monotherapy increased the risk of adverse events. Evidence on nursing home placement and outcomes reported at long-term follow-up was, in general, sparse. The overall quality of evidence at short- and long-term follow-up was very low. In most of the included trials, memantine was added to ongoing AChEI therapy, which is in line with the standard treatment course.
For the comparison of combination therapy versus memantine monotherapy, we could include only two trials. The overall quality of evidence for this comparison was very low at short- and long-term follow-up.
Although the effects of combination therapy compared to AChEI monotherapy on cognition and clinical global impression were statistically significant, they were small and their clinical relevance remains unclear. On a standardised scale, as a rule of thumb, an effect size of 0.2 may be considered a small clinically relevant effect [39, 40]. Hence, the effect estimates for cognition (SMD 0.20, 95% CI 0.05 to 0.35) and clinical global impression (SMD −0.15, 95% CI −0.28 to −0.01) included the possibility of such an effect. The clinical relevance of the small effects would be difficult to interpret even if they were reported on an original scale, and there is – to our knowledge – no current consensus regarding clinically relevant differences between treatment groups for these outcomes. Similarly, the effects of memantine monotherapy and particularly AChEI monotherapy compared to placebo have been reported to be small as well, and their clinical relevance has been questioned repeatedly, especially when put into relation with potential harms [4, 5, 41–47]. A possible approach that might facilitate the interpretation of the small effects would be to compare treatment response rates [48], based on pre-defined clinically relevant differences, i.e. “minimal clinically important difference (MCID).” Importantly, MCIDs represent the clinically important difference for an individual patient and therefore cannot be used to directly judge the clinical relevance of group differences [49]. Due to the degenerative nature of the disease, a dementia patient may be considered to be a treatment responder if the outcome measures remain stable over a clinically relevant follow-up period. To our knowledge, a consensus on relevant MCIDs for the different outcomes still needs to be established, even though some authors in the literature have proposed cut-offs for MCIDs for cognition [49, 50]. One of the two included RCTs that mentioned response rates, the EXPECT 2011 trial, did not pre-define MCIDs and finally did not report the response rates for their study population. The other RCT, Tariot 2004, reported the response rates for global impression, but for no other outcome. We consider the available evidence from these responder analyses and safety (see next paragraph) too sparse to support the interpretation of clinical relevance. The clinical relevance of the effects of combination therapy compared with monotherapy therefore remains unclear.
For the comparison of combination therapy with AChEI monotherapy, only four studies reported the number of patients that experienced an adverse event; hence, the small beneficial effects cannot be weighed against the potential harm. There was no evidence that the risk of adverse events was higher for combination therapy than for AChEI monotherapy. Adverse events frequently occur during the titration period of AChEI. For the comparison of combination therapy with AChEI monotherapy, most RCTs included patients who had already been on a stable dose of AChEI. Hence, adverse events that had occurred before study recruitment did not appear in the study reports and patients who did not tolerate AChEIs were already excluded. Consequently, our findings are more likely to reflect the adverse event profile of memantine added to ongoing AChEI therapy.
Evidence on patient-relevant outcomes such as nursing home placement was very limited. Nursing home placement was judged to be a critical outcome, because it is a proxy for the patient’s ability to live in his or her home environment with or without health care support [50]. The delay of nursing home placement may vary between different health care systems and depends on the availability of ambulatory care, cultural aspects, services to support families, institutional care facilities and financial resources. Due to these factors, it should be interpreted in conjunction with direct measures of function that reflect a patient’s ability to maintain independence. In this context, quality of life was rarely reported. This may be due to the difficulty of assessing quality of life from a patient’s perspective in Alzheimer’s disease due to progressing disease [51] and expected systematic underestimation of the patient’s quality of life by the caregivers [52].
This is the most up-to-date and comprehensive review of the evidence on combination therapy in patients with moderate to severe Alzheimer’s disease, and is the only review which has systematically assessed nursing home placement and quality of life. We are aware of several recently published systematic reviews that addressed a similar question. In brief, these reviews either did not include relevant RCTs that were available at the time when their review was conducted [11], included patients with mild Alzheimer’s disease [12] for which memantine is not approved or were limited to a single AChEI, namely donepezil, and hence a substance comparison [13]. Another systematic review, Kishi 2017 [53], primarily assessed the efficacy of memantine in patients with mild to severe Alzheimer’s disease. Kishi 2017 conducted subgroup analyses for moderate to severe Alzheimer’s disease and reported an effect estimate very similar to ours in this subgroup for cognition (SMD 0.17, 95% CI 0.03 to 0.31). In contrast to our findings, however, they reported a significant difference in behavioural and psychological symptoms of dementia (SMD 0.33, 95% CI 0.09 to 0.57). Kishi 2017 included two Japanese and one Chinese trial in this sub-group analysis, whereas they did not include EXPECT 2011 and Porsteinsson 2008; this might explain the discrepancy of the findings for the outcome behavioural and psychological symptoms of dementia compared to the present analysis. These Chinese or Japanese trials were not indexed in EMBASE, Medline or CENTRAL when we conducted our search, and we were unable to retrieve them. It is unclear how the inclusion of these studies would have affected our pooled effect estimates. Whereas the Chinese study [54] and one [55] of the two Japanese studies were relatively small (n = 32 and n = 35 patients) and probably would have had only little effect, the other Japanese study [56] (Nakamura 2016) would have been fairly large (n = 546). However, Kishi 2017 reported the effect of the Nakamura 2016 trial on cognition to be SMD 0.10, 95% CI 0.07 to 0.27. While Nakamura 2016 would have increased the power of our analysis, the effect largely overlaps with our pooled effect estimate. This would have resulted in a more precise effect estimate, but probably differing only marginally from our result.
A recently published extensive network meta-analysis on 110 RCTs by Tricco et al 2018 [57] reported the combination of donepezil with memantine as superior to donepezil alone for the outcome of behaviour (measured with the Neuropsychiatric Inventory [NPI]) (MD −3.9, 95% CI −7.23 to −0.56; 26 RCTs with 5138 patients, 7 nodes, 6 treatments and placebo) or galantamine alone (MD −4.17, 95% credible intervals −7.97 to −0.29; 26 RCTs with 5138 patients, 7 nodes, 6 treatments and placebo). Tricco 2018 found no significant differences for the other outcomes or for the comparison with memantine monotherapy. Finally, around half of the included 110 RCTs were done on patients with mild to moderate Alzheimer’s disease and it remains unclear how this affected the analysis. In our meta-analysis, we report behavioural and psychological symptoms of six RCTs as SMD because the studies used different NPI scales. However, if we do not standardise the mean difference, the resulting MD (−3.07, 95% CI −6.53 to 0.38) is similar to the MDs reported by Tricco 2018.
Our systematic review has limitations, which mainly relate to the study limitations of the included RCTs: (1) Attrition rates in the individual RCTs were high and most studies used “last observation carried forward (LOCF)” for the intention-to-treat analysis. Given that dementia is a progressive disease, results based on LOCF analysis seem inappropriate [58]. The number of withdrawals in AChEI monotherapy was slightly higher and we addressed missing data with LOCF. This may have shifted the effect in favour of AChEI monotherapy, and we may therefore have underestimated the combination therapy’s effect. (2) Our pooled results are limited because heterogeneity among studies was high and remained unexplained because too few studies were available for proper sub-group or sensitivity analyses. (3) Applied instruments like the MMSE or Alzheimer’s Disease Assessment Scale, cognitive subscale (ADAS-Cog) are known to be less sensitive for severe dementia because of floor effects [38] and may captured imprecisely the differences in treatment effects the further Alzheimer’s disease progressed. (4) Publication bias was not detected. v) Seven of the nine included RCTs were industry sponsored. Finally, we were unable to consider all available evidence because we could not retrieve one larger [56] and two smaller trials [54, 55].
Future researchers should consider conducting an individual patient data (IPD) meta-analysis, which would allow them (1) to perform post-hoc responder analyses based on MCIDs, which still need to be established, (2) to use accurate imputation methods (instead of LOCF) and (3) to assess heterogeneity among trials with subgroups analyses. However, all study authors would need to agree to share their trial data. Future RCTs should report on functional outcomes and clearly define how they will assess the clinical relevance (e.g. responder analyses). Further, they should investigate time to nursing home placement and assure complete safety reporting.
Trials with promising new agents (idalopirdine or intepirdine) have been stopped prematurely because of lack of benefit [59, 60], and although more than 28 Alzheimer’s disease agents are currently in phase III [61], the authorised pharmaceutical therapies are limited to AChEI, memantine and its combination. This highlights the importance of investigating the added benefit of the combination of the two authorised and widely administered therapies with well-designed RCTs with long-term follow-up.
To conclude, combination therapy compared to AChEI monotherapy showed statistically significant effects for cognition and clinical global impression at short term follow-up. The clinical relevance of these effects is uncertain. We have judged the overall quality of evidence to be very low, mainly because of unexplained heterogeneity among the included RCTs and inappropriate accounting in the analysis for the high attrition rates. The sparse available data for critical outcomes, the poor reporting of adverse events and evidence mainly based on short-term follow-up data highlight the need for large, investigator-initiated, pragmatic RCTs with long-term follow-up.
AppendixSupplement 1 ‒ Search strategies, conducted February 5th, 2018
Supplement 2 – Hierarchy of extraction of eligible instruments or further explanations
Supplement 3 – GRADE Summary of findings
The appendix is available as a separate file for downloading at https://smw.ch/en/article/doi/smw.2019.20093.
The research question of the original Health Technology Assessment report (HTA) on “Combination therapy compared to monotherapy for moderate to severe Alzheimer's disease” was selected from a pool of research questions submitted to the Swiss Medical Board (SMB) on the basis of pre-defined criteria. We defined the PICO question (eligible population, intervention, comparator and outcomes) and the relevance of the different outcomes according to GRADE in conjunction with clinical experts while taking into consideration feedback from a stakeholder process. Subsequently, we were commissioned by the SMB to conduct the systematic review including the systematic literature search, meta-analyses, presentation and interpretation of the results. The SMB did not influence the development and content of the assessment report or the preparation of this manuscript.
The complete HTA report including the report of the Appraisal Committee of the Swiss Medical Board is available on the SMB homepage.
The preparation of this manuscript, which included an update of the literature search, was covered by institutional funds of the ‘Stiftung Institut für klinische Epidemiologie’ and the Institute of Pharmaceutical Medicine at the University of Basel.
R.W.K. received advisor fees from Novartis, Merz and Lundbeck during the 36 months prior to the manuscript submission. H.R. has worked on projects in the last three years for the Swiss Medical Board, Bayer, and Grünenthal for which the respective institutes received funding. No other potential conflict of interest was reported.
1Maier WB. UB. Neurokognitive Störungen im DSM-5: Durchgreifende Änderungen in der Demenzdiagnostik. Nervenarzt. Berlin Heidelberg: Springer-Verlag, 2014:564–70.
2Siberski J. Dementia and DSM-5: Changes, Cost, and Confusion. Secondary Dementia and DSM-5: Changes, Cost, and Confusion 2012. http://www.todaysgeriatricmedicine.com/archive/110612p12.shtml.
3WHO World Health Organization. Dementia. Secondary Dementia March 2015 http://www.who.int/mediacentre/factsheets/fs362/en/.
4 Birks J . Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD005593.
5 Birks JS , Chong LY , Grimley Evans J . Rivastigmine for Alzheimer’s disease. Cochrane Database Syst Rev. 2015;9:CD001191.
6 Loy C , Schneider L . Galantamine for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst Rev. 2006;(1):CD001747. doi:.https://doi.org/10.1002/14651858.CD001747.pub3
7 Matsunaga S , Kishi T , Iwata N . Memantine monotherapy for Alzheimer’s disease: a systematic review and meta-analysis. PLoS One. 2015;10(4):e0123289. doi:.https://doi.org/10.1371/journal.pone.0123289
8 Monsch AU , Büla C , Hermelink M , Kressig RW , Martensson B , Mosimann U , et al., Alzheimer Forum Schweiz. Konsensus 2012 zur Diagnostik und Therapie von Demenzkranken in der Schweiz [Consensus 2012--diagnosis and treatment of patients with dementia in Switzerland]. Praxis (Bern 1994). 2012;101(19):1239–49. doi:.https://doi.org/10.1024/1661-8157/a001085
9European Medicines Agency (EMA). Refusal of the marketing authorisation for Acrescent (memantine hydrochloride / donepezil hydrochloride). http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002424/WC500134080.pdf, accessed 05.03.2018. EMA 2012.
10European Medicines Agency (EMA). Refusal of the marketing authorisation for Balaxur (memantine hydrochloride / donepezil hydrochloride). http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002708/WC500134081.pdf, accessed 05.03.2018. EMA 2012.
11 Schmidt R , Hofer E , Bouwman FH , Buerger K , Cordonnier C , Fladby T , et al. EFNS-ENS/EAN Guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur J Neurol. 2015;22(6):889–98. doi:.https://doi.org/10.1111/ene.12707
12 Tsoi KK , Chan JY , Leung NW , Hirai HW , Wong SY , Kwok TC . Combination Therapy Showed Limited Superiority Over Monotherapy for Alzheimer Disease: A Meta-analysis of 14 Randomized Trials. J Am Med Dir Assoc. 2016;17(9):863.e1–8. doi:.https://doi.org/10.1016/j.jamda.2016.05.015
13 Chen R , Chan PT , Chu H , Lin YC , Chang PC , Chen CY , et al. Treatment effects between monotherapy of donepezil versus combination with memantine for Alzheimer disease: A meta-analysis. PLoS One. 2017;12(8):e0183586. doi:.https://doi.org/10.1371/journal.pone.0183586
14Swiss Medical Board. Combination therapy compared to monotherapy for moderate to severe Alzheimer's Disease - Assessment report published 2017. https://www.swissmedicalboard.ch/fileadmin/public/news/2017/assessment_report_smb_antidementia_long_2017.pdf, accessed April 3rd, 2018.
15 Wong SS , Wilczynski NL , Haynes RB . Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. J Med Libr Assoc. 2006;94(1):41–7.
16Cochrane Work. The Cochrane highly sensitive search strategies for identifying randomized trials in PubMed, sensitivity- and precision-maximizing version (2008 revision), http://workcochraneorg/pubmed.
17 Guyatt G , Oxman AD , Kunz R , et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64(4):395–400.
18 Guyatt G , Oxman AD , Akl E , et al. GRADE guidelines 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94.
19 DerSimonian R , Laird N . Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi:.https://doi.org/10.1016/0197-2456(86)90046-2
20 da Costa BR , Nüesch E , Rutjes AW , Johnston BC , Reichenbach S , Trelle S , et al. Combining follow-up and change data is valid in meta-analyses of continuous outcomes: a meta-epidemiological study. J Clin Epidemiol. 2013;66(8):847–55. doi:.https://doi.org/10.1016/j.jclinepi.2013.03.009
21Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. www.cochrane.org/training/cochrane-handbook The Cochrane Collaboration last edited 20 March 2011
22 Guyatt GH , Oxman AD , Schünemann HJ , Tugwell P , Knottnerus A . GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. doi:.https://doi.org/10.1016/j.jclinepi.2010.09.011
23 Araki T , Wake R , Miyaoka T , Kawakami K , Nagahama M , Furuya M , et al. The effects of combine treatment of memantine and donepezil on Alzheimer’s disease patients and its relationship with cerebral blood flow in the prefrontal area. Int J Geriatr Psychiatry. 2014;29(9):881–9. doi:.https://doi.org/10.1002/gps.4074
24 Howard R , McShane R , Lindesay J , Ritchie C , Baldwin A , Barber R , et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med. 2012;366(10):893–903. doi:.https://doi.org/10.1056/NEJMoa1106668
25 Howard R , McShane R , Lindesay J , Ritchie C , Baldwin A , Barber R , et al. Nursing home placement in the Donepezil and Memantine in Moderate to Severe Alzheimer’s Disease (DOMINO-AD) trial: secondary and post-hoc analyses. Lancet Neurol. 2015;14(12):1171–81. doi:.https://doi.org/10.1016/S1474-4422(15)00258-6
26 Choi SH , Park KW , Na DL , Han HJ , Kim EJ , Shim YS , et al.; EXPECT Study Group. Tolerability and efficacy of memantine add-on therapy to rivastigmine transdermal patches in mild to moderate Alzheimer’s disease: a multicenter, randomized, open-label, parallel-group study. Curr Med Res Opin. 2011;27(7):1375–83. doi:.https://doi.org/10.1185/03007995.2011.582484
27 Yoon SJ , Choi SH , Na HR , et al. Effects on agitation with rivastigmine patch monotherapy and combination therapy with memantine in mild to moderate Alzheimer’s disease: a multicenter 24-week prospective randomized open-label study (the Korean EXelon Patch and combination with mEmantine Comparative Trial study). Geriatr Gerontol Int. 2017;17(3):494–9.
28 Grossberg GT , Manes F , Allegri RF , Gutiérrez-Robledo LM , Gloger S , Xie L , et al. The safety, tolerability, and efficacy of once-daily memantine (28 mg): a multinational, randomized, double-blind, placebo-controlled trial in patients with moderate-to-severe Alzheimer’s disease taking cholinesterase inhibitors. CNS Drugs. 2013;27(6):469–78. doi:.https://doi.org/10.1007/s40263-013-0077-7
29 Herrmann N , Gauthier S , Boneva N , Lemming OM ; 10158 Investigators. A randomized, double-blind, placebo-controlled trial of memantine in a behaviorally enriched sample of patients with moderate-to-severe Alzheimer’s disease. Int Psychogeriatr. 2013;25(6):919–27. doi:.https://doi.org/10.1017/S1041610213000239
30 Porsteinsson AP , Grossberg GT , Mintzer J , Olin JT ; Memantine MEM-MD-12 Study Group. Memantine treatment in patients with mild to moderate Alzheimer’s disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008;5(1):83–9. doi:.https://doi.org/10.2174/156720508783884576
31 Cummings JL , Schneider E , Tariot PN , Graham SM ; Memantine MEM-MD-02 Study Group. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology. 2006;67(1):57–63. doi:.https://doi.org/10.1212/01.wnl.0000223333.42368.f1
32 Feldman HH , Schmitt FA , Olin JT ; Memantine MEM-MD-02 Study Group. Activities of daily living in moderate-to-severe Alzheimer disease: an analysis of the treatment effects of memantine in patients receiving stable donepezil treatment. Alzheimer Dis Assoc Disord. 2006;20(4):263–8. doi:.https://doi.org/10.1097/01.wad.0000213859.35355.59
33 Schmitt FA , van Dyck CH , Wichems CH , Olin JT ; Memantine MEM-MD-02 Study Group. Cognitive response to memantine in moderate to severe Alzheimer disease patients already receiving donepezil: an exploratory reanalysis. Alzheimer Dis Assoc Disord. 2006;20(4):255–62. doi:.https://doi.org/10.1097/01.wad.0000213860.35355.d4
34 Tariot PN , Farlow MR , Grossberg GT , Graham SM , McDonald S , Gergel I ; Memantine Study Group. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–24. doi:.https://doi.org/10.1001/jama.291.3.317
35 van Dyck CH , Schmitt FA , Olin JT ; Memantine MEM-MD-02 Study Group. A responder analysis of memantine treatment in patients with Alzheimer disease maintained on donepezil. Am J Geriatr Psychiatry. 2006;14(5):428–37. doi:.https://doi.org/10.1097/01.JGP.0000203151.17311.38
36 Shao Z-Q . Comparison of the efficacy of four cholinesterase inhibitors in combination with memantine for the treatment of Alzheimer’s disease. Int J Clin Exp Med. 2015;8(2):2944–8.
37 Wilkinson D , Fox NC , Barkhof F , Phul R , Lemming O , Scheltens P . Memantine and brain atrophy in Alzheimer’s disease: a 1-year randomized controlled trial. J Alzheimers Dis. 2012;29(2):459–69. doi:.https://doi.org/10.3233/JAD-2011-111616
38 Robert P , Ferris S , Gauthier S , Ihl R , Winblad B , Tennigkeit F . Review of Alzheimer’s disease scales: is there a need for a new multi-domain scale for therapy evaluation in medical practice? Alzheimers Res Ther. 2010;2(4):24. doi:.https://doi.org/10.1186/alzrt48
39Cohen J. Statistical power analysis for the behavioral sciences. 2nd edition. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988.
40 King MT . A point of minimal important difference (MID): a critique of terminology and methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):171–84. doi:.https://doi.org/10.1586/erp.11.9
41Institut für Qualität and Wirtschaftlichkeit im Gesundheitswesen (IQWiG). Cholinesterasehemmer bei Alzheimer Demenz: Ergänzungsauftrag Rivastigmin-Pflaster und Galantamin. Bericht Nr. 18. https://www.iqwig.de/download/A09-05_Abschlussbericht_Cholinesterasehemmer_Ergaenzungsauftrag_Rivastigmin_Pflaster_Galantamin.pdf, accessed 16.05.2018. IQWiG 2012.
42 Rodda J , Walker Z . Ten years of cholinesterase inhibitors. Int J Geriatr Psychiatry. 2009;24(5):437–42. doi:.https://doi.org/10.1002/gps.2165
43 Blanco-Silvente L , Castells X , Saez M , Barceló MA , Garre-Olmo J , Vilalta-Franch J , et al. Discontinuation, Efficacy, and Safety of Cholinesterase Inhibitors for Alzheimer’s Disease: a Meta-Analysis and Meta-Regression of 43 Randomized Clinical Trials Enrolling 16 106 Patients. Int J Neuropsychopharmacol. 2017;20(7):519–28. doi:.https://doi.org/10.1093/ijnp/pyx012
44 Lemstra AW , Richard E , van Gool WA . Cholinesterase inhibitors in dementia: yes, no, or maybe? Age Ageing. 2007;36(6):625–7. doi:.https://doi.org/10.1093/ageing/afm117
45Haute Autorité de Santé (HAS). Alzheimer – Zoom sur les travaux de la HAS: parcours, évaluation des médicaments. Published 29.05.2018. https://www.has-sante.fr/portail/jcms/c_2680920/fr/medicaments-alzheimer-interet-medical-insuffisant, accessed June 5th, 2018.
46Institut für Qualität and Wirtschaftlichkeit im Gesundheitswesen (IQWiG). Memantin bei Alzheimer Demenz. Bericht Nr. 59. https://www.iqwig.de/download/A05-19C_Abschlussbericht_Memantin_bei_Alzheimer_Demenz.pdf, accessed 17.01.2017. IQWiG 2009.
47Institut für Qualität and Wirtschaftlichkeit im Gesundheitswesen (IQWiG). Responderanalysen zu Memantin bei Alzheimer Demenz - Rapid Report. Bericht Nr. 84. https://www.iqwig.de/de/projekte-ergebnisse/projekte/arzneimittelbewertung/2010-oder-frueher/a10-06-responderanalysen-zu-memantin-bei-alzheimer-demenz-rapid-report.1349.html, accessed 18.06.2018. IQWiG 2011.
48European Medicines Agency (EMA). Guideline on mdicinal products for the treatment of Alzheimer's disease and other dementias. Committee for medicinal products for human use (CHMP). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003562.pdf, accessed 09.05.2018. EMA 2008.
49 Dworkin RH , Turk DC , McDermott MP , Peirce-Sandner S , Burke LB , Cowan P , et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146(3):238–44. doi:.https://doi.org/10.1016/j.pain.2009.08.019
50 Geldmacher DS , Provenzano G , McRae T , Mastey V , Ieni JR . Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s disease. J Am Geriatr Soc. 2003;51(7):937–44. doi:.https://doi.org/10.1046/j.1365-2389.2003.51306.x
51 Riepe MW , Mittendorf T , Förstl H , Frölich L , Haupt M , Leidl R , et al. Quality of life as an outcome in Alzheimer’s disease and other dementias--obstacles and goals. BMC Neurol. 2009;9(1):47. doi:.https://doi.org/10.1186/1471-2377-9-47
52 Kahle-Wrobleski K , Ye W , Henley D , Hake AM , Siemers E , Chen YF , et al. Assessing quality of life in Alzheimer’s disease: Implications for clinical trials. Alzheimers Dement (Amst). 2017;6:82–90. doi:.https://doi.org/10.1016/j.dadm.2016.11.004
53 Kishi T , Matsunaga S , Oya K , Nomura I , Ikuta T , Iwata N . Memantine for Alzheimer’s Disease: An Updated Systematic Review and Meta-analysis. J Alzheimers Dis. 2017;60(2):401–25. doi:.https://doi.org/10.3233/JAD-170424
54 Zheng Y , Yu J . The efficacy and safety of combination therapy of memantine and donepezil among old Alzheimer’s disease patients (Translated from Chinese). Modern Practical Medicine. 2011;4:415–6.
55IE2201 (after 2001). Phase I study of SUNY7017 (memantine hydrochloride) in patients with dementia of Alzheimers type. Industry trial. Year published unknown.
56 Nakamura Y , Kitamura S , Nagakubo T , et al. Study of memantine hydrochloride in combination with donepezil hydrochloride in patients with moderate-to-severe alzheimer’s disease. Jpn J Geriatr Med. 2016;54:1147–58.
57 Tricco AC , Ashoor HM , Soobiah C , Rios P , Veroniki AA , Hamid JS , et al. Comparative Effectiveness and Safety of Cognitive Enhancers for Treating Alzheimer’s Disease: Systematic Review and Network Metaanalysis. J Am Geriatr Soc. 2018;66(1):170–8. doi:.https://doi.org/10.1111/jgs.15069
58European Medicines Agency (EMA). Consultation on revised guideline on medicines to treat Alzheimer’s disease. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/02/WC500200830.pdf accessed 19.01.2017. EMA 2016.
59 Bennett DA . Lack of Benefit With Idalopirdine for Alzheimer Disease: Another Therapeutic Failure in a Complex Disease Process. JAMA. 2018;319(2):123–5. doi:.https://doi.org/10.1001/jama.2017.19700
60 Simpson I . An industry update: the latest developments in therapeutic delivery. Ther Deliv. 2018;9(1):9–15. doi:.https://doi.org/10.4155/tde-2017-0109
61 Cummings J , Lee G , Mortsdorf T , Ritter A , Zhong K . Alzheimer’s disease drug development pipeline: 2017. Alzheimers Dement (N Y). 2017;3(3):367–84. doi:.https://doi.org/10.1016/j.trci.2017.05.002
D.G., V.G., H.R. and A.U.M were responsible for the study’s concept and design. D.G., V.G., H.R., R.W.K, M.S., K.A.M., Z.A., H.C.B. and Y.T. were responsible for data collection, data analysis and interpretation. All authors contributed to drafting the manuscript and preparing the figures. D.G. and V.L.G. contributed equally to the present publication.
The research question of the original Health Technology Assessment report (HTA) on “Combination therapy compared to monotherapy for moderate to severe Alzheimer's disease” was selected from a pool of research questions submitted to the Swiss Medical Board (SMB) on the basis of pre-defined criteria. We defined the PICO question (eligible population, intervention, comparator and outcomes) and the relevance of the different outcomes according to GRADE in conjunction with clinical experts while taking into consideration feedback from a stakeholder process. Subsequently, we were commissioned by the SMB to conduct the systematic review including the systematic literature search, meta-analyses, presentation and interpretation of the results. The SMB did not influence the development and content of the assessment report or the preparation of this manuscript.
The complete HTA report including the report of the Appraisal Committee of the Swiss Medical Board is available on the SMB homepage.
The preparation of this manuscript, which included an update of the literature search, was covered by institutional funds of the ‘Stiftung Institut für klinische Epidemiologie’ and the Institute of Pharmaceutical Medicine at the University of Basel.
R.W.K. received advisor fees from Novartis, Merz and Lundbeck during the 36 months prior to the manuscript submission. H.R. has worked on projects in the last three years for the Swiss Medical Board, Bayer, and Grünenthal for which the respective institutes received funding. No other potential conflict of interest was reported.