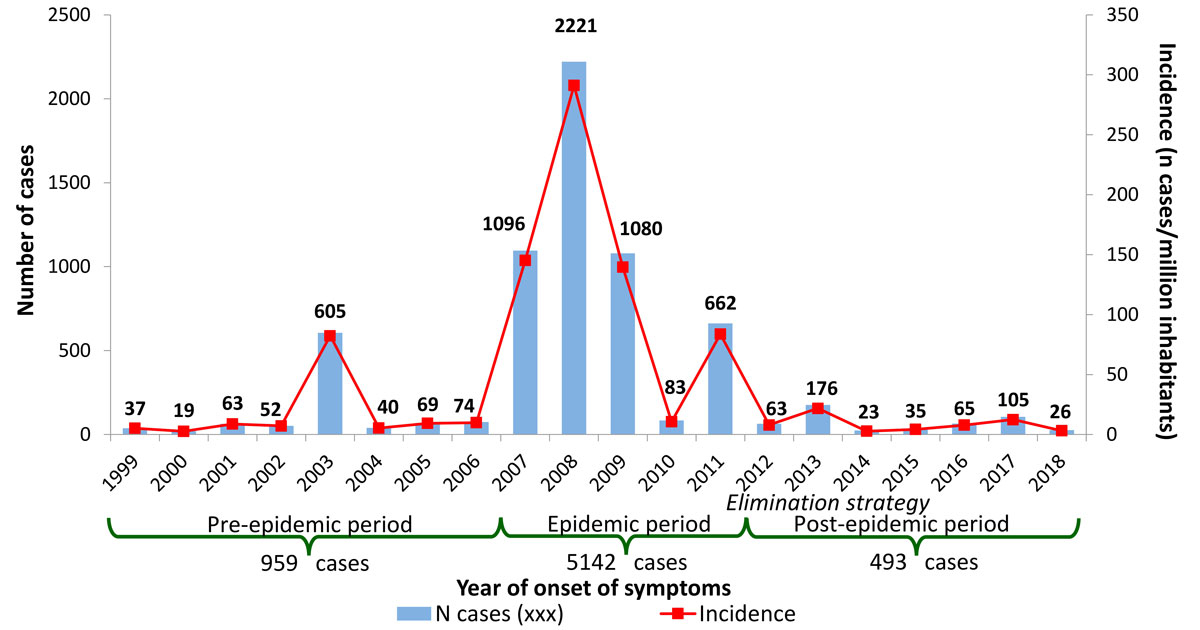
Figure 1 Number of cases of measles and incidence per million inhabitants, per year in Switzerland, 1999–July 2018.
DOI: https://doi.org/10.4414/smw.2019.20102
Switzerland, like all countries worldwide, aims to eliminate measles under the leadership of the World Health Organization (WHO) [1]. To strengthen primary prevention efforts and outbreak control, the Federal Office of Public Health (FOPH) and its partners implemented a national strategy for measles elimination between 2011 and 2015 [2], now covered by the national vaccination strategy [3]. The objective for measles is to achieve long-term vaccination coverage of at least 95% (elimination threshold) through two doses in children and adolescents. It is also recommended that whenever a patient born after 1963 comes into contact with the medical system, their vaccination status is checked and they are offered catch-up immunisation if necessary.
Measles elimination is defined as a sustained absence of endemic measles transmission in a defined country or region. Following this interruption, some (rare) cases can still occur – this concerns imported cases and potential import-related cases. In practice, for each country the WHO assesses whether no outbreak has lasted longer than 1 year in the presence of a well-performing measles surveillance system. It is therefore vital that this surveillance is highly sensitive (reporting any suspected case that meets the reporting criteria) and specific (confirmation of suspected cases in a laboratory), and that it is able to identify outbreaks and the importation status of cases. The surveillance should also be responsive (reporting within 24 hours in Switzerland) to allow early and effective intervention.
The general aim of this article is to evaluate Switzerland’s progress towards eliminating measles in terms of epidemiology and vaccination coverage. More specifically, it describes the recent evolution of measles epidemiology by comparing the characteristics of cases reported during the epidemic period (2007–2011) with those reported during the current post-epidemic period (2012–July 2018). It is expected that certain epidemiological characteristics will change as cases become rarer.
Measles has been continuously monitored in Switzerland since March 1999 within the framework of mandatory reporting of infectious diseases [4]. Physicians have to notify the cantonal officers of public health of any suspected cases of measles that meet the clinical case definition of a maculopapular rash and a fever accompanied by at least one of the following three symptoms: cough, rhinitis or conjunctivitis. The physician’s notification provides information on the clinical manifestations and place of infection, as well as the patient’s sex, age, nationality and vaccination status.
Laboratories are required to notify the cantonal officers of public health and the FOPH of any positive measles tests. In practice, this essentially involves detection of the antibody IgM in the blood and viral RNA using the polymerase chain reaction (PCR) from a nasopharyngeal swab. Laboratories also have to report all negative PCR tests, which provide data on the number of suspected cases of measles that are discarded following a laboratory test. In this regard, negative IgM results are unreliable and therefore do not need to be reported. Serological tests to detect IgM in addition to IgG are often used – wrongly – to assess a patient’s immune status.
In order to strengthen the molecular epidemiology of measles, the FOPH set up a National Measles and Rubella Reference Laboratory in January 2018 (CNRRR, Geneva University Hospital) and asked laboratories to submit a sample whenever they detect measles virus RNA by PCR [5]. The CNRRR then sequences the virus to determine the genotype and variant. When these data are linked to the epidemiological information supplied by physicians, they make it possible to determine the epidemiological link between cases and their importation status.
Reported cases are classified as:
Cases that fall into these three categories are used for the following analyses and are referred to as “measles cases”. All other suspected cases are discarded, particularly those that yield a negative laboratory test. The same applies to cases where the patients were not resident in Switzerland or the Principality of Liechtenstein.
The data on the classification of the Swiss population by sex, age, canton and nationality were provided by the Federal Statistical Office. Proportions were compared using the chi-squared test. To test differences in medians we used Mood’s median test. All analyses were performed using STATA 15.0 software.
Between 1999 and the end of July 2018, 6 594 cases of measles were reported. These 20 years of surveillance comprise three epidemiological periods (fig. 1):

Figure 1 Number of cases of measles and incidence per million inhabitants, per year in Switzerland, 1999–July 2018.
This article focuses on the 5635 cases of measles reported during the two most recent periods.
The average annual incidence rate of measles fell from 133 cases per million inhabitants during the epidemic period (range 11–291) to 9 cases per million inhabitants (3–22) during the post-epidemic period. This constitutes a decline in the incidence of measles of 94% between these two periods.
Between the epidemic and post-epidemic periods, the proportion of laboratory-confirmed cases increased from 46% to 71%, while that of epidemiologically-linked cases fell from 45% to 16% (table 1). The proportion of cases only clinically compatible was low during both periods.
Table 1 Characteristics of reported measles cases and annual incidence per million inhabitants by period in Switzerland, 2007–July 2018.
| Epidemic period 2007–2011 | Post-epidemic period 2012–2018 | p-value* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N cases | % | % known | Incidence/million * year | N cases | % | % known | Incidence/million * year | ||||
| Classification | Clinically compatible | 452 | 8.8 | 8.8 | 11.7 | 66 | 13.4 | 13.4 | 1.1 | <0.001 | |
| Epidemiologically-linked | 2305 | 44.8 | 44.8 | 59.7 | 79 | 16.0 | 16.0 | 1.4 | |||
| Laboratory-confirmed | 2385 | 46.4 | 46.4 | 61.7 | 348 | 70.6 | 70.6 | 6.0 | |||
| Sex | Male | 2580 | 50.2 | 50.3 | 135.9 | 267 | 54.2 | 54.2 | 9.3 | 0.099 | |
| Female | 2552 | 49.6 | 49.7 | 129.9 | 226 | 45.8 | 45.8 | 7.7 | |||
| Unknown | 10 | 0.2 | 0 | 0.0 | |||||||
| Nationality | Swiss | 4065 | 79.1 | 92.6 | 157.3 | 361 | 73.2 | 84.1 | 9.5 | <0.001 | |
| Foreign | 325 | 6.3 | 7.4 | 45.5 | 68 | 13.8 | 15.9 | 5.6 | |||
| Unknown | 752 | 14.6 | 64 | 13.0 | |||||||
| Importation status | Imported case | 100 | 1.9 | 1.9 | 2.6 | 76 | 15.4 | 17.0 | 1.3 | <0.001 | |
| Import-related case | n.a. | 82 | 16.6 | 18.3 | 1.4 | ||||||
| Endemic case | n.a. | 289 | 58.6 | 64.7 | 5.0 | ||||||
| Unknown | n.a. | 46 | 9.3 | ||||||||
| Imported from† | Europe | 74 | 74.0 | 75.5 | 1.9 | 34 | 44.7 | 44.7 | 0.6 | <0.001 | |
| ‒ France, Italy, Germany, Austria | 67 | 67.0 | 68.4 | 1.7 | 16 | 21.1 | 21.1 | 0.3 | |||
| Africa | 7 | 7.0 | 7.1 | 0.2 | 4 | 5.3 | 5.3 | 0.1 | |||
| Asia/Oceania | 12 | 12.0 | 12.2 | 0.3 | 35 | 46.1 | 46.1 | 0.6 | |||
| America | 5 | 5.0 | 5.1 | 0.1 | 3 | 3.9 | 3.9 | 0.1 | |||
| Unknown | 2 | 2.0 | 0 | 0.0 | 0.0 | ||||||
| Vaccination status‡ | Vaccinated | All | 368 | 7.2 | 8.1 | 83 | 16.8 | 18.9 | <0.001 | ||
| 1 dose | 222 | 4.3 | 4.9 | 30 | 6.1 | 6.8 | |||||
| ≥2 doses | 106 | 2.1 | 2.3 | 35 | 7.1 | 8.0 | |||||
| N doses unknown | 40 | 0.8 | 0.9 | 18 | 3.7 | 4.1 | |||||
| Not vaccinated | 4167 | 81.0 | 91.9 | 357 | 72.4 | 81.1 | |||||
| Unknown | 607 | 11.8 | 53 | 10.8 | |||||||
| Total | 5142 | 100.0 | 100.0 | 133.1 | 493 | 100.0 | 100.0 | 8.5 | |||
N = number; n.a. = not availlable * For proportions, calculated for categories with known information † 100% = total of imported cases ‡ The incidence cannot be calculated because the vaccination status of the whole population is unknown
During the epidemic period, the number of reported cases peaked in March and April, and was lowest during the third and particularly the fourth quarters of the year (fig. 2). During the post-epidemic period, the seasonality of virus transmission was less marked and the peak shifted towards the summer months, with the fewest cases occurring in the fourth quarter.
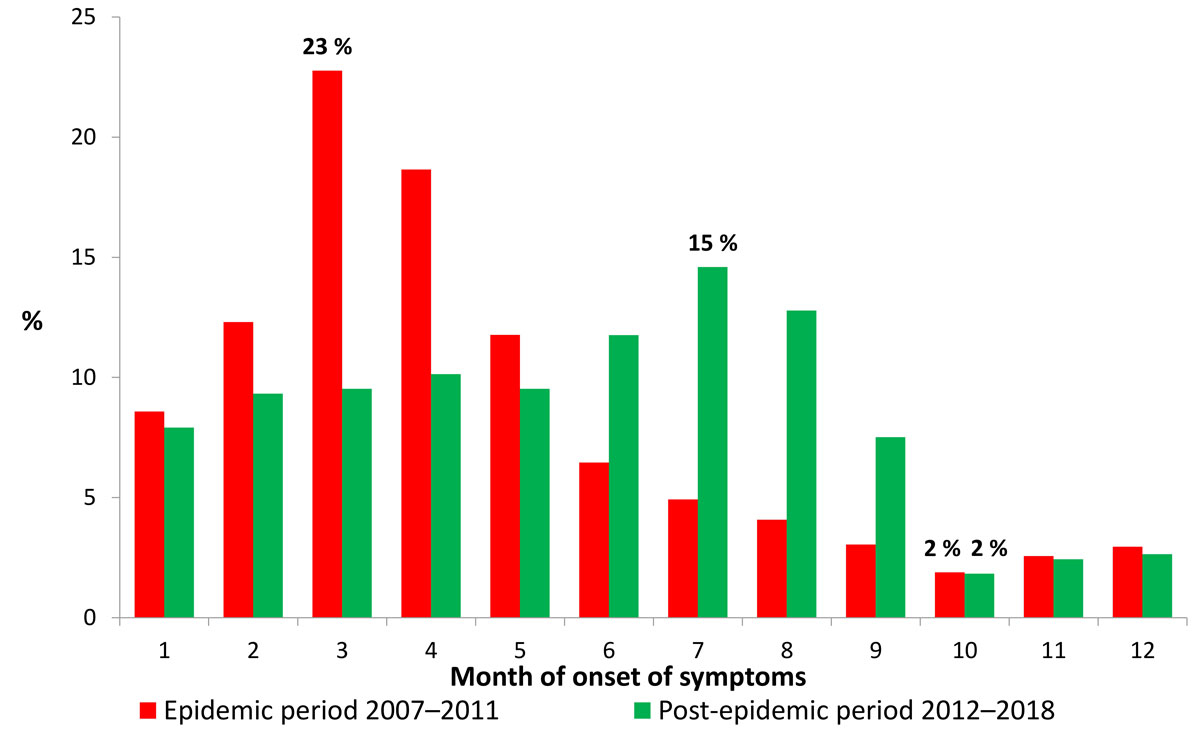
Figure 2 Proportion of measles cases per month and period in Switzerland, 2007–July 2018.
The proportion of measles cases by sex was similar during both the epidemic and the post-epidemic period (table 1). The median age of measles cases increased from 12 to 17 years (p <0.001) between the epidemic and the post-epidemic period, with the interquartile range rising from 8–18 years to 10–26 years. Figure 3 shows a shift towards older ages in the age structure of cases between the two periods. We note a peak in the 5–9 age group and 10–14 age group (each accounting for 23% of total cases) during the epidemic, and in the 15–19 age group and 20–29 age group in the post-epidemic period (20% and 21%, respectively), with the over-40s making up 10% of cases. Cases involving infants (<1 year old) were rare during both periods (2–3%), although infants are not usually vaccinated as they are too young. During the epidemic period, incidence was highest in the 5–9 year age group (609 cases per million inhabitants), and it was highest in the 15–19 age group (32/million) during the post-epidemic period.
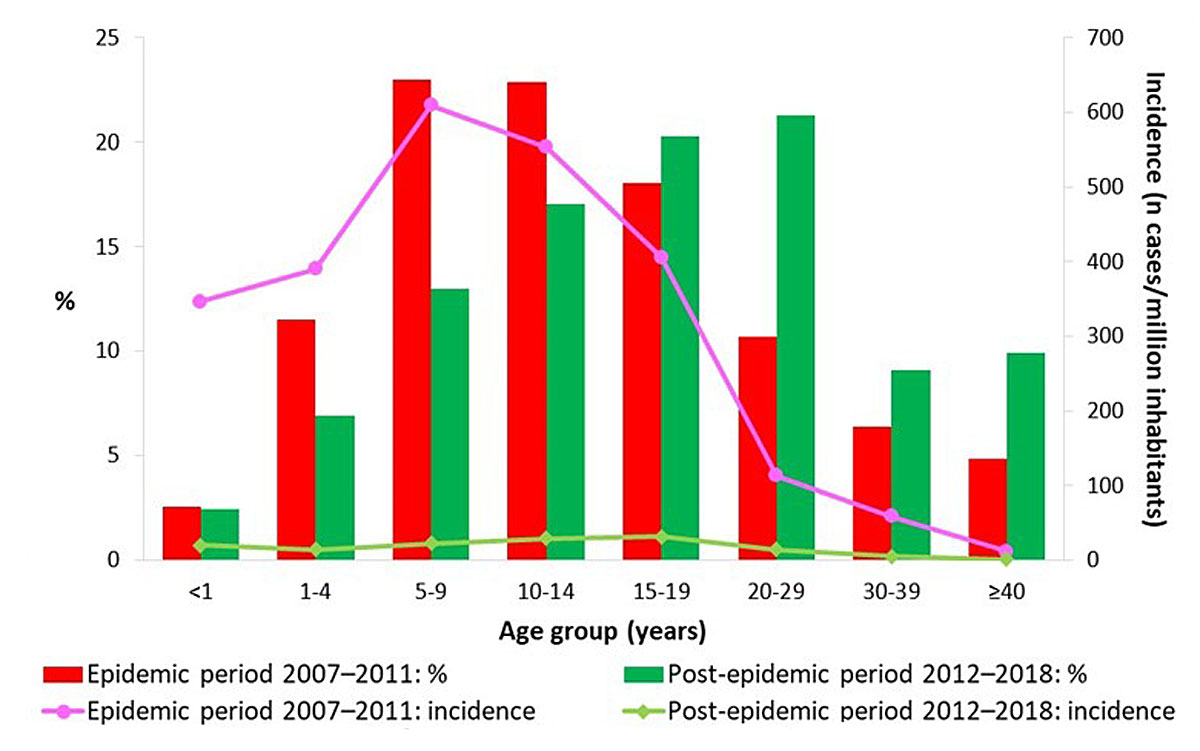
Figure 3 Proportion and incidence per million inhabitants of reported cases of measles by age group and period in Switzerland, 2007–July 2018.
The peak incidences of measles during the epidemic period were recorded along a strip stretching from central northern Switzerland to south-eastern Switzerland, passing through Central Switzerland, as well as in the cantons of Appenzell Innerrhoden, Appenzell Ausserrhoden, Schaffhausen and Geneva (fig. 4a). The cantons most affected were Appenzell Innerrhoden (1133 cases per million inhabitants) and Lucerne (564/million). A total of 1039 cases were reported in the canton of Lucerne alone during this period. The sharp decline in incidence which characterises the post-epidemic period is accompanied by a shift in the peak incidences to other regions: eastern central Switzerland (Schwyz: 83/million; Glarus: 68/million), followed by the south of the country and the Jura Mountains (fig. 4b).
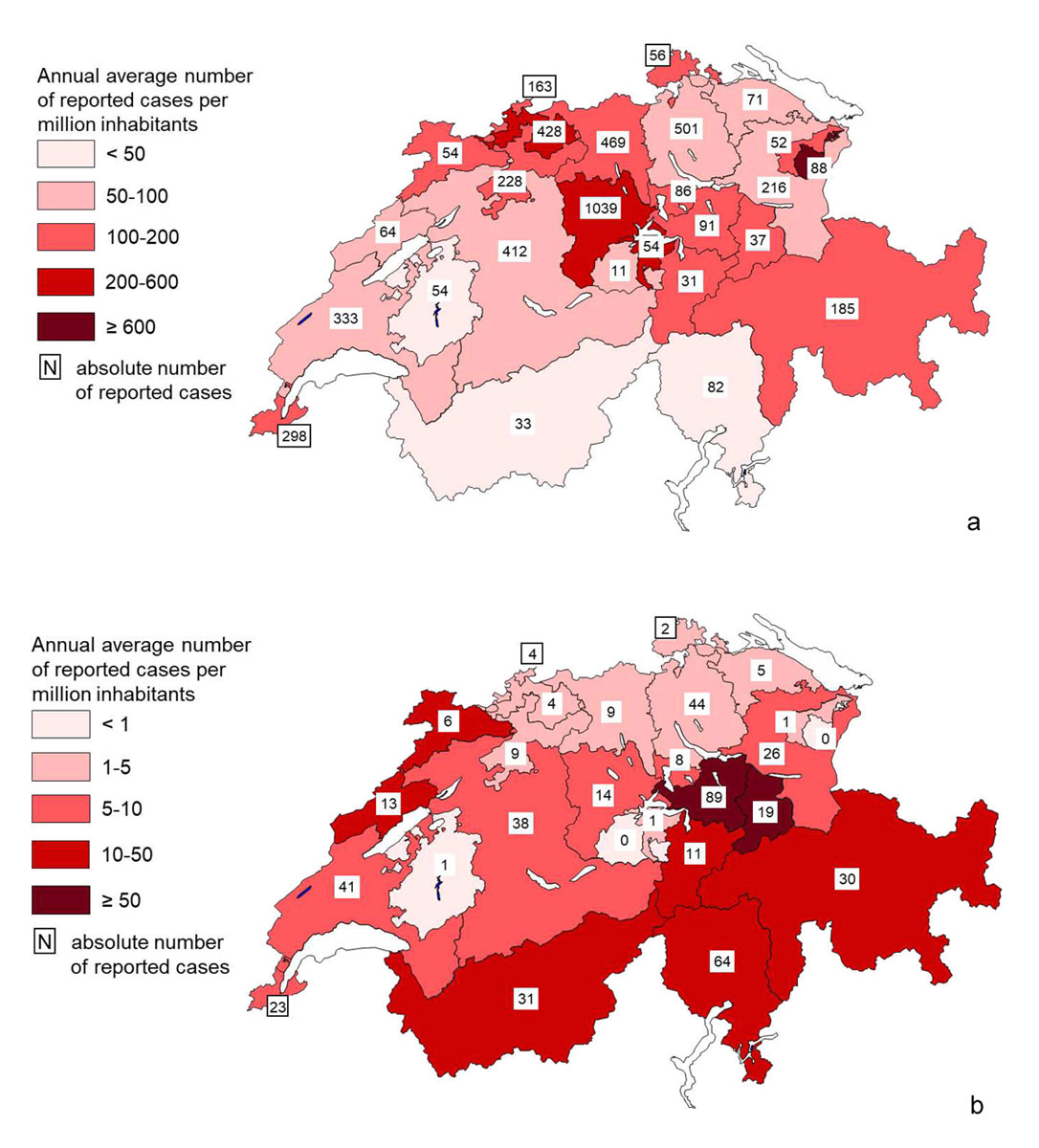
Figure 4 Total cases of measles and average annual incidence rate per million inhabitants in Switzerland, 2007–July 2018. (a) Epidemic period 2007–2011. (b) Post-epidemic period 2012–July 2018.
It should be noted that usually a single outbreak in a canton lasting for a limited period is the cause of the high cantonal incidences shown in figure 4, and this applies not only to the epidemic period but even more so to the post-epidemic period. The most notable exception is the canton of Lucerne which recorded three successive epidemic waves between 2007 and 2009.
In 2017, among the 8.5 million inhabitants in Switzerland there were 2.1 million foreign nationals (25%), of whom 83% were from European countries. The proportion of measles cases in foreign nationals increased from 7% to 16% (of total cases where nationality is known) between the epidemic period and the post-epidemic period (table 1). Incidence per million inhabitants in foreign nationals was 3.5 times lower than in Swiss nationals during the first period and 1.7 times lower during the second. The nationality of cases by continent was similar for both periods. Overall, from 2007 to July 2018, the nationality of 356 of a total of 393 cases affecting foreign nationals was known (91%). Of them, 83% were from Europe, 10% from Asia or Oceania, 4% from America and 3% from Africa.
The importation status of measles cases (imported case, import-related case, endemic case) has been detailed only since 2012 (table 1). Before that, only imported cases were singled out. More refined categorisation was introduced to respond to the needs of elimination progress monitoring by the WHO. Whereas imported cases were rare during the epidemic period (2%), they accounted for a much greater proportion during the post-epidemic period (17% of cases where importation status is known). During this latest period, each imported case generated on average a single secondary case. The majority of cases were thus considered endemic (65%).
The annual proportion of imported cases tended to be lower, the higher the number of measles cases. It was around 1% for each of the three years between 2007 and 2009 with major outbreaks (from 1080 to 2221 cases per year), culminating at 63% for the 24 cases with known importation status reported during the first seven months of 2018.
The epidemic curve from January 2016 to July 2018 illustrates the variability in importation status and in the recent temporal distribution of measles cases (fig. 5). We also noted several periods with no cases, the longest of which lasted 13 weeks, which is three to four times the maximum incubation period. Moreover, only imported cases were recorded during the five subsequent weeks.
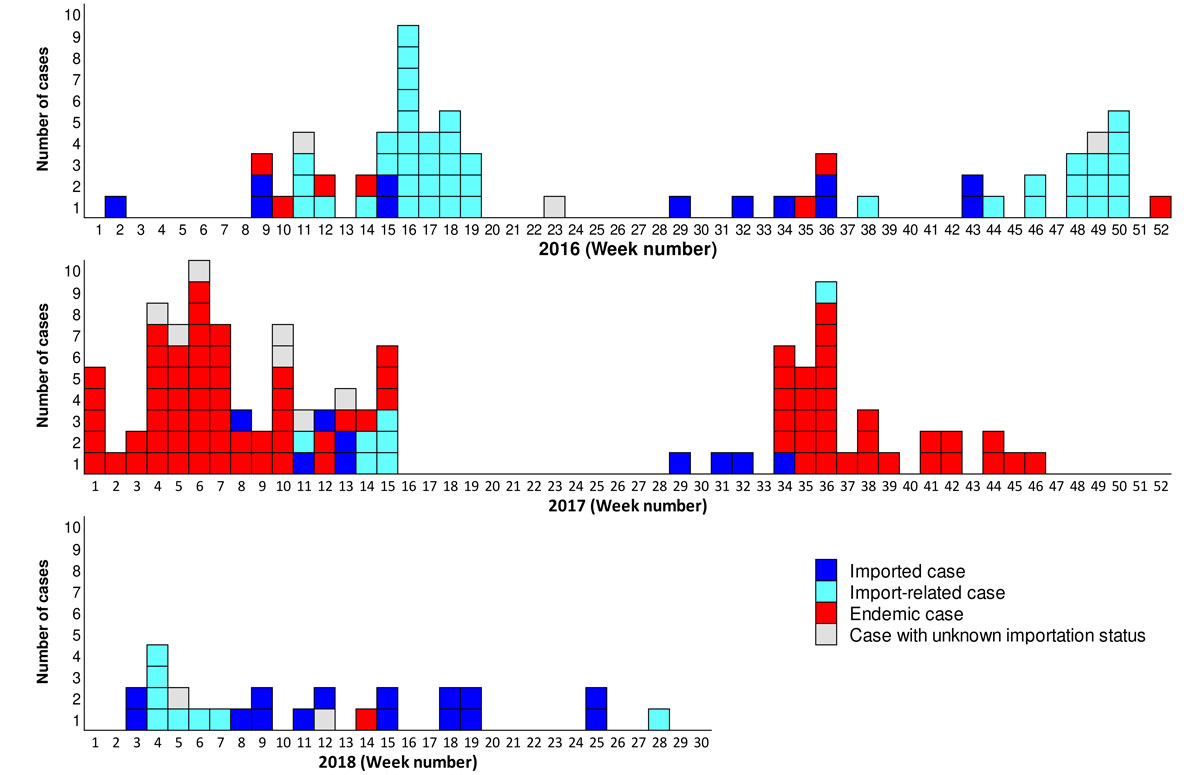
Figure 5 Epidemic curve of measles cases, by week and importation status in Switzerland, 2016–July 2018.
The majority of measles cases that were contracted abroad during the epidemic period were imported from Europe, mainly from Switzerland’s neighbouring countries (table 1). During the post-epidemic period, slightly more importations were from Asia/Oceania than Europe, including 10 cases from Thailand alone (13% of total importations).
During the epidemic period, the median age of imported cases was 17.5 versus 11 years (p = 0.002) for cases contracted in Switzerland. The median age of imported cases reached 25 years during the post-epidemic period and was also higher than for import-related cases (19 years; p = 0.001) and endemic cases (15 years; p ≤0.001).
Since 2012, non-sporadic measles cases have been grouped into outbreaks, defined as at least two epidemiologically linked cases. For the entire post-epidemic period, 55 outbreaks were identified (annual range 3–14), involving a total of 353 cases, or 72% of total reported cases (range 46–84%). Each outbreak involved between 2 and 97 cases (but only 22 for the second largest), with a median of 2 cases per outbreak (annual range 2–5). The outbreaks lasted between 1 and 19 weeks, with a median of 3 weeks (annual range 2–5). Outbreak cases were significantly younger than sporadic cases, with a median age of 15 and 25 years, respectively.
A genotyping result for the measles virus is available for 179 cases during the epidemic period (3% of total cases during the period) and 107 (22%) from the post-epidemic period. The viruses in circulation during these two periods were very different. The dominant viruses during the epidemic period belonged to genotypes D5 and D4, and to a lesser extent B3, whereas during the post-epidemic period, viruses of genotype D8 and less frequently B3 predominated. Between 2007 and July 2018, three other genotypes were exceptionally detected: D9, G3 and H1.
The genotype of the measles virus was known for 29 of 55 (53%) of outbreaks identified during the post-epidemic period. Genotypes B3 and D8 were detected virtually every year during this period, but it should be noted that the more refined genetic analyses revealed a wide variety of variants among these genotypes. For example, the outbreaks detected between 2016 and July 2018 originated every year from three to five variants of B3 and just as many of D8, meaning that a different variant caused most of the outbreaks occurring during the year.
The large majority of reported measles cases were not vaccinated according to the patients’ medical history or a written record, and this was the case both during the epidemic and post-epidemic period (table 1). Although the annual number of cases sharply declined between the two periods, the proportion of cases vaccinated with at least one dose increased from 8% to 19%.
The vaccination coverage of children and adolescents aged 2, 8 and 16 years is recorded in each canton in a three-year cycle. A national weighted average is calculated at the end of each cycle. For measles, national coverage has continually increased since the first survey in 1999–2003, irrespective of the age and number of doses considered (fig. 6). According to the latest national evaluation available, covering the years 2014–2016, it amounted to 94% at 2 years and 96% at 16 years for at least one dose. For two doses, these values were 87% and 93%, respectively.
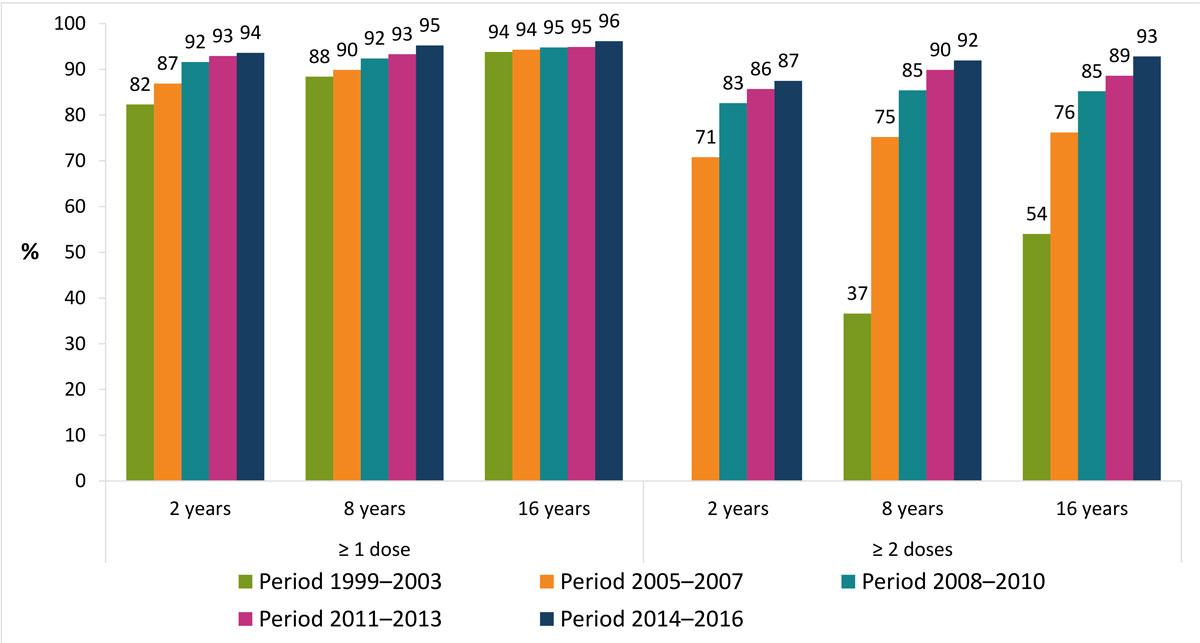
Figure 6 Evolution of measles vaccination coverage by survey period in Switzerland, 1999–2016.
Despite a gradual settling over time, the intercantonal disparities remained significant, especially for two doses, as shown in figure 7, which illustrates the latest available data on cantonal coverage. The extreme cantonal values for two doses ranged from 82% to 95% at age 2, and from 81% to 97% at age 16. Only one canton (Geneva) achieved the objective of vaccination coverage of 95% for two doses at age 2. Eight cantons in western and northern Switzerland achieved this coverage in adolescents at age 16.
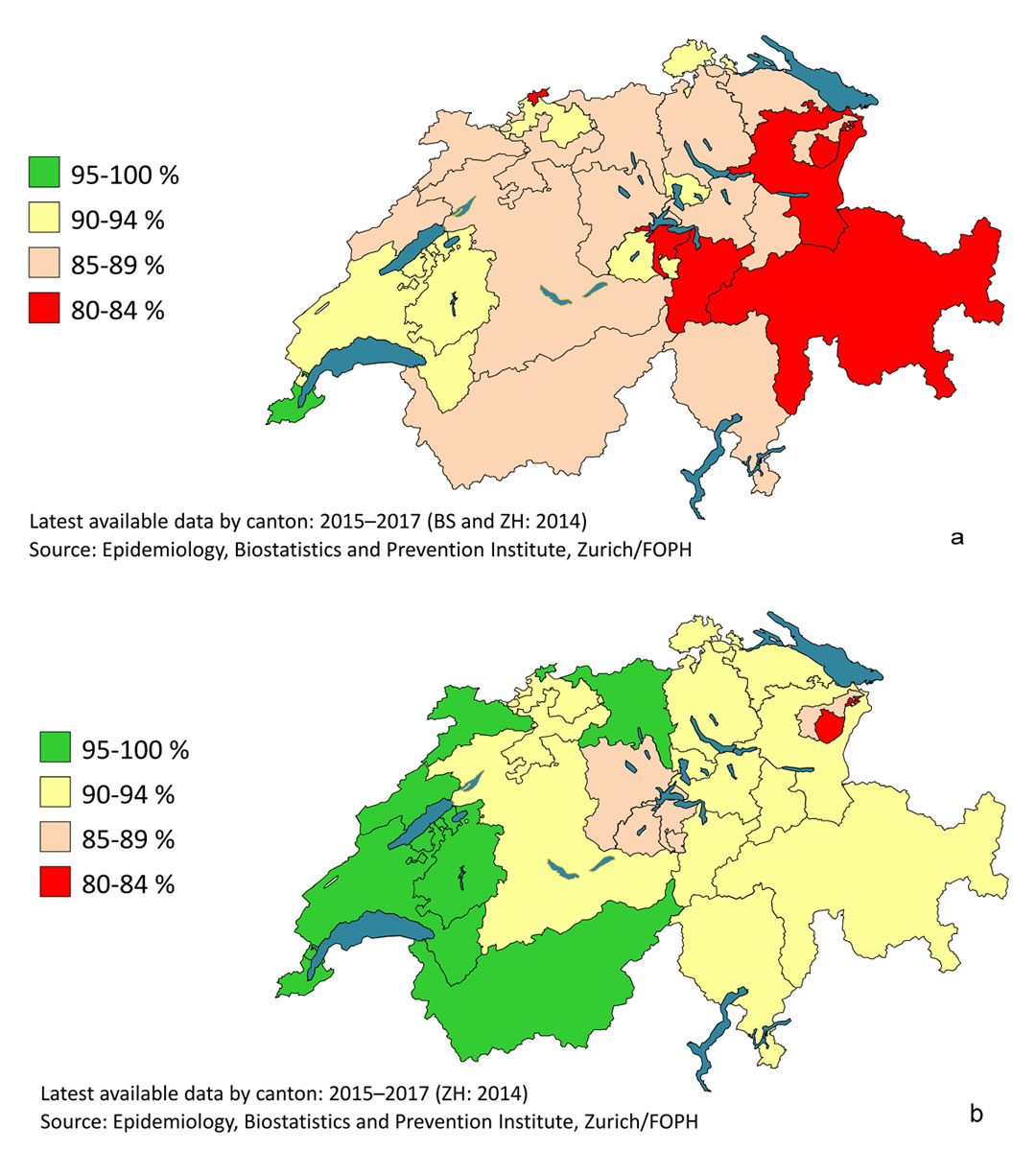
Figure 7 Measles vaccination coverage by canton in Switzerland, 2015–2017. (a) Two doses at age 2. (b) Two doses at age 16.
On the whole, measles vaccination coverage among foreign nationals resident in Switzerland was consistently higher than that of Swiss nationals. These differences tended to decrease as age and number of doses increased, as well as over time. Coverage of foreign nationals for one dose at age 2 was thus 10 percentage points higher than that of Swiss nationals in 2005–2007. The gap was just 5 percentage points in 2014–2016 at age 2, and 3 percentage points at age 16. For two doses at age 2, there was an 8-point gap in 2005–2007, and a gap of just 2 points in 2014–2016. There was therefore no longer a difference between Swiss and foreign nationals in 8- and 16-year-olds. These differences accumulated over many years suggest that non-Swiss children, adolescents and young adults in Switzerland are better protected against measles than Swiss nationals.
Cantonal incidence of measles during the epidemic in 2007–2011 tended to be lower the higher the cantonal vaccination coverage (measured in 2008–2010). The correlation coefficients thus ranged from −0.6 to −0.7 if we consider one or two doses at age 2, or two doses at age 16 (but only −0.1 for one dose at age 16). This correlation disappeared during the post-epidemic period (correlation coefficients from −0.4 to −0.1).
The incidence of measles in Switzerland is currently at its lowest level since surveillance of the disease was introduced in 1999. Between the epidemic period (2007–2011) [6] and the post-epidemic period (2012–July 2018), it fell by 94% to an annual average of 9 cases per million inhabitants, or even as low as 7/million if we exclude imported cases. It is worth noting that an incidence of the same magnitude (16/million) had already been achieved during the pre-epidemic period (1999–2006) [7]. However, the incidence was probably underestimated back then because of the gradual introduction of the notification system [8]. Furthermore, immunity was high among adults at the time owing to the widespread transmission of measles during their childhoods.
The proportion of laboratory-confirmed cases increased significantly between the epidemic and post-epidemic periods, to the detriment of epidemiologically linked cases. The former were then proportionally more numerous than in the European Union in 2017 (71% vs 61%, ranging from 37% to 100% depending on the country) [9]. During major outbreaks, physicians often refrain from confirming suspected cases of measles that meet the clinical case definition and are epidemiologically linked to one or more confirmed cases (in the family, at school, at nursery, etc.). On the other hand, they make a greater effort to investigate sporadic cases, which are proportionately more common during non-epidemic periods. In this respect, physicians largely follow the FOPH recommendations, which state that any suspected case of measles that does not have a known epidemiological link to another confirmed case should be confirmed in a laboratory [10]. This is also reflected in the continual increase in the number of suspected cases of measles per 100,000 inhabitants that are discarded following an investigation (data not provided), which is an important indicator of the quality of surveillance of this disease [1].
The sharp decline in incidence between the two periods is accompanied by epidemiological changes that are expected in such circumstances: an end to seasonality, an increase in the age of cases and an increase in the proportion of vaccinated cases. Endemic circulation of measles is characterised by marked seasonality, usually with a spike in late winter or early spring in the northern hemisphere. This was observed in Switzerland during the epidemic period, as well as recently in the European Union [9] and China [11], but no longer during the post-epidemic period. The relatively high proportion of imported cases, the limited number of secondary import-related cases and the brevity of outbreaks in general thus contributed to the random nature of the epidemic curve and the virtual disappearance of seasonality. It should be noted, however, that the moderate spike observed in the summer during the post-epidemic period is largely due to the year 2013 (35% of all cases in the post-epidemic period), which recorded outbreaks from June to August. In general, the holiday periods – particularly at the end of the year, Easter and in the summer – favour measles importation and import-related cases. On the other hand, the school holidays tend to interrupt outbreaks affecting children and adolescents.
The introduction of vaccination of young children against a disease usually considered a childhood illness, such as measles, generally goes hand in hand with a decline in the incidence of the disease and an increase in the age of remaining cases. Incidence decreases in particular in cohorts successively targeted by vaccination, but also in older age groups through a herd immunity effect. This difference in the impact of vaccination by age explains the increase in the median age of cases. The age continues to rise as vaccination coverage grows, as observed recently in Switzerland and also elsewhere in Europe [12, 13], North America [14], Japan [15], Taiwan [16] and China [11, 17]. Contrary to recent observations in Beijing, where increasing vaccination coverage coincided with a rising proportion of cases among infants below 1 year of age, this proportion remained low in Switzerland during both the epidemic and the post-epidemic period [11]. Similarly, during both periods, the incidence of measles in Switzerland was not highest among children under 1 year of age, nor even among those aged 1–4 years, in contrast to what was observed in the European Union in 2017 [9, 18]. The increase in age of cases in Switzerland also results in a significantly different mode of transmission between epidemic and post-epidemic periods. In the former, measles circulates in the form of medium- to large-scale outbreaks within communities without adequate protection, mainly comprising children and adolescents (schools, families, nurseries). In the latter, the proportion of sporadic cases, usually imported by young adults, is higher. Such importations occasionally lead to brief outbreaks, particularly within the social circles of index cases. With 61% of recent cases aged 15 or over and peak incidence in the 15–19 age group, measles can no longer be considered a childhood disease. Fears of an increase in the number of measles complications, of which adults are more at risk, are unfounded, however. Following the epidemic period, the incidence of measles fell by 87–91% according to age in those aged 20 or over. Unsurprisingly, the average number of annual hospitalisations and complications fell by similar proportions in adults (−81% for hospitalisations, −84% for pneumonia and −100% for encephalitis).
The increase in the proportion of vaccinated cases between the two periods, including those having received two doses, is not a cause for concern either [19]. As a minority of vaccinated persons are not protected against measles (around 5% after one dose and 1% after two doses), the proportion of vaccinated persons among the cases automatically increases as vaccination coverage grows. However, the total number of cases decreases as vaccination coverage grows and the risk of contracting measles remains much higher among persons who are not vaccinated. Therefore in 2014–2016, 4–6% of children aged between 2 and 16 (depending on their age) had not received a measles vaccination at all and 3–7% had received only one dose. During the post-epidemic period, this minority of children and adolescents who are not or only partially vaccinated accounted for 93% of measles cases occurring in this age group, while the majority who were fully vaccinated (87–93%) accounted for only 7%.
Since at least the middle of the 2000s, measles vaccination coverage was higher in foreign nationals than in Swiss nationals, although the latter tended to catch up over time. This probably explains in part why the incidence of measles was lower in foreign nationals, particularly during the epidemic period. In addition, the major outbreaks that characterised this period often affected circles that were somewhat resistant to vaccination, such as adherents of anthroposophy and homeopathy, mainly living in suburban or rural areas, in which foreign nationals are probably under-represented. This partial focalisation of cases within like-minded communities largely disappeared during the post-epidemic period, which reduced the gap in incidence with foreign nationals.
At cantonal level, variations in vaccination coverage partly explained the differences in measles incidence during the epidemic period. Local or regional gaps in vaccination coverage then allowed large-scale outbreaks to appear and develop over several months. This link between coverage and incidence disappeared during the post-epidemic period, which is characterised by a proliferation of sporadic cases and brief outbreaks that are often imported, hence the more random localisation of these rare cases.
The epidemic curve of the last three years, together with the genotyping data and information on the importation status of these cases, speak clearly in favour of an interruption of endemic measles circulation in Switzerland. Indeed, the overall number of cases was limited and transmission was discontinuous, with many periods without a single report. Furthermore, the outbreaks were brief and involved a small number of cases (a maximum of 22 over 11 weeks) and the proportion of sporadic cases was relatively high, as was that of imported cases. In addition, both the sporadic cases and the outbreaks showed a high level of genetic diversity.
Although the overall immunity of the population no longer allows sustainable circulation of the measles virus in Switzerland, we still note rare cases of imported measles, or cases that are import-related or even endemic. By default, we have classified as endemic those cases with a known exposure in Switzerland but without a known epidemiological link to an imported case, and cases with a suspected exposure in Switzerland in the absence of a stay abroad during the incubation period. Ultimately, all endemic cases should be linked to an importation that was impossible to trace. They include, for example, outbreak cases where the exposure of the index case could not be clarified and sporadic cases unwittingly exposed to an infected tourist in a public place.
The main limitation of this study is that mandatory reporting of measles, like any passive surveillance system, tends to underestimate the actual number of cases. However, this applies to both the epidemic and post-epidemic period and presumably has little influence on the observed epidemiological changes.
It should also be noted that endemic rubella transmission has been interrupted in Switzerland too, as recently recognised by the WHO [20]. This stems from use of the combined MMR vaccine (measles, mumps, rubella) for many years, the very high efficacy of this vaccine against rubella, and the fact that rubella is less contagious than measles.
Significant efforts have been undertaken in recent years by the federal and cantonal public health authorities, as well as by physicians and laboratories, to better protect the public against measles and to combat outbreaks in Switzerland. This has resulted in a decline in incidence of measles to a historically low level and an increase in vaccination coverage, which however remains significantly below the elimination threshold for young children. A coherent series of epidemiological indicators shows that endemic measles transmission in Switzerland has been interrupted for several years. This was confirmed for 2016 and 2017 by an independent evaluation conducted by the Regional Verification Commission for Rubella and Measles Elimination (WHO Europe) [20].
In order to consolidate this elimination status and to prevent a resurgence of measles, as observed recently in a number of European countries [21], these efforts must be continued, particularly within the framework of the national vaccination strategy [3]. In particular, this involves further increasing vaccination coverage, especially the second dose in young children, and closing the gaps by catching up on missing vaccinations for all non-immune persons born after 1963 [22].
7 Office fédéral de la santé publique. Epidémiologie récente et actuelle de la rougeole en Suisse. Bull OFSP. 2013;17:276–7.
The Federal Office of Public Health would like to thank the physicians and laboratories for reporting the measles cases. It also wishes to thank the National Reference Laboratory for Measles and Rubella (CNRRR, based at Geneva University Hospital), and the regional WHO laboratory for measles and rubella, based at the Robert Koch Institute, for the sequencing and genotyping of measles viruses. Thanks also go to the cantons and to Phung Lang, Carlotta Superti-Furga, Philipp Sinniger and Jan Fehr from the Epidemiology, Biostatistics and Prevention Institute (EBPI) at the University of Zurich for collecting and providing data on vaccination coverage.
No financial support and no other potential conflict of interest relevant to this article was reported.
1World Health Organization. Regional office for Europe. Eliminating measles and rubella: Framework for the verification process in the WHO European Region. Copenhagen: WHO; 2014. http://www.euro.who.int/en/health-topics/communicable-diseases/measles-and-rubella/publications/2014/eliminating-measles-and-rubella.-framework-for-the-verification-process-in-the-who-european-region [accessed on 10 Jan. 2019]
2Office fédéral de la santé publique. Stratégie nationale d’élimination de la rougeole 2011–2015. Berne: OFSP; 2012. www.bag.admin.ch/strategierougeole [accessed on 24 Sept. 2018]
3Office fédéral de la santé publique. Stratégie nationale de vaccination. Berne: OFSP; 2017. https://www.bag.admin.ch/bag/fr/home/strategie-und-politik/nationale-gesundheitsstrategien/nationale-strategie-impfungen-nsi.html [accessed on 10 Jan. 2019]
4Confédération suisse. Ordonnance du DFI sur la déclaration d'observations en rapport avec les maladies transmissibles de l'homme (RS 818.101.126). https://www.bag.admin.ch/infreporting [accessed on 10 Jan. 2019]
5Office fédéral de la santé publique. La Suisse se dote d’un centre national de référence pour la rougeole et la rubéole et réorganise le processus de génotypage de ces virus. Bull OFSP. 2017;51:15‒6. www.bag.admin.ch/bag-bulletin [accessed on 10 Jan. 2019]
6 Richard JL , Masserey Spicher V . Large measles epidemic in Switzerland from 2006 to 2009: consequences for the elimination of measles in Europe. Euro Surveill. 2009;14(50):19443.
8 Richard JL , Vidondo B , Mäusezahl M . A 5-year comparison of performance of sentinel and mandatory notification surveillance systems for measles in Switzerland. Eur J Epidemiol. 2008;23(1):55–65. doi:.https://doi.org/10.1007/s10654-007-9187-1
9European Centre for Disease Prevention and Control. Measles and rubella surveillance – 2017. Stockholm: ECDC; 2018. https://ecdc.europa.eu/sites/portal/files/documents/Measles-and-Rubella-Surveillance-2017.pdf [accessed on 17 Mar. 2019]
10Office fédéral de la santé publique. Directives de lutte contre la rougeole et les flambées de rougeole. Directives et recommandations. Berne: OFSP; 2013. https://www.bag.admin.ch/bag/fr/home/krankheiten/krankheiten-im-ueberblick/masern.html [accessed on 10 Jan. 2019]
11 Li J , Lu L , Pang X , Sun M , Ma R , Liu D , et al. A 60-year review on the changing epidemiology of measles in capital Beijing, China, 1951-2011. BMC Public Health. 2013;13(1):986. doi:.https://doi.org/10.1186/1471-2458-13-986
12 Woudenberg T , van Binnendijk RS , Sanders EAM , Wallinga J , de Melker HE , Ruijs WLM , et al. Large measles epidemic in the Netherlands, May 2013 to March 2014: changing epidemiology. Euro Surveill. 2017;22(3):30443. doi:.https://doi.org/10.2807/1560-7917.ES.2017.22.3.30443
13 Filia A , Bella A , Del Manso M , Baggieri M , Magurano F , Rota MC . Ongoing outbreak with well over 4,000 measles cases in Italy from January to end August 2017 - what is making elimination so difficult? Euro Surveill. 2017;22(37):30614. doi:.https://doi.org/10.2807/1560-7917.ES.2017.22.37.30614
14 Duclos P , Redd SC , Varughese P , Hersh BS . Measles in adults in Canada and the United States: implications for measles elimination and eradication. Int J Epidemiol. 1999;28(1):141–6. doi:.https://doi.org/10.1093/ije/28.1.141
15 Kaida A , Iritani N , Kanbayashi D , Yamamoto SP , Hirai Y , Hakui N , et al. Ten-year surveillance of measles virus from 2007–2016 in Osaka City, Japan. Jpn J Infect Dis. 2018;71(2):152–4. doi:.https://doi.org/10.7883/yoken.JJID.2017.322
16 Cheng WY , Wang HC , Wu HS , Liu MT . Measles surveillance in Taiwan, 2012-2014: Changing epidemiology, immune response, and circulating genotypes. J Med Virol. 2016;88(5):746–53. doi:.https://doi.org/10.1002/jmv.24392
17 Li S , Ma C , Hao L , Su Q , An Z , Ma F , et al. Demographic transition and the dynamics of measles in six provinces in China: A modeling study. PLoS Med. 2017;14(4):e1002255. doi:.https://doi.org/10.1371/journal.pmed.1002255
18Muscat M, Ben Mamou M, Singh S, de Kat C, Jankovic D, Huseynov S, et al. Eliminierung der Masern aus der Europäischen Region der WHO – Herausforderungen bleiben. Bundesgesundheitsbl. 2019. https://doi.org/https://doi.org/10.1007/s00103-019-02920-2
19 Althaus CL , Salathé M . Measles Vaccination Coverage and Cases among Vaccinated Persons. Emerg Infect Dis. 2015;21(8):1480–1. doi:.https://doi.org/10.3201/eid2108.150284
20World Health Organization. Regional office for Europe. Seventh meeting of the European regional verification commission for measles and rubella elimination (RVC). Copenhagen: WHO; 2018. http://www.euro.who.int/__data/assets/pdf_file/0008/378926/7th-RVC-Meeting-Report-FINAL.pdf?ua=1 [accessed on 10 Jan. 2019]
21World Health Organization. Regional office for Europe. Measles cases hit record high in the European Region; 20 August 2018. http://www.euro.who.int/en/media-centre/sections/press-releases/2018/measles-cases-hit-record-high-in-the-european-region [accessed on 10 Jan. 2019]
22Office fédéral de la santé publique, Commission fédérale pour les vaccinations. Plan de vaccination suisse 2018. Directives et recommandations. Berne: OFSP; 2018. www.bag.admin.ch/plandevaccination [accessed on 10 Jan. 2019]
No financial support and no other potential conflict of interest relevant to this article was reported.