Designing artificial senses: steps from physiology to clinical implementation
DOI: https://doi.org/10.4414/smw.2019.20061
Angélica Pérez
Fornosa, Raymond
Van de Bergbc, Jörg
Sommerhalderd, Nils
Guinanda
aDivision of Otorhinolaryngology and Head-and-Neck Surgery, Department of Clinical Neurosciences, Geneva University Hospitals, Geneva
bDepartment of Otorhinolaryngology and Head and Neck Surgery, Division of Balance Disorders, Maastricht University Medical Centre, School for Mental
Health and Neuroscience, Maastricht, Netherlands
cFaculty of Physics, Tomsk State Research University, Tomsk, Russian Federation
dOphthalmology Clinic, Department of Clinical Neurosciences, Geneva University Hospitals, Geneva
Summary
Our senses are the main information channels through which we perceive and interact with the world. Consequently, the physical and social functioning of patients suffering from severe sensory disabilities is limited on several levels. This has motivated the development of a novel therapeutic alternative: “artificial senses”, more commonly known as sensory neuroprostheses.
In order to restore lost function, sensory neuroprostheses attempt to take advantage of the information transfer pathway common to all senses: (i) transduction of the physical stimulus by sensory receptors, (ii) transmission of relevant information to primary sensory areas in the brain by sensory afferents, and (iii) analysis and integration of the information at multiple levels in the central nervous system. Neurosensory deficits might occur upon damage to any of the structures involved in this process. However, damage to the peripheral sensory receptor is often the cause of neurosensory loss. Most sensory neuroprostheses attempt to “replace” the malfunctioning or missing peripheral sensory organ by directly delivering basic sensory information to the brain using electrical currents. If the prosthesis is able to deliver enough consistent information, the brain will be able to correctly interpret it and useful rehabilitation can be achieved.
This review presents the main challenges related to the development, implementation and translation to clinical practice of these devices: (i) sensory information needs to be efficiently delivered to specific neural targets (e.g., peripheral afferents or specific central nuclei); (ii) then the expected physiological response must be evoked and quantified; (iii) the restoration of basic sensory abilities can lead to useful rehabilitation in meaningful everyday activities; (iv) optimal prospects require specific rehabilitation therapy and lifelong medico-technical follow-up.
To conclude, the current state and future of sensory neuroprostheses will be discussed. This will include current clinical and technical challenges, future prospects, and the potential of these devices to improve our fundamental knowledge of sensory physiology and neurosensory deficits.
Introduction
Our senses are the main means through which we perceive, understand, interact with and react to our environment. Consequently, sensory disabilities are a serious challenge that impacts all levels of physical and social functioning. This impact is even more pronounced in fragile populations, such as children, where general development is often impaired, leading to poor perceptions in adult life [1–4]. The elderly population is also seriously affected. Isolation and early cognitive decline are known comorbidities [5–7]. Finally, in addition to the impact on the individual, unaddressed disabilities also generate high costs for society [8]. This is related to, for example, healthcare system costs, loss of productivity, and special educational needs (see, e.g., [9]). A number of therapeutic alternatives exist, such as rehabilitation therapy and augmentation and substitution devices. These can be successful in certain circumstances. However, patients suffering from severe sensory disabilities often remain limited and have a poor quality of life.
Sensory neuroprostheses are new alternatives that have become available in the last 50 years. Their development and later translation to the clinic required multidisciplinary research efforts, including a deep understanding of primary sensory processing and fundamental investigations into the psychophysical basis of perception. The goal of this review is to highlight the multiple challenges involved in the development of sensory neuroprostheses, the current state of the technology, and to briefly discuss future clinical and research prospects.
Basic physiology of sensory systems
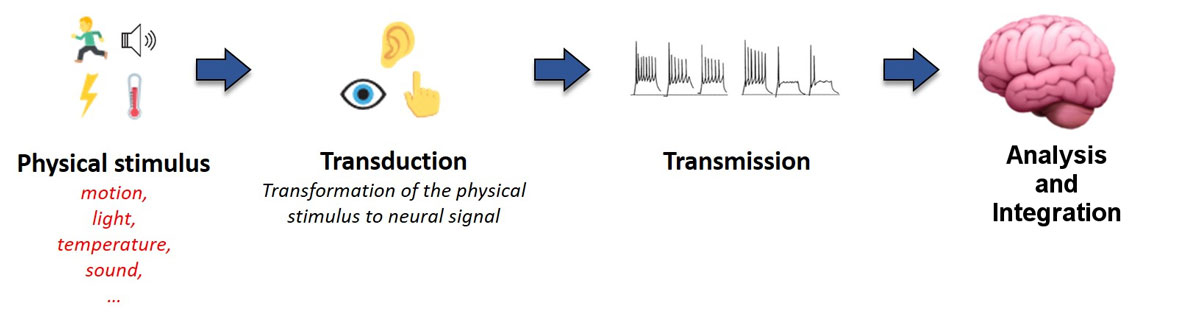
Perception and behaviour are fundamentally determined by internal (i.e., physiological) and external (i.e., environmental) events or stimuli. The nature of these stimuli can vary, for example mechanical in the case of sounds or chemical in the case of odorants, and thus require highly specialised sensory systems for effective detection, integration and processing. Despite some fundamental differences, all sensory systems rely on very similar processes which can be summarised in three fundamental steps (fig. 1).
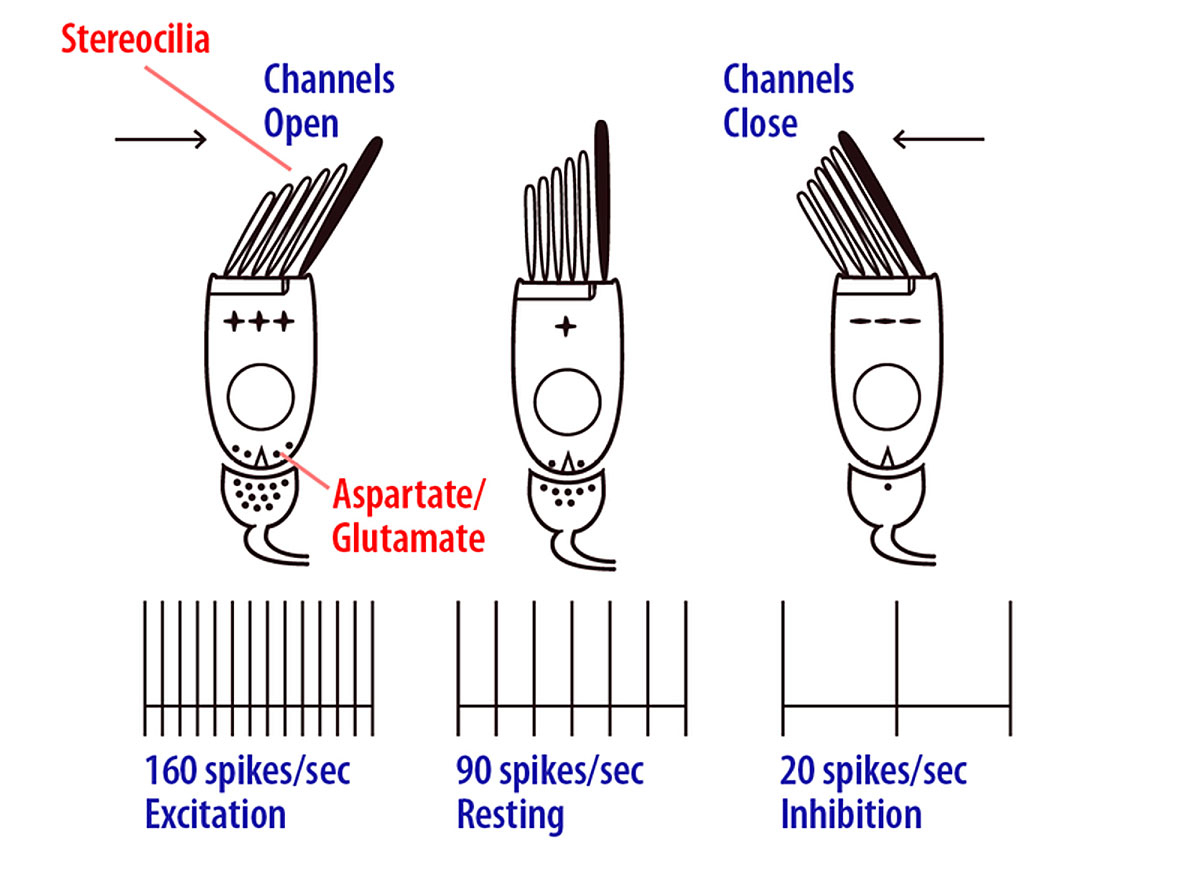
The first step is sensory transduction. This is achieved by a set of specialised receptors which detect stimuli (e.g., sound, light, temperature, motion) and transform them into neural electrical currents. In some cases, sensory receptors can be relatively simple (e.g., bare nerve endings reacting to changes in temperature). Other sensory modalities, such as hearing and vision, rely on complex, specialised cells that act as part of a network to enhance detection performance across a broad range of specific stimuli. The basic transduction process consists of a change in the membrane conductance of the sensory receptor upon physical/chemical stimulation. This leads to cell depolarisation (or hyperpolarisation) that excites (or inhibits) primary sensory neurons. The relevant features of the stimulus are represented by up- and down-modulations of the firing rate of the primary sensory neurons, compared to the rate when they are in their spontaneous or “at rest” state. Figure 2 illustrates these steps for hair cells in the vestibular system. When there is no physical stimulation (i.e., no motion), the hair cell’s potential is in its rest state and the vestibular nerve fires at its spontaneous firing rate. Upon mechanical vibration, the hair cell depolarises when the stereocilia deflect towards the kinocilium (the longest and most apical stereocilium), and hyperpolarises when the stereocilia deflect in the opposite direction. This results in a proportional increase or decrease in firing rate of the primary sensory neuron.
Once the sensory signal has been detected and transduced by the peripheral systems, the second step in the process is the transmission of the neural signal to the central nervous system (CNS). The axons of the primary sensory neurons form the afferent sensory nerve, whose compound activity encodes all pertinent characteristics of the stimulus. The information is then transmitted to the CNS through single or multiple parallel processing pathways, depending on the sensory modality. Note that each of these pathways involve a varying amount of neural tissue and nuclei, and consequently a varying number of “pre-processing” steps. However, it is important to highlight that, right from the initial transduction stage, sensory information is systematically organised in a specific and meaningful way (e.g., spectrally for sounds, spatially for images). This fundamental organisation is preserved all along the transmission pathway to the CNS (e.g., retinotopic for vision, tonotopic for hearing).
Finally, the third step in the sensory process is the analysis and integration of the information by the CNS. Each sensory pathway projects to specific subcortical and cortical areas. Primary sensory areas in the cortex receive information from each sensory system. From there, sensory information projects to the higher order sensory areas, where the information is segregated for the analysis of particular aspects of sensory stimuli. Finally, association areas integrate the information from different cortical areas to achieve the complex, multimodal representations of stimuli. It is interesting to note that the vestibular system is an example where information is directly projected to different cortical regions which are sensitive not only to vestibular input, but also receive inputs from other sensory systems which are fundamental to the overall sense of balance and equilibrium [10, 11]. Note that this analysis and integration step is of course not a sequential process, but is complex and dynamic: perception, cognitive functions and motor responses are also modulated by top-down processes (e.g., previous knowledge, attention, etc.), but this complexity is beyond the scope of this paper.
Sensory neuroprostheses: artificial restoration of lost sensory function
Neurosensory deficits can occur upon damage to any structure involved in the sensory perception process. However, if damage occurs only to the peripheral sensory receptor, the remaining structures involved in transmitting sensory information to the brain can remain functional. This is frequently observed, and is the foundation for the basic concept of sensory neuroprostheses: replace the absent sensory receptor by a prosthesis that is able to transmit enough basic features of the stimuli in a “language” that can be properly understood and interpreted by the CNS (fig. 3). In practice, lost function is replaced by an external sensor (e.g., a microphone, camera, gyroscope) that feeds a specific processing device able to translate this information into a consistent pattern of electrical currents. This “artificial” information is then delivered via implanted electrodes positioned in the vicinity of the sensory afferents (e.g., the auditory nerve, optic nerve, vestibular nerve). If delivered in the right way and to the correct structures, this information should reach the brain and be properly interpreted, potentially allowing the restoration of the lost sensory modality (e.g., hearing, vision, vestibular function).
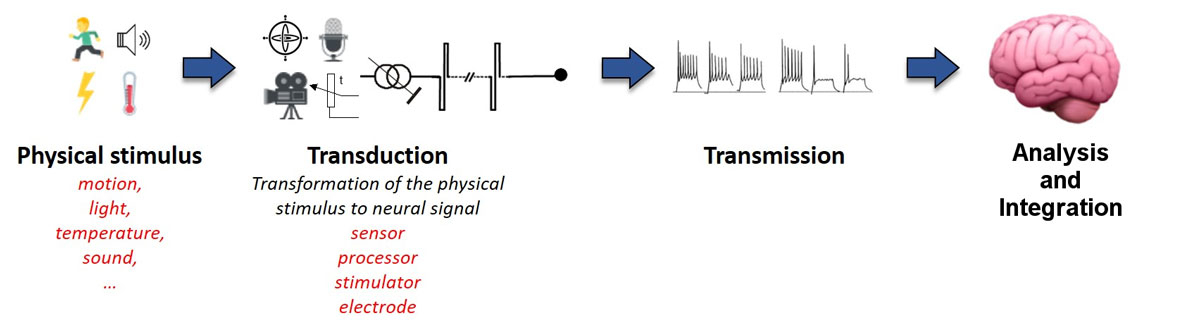
Figure 3
The “artificial senses”. When the sensory deficit is due to damage to the peripheral receptor, it can be replaced by external and implanted electronic components (sensor, processor, stimulator, electrodes in the vicinity of the sensory afferent). The concept is to transform the physical stimulus into a pattern of electrical currents that is consistent with the fundamental organization of the sensory system.
Today, a number of sensory neuroprosthetic devices exist. Some are widely available and have a proven track record of clinical success, such as cochlear implants [12]. Others, like retinal implants, have become available to blind patients suffering from retinal degenerations only in recent years [13]. Finally, newer devices to address other sensory deficits, like a vestibular implant to rehabilitate severe balance deficits, are being investigated and are only available as research devices.
Cochlear implants
The most frequent cause of severe to profound neurosensory deafness is damage to the sensory structures in the inner ear, with the rest of the auditory pathway remaining functional. Cochlear implants are devices designed to restore basic hearing abilities in patients suffering from this type of sensory deficit [14]. The idea is to bypass the damaged or missing sound transduction apparatus (i.e., inner hair cells in the cochlea) and directly stimulate the auditory nerve using electrical currents. Briefly, a microphone is used to capture sound stimuli. This information is fed to a speech processor that transforms it into a pattern of signals that is transmitted to an implanted stimulator transcutaneously using radio waves. The implanted stimulator decodes the signal and sends the corresponding pattern of electrical stimulation currents to the auditory nerve through an array of electrodes inserted in the cochlea. The distribution of currents along the electrode array respects the tonotopical organisation of the auditory system: signals generated from low frequency stimuli are transmitted via apical electrodes, while signals generated from high frequency stimuli are transmitted via basal electrodes [15, 16].
Cochlear implants are the most successful neuroprostheses to date, with a proven track record of success in more than 600,000 adults and children with profound bilateral deafness [17–19]. Today, cochlear implant recipients can understand spoken language and thus communicate effectively based exclusively on sound cues. Even children suffering from congenital or pre-lingual deafness can develop efficient speech comprehension and production skills, and consequently follow mainstream education along with their hearing peers if implanted early in life [20, 21]. The latter is probably the most impressive accomplishment of “artificial senses”.
Another example of an “artificial ear” is the auditory brainstem implant. This device is analogous in most aspects to the cochlear implant, except that it attempts to skip the auditory nerve and stimulate the next step in the auditory system: the cochlear nuclei in the brainstem [22]. These devices are clinically used in some centres to rehabilitate deaf patients who are not candidates for cochlear implantation, with variable outcomes [23].
Retinal implants
Retinal implants are devices that attempt to restore basic visual abilities to blind patients using electrical currents that directly activate bipolar and/or ganglion cells in the retina. This approach thus requires that the inner retinal layers still function relatively well so that visual information can be conveyed to the brain via the optic nerve. This appears to be the case, to a certain extent, in retinal degenerations such as retinitis pigmentosa and age-related macular degeneration [24–26]. Note that other types of visual prostheses besides retinal implants, such as cortical implants (which attempt to stimulate the primary visual cortex directly) and less invasive suprachoroidal and transcorneal implants are also currently being investigated.
Retinal implants have become commercially available in recent years. The technology has been demonstrated to be stable and safe for long-term implantation [27, 28]. All past and ongoing trials have demonstrated that these devices successfully elicit visual percepts. However, the real rehabilitation prospects of retinal implants are still a matter of discussion [13, 29].
The major clinical concern related to retinal implants is that the functional level provided by these devices remains limited [27, 30–33]. This is well illustrated by the relatively low use reported for retinal prosthesis wearers (maximum 2-3 h/day; [29, 31]) compared to the average for cochlear implant wearers (12 h/day; [34]). Implanted patients can, in the best cases, identify large and simple objects (e.g., light windows on a dark wall, dishes and cutlery) and use the rudimentary visual information provided by the device to improve their hand-eye coordination and mobility skills. “Star” performers are also capable of achieving more complex tasks, such as reading very large characters, one at a time [35, 36]. In everyday life, this translates to a very crude ability to recognise simple forms, which in most cases is limited to the “presence-or-absence” of a shape [30, 31] and the ability to distinguish between dynamic (moving) and static objects. The other major clinical issue is the substantial inter-subject variability in outcomes, with a relatively high rate of “bad” performers (see, e.g., [32]). Extensive research is therefore still needed in this field to improve device performance and candidate selection criteria. Special care should also be taken to ensure adequate patient information and to reach a consensus on the selection of pertinent outcomes [37]. This should be followed by reliable and meaningful studies providing quantitative evidence of functional improvements and significant quality of life metrics [38].
Vestibular implants
Vestibular implants are the most recent of the three sensory neuroprostheses presented here. These devices attempt to restore semicircular canal function in patients suffering from severe bilateral vestibulopathy. They use electrical currents delivered directly to the ampullary branches of the vestibular nerve. Motion information captured with a gyroscope is fed to a dedicated processor that communicates with the implanted device transcutaneously. Then, the implanted stimulator delivers the “artificial” motion signal to the vestibular system via electrodes implanted in the vicinity of the vestibular nerve branches. This concept is based on the pioneering work of Cohen and Suzuki, who were the first to demonstrate that electrical stimulation of the vestibular nerve branches resulted in eye and postural movements consistent with the stimulated branch [39–41]. Later, the team of Merfeld and Lewis laid the basis for actual vestibular implants through animal studies [42–47].
Today, vestibular implants are research only devices. They are being actively investigated by three groups: the Geneva-Maastricht group (Geneva University Hospitals and the University of Geneva, Geneva, Switzerland; Maastricht University Medical Centre, Maastricht, The Netherlands), the group at the Vestibular NeuroEngineering Laboratory (Johns Hopkins Medicine, Baltimore, United States of America), and the group at the Virginia Merrill Bloedel Hearing Research Center (University of Washington, Washington, United States of America). The Geneva-Maastricht group is the pioneer in human research related to vestibular implants. This group has achieved several fundamental milestones: (i) the development of special surgical approaches and their validation in acute intra-operative studies [48–52], (ii) the first chronic implantations in humans [53], (iii) the establishment of efficient stimulation strategies [54], and more recently (iv), the demonstration of useful rehabilitation with their vestibular implant prototype devices, both for basic vestibular reflexes [55–57] and for activities with clinical significance [58]. The Baltimore group started their investigations with animal models, where they addressed an important clinical issue by demonstrating that hearing could be preserved effectively during vestibular implantation with their particular device design and surgical technique [59]. They were also successful in restoring the vestibulo-ocular reflex in chinchillas and rhesus monkeys [60–63]. This group initiated a feasibility trial in humans during the summer of 2016 (https://clinicaltrials.gov/ct2/show/NCT02725463), but no results have been published yet. The initial concept of the group at the University of Washington was slightly different from the two preceding groups. They designed a device that functioned as a “vestibular pacemaker”. This was not intended to code motion, but to control the repeated, transient episodes of vertigo associated with Meniere’s disease. Unfortunately, they could not replicate the promising inner ear function preservation results of their animal studies in humans [64], and both the auditory and vestibular function of the patients implanted in their study deteriorated considerably [65, 66]. Recently, this group seems to have shifted focus to a motion coding device, as described in their latest publications [65–68].
From physiology to clinical application
Detecting meaningful sensory information and delivering it to the brain in the best possible form involves numerous challenges. These will be presented as a step-by-step approach highlighting the main aspects fundamental to the research, development and translation to the clinic of the “artificial senses”.
Transferring sensory information to the brain
The first step in the artificial restoration of a lost sensory function is to detect the physical stimulus and translate it into a pattern of meaningful electrical stimulation currents. The quality and amount of information that can be transmitted by the neuroprosthesis will be fundamentally limited by three aspects: (i) the characteristics of the external receptor (e.g., camera, microphone, motion sensor), (ii) the computing power of the processing device, and (iii) the finite number of electrodes available to transmit relevant information [69]. In addition, the pattern of electrical currents needs to be delivered to the primary sensory afferents in the most efficient way, minimising loss of information at the electrode-nerve interface. The choice of stimulation parameters is therefore critical.
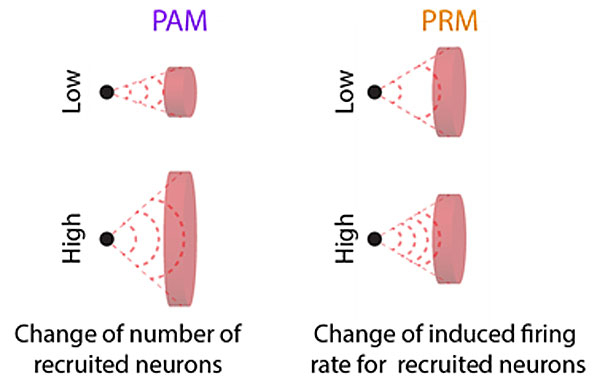
Simply speaking, primary sensory afferents encode the main characteristics of the stimulus via two complementary mechanisms. The first is the modulation of the firing rate of individual fibres (e.g., the firing rate of a fibre increases as stimulus intensity increases; see fig. 2). However, the dynamic range that can be encoded this way is limited because the firing rate of individual fibres is small (see, e.g., [70]). To increase the dynamic range, sensory systems employ a second mechanism, the progressive recruitment of larger populations of fibres with different sensitivities. Central neural relays can therefore encode stimulus characteristics based on the variations in individual firing rates that are induced in variable populations of fibres. Current sensory neuroprostheses attempt to mimic these “firing rate” and “fibre population” modulation mechanisms by using biphasic electrical pulse trains whose rate (number of pulses per second) or amplitude (current intensity) can be modulated to convey sensory information (fig. 4). Pulse rate modulation can indeed closely mimic individual firing rate variations, as demonstrated in electrophysiological experiments based on intracellular single cell recordings. However, current sensory neuroprostheses rely on distant extracellular stimulation. In this configuration, the range of responses that can be evoked using pulse rate modulation is insufficient for useful rehabilitation. Another possibility is to encode stimulus characteristics through the progressive recruitment of varying populations of fibres using amplitude modulation. In practice, this mode has been shown to be more efficient in generating a wider range of physiological responses, within the limited range available to the neuroprosthesis [71]. It is therefore routinely used in cochlear and retinal implant recipients. The advantage of amplitude modulation has recently also been verified experimentally in vestibular implant recipients [53, 54, 66].
At this point, there is a significant disadvantage to using amplitude modulation as the preferred stimulation paradigm that must be discussed. As introduced above, amplitude modulation recruits varying populations of fibres because of variation in the size of the induced electrical fields resulting from current intensity changes. In other words, the application of low electrical currents results in small electrical fields which recruit a small number of fibres, while high currents create large electrical fields which recruit a large number of fibres (fig. 4). This means that, as with high stimulation currents, the electrical field can be spread across a large neurosensory area, impeding the specific activation of a precise population of neurons. This fundamentally limits the amount of information that can be transmitted by the device. For example, current cochlear implants comprise 12-22 active electrodes, but in reality, it appears that only 8-10 distinct frequency percepts can be evoked with these [72–74]. This has also been demonstrated for retinal implants, where the situation appears to be aggravated by an additional mechanism, presumably of neural origin [75, 76]. In vestibular implants, the lack of selectivity of stimulation results, for example, in significant misalignments of the vestibulo-ocular response that could potentially hinder gaze stabilisation mechanisms [55, 63]. Alternative electrical stimulation paradigms which use co-modulation of amplitude and rate have been proposed to tackle this limitation [77, 78]. In theory, these new paradigms would better replicate normal sensory physiology and results from animal experiments appear promising. However, they still need to be validated in humans to determine if the benefit is clinically relevant despite the additional technological complexity.
Restoration of basic sensory functions
Once electrical stimulation has been delivered to the primary afferents, the expected physiological response must be objectively measured and quantified. This is necessary to evaluate the efficacy of the device and also to adapt the stimulation paradigm to each individual patient (“device fitting”). This step still requires fundamental knowledge of general and specific sensory physiology, as well as many quantitative and reproducible measures.
To achieve useful and meaningful function, sensory systems rely on some basic features that, when put together, allow complex behaviour. For example, in the case of vision, the ability to resolve detail in a visual image (i.e., visual acuity) mediates shape/object recognition, and is consequently the basis of complex but essential tasks such as reading. In the world of hearing intensity and frequency discrimination are the basis of language identification skills. A similar example for the vestibular system is the vestibulo-ocular reflex. Loss of this important reflex is thought to be correlated with clinical complaints of patients (e.g., oscillopsia). Furthermore, it is relatively simple to measure and quantify (example in fig. 5). Therefore, the vestibulo-ocular reflex has been the physiological response of choice for assessing the efficacy of vestibular implants [79].
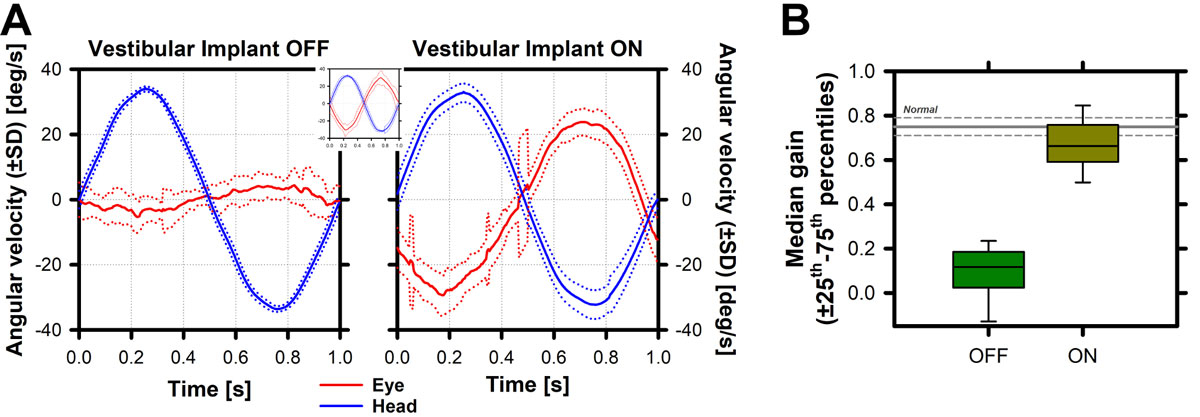
Figure 5
Vestibulo-ocular response measured in a vestibular implant recipient. A Left panel: When the vestibular implant is turned OFF, the vestibulo-ocular reflex (red plot) recorded upon a sinusoidal head movement (blue plot) is practically absent. When the vestibular implant is turned ON, a clear vestibulo-ocular reflex can be recorded, appearing to “mirror” the head movement, similar to what is observed in the healthy vestibulo-ocular reflex (inset between both panels) (57, 80-82). B The gain of the vestibulo-ocular reflex (peak head velocity / peak eye velocity) can then be computed for each experimental condition allowing, for example, the comparison of the efficacy of the “artificial” response with the “normal” vestibulo-ocular reflex (gray lines in figure 4B), the comparison between conditions/parameters (e.g., OFF vs ON), and/or to follow the evolution of responses.
Putting it all together: restoration of clinically relevant everyday activities
A successful sensory neuroprosthesis should restore useful function during complex tasks by combining the previously mentioned basic sensory functions. This must be quantified during tasks representative of patients’ everyday difficulties. In other words, in addition to the technical challenges directly related to the development of the device, specific tests related to patient complaints which correlate with quality of life measures must be developed [29, 80–82].
Once again, the recent field of vestibular implants provides a good illustration of this. One of the major complaints of patients suffering from a severe bilateral vestibulopathy is the lack of image stabilisation capabilities in dynamic situations [80, 81]. For example, they are unable to recognise the faces of people or cannot read information signs while they are walking. It is generally accepted that these dynamic image stabilisation abilities are mainly mediated by the vestibulo-ocular reflex. Therefore, if vestibular implants are an effective means of restoring the vestibulo-ocular reflex as presented above [57, 83–85], then this artificial reflex should be able to restore visual abilities in dynamic situations, under conditions representative of everyday life (e.g., walking). This should be verified experimentally in order to understand the rehabilitation prospects of the device [58]. Similar examples can be found in the cochlear and retinal implant fields for language comprehension [86] and reading [87], respectively.
Long-term rehabilitation
Finally, an important aspect for the success of sensory neuroprostheses is the need for specifically targeted rehabilitation therapy and long-term follow-up.
On one hand, the information provided by these “artificial senses” is limited and of a different nature to the one provided by healthy systems. For example, commercial cochlear implants have between 12 and 22 active contacts, while a healthy auditory system features approximately 3000 inner hair cells. Commercial retinal implants incorporate between 60 and 1500 electrodes to replace 100,000,000 rods and 3,000,000 cones. The amount and quality of information is further limited by electrical field and neural interactions, as already mentioned [73, 74, 88]. Therefore, patients must learn to interpret this limited (and potentially degraded) artificial information to maximise functional prospects. This requires specific rehabilitation therapy which can be relatively rapid, for example in cochlear implant recipients with post-lingual deafness. Such cases only require a few training sessions before patients can appropriately interpret the artificial information provided by the prosthesis and “match” it with the sensory percepts they had before disease. However, other cases require longer, intensive training periods that can last several years. For example, small children suffering from pre-lingual deafness require regular speech therapy so that they can develop language skills from the limited information provided by the implant.
On the other hand, sensory neuroprostheses require regular technical intervention from the specialised teams at implant centres to ensure proper functioning of the devices. In addition, the electrical stimulation parameters must be progressively adapted so that the optimum configuration can be achieved and maintained. For these reasons, it is important to highlight that sensory neuroprostheses are devices that require lifelong technical intervention, where success relies not only on the implantation surgery, but also on significant efforts from the patient together with the specialised group who carry out implant adaptation, maintenance and follow-up.
Discussion and perspectives
The goal of this paper was to present the main challenges surrounding the “artificial senses”, which attempt to replicate basic elements of the “normal” physiology of the different sensory systems. We saw that the same basic assumptions underlie the development of different sensory neuroprostheses. This is why success in one particular field (cochlear implants) has boosted and facilitated the development of others (retinal and vestibular implants, auditory brainstem implants). Indeed, the first prototypes of retinal, vestibular and auditory brainstem implants are “modified” versions of commercial cochlear implants.
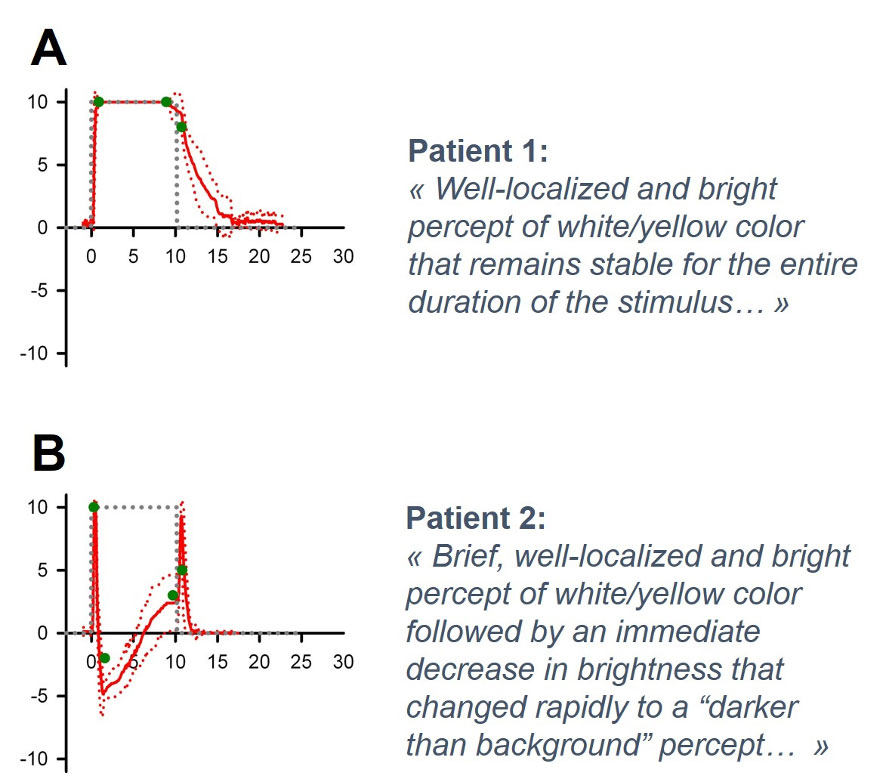
It is important to highlight that all the aspects mentioned above rely on the assumption that the basic organisation of the relevant sensory modality is preserved, allowing the brain to make sense of the “artificial” peripheral stimulation. Fortunately, this seems to be often the case in cochlear and vestibular implants, where the information is directly conveyed by sensory receptors to the vestibulo-cochlear nerve. The visual system is much more challenging in this respect, since information is conveyed to the optic nerve via a relatively complex retinal circuit. A good example of this additional complexity can be taken from a study conducted with a group of nine retinal implant recipients [89]. This paper showed that the time-course of visual percepts evoked by electrical stimulation was not as expected: it did not follow the time-course of the stimulus and was strikingly different between patients, leading to a range of performances from “good” (fig. 6A) to “poor” (fig. 6B; [89]). The reasons for this surprising observation are still poorly understood. One plausible explanation appears to be related to retinal remodelling in retinitis pigmentosa, with considerable inter-subject variation [90–92]. Another hypothesis points to the lack of selectivity of electrical stimulation, which could target bipolar cells, ganglion cells, or even ganglion cell axons. Manipulating some electrical stimulus parameters appears to improve the quality of visual percepts [93], but significant research in the field is still required to optimise outcomes and improve patient selection criteria [13].
Future perspectives
There are a number of fundamental issues that limit the artificial sensory function evoked by neuroprostheses that must still be addressed. Current devices use multichannel, monopolar extracellular stimulation configurations [94, 95] where efficacy is determined by two aspects. Firstly, the absolute amount of information that can actually be transmitted by the device is limited by the number of active channels (i.e., the number of stimulation electrodes). Secondly, as already mentioned, the selectivity of the stimulation is restricted to the size of the electrical fields generated upon stimulation. This latter aspect is the main factor limiting current neuroprostheses. This can be better understood by highlighting the main limitations of cochlear implants. Despite the success of these devices, they still do not provide sufficient information in several important everyday situations, such as speech perception in noisy environments, sound localisation and music perception. While some of these limitations can be improved with bilateral cochlear implantation or improved signal processing strategies, they cannot be eliminated because they are fundamentally related to the lack of selectivity of stimulation [96].
Besides the development of novel electrical stimulation paradigms, other promising alternatives to improve the selectivity of stimulation are currently being explored. The first alternative proposes the use of substitute stimulation methods with reduced spread and thus improved selectivity, such as optical stimulation. Short pulses of infrared light delivered from a focused source (i.e., laser) can be used to activate target neural tissue [97]. The applicability of this idea to cochlear and retinal implants is being actively investigated, with promising initial results from in vitro and in vivo experiments in animal models [98–100]. Optogenetics is another alternative, involving transgenic manipulations to introduce photosensitive ion channels (e.g., channelrhodopsin-2, ChR2) into neurons so that their activation can be directly controlled by specific light stimulation [101]. This particular approach has high significance in the field of vision. Indeed, if these viral vectors can be successfully and selectively induced in photoreceptor-deprived retinas, functional vision could potentially be restored without the need for an additional processing device [102]. Several obstacles remain before these approaches can be clinically applied to neurosensory restoration. The major hurdle of transgenic manipulations is obviously related to the inherent risks of inducing genetic modifications in humans. Other open issues are recurrent reports of the ineffectiveness of optical stimulation in evoking neural responses (see, e.g., [103]), minimisation of the effects of concurrent physical phenomena (e.g., pressure waves generated by the light stimulus, leading to the optoacoustic effect), solving the technical challenges involved in properly transducing the sensory stimulus (i.e., reaching the fast stimulation rates required for auditory nerve stimulation with relatively slow optical ion channels; [104]), and addressing the safety issues related to the long-term thermal stimulation of physiological systems [98, 105].
Another alternative to improve the selectivity of stimulation is to reduce the large distance between the stimulating electrodes and the neural targets using regenerative biology methods. The distance between the stimulating electrodes and the neural targets could potentially be reduced by novel penetrating electrode designs combined with neural growth factors to facilitate the migration of neural tissue towards the active surface of the stimulating electrodes (see, e.g., [106]). Furthermore, other, more focused stimulation methods that are not efficient with large electrode-to-nerve distances could also be implemented under these circumstances (i.e., bipolar stimulation). This idea seems appealing for retinal implants, but is hardly applicable in the field of cochlear and vestibular implants, where a physical barrier (the bony labyrinth) is present. Another idea is to promote the regeneration of sensory neuron dendrites that are then redirected towards the implant electrodes [107]. This could potentially lead to “gapless” electrode-to-nerve interfaces, even allowing one-to-one contact and “ideal” stimulation resolution [108]. The main challenges being investigated to bring the application of this interesting field one step closer are the potential difficulties related to altering the plasticity processes of the nervous system: how to precisely direct neural process growth towards the electrodes while preserving the primary organisation of the sensory system (e.g., retinotopy, tonotopy; see, e.g., [109]).
Closing remarks
This paper demonstrates the diverse, multidisciplinary nature of research on the “artificial senses”. Consequently, success in any specific field will undoubtedly have an important impact on all converging disciplines. For example, advances in the application of regenerative biology principles to sensory neuroprostheses will not only change the rehabilitation prospects of the devices, but will also promote fundamental research into the mechanisms of neural degeneration, which could open new avenues for the global treatment and prevention of these deficits. Another example of this cross-pollination is the ease with which any success in this field can be translated to treat other syndromes. For instance, the potential of electrical stimulation to treat a great variety of conditions, from various neurological disorders (Parkinson’s disease, epilepsy or dystonia) and psychiatric disorders (e.g., addiction and depression; [110, 111]) to sleep apnoea [112], is also being investigated. The potential of the concepts and technology derived from sensory neuroprostheses is so large that its actual potential impact is difficult to appreciate.
The development of sensory neuroprostheses relied on our fundamental knowledge of sensory physiology. Today, these devices also have the potential to become a powerful tool to increase our fundamental knowledge. Indeed, the “artificial senses” constitute a new experimental tool which allows us to interact directly with the nervous system in a way that has not been possible until recently. Therefore, the development of today’s sensory neuroprostheses will move our fundamental knowledge on physiology and disease forward. In turn, this knowledge will help pave the way for the “artificial senses” of the future.
This paper is an extract from a Privat-Docent thesis [
113
].
Acknowledgements
The authors thank Marco Pelizzone, Jean-Philippe Guyot and Herman Kingma for their comments, help and support. A special thankyou to the teams at the Geneva University Hospitals and at the Maastricht University Medical Center for their enthusiasm and collaboration. Last but not least, we want to thank the patients for their time and efforts: the field would not be able to progress without the pioneers that have made all this possible.
References
1
Solebo
AL
,
Teoh
L
,
Rahi
J
. Epidemiology of blindness in children. Arch Dis Child. 2017;102(9):853–7. doi:.https://doi.org/10.1136/archdischild-2016-310532
2
Quittner
AL
,
Leibach
P
,
Marciel
K
. The impact of cochlear implants on young deaf children: new methods to assess cognitive and behavioral development. Arch Otolaryngol Head Neck Surg. 2004;130(5):547–54. doi:.https://doi.org/10.1001/archotol.130.5.547
3
Bailly
D
,
Dechoulydelenclave
MB
,
Lauwerier
L
. [Hearing impairment and psychopathological disorders in children and adolescents. Review of the recent literature]. Encephale. 2003;29(4 Pt 1):329–37.
4
Wiener-Vacher
SR
,
Hamilton
DA
,
Wiener
SI
. Vestibular activity and cognitive development in children: perspectives. Front Integr Nuerosci. 2013;7:92. doi:.https://doi.org/10.3389/fnint.2013.00092
5
Cook
G
,
Brown-Wilson
C
,
Forte
D
. The impact of sensory impairment on social interaction between residents in care homes. Int J Older People Nurs. 2006;1(4):216–24. doi:.https://doi.org/10.1111/j.1748-3743.2006.00034.x
6
Fritze
T
,
Teipel
S
,
Óvári
A
,
Kilimann
I
,
Witt
G
,
Doblhammer
G
. Hearing Impairment Affects Dementia Incidence. An Analysis Based on Longitudinal Health Claims Data in Germany. PLoS One. 2016;11(7):e0156876. doi:.https://doi.org/10.1371/journal.pone.0156876
7
Iwasaki
S
,
Yamasoba
T
. Dizziness and Imbalance in the Elderly: Age-related Decline in the Vestibular System. Aging Dis. 2015;6(1):38–47. doi:.https://doi.org/10.14336/AD.2014.0128
8Morgon Banks L, Polack S. The Economic Costs of Exclusion and Gains of Inclusion of People with Disabilities: Evidence from Low and Middle Income Countries. CBM; 2013. Available at: http://disabilitycentre.lshtm.ac.uk/files/2014/07/Costs-of-Exclusion-and-Gains-of-Inclusion-Report.pdf.
9WHO. Global costs of unaddressed hearing loss and cost-effectiveness of interventions: a WHO report, 2017. Geneva, Switzerland; 2017.
10
Brandt
T
,
Dieterich
M
. The vestibular cortex. Its locations, functions, and disorders. Ann N Y Acad Sci. 1999;871(1 OTOLITH FUNCT):293–312. doi:.https://doi.org/10.1111/j.1749-6632.1999.tb09193.x
11
Lopez
C
,
Blanke
O
. The thalamocortical vestibular system in animals and humans. Brain Res Brain Res Rev. 2011;67(1-2):119–46. doi:.https://doi.org/10.1016/j.brainresrev.2010.12.002
12
Şahin
MI
,
Sagers
JE
,
Stankovic
KM
. Cochlear Implantation: Vast Unmet Need to Address Deafness Globally. Otol Neurotol. 2017;38(6):786–7. doi:.https://doi.org/10.1097/MAO.0000000000001416
13
Brandli
A
,
Luu
CD
,
Guymer
RH
,
Ayton
LN
. Progress in the clinical development and utilization of vision prostheses: an update. Eye Brain. 2016;8:15–25.
14
Rauschecker
JP
,
Shannon
RV
. Sending sound to the brain. Science. 2002;295(5557):1025–9. doi:.https://doi.org/10.1126/science.1067796
15
Kiang
NY
,
Moxon
EC
. Tails of tuning curves of auditory-nerve fibers. J Acoust Soc Am. 1974;55(3):620–30. doi:.https://doi.org/10.1121/1.1914572
16
Kiang
NY
,
Moxon
EC
. Physiological considerations in artificial stimulation of the inner ear. Ann Otol Rhinol Laryngol. 1972;81(5):714–30. doi:.https://doi.org/10.1177/000348947208100513
17
Gaylor
JM
,
Raman
G
,
Chung
M
,
Lee
J
,
Rao
M
,
Lau
J
, et al.
Cochlear implantation in adults: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(3):265–72. doi:.https://doi.org/10.1001/jamaoto.2013.1744
18
Peters
BR
,
Wyss
J
,
Manrique
M
. Worldwide trends in bilateral cochlear implantation. Laryngoscope. 2010;120(S2, Suppl 2):S17–44. doi:.https://doi.org/10.1002/lary.20859
19The Ear Foundation. Cochear Implant Information Sheet. In: Foundation TE, editor. Nottingham, United Kingdom2016.
20
Brennan-Jones
CG
,
White
J
,
Rush
RW
,
Law
J
. Auditory-verbal therapy for promoting spoken language development in children with permanent hearing impairments. Cochrane Database Syst Rev. 2014;(3):CD010100. doi:.https://doi.org/10.1002/14651858.CD010100.pub2
21
Deriaz
M
,
Pelizzone
M
,
Pérez Fornos
A
. Simultaneous development of 2 oral languages by child cochlear implant recipients. Otol Neurotol. 2014;35(9):1541–4. doi:.https://doi.org/10.1097/MAO.0000000000000497
22
Shannon
RV
. Auditory implant research at the House Ear Institute 1989-2013. Hear Res. 2015;322:57–66. doi:.https://doi.org/10.1016/j.heares.2014.11.003
23
Colletti
V
. Auditory outcomes in tumor vs. nontumor patients fitted with auditory brainstem implants. Adv Otorhinolaryngol. 2006;64:167–85. doi:.https://doi.org/10.1159/000094651
24
Humayun
MS
,
Prince
M
,
de Juan
E, Jr
,
Barron
Y
,
Moskowitz
M
,
Klock
IB
, et al.
Morphometric analysis of the extramacular retina from postmortem eyes with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1999;40(1):143–8.
25
Santos
A
,
Humayun
MS
,
de Juan
E, Jr
,
Greenburg
RJ
,
Marsh
MJ
,
Klock
IB
, et al.
Preservation of the inner retina in retinitis pigmentosa. A morphometric analysis. Arch Ophthalmol. 1997;115(4):511–5. doi:.https://doi.org/10.1001/archopht.1997.01100150513011
26
Stone
JL
,
Barlow
WE
,
Humayun
MS
,
de Juan
E, Jr
,
Milam
AH
. Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch Ophthalmol. 1992;110(11):1634–9. doi:.https://doi.org/10.1001/archopht.1992.01080230134038
27
da Cruz
L
,
Dorn
JD
,
Humayun
MS
,
Dagnelie
G
,
Handa
J
,
Barale
PO
, et al.; Argus II Study Group. Five-Year Safety and Performance Results from the Argus II Retinal Prosthesis System Clinical Trial. Ophthalmology. 2016;123(10):2248–54. doi:.https://doi.org/10.1016/j.ophtha.2016.06.049
28
Kitiratschky
VB
,
Stingl
K
,
Wilhelm
B
,
Peters
T
,
Besch
D
,
Sachs
H
, et al.
Safety evaluation of “retina implant alpha IMS”--a prospective clinical trial. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2015;253(3):381–7. doi:.https://doi.org/10.1007/s00417-014-2797-x
29Sommerhalder J, Pérez Fornos A. Prospects and Limitations of Spatial Resolution. In: Gabel VP, editor. Artificial Vision: A Clinical Guide. 1. Cham, Switzerland: Springer International Publishing Switzerland; 2017. p. 29-45.
30
Stingl
K
,
Schippert
R
,
Bartz-Schmidt
KU
,
Besch
D
,
Cottriall
CL
,
Edwards
TL
, et al.
Interim Results of a Multicenter Trial with the New Electronic Subretinal Implant Alpha AMS in 15 Patients Blind from Inherited Retinal Degenerations. Front Neurosci. 2017;11:445. doi:.https://doi.org/10.3389/fnins.2017.00445
31
Stingl
K
,
Bartz-Schmidt
KU
,
Besch
D
,
Chee
CK
,
Cottriall
CL
,
Gekeler
F
, et al.
Subretinal Visual Implant Alpha IMS--Clinical trial interim report. Vision Res. 2015;111(Pt B):149–60. doi:.https://doi.org/10.1016/j.visres.2015.03.001
32
Luo
YH
,
da Cruz
L
. The Argus(®) II Retinal Prosthesis System. Prog Retin Eye Res. 2016;50:89–107. doi:.https://doi.org/10.1016/j.preteyeres.2015.09.003
33
Ho
AC
,
Humayun
MS
,
Dorn
JD
,
da Cruz
L
,
Dagnelie
G
,
Handa
J
, et al.; Argus II Study Group. Long-Term Results from an Epiretinal Prosthesis to Restore Sight to the Blind. Ophthalmology. 2015;122(8):1547–54. doi:.https://doi.org/10.1016/j.ophtha.2015.04.032
34
Contrera
KJ
,
Choi
JS
,
Blake
CR
,
Betz
JF
,
Niparko
JK
,
Lin
FR
. Rates of Long-Term Cochlear Implant Use in Children. Otology & neurotology. 2014;35(3):426–30.
35
Zrenner
E
,
Bartz-Schmidt
KU
,
Benav
H
,
Besch
D
,
Bruckmann
A
,
Gabel
VP
, et al.
Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc Biol Sci. 2011;278(1711):1489–97. doi:.https://doi.org/10.1098/rspb.2010.1747
36
Ahuja
AK
,
Dorn
JD
,
Caspi
A
,
McMahon
MJ
,
Dagnelie
G
,
Dacruz
L
, et al.; Argus II Study Group. Blind subjects implanted with the Argus II retinal prosthesis are able to improve performance in a spatial-motor task. Br J Ophthalmol. 2011;95(4):539–43. doi:.https://doi.org/10.1136/bjo.2010.179622
37
Rizzo
JF, 3rd
,
Ayton
LN
. Psychophysical testing of visual prosthetic devices: a call to establish a multi-national joint task force. J Neural Eng. 2014;11(2):020301. doi:.https://doi.org/10.1088/1741-2560/11/2/020301
38Fontanarosa J, Treadwell JR, Samson DJ, VanderBeek BL, Schoelles K. AHRQ Technology Assessments. Retinal Prostheses in the Medicare Population. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016.
39
Cohen
B
,
Suzuki
JI
. Eye movements induced by ampullary nerve stimulation. Am J Physiol. 1963;204(2):347–51. doi:.https://doi.org/10.1152/ajplegacy.1963.204.2.347
40
Suzuki
JI
,
Cohen
B
,
Bender
MB
. Compensatory Eye Movements Induced by Vertical Semicircular Canal Stimulation. Exp Neurol. 1964;9(2):137–60. doi:.https://doi.org/10.1016/0014-4886(64)90013-5
41
Suzuki
JI
,
Cohen
B
. Head, Eye, Body and Limb Movements from Semicircular Canal Nerves. Exp Neurol. 1964;10(5):393–405. doi:.https://doi.org/10.1016/0014-4886(64)90031-7
42
Gong
W
,
Merfeld
DM
. Prototype neural semicircular canal prosthesis using patterned electrical stimulation. Ann Biomed Eng. 2000;28(5):572–81. doi:.https://doi.org/10.1114/1.293
43
Lewis
RF
,
Gong
W
,
Ramsey
M
,
Minor
L
,
Boyle
R
,
Merfeld
DM
. Vestibular adaptation studied with a prosthetic semicircular canal. J Vestib Res. 2002-2003;12(2-3):87–94.
44
Merfeld
DM
,
Haburcakova
C
,
Gong
W
,
Lewis
RF
. Chronic vestibulo-ocular reflexes evoked by a vestibular prosthesis. IEEE Trans Biomed Eng. 2007;54(6):1005–15. doi:.https://doi.org/10.1109/TBME.2007.891943
45
Gong
W
,
Haburcakova
C
,
Merfeld
DM
. Vestibulo-ocular responses evoked via bilateral electrical stimulation of the lateral semicircular canals. IEEE Trans Biomed Eng. 2008;55(11):2608–19. doi:.https://doi.org/10.1109/TBME.2008.2001294
46
Lewis
RF
,
Haburcakova
C
,
Gong
W
,
Makary
C
,
Merfeld
DM
. Vestibuloocular reflex adaptation investigated with chronic motion-modulated electrical stimulation of semicircular canal afferents. J Neurophysiol. 2010;103(2):1066–79. doi:.https://doi.org/10.1152/jn.00241.2009
47
Lewis
RF
,
Haburcakova
C
,
Gong
W
,
Karmali
F
,
Merfeld
DM
. Spatial and temporal properties of eye movements produced by electrical stimulation of semicircular canal afferents. J Neurophysiol. 2012;108(5):1511–20. doi:.https://doi.org/10.1152/jn.01029.2011
48
van de Berg
R
,
Guinand
N
,
Guyot
JP
,
Kingma
H
,
Stokroos
RJ
. The modified ampullar approach for vestibular implant surgery: feasibility and its first application in a human with a long-term vestibular loss. Front Neurol. 2012;3:18. doi:.https://doi.org/10.3389/fneur.2012.00018
49
Guyot
JP
,
Sigrist
A
,
Pelizzone
M
,
Feigl
GC
,
Kos
MI
. Eye movements in response to electrical stimulation of the lateral and superior ampullary nerves. Ann Otol Rhinol Laryngol. 2011;120(2):81–7. doi:.https://doi.org/10.1177/000348941112000202
50
Feigl
GC
,
Fasel
JH
,
Anderhuber
F
,
Ulz
H
,
Rienmüller
R
,
Guyot
JP
, et al.
Superior vestibular neurectomy: a novel transmeatal approach for a denervation of the superior and lateral semicircular canals. Otol Neurotol. 2009;30(5):586–91. doi:.https://doi.org/10.1097/MAO.0b013e3181ab9164
51
Feigl
G
,
Kos
I
,
Anderhuber
F
,
Guyot
JP
,
Fasel
J
. Development of surgical skill with singular neurectomy using human cadaveric temporal bones. Ann Anat. 2008;190(4):316–23. doi:.https://doi.org/10.1016/j.aanat.2008.05.001
52
Kos
MI
,
Feigl
G
,
Anderhuber
F
,
Wall
C
,
Fasel
JH
,
Guyot
JP
. Transcanal approach to the singular nerve. Otol Neurotol. 2006;27(4):542–6.
53
Guyot
JP
,
Sigrist
A
,
Pelizzone
M
,
Kos
MI
. Adaptation to steady-state electrical stimulation of the vestibular system in humans. Ann Otol Rhinol Laryngol. 2011;120(3):143–9. doi:.https://doi.org/10.1177/000348941112000301
54
Nguyen
TAK
,
DiGiovanna
J
,
Cavuscens
S
,
Ranieri
M
,
Guinand
N
,
van de Berg
R
, et al.
Characterization of pulse amplitude and pulse rate modulation for a human vestibular implant during acute electrical stimulation. J Neural Eng. 2016;13(4):046023. doi:.https://doi.org/10.1088/1741-2560/13/4/046023
55
Guinand
N
,
van de Berg
R
,
Cavuscens
S
,
Stokroos
RJ
,
Ranieri
M
,
Pelizzone
M
, et al.
Vestibular Implants: 8 Years of Experience with Electrical Stimulation of the Vestibular Nerve in 11 Patients with Bilateral Vestibular Loss. ORL J Otorhinolaryngol Relat Spec. 2015;77(4):227–40. doi:.https://doi.org/10.1159/000433554
56
Pérez Fornos
A
,
Cavuscens
S
,
Ranieri
M
,
van de Berg
R
,
Stokroos
R
,
Kingma
H
, et al.
The vestibular implant: A probe in orbit around the human balance system. J Vestib Res. 2017;27(1):51–61. doi:.https://doi.org/10.3233/VES-170604
57
Perez Fornos
A
,
Guinand
N
,
van de Berg
R
,
Stokroos
R
,
Micera
S
,
Kingma
H
, et al.
Artificial balance: restoration of the vestibulo-ocular reflex in humans with a prototype vestibular neuroprosthesis. Front Neurol. 2014;5:66. doi:.https://doi.org/10.3389/fneur.2014.00066
58
Guinand
N
,
Van de Berg
R
,
Cavuscens
S
,
Stokroos
R
,
Ranieri
M
,
Pelizzone
M
, et al.
Restoring Visual Acuity in Dynamic Conditions with a Vestibular Implant. Front Neurosci. 2016;10:577. doi:.https://doi.org/10.3389/fnins.2016.00577
59
Dai
C
,
Fridman
GY
,
Della Santina
CC
. Effects of vestibular prosthesis electrode implantation and stimulation on hearing in rhesus monkeys. Hear Res. 2011;277(1-2):204–10. doi:.https://doi.org/10.1016/j.heares.2010.12.021
60
Dai
C
,
Fridman
GY
,
Chiang
B
,
Davidovics
NS
,
Melvin
TA
,
Cullen
KE
, et al.
Cross-axis adaptation improves 3D vestibulo-ocular reflex alignment during chronic stimulation via a head-mounted multichannel vestibular prosthesis. Exp Brain Res. 2011;210(3-4):595–606. doi:.https://doi.org/10.1007/s00221-011-2591-5
61
Dai
C
,
Fridman
GY
,
Chiang
B
,
Rahman
MA
,
Ahn
JH
,
Davidovics
NS
, et al.
Directional plasticity rapidly improves 3D vestibulo-ocular reflex alignment in monkeys using a multichannel vestibular prosthesis. J Assoc Res Otolaryngol. 2013;14(6):863–77. doi:.https://doi.org/10.1007/s10162-013-0413-0
62
Dai
C
,
Fridman
GY
,
Davidovics
NS
,
Chiang
B
,
Ahn
JH
,
Della Santina
CC
. Restoration of 3D vestibular sensation in rhesus monkeys using a multichannel vestibular prosthesis. Hear Res. 2011;281(1-2):74–83. doi:.https://doi.org/10.1016/j.heares.2011.08.008
63
Fridman
GY
,
Davidovics
NS
,
Dai
C
,
Migliaccio
AA
,
Della Santina
CC
. Vestibulo-ocular reflex responses to a multichannel vestibular prosthesis incorporating a 3D coordinate transformation for correction of misalignment. J Assoc Res Otolaryngol. 2010;11(3):367–81. doi:.https://doi.org/10.1007/s10162-010-0208-5
64
Rubinstein
JT
,
Bierer
S
,
Kaneko
C
,
Ling
L
,
Nie
K
,
Oxford
T
, et al.
Implantation of the semicircular canals with preservation of hearing and rotational sensitivity: a vestibular neurostimulator suitable for clinical research. Otol Neurotol. 2012;33(5):789–96. doi:.https://doi.org/10.1097/MAO.0b013e318254ec24
65
Golub
JS
,
Ling
L
,
Nie
K
,
Nowack
A
,
Shepherd
SJ
,
Bierer
SM
, et al.
Prosthetic implantation of the human vestibular system. Otol Neurotol. 2014;35(1):136–47. doi:.https://doi.org/10.1097/MAO.0000000000000003
66
Phillips
JO
,
Ling
L
,
Nie
K
,
Jameyson
E
,
Phillips
CM
,
Nowack
AL
, et al.
Vestibular implantation and longitudinal electrical stimulation of the semicircular canal afferents in human subjects. J Neurophysiol. 2015;113(10):3866–92. doi:.https://doi.org/10.1152/jn.00171.2013
67
Phillips
C
,
Ling
L
,
Oxford
T
,
Nowack
A
,
Nie
K
,
Rubinstein
JT
, et al.
Longitudinal performance of an implantable vestibular prosthesis. Hear Res. 2015;322:200–11.
68
Phillips
C
,
Defrancisci
C
,
Ling
L
,
Nie
K
,
Nowack
A
,
Phillips
JO
, et al.
Postural responses to electrical stimulation of the vestibular end organs in human subjects. Exp Brain Res. 2013;229(2):181–95. doi:.https://doi.org/10.1007/s00221-013-3604-3
69Perez Fornos A. Minimum requirements for a retinal prosthesis to restore useful vision. Geneva, Switzerland: University of Geneva; 2006.
70Kasper A. Electrically evoked activity in the human auditory system. Geneva, Switzerland: Université de Genève; 1991.
71
DiGiovanna
J
,
Nguyen
TAK
,
Guinand
N
,
Pérez-Fornos
A
,
Micera
S
. Neural network model of vestibular nuclei reaction to onset of vestibular prosthetic stimulation. Front Bioeng Biotechnol. 2016;4:34. doi:.https://doi.org/10.3389/fbioe.2016.00034
72
Shannon
RV
. Multichannel electrical stimulation of the auditory nerve in man. II. Channel interaction. Hear Res. 1983;12(1):1–16. doi:.https://doi.org/10.1016/0378-5955(83)90115-6
73
de Balthasar
C
,
Boëx
C
,
Cosendai
G
,
Valentini
G
,
Sigrist
A
,
Pelizzone
M
. Channel interactions with high-rate biphasic electrical stimulation in cochlear implant subjects. Hear Res. 2003;182(1-2):77–87. doi:.https://doi.org/10.1016/S0378-5955(03)00174-6
74
Boëx
C
,
de Balthasar
C
,
Kós
MI
,
Pelizzone
M
. Electrical field interactions in different cochlear implant systems. J Acoust Soc Am. 2003;114(4):2049–57. doi:.https://doi.org/10.1121/1.1610451
75
Horsager
A
,
Boynton
GM
,
Greenberg
RJ
,
Fine
I
. Temporal interactions during paired-electrode stimulation in two retinal prosthesis subjects. Invest Ophthalmol Vis Sci. 2011;52(1):549–57. doi:.https://doi.org/10.1167/iovs.10-5282
76
Horsager
A
,
Greenberg
RJ
,
Fine
I
. Spatiotemporal interactions in retinal prosthesis subjects. Invest Ophthalmol Vis Sci. 2010;51(2):1223–33. doi:.https://doi.org/10.1167/iovs.09-3746
77
Davidovics
NS
,
Fridman
GY
,
Della Santina
CC
. Co-modulation of stimulus rate and current from elevated baselines expands head motion encoding range of the vestibular prosthesis. Exp Brain Res. 2012;218(3):389–400. doi:.https://doi.org/10.1007/s00221-012-3025-8
78
Davidovics
NS
,
Rahman
MA
,
Dai
C
,
Ahn
J
,
Fridman
GY
,
Della Santina
CC
. Multichannel vestibular prosthesis employing modulation of pulse rate and current with alignment precompensation elicits improved VOR performance in monkeys. J Assoc Res Otolaryngol. 2013;14(2):233–48. doi:.https://doi.org/10.1007/s10162-013-0370-7
79
Merfeld
DM
,
Lewis
RF
. Replacing semicircular canal function with a vestibular implant. Curr Opin Otolaryngol Head Neck Surg. 2012;20(5):386–92. doi:.https://doi.org/10.1097/MOO.0b013e328357630f
80
Guinand
N
,
Boselie
F
,
Guyot
JP
,
Kingma
H
. Quality of life of patients with bilateral vestibulopathy. Ann Otol Rhinol Laryngol. 2012;121(7):471–7. doi:.https://doi.org/10.1177/000348941212100708
81
Guinand
N
,
Pijnenburg
M
,
Janssen
M
,
Kingma
H
. Visual acuity while walking and oscillopsia severity in healthy subjects and patients with unilateral and bilateral vestibular function loss. Arch Otolaryngol Head Neck Surg. 2012;138(3):301–6. doi:.https://doi.org/10.1001/archoto.2012.4
82Kos I. The electrical stimulation of the internal ear at the University Hospitals of Geneva. Geneva: University of Geneva; 2010.
83
Sommerhalder
J
,
Rappaz
B
,
de Haller
R
,
Fornos
AP
,
Safran
AB
,
Pelizzone
M
. Simulation of artificial vision: II. Eccentric reading of full-page text and the learning of this task. Vision Res. 2004;44(14):1693–706. doi:.https://doi.org/10.1016/j.visres.2004.01.017
84
van de Berg
R
,
Guinand
N
,
Nguyen
TA
,
Ranieri
M
,
Cavuscens
S
,
Guyot
JP
, et al.
The vestibular implant: frequency-dependency of the electrically evoked vestibulo-ocular reflex in humans. Front Syst Neurosci. 2015;8:255. doi:.https://doi.org/10.3389/fnsys.2014.00255
85
Guinand
N
,
Van de Berg
R
,
Cavuscens
S
,
Ranieri
M
,
Schneider
E
,
Lucieer
F
, et al.
The Video Head Impulse Test to Assess the Efficacy of Vestibular Implants in Humans. Front Neurol. 2017;8(600):600. doi:.https://doi.org/10.3389/fneur.2017.00600
86
Rabinowitz
WM
,
Eddington
DK
,
Delhorne
LA
,
Cuneo
PA
. Relations among different measures of speech reception in subjects using a cochlear implant. J Acoust Soc Am. 1992;92(4):1869–81. doi:.https://doi.org/10.1121/1.405252
87
da Cruz
L
,
Coley
BF
,
Dorn
J
,
Merlini
F
,
Filley
E
,
Christopher
P
, et al.; Argus II Study Group. The Argus II epiretinal prosthesis system allows letter and word reading and long-term function in patients with profound vision loss. Br J Ophthalmol. 2013;97(5):632–6. doi:.https://doi.org/10.1136/bjophthalmol-2012-301525
88
de Balthasar
C
,
Patel
S
,
Roy
A
,
Freda
R
,
Greenwald
S
,
Horsager
A
, et al.
Factors affecting perceptual thresholds in epiretinal prostheses. Invest Ophthalmol Vis Sci. 2008;49(6):2303–14. doi:.https://doi.org/10.1167/iovs.07-0696
89
Pérez Fornos
A
,
Sommerhalder
J
,
da Cruz
L
,
Sahel
JA
,
Mohand-Said
S
,
Hafezi
F
, et al.
Temporal properties of visual perception on electrical stimulation of the retina. Invest Ophthalmol Vis Sci. 2012;53(6):2720–31. doi:.https://doi.org/10.1167/iovs.11-9344
90
Marc
RE
,
Jones
BW
,
Anderson
JR
,
Kinard
K
,
Marshak
DW
,
Wilson
JH
, et al.
Neural reprogramming in retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48(7):3364–71. doi:.https://doi.org/10.1167/iovs.07-0032
91
Marc
RE
,
Jones
BW
,
Watt
CB
,
Strettoi
E
. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003;22(5):607–55. doi:.https://doi.org/10.1016/S1350-9462(03)00039-9
92
Strettoi
E
,
Pignatelli
V
,
Rossi
C
,
Porciatti
V
,
Falsini
B
. Remodeling of second-order neurons in the retina of rd/rd mutant mice. Vision Res. 2003;43(8):867–77. doi:.https://doi.org/10.1016/S0042-6989(02)00594-1
93
Weitz
AC
,
Nanduri
D
,
Behrend
MR
,
Gonzalez-Calle
A
,
Greenberg
RJ
,
Humayun
MS
, et al.
Improving the spatial resolution of epiretinal implants by increasing stimulus pulse duration. Sci Transl Med. 2015;7(318):318ra203. doi:.https://doi.org/10.1126/scitranslmed.aac4877
94
Rubinstein
JT
. Analytical theory for extracellular electrical stimulation of nerve with focal electrodes. II. Passive myelinated axon. Biophys J. 1991;60(3):538–55. doi:.https://doi.org/10.1016/S0006-3495(91)82084-7
95
Rubinstein
JT
,
Spelman
FA
. Analytical theory for extracellular electrical stimulation of nerve with focal electrodes. I. Passive unmyelinated axon. Biophys J. 1988;54(6):975–81. doi:.https://doi.org/10.1016/S0006-3495(88)83035-2
96
Wilson
BS
. The cochlear implant and possibilities for narrowing the remaining gaps between prosthetic and normal hearing. World J Otorhinolaryngol Head Neck Surg. 2018;3(4):200–10. doi:.https://doi.org/10.1016/j.wjorl.2017.12.005
97
Chernov
M
,
Roe
AW
. Infrared neural stimulation: a new stimulation tool for central nervous system applications. Neurophotonics. 2014;1(1):011011. doi:.https://doi.org/10.1117/1.NPh.1.1.011011
98
Richardson
RT
,
Thompson
AC
,
Wise
AK
,
Needham
K
. Challenges for the application of optical stimulation in the cochlea for the study and treatment of hearing loss. Expert Opin Biol Ther. 2017;17(2):213–23. doi:.https://doi.org/10.1080/14712598.2017.1271870
99
Wang
J
,
Tian
L
,
Lu
J
,
Xia
M
,
Wei
Y
. Effect of shorter pulse duration in cochlear neural activation with an 810-nm near-infrared laser. Lasers Med Sci. 2017;32(2):389–96. doi:.https://doi.org/10.1007/s10103-016-2129-y
100
Matic
AI
,
Robinson
AM
,
Young
HK
,
Badofsky
B
,
Rajguru
SM
,
Stock
S
, et al.
Behavioral and electrophysiological responses evoked by chronic infrared neural stimulation of the cochlea. PLoS One. 2013;8(3):e58189. doi:.https://doi.org/10.1371/journal.pone.0058189
101
Guo
W
,
Hight
AE
,
Chen
JX
,
Klapoetke
NC
,
Hancock
KE
,
Shinn-Cunningham
BG
, et al.
Hearing the light: neural and perceptual encoding of optogenetic stimulation in the central auditory pathway. Sci Rep. 2015;5(1):10319. doi:.https://doi.org/10.1038/srep10319
102
Yue
L
,
Weiland
JD
,
Roska
B
,
Humayun
MS
. Retinal stimulation strategies to restore vision: Fundamentals and systems. Prog Retin Eye Res. 2016;53:21–47. doi:.https://doi.org/10.1016/j.preteyeres.2016.05.002
103
Verma
RU
,
Guex
AA
,
Hancock
KE
,
Durakovic
N
,
McKay
CM
,
Slama
MC
, et al.
Auditory responses to electric and infrared neural stimulation of the rat cochlear nucleus. Hear Res. 2014;310:69–75. doi:.https://doi.org/10.1016/j.heares.2014.01.008
104
Boyden
ES
,
Zhang
F
,
Bamberg
E
,
Nagel
G
,
Deisseroth
K
. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–8. doi:.https://doi.org/10.1038/nn1525
105
Senova
S
,
Scisniak
I
,
Chiang
CC
,
Doignon
I
,
Palfi
S
,
Chaillet
A
, et al.
Experimental assessment of the safety and potential efficacy of high irradiance photostimulation of brain tissues. Sci Rep. 2017;7(1):43997. doi:.https://doi.org/10.1038/srep43997
106Palanker DV, Huie P, Vankov AB, Freyvert Y, Fishman H, Marmor MF, et al., eds. Attracting retinal cells to electrodes for high-resolution stimulation. 2004.
107
Pinyon
JL
,
Tadros
SF
,
Froud
KE
,
Y Wong
AC
,
Tompson
IT
,
Crawford
EN
, et al.
Close-field electroporation gene delivery using the cochlear implant electrode array enhances the bionic ear. Sci Transl Med. 2014;6(233):233ra54. doi:.https://doi.org/10.1126/scitranslmed.3008177
108
Senn
P
,
Roccio
M
,
Hahnewald
S
,
Frick
C
,
Kwiatkowska
M
,
Ishikawa
M
, et al.
NANOCI-Nanotechnology Based Cochlear Implant With Gapless Interface to Auditory Neurons. Otol Neurotol. 2017;38(8):e224–31. doi:.https://doi.org/10.1097/MAO.0000000000001439
109
Landry
TG
,
Fallon
JB
,
Wise
AK
,
Shepherd
RK
. Chronic neurotrophin delivery promotes ectopic neurite growth from the spiral ganglion of deafened cochleae without compromising the spatial selectivity of cochlear implants. J Comp Neurol. 2013;521(12):2818–32. doi:.https://doi.org/10.1002/cne.23318
110
Yang
LZ
,
Shi
B
,
Li
H
,
Zhang
W
,
Liu
Y
,
Wang
H
, et al.
Electrical stimulation reduces smokers’ craving by modulating the coupling between dorsal lateral prefrontal cortex and parahippocampal gyrus. Soc Cogn Affect Neurosci. 2017;12(8):1296–302. doi:.https://doi.org/10.1093/scan/nsx055
111
Jiménez
F
,
Nicolini
H
,
Lozano
AM
,
Piedimonte
F
,
Salín
R
,
Velasco
F
. Electrical stimulation of the inferior thalamic peduncle in the treatment of major depression and obsessive compulsive disorders. World Neurosurg. 2013;80(3-4):30.e17–25. doi:.https://doi.org/10.1016/j.wneu.2012.07.010
112
Vanderveken
OM
,
Beyers
J
,
Op de Beeck
S
,
Dieltjens
M
,
Willemen
M
,
Verbraecken
JA
, et al.
Development of a Clinical Pathway and Technical Aspects of Upper Airway Stimulation Therapy for Obstructive Sleep Apnea. Front Neurosci. 2017;11:523. doi:.https://doi.org/10.3389/fnins.2017.00523
113Perez Fornos A. Sensory neuroprostheses: from physiology to clinical application. Université de Genève. Thèse de privat-docent, 2017. https://archive-ouverte.unige.ch/unige:102019