Adjuvant trastuzumab chemotherapy in early breast cancer: meta-analysis of randomised trials and cost-effectiveness analysis
DOI: https://doi.org/10.4414/smw.2019.20082
Tan Nhut
Doana, Jan
Barendregtb*
aDepartment of Medicine at The Royal Melbourne Hospital, University of Melbourne, Victoria, Australia
bEpiGear International, Noosa, Queensland, Australia
Summary
BACKGROUND
Trastuzumab has a large financial impact on the average cost of breast cancer treatment. This study reassessed the cost-effectiveness of listing the drug on the subsidised Australian Pharmaceutical Benefits Scheme.
METHODS
Using a continuous-time, discrete-event microsimulation model, we examined the effect of 1-year trastuzumab on the total number of disability-adjusted life-years (DALYs) averted among Australian women with human epidermal growth factor receptor-2 (HER2) positive early breast cancer. Target population was women aged 30–100 years and newly diagnosed with the disease in 2003. The model adjusted for tumour size and followed the women up until death or age 100 years. Uncertainty was examined in univariate and probabilistic multivariate sensitivity analyses.
RESULTS
The incremental cost-effectiveness ratio (ICER) was A$132,537 (95% confidence interval 91,172–200,485) per DALY averted. Results were sensitive to restriction of trastuzumab to high-risk (large tumour) and/or high-benefit (young) patients. Suitable combinations of tumour size and age restrictions would improve the cost-effectiveness of trastuzumab. Specifically, restricting trastuzumab to women aged 40 years or younger with tumour sizes 40+ mm reduced the ICER to A$35,290 per DALY averted.
CONCLUSION
Trastuzumab for HER2-positive early breast cancer had a high ICER. It is unclear why the Pharmaceutical Benefits Scheme listing does not use restrictions to improve the cost-effectiveness of the drug.
Background
Approximately 20% of incident breast cancers overexpress the human epidermal growth factor receptor-2 (HER2) [1]. HER2 overexpression and/or amplification of the HER2 oncogene is associated with a poor prognosis and an aggressive form of breast cancer [1]. Trastuzumab, a humanised anti-p185 HER2 monoclonal antibody, was registered in Australia in 2006 for the treatment of HER2-positive early breast cancer in combination with adjuvant chemotherapy [2]. It is subsidised for all Australian women with early breast cancer under the Pharmaceutical Benefits Scheme [3].
Clinical trials show that adjuvant trastuzumab and chemotherapy (ATC) improves disease-free and overall survival compared with standard adjuvant chemotherapy (SAC) alone in patients with early and metastatic HER2-positive breast cancer [4–8]. However, owing to the short follow-up of these trials, long-term effects of trastuzumab on overall survival in breast cancer women are unknown. The greater risk of cardiac toxicity, particularly when combined with an anthracycline-based regimen [8], and the increased risk of metastasis to the central nervous system [9] are further challenges associated with adjuvant trastuzumab chemotherapy that need to be taken into account.
A number of studies have recently evaluated the cost-effectiveness of adding trastuzumab to SAC [10–14]. However, these studies only included patients aged 50 or older, did not distinguish disease-specific fatality by tumour size, and did not include data on the effect size of trastuzumab from all clinical trials. This study provides additional evidence on the cost-effectiveness of ATC, compared with SAC, for the treatment of HER2-positive early breast cancer. We included all eligible patients irrespective of their age category, distinguished case-fatality of breast cancer by tumour size, and included data from all available clinical trials in the calculation of the effect size of trastuzumab.
Methods
Clinical and epidemiological data
Data on breast cancer survival by different tumour sizes were derived from a South Australian study (table 1) [15]. We calculated age-specific background mortality rates (to take into account deaths due to causes other than breast cancer) and disease-specific mortality rates from information available in the Australian Burden of Disease study [16]. Relative risk (RR) of recurrence, given HER2-positive disease, was assumed to be 1.47 (95% confidence interval [CI] 0.94–2.28), based on a cohort study [17].
Table 1 Survival from breast cancer by tumour size [15]
|
Tumour size
|
Survival
|
|
5-year (%)
|
10-year (%)
|
15-year (%)
|
20-year (%)
|
| <10 mm |
96 |
91 |
83 |
83 |
| 10–14 mm |
92 |
84 |
75 |
55 |
| 15–19 mm |
91 |
82 |
74 |
57 |
| 20–29 mm |
84 |
72 |
67 |
54 |
| 30–39 mm |
77 |
63 |
57 |
51 |
| 40+ mm |
62 |
51 |
43 |
40 |
The effect size for death (RR for death) was based on a meta-analysis of all published trials. A systematic electronic literature search of PubMed, Medline (via Ovid), Embase, CINAHL, and Cochrane databases was conducted to identify all randomised controlled trials (RCTs) of adjuvant trastuzumab in HER2-positive early breast cancer published between January 2004 and July 2018. The search included the following Medical Subject Headings terms or text key words: ‘trastuzumab’, ‘adjuvant therapy’, and ‘breast cancer’. The full texts of studies considered relevant after the initial abstract screening cycles were evaluated. Secondary searching of the reference lists of the studies was also performed for relevant reports. Studies were included in the meta-analysis if they were RCTs investigating the effectiveness of ATC in comparison with SAC alone in HER2-positive early breast cancer patients. A total of five RCTs were included in the meta-analysis. These trials included the Herceptin Adjuvant (HERA) [8], the North Central Cancer Treatment Group (NCCTG N9831) [5, 7, 18], the National Surgical Adjuvant Breast and Bowel Project (NSABP B31) [5, 7, 18], the Breast Cancer International Research Group (BCIRG 006) [19], and the Finland Herceptin (FinHer) [20] studies. The analysis was done using MetaXL (EpiGear, Brisbane, Queensland, Australia). Heterogeneity between trials was tested using the Q statistic at significance level of 10% according to the method outlined by Egger et al. [21]. If the p-value of the chi-square distribution of the Cochran’s Q is smaller than 0.1, heterogeneity between studies is statistically significant [21]. If there was evidence of heterogeneity between studies, a random-effect model was adopted for the meta-analysis; otherwise, a fixed-effect model was used.
We assumed the effect of trastuzumab remained unchanged for the first 5 years; after that it was attenuated exponentially such that there was only small effect in year 10 (RR 0.97) and no effect remained after year 13 (RR 1). Heart failure, occurring in 2.5% of women receiving trastuzumab and 0% in non-trastuzumab women, was taken from a meta-analysis [22]. Heart failure was assumed to have no impact on mortality.
Model
A continuous-time, discrete-event microsimulation model was constructed in Microsoft Excel, using the Ersatz add-in (EpiGear, Brisbane, Queensland, Australia). The model structure is described in appendix 1. Continuous-time, discrete-event microsimulation models are individual-level models that use a stochastic process to simulate sequences of events (instead of modelling events one cycle at a time) for a group of hypothetical individuals by drawing directly from probability distributions of event times [23]. Unlike Markov cohort models in which events are modelled as transitions from one state to another, discrete-event microsimulation models are concerned with the duration during which patients reside in one health state [23]. In these models, the length of time spent in a certain health state is directly drawn per patient from the corresponding cumulative survival distribution; whereas in Markov cohort models such distributions are used to estimate the transition probabilities over time [23]. Drawing directly from the cumulative survival distribution allows patients to move through the model, proceed to another health state and experience events at any discrete point in time. During such moving process, the occurrence of an event triggers the analysis. Individuals within a discrete-event microsimulation model can be assigned attributes (for example, age and tumour size). Costs and health effects can be incorporated into the model with respect to patient attributes and/or the events experienced within the model.
The hypothetical target population (n = 2447) was 20% of all newly diagnosed breast cancer cases in Australia [16], assuming that 20% of breast cancer cases were HER2-positive [1]. A lifetime horizon was adopted. Results were presented in terms of cost (Australian dollars A$, year 2017 values) per disability-adjusted life-year (DALY) averted.
We distinguished four different tumour sizes (<10 mm, 10–19 mm, 20–39 mm, and 40+ mm) by combining the 10–14 mm with 15–19 mm and the 20–29 mm with 30–39 mm categories of an Australian study [15]. To interpolate survival data to single years and extrapolate beyond the 20 years of follow-up in the Luke et al. [15] study, we assumed the relative survival by tumour size had a lognormal distribution. From the survival curves we calculated annual excess mortality risk by tumour size and time since diagnosis. We distinguished three different health states: (1) breast cancer diagnosis and primary treatment; (2) disease remission; and (3) disseminated and terminal disease. To calculate DALYs, we used breast cancer disability weights (by tumour size and age category, table 2) from a Dutch study [24] and age-specific rates of disability due to all other causes from the Australian Burden of Disease study [16]. Disease-specific and background disability weights were combined using a multiplicative formula. We assumed durations of the diagnosis and primary treatment stage of 0.22, 0.35 and 0.67 years for tumour sizes <20 mm, 20–39 mm and 40+ mm, respectively, based on expert advice provided to the Australian Burden of Disease study [16]. The duration of the disseminated and terminal stage was assumed to be 1.5 years [16]. We assumed death from breast cancer occurred only in metastatic patients. The probability of loco-regional recurrence and the transition probability of metastases to remission were considered to be the same in both the ATC and SAC groups.
Table 2 Breast cancer disability weights by tumour size and health state [24].
|
Health state
|
Tumour size
|
|
<10 mm
|
10–19 mm
|
20–39 mm
|
40+ mm
|
| Diagnosis and primary treatment |
0.26 |
0.26 |
0.69 |
0.80 |
| Remission |
0.26 |
0.26 |
0.26 |
0.26 |
| Disseminated and terminal disease |
0.80 |
0.80 |
0.80 |
0.80 |
Costs
We used an Australian health sector perspective. We included only direct medical costs. Because all of the patients received breast surgery, radiotherapy and standard chemotherapy, cost differences between the ATC and SAC groups were costs associated with trastuzumab use. These costs included drug cost of trastuzumab, trastuzumab infusion cost, and cost of cardiac screening and treatment.
Cost of trastuzumab was estimated based on the three-weekly regimen (8 mg/kg loading dose followed by 6 mg/kg every 3 weeks) for a total of 1 year. Trastuzumab is available in Australia as a single-dose vial containing 60 mg or 150 mg. Unit costs of trastuzumab (A$412 for a 60 mg vial and A$1030 for a 150 mg vial) were derived from the Australian Pharmaceutical Benefits Scheme (PBS) web site [25]. The two vial sizes were combined in the way that gave the desired dose with the lowest cost. The number of vials was rounded up. Partially used vials were wasted. Trastuzumab dose is dependent on patient weight, which was divided into three weight categories: <57 kg, 57–75 kg, and 75+ kg using weight distribution by age reported in Dunstan et al. [26]. For patients who did not develop heart failure, trastuzumab withdrawal rate was assumed to be zero; otherwise the treatment was discontinued when heart failure was diagnosed. Trastuzumab drug cost is detailed in table 3. Cost per infusion was derived from the Australian Medicare Benefits Schedule (MBS) web site [27]. We assumed the loading dose was infused over a period of 90 minutes and subsequent maintenance infusions were less than 60 minutes [28]. Trastuzumab infusion costs are shown in table 4.
Table 3 One-year trastuzumab drug cost.
| |
Weight category
|
|
<57 kg
|
57–75 kg
|
75+ kg
|
Loading dose
(8 mg/kg) |
Total loading dose (mg) |
<456 |
456–600 |
>600 |
| Vial combinations |
150 mg vial × 3 |
150 mg vial × 3 +
60 mg vial × 2 |
150 mg vial × 3 +
60 mg vial × 3 |
| Loading cost (A$, 1 course) |
3090.63 |
3914.79 |
4326.87 |
Continuing dose
(6 mg/kg) |
Total continuing dose (mg) |
<342 |
342–450 |
>450 |
| Vial combinations |
60 mg vial × 6 |
150 mg vial × 3 |
150 mg vial × 2 +
60 mg vial × 3 |
| Continuing cost (A$, 16 courses) |
39,559.68 |
49,450.08 |
52,746.56 |
|
Total trastuzumab cost/patient/year (A$)
|
42,650.31
|
53,364.87
|
57,073.43
|
Table 4 Costs of trastuzumab infusion, heart failure screening and treatment and metastasis.
| |
Unit cost (A$)
|
Number/year
|
Total cost A$/patient/year
|
| Drug infusion for loading dose |
92.41 |
1 |
92.41 |
| Drug infusion for continuing dose |
62.61 |
16 |
1001.76 |
| Metastasis cost |
|
|
47,674.00 |
| Baseline heart failure screening |
2-Dimentional echocardiography |
130.85 |
1 |
130.85 |
| 12-Lead electrocardiogram |
30.50 |
1 |
30.50 |
| General practitioner visit |
34.90 |
1 |
34.90 |
| Follow-up heart failure screening |
2-Dimentional echocardiography |
130.85 |
3 |
392.55 |
| 12-Lead electrocardiogram |
30.50 |
1 |
30.50 |
| General practitioner visit |
34.90 |
3 |
104.70 |
|
Total for heart failure screening
|
|
|
724.00
|
| Heart failure treatment |
2-Dimensional echocardiography |
130.85 |
4 |
523.40 |
| 12-lead electrocardiogram |
30.50 |
4 |
122.00 |
| Initial visit (cardiologist) |
82.33 |
1 |
82.33 |
| Follow-up visit (cardiologist) |
41.30 |
3 |
123.90 |
| General practitioner visit |
34.95 |
4 |
139.80 |
| Drugs |
Captopril |
|
|
416.59 |
| Furosemide |
|
|
31.65 |
| Spironolactone |
|
|
44.49 |
| Carvedilol |
|
|
768.69 |
|
Total for heart failure treatment
|
|
|
2252.85
|
|
Total for heart failure screening and treatment
|
|
|
2976.85
|
Unit costs of all cardiac monitoring tests were derived from the MBS web site [27]; unit costs of heart failure drugs were derived from the PBS web site [25]. The cost estimates of heart failure screening and treatment are detailed in table 4. We assumed those who developed heart failure did so halfway (that is, 6 months after using trastuzumab); therefore, total costs incurred by these people included costs of 6-month trastuzumab plus costs of heart failure screening and treatment.
Metastasis cost was derived from an Australian study [12]. Costs of HER2 status testing were not included because HER2 testing is a routine practice in Australia for all patients with breast cancer. Cost of future treatment for unrelated diseases of patients who survive as a result of the intervention was excluded from the analysis. All costs were calculated in A$ 2017 value, and adjusted for inflation where applicable using the consumer price index for medical care services. Both costs and DALYs were discounted at 3% to account of time preference [29].
Uncertainty and sensitivity analysis
We modelled parameter uncertainty around survival rates, effect size, RR of recurrence and weight distribution. The uncertainty analysis was performed with Monte Carlo simulation (2,000 iterations) using Ersatz (EpiGear, Brisbane, Queensland, Australia). We assumed the RRs of effect size and recurrence followed a lognormal distribution; survival followed a binomial distribution; and weight distribution had a multinomial distribution. The parameters of these distributions are shown in appendix 2.
Univariate sensitivity analysis was carried out to understand the sensitivity of results to changes in individual variables. The following variables were assessed: heart failure rate, RR of recurrence, yearly cost of metastasis, yearly cost of heart failure treatment, duration of treatment effect of trastuzumab, discount rate and the price of trastuzumab. Ranges of variation were based on published data where available, and otherwise varied within ±20% (table 5). As this study used published data in the literature, ethics approval was not required.
Table 5 Ranges of variation of variables in univariate sensitivity analysis.
|
Variable
|
Base case
|
Range
|
Reference
|
| Heart failure rate |
2.5% |
0.6–4.5% |
[8] |
| Yearly cost of metastasis |
A$47,674.00 |
±20% |
|
| Yearly cost of heart failure treatment |
A$2252.85 |
±20% |
|
| Duration of treatment effect of trastuzumab |
Diminish after 5 years |
Lifelong |
[14] |
| Relative risk of recurrence |
1.47 |
95% CI
0.94–2.28 |
[16] |
| Discount rate |
3% |
0–5% |
|
| Price of trastuzumab |
A$412.08 (60 mg vial)
A$1030.21 (150 mg vial) |
±20% |
|
Results
Meta-analysis
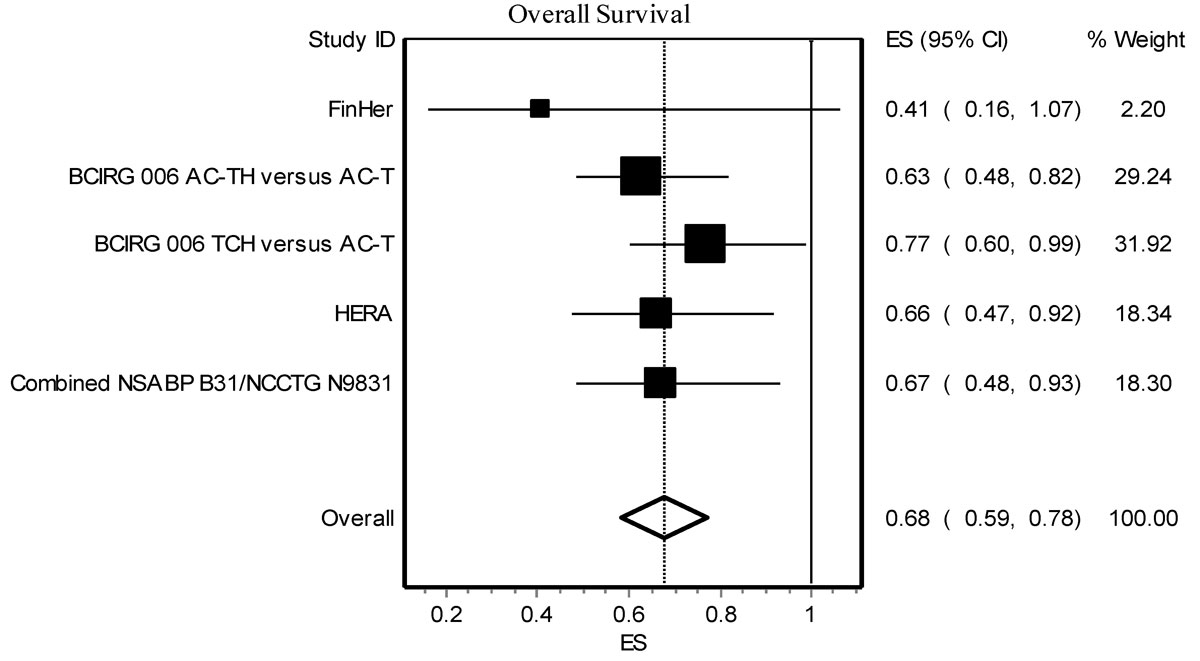
Result of the meta-analysis is shown in figure 1. The analysis shows that RR for death was significantly prolonged in the trastuzumab arm relative to the non-trastuzumab arm (pooled RR 0.68, 95% CI 0.59–0.78), without significant heterogeneity (p = 0.66).
Base case analysis
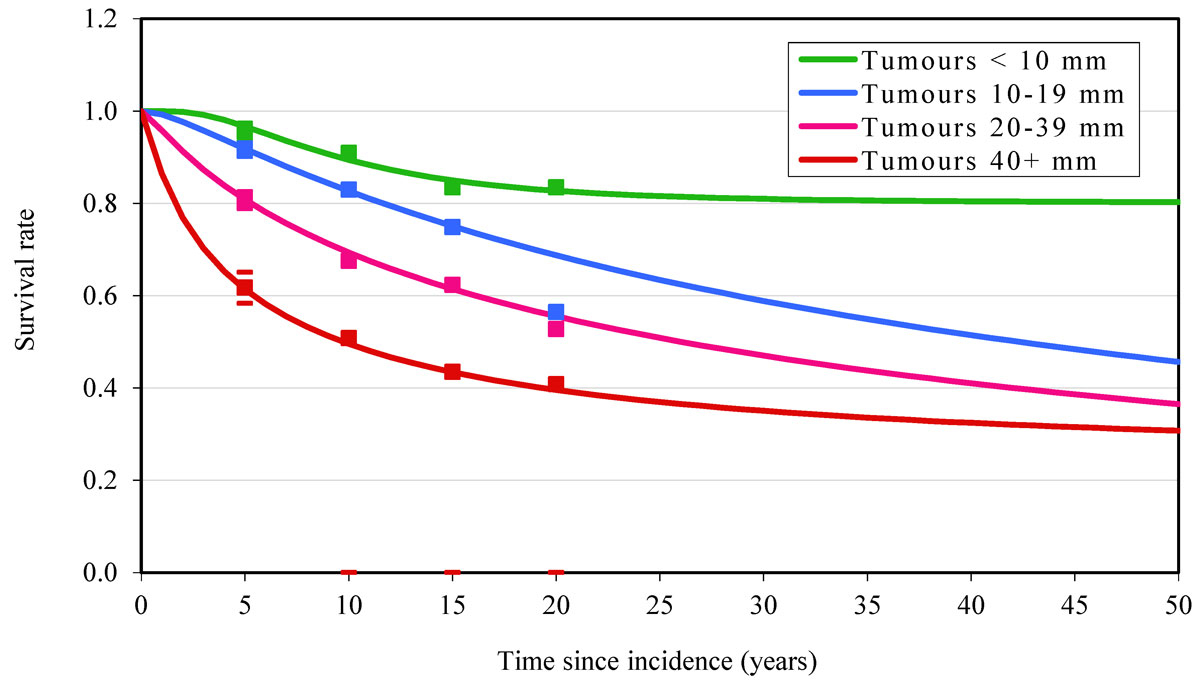
The survival curves (fig. 2) suggest that the lognormal distributions fitted the data well, with the exception of the 20-year follow-up point for 10–19 mm tumours. In the model, 28% (688 deaths) of women died from breast cancer in the ATC group compared with 31% (765 deaths) in the SAC group. With a total of 77 deaths avoided, adding trastuzumab to SAC led to a 10% reduction in the case fatality of breast cancer in Australian females. The 77 avoided deaths corresponded to 1265 life years (95% CI 812–1737) and 1005 DALYs (95% CI 645–1380). With a total incremental cost of A$128 million (95% CI 126–130 million), the ICER was estimated at A$132,537 (95% CI 91,172–200,485) per DALY averted or A$105,291 (95% CI 72,534–159,008) per life year gained (LYG). Every death avoided in the model cost approximately A$1.5 million.
Restricting trastuzumab to hig-risk (large tumours) and/or high-benefit patients (young women) had a major impact on the ICER. The ICER reduced from A$132,537 per DALY averted in the base case scenario to A$105,000 when the intervention was limited to patients with tumour sizes 20+ mm. Limiting trastuzumab to women aged less than 60 years reduced the ICER to A$103,000. Restricting trastuzumab to tumour sizes of 40+ mm reduced the ICER to A$58,400 for all ages; with an additional restriction to women aged less than 60 years, the ICER reduced to A$50,000. Limiting the treatment to women aged 40 years or younger with tumour sizes 40+ mm caused the ICER to drop further to A$35,290 per DALY averted.
Uncertainty and sensitivity analysis
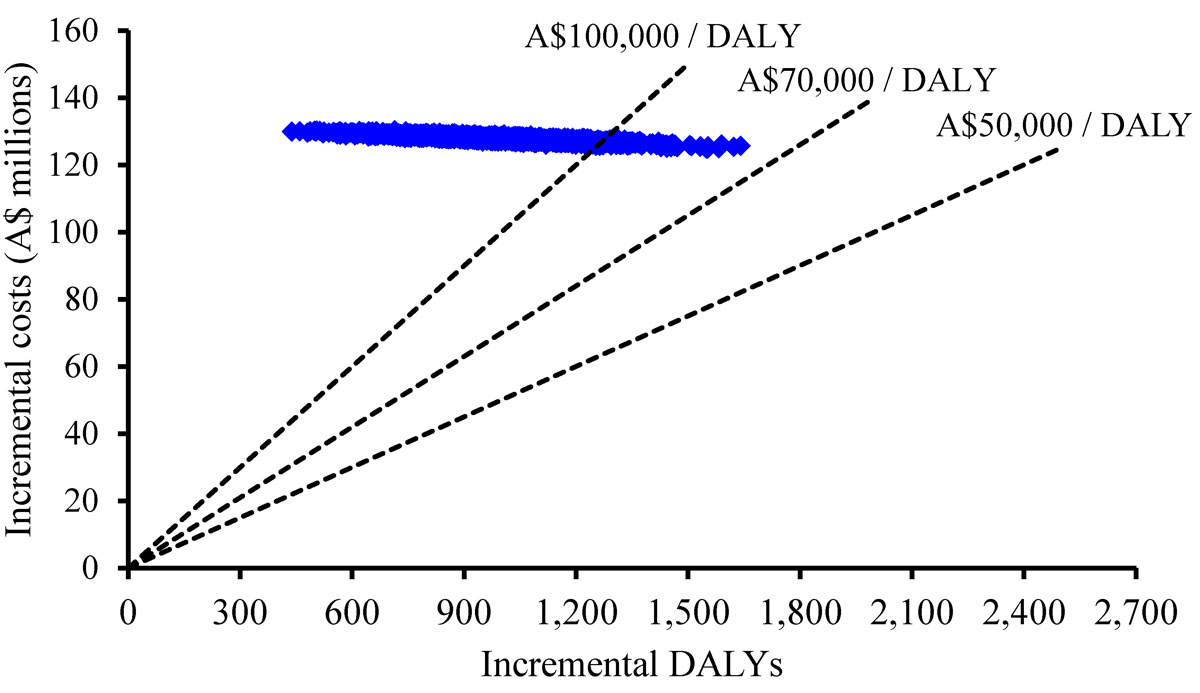
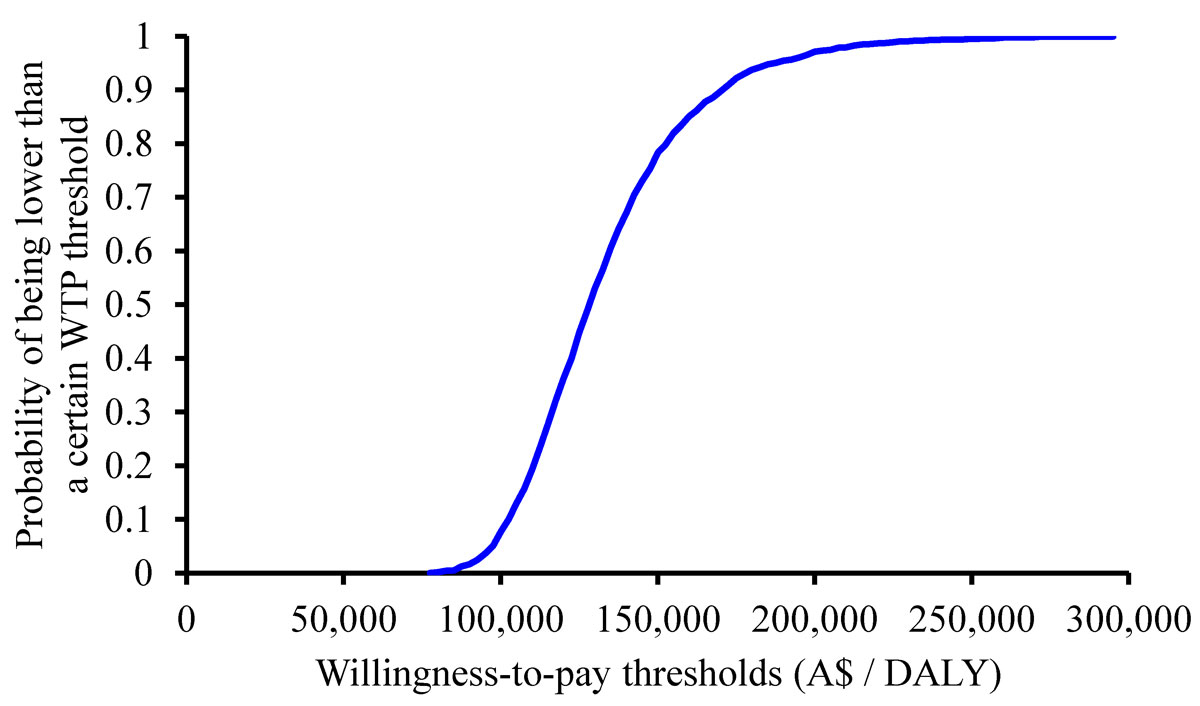
The cost-effectiveness plane is shown in figure 3. The graph shows that the 2000 simulated ICERs ranged from A$76,600 to A$296,790 per DALY averted. The cost-effectiveness acceptability curve (fig. 4) shows the probability that the ICER was lower than a particular willingness-to-pay (WTP) threshold. If a WTP threshold of A$50,000 per DALY averted was used, the probability of trastuzumab being cost effective was zero.
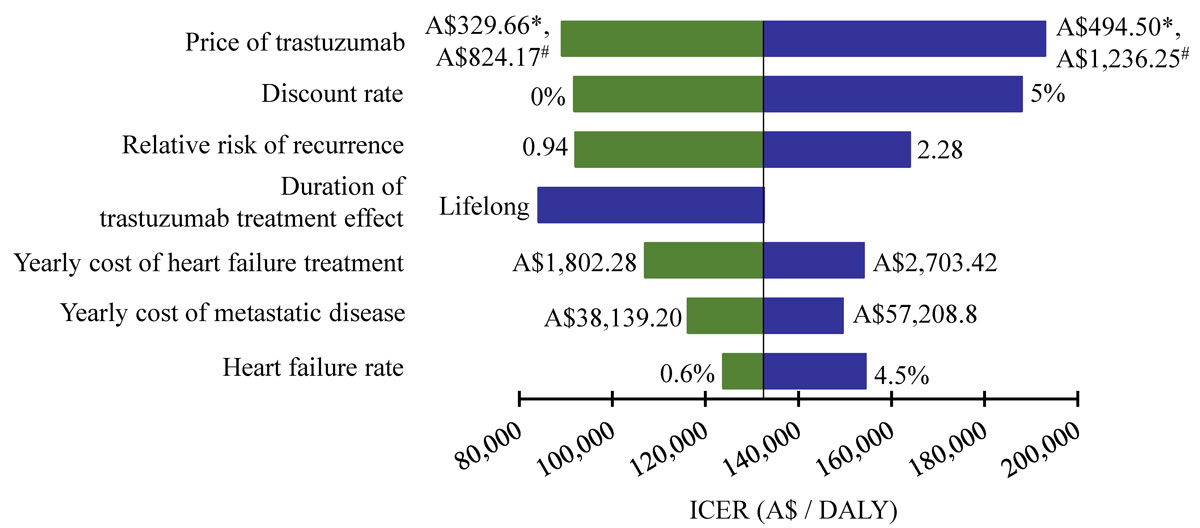
Univariate sensitivity analysis of key parameters is illustrated as a tornado diagram (fig. 5). Results were most sensitive to changes in the price of trastuzumab and discount rate. A 20% reduction in the price of trastuzumab would result in a 33% decrease in ICER (reducing from A$132,537 at baseline to A$89,172 per DALY averted). At a discount rate of 0%, the ICER reduced to A$91,768, compared with A$132,547 per DALY averted at baseline. Results were most robust to variations in cost of heart failure treatment, cost of metastatic disease and heart failure rate. None of the changes explored in the sensitivity analysis yielded an ICER of less than A$50,000 per DALY.
Discussion
The present study indicates that 1-year adjuvant trastuzumab administered every 3 weeks for Australian women with HER2-positive early breast cancer had a high ICER relative to standard chemotherapy alone from an Australian health sector perspective when patients of all age and of all tumour sizes were considered. For these patients, the cost effectiveness of the treatment was estimated to be A$132,537 per DALY averted. The cost effectiveness of trastuzumab was positively associated with tumour size and inversely associated with age. Restricting trastuzumab to tumour sizes of 40+ mm and to women aged less than 60 years reduced the ICER to A$50,000 per DALY averted. Results were most influenced by the price of trastuzumab and discount rate.
Previous studies consistently show that 1-year trastuzumab is a cost-effective regimen for women with HER2-positive early breast cancer in a number of countries. In the United Kingdom, a cost-effectiveness analysis conducted by the manufacturer estimated that the treatment costs £5,687 per quality-adjusted life-year (QALY) gained, suggesting that it is cost effective, assuming a threshold of £30,000 per QALY gained [30]. In Switzerland, the analysis by Dedes et al. [10] found that the ICER of 1-year trastuzumab ranged from €40,505 to €19,763 per LYG after 10 years and 15 years, respectively. The authors concluded that the treatment is cost effective in a long-term view, assuming a threshold of €50,000 per LYG [10]. In the United States, Garrison et al. [31] projected the cost effectiveness of trastuzumab to be US$26,417 per QALY from a healthcare system perspective and US$27,637 from a societal perspective. These ICERs are below the US$50,000 per QALY threshold commonly used in the United States. One-year trastuzumab was also found to be cost effective in other countries such as Taiwan [32], China [33], Japan [34] and Sweden [14]. Unlike these previous studies, trastuzumab in our study had an ICER that was higher than the WTP thresholds adopted in these studies. This is largely explained by the difference in effect size of trastuzumab which is less favourable in our analysis because of the inclusion of the latest results into the meta-analysis. Effect sizes were about 0.5 in these previous studies; with the latest trial results included it becomes 0.68 in our study. Our findings are consistent with previous studies in Iran [35], the United Kingdom [36] and Columbia [37] which also found that one-year adjuvant trastuzumab was not cost-effective.
To date there is only one published Australian study in this setting [12]. Unlike the present analysis, Millar and Millward [12] found that 1-year adjuvant trastuzumab had an ICER of A$22,793 per QALY. However, there are some noticeable differences between the present analysis and the one by Millar and Millward [12]. Millar and Millward [12] employed a Markov model; whereas we used a continuous-time, discrete-event microsimulation model. Disability weights of breast cancer used in the two studies are also different. This analysis used the disability weights estimated by Stouthard et al. [24], whereas Millar and Millward [12] used the values reported by Earle et al. [38] (0.02 in remission, 0.45 in metastasis disease). In addition, treatment effects differ between the two studies (0.68 in our study versus 0.48 in the Millar and Millward study). When the model was run for patients in the same age group (50–54 years) using the same utility weights (0.02 in remission and 0.45 in metastasis) and effect size (RR 0.48) as were used in the Millar and Millward study [12], the ICER fell to A$52,666 (95% CI 42,867–68,733) per DALY averted. However, this ICER was more than twofold higher than that reported by Millar and Millward (A$22,793 per QALY) [12]. Such disparity in results may be in part explained by the different model types employed in our study (discrete-event microsimulation model) and the study by Millar and Millward (Markov cohort model). Discrete-event models are superior to Markov cohort models in the following aspects. First, they allow for the incorporation of all kinds of patient characteristics and of enormous variability in the course of the disease and thereby for the natural presentation of the disease [23]. Second, by modelling individual patient pathways and allowing added flexibility in terms of data requirements, discrete-event models more flexible and more naturalistic than cohort models in modelling extended time horizons [23]. Third, because these models do not have the memory limitations of the Markov cohort model, they are likely to provide results with greater confidence [39].
The cost-effectiveness of trastuzumab is less favourable in older women because its ability to prevent relapse is less in older age groups who have lower risk of relapse due to lower overall survival [12]. In the elderly, the clinical advantages of ATC are highly influenced by the higher background mortality rate, which swamps the small effect of trastuzumab on the overall survival in these patients.
The optimal treatment duration of trastuzumab remains a subject of debate. This analysis estimated the cost-effectiveness of 1-year trastuzumab as per the licensed indication in Australia. However, it should be acknowledged that a shorter schedule (the 9-week regimen) has been tested and demonstrated to offer clinical benefit similar to that of the 1-year regimen [20]. In addition, the 9-week schedule was also found to be economically attractive in a number of studies [12, 36, 40]. Despite the above promising results from both an economic and clinical point of view, the 9-week regimen was not included in this analysis as a comparator because it has not been accepted as a clinically proven regimen in Australia.
In this analysis, heart failure was assumed to be reversible and have no direct impact on mortality. Although such assumptions are considered reasonable [41, 42], the study by Telli et al. [43] suggests that the assumed reversibility of trastuzumab-induced heart failure is questionable. In the sensitivity analysis, variations in the incidence and cost of heart failure had little impact on the ICERs. This is likely because of the relatively short follow-up time of this health state reported in the trials. It should be noted that long-term cardiac profile and its impact on the costs and health outcomes of patients treated with trastuzumab remain unknown. More severe/frequent cardiac events and/or more cardiac deaths can be observed when trastuzumab is in widespread clinical use. If this is the case, the assumptions of no additional cardiac mortality and of the reversibility of cardiac dysfunction associated with trastuzumab may lead to a bias in favour of the drug.
This analysis was conducted from a health sector perspective; as such, only direct medical costs were included. Knowing that the majority of early breast cancer patients are of working age and that trastuzumab improves overall survival and health-related quality of life [44], indirect cost savings and gains from potential greater labour force attachment and/or productivity due to trastuzumab could be significant. If a wider perspective were used and these benefits were included, the cost-effectiveness of trastuzumab would be more favourable than it is in this analysis.
Strengths of our study are the inclusion of all eligible patients irrespective of their age category, and the distinguishing of case-fatality of breast cancer by tumour size. Our estimation of trastuzumab cost, which was based on vials needed (which takes the wastage of the drug into account) rather than per kilogram provides more precise information on the cost-effectiveness of trastuzumab. The effect size in this analysis was derived from a meta-analysis of trials on adjuvant trastuzumab, rather than from one single trial. The meta-analysis combined the results of these studies into one estimate of effect using statistical strategy. It helped to increase the number of observations and the statistical power; and thus to improve the precision of the effect size estimates [21].
A limitation is that the lognormal curve of survival of tumour sizes 10–19 mm did not fit the data well, causing the modelled survival in this group to drop below the survival of the larger tumour sizes after almost 30 and 45 years of follow-up, respectively. However, the large majority of patients would not be followed-up for so many years, so the effect of this problem is not big.
To date, there remains a lack of international consensus about which variant of health-adjusted life-years should be used as a measure of health outcome in health economic evaluation. As such, some studies employ DALYs while others use QALYs. The DALY consists of two health measures: (1) years of life lost (YLL) due to premature death; and (2) years lived with disability (YLD) [45]. To calculate YLD, discounted present value of years lived in a condition is multiplied by the disability weight for that condition assigned on a scale from one (representing full health) to zero (representing death) [45]. Although DALY is commonly used in cost-effectiveness analysis, it faces several criticisms. DALY does not capture other aspects of a disease, such as psychological effects [46, 47]. The age weighting method used in DALY is another subject of debate [46]. By assigning the highest weights to 20–40 years old and lowest values to those younger than 5 or older than 90 years old, it is argued that DALY is merely an economic measure of productivity of the affected individuals [48]. QALY is another commonly used measure of health outcome that incorporates both length and quality of life. The QALY approach measures health outcome by assigning to each period of time a weight corresponding to the health-related quality-of-life during that period [49]. To calculate the number of QALYs relating to a health outcome, a quality weight assigned to a particular health state is multiplied by the length of time spent with that state. While QALY overcomes the aforementioned limitations associated with DALY, it is not without problems. The concept of “perfect health” is ambiguous and difficult to objectively quantify [49]. The effects of chronic diseases, where quality of life is compromised but survival is not, are also difficult to address using QALY [48].
Previous studies usually adopted a certain WTP threshold value to determine whether an intervention is cost effective. However, such thresholds are arbitrary, and the concept of WTP threshold is controversial [50]. When thresholds are treated as a decision rule for the cost effectiveness of an intervention, this approach assumes that decision makers have a maximum value they are willing to pay for a unit of health outcome or that there is a well-established shadow price of health improvements resulting from implementing a particular intervention [51]. However, given that healthcare markets are marked by significant degrees of market failure, which deters shadow prices from being truly prevailed, these assumptions are questionable [52]. Furthermore, it has been suggested that decision makers do not appear to maintain a fixed WTP for a unit of health outcome across different types and contexts of decisions [53, 54]. Therefore, we did not adopt a WTP threshold in our study. Rather, we present numerical results of the ICERs; as such, determination of the cost-effectiveness of trastuzumab can be made against any WTP value.
The Australian government agreed to list trastuzumab on the subsidised PBS for all women with early breast cancer, knowing that it would have a huge impact on breast cancer costs. In the 2004–2005 financial year, the total health expenditure on breast cancer in Australia was A$331 million, of which 15% was spent on prescription pharmaceuticals [55]. Given that the acquisition cost of trastuzumab is high compared with other chemotherapy agents, it is likely that a large part of the lifetime cost of breast cancer treatment is attributable to trastuzumab cost, which is estimated to be A$53,628 per patient per year in our analysis.
Trastuzumab is funded by the Australian government despite its poor cost-effectiveness. It has suggested that an important factor in listing drugs with poor cost-effectiveness might be the nature of the disease [56]. If this is the case, it would be of interest to know what makes breast cancer special. Whatever it is, the current listing of trastuzumab for early breast cancer on the PBS does not comply with the generally enforced criteria for cost-effectiveness in Australia.
Appendix 1 Model structure
Individuals who entered the model were Australian women with HER2-positive early breast cancer. The model was based on five main stages within the development process of early breast cancer:
Stage 1: Diagnosis and primary treatment with or without trastuzumab;
Stage 2: Disease-free;
Stage 3: Relapse;
Stage 4: Disseminated;
Stage 5: Dead (due to breast cancer or non-breast cancer causes).
Stage 1 was split into three mutually exclusive subdivisions including (i) HER2-positive early breast cancer with tumours smaller than 10 mm, (ii) HER2-positive early breast cancer with tumours of 10–20 mm, and (iii) HER2-positive early breast cancer with tumours of 20–40 mm. Stage 2 was split into two mutually exclusive subdivisions including (i) disease-free in the first 5 years, and (ii) disease-free after 5 years. Death from breast cancer was assumed to occur in stage 5 only; while death from other causes could occur in any stage.
The model first simulated age at incidence of HER2-positive early breast cancer by drawing a uniformly distributed random number between minimum and maximum HER2-positive incidence cases by age and tumour size. Incidence cases of HER2-positive early breast cancer were calculated by multiplying the incidence cases of breast cancer reported in the Burden of Disease and Injury in Australia 2003 [15] by the rate of HER2-positive early breast cancer (20%) [1].
The model then simulated whether a patient would die from breast cancer or from other causes using the cumulative survival distributions of breast cancer and other causes. Death due to breast cancer was determined based on age at death from other causes and breast cancer survival. If age at other causes death was lower than age at death from breast cancer, death was due to non-breast cancer causes and vice versa. Age at death from other causes was calculated by taking background mortality (mortality from non-breast cancer causes) probabilities by discrete time and returning a continuous survival time, conditional on having survived until the age at incidence.
Each incidence case (patient) entered the model and started with stage 1. The allocation of patient to one of the three mutually exclusive subdivisions of stage 1 was done with a random draw from a discrete distribution of incidence cases by tumour size. Patients then proceeded on to other stages of disease until they were cured and left the model thereafter or until they died (stage 5). The length of time spent in a certain health state was directly drawn per patient from the corresponding cumulative survival distribution, allowing people to move to another stage. Breast cancer survivals (number of years lived after having the disease until death) were calculated using excess mortality rate conditional on trastuzumab treatment, tumour size and having survived so far. The difference in survival between using and not using trastuzumab was determined by the effect size of the drug. Trastuzumab improves survival by decreasing excess mortality rate. If the patient received trastuzumab, the probability of that patient having trastuzumab-induced heart failure was determined by the probability of developing such cardiac toxicity derived from a meta-analysis (2.5%) [21]. Costs and health effects were assigned to each health state.
Appendix 2 Uncertainty distribution around parameters
Appendix 2 is available as a separate file for downloading at https://smw.ch/en/article/doi/smw.2019.20082/
References
1
Fabi
A
,
Malaguti
P
,
Vari
S
,
Cognetti
F
. First-line therapy in HER2 positive metastatic breast cancer: is the mosaic fully completed or are we missing additional pieces?
J Exp Clin Cancer Res. 2016;35(1):104. doi:.https://doi.org/10.1186/s13046-016-0380-5
2Pharmaceutical Benefits Advisory Committee. Public summary document (trastuzumab). 2006. Available at http://www.health.gov.au/internet/main/publishing.nsf/Content/C876BD009518915CCA2572110018BEC9/$File/Trastuzumab.pdf
3Australian Government Department of Health. Pharmaceutical Benefits Scheme. 2011. Available at http://pbs.gov.au/pbs/search?term=trastuzumab&search-type=medicines
4
Cameron
D
,
Piccart-Gebhart
MJ
,
Gelber
RD
,
Procter
M
,
Goldhirsch
A
,
de Azambuja
E
, et al.; Herceptin Adjuvant (HERA) Trial Study Team. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–205. doi:.https://doi.org/10.1016/S0140-6736(16)32616-2
5
Perez
EA
,
Romond
EH
,
Suman
VJ
,
Jeong
JH
,
Sledge
G
,
Geyer
CE, Jr
, et al.
Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744–52. doi:.https://doi.org/10.1200/JCO.2014.55.5730
6
Piccart-Gebhart
MJ
,
Procter
M
,
Leyland-Jones
B
,
Goldhirsch
A
,
Untch
M
,
Smith
I
, et al.; Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. doi:.https://doi.org/10.1056/NEJMoa052306
7
Romond
EH
,
Perez
EA
,
Bryant
J
,
Suman
VJ
,
Geyer
CE, Jr
,
Davidson
NE
, et al.
Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. doi:.https://doi.org/10.1056/NEJMoa052122
8
Smith
I
,
Procter
M
,
Gelber
RD
,
Guillaume
S
,
Feyereislova
A
,
Dowsett
M
, et al.; HERA study team. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369(9555):29–36. doi:.https://doi.org/10.1016/S0140-6736(07)60028-2
9
Balduzzi
S
,
Mantarro
S
,
Guarneri
V
,
Tagliabue
L
,
Pistotti
V
,
Moja
L
, et al.
Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2014;(6):CD006242.
10
Dedes
KJ
,
Szucs
TD
,
Imesch
P
,
Fedier
A
,
Fehr
MK
,
Fink
D
. Cost-effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: a model-based analysis of the HERA and FinHer trial. Ann Oncol. 2007;18(9):1493–9. doi:.https://doi.org/10.1093/annonc/mdm185
11
Kurian
AW
,
Thompson
RN
,
Gaw
AF
,
Arai
S
,
Ortiz
R
,
Garber
AM
. A cost-effectiveness analysis of adjuvant trastuzumab regimens in early HER2/neu-positive breast cancer. J Clin Oncol. 2007;25(6):634–41. doi:.https://doi.org/10.1200/JCO.2006.06.3081
12
Millar
JA
,
Millward
MJ
. Cost effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: a lifetime model. Pharmacoeconomics. 2007;25(5):429–42. doi:.https://doi.org/10.2165/00019053-200725050-00006
13
Hall
PS
,
Hulme
C
,
McCabe
C
,
Oluboyede
Y
,
Round
J
,
Cameron
DA
. Updated cost-effectiveness analysis of trastuzumab for early breast cancer: a UK perspective considering duration of benefit, long-term toxicity and pattern of recurrence. Pharmacoeconomics. 2011;29(5):415–32. doi:.https://doi.org/10.2165/11588340-000000000-00000
14
Lidgren
M
,
Jönsson
B
,
Rehnberg
C
,
Willking
N
,
Bergh
J
. Cost-effectiveness of HER2 testing and 1-year adjuvant trastuzumab therapy for early breast cancer. Ann Oncol. 2007;19(3):487–95. doi:.https://doi.org/10.1093/annonc/mdm488
15
Luke
C
,
Gill
G
,
Birrell
S
,
Humeniuk
V
,
Borg
M
,
Karapetis
C
, et al.
Treatment and survival from breast cancer: the experience of patients at South Australian teaching hospitals between 1977 and 2003. J Eval Clin Pract. 2007;13(2):212–20. doi:.https://doi.org/10.1111/j.1365-2753.2006.00678.x
16Begg S, Vos T, Barker B, Stevenson C, Stanley L, Alan D, et al. The burden of disease and injury in Australia 2003. PHE 82. Canberra: AIHW; 2007.
17
Muñoz
M
,
Fernández-Aceñero
MJ
,
Martín
S
,
Schneider
J
. Prognostic significance of molecular classification of breast invasive ductal carcinoma. Arch Gynecol Obstet. 2009;280(1):43–8. doi:.https://doi.org/10.1007/s00404-008-0867-1
18
Perez
EA
,
Romond
EH
,
Suman
VJ
,
Jeong
JH
,
Davidson
NE
,
Geyer
CE, Jr
, et al.
Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29(25):3366–73. doi:.https://doi.org/10.1200/JCO.2011.35.0868
19Dennis Slamon. BCIRG 006 phase III randomized trial comparing AC→T with AC→TH and with TCH in the adjuvant treatment of HER2-amplified early breast cancer patients: third planned efficacy analysis. 2009. Available at http://www.bcirg.org/NR/rdonlyres/eno7mvfpseiqi5g3pernz37zzeavin4f7o5hos4zwlu76clvwkfluhskusgcmnqvyqk7ksb4gdimpmt6xcmkxppnqce/945_GS5_02_+abst+62+Jan+10.pdf
20
Joensuu
H
,
Bono
P
,
Kataja
V
,
Alanko
T
,
Kokko
R
,
Asola
R
, et al.
Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27(34):5685–92. doi:.https://doi.org/10.1200/JCO.2008.21.4577
21Matthias E, George DS, Douglas GA. Systematic reviews in health care: meta‐analysis in context. BMJ Publishing Group. 2008.
22
Bria
E
,
Cuppone
F
,
Fornier
M
,
Nisticò
C
,
Carlini
P
,
Milella
M
, et al.
Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: the dark side of the moon? A meta-analysis of the randomized trials. Breast Cancer Res Treat. 2008;109(2):231–9. doi:.https://doi.org/10.1007/s10549-007-9663-z
23
Caro
JJ
. Pharmacoeconomic analyses using discrete event simulation. Pharmacoeconomics. 2005;23(4):323–32. doi:.https://doi.org/10.2165/00019053-200523040-00003
24Marlies EAS, Marie-Louise EB, Gouke JB, Jan JB, Pieter GNK, Harry PA, et al. Disability weights for diseases in the Netherlands. Rotterdam: Erasmus University Rotterdam. 1997.
25Australian Government Department of Health. The Pharmaceutical Benefits Scheme. 2018. Available at http://www.pbs.gov.au/pbs/home
26
Dunstan
DW
,
Zimmet
PZ
,
Welborn
TA
,
De Courten
MP
,
Cameron
AJ
,
Sicree
RA
, et al.
The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care. 2002;25(5):829–34. doi:.https://doi.org/10.2337/diacare.25.5.829
27Australian Government Department of Health. The Medicare Benefits Schedule. 2018. Available at http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Home
28National Breast and Ovarian Cancer Centre. Recommendations for use of Trastuzumab for the treatment of HER2-positive breast cancer. 2011. Available at http://guidelines.nbocc.org.au/guidelines/trastuzumab/
29
Smith
DH
,
Gravelle
H
. The practice of discounting in economic evaluations of healthcare interventions. Int J Technol Assess Health Care. 2001;17(2):236–43. doi:.https://doi.org/10.1017/S0266462300105094
30Roche. Herceptin (trastuzumab): achieving clinical excellence in the adjuvant treatment of HER-2 positive breast cancer. Roche submission to the National Institute for Health and Clinical Excellence 2006. Available at https://www.nice.org.uk/guidance/ta107/documents/roche2
31
Garrison
LP, Jr
,
Lubeck
D
,
Lalla
D
,
Paton
V
,
Dueck
A
,
Perez
EA
. Cost-effectiveness analysis of trastuzumab in the adjuvant setting for treatment of HER2-positive breast cancer. Cancer. 2007;110(3):489–98. doi:.https://doi.org/10.1002/cncr.22806
32
Lang
HC
,
Chen
HW
,
Chiou
TJ
,
Chan
AL
. The real-world cost-effectiveness of adjuvant trastuzumab in HER-2/neu-positive early breast cancer in Taiwan. J Med Econ. 2016;19(10):923–7. doi:.https://doi.org/10.1080/13696998.2016.1185013
33
Chen
W
,
Jiang
Z
,
Shao
Z
,
Sun
Q
,
Shen
K
. An economic evaluation of adjuvant trastuzumab therapy in HER2-positive early breast cancer. Value Health. 2009;12(Suppl 3):S82–4. doi:.https://doi.org/10.1111/j.1524-4733.2009.00634.x
34
Shiroiwa
T
,
Fukuda
T
,
Shimozuma
K
,
Ohashi
Y
,
Tsutani
K
. The model-based cost-effectiveness analysis of 1-year adjuvant trastuzumab treatment: based on 2-year follow-up HERA trial data. Breast Cancer Res Treat. 2008;109(3):559–66. doi:.https://doi.org/10.1007/s10549-007-9679-4
35
Aboutorabi
A
,
Hadian
M
,
Ghaderi
H
,
Salehi
M
,
Ghiasipour
M
. Cost-effectiveness analysis of trastuzumab in the adjuvant treatment for early breast cancer. Glob J Health Sci. 2014;7(1):98–106. doi:.https://doi.org/10.5539/gjhs.v7n1p98
36
Clarke
CS
,
Hunter
RM
,
Shemilt
I
,
Serra-Sastre
V
. Multi-arm Cost-Effectiveness Analysis (CEA) comparing different durations of adjuvant trastuzumab in early breast cancer, from the English NHS payer perspective. PLoS One. 2017;12(3):e0172731. doi:.https://doi.org/10.1371/journal.pone.0172731
37
Buendía
JA
,
Vallejos
C
,
Pichón-Rivière
A
. An economic evaluation of trastuzumab as adjuvant treatment of early HER2-positive breast cancer patients in Colombia. Biomedica. 2013;33(3):411–7.
38
Earle
CC
,
Chapman
RH
,
Baker
CS
,
Bell
CM
,
Stone
PW
,
Sandberg
EA
, et al.
Systematic overview of cost-utility assessments in oncology. J Clin Oncol. 2000;18(18):3302–17. doi:.https://doi.org/10.1200/JCO.2000.18.18.3302
39
Karnon
J
,
Brown
J
. Selecting a decision model for economic evaluation: a case study and review. Health Care Manage Sci. 1998;1(2):133–40. doi:.https://doi.org/10.1023/A:1019090401655
40
Neyt
M
,
Huybrechts
M
,
Hulstaert
F
,
Vrijens
F
,
Ramaekers
D
. Trastuzumab in early stage breast cancer: a cost-effectiveness analysis for Belgium. Health Policy. 2008;87(2):146–59. doi:.https://doi.org/10.1016/j.healthpol.2007.11.003
41
Ewer
MS
,
Vooletich
MT
,
Durand
JB
,
Woods
ML
,
Davis
JR
,
Valero
V
, et al.
Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23(31):7820–6. doi:.https://doi.org/10.1200/JCO.2005.13.300
42
Perez
EA
,
Suman
VJ
,
Davidson
NE
,
Sledge
GW
,
Kaufman
PA
,
Hudis
CA
, et al.
Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26(8):1231–8. doi:.https://doi.org/10.1200/JCO.2007.13.5467
43
Telli
ML
,
Hunt
SA
,
Carlson
RW
,
Guardino
AE
. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol. 2007;25(23):3525–33. doi:.https://doi.org/10.1200/JCO.2007.11.0106
44
Norum
J
,
Olsen
JA
,
Wist
EA
,
Lønning
PE
. Trastuzumab in adjuvant breast cancer therapy. A model based cost-effectiveness analysis. Acta Oncol. 2007;46(2):153–64. doi:.https://doi.org/10.1080/02841860601096841
45
Grosse
SD
,
Lollar
DJ
,
Campbell
VA
,
Chamie
M
. Disability and disability-adjusted life years: not the same. Public Health Rep. 2009;124(2):197–202. doi:.https://doi.org/10.1177/003335490912400206
46Fox-Rushby JA. Disability-adjusted life years (DALYs) for decision-making? An overview of the literature. Office of Health Economics. 2002. Available at https://www.ohe.org/publications/disability-adjusted-life-years-dalys-decision-making
47
Thacker
SB
,
Stroup
DF
,
Carande-Kulis
V
,
Marks
JS
,
Roy
K
,
Gerberding
JL
. Measuring the public’s health. Public Health Rep. 2006;121(1):14–22. doi:.https://doi.org/10.1177/003335490612100107
48Lajoie J. Understanding the measurement of Global Burden of Disease. 2015. Available at https://nccid.ca/publications/understanding-the-measurement-of-global-burden-of-disease/
49Brazier J, Ratcliffe J, Salomon J, Tsuchiya A. Measuring and valuing health benefits for economic evaluation. 2016. Oxford: Oxford University Press.
50Cairns J. Using cost-effectiveness evidence to inform decisions as to which health services to provide. Health Systems Reform. 2016;2??
51
Grosse
SD
. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8(2):165–78. doi:.https://doi.org/10.1586/14737167.8.2.165
52Elliott R, Payne K. Essentials of economic evaluation in healthcare. 2004. London: Pharmaceutical Press.
53
Corso
PS
,
Hammitt
JK
,
Graham
JD
,
Dicker
RC
,
Goldie
SJ
. Assessing preferences for prevention versus treatment using willingness to pay. Med Decis Making. 2002;22(1, Suppl):S92–101. doi:.https://doi.org/10.1177/027298902237713
54
Franic
DM
,
Pathak
DS
,
Gafni
A
. Quality-adjusted life years was a poor predictor of women’s willingness to pay in acute and chronic conditions: results of a survey. J Clin Epidemiol. 2005;58(3):291–303. doi:.https://doi.org/10.1016/j.jclinepi.2004.10.005
55Australian Institute of Health and Welfare. Breast cancer in Australia: an overview. Canberra; AIHW: 2009.
56
Raftery
JP
. Paying for costly pharmaceuticals: regulation of new drugs in Australia, England and New Zealand. Med J Aust. 2008;188(1):26–8.