
Figure 1 The different degrees of burn wounds according to skin structure.
DOI: https://doi.org/10.4414/smw.2019.20079
Throughout and across history, allusions to the artificial generation of tissues and organs can be found in mythology, literature or art, such as that illustrated in the “Healing of Justinian” painting (278 AD), where the transplantation of an artificial limb by Cosmas and Damien [1] is depicted. About four decades ago, technological advances in biomedical sciences achieved the translation of this fundamental concept to practical approaches, thus giving birth to the modern fields of tissue engineering and regenerative medicine [2]. Langer and Vacanti, who are often referred to as tissue engineering pioneers, defined tissue engineering as the interdisciplinary field that applies the principles of engineering and life sciences to the development of biological substitutes that restore, maintain or improve the function of tissues or whole organs [3]. Tissue engineering has evolved from the field of biomaterials and now refers to the combination of scaffolds, cells and biologically active molecules into functional tissue constructs designed to be implanted into damaged tissue [4]. Regenerative medicine has a broader research scope that not only includes the concepts of tissue engineering, but also incorporates the notion of self-healing, where the body uses its own systems with the help of foreign biomaterials to rebuild tissues and organs. Tissue engineering and regenerative medicine have become largely interchangeable, as both fields use cell therapy to cure complex wounds or diseases [5].
Because of their two-dimensional structure, skin and cartilage were considered good candidates to be the first tissues to be engineered or regenerated using cell-based products [6]. Although several examples of cell-based engineered tissues have been approved in the USA by the Food and Drug Administration (FDA), a lack of funding has limited their evaluation in extensive clinical trials [7]. For burn care, cell-based therapies have been implemented worldwide for many years, and improved techniques have helped to save many lives since they were first used in 1981 [8, 9].
There is a long history of using cell therapy in Switzerland. Famous pioneering examples of Swiss-based therapy were performed around 1930 by Paul Niehans, who harvested embryonic cow cells to treat illustrious figures such as Charlie Chaplin or Pope Pius XII [10]. With increased regulation for security, Swiss authorities (Federal Health Office and Swissmedic) have regulated such practices to ensure the overall safety of patients. With a high incidence of cellular therapy use (>40 per 10 million population), Switzerland had one of the highest rates of cell transplantation in Europe in 2009 [11]. Cell therapies are particularly common for skin regeneration, especially at the University Hospital of Lausanne (CHUV), which is one of only two national centres specialised in burn care. Here, we aim to give an overview of the history of the different cell therapies used for skin regeneration in the burn unit at the CHUV, and describe the techniques and the conditions under which they are used, as well as highlight the regulatory challenges and perspectives of cell therapies.
The skin is composed of three layers: the epidermis, dermis and hypodermis (fig. 1). The outermost protective layer is the epidermis, which is composed mainly of keratinocytes and provides a barrier against pathogens [12]. The dermis, the layer beneath the epidermis, contains the vasculature, nerves and glands and is mainly composed of fibroblasts surrounded by an extracellular matrix of collagen and proteoglycan, which provides the elasticity and tensile strength of the skin [13]. Finally, the hypodermis or the subcutaneous tissue is connective tissue that attaches the skin to bone and muscles, and is mainly composed of adipose tissue. Burn severity is classified according to the depth of the wound and layers of skin that are effected: a first degree burn involves only the epidermis, second degree superficial is a wound of the upper half of the dermis, second degree deep involves almost all of the dermis, and third degree burns reach the hypodermis. When a burn wound is second degree deep / third degree, the vasculature is destroyed and the body loses its capacity to self-regenerate. Surgical procedures combined with cell therapies thus become indispensable.

Figure 1 The different degrees of burn wounds according to skin structure.
For a further understanding of the intricacies of cell therapy approaches in skin regeneration for burns, we first introduce the definitions of various cell types. Several cell types from different stages of development that have different properties can be used in a cell-based therapy (fig. 2).
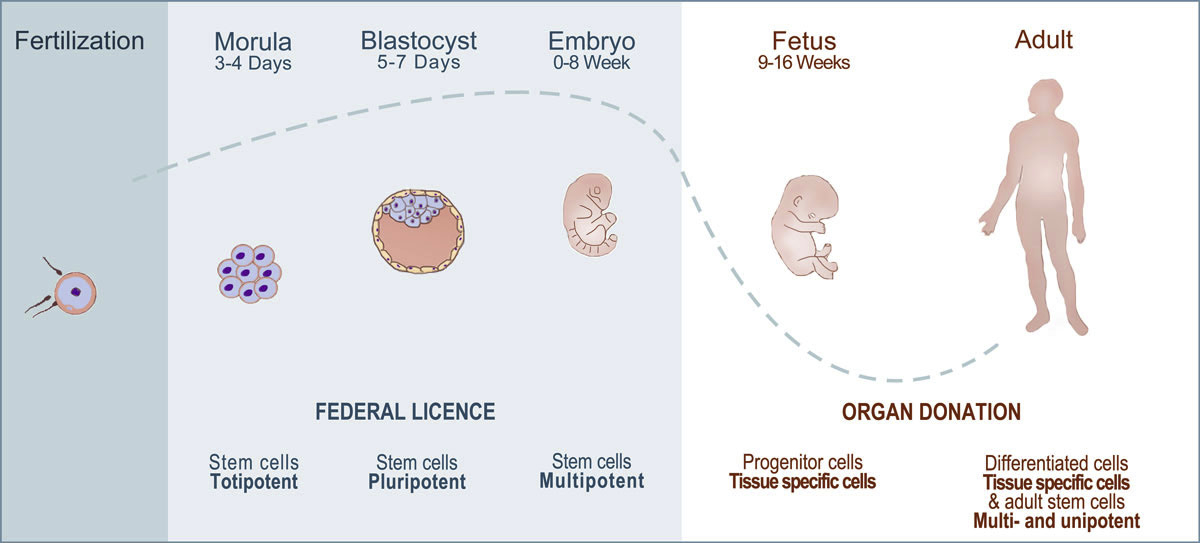
Figure 2 Different stages of development. In Switzerland, the use of human fetal cells and adult cells is part of a Federal Transplantation Programme (blue), whereas the use of human cells at earliest stage requires a license (red).
In cell therapy, autologous transplants are where the cell donor and recipient are the same person. For allogeneic transplants, the cell donor and recipient are different individuals. One of the specialisations at the CHUV is the use of both autologous and allogeneic progenitor cells for the regeneration of skin in burn patients (fig. 3).
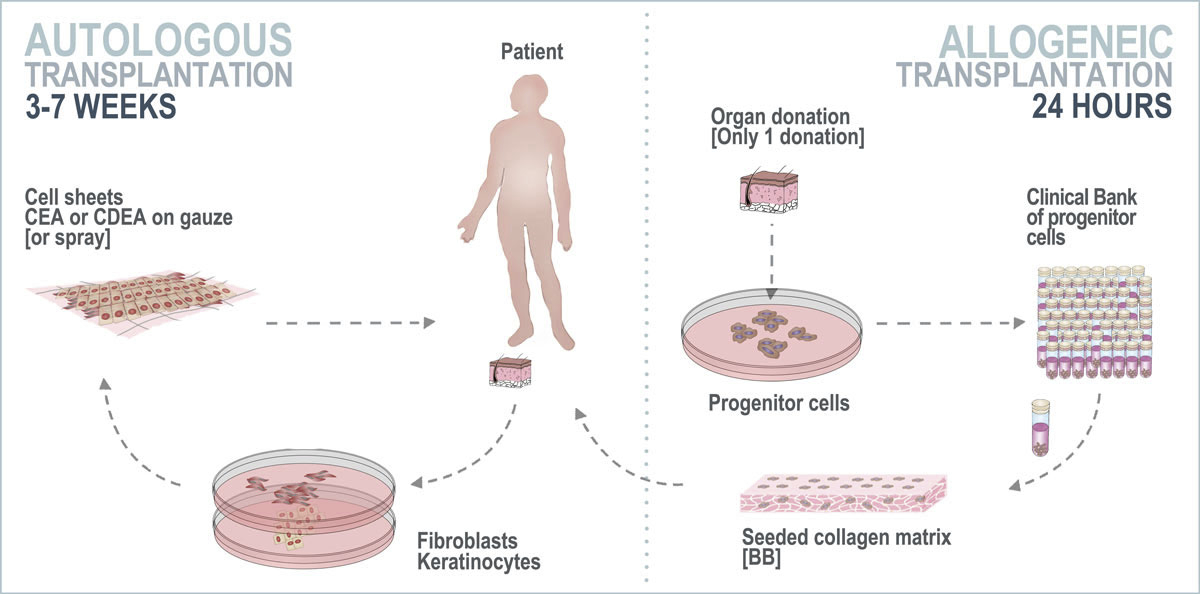
Figure 3 Autologous and allogeneic cell therapy performed at the CHUV Burn Centre. Cell culture is required for each patient for autologous transplants, whereas for an allogeneic transplant, a single organ donation creates a cell bank that can treat multiple patients and significantly reduce the time-to-treatment. CEA = cultured epithelial autograft; CDEA = cultured dermal-epithelial autograft; BB = biological bandage
The use of progenitor cells has been part of the Federal Organ Transplant Programme since 1991 and is registered with the Swiss Federal Office of Public Health. Progenitor cells do not induce a rejection reaction, unlike other sources of allogeneic cells [23], which means that it could be possible to create a universal cell bank allowing the treatment of several patients from only one organ donation [24]. Hundreds of biological bandages can be applied to the patient within 24 hours, whereas an autologous cell therapy requires a biopsy for each patient and culture for several weeks (3–7, depending on technique).
Cell therapy has a long history at the burn unit of the CHUV: the first cultured epithelial autograft (CEA) was introduced into clinics as early as in 1985. For CEA, a biopsy of the patient's epidermis (approximately 10 cm2) is taken and digested to obtain keratinocytes. Cells expand on a feeder layer and form stratified cell sheets in 14–21 days when cultured in the appropriate medium. This thin layer of skin (keratinocyte sheet) can then be transferred on Vaseline gauze and applied topically to the patient for wound closure [25] (fig. 4a). In exceptional cases (1–2 cases per year), where the patient’s condition does not allow for a long delay in treatment, it is possible to apply keratinocytes by spray instead of as a sheet in order to reduce preparation time [26].
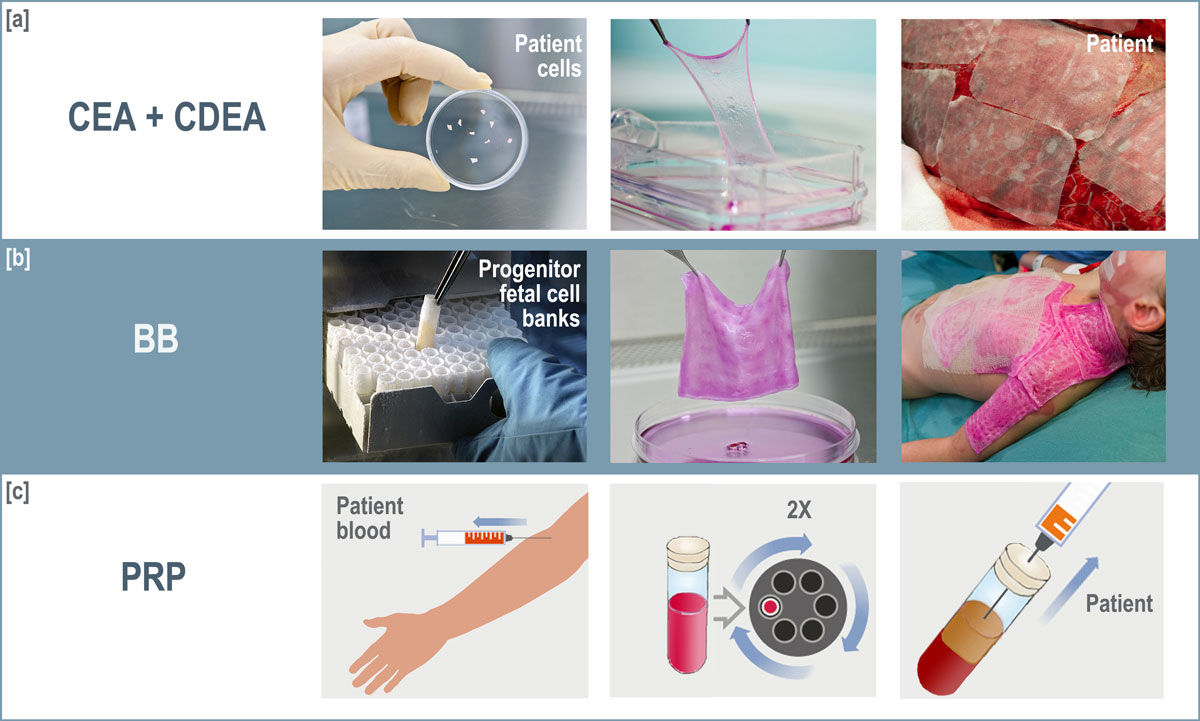
Figure 4 Different types of cell therapy used at the CHUV Burn Centre. [a] Cultured epidermal autograft and cultured dermal-epidermal autograft (CEA and CDEA) – keratinocytes are cultured on feeder cell layers for cell expansion, fibroblasts are retrieved from dermis explants (left panel). In the case of a CDEA, parallel culture of fibroblasts is done, and the keratinocytes are applied on the fibroblast layer after expansion. Finally, the cell sheet (middle panel) is washed with PBS and applied on patient with gauze (right panel). [b] Biological bandage (BB) – a vial from the cell bank (left panel) is thawed, and cells are seeded on a collagen matrix and incubated overnight in culture medium (middle panel), then washed and applied to patient (right panel). [c] Platelet rich plasma (PRP) – blood is collected, centrifuged in a two-step process to isolate platelets, which are then applied to the patient.
Cultured dermal-epidermal autograft (CDEA), which was implemented in the mid-1990s, is based on the principle of CEA, but allows re-creation of a thicker skin-graft, because a double layer composed of autologous fibroblasts and keratinocytes is used [27]. CDEA provides a better clinical outcome, with a skin graft having more functional mechanical properties than a CEA. However, CDEA is less commonly used since it requires a full thickness biopsy and longer culture times, typically 6–7 weeks. CDEA is therefore used exclusively in severely burned patients for whom hospitalisation duration is longer and multiple surgical and grafting operations are needed. It is worth mentioning that systematic prophylactic use of antibiotics prior to a skin autografting is not recommended, to avoid promoting resistance; however, in the case of a cell-based therapy such as CEA or CDEA, it is common practice at CHUV to prepare recipient sites 2 days prior to grafting by a daily application of gentamicin and/or terbinafine, according to the targeted pathogen.
During the 3–7 weeks required for preparation of CEA and CDEA, patients are covered with temporary skin substitutes to prevent fluid loss, reduce infection risk and decrease pain by nerve covering [28]. Over the years, temporary skin substitutes have included xenogeneic decellularised porcine skin, allogenic cadaveric human skin and synthetic dressings [29], all of which carry some risk of rejection and disease transfer. Furthermore, the frequent integration of temporary covers by the recipient renders ablation difficult with painful eschars [27]. In early 2000, a new allogenic cell therapy was developed in the Regenerative Therapy Unit at the CHUV: use of biological bandages made of human progenitor skin fibroblasts seeded onto biodegradable equine collagen scaffolds (fig. 4b). The function of these bandages was to replace other temporary dressings that had many clinical complications. These bioactive bandages allow simultaneous enhancement of skin regeneration, which is most likely related to their release of growth factors and cytokines [30]. The first clinical trials, begun in 2000, demonstrated that biological bandages provided efficient treatment of second degree superficial and deep burns without autografting, showing that progenitor skin cells may have great potential to treat burns and, eventually, acute and chronic wounds of other types [20]. Biological bandages have since been used routinely at the CHUV, particularly for paediatric burns.
Beginning in the early 2000s, use of platelet rich plasma [31] was integrated into patient care. Local application of platelet rich plasma has been shown to improve healing in donor sites [32] (fig. 4c).
It is worth mentioning that a new Federal Act on Transplantation of Organs, Tissues and Cells came into force in 2007.This involved implementation of good manufacturing practice (GMP) procedures for cell therapy preparation [33]. Thus, a cell production centre was initiated as part of the CHUV’s 2009–2013 strategic plan. Since 2015, all types of cell therapy are manufactured at the Cell Production Centre, which is an accredited GMP facility in Switzerland for cell therapy production, not only for clinical trials but also for routine preparation of cell therapies for burn patients. A summary of the different cell therapies for burns can be found in table 1.
Table 1 Summary of different cell therapies used at the CHUV Burn Centre.
| Type of therapy | Description | Type of biopsy |
Type of cells
(type of graft) |
|---|---|---|---|
| CEA | Cultured epithelial autograft | Epidermal | Keratinocytes (autologous) |
| CDEA | Cultured dermal-epithelial autograft | Epidermal and dermal | Fibroblasts and keratinocytes (autologous) |
| BB | Biological bandage | No biopsy: off-the-shelf cell bank | Human progenitor fibroblasts (allogenic) |
| PRP | Platelet rich plasma | Blood | Platelets (autologous) |
When burns are deep (second and third degree burns), the skin loses its ability to regenerate and a skin graft is required. Split-thickness autografting is still the gold standard for the treatment of deep burn wounds [27]. This consists of taking from the patient epidermis with a small portion of the dermis in order to cover the burn wound (the donor site heals spontaneously by re-epithelialisation because of the remaining dermis portion). However, for severely burned patients, when the damaged surface is too large to be covered by autografting, cell therapy is needed. The appropriate type of cell therapy depends on the total body surface area (TBSA) and the depth of the burn. A CEA strategy is used for adult burn patients with a deep burn surface that exceeds 40–50% of the TBSA. The surgeon may also decide to use a CDEA for the areas where he/she considers it to be necessary, such as the hands or other body areas that require a certain functionality. However, this is only done in patients where it is known that hospitalisation will be for a long time, permiting cell culturing (generally for very severe burns).
Biological bandages serve as a temporary cover, allowing the time to prepare CEAs and/or CDEAs. They can also be used to cover donor graft sites to stimulate wound closure and in combination with a mesh graft in a “sandwich-like” configuration. Likewise, platelet rich plasma is used in burns to stimulate and accelerate the healing of wounds with large surfaces.
For paediatric burn patients at the CHUV, it is standard to use biological bandages to cover burns of 10% TBSA or more, since this is the definition of a severe burn in paediatric patients. Every 2 days the biological bandages are replaced by new ones, and this process is normally repeated up to four times. After 10 days, the surgeon assesses the wound closure and decides if a skin autograft is required for surface areas that are not completely closed. Most paediatric burns at CHUV are second degree superficial burns that are appropriately treated with biological bandages, with complete wound closure usually within 10 days, thus decreasing the necessity of skin autografting. In addition, CEA and CDEA can also be used in paediatric burn patients (>40% TBSA), but these are fortunately rare cases in the CHUV. A summary of the different cases for cell therapies used can be found in table 2.
Table 2 Different indications for the use of cell therapies at the CHUV Burn Centre, as well as the average number of patient per year that benefited from each therapy for the past 5 years. The time delay before receiving a cell therapy varies depending on the type of treatment: the need for a cell culture step considerably prolongs the preparation time before transfer to the patient. Note that biological bandages and platelet rich plasma can be transferred within 24–72 hours and less than 24 hours, respectively, after preparation.
| Adult | Child | Use and indication | Burned surface | Duration of preparation | Number of patients/year | |
|---|---|---|---|---|---|---|
| Cultured epithelial autograft | + | + | Applied directly on wounds Applied in “sandwich” with skin mesh grafts On autograft donor sites |
>40–50% | 2–3 weeks* | 11 |
| Cultured dermal-epithelial autograft | + | + | Very rare cases, for long hospital stays only | >70–80% | 6-8 weeks | 2 |
| Biological bandage | + | + | Temporary cover on areas needing further grafting On autograft donor sites |
Starting from: >20% for adults; >10% for children | 24–72 hours | 9 |
| Platelet rich plasma | + | – | For any type of burn On autograft donor sites |
>1–2% | <24 hours | 115 |
* This preparation time can be reduced to 1 week if cultured epithelial autograft is delivered by spray. In general, the spray alternative is used when the condition of the patient does not allow longer wait periods.
Skin wounds are major contributors to global disease burden, affecting millions of people worldwide [34]. This burden is expected to increase, with serious consequences in the future as over half of all chronic and acute skin wounds become infected [35]. Such infections are particularly worrisome in the context of the rise in resistance of bacterial pathogens to antibiotic treatments [36, 37]. In this context, research efforts are ongoing at the CHUV to develop a new generation of biological bandages with antimicrobial properties to treat bacterial infections topically. For biological bandage production, the use of collagen scaffolds for progenitor cell support may be more prone to infection because of their high hydroxyproline content, which is a source of nutrients for opportunistic bacterial pathogens such as Pseudomonas aeruginosa [38]. In 2016, Abdel-Sayed and co-authors showed that coupling the collagen matrix with a certain class of antimicrobial peptide dendrimers not only inhibited the growth of multi-drug resistant Pseudomonas aeruginosa, but also could have pro-angiogenic effects, which are involved in improved wound healing [39]. A preclinical study testing the safety and efficacy of these new class of bandages is currently ongoing. Meanwhile, the CHUV also has other successful cell therapy endeavours. For instance, in 2018 the first patient was treated with a matrix-assisted autologous chondrocyte transplant. This was the first clinical protocol for cell therapy to be accepted in Switzerland with full GMP processing in association with the Cell Production Centre of the CHUV. Cell therapy research is also undertaken in Zurich, where the second national centre of “highly specialised medicine” for burn care management is situated; an example is experimentation of bio-printing in order to one day be able to produce skin for patients in some sort of skin factory, in a fast, accurate and efficient way.
The European Directives and Regulations (2001/83/EC and 1394/2007) classified cell therapies as “Advanced Therapy Medicinal Products” (ATMP) as of 2008, which imposes industrial GMP requirements, as well as marketing authorisation, for cell therapy production in hospital settings. This in turn has increased the costs considerably and could lengthen the process of introducing new treatments for burn patients [33]. Hence, it is important to discuss with health authorities about hospital exemptions to allow compassionate use of cell therapies, without the requirement for marketing authorisation, to make these customised and life-saving treatments available to patients [40–42]. From January 2019, revisions of the Therapeutic Products Act (Stage 2), which aim to simplify and facilitate patient access to medicinal products and improve conditions for biomedical research, have come into force. In particular, this allows a 2-year authorisation for the “compassionate use” of cell therapies provided that a clinical study has been approved in Switzerland prior to the request. Scientific research continues to advance therapeutic possibilities in tissue engineering and regenerative medicine, even as current possibilities for patient care are hampered by the hurdles of GMP procedures, increased administrative load and costs. It is of course in the best interest of physicians and scientists that these hurdles are removed by the competent authorities to ensure that patients have access to the best treatment options and with the highest security at all times.
A thank you to Dr Shawna McCallin for reviewing this manuscript for a spell check.
No financial support and no other potential conflict of interest relevant to this article was reported.
1Meyer U, Meyer T, Handschel J, Wiesmann HP. Fundamentals of Tissue Engineering and Regenerative Medicine. Berlin, Heidelberg: Springer; 2009.
2 Vacanti JP , Langer R . Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354(Suppl 1):S32–4. doi:.https://doi.org/10.1016/S0140-6736(99)90247-7
3 Langer R , Vacanti JP . Tissue engineering. Science. 1993;260(5110):920–6. doi:.https://doi.org/10.1126/science.8493529
4 O’Brien FJ . Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14(3):88–95. doi:.https://doi.org/10.1016/S1369-7021(11)70058-X
5 Berthiaume F , Maguire TJ , Yarmush ML . Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng. 2011;2(1):403–30. doi:.https://doi.org/10.1146/annurev-chembioeng-061010-114257
6 Huey DJ , Hu JC , Athanasiou KA . Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–21. doi:.https://doi.org/10.1126/science.1222454
7 Khademhosseini A , Vacanti JP , Langer R . Progress in tissue engineering. Sci Am. 2009;300(5):64–71. doi:.https://doi.org/10.1038/scientificamerican0509-64
8 Bell E , Ehrlich HP , Buttle DJ , Nakatsuji T . Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science. 1981;211(4486):1052–4. doi:.https://doi.org/10.1126/science.7008197
9 Hernon CA , Dawson RA , Freedlander E , Short R , Haddow DB , Brotherston M , et al. Clinical experience using cultured epithelial autografts leads to an alternative methodology for transferring skin cells from the laboratory to the patient. Regen Med. 2006;1(6):809–21. doi:.https://doi.org/10.2217/17460751.1.6.809
10 Lefrère JJ , Berche P . [Doctor Brown-Sequard’s therapy]. Ann Endocrinol (Paris). 2010;71(2):69–75. Article in French. doi:.https://doi.org/10.1016/j.ando.2010.01.003
11 Martin I , Baldomero H , Bocelli-Tyndall C , Slaper-Cortenbach I , Passweg J , Tyndall A . The survey on cellular and engineered tissue therapies in Europe in 2009. Tissue Eng Part A. 2011;17(17-18):2221–30. doi:.https://doi.org/10.1089/ten.tea.2011.0131
12 Madison KC . Barrier function of the skin: “la raison d’être” of the epidermis. J Invest Dermatol. 2003;121(2):231–41. doi:.https://doi.org/10.1046/j.1523-1747.2003.12359.x
13 Breitkreutz D , Mirancea N , Nischt R . Basement membranes in skin: unique matrix structures with diverse functions? Histochem Cell Biol. 2009;132(1):1–10. doi:.https://doi.org/10.1007/s00418-009-0586-0
14 Thomson JA , Itskovitz-Eldor J , Shapiro SS , Waknitz MA , Swiergiel JJ , Marshall VS , et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. doi:.https://doi.org/10.1126/science.282.5391.1145
15 Maleki M , Ghanbarvand F , Reza Behvarz M , Ejtemaei M , Ghadirkhomi E . Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells. 2014;7(2):118–26. doi:.https://doi.org/10.15283/ijsc.2014.7.2.118
16 Western P . Foetal germ cells: striking the balance between pluripotency and differentiation. Int J Dev Biol. 2009;53(2-3):393–409. doi:.https://doi.org/10.1387/ijdb.082671pw
17 Blanpain C , Fuchs E . Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22(1):339–73. doi:.https://doi.org/10.1146/annurev.cellbio.22.010305.104357
18 Lv FJ , Tuan RS , Cheung KM , Leung VY . Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408–19. doi:.https://doi.org/10.1002/stem.1681
19 Applegate LA , Scaletta C , Hirt-Burri N , Raffoul W , Pioletti D . Whole-cell bioprocessing of human fetal cells for tissue engineering of skin. Skin Pharmacol Physiol. 2009;22(2):63–73. doi:.https://doi.org/10.1159/000178865
20 Ramelet AA , Hirt-Burri N , Raffoul W , Scaletta C , Pioletti DP , Offord E , et al. Chronic wound healing by fetal cell therapy may be explained by differential gene profiling observed in fetal versus old skin cells. Exp Gerontol. 2009;44(3):208–18. doi:.https://doi.org/10.1016/j.exger.2008.11.004
21 Darwiche S , Scaletta C , Raffoul W , Pioletti DP , Applegate LA . Epiphyseal Chondroprogenitors Provide a Stable Cell Source for Cartilage Cell Therapy. Cell Med. 2012;4(1):23–32. doi:.https://doi.org/10.3727/215517912X639324
22 Vuadens F , Crettaz D , Scelatta C , Servis C , Quadroni M , Bienvenut WV , et al. Plasticity of protein expression during culture of fetal skin cells. Electrophoresis. 2003;24(7-8):1281–91. doi:.https://doi.org/10.1002/elps.200390165
23 Montjovent MO , Bocelli-Tyndall C , Scaletta C , Scherberich A , Mark S , Martin I , et al. In vitro characterization of immune-related properties of human fetal bone cells for potential tissue engineering applications. Tissue Eng Part A. 2009;15(7):1523–32. doi:.https://doi.org/10.1089/ten.tea.2008.0222
24 De Buys Roessingh AS , Hohlfeld J , Scaletta C , Hirt-Burri N , Gerber S , Hohlfeld P , et al. Development, characterization, and use of a fetal skin cell bank for tissue engineering in wound healing. Cell Transplant. 2006;15(8-9):823–34. doi:.https://doi.org/10.3727/000000006783981459
25 Rheinwald JG , Green H . Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6(3):331–43. doi:.https://doi.org/10.1016/S0092-8674(75)80001-8
26 Hartmann B , Ekkernkamp A , Johnen C , Gerlach JC , Belfekroun C , Küntscher MV . Sprayed cultured epithelial autografts for deep dermal burns of the face and neck. Ann Plast Surg. 2007;58(1):70–3. doi:.https://doi.org/10.1097/01.sap.0000250647.39784.bb
27 Koch N , Erba P , Benathan M , Raffoul W . [New developments in skin reconstruction - cell cultures and skin substitutes plus review of the literature]. Ann Burns Fire Disasters. 2010;23(3):131–6. Article in French.
28 Sheridan R . Closure of the excised burn wound: autografts, semipermanent skin substitutes, and permanent skin substitutes. Clin Plast Surg. 2009;36(4):643–51. doi:.https://doi.org/10.1016/j.cps.2009.05.010
29 Sun BK , Siprashvili Z , Khavari PA . Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346(6212):941–5. doi:.https://doi.org/10.1126/science.1253836
30 Hohlfeld J , de Buys Roessingh A , Hirt-Burri N , Chaubert P , Gerber S , Scaletta C , et al. Tissue engineered fetal skin constructs for paediatric burns. Lancet. 2005;366(9488):840–2. doi:.https://doi.org/10.1016/S0140-6736(05)67107-3
31 Akhundov K , Pietramaggiori G , Waselle L , Darwiche S , Guerid S , Scaletta C , et al. Development of a cost-effective method for platelet-rich plasma (PRP) preparation for topical wound healing. Ann Burns Fire Disasters. 2012;25(4):207–13.
32 Guerid S , Darwiche SE , Berger MM , Applegate LA , Benathan M , Raffoul W . Autologous keratinocyte suspension in platelet concentrate accelerates and enhances wound healing - a prospective randomized clinical trial on skin graft donor sites: platelet concentrate and keratinocytes on donor sites. Fibrogenesis Tissue Repair. 2013;6(1):8. doi:.https://doi.org/10.1186/1755-1536-6-8
33 Dimitropoulos G , Jafari P , de Buys Roessingh A , Hirt-Burri N , Raffoul W , Applegate LA . Burn patient care lost in good manufacturing practices? Ann Burns Fire Disasters. 2016;29(2):111–5.
34 Hay RJ , Johns NE , Williams HC , Bolliger IW , Dellavalle RP , Margolis DJ , et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134(6):1527–34. doi:.https://doi.org/10.1038/jid.2013.446
35 Armstrong DG , Boulton AJM , Bus SA . Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017;376(24):2367–75. doi:.https://doi.org/10.1056/NEJMra1615439
36 Ventola CL . The antibiotic resistance crisis: part 2: management strategies and new agents. P&T. 2015;40(5):344–52.
37 Ventola CL . The antibiotic resistance crisis: part 1: causes and threats. P&T. 2015;40(4):277–83.
38 Gonzalez MR , Ducret V , Leoni S , Fleuchot B , Jafari P , Raffoul W , et al. Transcriptome Analysis of Pseudomonas aeruginosa Cultured in Human Burn Wound Exudates. Front Cell Infect Microbiol. 2018;8:39. doi:.https://doi.org/10.3389/fcimb.2018.00039
39 Abdel-Sayed P , Kaeppeli A , Siriwardena T , Darbre T , Perron K , Jafari P , et al. Anti-Microbial Dendrimers against Multidrug-Resistant P. aeruginosa Enhance the Angiogenic Effect of Biological Burn-wound Bandages. Sci Rep. 2016;6(1):22020. doi:.https://doi.org/10.1038/srep22020
40 Hirsch T , Rothoeft T , Teig N , Bauer JW , Pellegrini G , De Rosa L , et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551(7680):327–32. doi:.https://doi.org/10.1038/nature24487
41 Marks P , Gottlieb S . Balancing Safety and Innovation for Cell-Based Regenerative Medicine. N Engl J Med. 2018;378(10):954–9. doi:.https://doi.org/10.1056/NEJMsr1715626
42 Mavilio F , Pellegrini G , Ferrari S , Di Nunzio F , Di Iorio E , Recchia A , et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006;12(12):1397–402. doi:.https://doi.org/10.1038/nm1504
No financial support and no other potential conflict of interest relevant to this article was reported.