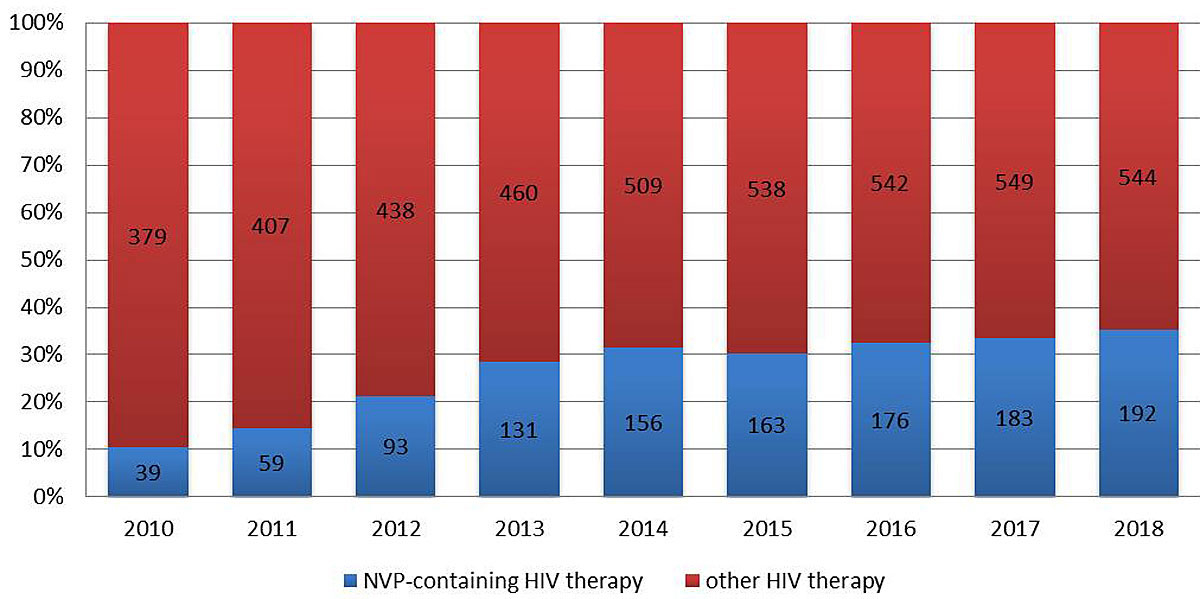
Figure 1 Percentage of patients on nevirapine (NVP)-containing HIV therapy.
DOI: https://doi.org/10.4414/smw.2019.20053
Combined antiretroviral treatment (cART) with three antiretroviral compounds was established in 1996 and is able to achieve a complete and continued suppression of human immunodeficiency virus (HIV) replication within the body. The main reason to combine three compounds to treat HIV infection is to prevent the escape of drug resistant mutants. This risk is especially high during the initial phase of first-line therapy, when massive viral replication occurs. Once the virus is suppressed, no further viral replication and evolution occurs as long as the treatment is maintained [1]. As a result, life expectancy in people living with HIV is now similar to that of the general population [2]. All international guidelines specify antiretroviral combinations to initiate cART [3]. However, the distinct requirements of long-term maintenance therapy once viral replication has been stably suppressed has not been studied intensively. The most important requirement during the maintenance phase is to sustain viral suppression using compounds with as few as possible long-term side effects.
Nevirapine, the first NNRTI (non-nucleoside reverse transcriptase inhibitor), which entered the market in the early 1990s, is started less and less, but still continued today, and thus has an exceptional record for long-term tolerability [4–6]. Typical side effects of all NNRTIs include rash and hepatitis, as well as neuropsychological side effects and dyslipidaemia with efavirenz and rilpivirine. Dyslipidaemia, nausea and diarrhoea are frequent side effects of protease inhibitors. Among all the NNRTIs and protease inhibitors, nevirapine is the only one that has no negative effect on lipids [7]. One outstanding characteristic of nevirapine is its high penetration into sanctuaries. Among all antiretroviral drugs, nevirapine is probably the one with the best documented penetration rate in the genital tract and in the central nervous system including the brain tissue, and the drug is used with excellent results in HIV encephalopathy / HIV dementia [8, 9]. Additionally, it has been shown to be strongly correlated to undetectable HIV-DNA concentrations [10].
The reason, why nevirapine is not frequently started anymore despite its favourable price (see below) is the relatively frequent (15–25%) occurrence of hypersensitivity reactions (either skin rash or elevated liver enzymes) in the first 3 months after treatment initiation, especially in patients with a high CD4 count (>250/µl in women and >400/µl in men) [11, 12]. Undetected elevation of liver enzymes may cause liver failure during continued treatment with nevirapine. Interestingly, the rate of adverse events in HIV-positive individuals initiating nevirapine is lower if they are already under a suppressing antiretroviral regimen [13, 14].
On the basis of the latter observation, we started to evaluate the use of nevirapine for antiviral maintenance therapy at our centre, the infectious diseases clinic of the Cantonal Hospital St Gallen. Since 2010, we have systematically evaluated the option to switch patients on stable antiretroviral therapy to a nevirapine-containing regimen, including patients with high CD4 cell counts. As a consequence, nevirapine is now the most frequently used third agent in our centre, with more than 200 patients on nevirapine.
Within the Swiss HIV Cohort Study (SHCS) [15], a multicentre prospective observational study initiated in 1988, more than 850 patients are stably treated with nevirapine-containing regimens. Compared with the drug costs for the ten most common antiretroviral combinations used in the SHCS in 2017, nevirapine-based therapies are on average 22% or 300 CHF/month less expensive than the average costs of the other cART regimens.
Based on a retrospective chart review, we explored whether switching to a nevirapine-containing regimen could represent an option for maintenance therapy in current HIV management.
The Infectious Diseases Clinic of the Cantonal Hospital St Gallen is one of seven centres of the Swiss HIV Cohort Study (SHCS) and is the biggest non-university centre. It has a catchment area of about 600,000 people. The majority of Swiss HIV patients are treated at one of the SHCS centres, with the rest being treated at infectious disease units at smaller hospitals or in private practice. All adult HIV-infected patients followed up at the Infectious Diseases Clinic of the Cantonal Hospital St Gallen between 2010 and 2018 were considered for the analysis. Of these, all patients on a nevirapine-containing regimen were included in the analysis. Data in the clinical database were collected prospectively according to the standard defined by the SHCS [15]. The initial dose of nevirapine was 200 mg once daily, with an increase to one 400-mg prolonged release tablet daily after 2 weeks if there was no sign of adverse drug effects (e.g., rash, elevated liver enzymes). All treatment starts and stops were evaluated, and reasons for treatment interruption were obtained from chart review. This study was approved by the local ethics committee (Ethikkommission Ostschweiz, Project-ID 2018-01595).
Hypersensitivity reaction (HSR) was defined as any rash or elevation of liver enzymes within the first 90 days of nevirapine treatment that led to nevirapine stop. Nevirapine interruption was defined as nevirapine stop followed by restart within the study period. A CD4+ lymphocyte count of >250/µl in females and >400/µl in males was considered as a “high CD4 count” and a CD4 count of ≤250/µl in females and ≤400/µl in males as “low CD4 count”. Undetectable viraemia was defined as plasma HIV-RNA <50 copies/ml.
Descriptive statistics were used to characterise the study population.
Every 6 months between 2010 and 2018 (1 January and 1 July of each year), we determined the total number of patients on ART and nevirapine, the proportion of ART patients a on nevirapine-containing regimen and the number of nevirapine starts and stops in the past 6 months.
For the time-to-event analysis, we used only the first nevirapine treatment in each patient. Follow-up time was defined as time between nevirapine start and nevirapine stop. For patients in whom nevirapine was not stopped, follow-up time was censored at the date of last information (the latest of the following four dates: last follow-up date, last laboratory date, latest date on which the patient was known to be alive or date of death).
To calculate stop rates, the number of stops was divided by the total number of person-years. Associations between sex, age at nevirapine-start, hepatitis C virus (HCV) co-infection, time on nevirapine, ethnicity, CD4 nadir, CD4 cell count, HIV viral load and ART backbone at nevirapine start and stop rate were evaluated by calculation of rate ratios. Stop rates were considered significantly different at the 5% level, if the 95% confidence interval (CI) of the stop ratio did not include 1.
To investigate the effect of time under nevirapine on the stop rate, Lexis expansion was used. The Lexis expansion is a method that splits the follow-up time of individuals in a cohort study. Using this manipulation of cohort data, we can examine the effect of variables that change over time [16]. For each individual, the time on nevirapine was split into ≤90 days and >90 days, since hypersensitivity reactions to nevirapine usually occur within the first 90 days of therapy. Thus, separate stop rates for the first 90 days on nevirapine and thereafter could be calculated and compared.
Kaplan-Meier survival curves for the probability of remaining on nevirapine, stratified by sex, age at nevirapine start (<45 years versus ≥45 years), HCV co-infection, ethnicity, CD4 nadir, CD4 count, HIV-RNA and ART backbone at nevirapine-start were compared using log-rank tests.
Stata Version 12.0 was used for statistical analyses.
In 2010, 10% (39/379) of all HIV-patients treated at our clinic in St Gallen were on a nevirapine-containing regimen. By 2014, this number had increased to 33% (156/509). Since then, the percentage of patients treated with nevirapine remained stable at one third of the total number of patients treated, with 35% (192/544) in 2018 (fig.1).

Figure 1 Percentage of patients on nevirapine (NVP)-containing HIV therapy.
Between 1 January 2010 and 31 July 2018, there were 307 nevirapine starts. In 277 cases, it was the first nevirapine start for that patient, in 26 the second, in 3 the third and in 1 the fourth. Thirty-three percent (101/307) of the nevirapine therapies were stopped during follow up; 19% (19/101) of the stops were actually interruptions and nevirapine was restarted at a later time-point during follow up. Baseline characteristics of 277 patients with a first nevirapine-containing cART are summarised in table 1.
Table 1 Baseline characteristics of patients using nevirapine.
|
All first NVP therapies since 1 Jan 2010
(n = 277; 92 stops) |
Duration ≤90 days
(n = 56; 48 stops) |
Duration >90 days
(n = 221; 44 stops) |
|||
|---|---|---|---|---|---|
| Sex | Female | 24% (66) | 20% (11) | 25% (55) | |
| Male | 76% (211) | 80% (45) | 75% (166) | ||
| Age (y) | Median (IQR) | 47 (38–54) | 48 (38–56) | 47 (38–53) | |
| <45 | 43% (119) | 34% (19) | 45% (100) | ||
| ≥45 | 57% (158) | 66% (37) | 55% (121) | ||
| Ethnicity | White | 85% (235) | 82% (46) | 86% (189) | |
| Black | 12% (32) | 7% (4) | 13% (28) | ||
| Hispanic | 1% (3) | 0% (0) | 1% (3) | ||
| Asian | 3% (7) | 11% (6) | 0.5% (1) | ||
| HCV co-infection | 18% (50) | 21% (12) | 17% (38) | ||
| CD4 nadir (…/µl) | Median (IQR) | All | 240 (120–360) | 220 (145–340) | 240 (120-360) |
| Women | 212 (110–320) | 220 (110–420) | 210 (109-320) | ||
| Men | 240 (140–380) | 220 (153–310) | 240 (130-390) | ||
| <200 | 39% (109) | 39% (22) | 39% (87) | ||
| ≥200 and <350 | 34% (94) | 36% (20) | 33% (74) | ||
| ≥350 and <500 | 16% (45) | 14% (8) | 17% (37) | ||
| ≥500 | 10% (29) | 11% (6) | 10% (23) | ||
| Low* | 75% (208) | 80% (45) | 74% (163) | ||
| High† | 25% (69) | 20% (11) | 26% (58) | ||
| CD4 at NVP start (…/µl) | Median (IQR) | All | 590 (400–840) (n = 275) |
585 (415–753) (n = 56) |
590 (380-870) (n = 219) |
| Women | 620 (380–860) (n = 65) |
755 (600–1060) (n = 11) |
585 (370-820) (n = 54) |
||
| Men | 580 (400–840) (n = 210) |
540 (410–730) (n = 45) |
590 (400-890) (n = 165) |
||
| <200 | 5% (14/275) | 4% (2/56) | 5% (12/219) | ||
| ≥200 and <350 | 12% (32/275) | 5% (3/56) | 13% (29/219) | ||
| ≥350 and <500 | 20% (54/275) | 29% (16/56) | 17% (38/219) | ||
| ≥500 | 62% (175/275) | 63% (35/56) | 64% (140/219) | ||
| Low* | 22% (61/275) | 20% (11/56) | 23% (50/219) | ||
| High† | 78% (214/275) | 80% (45/56) | 77% (169/219) | ||
| HIV-RNA (cop/ml) |
<50 | 81% (223/275) | 86% (48) | 80% (175/219) | |
| ≥50 | 19% (52/275) | 14% (8) | 20% (44/219) | ||
| Low-low-detectable | 8% (21/275) | 9% (5/56) | 7% (16/219) | ||
| Low-low-undetectable | 15% (40/275) | 11% (6/56) | 16% (34/219) | ||
| Low-high-detectable | 6% (16/275) | 2% (1/56) | 7% (15/219) | ||
| Low-high-undetectable | 47% (130/275) | 59% (33/56) | 44% (97/219) | ||
| High-high-detectable | 5% (15/275) | 4% (2/56) | 6% (13/219) | ||
| High-high-undetectable | 19% (53/275) | 16% (9/56) | 20% (44/219) | ||
| NVP-start | 2010–2011 | 28% (77) | 21% (12) | 29% (65) | |
| 2012–2013 | 34% (93) | 29% (16) | 35% (77) | ||
| 2014–2015 | 17% (48) | 23% (13) | 16% (35) | ||
| 2016–2017 | 17% (47) | 7% (4) | 19% (43) | ||
| 01–07/2018 | 4% (12) | 20% (11) | 0.5% (1) | ||
| Backbone at NVP start | 3TC/ABC | 74% (204) | 79% (44) | 72% (160) | |
| ETC/TDF | 21% (59) | 16% (9) | 23% (50) | ||
| ETC/TAF | 2% (6) | 4% (2) | 2% (4) | ||
| 3TC/TDF | 1% (3) | 0% (0) | 1% (3) | ||
| Other | 2% (5) | 2% (1) | 2% (4) | ||
3TC = lamivudine; ABC = abacavir; ETC = emtricitabine; IQR = interquartile range; NVP = nevirapine; TAF = tenofovir alafenamide; TDF = tenofovir disoproxil fumarate * ≤250/µl in women and ≤400/µl in men † >250/µl in women and ≥400/µl in men “Low/high-low/high-detectable/undetectable” refers to “(CD4 nadir)–(CD4 at NVP-start)–(HIV RNA at NVP start)” with “low” for CD4 ≤250/µl in women and ≤400/µl in men, “high” for CD4 >250/µl in women and >400/µl in men, “detectable” for HIV-RNA ≥50 cop/ml and “undetectable” for HIV-RNA <50 cop/ml
Total observation time was 878 years (mean 3.2 years per patient), with the longest observation period of 8.2 years. Thirty-three percent (92/277) of first nevirapine therapies were discontinued; 17% (16/92) of these patients resumed nevirapine later during follow up. Sixty-seven percent (185/277) maintained nevirapine until their last contact with us. Overall, nevirapine was used for a median of 2.9 years with an interquartile range (IQR) of 0.5–5.6 years.
Of patients who continued nevirapine for more than 90 days (n = 221), the total observation time was 819 years (an average of 3.7 years per patient), with the longest observation period of 7.9 years. Twenty percent (44/221) discontinued nevirapine, of whom 32% (14/44) resumed it later during follow up. Eighty percent (177/221) maintained nevirapine until their last contact with us. Overall, nevirapine-therapies that were continued for more than 90 days were used for a median of 4.2 years (IQR 1.5–5.9).
The nevirapine stop rate in the 277 patients with a first nevirapine therapy was 10.5/100 person years (95% CI 8.5–12.9) (table 2). Nevirapine stop rates in women and men were comparable. There was also no difference between patients starting their first nevirapine therapy at the age of ≥45 versus <45 years. However, the nevirapine stop rate was almost three times higher in HCV co-infected than in HIV mono-infected patients, and 15-fold higher during the first 90 days of nevirapine-containing cART than thereafter; 52% of nevirapine stops (48/92) occurred in the first 90 days of the treatment.
Table 2 Nevirapine stop rates in all nevirapine first therapies by sex, age, HCV-serostatus and duration on nevirapine.
|
Stops
(n) |
FU time
(y) |
Stop rate (per 100 PY)
(95% CI) |
Crude rate ratio
(95% CI) |
p-value | ||
|---|---|---|---|---|---|---|
| (A) All patients on NVP (n = 277) | ||||||
| Overall | 92 | 878.1 | 10.5 (8.5–12.9) | – | – | |
| Sex | Men | 69 | 653.9 | 10.6 (8.3–13.4) | 1.00 (ref.) | 0.907 |
| Women | 23 | 224.2 | 10.3 (6.8–15.4) | 0.97 (0.61–1.56) | ||
| Age at NVP start | <45 years | 39 | 376.4 | 10.4 (7.6–14.2) | 1.0 (ref.) | 0.927 |
| ≥45 years | 53 | 501.6 | 10.6 (8.1–13.8) | 1.02 (0.67–1.54) | ||
| HCV status | Negative | 63 | 757.3 | 8.3 (6.5–10.6) | 1.00 (ref.) | <0.001 |
| Positive | 29 | 120.7 | 24.0 (16.7–34.6) | 2.89 (1.86–4.48) | ||
| Days on NVP | ≤90 | 48 | 59.4 | 80.8 (60.9–107.3) | 1.00 (ref.) | <0.001 |
| >90 | 44 | 818.6 | 5.4 (4.0–7.2) | 0.066 (0.044–0.100) | ||
| Ethnicity – all | White | 75 | 769.6 | 9.7 (7.8–12.2) | 1.00 (ref.) | |
| Black | 11 | 88.6 | 12.4 (6.9–22.4) | 1.27 (0.68–2.40) | 0.452 | |
| Hispanic | 0 | 14.5 | 0 | 0.00 | 0.235 | |
| Asian | 6 | 5.4 | 111.5 (50.1–248.1) | 11.44 (4.98–26.27) | <0.001 | |
| Ethnicity – non-Asian vs Asian | Non–Asian | 86 | 872.6 | 9.9 (8.0–12.2) | 1.00 (ref.) | <0.001 |
| Asian | 6 | 5.4 | 111.5 (50.1–248.1) | 11.31 (4.94–25.88) | ||
| CD4 nadir (…/µl) | <200 | 45 | 318.3 | 14.1 (10.6–18.9) | 1.00 (ref.) | 0.012 |
| ≥200 | 47 | 559.7 | 8.4 (6.3–11.2) | 0.59 (0.34–0.89) | ||
| High CD4 nadir* | No | 75 | 648.5 | 11.6 (9.2–14.5) | 1.00 (ref.) | 0.095 |
| Yes | 17 | 229.5 | 7.4 (4.6–11.9) | 0.64 (0.38–1.08) | ||
| High CD4 at NVP start* | No | 26 | 171.0 | 15.2 (10.3–22.3) | 1.00 (ref.) | 0.030 |
| Yes | 65 | 922.9 | 9.2 (7.2–11.8) | 0.61 (0.39–0.96) | ||
| HIV-RNA (cop/ml) | <50 | 72 | 709.1 | 10.2 (8.1–12.8) | 1.00 (ref.) | 0.646 |
| ≥50 | 19 | 166.3 | 11.4 (7.3–17.9) | 1.13 (0.68–1.87) | ||
| Low–low–detectable | 12 | 40.5 | 29.7 (16.8–52.2) | 2.50 (1.32–4.70) | 0.003 | |
| Low–low–undetectable | 14 | 130.6 | 10.7 (6.4–18.1) | 0.90 (0.50–1.64) | 0.735 | |
| Low–high–detectable | 2 | 81.4 | 2.5 (0.6–9.8) | 0.21 (0.05–0.85) | 0.016 | |
| Low–high–undetectable | 47 | 395.5 | 11.9 (8.9–15.8) | 1.00 (ref.) | 0.908 | |
| High–high–detectable | 5 | 44.4 | 11.3 (4.7–27.0) | 0.95 (0.38–2.38) | 0.038 | |
| High–high–undetectable | 11 | 183.1 | 6.0 (3.3–10.9) | 0.51 (0.26–0.98) | 0.003 | |
| 3TC/ABC-backbone at NVP start | Yes | 68 | 648.2 | 10.5 (8.3–13.3) | 1.00 (ref.) | 0.985 |
| No | 24 | 229.8 | 10.4 (7.0–15.6) | 1.00 (0.63–1.59) | ||
| (B) Patients who were on NVP >90 days (n = 221) | ||||||
| Overall | 44 | 818.6 | 5.4 (4.0–7.2) | – | – | |
| Sex | Men | 30 | 608.8 | 4.9 (3.4–7.0) | 1.00 (ref.) | 0.347 |
| Women | 14 | 209.8 | 6.7 (4.0–11.3) | 1.35 (0.72–2.55) | ||
| Age at NVP start | <45 years | 22 | 350.4 | 6.3 (4.1–9.5) | 1.00 (ref.) | 0.335 |
| ≥45 years | 22 | 468.2 | 4.7 (3.1–7.1) | 0.75 (0.42–1.35) | ||
| HCV status | Negative | 27 | 708.4 | 3.8 (2.6–5.6) | 1.00 (ref.) | <0.001 |
| Positive | 17 | 110.3 | 15.4 (9.6–24.8) | 4.04 (2.20–7.42) | ||
| Ethnicity | White | 36 | 718.8 | 5.0 (3.6–6.9) | 1.00 (ref.) | |
| Black | 8 | 81.3 | 9.8 (4.9–19.7) | 1.97 (0.91–4.23) | 0.078 | |
| Hispanic | 0 | 13.7 | 0.0 | 0.00 | 0.407 | |
| Asian | 0 | 4.9 | 0.0 | 0.00 | 0.621 | |
| Non–Asian | 44 | 813.8 | 5.4 (4.0–7.3) | 1.00 (ref.) | 0.608 | |
| Asian | 0 | 4.9 | 0.0 | 0.00 | ||
| CD4 nadir (…/µl) | <200 | 25 | 295.0 | 8.5 (5.7–12.5) | 1.00 (ref.) | 0.004 |
| ≥200 | 19 | 523.6 | 3.6 (2.3–5.7) | 0.43 (0.24–0.78) | ||
| High CD4 nadir* | No | 35 | 604.3 | 5.8 (4.2–8.1) | 1.00 (ref.) | 0.388 |
| Yes | 9 | 214.3 | 4.2 (2.2–8.1) | 0.73 (0.35–1.51) | ||
| High CD4 at NVP start* | No | 16 | 157.9 | 10.1 (6.2–16.5) | 1.00 (ref.) | 0.003 |
| Yes | 27 | 658.6 | 4.1 (2.8–6.0) | 0.40 (0.22–0.75) | ||
| HIV-RNA (cop/ml) | <50 ≥50 |
31 | 661.6 | 4.7 (3.3–6.7) | 1.00 (ref.) | 0.135 |
| 12 | 154.8 | 7.8 (4.4–13.6) | 1.65 (0.85–3.22) | |||
| Low–low–detectable | 8 | 36.2 | 22.1 (11.1–44.2) | 4.53 (1.97–10.41) | ||
| Low–low–undetectable | 8 | 121.7 | 6.8 (3.3–13.1) | 1.35 (0.59–3.10) | <0.001 | |
| Low–high–detectable | 1 | 77.6 | 1.3 (0.2–9.1) | 0.26 (0.04–1.98) | 0.483 | |
| Low–high–undetectable | 18 | 368.5 | 4.9 (3.1–7.8) | 1.00 (ref.) | 0.163 | |
| High–high–detectable | 3 | 41.1 | 7.3 (2.4–22.7) | 1.50 (0.44–5.08) | 0.516 | |
| High–high–undetectable | 5 | 171.5 | 2.9 (1.2–7.0) | 0.56 (0.22–1.61) | 0.302 | |
| 3TC/ABC backbone at NVP-start | Yes | 31 | 604.8 | 5.1 (3.6–7.3) | 1.00 (ref.) | 0.605 |
| No | 13 | 213.9 | 6.1 (3.5–10.5) | 1.19 (0.62–2.27) | ||
NVP = nevirapine; 3TC = lamivudine; ABC = abacavir; HCV = Hepatitis C Virus; HIV = Human Immunodeficiency Virus; FU = follow-up; PY = person years; CI = confidence interval; ref. = reference * >250/µl in women and >400/µl in men “Low/high-low/high-detectable/undetectable” refers to “(CD4 nadir)–(CD4 at NVP start)–(HIV-RNA at NVP start)” with “low” for CD4 ≤250/µl in women and ≤400/µl in men, “high” for CD4 >250/µl in women and >400/µl in men, “detectable” for HIV-RNA ≥50 cop/ml and “undetectable” for HIV-RNA <50 cop/ml.
In the analysis of nevirapine therapies with a duration of >90 days (n = 221), the overall stop rate was reduced to 5.4/100 person years (table 2B). Again, there was no difference between women and men, and age ≥45 and <45 years at nevirapine start, but the nevirapine stop rate in HCV co-infected was 4-fold higher than in HIV-mono-infected patients.
Six out of seven Asian patients experienced HSR (hepatotoxicity / skin rash) within the first month after nevirapine-start. Among all HSR cases, patients with a CD4 count ≥350 and <500/µl at nevirapine start were overrepresented (32% [12/38] vs 18% [42/237], p = 0.046). At 4.3% (2/46), the proportion with HSR was lowest in patients with CD4 count <350/µl at nevirapine start. It was higher in patients with a CD4 count ≥500/µl (13.7% [24/175], p = 0.079), and highest in patients with CD4 count ≥200 and <350/µl at nevirapine start (22.2% [12/54], p = 0.010). In fully suppressed patients with high CD4 counts at nevirapine start, a high CD4 nadir was not a risk factor for HSR. Overall, 9.4% (5/53) of patients classified as “high-high-undetectable” versus 17.7% (23/130) of patients classified as “low-high-undetectable” experienced HSR (p = 0.159). The overall nevirapine discontinuation rate was also lower in the "high-high-undetectable" group (fig. 2).
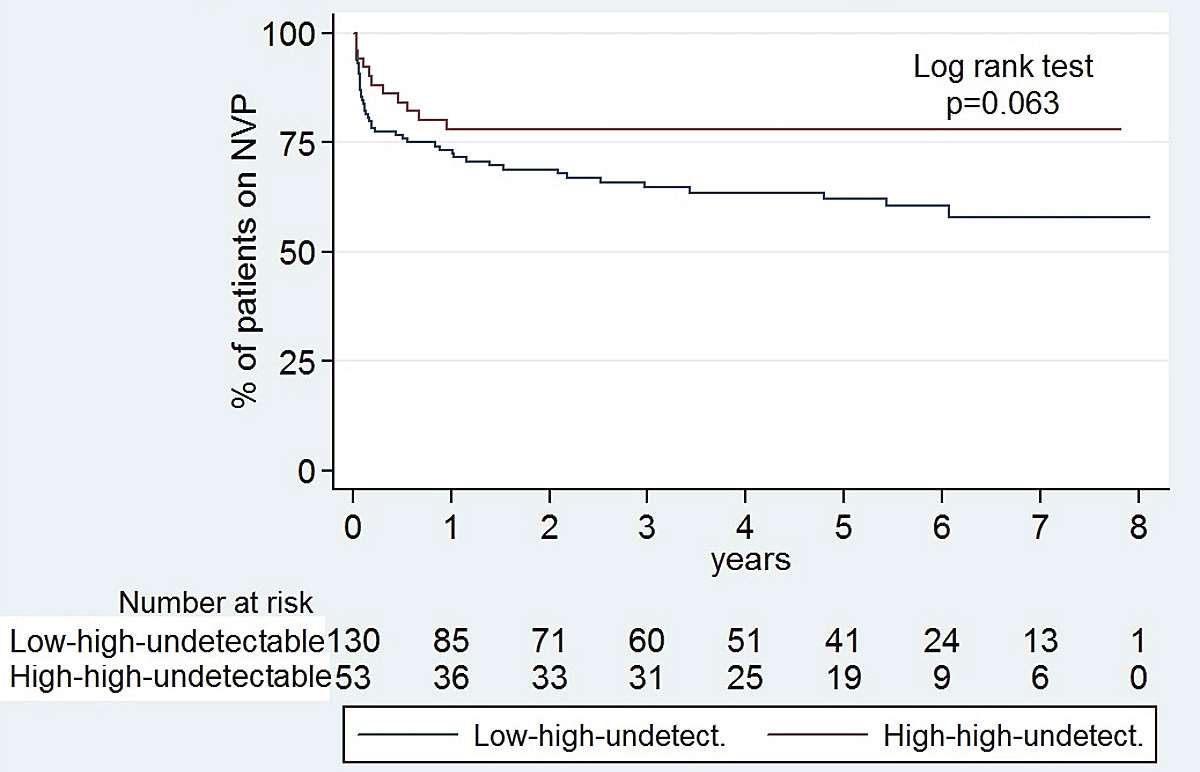
Figure 2 Kaplan-Meier survival curves: probability of remaining on nevirapine (NVP) in high-high-undetectable patients versus low-high-undetectable patients. “Low/high-low/high-detectable/undetectable” refers to “(CD4 nadir)–(CD4 at NVP start)–(HIV-RNA at NVP start)” with “low” for CD4 ≤250/µl in women and ≤400/µl in men, “high” for CD4 >250/µl in women and >400/µl in men, “detectable” for HIV-RNA ≥50 cop/ml and “undetectable” for HIV-RNA <50 cop/ml.
In a population with 74% lamivudine/abacavir backbone, 81% fully suppressed, median CD4 nadir 240/µl (IQR 120–360) and median CD4-count at nevirapine start 590/µl (IQR 400–840) (refer to the baseline characteristics, table 1), both high CD4 nadir and high CD4 count at nevirapine start were associated with lower rather than higher discontinuation rates (figs 3 and 4 , table 2).
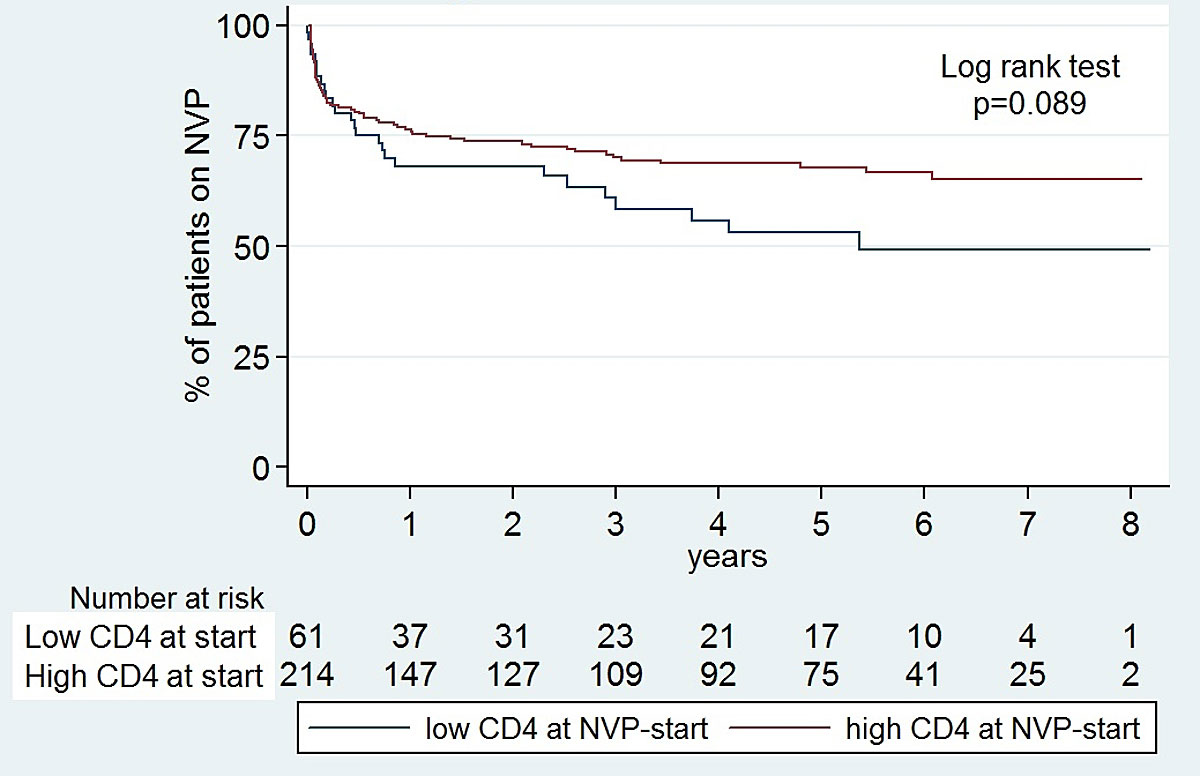
Figure 3 Kaplan-Meier survival curves: probability of remaining on nevirapine (NVP) with high CD4 at NVP start versus low CD4 at NVP start.
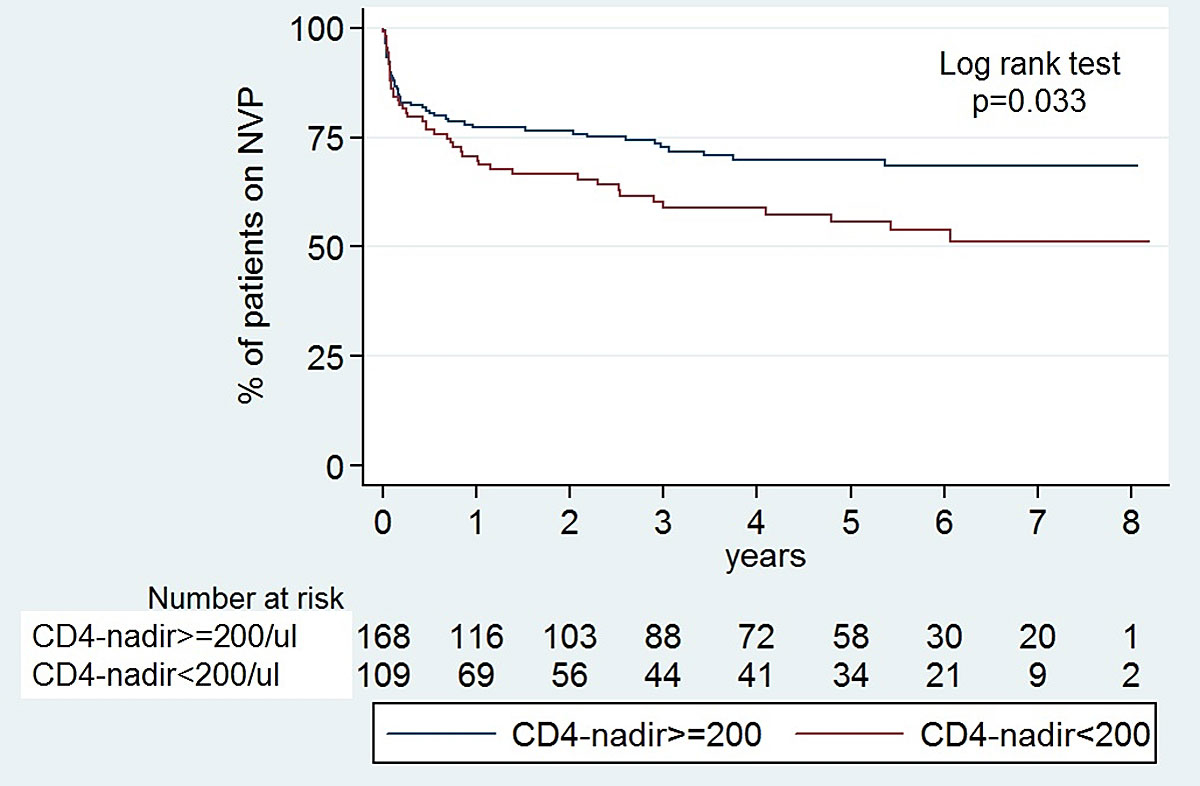
Figure 4 Kaplan-Meier survival curves: probability of remaining on nevirapine (NVP) with CD4 nadir ≥200/µl versus <200/µl.
Major reasons for the 92 discontinuations of nevirapine were: hypersensitivity reactions during the first 90 days (liver toxicity and/or skin rash) in 38 cases (41%) after a median time on nevirapine of 28 days (IQR 17–44, ange 1–81 days); other adverse drug reactions in 17 cases (19%); and non-adherence in 14 (15%). Detailed reasons are listed in table 3 . Notably, five patients died while on nevirapine therapy. None of the five deaths was associated with a known adverse effect of nevirapine. Causes of death were myocardial infarction (two), drug overdose (one), oropharyngeal carcinoma (one) and pneumonia with respiratory failure (one); four of the five patients were co-infected with HCV.
Table 3 Reasons for stopping or interrupting nevirapine therapies (after 1 Jan 2010).
| (A) All Nevirapine stops (n = 92/277, 33%) | |||
|---|---|---|---|
| All (n = 92) interruptions: 17% (16) |
HCV-negative (n = 63) interruptions:
16% (10) |
HCV-positive (n = 29) interruptions:
21% (6) |
|
| Hypersensitivity reaction* | 41% (38) | 51% (32) | 21% (6) |
| Other adverse drug reaction† | 19% (17) | 16% (10) | 24% (7) |
| Non-Adherence | 15% (14) | 14% (9) | 17% (5) |
| Skin rash or elevated liver enzymes‡ | 5% (5) | 5% (3) | 7% (2) |
| Interaction HCV therapy | 4% (4) | 0% (0) | 14% (4) |
| Other interaction§ | 2% (2) | 2% (1) | 3% (1) |
| Patient’s wish | 4% (4) | 6% (4) | 0% (0) |
| Interruption of insurance coverage | 1% (1) | 2% (1) | 0% (0) |
| Death | 5% (5) | 2% (1)¶ | 14% (4)‖ |
| Drop-out | 2% (2) | 3% (2) | 0% (0) |
| (B) Nevirapine-stops after 90 days of therapy (n = 44/221, 20%) | |||
|---|---|---|---|
| All (n = 44) interruptions: 32% (14) | HCV-negative (n = 27) interruptions: 33% (9) | HCV-positive (n = 17) Interruptions: 29% (5) | |
| Skin rash or elevated liver enzymes‡ | 11% (5) | 11% (3) | 12% (2) |
| Other adverse drug reaction† | 27% (12) | 30% (8) | 24% (4) |
| Non-adherence | 27% (12) | 33% (9) | 18% (3) |
| Interaction HCV therapy | 9% (4) | 0% (0) | 24% (4) |
| Other interaction§ | 2% (1) | 4% (1) | 0% (0) |
| Patient’s wish | 7% (3) | 11% (3) | 0% (0) |
| Interruption of insurance coverage | 2% (1) | 4% (1) | 0% (0) |
| Death | 9% (4) | 0% (0) | 24% (4)‖ |
| Drop-out | 5% (2) | 7% (2) | 0% (0) |
* Skin rash and/or elevated liver enzymes including the 5 patients in part 2 specified as “skin rash or elevated liver enzymes” † Other than hypersensitivity reactions ‡ Not clearly associated with nevirapine use: 3 cases had elevated liver enzymes, 2 cases of intercurrent skin rash § For example, chemotherapy, methadone ¶ Myocardial infarction ‖ Myocardial infarction, oropharyngeal carcinoma, pneumonia, probable drug overdose
For the 44 patients who stopped nevirapine after more than 90 days, major reasons were: non-adherence in 12 cases (27%), including two patients with low-level viraemia; and “other adverse drug reactions” in another 12 (27%). Additionally, three patients had elevated liver enzymes and two a skin rash. However, according to the chart review, these adverse events were mild and not clearly associated with nevirapine. In 4 of the 50 (9%) patients co-infected with HCV, therapy was stopped due to interactions with a planned Hepatitis C-therapy. In a Kaplan-Meier analysis (fig. 5), the probability of remaining on the first nevirapine therapy was significantly higher in HIV mono-infected patients compared to HIV-HCV co-infected patients.
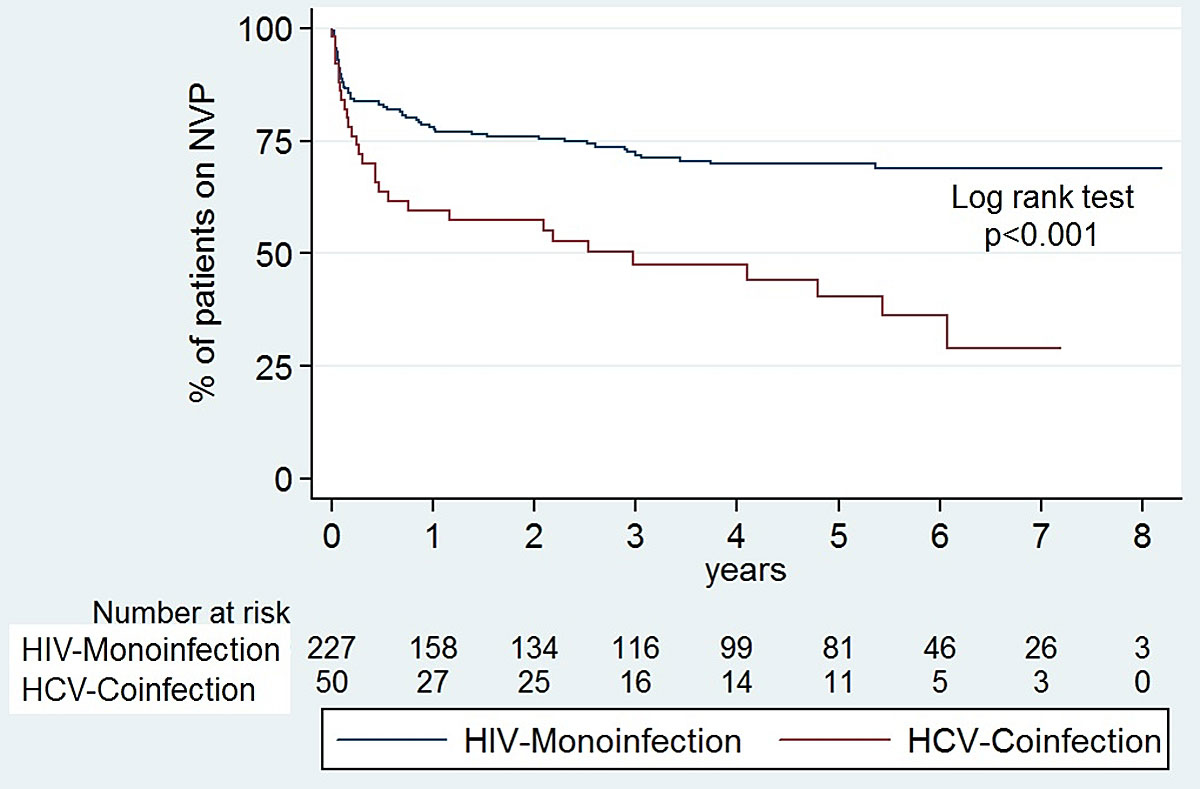
Figure 5 Kaplan-Meier survival curves: probability of remaining on nervirapine (NVP) with hepatitis C virus (HCV) co-infection versus HIV monoinfection.
This retrospective study examined the potential of nevirapine as a switch option for maintenance therapy in our cohort of HIV patients. A switch to nevirapine was offered after reaching stable viral suppression and included patients with high CD4 counts (>250/µl in women and >400/µl in men) both before ART and at nevirapine start (high-high-undetectable), which is a growing population since nowadays ART is recommended irrespective of CD4 count [17]. Overall, 38/277 patients (14%) had to discontinue nevirapine because of HSR, which is within the expected range known from previous studies [11, 12]. Liver enzyme elevations or skin rashes observed after 90 days of nevirapine use were mild and could not clearly be associated with the therapy. There was no significant difference between stop rates of male and female patients, even though previous results from the ATHENA cohort in patients starting nevirapine as first-line cART demonstrated female sex to be a risk factor for nevirapine associated HSR [13]. In accordance with the results of this latter study, 6/7 of our patients from Asia experienced HSR, which indicates Asian ethnicity is a risk factor. Remarkably, they also observed that in ART-experienced patients with an undetectable viral load and a high CD4 count at nevirapine start, a high CD4 count before ART significantly increased the HSR risk from 5.2% (31/596) to 9.3% (24/258) (p = 0.025). In contrast, our results do not suggest such a difference, with a HSR prevalence of 17.7% (23/130) in the low-high-undetectable and 9.4% (5/53) in the high-high-undetectable group (p = 0.159). The higher HSR prevalence in our population (overall 38/277, 14%) might be in part due to the fact that three quarters of the patients had lamivudine/abacavir as ART backbone compared with only ≤11% in earlier studies, where zidovudine/abacavir was still dominating [13, 18]. Thus, abacavir-related hypersensitivity might have been misclassified as HSR due to nevirapine, unless patients had a screening test for the HLA-B*5701 allele prior to treatment start [19]. Another difference between the study populations is that in our study, the median CD4 count at nevirapine start was >500/µl, whereas it was only 350–400/µl in the ATHENA study. This is of note because in our study patients with HSR had more often CD4 counts at nevirapine start of between 350/µl and 500/µl.
Inarguably, nevirapine has the potential for severe side effects and starting nevirapine therapy requires close follow up, especially during the first 3 months as the majority of hypersensitivity reactions occur within this time-frame. However, for long-term use, nevirapine has an exceptional record of tolerability and few side effects, which makes it a good choice for cART maintenance. In the ATHENA cohort patients who switched their first-line highly active ART despite successful viral suppression, switching from a protease inhibitor to nevirapine while continuing the other antiretroviral drugs was associated with a risk of a subsequent toxicity-driven switch five-fold lower than changing to another protease inhibitor-containing regimen [5]. In patients of the French Nationwide Cohort Study who received stable antiretroviral regimens for at least 6 months, treatment discontinuation due to adverse events was two- to –three-fold lower with abacavir/lamivudine/nevirapine (97% given as a switch regimen) compared with other standard antiretroviral regimens before 2013 [20]. In EuroSIDA patients starting nevirapine-, efavirenz- or lopinavir-based cART for the first time, follow up started ≥3 months after initiation of treatment if HIV-RNA was <500 copies/ml. Compared with the nevirapine group, those on efavirenz had a 31% (p = 0.01) and those on lopinavir a 66% (p <0.0001) higher risk of discontinuation because of toxicities or patient/physician choice [5].
In general, the advantage of “old drugs” that have been used for many years are that potential side effects – especially rare events and those occurring after long-term exposure – are often better known as compared with “new drugs”. Moreover, the drug costs are favourable.
There were only two discontinuations of nevirapine due to low-level viraemia in our patients, with probable non-adherence in both cases. Importantly, we did not see an emergence of viral resistance. This is remarkable because nevirapine has a low barrier to resistance as it requires only a single mutation in the reverse transcriptase genome, as opposed to, for example, protease inhibitors, which generally require four to six mutations in the protease gene to develop viral failure, or integrase inhibitors. However, as argued in the Background section, this risk is highest in the initial phase of therapy when massive viral replication occurs.
As with most cART regimens, the most common reason for stopping nevirapine was poor adherence, which often resulted in a complete ART stop. This may not be directly linked to nevirapine, but may rather be a general challenge in patients using ART. A cohort analysis in Sweden [21] showed discontinuation rates for efavirenz of 35% in ART-naïve patients and 44% in ART-experienced patients after 3 years. Although the discontinuation rate is similar in our study (33%), significantly fewer patients (20%) stopped nevirapine after more than 90 days, which supports the good tolerability and the occurrence of few long-term side effects once the risk period for hypersensitivity reaction has passed.
We found a significantly higher discontinuation rate in patients co-infected with HCV. However, hypersensitivity or other adverse drug reactions were not more frequent than in HIV mono-infected patients. The two main different reasons for nevirapine discontinuation in the co-infected group were interactions with a planned HCV therapy and death. Interactions with direct-acting antivirals (DAAs) for hepatitis C treatment that limit the use of nevirapine in co-infected patients has become an important consideration in recent years, especially since October 2017 when DAAs became more widely used. However, for patients switching to a different cART-regimen because of HCV therapy, switching back to a nevirapine-containing regimen after completion could be an option and was performed in two of our cases. Higher mortality of co-infected individuals has been described in the literature [22], similarly to the results in our cohort.
When switching to nevirapine, good patient instruction is vital, including the instruction to immediately contact the clinic in the case of pruritus or skin rash, and 2-weekly liver enzyme tests. The US Centers for Disease Control and Prevention (CDC) reviewed 22 reports of serious adverse events related to nevirapine taken for post-exposure prophylaxis after HIV exposure. Routine 2-weekly measurement of liver enzymes was not described in the review, and in six patients, a starting dose of 200 mg, as proposed for nevirapine, was not used [23]. In Stevens-Johnson syndrome and toxic epidermal necrolysis, prompt discontinuation of the causative agent is crucial to decrease mortality [24]. This underscores the importance of a proper follow up and good patient instruction. Thus, for the first 3 months, patients are required to comply with the somewhat time-consuming follow up, which is, however, feasible with properly motivated and informed patients.
This was a retrospective and monocentric analysis with inherent limitations. First, there is a selection bias, as patients with already elevated liver enzymes or poor adherence were probably not offered a switch to nevirapine-containing cART, which might have led to an overestimation of tolerability. Second, the design did not include a control group to compare tolerability to an alternative regimen. Third, we did not perform a multivariate analysis to investigate specific risk factors for stopping nevirapine. Fourth, the number of patients included did not allow any conclusion about the occurrence of rare severe side effects. Finally, we did not perform a cost-effectiveness analysis to compare the total of nevirapine-containing treatment costs to other cART regimens. As a result of these limitations, it is not possible to make any claim of superiority of a nevirapine-based regimen over any other cART. However, this is the largest cohort of nevirapine treated patients in a Swiss HIV centre providing data on current efficacy and safety of a treatment alternative in current HIV management.
In conclusion, two third of the patients continued nevirapine with favourable long-term tolerability and efficacy. Thus, our results suggest that this low-cost “old drug” may still represent a valid switch option for maintenance therapy in selected patients with a fully suppressed viral load. Initial close follow-up ensures early detection of potential side effects and avoids irreversible adverse events in patients. Further evaluation is needed to confirm the results.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Joos B , Fischer M , Kuster H , Pillai SK , Wong JK , Böni J , et al.; Swiss HIV Cohort Study. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc Natl Acad Sci USA. 2008;105(43):16725–30. doi:.https://doi.org/10.1073/pnas.0804192105
2 Gueler A , Moser A , Calmy A , Günthard HF , Bernasconi E , Furrer H , et al.; Swiss HIV Cohort Study, Swiss National Cohort. Life expectancy in HIV-positive persons in Switzerland: matched comparison with general population. AIDS. 2017;31(3):427–36.
3 Ryom L , Boesecke C , Bracchi M , Ambrosioni J , Pozniak A , Arribas J , et al.; EACS Governing Board. Highlights of the 2017 European AIDS Clinical Society (EACS) Guidelines for the treatment of adult HIV-positive persons version 9.0. HIV Med. 2018;19(5):309–15. doi:.https://doi.org/10.1111/hiv.12600
4 Dieleman JP , Sturkenboom MC , Wit FW , Jambroes M , Mulder JW , Ten Veen JH , et al.; AIDS Therapy Evaluation, The Netherlands Study Group (ATHENA). Low risk of treatment failure after substitution of nevirapine for protease inhibitors among human immunodeficiency virus-infected patients with virus suppression. J Infect Dis. 2002;185(9):1261–8. doi:.https://doi.org/10.1086/340131
5 Dieleman JP , Jambroes M , Gyssens IC , Sturkenboom MC , Stricker BH , Mulder WM , et al.; ATHENA Study Group. Determinants of recurrent toxicity-driven switches of highly active antiretroviral therapy. The ATHENA cohort. AIDS. 2002;16(5):737–45. doi:.https://doi.org/10.1097/00002030-200203290-00009
6 Reekie J , Reiss P , Ledergerber B , Sedlacek D , Parczewski M , Gatell J , et al.; EuroSIDA study group. A comparison of the long-term durability of nevirapine, efavirenz and lopinavir in routine clinical practice in Europe: a EuroSIDA study. HIV Med. 2011;12(5):259–68. doi:.https://doi.org/10.1111/j.1468-1293.2010.00877.x
7 Kamara DA , Smith C , Ryom L , Reiss P , Rickenbach M , Phillips A , et al. Longitudinal analysis of the associations between antiretroviral therapy, viraemia and immunosuppression with lipid levels: the D:A:D study. Antivir Ther. 2016;21(6):495–506. doi:.https://doi.org/10.3851/IMP3051
8 Calcagno A , Di Perri G , Bonora S . Pharmacokinetics and pharmacodynamics of antiretrovirals in the central nervous system. Clin Pharmacokinet. 2014;53(10):891–906. doi:.https://doi.org/10.1007/s40262-014-0171-0
9 Rodríguez-Arrondo F , Aguirrebengoa K , Portu J , Muñoz J , García MA , Goikoetxea J , et al. Long-term effectiveness and safety outcomes in HIV-1-infected patients after a median time of 6 years on nevirapine. Curr HIV Res. 2009;7(5):526–32. doi:.https://doi.org/10.2174/157016209789346246
10 Sarmati L , Parisi SG , Montano M , Andreis S , Scaggiante R , Galgani A , et al. Nevirapine use, prolonged antiretroviral therapy and high CD4 nadir values are strongly correlated with undetectable HIV-DNA and -RNA levels and CD4 cell gain. J Antimicrob Chemother. 2012;67(12):2932–8. doi:.https://doi.org/10.1093/jac/dks331
11 Montaner JS , Reiss P , Cooper D , Vella S , Harris M , Conway B , et al.; for the INCAS Study Group. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS Trial. Italy, The Netherlands, Canada and Australia Study. JAMA. 1998;279(12):930–7. doi:.https://doi.org/10.1001/jama.279.12.930
12 de Jong MD , Vella S , Carr A , Boucher CA , Imrie A , French M , et al. High-dose nevirapine in previously untreated human immunodeficiency virus type 1-infected persons does not result in sustained suppression of viral replication. J Infect Dis. 1997;175(4):966–70. doi:.https://doi.org/10.1086/514002
13 Wit FWNM , Kesselring AM , Gras L , Richter C , van der Ende ME , Brinkman K , et al. Discontinuation of nevirapine because of hypersensitivity reactions in patients with prior treatment experience, compared with treatment-naive patients: the ATHENA cohort study. Clin Infect Dis. 2008;46(6):933–40. doi:.https://doi.org/10.1086/528861
14 Patterson P , Socías E , Pryluka D , Lapadula P , Pérez H , Cahn P . Switching to nevirapine-based regimens after undetectable viral load is not associated with increased risk of discontinuation due to toxicity. J Int AIDS Soc. 2014;17(4, Suppl 3):19794. doi:.https://doi.org/10.7448/IAS.17.4.19794
15 Schoeni-Affolter F , Ledergerber B , Rickenbach M , Rudin C , Günthard HF , Telenti A , et al.; Swiss HIV Cohort Study. Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol. 2010;39(5):1179–89. doi:.https://doi.org/10.1093/ije/dyp321
16 Nitika N , Mishra SS , Lohani P . Lexis Expansion: a prerequisite for analyzing time changing variables in a cohort study. Nepal J Epidemiol. 2017;7(2):681–4. doi:.https://doi.org/10.3126/nje.v7i2.17974
17 Saag MS , Benson CA , Gandhi RT , Hoy JF , Landovitz RJ , Mugavero MJ , et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2018 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320(4):379–96. doi:.https://doi.org/10.1001/jama.2018.8431
18 Kesselring AM , Wit FW , Sabin CA , Lundgren JD , Gill MJ , Gatell JM , et al.; Nevirapine Toxicity Multicohort Collaboration. Risk factors for treatment-limiting toxicities in patients starting nevirapine-containing antiretroviral therapy. AIDS. 2009;23(13):1689–99. doi:.https://doi.org/10.1097/QAD.0b013e32832d3b54
19 Ma JD , Lee KC , Kuo GM . HLA-B*5701 testing to predict abacavir hypersensitivity. PLoS Curr. 2010;2:RRN1203. doi:.https://doi.org/10.1371/currents.RRN1203
20 de Boissieu P , Dramé M , Raffi F , Cabie A , Poizot-Martin I , Cotte L , et al.; Dat’AIDS Study Group. Long-term efficacy and toxicity of abacavir/lamivudine/nevirapine compared to the most prescribed ARV regimens before 2013 in a French Nationwide Cohort Study. Medicine (Baltimore). 2016;95(37):e4890. doi:.https://doi.org/10.1097/MD.0000000000004890
21 Häggblom A , Lindbäck S , Gisslén M , Flamholc L , Hejdeman B , Palmborg A , et al. HIV drug therapy duration; a Swedish real world nationwide cohort study on InfCareHIV 2009-2014. PLoS One. 2017;12(2):e0171227. doi:.https://doi.org/10.1371/journal.pone.0171227
22 May MT , Justice AC , Birnie K , Ingle SM , Smit C , Smith C , et al. Injection drug use and Hepatitis C as risk factors for mortality in HIV-infected individuals: the Antiretroviral Therapy Cohort Collaboration. J Acquir Immune Defic Syndr. 2015;69(3):348–54. doi:.https://doi.org/10.1097/QAI.0000000000000603
23 Centers for Disease Control and Prevention (CDC). Serious adverse events attributed to nevirapine regimens for postexposure prophylaxis after HIV exposures--worldwide, 1997-2000. MMWR Morb Mortal Wkly Rep. 2001;49(51-52):1153–6.
24 Garcia-Doval I , LeCleach L , Bocquet H , Otero XL , Roujeau JC . Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136(3):323–7. doi:.https://doi.org/10.1001/archderm.136.3.323
JN and CRK collected the data, JN wrote the first version of the manuscript. AB performed all statistical analyses. All authors contributed to the conception of the study, choice of statistical analyses, and interpretation of the findings and were involved in the preparation and review of the final manuscript.
No financial support and no other potential conflict of interest relevant to this article was reported.