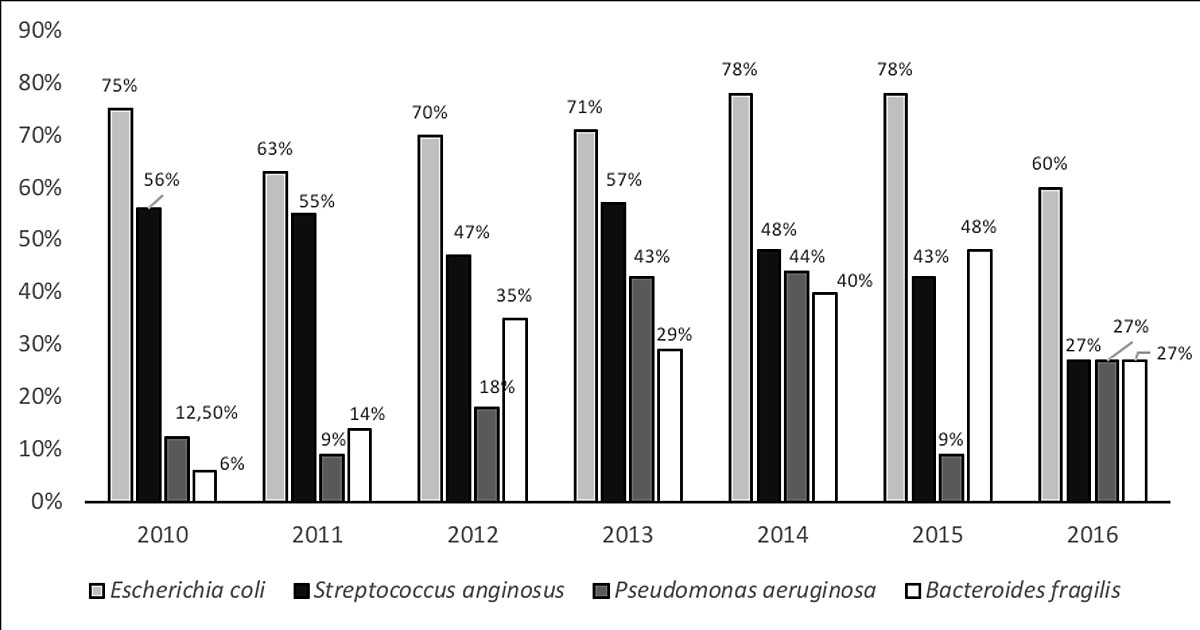
Figure 1 Microbiological results by year of identification.
DOI: https://doi.org/10.4414/smw.2019.20055
Appendicitis is the most common abdominal surgical emergency in children. Complicated appendicitis accounts for 25% of all appendicitis cases in paediatric populations, and the infectious postoperative complication rate is 3–25% [1, 2]. Case management, including the choice and duration of postoperative antibiotic therapy, varies among centres and surgeons [3–5]. Several authors have tried to suggest standardised management, but no real consensus has emerged [1, 5–7].
Combined triple therapy (e.g., amoxicillin + aminoglycoside + metronidazole) has been widely used [1, 8, 9]. However, recent evidence suggests that a broad-spectrum, single antibiotic therapy (e.g., piperacillin-tazobactam) [10–12] or a combination of ceftriaxone and metronidazole [13–15] are at least as effective and economical [16].
The emergence of resistant pathogens like Pseudomonas aeruginosa, which have been found in 15–35% of complicated appendicitis cases [3, 4, 8, 9, 17–19], should be considered when choosing the appropriate antibiotic regimen. The initial choice of antibiotics is important, as studies have shown that the risk of complications increases when bacteria appear resistant to the chosen antibiotics [4, 9, 10, 16]. However, evidence that empirical coverage of P. aeruginosa decreases the risk of post-surgical infectious complications is lacking [20].
In our institution, triple antibiotherapy (co-amoxicillin (amoxicillin + clavulanic acid) + aminoglycoside + metronidazole) used to be prescribed for complicated appendicitis. Since 2013, after an evaluation of regional resistances showed that fewer than 15% of Escherichia coli cases were resistant to co-amoxicillin, and with regard to the IDSA guidelines [21, 22], we reduced the antibiotic spectrum to limit the emergence of antibiotic resistance. Co-amoxicillin was prescribed alone for pre- and post-surgical management.
In the months following the implementation of our new antibiotic strategy, an increase in P. aeruginosa documented in peritoneal swabs was observed.
Antibiotic prescriptions were adjusted according to microbiological findings when fever persisted 48 hours after surgery or when a bad clinical evolution showed signs of postoperative infection. Until 2015, the initial empirical therapy remained co-amoxicillin alone in the majority of cases. Since then, the choice of antibiotics has been more variable and dependent on the surgeon.
This study’s objective was to describe the bacteriology of the peritoneal swabs taken from cases of complicated appendicitis in our paediatric patients, and to correlate the risk of infectious complications (wound or intra-abdominal abscesses) with regards to the initial empirical antibiotics prescribed and the resistance profile of the bacteria.
This retrospective, single-centre cohort study was carried out in a tertiary care hospital (Lausanne University Hospital, Lausanne, Switzerland). Children and adolescents under 18 years old who underwent an appendectomy between 1 January 2010 and 31 December 2016 for a complicated appendicitis (perforated, peritonitis or abscess) were eligible for inclusion if they also had a peritoneal swab culture. Due to its retrospective, anonymous design, with no anticipated harm to the patients, informed consent was not requested. The study was approved by the Human Research Ethics Committee of the canton of Vaud and conducted in accordance with the principles of the Declaration of Helsinki, the standards of Good Clinical Practice and Swiss regulatory requirements.
A paediatric surgeon established the indication for surgery based on his/her clinical diagnosis of appendicitis or, when in doubt, with the help of a radiological examination (echography or CT scan). Surgical intervention consisted of appendectomy by open or laparoscopic surgery. The first dose of antibiotics was given by the anaesthetist at induction or, when surgery was delayed, at diagnosis (less than 24 hours before surgery in every case). The diagnosis of complicated appendicitis was made by the surgeon during the procedure. The usual duration of antibiotics was four to seven days when post-surgical evolution was uneventful, but could be changed according to the microbiological results and extended by up to two weeks when infectious complications occurred. Management of complications was decided individually for each patient and when possible, a conservative treatment was preferred. If not, we preferred a percutaneous drainage by the interventional radiologist but when this was not technically feasible, we decided to perform a second surgery.
All eligible patients were identified from our surgical database. Relevant information relating to demographic characteristics, clinical presentation, microbiological investigations, management and evolution were retrospectively retrieved from medical records. Children who had not had bacterial cultures were subsequently excluded.
Demographic information (e.g., gender, age), clinical characteristics (e.g., duration of symptoms), microbiological information (e.g., bacteria isolated, antibacterial resistance) and management (e.g., surgical procedure, antibiotic treatment) were compared between groups using either the Student’s t-test or the Kruskal–Wallis test for continuous variables, and the chi-squared test or Fisher’s exact test for categorical variables. All variables with a p-value <0.05 were then included in a multivariable logistic regression model for which adjusted odds ratios (OR) and 95% confidence intervals (CI) were calculated. All tests were two-tailed, and a p-value <0.05 was considered statistically significant. Statistical analyses were computed using Stata software (Stata/IC 11.2 for Mac; StataCorp, Lakeway, TX).
Between 1 January 2010 and 31 December 2016, 742 appendectomies were performed in our institution. Diagnosis was made by clinical examination, without ultrasound or CT-scan. One hundred and fifty-one patients (20%) had complicated appendicitis, confirmed surgically. After the exclusion of 18 cases due to the absence of peritoneal swab cultures, 133 patients were available for analyses (table 1). Median age was 9.5 years old (interquartile range [IQR] 5.7–12.4); there were 53 girls (40%) and 80 boys (60%). The median duration of symptoms before surgery was two days (IQR 2–5 days). There was an abscess at presentation which was confirmed by the primary surgery in 23 patients (17%). A laparoscopic appendectomy was performed in 75 cases (56%), whereas the other 58 appendectomies (44%) were carried out by laparotomy. A washout of the peritoneal cavity was performed in all cases. Forty-seven patients (35%) were given a triple antibiotherapy (co-amoxicillin, amikacin and metronidazole), 63 (48%) a monotherapy using co-amoxicillin, 15 (11%) were treated with piperacillin-tazobactam and 8 (6%) a dual therapy (ceftriaxone + metronidazole) because of an allergy to co-amoxicillin.
Table 1 Patient characteristics and risks factors for infectious complications.
| Entire population (n = 133) | Complications (wound, n = 7, or intra-abdominal abscess, n = 21) | No infectious complications (n = 105) | p-value* | |
|---|---|---|---|---|
| Gender, n female (%) | 53 (40) | 12 (43) | 41 (39) | 0.7 |
| Age, median years (IQR) | 9.5 (5.7–12.4) | 9.8 (5.3–12.4) | 9.5 (5.8–12.4) | 0.4 |
| Duration of symptoms, median days (IQR) | 2 (2–5) | 4 (2–5) | 2 (2–5) | 0.04 |
| Abscess at presentation, n (%) | 23 (17) | 7 (25) | 16 (15) | 0.2 |
| Laparoscopy, n (%) | 75 (56) | 17 (61) | 58 (55) | 0.6 |
| P. aeruginosa positive, n (%) | 31 (23) | 8 (29) | 23 (22) | 0.5 |
| Co-amoxicillin-resistant E. coli, n/total (%) | 13/94 (14) | 7/25 (28) | 6/69 (9) | 0.02 |
| Empirical antibiotic chosen | 0.8 | |||
| Co-amoxicillin | 63 (48) | 15 (24) | 48 (76) | |
| Piperacillin-tazobactam | 15 (11) | 2 (13) | 13 (87) | |
| Triple antibiotherapy | 47 (35) | 9 (19) | 38 (81) | |
| Ceftriaxone-metronidazole | 8 (6) | 2 (25) | 6 (75) |
IQR = interquartile range Triple antibiotherapy: co-amoxicillin, aminoglycoside and metronidazole * Univariable analysis comparing patients with and without infectious complications: Student’s t-test or the Kruskal-Wallis test for continuous variables and chi-squared test or Fisher’s exact test for categorical variables
E. coli was the most common bacterium, found in 94 patients (71%) (fig. 1). Thirteen cases (14%) were resistant or had intermediate resistance to co-amoxicillin. Only three strains (3%) were resistant to cephalosporins owing to the production of broad-spectrum beta-lactamase (ESBL), and only one strain was resistant to amikacin. The resistance rate to co-amoxicillin varied from 0% (0/9) in 2013 to 33% (6/18) in 2015.

Figure 1 Microbiological results by year of identification.
The second most common bacterium was Streptococcus anginosus, found in 64 patients (48%). They were all sensitive to co-amoxicillin. P. aeruginosa was retrieved from the peritoneal fluids of 31 patients (23%) and Bacteroides fragilis from the peritoneal fluids of 40 patients (30%). B. fragilis appeared to have an intermediate or complete resistance to co-amoxicillin in only four patients (10%). These four cases were all sensitive to piperacillin-tazobactam and metronidazole. The rate of P. aeruginosa prevalence increased significantly between 2010 and 2016, from 9% to 44%. (fig. 1).
Overall, 31% (41/133), 26% (34/133), 3% (4/133) and 1% (1/133) of our patients had cultures of peritoneal samples which showed resistance of at least one bacterium (E. coli, P. aeruginosa and/or B. fragilis) to co-amoxicillin, dual therapy of ceftriaxone + metronidazole, piperacillin-tazobactam and triple therapy of co-amoxicillin + amikacin + metronidazole, respectively (table 2). Thirty-one of the 34 ceftriaxone-resistant bacteria were P. aeruginosa. Resistances more than doubled between 2010 and 2013/2014 before decreasing slightly in 2015-2016 (table 2). The empirical coverage of co-amoxicillin-resistant E. coli and P. aeruginosa was adequate in 31% (4/13) and 32% (10/31) of cases, respectively.
Table 2 Antimicrobial resistant rates of peritoneal samples over time.
| Year | Entire population (absolute numbers) | Co-amoxicillin resistance rate (number of resistant samples) |
Dual antibiotherapy resistance rate
(number of resistant samples) |
Piperacillin-tazobactam resistance rate
(number of resistant samples) |
Triple antibiotherapy resistance rate
(number of resistant samples) |
|---|---|---|---|---|---|
| 2010 | 16 | 19% (n = 3) | 12% (n = 2) | 0% | 0% |
| 2011 | 22 | 14% (n = 3) | 9% (n = 2) | 0% | 0% |
| 2012 | 17 | 24% (n = 4) | 24% (n = 4) | 0% | 0% |
| 2013 | 13 | 46% (n = 6) | 46% (n = 6) | 0% | 0% |
| 2014 | 27 | 44% (n = 12) | 41% (n = 11) | 4% (n = 1) | 0% |
| 2015 | 23 | 39% (n = 9) | 22% (n = 5) | 13% (n = 3) | 4% (n = 1) |
| 2016 | 15 | 27% (n = 4) | 27% (n = 4) | 0% | 0% |
| 2010-2016 | 133 | 31% (n = 41) | 26% (n = 34) | 3% (n = 4) | 1% (n = 1) |
Dual antibiotherapy: ceftriaxone and metronidazole, triple antibiotherapy: co-amoxicillin, amikacin and metronidazole. Co-amoxicillin resistance: culture positive for P. aeruginosa, co-amoxicillin-resistant E. coli or co-amoxicillin-resistant B. fragilis. Dual antibiotherapy resistance: culture positive for P. aeruginosa or ESBL- (extended spectrum beta-lactamase) producing E. coli. Piperacillin-tazobactam resistance: culture positive for ESBL-producing E. coli or piperacillin-tazobactam-resistant P. aeruginosa. Triple-therapy resistance: amikacin-resistant E. coli. All P. aeruginosa were sensitive to amikacin. All B. fragilis were sensitive to metronidazole and to piperacillin-tazobactam.
Postoperative, intra-abdominal abscesses occurred in 21 patients (16%) (table 1). Fourteen patients required follow-up surgery (nine laparotomies and five laparoscopies). Four initial laparoscopies were extended to laparotomies. Three required drainage under radiological guidance, and four were treated conservatively with antibiotics alone. Seven other patients were treated for a wound abscess. We did not document new bacteriologic species in the abscesses. The rate of postoperative abscesses was not correlated to the presence of a preoperative abscess. These abscesses are drained surgically during the appendectomy and do not recur in most patients.
A longer duration of symptoms before surgery and the presence of co-amoxicillin-resistant E. coli were positively correlated with the occurrence of infectious complications (table 1). In a multivariable analysis, only co-amoxicillin-resistant E. coli remained significantly associated with infectious complications (OR 4.8; 95% CI 1.4–16.8; p = 0.013) (table 3).
Table 3 Multivariable analysis.
| Predictor | Contrast | Odds ratio (95% CI) for infectious complications | p-value |
|---|---|---|---|
| Co-amoxicillin-resistant E. coli | Yes vs no | 4.82 (1.38–16.77) | 0.013 |
| Duration of symptoms | One-day increase | 1.22 (0.99–1.51) | 0.067 |
| Intercept | - | 0.14 (0.05–0.37) | <0.001 |
CI = confidence interval
The initial presentation (abscess, peritonitis or simple perforation) and the surgical technique (laparotomy vs laparoscopy) had no significant impact on the complication rate.
The presence of P. aeruginosa had no impact on the rate of infectious complications (table 1). However, in patients infected with P. aeruginosa, the absence of adequate antibiotic coverage may increase the risk of a wound or intra-abdominal abscess. The rate of infectious complications was 38% (8/21 patients) when the empiric antibiotic did not cover P. aeruginosa and 0% (0/10 patients) when it was covered adequately (p = 0.03).
The median duration of hospitalisation for the entire population was six days (IQR 5–9), but it was significantly longer when infectious complications occurred. The median duration of hospitalisation in patients with and without infectious complications was 14 days (IQR 7.5–18.5) and 6 days (IQR 5–7) respectively (p <0.001). Nine patients (7%) were rehospitalised, and in eight cases this was for infectious complications. Eight out of 28 patients (29%) with infectious complications were rehospitalised, compared to one of 105 patients (1%) without infectious complications (p < 0.001).
The choice of antibiotics for complicated appendicitis should address local patterns of E. coli, which is the most frequently identified bacterium [9, 21]. In our study, we confirm the increased risk of complications in the presence of co-amoxicillin-resistant E. coli.
The indication for covering P. aeruginosa is not clear, even if there is some evidence of a correlation between the presence of P. aeruginosa and the risk of postoperative infectious complications [19]. Skarda et al. compared the rates of infectious complications before and after the implementation of a new protocol [20]. The new protocol implemented the use of a dual therapy using ceftriaxone and metronidazole instead of monotherapy with piperacillin-tazobactam. In their population of 306 children with ruptured appendicitis (152 under the new protocol), they found no increase in infectious complications associated with the new protocol. The complication rate was 1.3% after the implementation of the new protocol, compared to 5.2% before. The difference was not statistically significant. The overall rate of P. aeruginosa was not reported. However, in a previous report [10] the same authors reported a P. aeruginosa rate of 32%. In recent reviews or guidelines, the question of how to cover P. aeruginosa has not been specifically addressed, even when coverage for this bacterium uses a regimen including aminoglycoside (mostly in a triple therapy regimen) or piperacillin-tazobactam (monotherapy) [6, 14].
Our results showed that, even though the absence of coverage for P. aeruginosa increased the risk of complications when this bacterium was identified, its presence alone did not increase the risk of infectious complications. The adequate coverage of co-amoxicillin-resistant E. coli (4/13, 31%) and P. aeruginosa (10/31, 32%) were comparable in our study. However, the proportion of complications was higher with co-amoxicillin-resistant E. coli (7/13, 54%) than with P. aeruginosa (8/31, 26%). It is difficult to draw definitive conclusions with so few cases, but we can assume that in ruptured appendicitis, the relative pathogenic role of E. coli is higher than that of P. aeruginosa. A low inoculum for P. aeruginosa in comparison to E. coli might also explain this difference. Unfortunately, the bacterial cultures were not quantitative and could not determine the inoculum.
If we wanted to empirically cover P. aeruginosa and co-amoxicillin-resistant E. coli, our best choice would be piperacillin-tazobactam or triple antibiotherapy (table 2). However, because the benefit of empiric coverage of P. aeruginosa is not definitively proven and in the absence of a stable, high identification rate of P. aeruginosa, as was the case at our institution in 2013–2014, there is currently insufficient evidence to widen the antibiotic spectrum to cover P. aeruginosa. However, with the rate of co-amoxicillin resistance of E. coli peaking at more than 30% in 2015, we decided to change our choice of antibiotic from co-amoxicillin to a dual therapy of ceftriaxone and metronidazole. In the case of bad clinical evolution or cultures positive for P. aeruginosa, the treatment was changed to piperacillin-tazobactam as soon as possible to reduce the risk of postoperative complications.
Our study has some limitations. First, its design is retrospective, and a significant proportion of patients had not had peritoneal swab cultures done. Second, our surgical and antibiotic strategies were not uniform throughout the study period. Finally, the small number of patients may have limited our ability to prove a significant impact of P. aeruginosa or other variables (such as the presence of abscess at presentation) on the risk of infectious complications, and the local conditions may limit the generalisability of our findings to other settings.
The multivariable analysis of this small, retrospective study revealed a statistically significant increase in the risk of postoperative complications in the presence of resistant E. coli. Knowledge of local resistance patterns should guide antibiotic strategies. The impact of the presence of P. aeruginosa and the absence of its antibiotic coverage on the rate of infectious complications remains unclear. More data would be needed to justify the systematic coverage of P. aeruginosa in children with ruptured appendicitis.
No financial support and no other potential conflicts of interest relevant to this article were reported. The views expressed in the submitted article are our own and not an official position of the institution or funder.
1 Emil S , Laberge JM , Mikhail P , Baican L , Flageole H , Nguyen L , et al. Appendicitis in children: a ten-year update of therapeutic recommendations. J Pediatr Surg. 2003;38(2):236–42. doi:.https://doi.org/10.1053/jpsu.2003.50052
2 Emil S , Elkady S , Shbat L , Youssef F , Baird R , Laberge JM , et al. Determinants of postoperative abscess occurrence and percutaneous drainage in children with perforated appendicitis. Pediatr Surg Int. 2014;30(12):1265–71. doi:.https://doi.org/10.1007/s00383-014-3617-4
3 Adibe OO , Barnaby K , Dobies J , Comerford M , Drill A , Walker N , et al. Postoperative antibiotic therapy for children with perforated appendicitis: long course of intravenous antibiotics versus early conversion to an oral regimen. Am J Surg. 2008;195(2):141–3. doi:.https://doi.org/10.1016/j.amjsurg.2007.10.002
4 Guillet-Caruba C , Cheikhelard A , Guillet M , Bille E , Descamps P , Yin L , et al. Bacteriologic epidemiology and empirical treatment of pediatric complicated appendicitis. Diagn Microbiol Infect Dis. 2011;69(4):376–81. doi:.https://doi.org/10.1016/j.diagmicrobio.2010.11.003
5 Slusher J , Bates CA , Johnson C , Williams C , Dasgupta R , von Allmen D . Standardization and improvement of care for pediatric patients with perforated appendicitis. J Pediatr Surg. 2014;49(6):1020–4, discussion 1024–5. doi:.https://doi.org/10.1016/j.jpedsurg.2014.01.045
6 Rentea RM , Peter SD , Snyder CL . Pediatric appendicitis: state of the art review. Pediatr Surg Int. 2017;33(3):269–83. doi:.https://doi.org/10.1007/s00383-016-3990-2
7 Chen C , Botelho C , Cooper A , Hibberd P , Parsons SK . Current practice patterns in the treatment of perforated appendicitis in children. J Am Coll Surg. 2003;196(2):212–21. doi:.https://doi.org/10.1016/S1072-7515(02)01666-6
8 Chan KW , Lee KH , Mou JW , Cheung ST , Sihoe JD , Tam YH . Evidence-based adjustment of antibiotic in pediatric complicated appendicitis in the era of antibiotic resistance. Pediatr Surg Int. 2010;26(2):157–60. doi:.https://doi.org/10.1007/s00383-009-2540-6
9 Obinwa O , Casidy M , Flynn J . The microbiology of bacterial peritonitis due to appendicitis in children. Ir J Med Sci. 2014;183(4):585–91. doi:.https://doi.org/10.1007/s11845-013-1055-2
10 Fallon SC , Hassan SF , Larimer EL , Rodriguez JR , Brandt ML , Wesson DE , et al. Modification of an evidence-based protocol for advanced appendicitis in children. J Surg Res. 2013;185(1):273–7. doi:.https://doi.org/10.1016/j.jss.2013.05.088
11 Nadler EP , Reblock KK , Ford HR , Gaines BA . Monotherapy versus multi-drug therapy for the treatment of perforated appendicitis in children. Surg Infect (Larchmt). 2003;4(4):327–33. doi:.https://doi.org/10.1089/109629603322761382
12 Goldin AB , Sawin RS , Garrison MM , Zerr DM , Christakis DA . Aminoglycoside-based triple-antibiotic therapy versus monotherapy for children with ruptured appendicitis. Pediatrics. 2007;119(5):905–11. doi:.https://doi.org/10.1542/peds.2006-2040
13 St Peter SD , Little DC , Calkins CM , Murphy JP , Andrews WS , Holcomb GW, 3rd , et al. A simple and more cost-effective antibiotic regimen for perforated appendicitis. J Pediatr Surg. 2006;41(5):1020–4. doi:.https://doi.org/10.1016/j.jpedsurg.2005.12.054
14 Holcomb GW , III, St Peter SD . Current management of complicated appendicitis in children. Eur J Pediatr Surg. 2012;22(3):207–12. doi:.https://doi.org/10.1055/s-0032-1320016
15 Desai AA , Alemayehu H , Holcomb GW , III, St Peter SD . Safety of a new protocol decreasing antibiotic utilization after laparoscopic appendectomy for perforated appendicitis in children: A prospective observational study. J Pediatr Surg. 2015;50(6):912–4. doi:.https://doi.org/10.1016/j.jpedsurg.2015.03.006
16 Lee SL , Islam S , Cassidy LD , Abdullah F , Arca MJ ; 2010 American Pediatric Surgical Association Outcomes and Clinical Trials Committee. Antibiotics and appendicitis in the pediatric population: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J Pediatr Surg. 2010;45(11):2181–5. doi:.https://doi.org/10.1016/j.jpedsurg.2010.06.038
17 Dumont R , Cinotti R , Lejus C , Caillon J , Boutoille D , Roquilly A , et al. The Microbiology of Community-acquired Peritonitis in Children. Pediatr Infect Dis J. 2011;30(2):131–5. doi:.https://doi.org/10.1097/INF.0b013e3181eed7a4
18 Schmitt F , Clermidi P , Dorsi M , Cocquerelle V , Gomes CF , Becmeur F . Bacterial studies of complicated appendicitis over a 20-year period and their impact on empirical antibiotic treatment. J Pediatr Surg. 2012;47(11):2055–62. doi:.https://doi.org/10.1016/j.jpedsurg.2012.04.025
19 Chen CY , Chen YC , Pu HN , Tsai CH , Chen WT , Lin CH . Bacteriology of acute appendicitis and its implication for the use of prophylactic antibiotics. Surg Infect (Larchmt). 2012;13(6):383–90. doi:.https://doi.org/10.1089/sur.2011.135
20 Skarda DE , Schall K , Rollins M , Andrews S , Olson J , Greene T , et al. Response-based therapy for ruptured appendicitis reduces resource utilization. J Pediatr Surg. 2014;49(12):1726–9. doi:.https://doi.org/10.1016/j.jpedsurg.2014.09.012
21 Solomkin JS , Mazuski JE , Bradley JS , Rodvold KA , Goldstein EJ , Baron EJ , et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133–64. doi:.https://doi.org/10.1086/649554
22 Mazuski JE , Sawyer RG , Nathens AB , DiPiro JT , Schein M , Kudsk KA , et al.; Therapeutic Agents Committee of the Surgical Infections Society. The Surgical Infection Society guidelines on antimicrobial therapy for intra-abdominal infections: an executive summary. Surg Infect (Larchmt). 2002;3(3):161–73. doi:.https://doi.org/10.1089/109629602761624171
VA and PAC contributed equally to the work and should be considered first co-authors
No financial support and no other potential conflicts of interest relevant to this article were reported. The views expressed in the submitted article are our own and not an official position of the institution or funder.