Establishing a non-intubated thoracoscopic surgery programme for bilateral uniportal sympathectomy
DOI: https://doi.org/10.4414/smw.2019.20064
Claudio
Caviezela, Rolf
Schuepbachb, Bastian
Grandeb, Isabelle
Opitza, Marco
Zalunardob, Walter
Wedera, Sven
Hillingera
aDepartment of Thoracic Surgery, University Hospital Zurich, Switzerland
bInstitute of Anaesthesiology, University Hospital Zurich, Switzerland
Summary
AIM OF THE STUDY
Non-intubated, video-assisted thoracoscopic surgery (NiVATS) has been successfully developed in several centres worldwide. Local anaesthesia techniques and techniques to perform thoracoscopic surgery on a spontaneously breathing lung are the two key elements which must be adopted to establish a NiVATS programme. We established NiVATS by performing bilateral, uniportal sympathectomies, and compared it to classical video-assisted thoracoscopic surgery (VATS) under general anaesthesia with double-lumen intubation.
METHODS
Ten consecutive bilateral VATS sympathectomies were compared with ten consecutive NiVATS procedures. Nineteen of the procedures were for palmar hyperhidrosis and one was for facial blushing. Duration of anaesthesia, surgery and hospitalisation, perioperative complications, side effects and quality of life before and after sympathectomy were analysed.
RESULTS
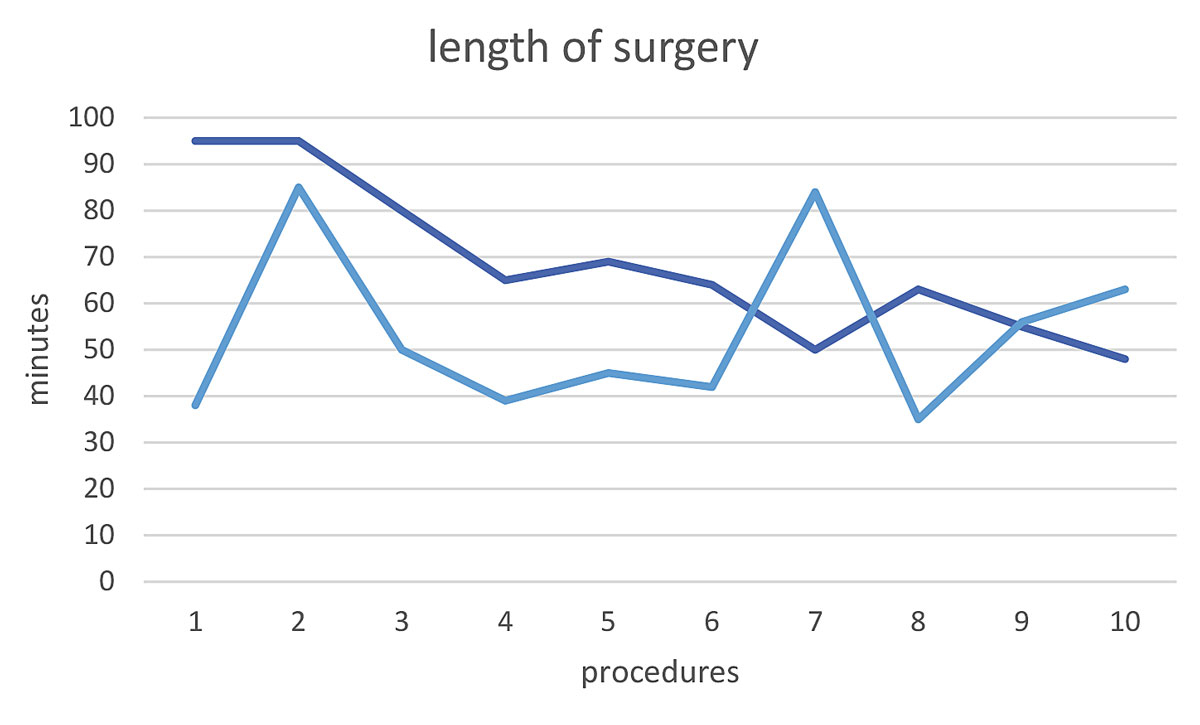
Median age was 26.5 years (range 17–55) and mean BMI in the NiVATS group was 21.8 (range 19.1–26.3). NiVATS sympathectomies were performed as outpatient procedures significantly more often (9/10 vs 3/10, p = 0.008). Quality of life was significantly increased after sympathectomy in all patients, with no significant differences between the NiVATS and the VATS groups. There were no differences between the two groups regarding compensatory sweating (40 vs 50%, p = 0.66). The duration of anaesthesia, not including the time required for the surgery, was significantly shorter in the NiVATS group (p <0.001). The duration of surgery, from the first local anaesthesia until the last skin suture, was significantly longer in the NiVATS group (p = 0.04), but showed a constant decline during the learning curve, from 95 minutes initially to 48 minutes for the last procedure. Costs were significantly lower in the NiVATS group (p = 0.04).
CONCLUSION
Thoracoscopic sympathectomy is a suitable procedure with which to establish a NiVATS programme. Patients are usually young and of healthy weight, facilitating the learning curve for the local anaesthesia techniques and the surgery. Compared to VATS, sympathectomy is more likely to be performed as an outpatient procedure and has a lower cost, while safety and efficacy are maintained.
Introduction
During the past 15 years, non-intubated, video-assisted thoracoscopic surgery (NiVATS) has been successfully introduced and developed in several centres worldwide [1–3]. Although there is a wide variety of non-intubated, sometimes called “awake”, techniques, they all have the same advantages. The shortcomings of intubation with a double-lumen tube, necessitating neuromuscular blockade to facilitate intubation and provide complete relaxation throughout VATS, can be avoided. The use of a simple, intravenous drug regime for analogue sedation, rigorous local infiltration anaesthesia and intercostal nerve blocks prevents postoperative discomforts such as pain, sore throat and coughing, and shortens the recovery time [4–6]. A recent randomised study compared NiVATS procedures under epidural anaesthesia with thoracic surgery operations under general anaesthesia [7]. The procedures included bullae resection, pulmonary wedge resection and lobectomy in 354 patients. The authors showed a shorter postoperative fasting time, a shorter duration of antibiotic use and shorter hospital stay for the 167 patients who underwent NiVATS. Besides NiVATS with epidural anaesthesia, intravenous analgosedation with propofol and remifentanil in combination with surgical local anaesthesia was proven feasible [8]. Intrathoracic vagal block by infiltration of bupivacaine or ropivacaine near the vagal nerve is necessary to inhibit the cough reflex during thoracoscopic manipulation of the lung [8, 9]. Several studies have described the feasibility, safety and effectiveness of NiVATS sympathectomy for primary hyperhidrosis [9, 10]. So far, only Elia and colleagues have compared NiVATS sympathectomy with sympathectomy under general anaesthesia [9]. They found no difference in postoperative quality of life, but better patient satisfaction and reduced costs after the NiVATS procedures. Artificial pneumothorax, intercostal nerve block and vagal block are the techniques of importance for the NiVATS procedure learning curve. Needless to say, the surgeon might feel an increased level of stress while operating on an awake or moderately sedated patient. Because bilateral sympathectomy is a short and technically relatively easy procedure, usually performed in young and otherwise healthy patients, we started our NiVATS programme with this intervention. The aim of this study is to present the learning curve and results of NiVATS by comparing bilateral, thoracoscopic sympathectomy either with spontaneous ventilation or under general anaesthesia with intubation requiring a double-lumen tube.
Methods
The institutional NiVATS programme of the Thoracic Surgery Department at the University Hospital Zurich was initiated in May 2017. For this study, the first ten NiVATS sympathectomies were retrospectively analysed and compared with ten preceding VATS sympathectomies performed under general anaesthesia with double-lumen intubation. In total, therefore, 20 consecutive patients who underwent bilateral sympathectomy from December 2016 to December 2017 were included in this analysis, regardless of their diagnosis (hyperhidrosis or facial blushing).
Indications for thoracoscopic sympathetic surgery
Palmar hyperhidrosis patients were accepted for sympathectomy after they had undergone topical treatments with aluminium chloride hexahydrate and tap water iontophoresis without success. Treatment with botulinum toxin injections was voluntary.
Facial blushing: these patients required an assessment by a psychological specialist to evaluate the severity of social phobia. They also must have completed a cognitive behavioural therapy programme.
Surgery
VATS sympathectomy
The operation was performed under general anaesthesia. Intubation was performed with a double-lumen tube to provide one-lung ventilation. The patient was in a supine position with both arms raised over the head. Usually, the operation started on the right side. The ten procedures were performed by two different surgeons (CC and SH). A two-port approach was used, utilising two 5 mm ports, all of them in the submammary and axillary line in the fourth or fifth intercostal space. Sympathectomy was performed by resection of a 2 mm segment at level T3 (palmar hyperhidrosis) or T2 (facial blushing) with electrocautery. Following sympathectomy, the anaesthesiologist was asked to re-ventilate the lung after a 16 Charrière chest tube was put in situ. The chest tube was connected to a digital suction device (Thopaz™, Medela AG, Baar, Switzerland) with 10 cm H2O suction. After showing no airflow for one to two minutes, the chest tube was removed.
NiVATS – anaesthesia
Sedation was started with a loading dose of 4 µg/kg of dexmedetomidine over 15 min, followed by continuous infusion of 0.5–1.0 µg/kg/h. A target-controlled infusion (TCI) of 1–2 µg/ml plasma concentration of supplemental propofol and 0.05 µg/kg/min of remifentanil, starting at the time of local anaesthesia by the surgeon, were used for the first eight procedures. The last two procedures were performed without remifentanil.
All patients received supplementary oxygen (2–5 l/min) via a facial mask throughout the procedure.
Before initiating the NiVATS programme, anaesthesiological management was discussed at an interdisciplinary, round table discussion between anaesthesiologists and thoracic surgeons. The three most experienced anaesthesiologists performed all anaesthesia for the NiVATS operations. If there was any intraoperative need for intubation and muscle relaxation, a double-lumen tube was inserted.
NiVATS – surgery and local anaesthesia
After the first NiVATS sympathectomy patient, all further 9 consecutive sympathectomy patients were accepted for NiVATS.
The operation was performed as uniportal thoracoscopy. All ten procedures were performed by the same surgeon (CC). The skin and subcutis were anaesthetised with 5 ml lidocaine 1%, and an intercostal block with 5 ml ropivacaine 0.5% was added. One 1.5 cm incision was made in the submammary line, towards the axilla (fig. 1A), in the fourth or fifth intercostal space, and an XX-small Alexis Wound Protector/Retractor (1–3 cm, Applied Medical Resources Corporation, Rancho Santo Margarita, CA, USA) was installed. After awaiting the artificial pneumothorax, the vagal block was performed with a puncture needle (three-part, length 28 cm, diameter 1.3 mm; Angiomed, Karlsruhe, Germany), and 3 ml of lidocaine 1% were injected. The syringe was connected to the needle with an arterial pressure measuring extension set (Codan Medical AG, Switzerland). Figure 2 shows the needle and connected syringe. On the right side, the nerve was targeted on the trachea, behind the cava superior vein (fig. 1B). On the left side, it was targeted on the aortic arch (fig. 1C). An additional local anaesthesia (lidocaine 5 ml) was applied to the approached subpleural space, just beside the sympathetic chain (fig. 1D). Sympathectomy was performed by resection of a 2 mm segment at level T3 (palmar hyperhidrosis) or T2 (facial blushing) with electrocautery. The resection was not different from that with general anaesthesia. After sympathectomy, a 16 Charrière chest tube was inserted. Re-expansion of the lung was achieved by connecting the chest tube to the surgical suction device under thoracoscopic visualisation. After removing the camera, the tube was connected to a digital suction device (Thopaz™, Medela AG, Baar, Switzerland) with 10 cm H2O suction. After showing no airflow for one to two minutes, the chest tube was removed.
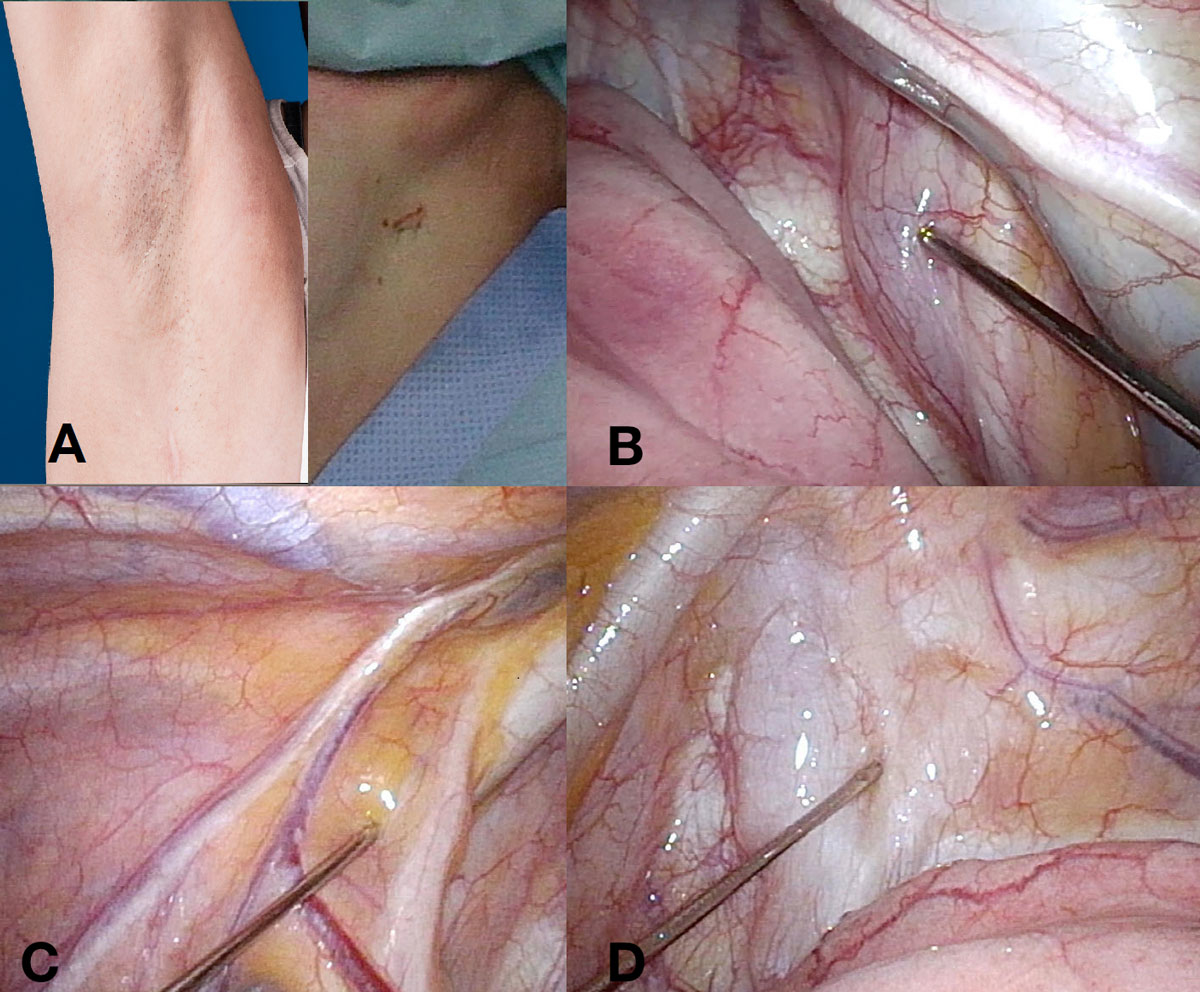
Figure 1 A: axillary single incision on the right side one year after the operation (right) and on the left side just after skin suture (left). B: local anaesthesia for vagal nerve on the right side. C: local anaesthesia for vagal nerve on the left side. D: local anaesthesia for parietal pleura on the left side.
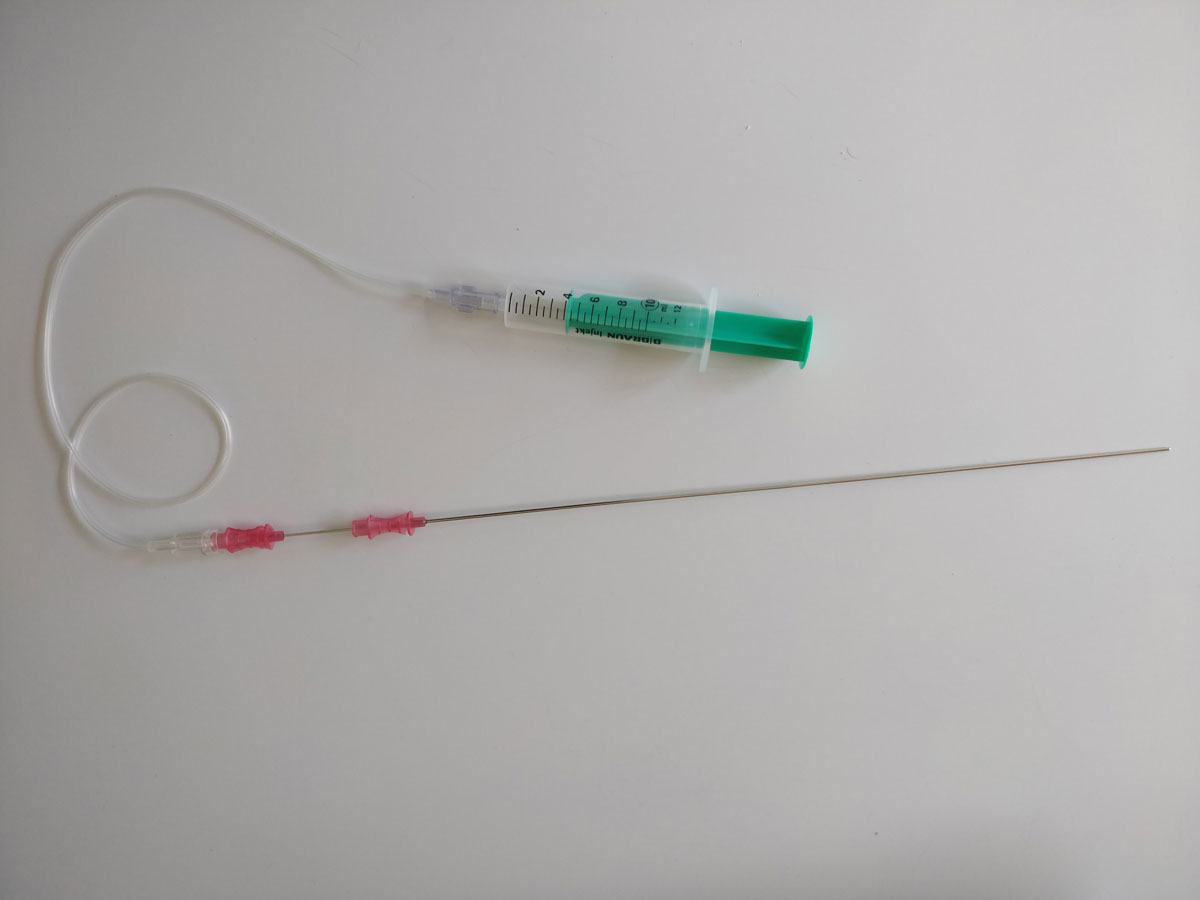
Figure 2 Three-part puncture needle with connected syringe. The innermost part of the needle was removed.
Outcomes
The primary outcome was the learning curve of the NiVATS procedure, expressed as the length of the procedure. Length of anaesthesia, length of hospitalisation, perioperative complications, side effects and quality of life before and after sympathectomy were analysed as secondary outcomes. All outcomes were compared between the two groups.
Learning curve
To define the learning curve, the following time points were analysed and compared:
- beginning of induction of anaesthesia until the end of anaesthesia care
- length of all surgical interventions from the first local anaesthesia until the last skin suture
- total time of anaesthesia care by the anaesthesiologist without surgical intervention
The surgical learning curve was defined as the duration of the operation. Surgical complications like air leaking, chest tube in situ after termination of operation and feasibility of NiVATS – also sometimes used as markers of surgical learning curves – were described separately. Feasibility of the NiVATS procedure was assessed with the feasibility score, developed by Pompeo and colleagues (table 1, [11]). This score shows whether the operation was feasible with non-intubated anaesthesia or whether it was necessary to convert to intubation.
Table 1 Feasibility Score for NiVATS procedures.
|
Score
|
Description
|
| 4 |
Excellent. No adverse events; no need for adjunctive drugs; no conversion to general anaesthesia or thoracotomy. |
| 3 |
Good. Minor adverse events requiring adjunctive measures or drugs; no need for conversion to general anaesthesia or thoracotomy. |
| 2 |
Satisfactory. Adverse events or findings requiring adjunctive measures or drugs, or thoracotomy without conversion to general anaesthesia. |
| 1 |
Unsatisfactory. Procedure converted to general anaesthesia. |
| 0 |
Procedure not performed. |
Quality of life
A quality of life (QoL) questionnaire was filled out by every patient a few weeks before surgery (usually during the outpatient visit and when obtaining informed consent), and also 30 days after surgery (during the postoperative outpatient visit). This questionnaire was adapted from Milanez de Campos and colleagues [12]. Table 2 shows the English version. All our patients used a German version.
Table 2 Quality of life questionnaire.
|
QoL in general
|
Before surgery
|
30 days after surgery
|
| Excellent |
1 |
1 |
| Very good |
2 |
2 |
| Good |
3 |
3 |
| Poor |
4 |
4 |
| Very poor |
5 |
5 |
|
For patients with hyperhidrosis
|
10 excellent – 50 very poor
|
| Writing |
1 2 3 4 5 |
1 2 3 4 5 |
| Hand shaking |
1 2 3 4 5 |
1 2 3 4 5 |
| Socialising |
1 2 3 4 5 |
1 2 3 4 5 |
| Grasping objects |
1 2 3 4 5 |
1 2 3 4 5 |
| Dancing |
1 2 3 4 5 |
1 2 3 4 5 |
| Manual work |
1 2 3 4 5 |
1 2 3 4 5 |
| Leisure |
1 2 3 4 5 |
1 2 3 4 5 |
| Sports |
1 2 3 4 5 |
1 2 3 4 5 |
| Holding hands |
1 2 3 4 5 |
1 2 3 4 5 |
| Intimate touching |
1 2 3 4 5 |
1 2 3 4 5 |
|
For patients with facial blushing
|
2 excellent – 10 very poor
|
| I always justify myself |
1 2 3 4 5 |
1 2 3 4 5 |
| People rejected me slightly |
1 2 3 4 5 |
1 2 3 4 5 |
Follow-up
Follow-up after sympathectomy consisted of a clinical outpatient visit four weeks after the operation. This included a thoracic x-ray. The QoL questionnaire was completed during this visit. Usually, patients are contacted again by telephone one year after the operation, when compensatory sweating or other side effects are checked again. For this study, the last included patient was contacted six months after the operation.
Costs
Costs analysis in Swiss francs (CHF) was split into costs for doctors, costs for nurses, and all other costs (facility management, catering, administrative support).
Statistics
Descriptive statistics were used to summarise patients’ characteristics. Continuous variables were reported as mean and standard deviation (SD) or median and range, and were compared between the two groups using two-sample independent t-tests or Mann-Whitney U tests (non-normal data). Comparisons of pre- and postoperative values originating from the same patient were compared using the Wilcoxon signed rank test (non-normal data). Categorical variables were summarised as frequencies (%) and compared using Pearson’s chi-squared test or Fisher’s exact test where applicable. All reported p-values are two-sided, with a significance level of 0.05. SPSS version 22 [IBM SPSS Statistics for Windows, Armonk, NY, USA: IBM Corp., 2013] was used for data analysis.
Ethics approval
The institutional review board approved this study (BASEC-# 2018-00497).
Results
Twenty consecutive patients were analysed. Fourteen patients were female (70%) and the mean age of all patients was 28.5 years (median 26.5, range 17–55).
Nineteen patients were operated on for primary palmar hyperhidrosis with bilateral sympathectomy on level T3, and one (female) patient for facial blushing with bilateral sympathectomy on level T2.
One (female) patient had had surgery for scoliosis five and ten years before the sympathectomy for hyperhidrosis. All other patients had no relevant comorbidities.
Table 3 shows the characteristics of all procedures. NiVATS sympathectomies were performed as an outpatient procedure significantly more often (p = 0.008).
Table 3 Characteristics of all procedures.
| |
VATS
|
NiVATS
|
p-value
|
| Patients |
10 |
10 |
|
| Mean age in years (range) |
28.6 (17–46) |
28.5 (20–55) |
0.912 |
| Female patients, n (%) |
6 (60%) |
8 (80%) |
|
| Mean BMI in kg/m2 (range) |
23.6 (17–30.4) |
21.8 (19.1–26.3) |
0.545 |
| Median duration of procedure in minutes (range) |
125 (110–240) |
117.5 (90–150) |
0.247 |
| Median duration of anaesthesia in minutes (range) |
81 (62–86) |
52.5 (35 -65) |
<0.001
|
| Median duration of surgery in minutes (range) |
47.5 (35–85) |
64.5 (48–95) |
0.041
|
| Mean feasibility score |
– |
4 |
–
|
| Postoperative pneumothorax without chest tube, n (%) |
3 (30%) |
5 (50%) |
0.648 |
| Postoperative chest tube, n (%) |
0 |
1 (10%) |
0.317 |
| Outpatient procedure, n (%) |
3 (30%) |
9 (90%) |
0.008
|
| Compensatory sweating, n (%) |
4 (40%) |
5 (50%) |
0.661 |
The proportion of patients who showed postoperative pneumothorax upon chest x-raywithout the need for a chest tube upon chest x-ray did not differ significantly between the two groups (five in the NiVATS group and three in the VATS group; p = 0.64). Both procedures showed no differences regarding compensatory sweating.
Learning curve
The duration of the total procedure, including induction of anaesthesia and surgery and until the termination of anaesthesia, did not differ between the groups (VATS median 125 min (range 110–240), NiVATS 117.5 (90–150), p = 0.247). The length of anaesthesia not including the time for surgery was significantly shorter in the NiVATS group (VATS median 81 min (range 62–86), NiVATS 52.5 (35–65), p <0.001). The duration of surgery, from the first local anaesthesia until the last skin suture, was significantly longer in the NiVATS group (VATS median 47.5 min (range 35–85), NiVATS 64.5 (48–95), p = 0.04). The duration of surgery showed a decline during the learning curve (fig. 3).
Two VATS procedures had an operation time of longer than 80 minutes. In both cases, adhesiolysis was necessary.
Only one patient left the operation room with a chest tube in situ due to air leak (table 3). This was the second patient operated on with the NiVATS technique. The needle for vagal local anaesthesia caused a lesion in the lung parenchyma. We changed the technique and used a thinner calibre needle for the following procedures.
Anaesthesia
Initially, dexmedetomidine was used in combination with remifentanil. For the last two NiVATS procedures, we switched to dexmedetomidine in combination with propofol bolus 10–20 mg if required. We used this regimen with patients complaining about discomfort or showing agitation, and to avoid the deep breathing pattern often seen under anaesthesia with opioids (remifentanil), which hinders precise surgical work. One patient had hallucinations after skin closure during termination of anaesthesia. No other anaesthesiological side effects were noticed. The patient who had hallucinations received a combination of dexmedetomidine, propofol and remifentanil. A few hours after the procedures, the patient had no more complaints and could leave the hospital as an outpatient.
Costs
A cost analysis comparing NIVATS to VATS revealed a statistically significant advantage for the NIVATS group (table 4). Doctor-related costs were CHF 854.33 per patient (standard deviation CHF 115.55) for NiVATS and CHF 1,505.25 (94.08) for VATS procedures (p = 0.024). Nurse-related costs were CHF 435 (77.29) for NiVATS and CHF 609.62 (26.62) for VATS (p = 0.048). All patient-related costs were CHF 3,290.33 (337.56) for NiVATS and CHF 4,482.12 (207.75) for VATS procedures (p = 0.048).
Table 4 Cost comparison between NIVATS and VATS sympathectomy.
|
Costs
|
NIVATS
|
VATS
|
p-value
|
| Doctors |
854.33 (115.55) |
1505.25 (94.08) |
0.024 |
| Nurses |
435 (77.29) |
609.62 (26.62) |
0.048 |
| All patient-related costs |
3,290.33 (337.56) |
4482.12 (207.75) |
0.048 |
Quality of life
All patients with compensatory sweating (40% in the VATS group, 50% in the NiVATS group, p = 0.66) declared it as mild, and would undergo the sympathectomy again, being aware of the actual severity of this side effect. Table 5 shows the results of the questionnaire. All patients answered the questions, 19 patients concerning their hyperhidrosis, one patient concerning her facial blushing. Quality of life was significantly increased after sympathectomy, both in general and in specific areas. The only preoperative points that showed any significant difference between the groups concerned functional and social domains among hyperhidrosis patients (the VATS group had a mean of 35.3 points compared to the NiVATS group, who had a mean of 42.33 points, p = 0.033). All other comparisons did not differ significantly between the two groups, neither preoperative nor postoperative.
Table 5 Quality of life results.
| |
Before surgery
|
30 days after surgery
|
|
QoL in general
|
1 excellent – 5 very poor
|
| VATS (10/10 patients) |
3.9 |
1.3 |
| NiVATS (10/10 patients) |
4.33 |
1.33 |
|
For patients with hyperhidrosis
|
10 excellent – 50 very poor
|
| VATS (10/10 patients) |
35.3 |
11.2 |
| NiVATS (9/10 patients) |
42.33 |
11.11 |
|
For patients with facial blushing
|
2 excellent – 10 very poor
|
| NiVATS (1/10 patients) |
6 |
2 |
Discussion
This retrospective analysis compared thoracoscopic sympathectomy under general anaesthesia with the same operation using a NiVATS approach. The primary outcome was the learning curve, expressed as the operation time. This was significantly longer for the NiVATS group, but declined over time. Outcomes regarding postoperative complications and quality of life were comparable between the groups, but costs were clearly lower in the NiVATS group. Based on these results, the NiVATS technique was successfully established in our department and is currently applied to other procedures as well.
Only one study has previously compared the two different methods for this specific operation [9]. This study was 14 years ago but, among others, it initiated the mainstream use of NiVATS today. Our results are comparable with the results from Elia and Pompeo et al. The main advantages of the NiVATS procedure are fewer side effects from anaesthesia because muscle relaxants are avoided, having no potential for tissue irritation and damage to the upper and lower airway because of intubation, and the lower costs due to the exclusion of flexible bronchoscopy and not needing to reverse muscle relaxation at the end of the procedure [13].
In our experience, patients often wanted to stay in the hospital for the first postoperative night after VATS sympathectomy due to slight discomfort and nausea, perhaps due to the use of strong opioids (fentanyl) during anaesthesia. There is evidence that (non-smoking) young females have a higher risk of postoperative nausea [14] and our patient group primarily consisted of young females.
Patients left the hospital as an outpatient significantly more often after NiVATS. This phenomenon might be caused by a bias as the NiVATS patients are already primed to be outpatients, while patients who underwent the VATS procedures under general anaesthesia were kept in the hospital one day longer. Nevertheless, from a medical point of view, the less invasive regime of anaesthesiological airway management and consecutive sedation with short-acting medication may anyway be regarded as advantageous. Liu and colleagues, for example, showed that there was significantly less tumour necrosis factor α (TNF-α) in the bronchoalveolar lavage fluid and less C-reactive protein (CRP) in the serum in their patients after NiVATS compared to after VATS [7]. The NiVATS patients suffered from fewer postoperative respiratory complications and used postoperative antibiotics for a shorter period of time.
As the results regarding, for example, dry hands or compensatory sweating and postoperative quality of life are equal after NiVATS and VATS sympathectomies, the costs become more important. Our cost analysis shows a statistically significant advantage of NiVATS, which is probably mostly influenced by the outpatient setting. During the twelve months studied until December 2017, we performed 20 sympathectomies. Since the NiVATS programme and the outpatient sympathectomy were initiated, we have experienced an increase in the number of hyperhidrosis patients admitted. We now operate on approximately 30 patients per year. This might be the highest rate of sympathectomies in centres in Switzerland.
The insurance companies seem to cover this non-obligatory treatment more liberally, provided it is performed as an outpatient procedure. However, our cost analysis showed a small profit for the hospital in the case of an outpatient operation due to the savings. To summarise, the lower costs and the rise in referrals might be due to the outpatient management, or might just be part of a larger trend as sympathectomies are finally given more structure and are managed in a more straightforward way.
Needless to say, despite the more liberal coverage of this treatment, hyperhidrosis patients still need a rigorous preoperative assessment to identify the candidates suitable for operation [16, 17].
Sympathectomy is a very short procedure, but in our series, its operation time is prolonged in the NiVATS group. The additional time is because it is necessary to wait for the local anaesthesia to take effect and for the artificial pneumothorax. It also takes time for the local anaesthesia for the vagal nerve and the parietal pleura to take effect. These procedures require a learning curve for both anaesthesiologists and surgeons, who must adapt to the unusual situation of spontaneous ventilation in a thoracoscopic procedure with several intrathoracic punctures [8]. The learning curve demonstrated in this study shows a decline in operation time, defined as from the first local anaesthesia until skin closure. This seems to reflect the additional time caused by special circumstances in non-intubated patients.
The only patient with a chest tube in place was the second one to undergo a NiVATS operation. A chest tube was required because the lung parenchyma was accidently punctured. This was caused by the first two vagal block procedures being performed with a larger, more cumbersome needle. After changing to a 17.5 gauche needle with three parts (the innermost part was removed), the later blocks were uneventful. The NiVATS procedures were performed as uniportal surgeries, while the VATS operations were done with two ports. Successful uniportal, thoracoscopic sympathectomies are already described in case reports and case series [10, 18]. Puncturing the vagal nerve is the most unusual procedure. This probably needs the most training, as the lungs are totally relaxed and easy to handle once the vagal nerve has been blocked. No differences from a double-lumen intubation with one lung ventilation were detectable. On the right side, the nerve seems to be always visible, but on the left side it is sometimes hidden by the mediastinal/para-aortic fat. Therefore, a BMI of less than 28 seems reasonable when considering patients for NiVATS. Obesity, with a BMI >30, is suggested as a contraindication by some authors [19]. Patients scheduled for bilateral sympathectomy usually have a slender habitus. The intercostal local anaesthesia, operating through one port and the intrathoracic punctures are all easier in slender patients. Sympathectomy is therefore a good choice of operation with which to start a (uniportal) NiVATS programme.
Performing sympathectomy as a NiVATS procedure immediately showed us the benefits of non-intubated anaesthesia. Patients woke significantly faster and left the hospital soon after the operation. A patient with, for example, a simple pulmonary wedge resection, might stay in hospital for two or three days because of the chest tube. Therefore, the anaesthetic side effects are not present long before dismissal, and measuring the benefits of NiVATS becomes more difficult. This advantage might therefore be biased, institution-specific and only true for this minor operation. So far, NiVATS can be proposed for this particular patient population. Other populations still need further research.
Several centres already perform sympathectomies as an outpatient procedure, even under general anaesthesia with double-lumen intubation. Nevertheless, in our institution, the switch from VATS under general anaesthesia to NiVATS facilitated the establishment of this technique particularly, and enabled sympathectomy to be performed as an outpatient operation more often.
As well as local anaesthesia, intravenous sedation plays a major role. We started with dexmedetomidine and remifentanil. Patients with opioids show a typical deep breathing pattern, which made the surgery more difficult because of the heavily moving diaphragm. The change to dexmedetomidine in combination with TCI-propofol minimised these movements.
The main limitations of this study are the small sample size, the single centre setting and the bias mentioned above. Future research should focus on detecting any real advantages or disadvantages of NiVATS, preferably in a randomised setting.
In conclusion, NiVATS sympathectomy is a safe, feasible and effective operation compared to VATS sympathectomy under general anaesthesia. It might help to quicken the patient’s postoperative recovery and therefore transform the operation into an outpatient procedure. Nevertheless, this statement needs further research, preferably with a randomised controlled trial. The above-mentioned bias might be reduced by treating all patient groups as outpatients from the beginning on.
On the other hand, NiVATS sympathectomy is a suitable procedure with which to establish a non-intubated, thoracic surgery programme. The learning curve is short and the different local anaesthesiological techniques are relatively easy to implement, especially with the slender anatomy typical of patients with this disease.
References
1
Pompeo
E
,
Mineo
D
,
Rogliani
P
,
Sabato
AF
,
Mineo
TC
. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg. 2004;78(5):1761–8. doi:.https://doi.org/10.1016/j.athoracsur.2004.05.083
2
Chen
JS
,
Cheng
YJ
,
Hung
MH
,
Tseng
YD
,
Chen
KC
,
Lee
YC
. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg. 2011;254(6):1038–43. doi:.https://doi.org/10.1097/SLA.0b013e31822ed19b
3
Guo
Z
,
Shao
W
,
Yin
W
,
Chen
H
,
Zhang
X
,
Dong
Q
, et al.
Analysis of feasibility and safety of complete video-assisted thoracoscopic resection of anatomic pulmonary segments under non-intubated anesthesia. J Thorac Dis. 2014;6(1):37–44.
4
Gal
TJ
. Bronchial hyperresponsiveness and anesthesia: physiologic and therapeutic perspectives. Anesth Analg. 1994;78(3):559–73. doi:.https://doi.org/10.1213/00000539-199403000-00025
5
Whitehead
T
,
Slutsky
AS
. The pulmonary physician in critical care * 7: ventilator induced lung injury. Thorax. 2002;57(7):635–42. doi:.https://doi.org/10.1136/thorax.57.7.635
6
Wang
B
,
Ge
S
. Nonintubated anesthesia for thoracic surgery. J Thorac Dis. 2014;6(12):1868–74.
7
Liu
J
,
Cui
F
,
Li
S
,
Chen
H
,
Shao
W
,
Liang
L
, et al.
Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov. 2015;22(2):123–30. doi:.https://doi.org/10.1177/1553350614531662
8
Chen
KC
,
Cheng
YJ
,
Hung
MH
,
Tseng
YD
,
Chen
JS
. Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation. J Thorac Dis. 2014;6(1):31–6.
9
Elia
S
,
Guggino
G
,
Mineo
D
,
Vanni
G
,
Gatti
A
,
Mineo
TC
. Awake one stage bilateral thoracoscopic sympathectomy for palmar hyperhidrosis: a safe outpatient procedure. Eur J Cardiothorac Surg. 2005;28(2):312–7, discussion 317. doi:.https://doi.org/10.1016/j.ejcts.2005.03.046
10
Ning
Y
,
Wang
Y
,
Tao
X
,
Tang
H
,
Jiang
J
,
Li
Y
, et al.
Single-Port Endoscopic Thoracic Sympathectomy with Monitored Anesthesia Care: A More Promising Procedure for Palmar Hyperhidrosis. World J Surg. 2015;39(9):2269–73. doi:.https://doi.org/10.1007/s00268-015-3104-7
11
Pompeo
E
,
Rogliani
P
,
Cristino
B
,
Schillaci
O
,
Novelli
G
,
Saltini
C
. Awake thoracoscopic biopsy of interstitial lung disease. Ann Thorac Surg. 2013;95(2):445–52. doi:.https://doi.org/10.1016/j.athoracsur.2012.10.043
12
Milanez de Campos
JR
,
Kauffman
P
,
Werebe
EC
,
Andrade Filho
LO
,
Kusniek
S
,
Wolosker
N
, et al.
Quality of life, before and after thoracic sympathectomy: report on 378 operated patients. Ann Thorac Surg. 2003;76(3):886–91. doi:.https://doi.org/10.1016/S0003-4975(03)00895-6
13
Yang
JT
,
Hung
MH
,
Chen
JS
,
Cheng
YJ
. Anesthetic consideration for nonintubated VATS. J Thorac Dis. 2014;6(1):10–3.
14
Apfel
CC
,
Heidrich
FM
,
Jukar-Rao
S
,
Jalota
L
,
Hornuss
C
,
Whelan
RP
, et al.
Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109(5):742–53. doi:.https://doi.org/10.1093/bja/aes276
16
Cerfolio
RJ
,
De Campos
JR
,
Bryant
AS
,
Connery
CP
,
Miller
DL
,
DeCamp
MM
, et al.
The Society of Thoracic Surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg. 2011;91(5):1642–8. doi:.https://doi.org/10.1016/j.athoracsur.2011.01.105
17
Sternbach
JM
,
DeCamp
MM
. Targeting the Sympathetic Chain for Primary Hyperhidrosis: An Evidence-Based Review. Thorac Surg Clin. 2016;26(4):407–20. doi:.https://doi.org/10.1016/j.thorsurg.2016.06.005
18
Obeso Carillo
GA
,
Cañizares Carretero
MA
,
Padín Barreiro
L
,
Soro García
J
,
Blanco Tuimil
L
. Nonintubated bilateral single port thoracoscopic sympathectomy in the context of an outpatient program, the least invasive management for hyperhidrosis surgery. Ann Transl Med. 2015;3(22):357.
19
Hung
MH
,
Hsu
HH
,
Cheng
YJ
,
Chen
JS
. Nonintubated thoracoscopic surgery: state of the art and future directions. J Thorac Dis. 2014;6(1):2–9.