Outcome of inter-hospital transfer of patients on extracorporeal membrane oxygenation in Switzerland
DOI: https://doi.org/10.4414/smw.2019.20054
Markus J.
Wilhelma, Devdas T.
Inderbitzina, Diana
Resera, Maximilian
Halbea, Koen
Van Tillburga, Roland
Albrechtb, Stefan M.
Müllerc, Urs
Wengerd, Marco
Maggiorinid, Alain
Rudigerd, Dominique
Bettexd, Reto
Schüpbachd, Alberto
Webera, Stefano
Benussia, Ludwig K.
Von Segessera, Andreas J.
Flammere, Francesco
Maisanoa, Frank
Ruschitzkae
aClinic for Cardiovascular Surgery, University Heart Centre, Zurich, Switzerland
bREGA, Schweizerische Rettungsflugwacht, Zurich Flughafen, Switzerland
cSchutz und Rettung Zurich, Switzerland
dInstitute for Intensive Care Medicine, University Hospital Zurich, Switzerland
eClinic for Cardiology, University Heart Centre, Zurich, Switzerland
Summary
AIMS OF THE STUDY
An extracorporeal membrane oxygenation system (ECMO), as a bridge to either recovery, a ventricular assist device (VAD), or heart or lung transplantation, may be the only lifesaving option for critically ill patients suffering from refractory cardiac, respiratory or combined cardiopulmonary failure. As peripheral hospitals may not offer ECMO treatment, tertiary care centres provide specialised ECMO teams for on-site implantation and subsequent patient transfer on ECMO to the tertiary hospital. This study reports the results of the largest ECMO transportation programme in Switzerland and describes its feasibility and safety.
METHODS
Patients transported on ECMO by our mobile ECMO team to our tertiary centre between 1 September 2009 and 31 December, 2016 underwent retrospective analysis. Implantation was performed by our specialised ECMO team (primary transport) or by the medical staff of the referring hospital (secondary transport) with subsequent transfer to our institution. Type of ECMO, transport data, patient baseline characteristics, operative variables and postoperative outcomes including complications and mortality were collected from medical records.
RESULTS
Fifty-eight patients were included (three patients excluded: one repatriation, two with incomplete medical records). Thirty-five patients (60%) received veno-venous, 22 (38%) veno-arterial and one patient (2%) veno-venoarterial ECMO. Forty-nine (84%) patients underwent primary and nine (16%) secondary transport. Thirty-five (60%) patients were transferred by helicopter and 23 (40%) by ambulance, with median distances of 38.1 (13–225) km and 21 (3-71) km respectively. No clinical or technical complications occurred during transportation. During hospitalisation, three patients had ECMO-associated complications (two compartment syndrome of lower limb, one haemothorax after central ECMO upgrade). Median days on ECMO was 8 (<1–49) and median days in hospital was 17 (<1–122). ECMO weaning was successful in 41 patients (71%), on-transport survival was 100%, 40 patients survived to discharge (69%), and overall survival was 67% (39 patients) at a median follow-up of 58 days (<1–1441). Cumulative survival was significantly affected by cardiogenic shock vs. ARDS (p = 0.001), veno-arterial and veno-venoarterial vs. veno-venous ECMO (p = 0.001) and after secondary vs. primary transport (p <0.001). The ECMO weaning rate was significantly lower after secondary transfer (22%, two patients, both vaECMO) vs. primary transfer (80%, p = 0.002, 39 patients of which 35 (71%) had vvECMO).
CONCLUSIONS
The first results of our ECMO transportation programme show its feasibility, safety and efficacy without on-site implant or on-transport complications or mortality. The favourable early survival may justify the large effort with respect to logistics, costs and manpower. With rising awareness, referring centres may increasingly consider this lifesaving option at an early stage, which may further improve outcomes.
Introduction
Extracorporeal membrane oxygenator support for cardiopulmonary failure was first described by Bartlett and colleagues in 1977 and has emerged as a lifesaving option in isolated or combined refractory respiratory and cardiac failure [1–4]. In patients in whom conventional ventilatory and haemodynamic support does not allow for safe transfer to a tertiary care centre, extracorporeal membrane oxygenation (ECMO) is an option for cardiopulmonary stabilisation [5–9]. Since ECMO implantation requires particular expertise and technical equipment that might not be available in primary medical institutions, highly specialised ECMO teams can offer on-site ECMO implantation at the referring hospital, with subsequent patient transport to their tertiary care centre base [1–3, 10, 11].
Since Bartlett’s description, the concept has developed further, mainly in paediatric patients [12, 13]. It became of major interest in adults during the H1N1 pandemic in 2009/2010, which had a high incidence of acute respiratory distress syndrome (ARDS) due to pneumonia [14, 15]. Meanwhile, larger studies and reviews have shown the safety and efficacy of patient transfers on ECMO, with a negligible on-transport mortality of up to 0.5% and an overall survival until hospital discharge of >50% [1, 3, 10, 11].
The largest studies, of over 100 transportations of patients on ECMO, were published by the University of Michigan (Ann Arbor), University of Arkansas for Medical Sciences College of Medicine (Little Rock), the Columbia University Medical Center (New York) and Karolinska University Hospital (Stockholm) [2, 10, 11, 16]. These studies describe the safety of their transportation on ECMO without any on-transport mortality and with an overall survival rate of 60–70%. These outcomes are comparable to the survival of patients with in-hospital ECMO implantation and to the results published by the Extracorporeal Life Support Organization (ELSO, Ann Arbor, MI, USA, www.elso.org) which has over 400 member-units worldwide and maintains a registry on ECMO. Guidelines on ECMO transportation (www.elso.org: “Guidelines for ECMO Transport”) have been published by the ELSO and revised based on this evidence.
Following these guidelines, the Clinic for Cardiovascular Surgery at the University Hospital Zurich built up a dedicated ECMO transport team in 2009, the first of its kind in Switzerland. Since then, it has grown to become the largest such institution in Switzerland. The objective of this study is to report on the feasibility, safety and outcomes of patient transportations on ECMO to our tertiary care centre by our specialised team from 2009 to 2016.
Materials and methods
Patient cohort and primary endpoint
All patients transported on ECMO by our mobile ECMO team between September 2009 and December 2016 underwent retrospective analysis. Of the 61 patients identified, three were excluded. One patient received ECMO in our hospital and was repatriated to Italy, and two other patients’ medical records were incomplete. Thus, 58 patients with ECMO implantation in a peripheral hospital and subsequent airborne or ground transportation to our centre were included in this retrospective analysis. The Clinic for Cardiovascular Surgery, together with the Clinic for Cardiology, constitute the University Heart Centre Zurich, which offers all treatment modalities for acute and chronic heart failure. The specialised ECMO team of perfusionists maintain proper function of all ECMO running in-house and also provide a perfusionist for the transport of patients on ECMO, including on-site implantation.
The primary endpoint was overall (implant, on- and post-transport) survival. Secondary endpoints were overall (implant, on- and post-transport) complications and potential predictive risk factors identified in a regression analysis.
Protocol: indication and criteria for ECMO implantation
Intensivists from regional hospitals consult our cardiac surgeon on call to request extracorporeal circulation with subsequent transportation to our centre for a critically ill patient whose condition does not allow conventional transportation. The patient’s clinical data are discussed by the ECMO team, which consists of a cardiac surgeon, an intensivist and a perfusionist. Indications for transportation on ECMO are potentially reversible cardiac (cardiogenic shock; CS) or respiratory (acute respiratory distress syndrome: ARDS) failure or combined cardiopulmonary failure which is refractory to conservative intensive care treatment. Detailed diagnoses for CS and ARDS are given in table 1. Criteria for ECMO generally follow the “Stockholm Experience” [2]. The most important respiratory failure criteria are an oxygen partial pressure <80 mm Hg at a fraction of inspired oxygen (FiO2) of 100% and respiratory acidosis (pH <7.1). Cardiac failure criteria are acidaemia with or without lactataemia, central venous saturation <55% and cardiac index (CI) < 2 l/min/kg bodyweight despite administration of vasoactive drugs. Contraindications for ECMO implant are evident irreversibility of CS or ARDS, irreversible brain injury, intracranial bleeding/haematoma and other comorbidities with a detrimental prognosis and a suspected life expectancy <1 year. However, the final decision on the indication for ECMO and the type of ECMO is made by the ECMO team on-site after arrival at the peripheral hospital, together with the local treatment team, based on the patient’s clinical condition.
Table 1 Demographic data of study population.
| Total number of patients (n) |
58 |
| Age (years; mean ± SD) |
49.3 ± 13.2 |
| Male gender (n; %) |
34 (58.6%) |
| Body weight (kg; mean ± SD) |
81.8 ± 23.2 |
| Height (cm; mean ± SD) |
170.5 ± 9 |
| Body mass index (kg/m2; mean ± SD) |
28.1 ± 7.2 |
| Body surface area (m2; mean ± SD) |
1.97 ± 0.35 |
| Resuscitation prior to ECMO implantation (n) |
2 |
| Isolated cardiogenic shock (n; %): |
18 (31%) |
| ‒ Myocardial infarction |
8 |
| ‒ Myocarditis |
2 |
| ‒ Dilative cardiomyopathy |
2 |
| ‒ Unknown aetiology of cardiac failure |
6 |
| Isolated respiratory failure (ARDS) (n; %): |
36 (62%) |
| ‒ Pneumonia (aspiration, bacterial) |
16 |
| ‒ Pneumonia (H1N1 virus) |
2 |
| ‒ Sepsis |
8 |
| ‒ Near drowning |
1 |
| ‒ Trauma |
1 |
| ‒ COPD exacerbation |
1 |
| ‒ Asthma with respiratory failure |
1 |
| ‒ Anaphylactic respiratory failure |
1 |
| ‒ Open tuberculosis |
1 |
| ‒ Unknown aetiology for respiratory failure |
4 |
| Combined cardiopulmonary failure (n; %) |
4 (7%) |
| ‒ Recurrent pulmonary embolism |
1 |
| ‒ Pulmonary embolism and acute right ventricular failure |
1 |
| ‒ Sepsis with cardiac failure |
1 |
| ‒ Pneumonia with cardiac failure |
1 |
Type of transport
Two types of patient transfer were defined: primary transfer was defined as an on-site ECMO implantation at the peripheral hospital by our ECMO team, with subsequent patient transfer to our tertiary care centre. The transport was accompanied by a cardiac surgeon, an anaesthesiologist and a perfusionist. If the ECMO was implanted by a team from the peripheral hospital prior to the transfer, it was defined as a secondary transfer. In this case, the ECMO team consisted of an anaesthesiologist and a perfusionist, without a cardiac surgeon. All ECMO systems were implanted and initiated at the peripheral intensive care unit (ICU), and the patient was stabilised for 30 minutes on ECMO prior to transportation.
ECMO type, materials and equipment, implantation
In primary transports, the ECMO implantation was a collaboration between our ECMO team and an on-site team of intensivists, nurses, scrub nurses and a cardiologist providing transoesophageal echocardiography (TEE).
The required equipment, including cannulas, wires, tubing, sutures, draping and surgical instruments, was provided by our ECMO team. Both veno-venous (vv) and veno-arterial (va) ECMOs were implanted. In one case, an additional afferent cannula was implanted in the right internal jugular vein (JV), resulting in a veno-venoarterial ECMO circuit. This case was assigned to the vaECMO group for analysis. The systems used were LiveBox (Sorin, LivaNova SA, Lausanne, Switzerland) until 2015 and thereafter CardiohelpTM (Maquet Inc., Rastatt, Germany). The CardiohelpTM all-inclusive kit consists of the oxygenator, which includes the centrifugal pump. The HLS cannula by Maquet (Maquet Cardiopulmonary AG, Rastatt, Germany) was used for venous and arterial cannulas, sized 15 to 29.
Implantation took place in the ICU with the assistance of a surgical fellow and/or scrub nurse from the referral unit. After sterile draping, the ECMO implant was started following the administration of a heparin bolus (100 IU/kg). Depending on bodyweight and size, venous drainage was performed by a long heparin-coated 19, 23, 25 or 29 French (F) cannula (Venous HLS Cannula by Maquet Cardiopulmonary AG, Rastatt, Germany). The cannula was placed percutaneously via the right femoral vein (VFC) through the right atrium, with its tip in the superior vena cava (SVC) in the case of vaECMO, and in the inferior vena cava (IVC), just below its inflow into the right atrium, in the case of vvECMO. The venous cannulas were implanted under TEE guidance by a local cardiologist using the bicaval view or alternatively, by fluoroscopy. For afferent access of vvECMO, the right internal JV was punctured and a heparin coated 15, 17 or 19 French arterial cannula (Arterial HLS Cannula by Maquet Cardiopulmonary AG, Rastatt, Germany) was advanced so that its tip was located at the SVC-right atrial (RA) junction. Afferent access of vaECMO was achieved by percutaneous implantation of a heparin-coated 15, 17 or 19 F cannula (Arterial HLS Cannula by Maquet Cardiopulmonary AG, Rastatt, Germany) into the common femoral artery (CFA) or into the right subclavian artery (RSA) by a surgical cut down. The arterial femoral access was pre-closed using a ProGlide Perclose Vascular Closure Device (Abbott Vascular, Altishofen, Switzerland). Prior to insertion of the femoral cannula, a 7–9 F introducer sheath was percutaneously inserted into the superficial femoral artery (AFS) for peripheral arterial limb perfusion. Adequate arterial puncture was verified by TEE or fluoroscopic visualisation of the guide wire in the descending aorta. Trans-cutaneous duplex sonography was available to visualise inguinal vessels and to facilitate the access. In challenging cases, both super-stiff guide wires and head-hunter catheters were used for the safe placement of the efferent venous cannula. Cannulas were securely fixed to the skin by multiple sutures for safe transportation. The cannulas were flushed and connected to the ECMO device under de-airing. The ECMO then was initiated.
Means of transportation
Means of transportation included a conventional ground ambulance and a helicopter (REGA, Rettungsflugwacht, Switzerland). The choice of appropriate means of transfer is based on the distance to the referring unit and the weather conditions, which occasionally make a helicopter flight impossible due to restricted vision. Both helicopter and ambulance were equipped with a custom-made fixation plate for the ECMO device to avoid dislocation during transfer, and the ECMO was connected to the electrical power of the transport vehicle or helicopter to avoid battery exhaustion. The anaesthesiologist confirmed cardiopulmonary stability and stable blood gas analysis prior to departure and managed ventilator settings and vasoactive drug administration during transportation, while the perfusionist controlled the ECMO.
Outcome: complications, ECMO weaning, survival, destination of ECMO treatment
Clinical and technical complications (during on-site implantation, during transport, post-transport and ECMO-related) were looked up in the documents of the referring hospital, the implant and transportation protocol and the medical records of our hospital. However, it was not always possible to retrieve medicals records prior to ECMO implantation and transport from the referring hospitals. Therefore, risk scores (e.g. APACHE, SAVE) could not be reliably evaluated for respective subgroup analyses. No further sub-categorisation of complications was defined. Needing to change cannulas or an ECMO upgrade was analysed and listed separately, and not classified as a complication (table 2).
Table 2 ECMO implantation and transportation characteristics.
| ECMO configuration |
Veno-arterial (va) |
22 (38%), 9 implanted by referral hospital*
|
| Veno-venous (vv) |
35 (60%)†
|
| Veno-venoarterial (v-va) |
1 (2%)†
|
| Implanted afferent cannula |
CFA |
21 |
| JV |
31 |
| CFV |
4 |
| RSA |
2 |
| Implanted efferent inguinal cannula (CFV) |
Unilateral |
57 (98%) |
| Bilateral |
1 (2%) |
| Type of transportation |
Primary (ECMO implant by our team) |
49 (84%) of which 35 (71%) vv and 14 (29%) va |
| Secondary (ECMO implant by referring hospital) |
9 (16%) of which 9 (100%) va |
| Means of transportation: |
Helicopter (airborne transportation) |
35 (60%) |
| Ambulance (ground transportation) |
23 (40%) |
| Transport distance (Km; median (min, max)) |
Helicopter |
38.1 (13, 225) |
| Ambulance |
21 (3, 71) |
| Clinical and technical complications |
During implant at referral unit |
0 |
| During transport |
0 |
| After arrival, ECMO implant related |
3‡
|
The ECMO weaning rate was retrieved from the medical records at our hospital (operations notes and postoperative course). The principal endpoint of overall survival (during ECMO implant, during transport, post-transport until discharge and up to the documented post-hospital follow-up) was evaluated.
Days on ECMO was calculated from the dates of ECMO implantation and explantation or of death. ICU- and in-hospital-days were retrieved from medical records. The follow-up was retrieved from available post-discharge, external or in-house follow-up records.
The ECMO destination was grouped as bridge to recovery, bridge to ventricular assist device or bridge to heart or lung transplantation.
Data management and statistical analysis
In this retrospective study, data on transports were collected throughout the duration of the study in a prospective manner, with post-hoc retrospective analysis by an independent statistician using IBM SPSS Statistics (IBM, Armonk, New York, USA). Continuous variables are given as mean ± standard deviation or median with range. Categorical variables are listed as numbers and proportions (%). Data were compared between groups (diagnosis, type of ECMO, type of transport, means of transport) using a Mann-Whitney U test, chi square test or Fisher’s exact test as appropriate. Overall survival was analysed using Kaplan-Meier curves and compared between groups using a log-rank (Mantel Cox) test. Intergroup differences were considered statistically significant if p <0.05.
Ethical considerations
The study was approved by the local ethical committee (Kantonale Ethikkommission Zurich, Project number BASEC No. 2017-00517) in accordance with the principles of the declaration of Helsinki. All participating investigators signed a declaration of confidentiality. All authors have read and agreed to the manuscript as written.
Results
Fifty-eight patients were included in our analysis. The caseload grew exponentially over the duration of the study (fig. 1).
Demographic baseline data are summarised in table 1. Two patients were resuscitated prior to ECMO implant. The majority of patients (62%, 36 patients) suffered from isolated respiratory failure, while 18 patients (31%) were in isolated cardiogenic shock. In four patients (7%), combined cardiopulmonary failure required vaECMO. In one patient with isolated cardiogenic shock after hypoxic cardiac arrest due to a status asthmaticus, a vvECMO was sufficient due to haemodynamic stabilisation following adequate oxygenation through the ECMO. In two patients with isolated respiratory failure, a vaECMO was implanted on-site due to marginal cardiac function in echocardiography.
ECMO implant data and transportation characteristics are shown in table 2. Of 58 ECMO implants, 35 (60%) were veno-venous, 22 (38%) were veno-arterial and one (2%) was veno-venoarterial. Primary transfer was provided in 49 (84%) patients and secondary transfer in 9 (16%). Thirty-five patients (60%) were transported by helicopter (median distance 38.1 km) and 23 patients (40%) by ambulance (median distance 21 km). No clinical or technical complications occurred at the referral hospital or during ECMO transport. After arrival at our hospital, three ECMO-related complications were recorded. One patient who had been transferred with vvECMO after nearly drowning needed an upgrade to veno-venoarterial ECMO two days later due to cardiac failure using bilateral inguinal veins for drainage and both the right subclavian artery and the right internal jugular vein as afferent vessels. After further deterioration, a central ECMO had to be implanted. The patient developed postoperative haemothorax needing surgical revision. The second patient (secondary transfer) developed a compartment syndrome due to ischaemia of the lower limb caused by the lack of a peripheral arterial perfusion cannula until arrival in our hospital. The third patient needed a change of the arterial femoral cannula to the other inguinal side due to continuous bleeding at the cannulation site. The patient developed a compartment syndrome on the side of initial cannulation.
No significant difference between the patient groups “type of ECMO” or “type of transport” were found with reference to age, gender, bodyweight, body size and body mass index (table 3).
Table 3 Demographic data for study groups by type of extracorporeal membrane oxygenation (ECMO) and type of transport.
| |
VV ECMO
n = 35
|
VA ECMO*
n = 23
|
p-value
|
Primary transport
n = 49
|
Secondary transport
n = 9
|
p-value
|
| Age (years; mean ± SD) |
48.5 ± 13 |
50.6 ± 13 |
0.62†
|
48.3 ± 13.6 |
55.3 ± 9.3 |
0.27†
|
| Male gender (n; % of group) |
19 (54) |
15 (65) |
0.43‡
|
28 (57) |
6 (67) |
0.72‡
|
Body weight
(kg; mean ± SD) |
78 ± 20 |
87 ± 26 |
0.13†
|
80.7 ± 24.2 |
87.8 ± 16.7 |
0.13†
|
| Height (cm; mean ± SD) |
170 ± 9 |
171 ± 7 |
0.56†
|
170 ± 9 |
175 ± 7 |
0.08†
|
| Body mass index (kg/m2; mean ± SD) |
27.1 ± 5.5 |
29.6 ± 9 |
0.28†
|
28 ± 7.7 |
28.6 ± 4.2 |
0.38†
|
Differences between patients with vvECMO versus vaECMO and primary versus secondary transport are shown in tables 4A and 4B
. Duration of ECMO therapy (median 8 days (1–49)) and ICU stay (median 13 days (1–101)) were comparable for both type of ECMO and type of transport. Hospital stay (median 17 days (1–122)) was also similar in vvECMO and vaECMO, but significantly longer after primary transfer than after secondary transport (19 days (1–122) versus 7 days (1–35), p = 0.048)). Median follow-up after ECMO implant was 58 days (1–1441). This was significantly longer after vvECMO than after vaECMO, and also significantly longer after primary transport than after secondary transport (Tables 4A/B). There was no significant difference between vvECMO and vaECMO regarding the need for an ECMO upgrade, but adjustments of the initial cannulation were significantly more frequent after vaECMO than after vvECMO (n = 6 (26%) versus n = 2 (6%), p = 0.048). Weaning from ECMO was possible in 71% of patients, with a significant difference between vvECMO (83%) and vaECMO (52%) (p = 0.041). The weaning rate after primary transfer was 80% compared to 22% after secondary transfer (p = 0.002). Survival during transport was 100%. Survival to discharge was 69% in the total cohort. It was 83% in vvECMO compared to 48% in vaECMO (p = 0.008), and 80% after primary transport versus 11% after secondary transport (p<0.001). Overall survival was 67%, with a rate of 83% in vvECMO versus 43% in vaECMO (p = 0.001) and 78% after primary transfer versus 11% after secondary transfer (p <0.001). One patient died after hospital discharge due to cerebral haemorrhage on oral anticoagulation, while all other (n = 18) documented deaths occurred during hospitalisation (multi-organ failure (n = 13), respiratory failure (n = 2) and cerebral ischa emia after resuscitation (n = 3)). Completeness of follow-up after hospital discharge was 88% (35 patients).
Table 4A Follow-up data by type of extracorporeal membrane oxygenation (ECMO).
| |
Total
n = 58
|
VV ECMO
n = 35
|
VA ECMO*
n = 23
|
p-value
|
Days on ECMO
(median (min, max)) |
8 (<1, 49) |
7 (<1, 49) |
8 (<1, 35) |
0.82†
|
Days on ICU
(median (min, max)) |
13 (<1, 101) |
14, <1, 101) |
12 (<1, 41) |
0.15†
|
Days in hospital
(median (min, max)) |
17 (<1, 122) |
24 (<1, 115) |
15 (<1, 122) |
0.15†
|
Days of follow-up
(median (min, max)) |
58 (<1, 1441) |
127 (<1, 1441) |
19 (<1, 790) |
0.01†
|
Upgrading of ECMO
(n; % of group) |
9 (16) |
5 (14) |
4 (17) |
1.0‡
|
Cannula change ECMO
(n; % of group) |
8 (14) |
2 (6) |
6 (26) |
0.048‡
|
Weaning from ECMO
(n; % of group) |
41 (71) |
29 (83) |
12 (52) |
0.041‡
|
Survival during transport
(n; % of group) |
58 (100) |
35 (100) |
23 (100) |
– |
Survival to hospital discharge
(n; % of group) |
40 (69) |
29 (83) |
11 (48) |
0.008‡
|
Overall survival
(n; % of group) |
39 (67) |
29 (83) |
10 (43) |
0.001‡
|
Table 4B Follow-up data by type of transport.
| |
Total
n = 58
|
Primary transport
n = 49
|
Secondary transport
n = 9
|
p-value
|
Days on ECMO
(median (min, max)) |
8 (<1, 49) |
8 (<1, 49) |
7 (1, 35) |
0.54*
|
Days on ICU
(median (min, max)) |
13 (<1, 101) |
15 (<1, 101) |
7 (1, 35) |
0.1*
|
Days in hospital
(median (min, max)) |
17 (<1, 122) |
19 (<1, 122) |
7 (1, 35) |
0.048*
|
Days of follow-up
(median (min, max)) |
58 (<1, 1441) |
79 (<1, 1441) |
7 (1, 790) |
0.01*
|
Upgrading of ECMO
(n; % of group) |
9 (16) |
8 (16) |
1 (11) |
1.0†
|
Cannula change ECMO
(n; % of group) |
8 (14) |
6 (12) |
2 (22) |
0.6†
|
Weaning from ECMO
(n; % of group) |
41 (71) |
39 (80) |
2 (22) |
0.002†
|
Survival during transport
(n; % of group) |
58 (100) |
49 (100) |
9 (100) |
– |
Survival to hospital discharge
(n; % of group) |
40 (69) |
39 (80) |
1 (11) |
<0.001†
|
Overall survival
(n; % of group) |
39 (67) |
38 (78) |
1 (11) |
<0.001†
|
Outcomes of ECMO therapy are displayed in table 5. ECMO served as a bridge to recovery in 38 (66%) patients and to a ventricular assist device (VAD) in two (3%) patients. One of the VAD patients died 25 days after discharge due to cerebral bleeding under oral anticoagulation. Another patient was bridged to salvage cardiac surgery. After resuscitation in the peripheral coronary angiography laboratory and stenting of the right coronary artery, vaECMO was implanted, followed by primary transfer to our hospital. After emergency mitral valve replacement upon arrival due to acute mitral regurgitation in ischemic papillary muscle rupture, the patient died after surgery due to multi-organ failure.
Table 5 Outcomes of ECMO treatment in all patients.
| Total number of patients (n) |
58 |
| Bridge to recovery (n; %) |
38 (66%) |
| Bridge to ventricular assist device (n; %)*
|
2 (3%) |
| Bridge to salvage surgery (n; %)†
|
1 (2%) |
| Death‡ (n; %) |
17 (29%) |
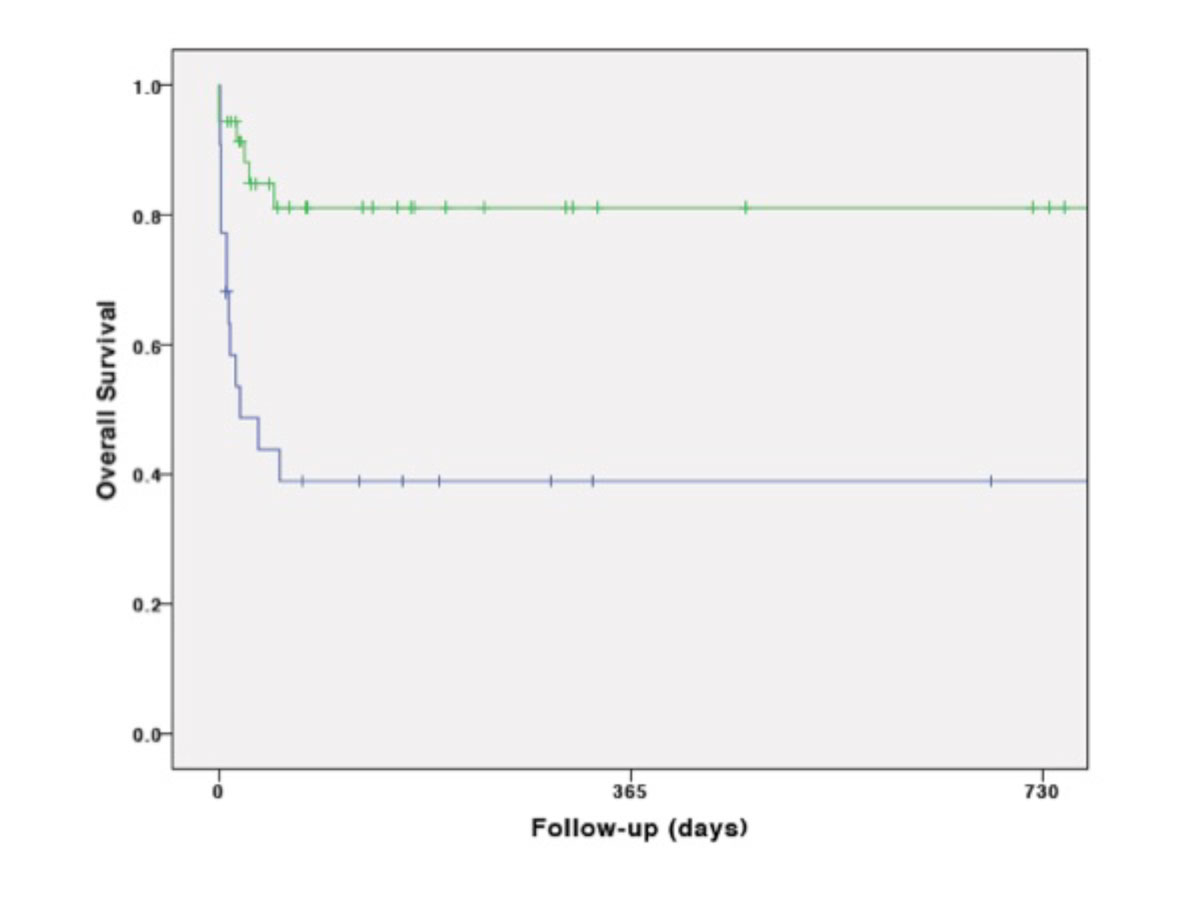
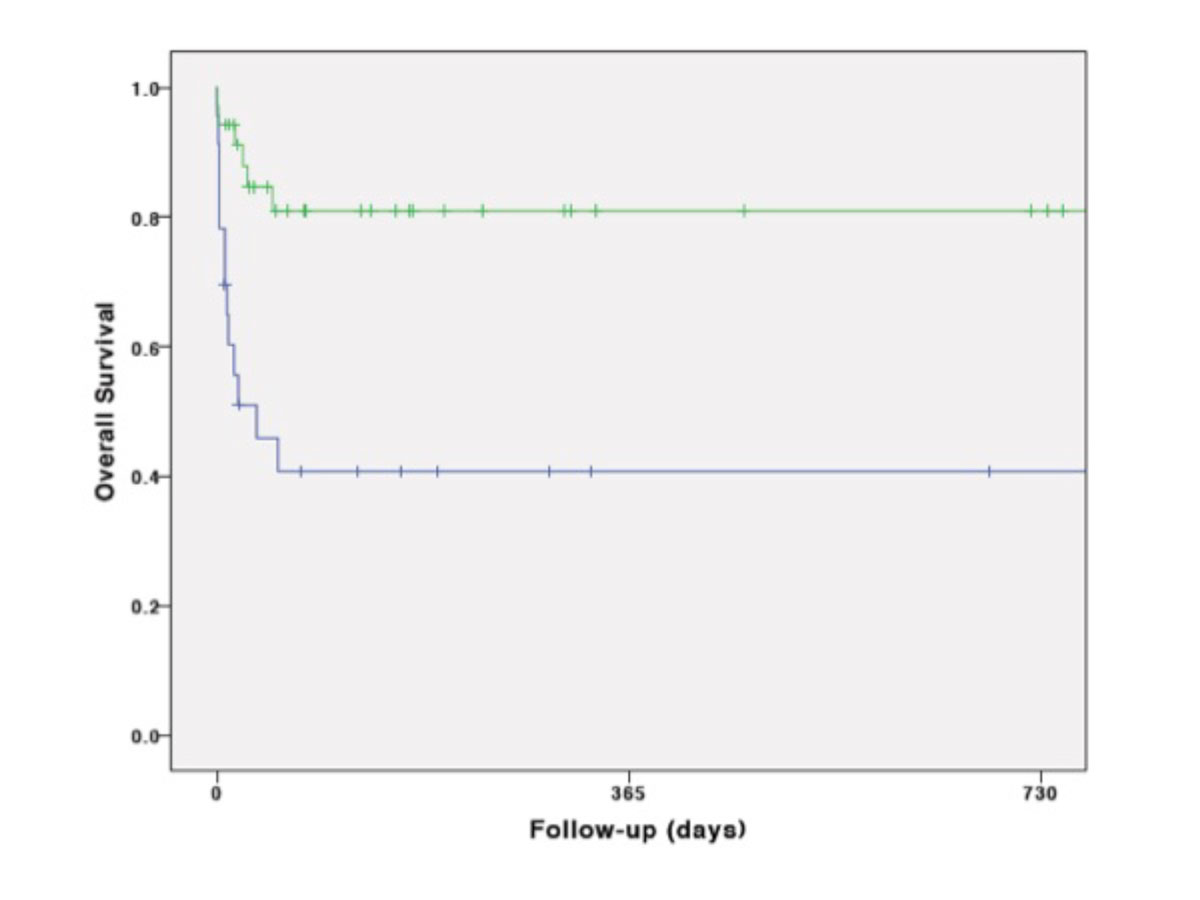
Kaplan-Meier cumulative survival was mainly affected by the first days after ECMO transportation, during hospitalisation (fig. 2A). It was significantly lower in CS than in ARDS (p = 0.001) fig. 2B), in vaECMO than in vvECMO (p = 0.001) (fig. 2C) and in secondary than in primary transport (p <0.001) (fig. 2D). However, survival was not significantly influenced by means of transportation (p = 0.75) (fig. 2E).
Discussion
The principle of transporting critically ill patients to a tertiary care centre with a highly specialised intensive care unit and a broad experience in ECMO treatment has been recently demonstrated to reduce mortality [2, 3, 10, 11, 16, 17]. The transfer of a haemodynamically or respiratorily unstable patient without extracorporeal circulatory support may be an incalculable risk. It can be reduced by ECMO implementation on-site at the referring hospital and subsequent patient transport on ECMO. Several authors have reported a very low on-transport mortality of patients transported on ECMO, and a similar overall mortality compared to patients receiving an ECMO at the ECMO centre. This supports the concept of an ECMO transportation programme [1, 3, 10, 11].
Based on the current literature and the data of the international ECMO registry, the ELSO published “Guidelines for ECMO Transport” addressing standardised aspects of medical personnel, equipment, anticoagulation and transport logistics (www.elso.org: “Guidelines for ECMO Transport”). The organisation of our ECMO transport team and equipment is in accordance with those recommendations. For in-centre ECMO implantation, the International ECMO Network has recommended a minimum of 20 ECMO implantations per year for acute respiratory failure [18]. An analysis of the ELSO registry identified a volume of 30 annual ECMO implantations per centre to be associated with reduced mortality [19]. However, no such caseloads have been defined in ECMO transportation so far. In 2016, we have reached numbers of ECMO transportation which ECMO network and ECMO registry recommend for in-centre ECMO implantation.
Our overall outcomes reflect a successful implementation of the ECMO transportation programme at our institution, with a 100% implant and on-transport survival, a 100% absence of on-site or on-transport technical and clinical complications, a 69% survival to discharge rate and a 67% overall survival rate. These results are in line with the recently published data from larger cohorts and reviews summarising smaller collectives, which describe a survival to discharge rate of approximately 61–63% for all transported ECMO patients (vv and va) [1–3, 10, 11]. This does not differ much from the survival of ECMO patients without transfer of 58%, published by the ELSO (www.elso.org: ECLS Registry Report 2016).
Veno-venous ECMO resulted in a significantly better weaning rate, survival to discharge rate and overall survival rate compared to vaECMO. These findings are comparable to the literature and reflect the underlying diagnosis, with a significantly higher risk in patients with cardiac or combined failure compared to patients with stand-alone respiratory failure [1–3, 10, 11]. The higher rate of cannulation change in patients on vaECMO compared to patients on vvECMO is explained by the fact that downgrading from vaECMO to vvECMO was included, which was part of the weaning process in some vaECMO patients.
The significantly shorter hospital stay and follow-up of patients after secondary transfer compared to after primary transport is because of the low survival to discharge rate in this group. All secondary transport patients were on vaECMO which, as we have shown, was associated with a less favourable prognosis and may explain, at least partly, the poor outcome in this subgroup.
Some deaths prior to transport have been described, but the exact number of patients that were lost due to the late arrival of the ECMO team are lacking and are most probably underestimated [1]. Broman et al. identified 13 ECMO candidates in their own cohort within a period of four years who died before ECMO was installed, indicating the problem of delays and the importance of immediate alert by the referring hospital in the case of haemodynamic or respiratory deterioration [1]. This emphasises the utmost importance of peripheral hospitals contacting the ECMO centre early.
While some rare cases of death during transport have been previously described, there were no deaths during implant or transport in our cohort [1, 3, 10, 11]. The absence of technical or clinical complications during implant and transport compares favourably to the data in the literature [1, 11, 20, 21]. The incidence of on-transport complications has been reported to be as high as 31.7% [20]. However, such studies also documented adverse events not directly related to the patient or the ECMO which might explain the difference [20, 21]. The most frequently described complications are patient-related, such as loss of tidal volume, followed by staff-related complications and technical and vehicle malfunctions.
The appropriate choice of ECMO type may prevent fatal events during transportation. Patients with acute respiratory failure and borderline haemodynamic stability should be considered for straight vaECMO implantation. Broman et al. cite two cases of patients taken on vvECMO who died during transport due to acute cardiac failure [2, 11]. Thus, two of our patients with a primarily respiratory failure were transported on vaECMO upon the decision of the ECMO team due to an expected risk of on-transport cardiac deterioration.
A variety of technical equipment and systems have been tested for feasibility and safety in various studies [21–24]. The miniaturisation of systems has certainly facilitated the transport of patients on ECMO. Maintenance and constant updates to the current technical standards must be approved by the team of perfusionists. Fast and safe transportation is a key factor for success. Both were professionally provided by REGA and “Schutz und Rettung Zürich” which supplied us with a helicopter and ambulance, respectively. Paramedics from both institutions developed a custom-made fixation plate for the ECMO together with the perfusionists to avoid dislocation during transfer.
Percutaneous cannulation at a peripheral institution is a particular challenge. The cardiac surgeon performing the procedure is confronted with an unfamiliar environment and unknown staff to work with, although, in our experience, the personnel in other hospitals are always very helpful. Thus, the implantation can only be successful if the operator is well trained, equipped with sufficient interventional skills and capable of solving potential problems by eventual surgical cut-down. The early implant of a peripheral perfusion cannula into the femoral artery distally to the afferent ECMO cannula is crucial to avoid peripheral limb ischaemia. Due to haemodynamic instability, an implant prior to ECMO cannulation is not always feasible. After arrival at the tertiary care centre at the latest, a peripheral perfusion cannula should be installed under duplex sonography or fluoroscopy.
Conclusions
The first results of the largest ECMO transportation programme in Switzerland show its feasibility, safety and efficacy, without on-site implant or on-transport complications or mortality. The outcomes of patients on vvECMO and vaECMO are at least as good as in patients who receive the ECMO directly in the specialised ECMO centre without the need for transportation. This may justify the enormous level of logistics, expenditure and manpower required. Primary care centres are invited to use the option of on-site ECMO implantation with subsequent transportation to a specialised ECMO centre in patients with critical acute cardiac or respiratory failure. With rising awareness, referring centres will begin to consider this service at an early stage when the patient’s condition still leaves all therapeutic options open. Early communication will facilitate logistic organisation and accelerate the onset of ECMO treatment.
Acknowledgements
We kindly thank Prof. B. Seifert, Statistician, Institute of Biostatistics, University of Zurich, Zurich, Switzerland for his independent statistical analysis. He chose to refrain from co-authorship.
Author contributions
MJW and DTI contributed equally to the present study and publication.
References
1
Broman
LM
,
Frenckner
B
. Transportation of Critically Ill Patients on Extracorporeal Membrane Oxygenation. Front Pediatr. 2016;4:63. doi:.https://doi.org/10.3389/fped.2016.00063
2
Broman
LM
,
Holzgraefe
B
,
Palmér
K
,
Frenckner
B
. The Stockholm experience: interhospital transports on extracorporeal membrane oxygenation. Crit Care. 2015;19(1):278. doi:.https://doi.org/10.1186/s13054-015-0994-6
3
Mendes
PV
,
de Albuquerque Gallo
C
,
Besen
BAMP
,
Hirota
AS
,
de Oliveira Nardi
R
,
Dos Santos
EV
, et al.
Transportation of patients on extracorporeal membrane oxygenation: a tertiary medical center experience and systematic review of the literature. Ann Intensive Care. 2017;7(1):14. doi:.https://doi.org/10.1186/s13613-016-0232-7
4
Bartlett
RH
,
Gazzaniga
AB
,
Fong
SW
,
Jefferies
MR
,
Roohk
HV
,
Haiduc
N
. Extracorporeal membrane oxygenator support for cardiopulmonary failure. Experience in 28 cases. J Thorac Cardiovasc Surg. 1977;73(3):375–86.
5
Abrams
D
,
Combes
A
,
Brodie
D
. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol. 2014;63(25, 25 Pt A):2769–78. doi:.https://doi.org/10.1016/j.jacc.2014.03.046
6
Boedy
RF
,
Howell
CG
,
Kanto
WP, Jr
. Hidden mortality rate associated with extracorporeal membrane oxygenation. J Pediatr. 1990;117(3):462–4. doi:.https://doi.org/10.1016/S0022-3476(05)81098-4
7
Brodie
D
,
Bacchetta
M
. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365(20):1905–14. doi:.https://doi.org/10.1056/NEJMct1103720
8
Fromm
RE, Jr
,
Dellinger
RP
. Transport of critically ill patients. J Intensive Care Med. 1992;7(5):223–33. doi:.https://doi.org/10.1177/088506669200700503
9
Peek
GJ
,
Mugford
M
,
Tiruvoipati
R
,
Wilson
A
,
Allen
E
,
Thalanany
MM
, et al.; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–63. doi:.https://doi.org/10.1016/S0140-6736(09)61069-2
10
Biscotti
M
,
Agerstrand
C
,
Abrams
D
,
Ginsburg
M
,
Sonett
J
,
Mongero
L
, et al.
One Hundred Transports on Extracorporeal Support to an Extracorporeal Membrane Oxygenation Center. Ann Thorac Surg. 2015;100(1):34–9, discussion 39–40. doi:.https://doi.org/10.1016/j.athoracsur.2015.02.037
11
Bryner
B
,
Cooley
E
,
Copenhaver
W
,
Brierley
K
,
Teman
N
,
Landis
D
, et al.
Two decades’ experience with interfacility transport on extracorporeal membrane oxygenation. Ann Thorac Surg. 2014;98(4):1363–70. doi:.https://doi.org/10.1016/j.athoracsur.2014.06.025
12
Carter
JM
,
Gerstmann
DR
,
Clark
RH
,
Snyder
G
,
Cornish
JD
,
Null
DM, Jr
, et al.
High-frequency oscillatory ventilation and extracorporeal membrane oxygenation for the treatment of acute neonatal respiratory failure. Pediatrics. 1990;85(2):159–64.
13
Cornish
JD
,
Carter
JM
,
Gerstmann
DR
,
Null
DM, Jr
. Extracorporeal membrane oxygenation as a means of stabilizing and transporting high risk neonates. ASAIO Trans. 1991;37(4):564–8.
14
Bagdure
D
,
Curtis
DJ
,
Dobyns
E
,
Glodé
MP
,
Dominguez
SR
. Hospitalized children with 2009 pandemic influenza A (H1N1): comparison to seasonal influenza and risk factors for admission to the ICU. PLoS One. 2010;5(12):e15173. doi:.https://doi.org/10.1371/journal.pone.0015173
15
Bai
L
,
Gu
L
,
Cao
B
,
Zhai
XL
,
Lu
M
,
Lu
Y
, et al.
Clinical features of pneumonia caused by 2009 influenza A(H1N1) virus in Beijing, China. Chest. 2011;139(5):1156–64. doi:.https://doi.org/10.1378/chest.10-1036
16
Clement
KC
,
Fiser
RT
,
Fiser
WP
,
Chipman
CW
,
Taylor
BJ
,
Heulitt
MJ
, et al.
Single-institution experience with interhospital extracorporeal membrane oxygenation transport: A descriptive study. Pediatr Crit Care Med. 2010;11(4):509–13.
17
Starck
CT
,
Hasenclever
P
,
Falk
V
,
Wilhelm
MJ
. Interhospital transfer of seriously sick ARDS patients using veno-venous Extracorporeal Membrane Oxygenation (ECMO): Concept of an ECMO transport team. Int J Crit Illn Inj Sci. 2013;3(1):46–50. doi:.https://doi.org/10.4103/2229-5151.109420
18
Combes
A
,
Brodie
D
,
Bartlett
R
,
Brochard
L
,
Brower
R
,
Conrad
S
, et al.; International ECMO Network (ECMONet). Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190(5):488–96. doi:.https://doi.org/10.1164/rccm.201404-0630CP
19
Barbaro
RP
,
Odetola
FO
,
Kidwell
KM
,
Paden
ML
,
Bartlett
RH
,
Davis
MM
, et al.; Analysis of the Extracorporeal Life Support Organization Registry. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191(8):894–901. doi:.https://doi.org/10.1164/rccm.201409-1634OC
20
Ericsson
A
,
Frenckner
B
,
Broman
LM
. Adverse Events during Inter-Hospital Transports on Extracorporeal Membrane Oxygenation. Prehosp Emerg Care. 2017;21(4):448–55. doi:.https://doi.org/10.1080/10903127.2017.1282561
21
Raspé
C
,
Rückert
F
,
Metz
D
,
Hofmann
B
,
Neitzel
T
,
Stiller
M
, et al.
Inter-hospital transfer of ECMO-assisted patients with a portable miniaturized ECMO device: 4 years of experience. Perfusion. 2015;30(1):52–9. doi:.https://doi.org/10.1177/0267659114531611
22
Javidfar
J
,
Brodie
D
,
Takayama
H
,
Mongero
L
,
Zwischenberger
J
,
Sonett
J
, et al.
Safe transport of critically ill adult patients on extracorporeal membrane oxygenation support to a regional extracorporeal membrane oxygenation center. ASAIO J. 2011;57(5):421–5. doi:.https://doi.org/10.1097/MAT.0b013e3182238b55
23
Lucchini
A
,
De Felippis
C
,
Elli
S
,
Gariboldi
R
,
Vimercati
S
,
Tundo
P
, et al.
Mobile ECMO team for inter-hospital transportation of patients with ARDS: a retrospective case series. Heart Lung Vessel. 2014;6(4):262–73.
24
Roncon-Albuquerque
R, Jr
,
Basílio
C
,
Figueiredo
P
,
Silva
S
,
Mergulhão
P
,
Alves
C
, et al.
Portable miniaturized extracorporeal membrane oxygenation systems for H1N1-related severe acute respiratory distress syndrome: a case series. J Crit Care. 2012;27(5):454–63. doi:.https://doi.org/10.1016/j.jcrc.2012.01.008