Tissue engineering for paediatric patients
DOI: https://doi.org/10.4414/smw.2019.20032
Marcus
Mummeab, Anke
Wixmertena, Sylvie
Miota, Andrea
Barberoa, Alexandre
Kaempfenac, Franziska
Saxera, Sebastian
Gehmertb, Andreas
Kriegb, Dirk J.
Schaefera, Marcel
Jakoba, Ivan
Martina
aDepartment of Surgery and Department of Biomedicine, University Hospital Basel, University of Basel, Switzerland
bOrthopaedic Unit, University Children’s Hospital, Basel, Switzerland
cPaediatric Surgery Unit, University Children’s Hospital, Basel, Switzerland
Summary
The effects of oncological treatment, congenital anomalies, traumatic injuries and post-infection damage critically require sufficient amounts of tissue for structural and functional surgical reconstructions. The patient’s own body is typically the gold standard source of transplant material, but in children autologous tissue is available only in small quantities and with severe morbidity at donor sites. Engineering of tissue grafts starting from a small amount of autologous material, combined with suitable surgical manipulation of the recipient site, is expected to enhance child and adolescent health, and to offer functional restoration for long-term wellbeing. Moreover, engineered tissues based on patient-derived cells represent invaluable models to investigate mechanisms of disease and to develop/test novel therapeutic approaches. In view of these great opportunities, here we introduce the currently limited successful implementation of tissue engineering in paediatric settings and discuss the open challenges in the field. A particular focus is on the specific needs and envisioned strategies in the areas of bone and osteochondral regeneration in children.
Introduction
The field of tissue engineering, originally conceptualised about 25 years ago [1, 2], has resulted in some pioneering examples of clinical success, but has clearly not lived up to initial expectations. This can be attributed to a variety of challenges, rooted in the complexity of processes that need to be understood and controlled in tissue/organ regeneration [3].
Implementation of tissue engineering paradigms in the context of a paediatric population is particularly relevant because of the critical need for transplant material and the small amount of accessible autologous graft material, which is available only at the expense of significant morbidity at donor sites. The natural propensity to healing in younger individuals represents an opportunity for tissue engineering strategies. This has led to a few relevant reports of engineered tissue transplantation in some clinical settings, as detailed below.
Engineered urethral tissues have been used for hypospadias, a genitourinary congenital malformation [4]. Authors have reported variable degrees of success, as well as the importance of refining strategies in order to enable the natural growth of penile size at puberty. An engineered autologous vagina was implanted in four adolescent patients with vaginal aplasia caused by Mayer-Rokitansky-Küster-Hauser syndrome, with successful structural and functional results in follow-up assessments up to 8 years later [5]. Ear-shaped engineered cartilage tissues successful auricular reconstruction was shown in five patients with microtia, despite the need to define a standard surgical procedure [6]. For the regeneration of epidermis after burn injuries, sheets with cultured autologous keratinocytes (e.g. Epicel®) have been introduced [7], but the major challenge of a complete dermo-epidermal reconstruction remains. The combination of tissue engineering and gene therapy led to the regeneration of the entire human epidermis in a seven-year-old boy with junctional epidermolysis bullosa, a genetic disease, by means of autologous transgenic keratinocytes [8]. Further approaches to engineering tissues for the treatment of congenital cardiovascular abnormalities [9], short bowel syndrome [10] and tracheal replacement [11], or to recover motor/sensory nerve function [12] are highly promising, though they are not yet in clinical use beyond single case studies.
Apart from the opportunities offered and the encouraging results, paediatric patients present a set of specific scientific and technical challenges to successful tissue engineering, which have so far slowed down progress in this promising field. For example, the amount of starting material for a pool of suitable autologous cells is often extremely limited. Moreover, the ongoing structural (e.g., due to physical growth), biological (e.g., due to hormonal changes) and psychological development of a child means that the patient is never in a steady state. Additional challenges in the planning and execution of clinical trials in paediatric cases are related to the, correctly, rigorous ethical constraints needed to guarantee safety of the patients. The number of cases that can be treated for specific indications, especially for congenital diseases, is also generally limited, and it is difficult to derive from trials with small cohorts the general conclusions that are required to warrant introduction of novel procedures in routine clinical practice.
In this review, we first introduce regulatory considerations for clinical trials using engineered tissues in children and then discuss clinical needs and envisioned strategies in the specific context of paediatric tissue engineering for bone and osteochondral regeneration.
Regulatory landscape for use of tissue engineered products in paediatric populations
Children are considered a vulnerable population and special legal requirements are laid down in the directive for clinical trials 2001/20/EC, with additional regulations such as ICH guideline E11 (Clinical Investigations of Medicinal products in the Pediatric Population). These measures protect children from unjustified risk in clinical trials, and participants in clinical studies are expected to potentially benefit. Purely pharmacokinetic studies are not allowed in healthy children and, whenever appropriate, data on safety need to be taken from adult exposure. Moreover, depending on the disease, paediatric studies are often initiated in the later stages of the clinical development of medicines (e.g., after phase II in adults).
As a consequence, generally about 50–90% of medications used to treat children are used off-label, based on results from studies in adults. However, since “children are not small adults” [13], off-label use could potentially lead to increased risk of inefficacy or adverse reactions [14, 15]. Even though it is often questioned whether paediatric clinical trials are ethical, they are crucial to provide safe and high quality medicine for children.
There are many reasons for the low number of clinical studies in the paediatric population, including ethical, legal, economic, social and organisational challenges. The economic incentive for companies to perform paediatric trials, with high costs for a small market, is low. There is often a lack of a suitable infrastructure and competence for conducting paediatric clinical trials [16, 17]. To provide more support for paediatric trials, several national and EU networks have been established over the years (Enpr-EMA, SwissPedNet, PAED-Net, MCRN etc.). This avoids unnecessary replication of studies and fosters high-quality, ethical research on the quality, safety and efficacy of medications used in children.
Because of all these challenges, it became evident that market forces alone are insufficient to stimulate adequate research, development and authorisation of medicinal products for the paediatric population. To fill this lack of evidence and availability of authorised medicines for children, the Pediatric Regulation (EC) No 1901/2006 has been adopted, with the goals (i) “to encourage and enable high-quality research into the development of medicines for children”, (ii) “to ensure, over time, that most medicines used by children are specifically authorized for such use with age-appropriate forms and formulations”, and (iii) “to increase the availability of high-quality information about medicines used by children.” The new Pediatric Regulation resulted in an increase of trials including paediatric cases from 8.25% in 2007 to 12.4% in 2016 (EudraCT database) and in the successful authorisation of over 260 new medicines for use in children [14].
Beyond the well-established bone marrow transplantation using haematopoietic stem cells, cellular therapies are typically classified (according to the EC Regulation No 1394/2007 on advanced therapies) as Advanced Therapy Medicinal Products (ATMPs). In this context, it is worth mentioning approval of Prochymal, allogeneic mesenchymal stromal/stem cells for the treatment of graft versus host disease (GvHD). Interestingly, no overall superiority of Prochymal was demonstrated in a phase III placebo-controlled trial [18]. However, a stratification of patients by age indicated that the primary outcome, remission after 28 days, was significantly increased in paediatric GvHD [19]. These findings emphasise that also for ATMPs safety and efficacy cannot be extrapolated from adult data, but need to be specifically tested and established for children.
In the even more restricted area of tissue engineering, currently only two products are authorised by the European Medicines Agency (EMA), namely Holoclar (conditional approval and orphan designation received in 2015) and Spherox (approval obtained in 2017). Holoclar (Holostem Terapie Avanzate S.r.l., Modena/Italy), consisting of a patient’s own limbal cells grown in a laboratory, is indicated for ocular burns. There is no information on the safety of Holoclar in children up to 7 years old and only limited information in patients 8 to 17 years old. Although the safety and efficacy have not yet been established for paediatric patients, in studies HLSTM01 and HLSTM02, which included three children (aged 13, 14 and 16 years) and two children (aged 8 and 14 years), respectively, the profile of adverse reactions was not different from that in the adult population. The EMA has deferred the obligation to submit the results of studies with Holoclar in one or more subsets of the paediatric population. A phase IV clinical trial is ongoing, with five patients between 2 and 17 years old planned.
Spherox (Co.don AG, Berlin/Germany), consisting of spheroids of human autologous matrix-associated chondrocytes, is currently the only available tissue engineered product for articular cartilage repair with market authorisation by EMA. Nonetheless, safety and efficacy of Spherox have not been established in patients aged less than 18 years and open physis is defined as contraindication. Furthermore, the positive recommendation for reimbursement by the UK National Institute for Health and Care Excellence (NICE) has only been granted for adult patients, thus the cost benefit ratio is still unclear for children. An investigation plan to evaluate safety and efficacy in paediatric patients is ongoing.
It is important to mention that when a medical device is combined with tissue engineering technologies, it is considered to be a combined ATMP. In these cases, the grafts have to comply with both ATMP and medical device regulations.
In order to develop suitable tissue engineered products for paediatric use, we advocate the importance of starting by targeting specific paediatric unmet clinical needs, which should lead to the envisioning of possible new solutions to improve treatment possibilities. This path will be proposed in the next section in the context of bone and osteochondral tissue engineering.
Targeting unmet clinical needs in paediatric patients
Bone regeneration
Repair of critical bone defects generally requires consideration of an interplay of different actors and factors, namely the presence of bone forming cells, the availability of osteogenic cytokines, the provision of vascularity, and support by a guiding structure that is compatible with mechanical stability. In paediatric cases, some additional factors and challenges need to be considered. In this situation, the skeleton is still developing and therefore any strategy should allow continued increase in size, with an appropriate interface with connected soft tissues and adaptation to evolving mechanical requirements.
Treatment of congenital cleft lip and palate illustrates these challenges. This congenital fusion defect of the maxilla has a major impact on the affected children. It impairs feeding, speech development, dentition, facial aesthetics and thereby psychosocial development. The aim of treatment, apart from closing the lip, is to close the connection between oro- and nasopharyngeal cavities and to restore the upper jaw. The latter requires a significant amount of adequately shaped bone with considerable mechanical resilience and the ability to grow. Autologous bone grafts are associated with complications and a notable failure rate, and are therefore often only used before final dentition at the age of seven or eight. The combination of cells and materials in a tissue engineering approach has been planned and tested in a variety of configurations [20]. These include the use of different sources of stem and progenitor cells, of scaffolds functioning also as barrier membranes for guiding the soft tissue interface, and of growth factors, typically in the form of platelet-rich plasma. The plethora of possible combinations and the difficulty of running well-powered randomised controlled trials in this indication, however, have resulted in a lack of evidence despite promising results in single patients [21].
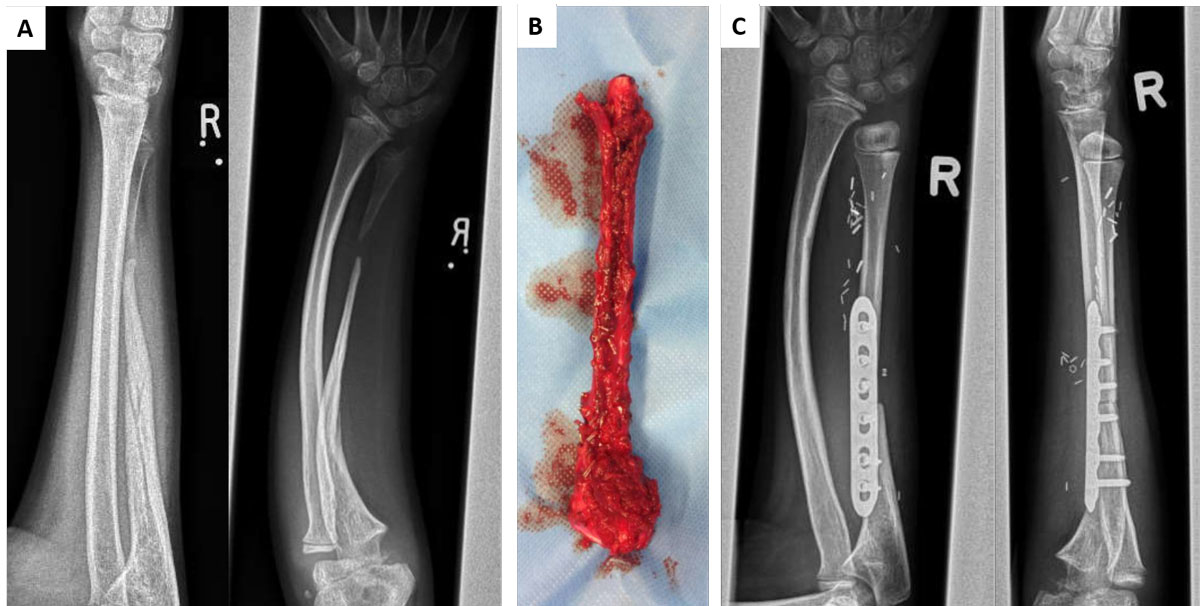
Defects in long bones also represent an unmet clinical need in children. These can be the consequence of cancer surgery, of congenital pseudoarthrosis of the tibia [22, 23] or abnormal bone mineralisation in genetic diseases such as neurofibromatosis. Even bagatelle traumas can result in pseudoarthrosis, with the need for bony reconstruction in an appropriate size. To date, autologous bone grafts harvested from another region of the body, such as the fibula, represent the state of the art (fig. 1). Depending on the size of the defect and, accordingly, the amount of graft material required, either donor site morbidity increases or the autologous bone available is not sufficient to treat defects with a reasonable risk-benefit ratio. For these high-risk bone defects, implantation of osteoprogenitor cells from various sources in combination with different materials has been tested by several groups [24, 25]. These approaches have been reported to be successful in a number of cases, though mechanical stability remains an important challenge.
Depending on the patient’s age, the maturity of tissue is of relevance and thus, for example, solutions need to be compatible with the presence of hypertrophic cartilage. This is well illustrated by the challenge of physeal fractures. Growth of long bones usually occurs at both extremities in a region called the epiphysis. This is a highly structured complex zone composed of cartilaginous cells. The maturity of these chondrocytes increases towards the end of the bone from a proliferative to a hypertrophic, a calcifying, and finally an ossifying layer as part of the enchondral ossification process. Disturbance of this structure, such as in the context of fractures, can result in premature growth stop. Depending on the fracture morphology, this leads to complete growth arrest or asymmetric growth, both with disabling consequences. For this indication, osteoprogenitor cells or chondrocytes, as well as the modulation of specific pathways, are currently being evaluated in animal models, but clinical applications are not yet consolidated [26].
In a nutshell, the promise for bone tissue engineering in paediatric patients is immense. However, given the high degree of individualisation necessary, the current literature cannot yet provide standard approaches or high-level evidence on specific procedures.
Osteochondral/joint reconstruction
Large defects involving cartilage/bone interfaces represent a severe clinical challenge in children. Reconstruction with free vascularised epiphyseal transfers, from the proximal fibula, for example, has been well described [27], but the surgery is complex and donor site morbidity problematic.
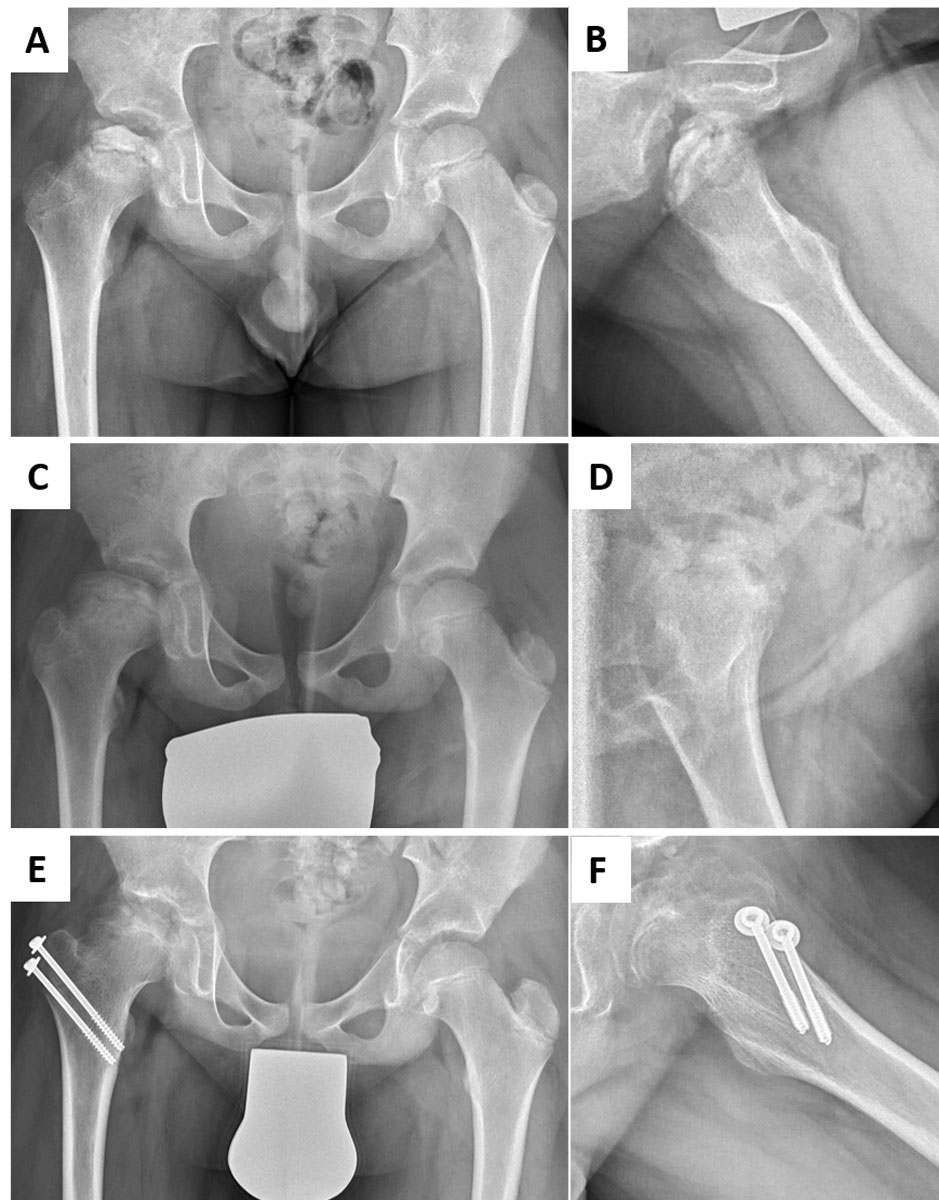
Osteochondral defects of the lower extremity can impact children’s mobility, and participation in leisure activities and sports in particular. Legg-Calvé-Perthes is a typical paediatric disease in this category, with the peak onset of symptoms between 5 and 10 years of age. The exact aetiology remains elusive, but compromised vascularisation and repetitive mechanical stress, amongst others, are discussed as potential causes. The disease can lead to subchondral fractures of variable severity, and eventually to complete destruction of the femoral head [13, 28]. Affected children are characterised by a shortened leg and functional impairment with limping. Moreover, incongruent articular surfaces at skeletal maturity are strongly associated with premature osteoarthritis of the hip joint [29]. Existing treatment modalities depend on the patient’s age and the stage of the disease. In younger patients or mild cases, observation, bed rest or medication with anti-inflammatory compounds, bisphosphonates or bone morphogenetic proteins have been used with success. In more severe cases, surgical treatment is necessary, with greater trochanteric epiphysiodesis to compensate for loss of epiphyseal height (fig. 2), or derotational osteotomy of the femur or osteotomy of the acetabulum, with the aim to protect the weak fragmented head from deformity [30]. Reconstruction of such osteochondral defects with tissue engineered grafts could restore articular congruence and delay or even avoid early osteoarthritic changes. However, to date no reports of the implantation of engineered osteochondral composite solutions have been identified.
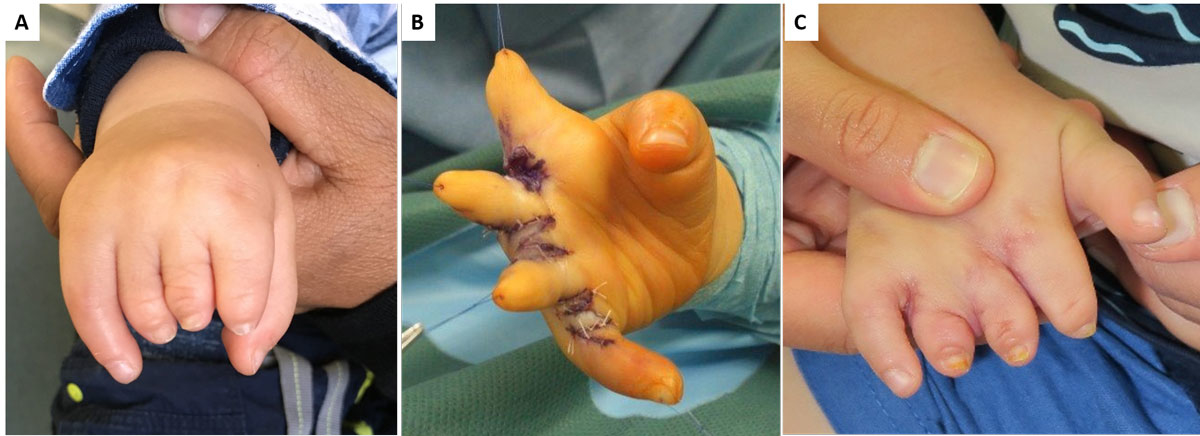
Defects in the upper extremity are often less dramatic as children adapt quickly and learn to deal with a deficiency much faster than adults do. However, if bilateral use of the hands is impaired, intervention is needed. For defects related to avascular necrosis in the hand (e.g., Kienbock, Preiser, or Dietrich disease) the same perspectives apply as for the lower extremity described above (e.g., Perthes disease). Different considerations apply for congenital malformations of the hand and upper extremity. These are present in about 1 in 200 to 1 in 5000 live births, depending on severity grading and geographic region. In about 60 to 80% of cases, these children do not have any associated syndromes. Surgery for congenital hand defects typically employs autologous tissue to improve hand function. However, many families will not agree to “mutilate” another part of the body in an already imperfect individual. Reconstruction without a donor site is therefore important. This dilemma is well illustrated in cases of symbrachydactyly, where the deformity involves short fingers but a full hand plate (fig. 3). Traditional treatment was transfer of a toe phalanx into the soft tissue pocket and later distraction to create digits [31]. Effects on the donor site at first seemed acceptable, but long-term results in the feet were poor [32] and the functional gain on the hand was insufficient [33]. We propose that filling the empty, well vascularised soft tissue pocket at the end of the phalanges with tissue engineered hypertrophic cartilage is a possible strategy to be developed. This approach would mimic endochondral bone growth [34] and lead to only minor differences from a non-vascularised toe phalangeal transfer. This would be the first application of tissue engineering in congenital deficiency of bone and cartilage.
Scientific and clinical perspectives
Tissue reconstruction in children in a variety of possible contingencies might greatly benefit from tissue engineering approaches, in view of (i) prevention of donor site morbidity, (ii) avoidance of implantation of synthetic substitutes that cannot grow with the individual and (iii) capitalisation on the biological potency of cells from younger individuals.
Further research on the interface between fundamental stem cell / developmental biology, material science and surgical technology is, however, necessary to the introduction of skeletal tissue engineering in children. Open challenges include selection of suitable cell sources, better understanding and control of biological processes (e.g., isolation, expansion and differentiation of stem/progenitor cells), generation of complex customised shapes (e.g., by 3D bioprinting), enhancement of robustness in manufacturing (e.g., by superior standardisation within bioreactor systems), and promotion of functional engraftment (e.g., the possibility for the graft to grow with patient age through physiological interfaces with surrounding soft tissues).
Because of the typically small patient populations, especially in orphan paediatric diseases, and the important ethical need to save children from the unnecessary risks of a trial, in silico approaches offer a useful technique through individualised computer simulation. Carlier et al. investigated, in 200 virtual subjects generated from a previously established model of murine bone regeneration, the effect of bone morphogenetic protein treatment in congenital pseudarthrosis of the tibia [35]. The potential of in silico medical technologies for tissue engineering obviously needs to be reconciled with the complexity of the processes, which can be modelled into machine learning algorithms only very approximately.
As for other clinical implementations of tissue engineering approaches [36, 37], we believe that it is important to switch from the classical view centred around the manufacture of a suitable graft, towards a more comprehensive paradigm, which can be referred to as “regenerative surgery”. The phrase captures that surgical manipulation of an engineered graft, preparation and conditioning of the environment at the recipient site and reaction of the surrounding soft tissue over time, are at least as critical as the manufactured implant and its designed functionalities [38].
Finally, the field of paediatric tissue engineering could go beyond the generation of implantable grafts. In fact, three-dimensional tissues generated using cells of children with genetic diseases (e.g., neurofibromatosis) represent invaluable advanced models for fundamental science. These would allow investigating the effect of specific genetic mutations on skeletal tissue development, growth and regeneration, possibly leading to the identification of innovative treatments, eventually bypassing the need of extensive implant material.
References
1
Langer
R
,
Vacanti
JP
. Tissue engineering. Science. 1993;260(5110):920–6. doi:.https://doi.org/10.1126/science.8493529
2
Cancedda
R
,
De Luca
M
. Tissue engineering for clinical application. Year Immunol. 1993;7:193–8. [Review].
3
Qu
D
,
Mosher
CZ
,
Boushell
MK
,
Lu
HH
. Engineering complex orthopaedic tissues via strategic biomimicry. Ann Biomed Eng. 2015;43(3):697–717. doi:.https://doi.org/10.1007/s10439-014-1190-6
4
Abbas
TO
,
Mahdi
E
,
Hasan
A
,
AlAnsari
A
,
Pennisi
CP
. Current Status of Tissue Engineering in the Management of Severe Hypospadias. Front Pediatr. 2018;5:283. doi:.https://doi.org/10.3389/fped.2017.00283
5
Raya-Rivera
AM
,
Esquiliano
D
,
Fierro-Pastrana
R
,
López-Bayghen
E
,
Valencia
P
,
Ordorica-Flores
R
, et al.
Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet. 2014;384(9940):329–36. doi:.https://doi.org/10.1016/S0140-6736(14)60542-0
6
Zhou
G
,
Jiang
H
,
Yin
Z
,
Liu
Y
,
Zhang
Q
,
Zhang
C
, et al.
In Vitro Regeneration of Patient-specific Ear-shaped Cartilage and Its First Clinical Application for Auricular Reconstruction. EBioMedicine. 2018;28:287–302. doi:.https://doi.org/10.1016/j.ebiom.2018.01.011
7Schiestl C, Stark GB. Plastische Chirurgie bei Kindern und Jugendlichen. Springer-Verlag 2007.
8
Hirsch
T
,
Rothoeft
T
,
Teig
N
,
Bauer
JW
,
Pellegrini
G
,
De Rosa
L
, et al.
Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551(7680):327–32. doi:.https://doi.org/10.1038/nature24487
9
Heydrick
S
,
Roberts
E
,
Kim
J
,
Emani
S
,
Wong
JY
. Pediatric cardiovascular grafts: historical perspective and future directions. Curr Opin Biotechnol. 2016;40:119–24. doi:.https://doi.org/10.1016/j.copbio.2016.03.013
10
Martin
LY
,
Ladd
MR
,
Werts
A
,
Sodhi
CP
,
March
JC
,
Hackam
DJ
. Tissue engineering for the treatment of short bowel syndrome in children. Pediatr Res. 2018;83(1-2):249–57. doi:.https://doi.org/10.1038/pr.2017.234
11
Hamilton
NJ
,
Kanani
M
,
Roebuck
DJ
,
Hewitt
RJ
,
Cetto
R
,
Culme-Seymour
EJ
, et al.
Tissue-Engineered Tracheal Replacement in a Child: A 4-Year Follow-Up Study. Am J Transplant. 2015;15(10):2750–7. doi:.https://doi.org/10.1111/ajt.13318
12
Fan
W
,
Gu
J
,
Hu
W
,
Deng
A
,
Ma
Y
,
Liu
J
, et al.
Repairing a 35-mm-long median nerve defect with a chitosan/PGA artificial nerve graft in the human: a case study. Microsurgery. 2008;28(4):238–42. doi:.https://doi.org/10.1002/micr.20488
13Hefti F. Kinderorthopädie in der Praxis. 3rd ed, Berlin Heidelberg: Springer-Verlag; 2015.
14Commission to the European Parliament and Council. State of Paediatric Medicines in the EU. 10 years of the EU Paediatric Regulation, COM (2017)626. Available from: https://ec.europa.eu/health/sites/health/files/files/paediatrics/docs/2017_childrensmedicines_report_en.pdf
15Regulation (EC) No 1901/2006 of the European Parliament and of the Council of 12 December 2006.
16
Greenberg
RG
,
Corneli
A
,
Bradley
J
,
Farley
J
,
Jafri
HS
,
Lin
L
, et al.
Perceived barriers to pediatrician and family practitioner participation in pediatric clinical trials: Findings from the Clinical Trials Transformation Initiative. Contemp Clin Trials Commun. 2018;9:7–12. doi:.https://doi.org/10.1016/j.conctc.2017.11.006
17
Greenberg
RG
,
Gamel
B
,
Bloom
D
,
Bradley
J
,
Jafri
HS
,
Hinton
D
, et al.
Parents’ perceived obstacles to pediatric clinical trial participation: Findings from the clinical trials transformation initiative. Contemp Clin Trials Commun. 2018;9:33–9. doi:.https://doi.org/10.1016/j.conctc.2017.11.005
18
Martin
PJ
,
Uberti
JP
,
Soiffer
RJ
,
Klingemann
H
,
Waller
EK
,
Daly
AS
, et al.
Prochymal Improves Response Rates In Patients With Steroid-Refractory Acute Graft Versus Host Disease (SR-GVHD) Involving The Liver And Gut: Results Of A Randomized, Placebo-Controlled, Multicenter Phase III Trial In GVHD. Biol Blood Marrow Transplant. 2010;16(2):S169–70. doi:.https://doi.org/10.1016/j.bbmt.2009.12.057
19Mesoblast Inc. [Internet]. Melbourne: Mesoblast Ltd.; c2016 [cited 2018 Jul 31]. 2018 ASX Announcements 2018 Feb 22: Primary Endpoint Successfully Achieved in MSB P3 GVHD Trial. Available from: http://investorsmedia.mesoblast.com/phoenix.zhtml?c=187006&p=irol-asxnews&nyo=0
20
Gładysz
D
,
Hozyasz
KK
. Stem cell regenerative therapy in alveolar cleft reconstruction. Arch Oral Biol. 2015;60(10):1517–32. doi:.https://doi.org/10.1016/j.archoralbio.2015.07.003
21
Wu
C
,
Pan
W
,
Feng
C
,
Su
Z
,
Duan
Z
,
Zheng
Q
, et al.
Grafting materials for alveolar cleft reconstruction: a systematic review and best-evidence synthesis. Int J Oral Maxillofac Surg. 2018;47(3):345–56. doi:.https://doi.org/10.1016/j.ijom.2017.08.003
22
Dufrane
D
,
Docquier
PL
,
Delloye
C
,
Poirel
HA
,
André
W
,
Aouassar
N
. Scaffold-free Three-dimensional Graft From Autologous Adipose-derived Stem Cells for Large Bone Defect Reconstruction: Clinical Proof of Concept. Medicine (Baltimore). 2015;94(50):e2220. doi:.https://doi.org/10.1097/MD.0000000000002220
23
Vériter
S
,
André
W
,
Aouassar
N
,
Poirel
HA
,
Lafosse
A
,
Docquier
PL
, et al.
Human Adipose-Derived Mesenchymal Stem Cells in Cell Therapy: Safety and Feasibility in Different “Hospital Exemption” Clinical Applications. PLoS One. 2015;10(10):e0139566. doi:.https://doi.org/10.1371/journal.pone.0139566
24
Tikkanen
J
,
Leskelä
HV
,
Lehtonen
ST
,
Vähäsarja
V
,
Melkko
J
,
Ahvenjärvi
L
, et al.
Attempt to treat congenital pseudarthrosis of the tibia with mesenchymal stromal cell transplantation. Cytotherapy. 2010;12(5):593–604. doi:.https://doi.org/10.3109/14653249.2010.487898
25
Granchi
D
,
Devescovi
V
,
Baglio
SR
,
Magnani
M
,
Donzelli
O
,
Baldini
N
. A regenerative approach for bone repair in congenital pseudarthrosis of the tibia associated or not associated with type 1 neurofibromatosis: correlation between laboratory findings and clinical outcome. Cytotherapy. 2012;14(3):306–14. doi:.https://doi.org/10.3109/14653249.2011.627916
26
Shaw
N
,
Erickson
C
,
Bryant
SJ
,
Ferguson
VL
,
Krebs
MD
,
Hadley-Miller
N
, et al.
Regenerative Medicine Approaches for the Treatment of Pediatric Physeal Injuries. Tissue Eng Part B Rev. 2018;24(2):85–97. doi:.https://doi.org/10.1089/ten.teb.2017.0274
27
Innocenti
M
,
Delcroix
L
,
Romano
GF
,
Capanna
R
. Vascularized epiphyseal transplant. Orthop Clin North Am. 2007;38(1):95–101. doi:.https://doi.org/10.1016/j.ocl.2006.10.003
28
Stulberg
SD
,
Cooperman
DR
,
Wallensten
R
. The natural history of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 1981;63(7):1095–108. doi:.https://doi.org/10.2106/00004623-198163070-00006
29
Joseph
B
. Natural history of early onset and late-onset Legg-Calve-Perthes disease. J Pediatr Orthop. 2011;31(2, Suppl):S152–5. doi:.https://doi.org/10.1097/BPO.0b013e318223b423
30
Chaudhry
S
,
Phillips
D
,
Feldman
D
. Legg-Calvé-Perthes disease: an overview with recent literature. Bull Hosp Jt Dis (2013). 2014;72(1):18–27.
31
Bourke
G
,
Ka
SPJ
. Free phalangeal transfer: donor-site outcome. Br J Plast Surg. 2002;55(4):307–11. doi:.https://doi.org/10.1054/bjps.2002.3836
32
Garagnani
L
,
Gibson
M
,
Smith
PJ
,
Smith
GD
. Long-term donor site morbidity after free nonvascularized toe phalangeal transfer. J Hand Surg Am. 2012;37(4):764–74. doi:.https://doi.org/10.1016/j.jhsa.2011.12.010
33
Kawabata
H
,
Tamura
D
. Five- and 10-Year Follow-Up of Nonvascularized Toe Phalanx Transfers. J Hand Surg Am. 2018;43(5):485.e1–5. doi:.https://doi.org/10.1016/j.jhsa.2017.10.034
34
Scotti
C
,
Piccinini
E
,
Takizawa
H
,
Todorov
A
,
Bourgine
P
,
Papadimitropoulos
A
, et al.
Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci USA. 2013;110(10):3997–4002. doi:.https://doi.org/10.1073/pnas.1220108110
35
Carlier
A
,
Vasilevich
A
,
Marechal
M
,
de Boer
J
,
Geris
L
. In silico clinical trials for pediatric orphan diseases. Sci Rep. 2018;8(1):2465. doi:.https://doi.org/10.1038/s41598-018-20737-y
36
Fulco
I
,
Miot
S
,
Haug
MD
,
Barbero
A
,
Wixmerten
A
,
Feliciano
S
, et al.
Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet. 2014;384(9940):337–46. doi:.https://doi.org/10.1016/S0140-6736(14)60544-4
37
Mumme
M
,
Barbero
A
,
Miot
S
,
Wixmerten
A
,
Feliciano
S
,
Wolf
F
, et al.
Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet. 2016;388(10055):1985–94. doi:.https://doi.org/10.1016/S0140-6736(16)31658-0
38
Martin
I
,
Jakob
M
,
Schaefer
DJ
. From Tissue Engineering to Regenerative Surgery. EBioMedicine. 2018;28:11–2. doi:.https://doi.org/10.1016/j.ebiom.2018.01.029