The rate of mother-to-child transmission of antiretroviral drug-resistant HIV strains is low in the Swiss Mother and Child HIV Cohort Study
DOI: https://doi.org/10.4414/smw.2019.20059
Francesca
Compagnoa, Klaudia
Naegeleab, Christian R.
Kahlertc, Irene
Höslid, Karoline
Aebi-Poppe, Begona
Martinez de Tejadaf, Paolo
Paionig, Sabine
Yerlyh, Jürg
Bönii, Manuel
Battegayj, Christoph
Rudink, Hans H.
Hirschabj, the Swiss HIV Cohort Studyl
aTransplantation and Clinical Virology, Department of Biomedicine, University of Basel, Switzerland
bClinical Virology, Laboratory Medicine, University Hospital Basel, Switzerland
cChildren’s Hospital of Eastern Switzerland and Cantonal Hospital, St Gallen, Switzerland
dUniversity Women`s Hospital Basel, Switzerland
eDepartment of Infectious Diseases, Bern University Hospital, Switzerland
fDepartment of Obstetrics and Gynaecology, University Hospitals of Geneva and Faculty of Medicine, Geneva, Switzerland
gUniversity Children's Hospital Zurich, Switzerland
hLaboratory of Virology, University Hospital Geneva, Switzerland
iInstitute of Medical Virology, University of Zurich, Switzerland
jInfectious Diseases and Hospital Epidemiology, University Hospital Basel, and University of Basel, Switzerland
kUniversity Children’s Hospital, Basel, Switzerland
lMembers of the Swiss HIV Cohort Study and the Swiss Mother and Child HIV Cohort Study: Aebi-Popp K, Anagnostopoulos A, Asner S, Battegay M, Baumann M, Bernasconi E, Böni J, Braun DL, Bucher HC, Calmy A, Cavassini M, Ciuffi A, Duppenthaler A, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J,
Francini K, Furrer H, Fux CA, Grawe C, Günthard HF (President of the SHCS), Haerry D (deputy of "Positive Council"), Hasse B, Hirsch HH, Hoffmann M,
Hösli I, Huber M, Kahlert CR (Chairman of the Mother & Child Substudy), Kaiser L, Keiser O, Klimkait T, Kottanattu L, Kouyos RD, Kovari H, Ledergerber B,
Martinetti G, Martinez de Tejada B, Marzolini C, Metzner KJ, Müller N, Nicca D, Paioni P, Pantaleo G, Perreau M, Polli Ch, Rauch A (Chairman of the Scientific
Board), Rudin C, Scherrer AU (Head of Data Centre), Schmid P, Speck R, Stöckle M (Chairman of the Clinical and Laboratory Committee), Tarr P, Thanh
Lecompte M, Trkola A, Vernazza P, Wagner N, Wandeler G, Weber R, Wyler CA, Yerly S
Summary
AIMS OF THE STUDY
Combination antiretroviral therapy (cART) has reduced mother-to-child transmissions (MTCT) and improved the prognosis of HIV-infected newborns. However, drug resistance mutations (DRM) in HIV-infected children, either transmitted by MTCT (HIV-tDRM) or selected by suboptimal adherence and drug levels (HIV-sDRM), remain a concern. We sought to determine the rate of HIV-tDRM and HIV-sDRM in MTCT pairs in Switzerland.
METHODS
We performed a retrospective analysis of prospectively collected clinical data and available stored samples from MTCT pairs participating in the Swiss Mother-Child HIV (MoCHIV) cohort.
RESULTS
We identified 22 HIV-infected mother-child pairs with delivery between 1989 and 2009 who had 15 years of follow-up (33% white ethnicity). Twenty-one women (96%) were treatment-naïve before pregnancy, 8 (36%) had an unknown HIV status and delivered vaginally, 2 were diagnosed but not treated, and 11 (50%) received antiretrovirals during pregnancy or at delivery, of whom only 6 cases (27%) had cART. HIV subtypes were concordant in all mother-child pairs (subtype B 13/22 [59%]). Using stored plasma (n = 66) and mononuclear cell (n = 43) samples from the children, HIV-tDRM (M184V) was identified in 1 of 22 (4.5%) mothers (1/11 treated, 9%) and was followed by HIV-sDRM at 10 months of age. HIV-sDRM (M184V 23%; K103N 4.5%; D67N 13.6%) occurred in 16/22 (73%) after 4 years, half of whom were treatment naïve. HIV-sDRM were associated with a lower CD4 T-cell nadir (p <0.05) and tended to have higher viral loads and more frequent cART changes.
CONCLUSIONS
HIV-tDRM were low in this Swiss MoCHIV cohort, making them a minor yet preventable complication of prenatal HIV care, whereas HIV-sDRM are a significant challenge in paediatric HIV care.
Introduction
Mother-to-child transmission (MTCT) accounts for more than 90% of HIV infections during childhood according to UNAIDS reports [1, 2]. Missed maternal diagnosis and non-treatment are the main causes of MTCT. These are preventable by a combination of measures consisting of early HIV testing for all women as part of routine prenatal care, immediate combined antiretroviral treatment (cART) and regular monitoring throughout pregnancy. Complete HIV load suppression by the third trimester, and specifically at the time of delivery, reduces the risk of transmission to almost zero. As outlined by the WHO (Prevention of MTCT – PMTCT) and European guidelines [3, 4], treatment-naïve women should start cART immediately, with an integrase strand transfer inhibitor (INSTI)-containing regimen being the preferred treatment. Pregnant women already under suppressive cART should continue, but contraindicated drugs such as didanosine, stavudine or triple nucleoside reverse transcriptase inhibitor (NRTI) combinations should be replaced. The latest data from the IMPAACT (International Maternal Pediatric Adolescent AIDS Clinical Trials Network) study suggest that exposure of mothers to dolutegravir (DTG) at the time of conception could be associated with a higher risk of neural tube defects. Therefore, the Swiss HIV Cohort Study (SHCS) advises against prescribing DTG for women who are trying to become pregnant [5].
In case of incomplete suppression (HIV-VL >50 copies (cp)/ml) at 34-36 weeks of gestation, intravenous zidovudine administration should be considered during labour and Caesarean section. In the case of ongoing maternal viral replication, international guidelines recommend starting cART in high-risk newborns as soon as possible and advocate lifelong continuation in the case of confirmed MTCT [4, 6, 7]. Recent studies indicate that starting cART at birth is associated with improved long-term outcomes for HIV-infected children [8, 9]. Accordingly, morbidity and mortality in HIV-infected children have significantly decreased in the recent decades [10].
HIV-infected children in Western Europe and North America benefit from easy access to specialised medical care which provides laboratory monitoring and cART [11]. Moreover, novel drugs and combinations have facilitated cART for pregnant women and children alike, allowing the goal of long-term HIV suppression with outstanding benefits for health at the individual, societal, and epidemiological levels to be reached.
Nevertheless, virological failure remains a key challenge in the management of HIV-infected children. The rates of virological failure range from 12% to 32% in high and limited-resource settings, respectively. Rates peak during infancy, again during adolescence, and then when transitioning to adult medical care [12–14]. Although dosing of cART in children is sometimes difficult because a suitable drug formulation (e.g. suspension) is not available, virological failure reflects not only a lack of access to care and cART or toxicity concerns, but often also adherence issues of both mothers and children. Thus, a main challenge in HIV-infected children is the emergence of HIV variants with drug resistance mutations (DRM), either selected by suboptimal adherence and insufficient drug combinations and levels (HIV-sDRM), or originating from MTCT (HIV-tDRM). For the reasons pointed out earlier, the risk of HIV-sDRM increases during the HIV-infected child’s lifetime [14]. Conversely, MTCT may involve HIV-tDRM [15], which may also impact cART efficacy and favour the emergence of HIV-sDRM. Here, we report the results of a retrospective analysis of data and blood samples collected prospectively from MTCT pairs participating in the Swiss Mother and Child HIV Cohort Study (MoCHIV) in order to evaluate the rate of transmitted and selected HIV drug resistance in Switzerland.
Materials and methods
Study design
Data and blood samples were collected prospectively by MoCHIV, in which 277 HIV-infected children have been enrolled since 1989. These children were followed in clinics in Basel, Bern, Genève, St Gallen, Zurich and Ticino [16]. MoCHIV is approved by the Swiss Federal Office of Public Health and by the institutional review boards of the participating centres. All women gave their written informed consent to participate in the study, including the retrospective analysis of their and their children’s anonymised data and stored material. In June 2017, 274
ldren had been registered in Switzerland as being born to HIV-infected mothers (see MoCHIV on SHCS website). Of these, 1559 children were diagnosed as being not infected with HIV-1, 145 children had an undefined HIV-status, and 277 children were HIV-1 infected and participated in the Swiss MoCHIV. Of the HIV-positive children, 72 were lost from follow-up or are being treated by a non-cohort physician, 62 have died, and 59 are currently under follow-up in MoCHIV.
For inclusion in this virological DRM study, we identified all MTCT pairs enrolled in the MoCHIV cohort from whom samples were available from the mothers at the time of pregnancy and/or within one year postnatally, and from the corresponding MoCHIV follow-up visits of the children. We analysed demographic, clinical and laboratory parameters including plasma HIV load, CD4 T-cell counts, cART, adherence and clinical conditions, as well as prospectively cryopreserved plasma and whole-blood samples. All available genotype and drug resistance profiles previously performed were included in the analysis. In addition, HIV-DRM were identified by HIV genotyping and resistance testing of the samples from mothers and children at the time of, or shortly after, birth in the Laboratory for Genotyping and Resistance Testing (formerly Division of Infection Diagnostics, Department Biomedicine, University of Basel, accreditation STS219; from 1 Jan 2019: Clinical Virology, Laboratory Medicine, University Hospital Basel, STS0568) to identify tDRM. HIV-tDRM were defined as DRM detected post-natally in RNA or proviral DNA samples that had been obtained before any antiretroviral treatment was administered and which matched the mother’s viral genotype. HIV-sDRM were defined as new DRM arising in children with virological failure.
HIV genotyping and resistance testing
The standard of care of HIV genotyping and resistance testing in our institution is Sanger sequencing of amplicons generated from the HIV protease, reverse transcriptase and, since 2012, the integrase genes. This analysis was performed on both plasma and/or peripheral blood mononuclear cell (PBMC) samples by generating amplicons using the Abbott ViroSeq HIV-1 Genotyping kit (Abbott, IL, USA) or in-house methods validated and accredited in the Division of Infection Diagnostics, Department Biomedicine, University of Basel (STS219). Briefly, following nested PCR and quality control by agarose gel electrophoresis, the amplicons were purified with illustra ExoProStar 1-Step (GE Healthcare, England). The sequencing was performed using the BigDye Terminator v3.1 cycle sequencing kit (Thermo Fisher Scientific, MA, USA), purification with Sephadex G-50 (GE Healthcare, England) and capillary electrophoresis on a 3500 Genetic Analyzer (Thermo Fisher Scientific, MA, USA). The sequences were analysed using SmartGene (Lausanne, Switzerland) [17] and the results interpreted using the HIV Drug Resistance Database of Stanford University (CA, USA). As an accredited regional HIV reference laboratory, the Laboratory for Genotyping and Resistance Testing participates in an external quality assurance program using the French ANRS.
Additionally, deep sequencing using the Illumina MiniSeq platform (CA, USA) was performed on 10 samples from three mother and child pairs. HIV protease, reverse transcriptase and integrase were first amplified using nested PCR primers, followed by library preparation using the Illumina Nextera XT kit (CA, USA). Paired-end sequencing was performed using the Illumina MiniSeq MidOutput Kit and a read length of 150bp. Reports were generated by DeepChek-HIV (ABL, Luxembourg) [18]. Proviral load was determined using DNA extracted from the collected PBMCs using a quantitative, real-time PCR assay with normalisation to 150,000 cells using aspartoacylase, a human diploid housekeeping gene routinely used for this purpose [19].
Data analysis
Patient demographics and laboratory parameters were analysed using descriptive statistics. Data from each individual child were described longitudinally over time. Demographic characteristics of mother-child pairs are presented as proportions (percentages) or as medians (25th, 75th; range), using box and whisker plots where indicated. Categorical variables were compared using the unpaired t-test and Fisher’s exact test, whereas continuous variables were analysed using the Wilcoxon rank-sum test. Two-sided p-values of <0.05 were considered statistically significant. Analyses were performed using the statistical software package SPSS version 24 (IBM® SPSS Statistics 24.0.0.1) and Excel version 15.37 (Microsoft® Excel 2016 for Mac).
Results
We identified 22 MTCT pairs which fulfilled the enrolment criteria in the Swiss MoCHIV study. The children were born between 1989 and 2009, thereby covering 20 years of different treatments and antiretroviral drug availabilities. Nineteen children are still enrolled in this national cohort and three have moved to the adult Swiss HIV Cohort Study (SHCS). Median gestational age at delivery was 38 weeks (25th percentile 28, 75th percentile 39, range 27–42 weeks) and 27% of the children were born prematurely (<37 weeks) (table 1). Eight of the 22 children (36%) were delivered vaginally. All but one of the mothers (21/22, 95%) reported as treatment-naïve before pregnancy. During pregnancy, only 11 (50%) mothers received any kind of antiretroviral therapy (zidovudine in five cases, table 1), and cART was administered to only 6 (27%) mothers. Plasma HIV load at the time of delivery was only available for 10 mothers, and none of them were suppressed to <50 cp/ml (median 71,249 cp/ml; table 1).
Table 1 Clinical characteristics of mothers in MTCT pairs.
|
Variables
|
n (%) or median
|
25th, 75th percentiles (range)
|
| Gestational age at delivery (weeks) |
38 |
28, 39 (27–42) |
| Type of delivery |
Vaginal |
8 (36) |
|
| Caesarean |
11 (50) |
| Unknown |
3 (14) |
| Maternal complication at delivery |
Haemorrhage |
2 (9) |
|
| Infection |
4 (18) |
| Other |
2 (9) |
| ART during pregnancy |
Yes |
11 (50) |
|
| Duration ≤1month |
8 (73) |
| Duration >1month |
3 (27) |
| Type of therapy / prophylaxis |
cART |
6 (27) |
|
| ZDV |
3 (14) |
| ZDV intrapartum |
2 (9) |
| None |
11 (50) |
| CD4 T cell at birth (cells/μl) |
308 |
72, 580 (32–822) |
| HIV load at birth (copies/ml) |
71,249 |
9840, 132,621 (204–284,000) |
The demographics of the HIV-infected children are summarised in table 2. To the best of our knowledge, there were no siblings in this analysis, but this was not due to explicit exclusion. Five (23%) children had detectable plasma HIV loads at birth or within the first month, suggesting in utero transmission (median HIV load 13,845 cp/ml; range 6880–2,531,122). In another 14 children, the first plasma HIV loads were detected within the first nine months of life. Only one child had undetectable HIV loads at six months of age, whereas the other children had median plasma HIV loads of 200,000 cp/ml (IQR 16,274–765,085; range 748–2,531,122). During follow-up visits, the nadir CD4 T-cell count varied widely, between 14 and 1,282/μl (median 280; IQR 146–627). Peak HIV loads at the time of diagnosing virological failure ranged from 1160 to 7,400,000 cp/ml (median 424,913; IQR 64,831–958,025). At last follow-up (median 14 years old; IQR 14–18 years; range 6–23 years), 19 of the 22 children were alive and attending regular follow-up (one child died at one year of age, two children were lost to follow-up at 9 and 10 years old), and 16 of these 19 children were on cART. The CD4 cell counts had a median of 709 cell/mmc (IQR 555–848; range 240–1,300). Plasma HIV loads were below the limit of detection (<20 cp/ml) in 11/19 children (median 60 cp/ml; IQR 0–77; range 0–10,000).
Table 2 Demographics of the HIV-infected children.
|
Variables
|
n (%) or median
|
25th, 75th percentiles (range)
|
| Total |
22 (100) |
|
| Gender (female) |
11 (50) |
|
| Ethnicity |
White |
7 (32) |
|
| Black |
8 (36) |
| Other |
7 (32) |
| HIV-1 subtype |
B |
13 (59) |
|
| Non-B |
9 (41) |
| Peak HIV load (copies/ml) |
424,914 |
64,831, 958,025
(1160–7,400,000) |
| Nadir CD4 T cell (cells/μl) |
280 |
146,627 (14–1,282) |
| Antenatal prophylaxis (yes) |
8 (36) |
|
| Age of the children at ART start |
At birth |
10 (45) |
|
| Not at birth but ≤1year |
7 (32) |
| Later >1year |
4 (18) |
| Never |
1 (5) |
| Initial HIV treatment |
ART |
11 (50) |
|
| cART |
10 (45) |
| No therapy |
1 (5) |
| CD4 T-cells at ART start (counts/μl) |
1,579 |
379, 2,45
(65–2,649) |
| HIV load at ART start (copies/ml) |
181,256 |
13,278, 602,277
(0–8,829,994) |
| ART changes (number) |
5 |
3, 8 (2–16) |
Antiviral treatment and virological failure
Thirteen (59%) children were born before the year 2000. Individual infant treatment history from the start of ART is summarised in figure 1
. All children, except one who died within the first year of life, had a follow-up longer than two years. Among children who received cART, there was an average of five treatment changes (median; IQR 3–8; range 2–16) (table 2). Virological failure occurred in all children during follow-up (median 2; IQR 2–3.5; range 1–6). Adjusting the observational data for the year of effective follow-up indicated an average of 0.18 virological failure episodes per year and child, i.e., a virological failure occurred once every five years of observation time on average. Approximately half the virological failure events were among those older than eight years of age. In three children, plasma HIV loads were detectable over prolonged periods of more than 10 years.
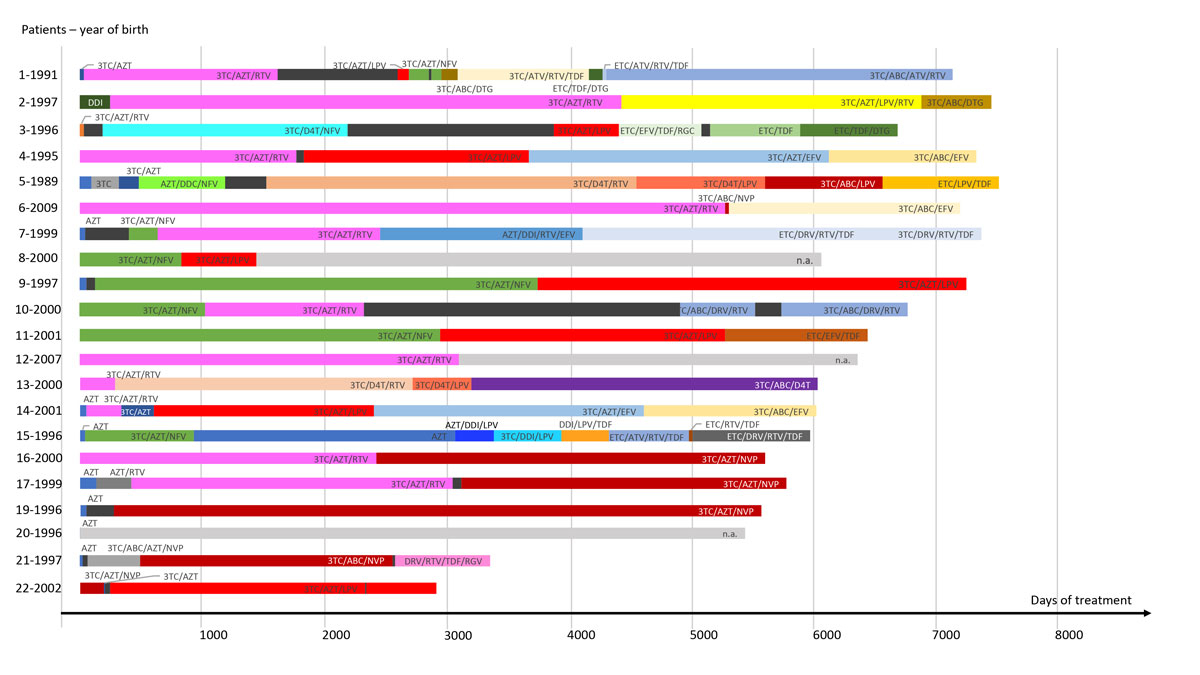
Figure 1
Individual antiretroviral treatment history of vertically HIV-infected children. HIV treatment history over eight years of follow-up is shown. Patients 18 and 21 were analysed by deep sequencing, as described in more detail in the results section, and patient 20 is shown in figure 4. The date of birth is indicated in front of the corresponding time line. Each coloured bar represents a type of treatment. A black bar means no treatment and a grey bar means data not available. Time is represented on the x axis, and the length of a bar corresponds to the interval of treatment, including when it was started.
3TC = lamivudine; AZT = zidovudine; FTC = emtricitabine; ABC = abacavir; DDI = didanosine; DDC = zalcitabine; D4T = stavudine; TDF = tenofovir; EFV = efavirenz; ETV = etravirine; DRV = darunavir; LPV = lopinavir; NFV = nelfinavir; ATV = atazanavir; RTV/r = ritonavir; DTG = dolutegravir; RGV = raltegravir
Genotype and drug resistance profile
A total of 165 genotypic resistance profiles were analysed. One third of them (56/165) had been performed previously during a routine visit. For 33 profiles, only partial sequences were available, and these needed to be repeated. Complete sequences of sufficient quality were available for a total of 109 samples, consisting of 66 plasma- and 43 PBMC-derived sequences. Concordant HIV-1 subtypes were observed in all mother-child pairs, the most prevalent being subtype B in 13/22 (59%) (fig. 2). Other subtypes and recombinant strains included C in 18% (4/22) of mother-child pairs and AC, A1C, A1, CRF18_cpx and CRF67_01B in 4.5% (1/22) each. Since protease inhibitors were the first antiretrovirals to be used in cART in Switzerland in 1996 [20], we used HIV-protease sequences as a surrogate of diversity and evolution over time. In the circular phylogram of mother and child viral RNA and proviral DNA sequences (fig. 2), examples of viral evolution are visualised in the distance between branches, which are still clustered within their respective HIV-1 subtypes. HIV polymorphisms and sequences not conferring intermediate to high-level antiretroviral resistance were detected in all children (not shown). HIV resistance genotypes conferring relevant intermediate to high-level antiretroviral resistance were found in 45% (10/22) of the mothers, affecting susceptibility to protease inhibitors (PI) in seven (70%), to NRTI in one (10%), to non-nucleo(s/t)ide reverse transcriptase inhibitors (NNRTI) in three (30%), and to INSTI-containing therapy in one (10%).

Figure 2
Circular phylogram of mother and child viral RNA and proviral DNA sequences. The diversity and evolution of HIV-1 protease sequences from RNA and proviral DNA over time is shown. Sequences that cluster together due to common origin and subtype are marked in different colours for subtype B, subtype C, subtype CRF18_cpx, subtype A1, subtype A1C, and subtype CRF67_01B.
M = mother; C = child; R = RNA; P = proviral DNA; date = sampling date
HIV-tDRM were identified in one of the 22 MTCT pairs (4.5%), showing matched mother- and child-derived HIV sequences (1 of 11 ART-exposed mothers, 9%). The presence of HIV-tDRM was associated with early emergence of HIV-sDRM (0.83 vs 3.5 years after birth), but the small numbers precluded a meaningful statistical analysis.
The emergence of HIV-sDRM was seen in 16/22 (73%) children, at a mean age of 4.3 years (median 2.8; IQR 0.8–5.9; range 0.25–14.8), and 10/22 (45%) had two or more mutations. Thirteen of these 16 (81%) were on treatment at the time of HIV-sDRM emergence. Children with HIV-sDRM showed a trend towards a greater number of treatment changes over the entire follow-up period compared to children without HIV-sDRM (7 vs 4 times, respectively; p = 0.104). The CD4 T-cell nadir was lower and associated with higher HIV loads in children with HIV-sDRM (p <0.05 and p = 0.06, respectively; fig. 3).
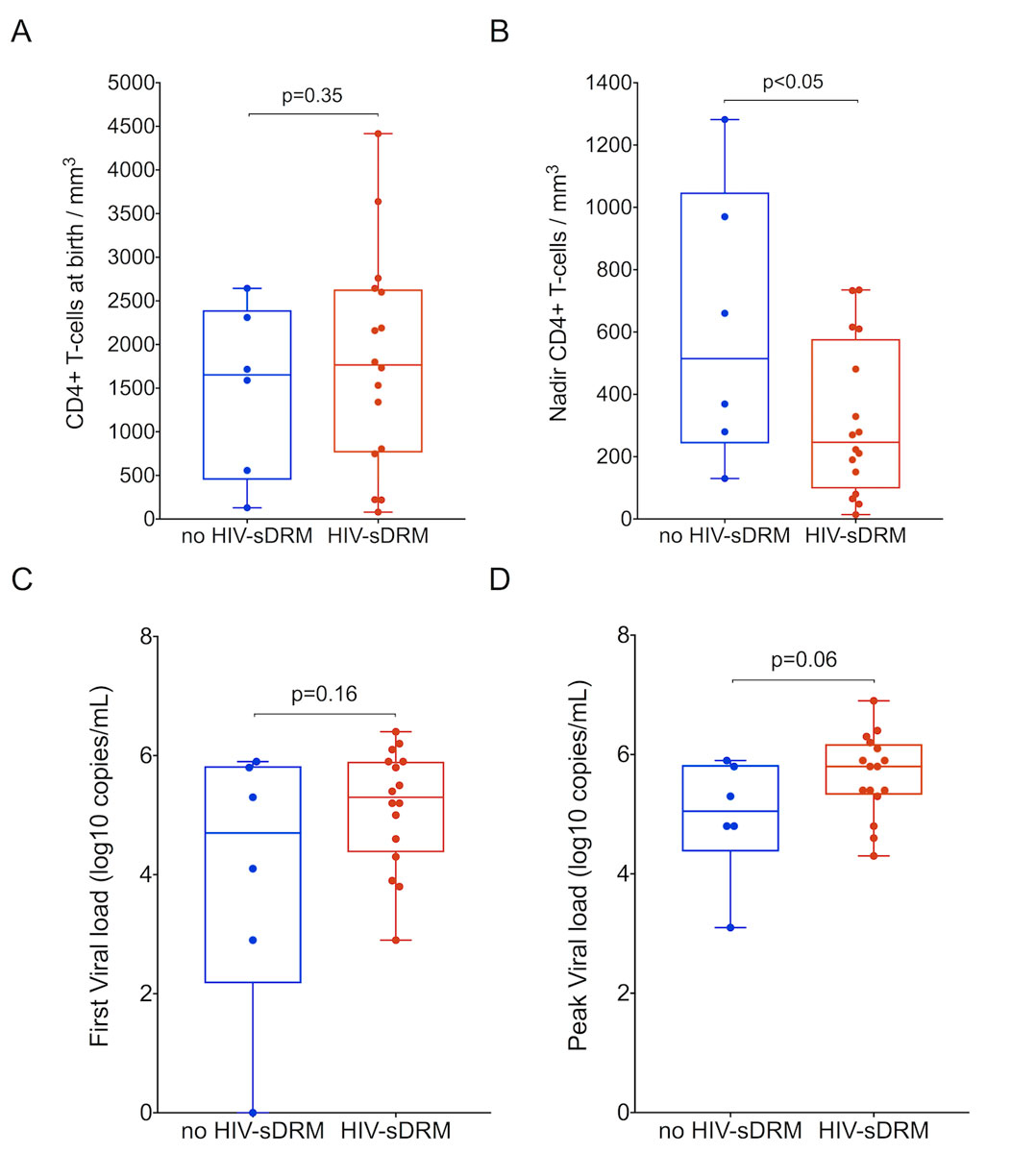
Figure 3
HIV load and CD4 T-cell count in vertically HIV-infected children. A. CD4 T-cell count in MTCT children at birth with and without selected HIV drug resistance mutations (HIV-sDRM). B. Nadir CD4 T-cell count in MTCT children with and without HIV-sDRM. C. First HIV-1 load in MTCT children with and without HIV-sDRM. D. Peak HIV-1 load in MTCT children with and without HIV-sDRM. Box and whiskers plots showing median and 25th and 75th percentiles.
The most frequent DRM was M184V, detected in 7/22 (32%), while other major NRTI mutations (M41L, L210W, T215FY, L219E, L74V, V75M) were seen only in one or two patients. Considering DRM associated with low-level or intermediate resistance, D67N was the most prevalent, detected in 14% of children (3/22), followed by K70R, detected in only one patient (4.5%). A wide range of NNRTI DRM were found (K101E, K103N, V106M, G190E, P225H, M230L) at a rate of 4.5% each. PI DRM were most commonly M46I, followed by V82A and I54V. Only one major INSTI DRM (R263K) was detected, in one child in a proviral sequence, while minor INSTI DRM were found in a total of six patients. However, clinical evidence of associated virological failure was lacking, as none of the patients were exposed to this class of drugs. In all but two cases, HIV-DRM emerged after previous exposure ranging from six months to 10 years. DRM were already present at the time of the first genotypic analysis (M184V, T215Y, L210W, M230I) in only two children without medical history of prior ART.
In three MTCT pairs, 10 sample materials from the time of delivery were available in sufficient amounts to complement the conventional Sanger sequencing by next-generation sequencing (NGS). In pair 21, no additional DRM were found. In pair 18, DRM M184I and V, 41L, 65R, 67N, 70R, 74I, 210W and 215Y were detected at frequencies of 53.7% (1,300 reads) and 22.7% (551 reads), 63.7% (557 reads), 1.2% (22 reads), 70.6% (1,282 reads), 1.2% (25 reads), 3% (64 reads), 4.1% (46 reads) and 70% (609 reads), respectively. None of the variants with frequencies of <4% were seen by amplicon-directed Sanger sequencing. In pair 20, NGS identified the PI resistance 82A at a rate of 16.5% (44,670 reads) during the time when the child had persistent high HIV loads having been exposed to this drug class. The PI resistance 82A increased to a detection rate of 99.6% (147,899 reads) two years later with the same method, and at that time was also identified by Sanger sequencing (fig. 4).
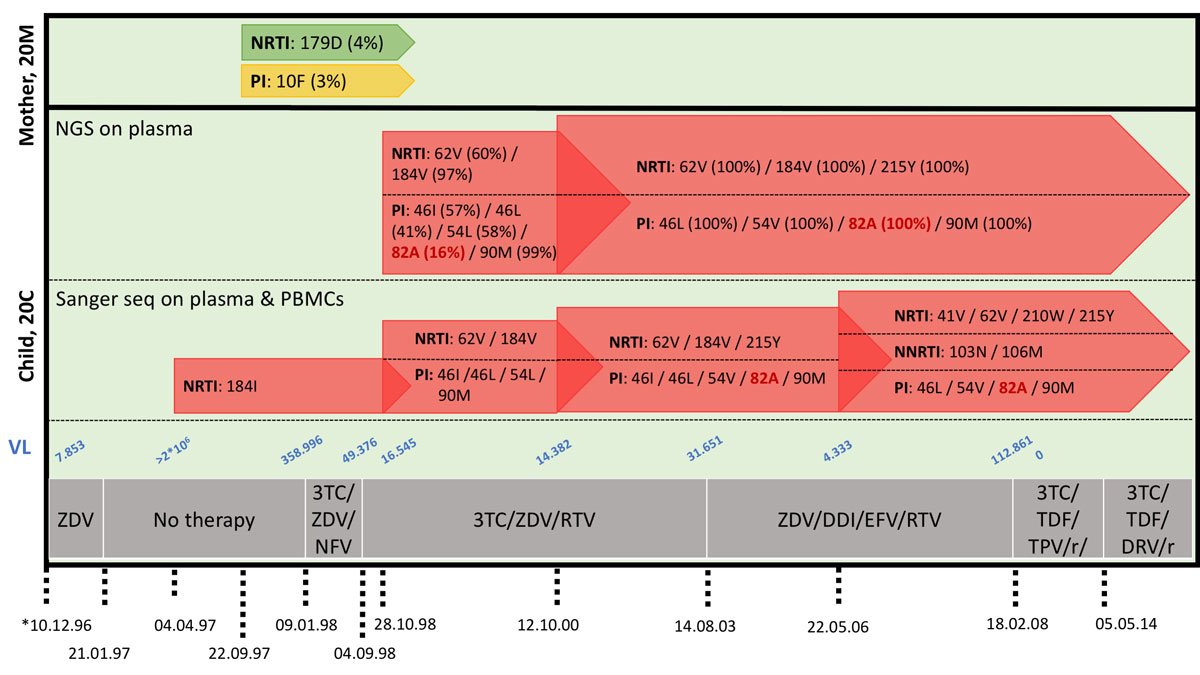
Figure 4
Deep sequencing of samples from mother-child pair 20. Comparison of Sanger sequencing and deep sequencing results of HIV-1 RNA from mother-child pair 20. Sampling dates and treatment history are indicated, as well as HIV-1 loads (copies / mL). Deep sequencing results are additionally shown as mutation frequencies (%). No material was available from the first time point at which the reported M184I was detected.
NRTI = nucleo(s/t)ide reverse transcriptase inhibitor; NNRTI = non-nucleo(s/t)ide reverse transcriptase inhibitor; PI = protease inhibitor; 3TC = lamivudine; ZDV = zidovudine; DDI = didanosine; TDF = tenofovir; EFV = efavirenz; NFV = nelfinavir; DRV = darunavir; TPV = tipranavir; RTV/r = ritonavir/boosted ritonavir
Discussion
In this study of the Swiss MoCHIV cohort, we analysed HIV-1 genotype and drug resistance profiles in data and blood samples collected prospectively from 22 HIV infected mother-child pairs. The rate of HIV-tDRM was one out of all 22 MTCT pairs (4.5%) and 1/11 (9%) MTCT pairs where mothers had been previously exposed to antiretrovirals. Although the overall study population was too small for more robust conclusions, other studies have reported rates between 4.9% and 17% [21–23]. The HIV-tDRM detected affected the HIV reverse transcriptase through the M184V mutation.
Conversely, the rate of HIV-sDRM was 16/22 (73%), emphasising that HIV-sDRM represent a significant issue in paediatric HIV care. The predominant mutation among HIV-sDRM was M184V (32%), but other mutations affecting other drugs and classes were also found at varying rates, including TAMs (M41L, L210W, T215FY, L219E in 4.5 to 9%; D67N in 14%; K70R in 4.5%), discriminatory and nonpolymorphic NRTI mutations L74V and V75M, mutations affecting PIs (M46I in 18%; V82A and I54V in 9%) and NNRTIs (K101E, K103N, V106M, G190E, P225H, M230L in 4.5%). Only one case of INSTI DRM was found, in line with the study period of up to 2014, an era before the widespread availability of INSTIs in Switzerland. Although antenatal screening for HIV infection is recommended in Switzerland, eight women were not known to be HIV-infected during pregnancy and delivery, and the three remaining women did not receive antiretroviral prophylaxis during pregnancy for unknown reasons. Revisiting the MoCHIV data from the last 20 years, there was only one registered case of vertical HIV transmission after 2009, which occurred in 2011 (see MoCHIV on SHCS website, fig. 3). Together with the reduced rate of MTCT, the rate of HIV-tDRM is expected to decrease in Switzerland, but this cannot be generalised to other settings, e.g. resource-limited countries.
HIV-sDRM emerged on average three years after birth and was associated with more frequent treatment changes (p = 0.104), lower CD4 T-cell nadir (p <0.05) and higher peak plasma HIV loads (p = 0.06). In the one case of HIV-tDRM, HIV-sDRM were subsequently detected after 10 months, suggesting that transmitted drug resistance might facilitate much earlier HIV-sDRM emergence and virological failure, although further analyses are needed due the low sample size. The first report on HIV-tDRM was published in 1994, describing a case of neonatal infection by a zidovudine-resistant HIV-1 strain [24]. In a systematic review published in 2014, HIV-tDRM data among treatment-naïve children were only available from 14 countries, but the overall number of cases analysed was low [25]. As expected, the type and prevalence of HIV-tDRM reflected the choice and availability of antiviral treatments in the respective countries. Historically, the low cost of nevirapine administered as a single dose to the mother during labour and to the child right after delivery allowed its widespread use and improved the prevention of MTCT in low resource settings. Unfortunately, rapid selection of resistant viral mutants was soon observed [26, 27]. In high-income countries, HIV-tDRM of 10% to 17% have been estimated [15]. A recent study investigated both selected and transmitted HIV drug resistance and indeed found a rate of 4.9% in the latter group [22]. However, the data originated from a small cross-sectional study of 19 families and did not compare HIV-tDRM and -sDRM. There are few data on the role of HIV-tDRM, even though they potentially have effects on virological failure and the emergence of HIV-sDRM when only limited options for new antiretroviral drug classes exist.
Our in-depth analysis of HIV-DRM by NGS was limited to three patients with sufficient sample materials. The three cases were representative of three different scenarios. In two cases, HIV-DRM at frequencies below 20% that were not detected by Sanger sequencing were detectable by deep sequencing. However, in one case the HIV-sDRM emerged as a majority variant after two years and was then detected by Sanger sequencing. Thus, NGS could be helpful for the identification of DRM-minority variants and for monitoring them over time. In the absence of DRM-informed decisions about effective cART, at least two new antiretrovirals that have not been used previously to treat either mother or child should be considered for children with virological failure [28].
Thus, the results of this study indicate that in the reported group of children, HIV-tDRM were found in only one out of 22 HIV-infected children born to mothers not receiving ART before and receiving it rarely during pregnancy (six with cART). In contrast, the frequency of HIV-sDRM was high and occurred with three to five years, despite the fact that half the children were treatment naïve.
This study has several limitations. Firstly, the number of available mother-child pairs was small, limiting the statistical analysis to the descriptive level, even though a long follow-up of 15 years was available. Secondly, this is a retrospective evaluation which included patients from a range of different eras of HIV diagnosis and management from the years 1989–2009, including the pre-ART and pre-cART eras. Thirdly, apparently treatment-naïve women appeared overrepresented among the 22 MTCT pairs, but this result emerged only because of this detailed analysis. However, we remain cautious by not directly attributing a low pre-test risk of HIV-tDRM to a patient history of no prior ART exposure. In some cases, there was no specific ART history available, whereas in others, this information may not have been reliable, given the stigma in patients with a difficult psycho-social history, including a migration background. Moreover, the transmission of drug-resistant viruses remains a possibility, especially in the early era of ART when insufficient suppression was usual. In this respect, it is notable that 60% of the MTCT pairs had the B subtype (fig. 2), but the 68% were of non-white ethnicity. Fourthly, HIV load and resistance testing could not be performed at all time points of interest, even though a significant effort was made by processing 109 samples of plasma and PBMC in addition to the HIV load data and resistance data that had been obtained at the time of the historic follow-up in the routine clinic. As the blood samples from these new-borns and infants were limited in size, additional next generation deep sequencing could not be performed except in 10 samples obtained from three cases. Nevertheless, the case studies were informative in principle, demonstrating that either no additional DRM were detectable (scenario 1), that DRM were detectable as minority species, but did not emerge as majority species (scenario 2), or that DRM were detectable as minority species and emerged as majority species during follow-up (scenario 3). Thus, HIV genotyping and resistance testing may provide relevant guidance for treatment, but access and adherence to appropriately dosed antiretrovirals, including new, not previously prescribed drug classes such as integrase inhibitors and combinations, remains an important consideration throughout paediatric HIV care.
Conclusions
While acknowledging these limitations, we conclude that HIV-tDRM were low in this Swiss study of mother-to child HIV transmission, making it a minor yet today preventable complication of HIV care [29, 30]. However, HIV-sDRM remain an important issue in paediatric HIV care, underpinned by the fact that all options were available for the treatment-naïve children in our study. Indeed, similar to the paediatric care of other chronic medical conditions [31] such diabetes mellitus [32] and transplantation [33], non-adherence in paediatric HIV care remains challenging despite more potent drugs, lower pill counts and fewer side effects in the current era of cART [14].
Author contributions
Francesca Compagno and Klaudia Naegele contributed equally to the manuscript
References
1UNAIDS. The Gap Report 2014. Geneva: UNAIDS; 2014.
2AIDSInfo. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. http://aidsinfo.nih.gov/guidelines2015.
3WHO. Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Geneva: World Health Organization; 2015.
4EACS EACS. EACS Guidelines Version 9.0 Version 9.0 ed. http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html2017.
5IMPAACT. Network IMPAACT. IMPAACT Press Releases and other Research News. https://impaactnetwork.org/news_/index.html 2018.
6
Bamford
A
,
Turkova
A
,
Lyall
H
,
Foster
C
,
Klein
N
,
Bastiaans
D
, et al.
Paediatric European Network for Treatment of AIDS (PENTA) guidelines for treatment of paediatric HIV-1 infection 2015: optimizing health in preparation for adult life. HIV Med. 2018;19(1):e1–42.
7WHO. Global Health Sector Response to HIV, 2000-2015: Focus on Innovations in Africa: Progress Report. Geneva: World Health Organization; 2015.
8
Klein
N
,
Palma
P
,
Luzuriaga
K
,
Pahwa
S
,
Nastouli
E
,
Gibb
DM
, et al.
Early antiretroviral therapy in children perinatally infected with HIV: a unique opportunity to implement immunotherapeutic approaches to prolong viral remission. Lancet Infect Dis. 2015;15(9):1108–14. doi:.https://doi.org/10.1016/S1473-3099(15)00052-3
9
Van Dyke
RB
,
Patel
K
,
Siberry
GK
,
Burchett
SK
,
Spector
SA
,
Chernoff
MC
, et al.; Pediatric HIV/AIDS Cohort Study. Antiretroviral treatment of US children with perinatally acquired HIV infection: temporal changes in therapy between 1991 and 2009 and predictors of immunologic and virologic outcomes. J Acquir Immune Defic Syndr. 2011;57(2):165–73. doi:.https://doi.org/10.1097/QAI.0b013e318215c7b1
10
Penazzato
M
,
Prendergast
A
,
Tierney
J
,
Cotton
M
,
Gibb
D
. Effectiveness of antiretroviral therapy in HIV-infected children under 2 years of age. Cochrane Database Syst Rev. 2012;7(7):CD004772. doi:.https://doi.org/10.1002/14651858.CD004772.pub3
11
Berti
E
,
Thorne
C
,
Noguera-Julian
A
,
Rojo
P
,
Galli
L
,
de Martino
M
, et al.
The new face of the pediatric HIV epidemic in Western countries: demographic characteristics, morbidity and mortality of the pediatric HIV-infected population. Pediatr Infect Dis J. 2015;34(5, Suppl 1):S7–13. doi:.https://doi.org/10.1097/INF.0000000000000660
12
Fitzgerald
F
,
Penazzato
M
,
Gibb
D
. Development of antiretroviral resistance in children with HIV in low- and middle-income countries. J Infect Dis. 2013;207(Suppl 2):S85–92. doi:.https://doi.org/10.1093/infdis/jit115
13
Davies
MA
,
Boulle
A
,
Eley
B
,
Moultrie
H
,
Technau
K
,
Rabie
H
, et al.; International epidemiologic Databases to Evaluate AIDS Southern Africa (IeDEA‐SA) Collaboration. Accuracy of immunological criteria for identifying virological failure in children on antiretroviral therapy - the IeDEA Southern Africa Collaboration. Trop Med Int Health. 2011;16(11):1367–71. doi:.https://doi.org/10.1111/j.1365-3156.2011.02854.x
14
Castro
H
,
Judd
A
,
Gibb
DM
,
Butler
K
,
Lodwick
RK
,
van Sighem
A
, et al., Pursuing Later Treatment Options II (PLATO II) project team for the Collaboration of Observational HIV Epidemiological Research Europe (COHERE). Risk of triple-class virological failure in children with HIV: a retrospective cohort study. Lancet. 2011;377(9777):1580–7. doi:.https://doi.org/10.1016/S0140-6736(11)60208-0
15
Persaud
D
,
Palumbo
P
,
Ziemniak
C
,
Chen
J
,
Ray
SC
,
Hughes
M
, et al.; Pediatric AIDS Clinical Trials Group P1030 Team. Early archiving and predominance of nonnucleoside reverse transcriptase inhibitor-resistant HIV-1 among recently infected infants born in the United States. J Infect Dis. 2007;195(10):1402–10. doi:.https://doi.org/10.1086/513871
16
Schoeni-Affolter
F
,
Ledergerber
B
,
Rickenbach
M
,
Rudin
C
,
Günthard
HF
,
Telenti
A
, et al.; Swiss HIV Cohort Study. Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol. 2010;39(5):1179–89. doi:.https://doi.org/10.1093/ije/dyp321
17
Bellecave
P
,
Recordon-Pinson
P
,
Fleury
H
. Evaluation of Automatic Analysis of Ultradeep Pyrosequencing Raw Data to Determine Percentages of HIV Resistance Mutations in Patients Followed-Up in Hospital. AIDS Res Hum Retroviruses. 2016;32(1):85–92. doi:.https://doi.org/10.1089/aid.2015.0201
18
Mohamed
S
,
Penaranda
G
,
Gonzalez
D
,
Camus
C
,
Khiri
H
,
Boulmé
R
, et al.
Comparison of ultra-deep versus Sanger sequencing detection of minority mutations on the HIV-1 drug resistance interpretations after virological failure. AIDS. 2014;28(9):1315–24. doi:.https://doi.org/10.1097/QAD.0000000000000267
19
Lautenschlager
I
,
Jahnukainen
T
,
Kardas
P
,
Lohi
J
,
Auvinen
E
,
Mannonen
L
, et al.
A case of primary JC polyomavirus infection-associated nephropathy. Am J Transplant. 2014;14(12):2887–92. doi:.https://doi.org/10.1111/ajt.12945
20
Egger
M
,
Hirschel
B
,
Francioli
P
,
Sudre
P
,
Wirz
M
,
Flepp
M
, et al.
Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. Swiss HIV Cohort Study. BMJ. 1997;315(7117):1194–9. doi:.https://doi.org/10.1136/bmj.315.7117.1194
21
Rogo
T
,
DeLong
AK
,
Chan
P
,
Kantor
R
. Antiretroviral treatment failure, drug resistance, and subtype diversity in the only pediatric HIV clinic in Rhode Island. Clin Infect Dis. 2015;60(9):1426–35. doi:.https://doi.org/10.1093/cid/civ058
22
Vaz
SN
,
Giovanetti
M
,
Rego
FF
,
de Oliveira
T
,
Danaviah
S
,
Gonçalves
ML
, et al.
Molecular Characterization of the Human Immunodeficiency Virus Type 1 in Women and Their Vertically Infected Children. AIDS Res Hum Retroviruses. 2015;31(10):1046–51. doi:.https://doi.org/10.1089/aid.2015.0166
23
Lel
R
,
Ngaira
J
,
Lihana
R
,
Khamadi
S
. HIV-1 drug resistance mutations among infants born to HIV-positive mothers in Busia, Kenya. AIDS Res Hum Retroviruses. 2014;30(12):1236–8. doi:.https://doi.org/10.1089/aid.2014.0158
24
Siegrist
CA
,
Yerly
S
,
Kaiser
L
,
Wyler
CA
,
Perrin
L
. Mother to child transmission of zidovudine-resistant HIV-1. Lancet. 1994;344(8939-8940):1771–2. doi:.https://doi.org/10.1016/S0140-6736(94)92911-4
25
Rojas Sánchez
P
,
Holguín
A
. Drug resistance in the HIV-1-infected paediatric population worldwide: a systematic review. J Antimicrob Chemother. 2014;69(8):2032–42. doi:.https://doi.org/10.1093/jac/dku104
26
Loubser
S
,
Balfe
P
,
Sherman
G
,
Hammer
S
,
Kuhn
L
,
Morris
L
. Decay of K103N mutants in cellular DNA and plasma RNA after single-dose nevirapine to reduce mother-to-child HIV transmission. AIDS. 2006;20(7):995–1002. doi:.https://doi.org/10.1097/01.aids.0000222071.60620.1d
27
Flys
TS
,
Donnell
D
,
Mwatha
A
,
Nakabiito
C
,
Musoke
P
,
Mmiro
F
, et al.
Persistence of K103N-containing HIV-1 variants after single-dose nevirapine for prevention of HIV-1 mother-to-child transmission. J Infect Dis. 2007;195(5):711–5. doi:.https://doi.org/10.1086/511433
28
Hirsch
HH
,
Drechsler
H
,
Holbro
A
,
Hamy
F
,
Sendi
P
,
Petrovic
K
, et al.
Genotypic and phenotypic resistance testing of HIV-1 in routine clinical care. Eur J Clin Microbiol Infect Dis. 2005;24(11):733–8. doi:.https://doi.org/10.1007/s10096-005-0044-4
29
Camacho-Gonzalez
AF
,
Kingbo
MH
,
Boylan
A
,
Eckard
AR
,
Chahroudi
A
,
Chakraborty
R
. Missed opportunities for prevention of mother-to-child transmission in the United States. AIDS. 2015;29(12):1511–5. doi:.https://doi.org/10.1097/QAD.0000000000000710
30
Aebi-Popp
K
,
Mulcahy
F
,
Glass
TR
,
Rudin
C
,
Martinez de Tejada
B
,
Bertisch
B
, et al.; European Collaborative Study in EuroCoord; Swiss Mother & Child HIV Cohort Study. Missed opportunities among HIV-positive women to control viral replication during pregnancy and to have a vaginal delivery. J Acquir Immune Defic Syndr. 2013;64(1):58–65. doi:.https://doi.org/10.1097/QAI.0b013e3182a334e3
31
Graves
MM
,
Roberts
MC
,
Rapoff
M
,
Boyer
A
. The efficacy of adherence interventions for chronically ill children: a meta-analytic review. J Pediatr Psychol. 2010;35(4):368–82. doi:.https://doi.org/10.1093/jpepsy/jsp072
32
Hood
KK
,
Peterson
CM
,
Rohan
JM
,
Drotar
D
. Association between adherence and glycemic control in pediatric type 1 diabetes: a meta-analysis. Pediatrics. 2009;124(6):e1171–9. doi:.https://doi.org/10.1542/peds.2009-0207
33
Dobbels
F
,
Ruppar
T
,
De Geest
S
,
Decorte
A
,
Van Damme-Lombaerts
R
,
Fine
RN
. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant. 2010;14(5):603–13. doi:.https://doi.org/10.1111/j.1399-3046.2010.01299.x



