Management of brainstem haemorrhages
DOI: https://doi.org/10.4414/smw.2019.20062
Sophie S.
Wanga, Yang
Yanga, Julia
Velza, Emanuela
Kellera, Andreas R.
Luftbc, Luca
Reglia, Marian C.
Neiderta, Oliver
Bozinova
aDepartment of Neurosurgery, University Hospital Zurich, University of Zurich, Switzerland
bDepartment of Neurology, University Hospital Zurich, University of Zurich, Switzerland
cCereneo, Centre for Neurology and Rehabilitation, Vitznau, Switzerland
Summary
Among spontaneous intracranial haemorrhages, primary non-traumatic brainstem haemorrhages are associated with the highest mortality rate. Patients classically present with rapid neurological deterioration. Previous studies have found that the severity of initial neurological symptoms and hydrocephalus are predictors of poor outcomes. In addition, radiological parameters aim to classify brainstem haematomas according to volume, extension and impact on prognosis. However, previous studies have failed to agree on a differentiated radiological classification for outcome and functional recovery. Electrophysiology, including motor, auditory and somatosensory evoked potentials, is used to estimate the extent of the initial injury and predict functional recovery. The current management of brainstem haematomas remains conservative, focusing on initial close neurocritical care monitoring. Surgical treatment concepts exist, but similarly to general intracranial haemorrhage management, they continue to be controversial and have not been sufficiently investigated. This is especially the case for haematomas in the posterior fossa, as these are excluded from most current clinical trials. Existing studies were mostly carried out before the present millennium began, and limitations are evident in the adaptation of those results and recommendations to current management, with today’s technological and diagnostic possibilities. We therefore recommend the re-evaluation of brainstem haemorrhages in the modern neurosurgical and intensive care environment.
Introduction
Terminology
The American Heart Association/American Stroke Association (AHA/ASA) has defined an intracerebral haemorrhage (ICH) as a focal collection of blood within the brain parenchyma or ventricular system that is not the result of trauma. ICHs are often referred to as “spontaneous”, “non-traumatic” or “primary” [1]. The same terminology applies to brainstem haemorrhages. Some terms focus on the pons only, and therefore the label “primary pontine haemorrhage” is applied. Others also include tegmental parts. However, we have noticed an inconsistency: some groups use the term “pontine haemorrhage” for bleedings that also expand into the tegmentum (mesencephalon). Therefore, we suggest using the term “brainstem haemorrhage” to address the brainstem as one functional and anatomical unit.
Epidemiological features – incidence, risk factors and mortality
In developed countries, stroke remains one of the leading causes of death and morbidity. Spontaneous ICH is the underlying cause of up to 19.6% of all strokes [2, 3]. Six to ten percent of spontaneous ICHs are localised in the brainstem. The risk of ICH increases continuously with age. However, brainstem haemorrhages occur in a younger patient group, with the highest incidence occurring in patients between 40 years and 60 years old [4, 5]. The most common cause of primary bleeding into the brainstem is hypertension [4]. Previous studies have noted a higher distribution of ICHs in cocaine users, but no significance was accorded to the relationship between this risk factor and localisation to the brainstem [6] (fig. 1 presents an illustrative case).
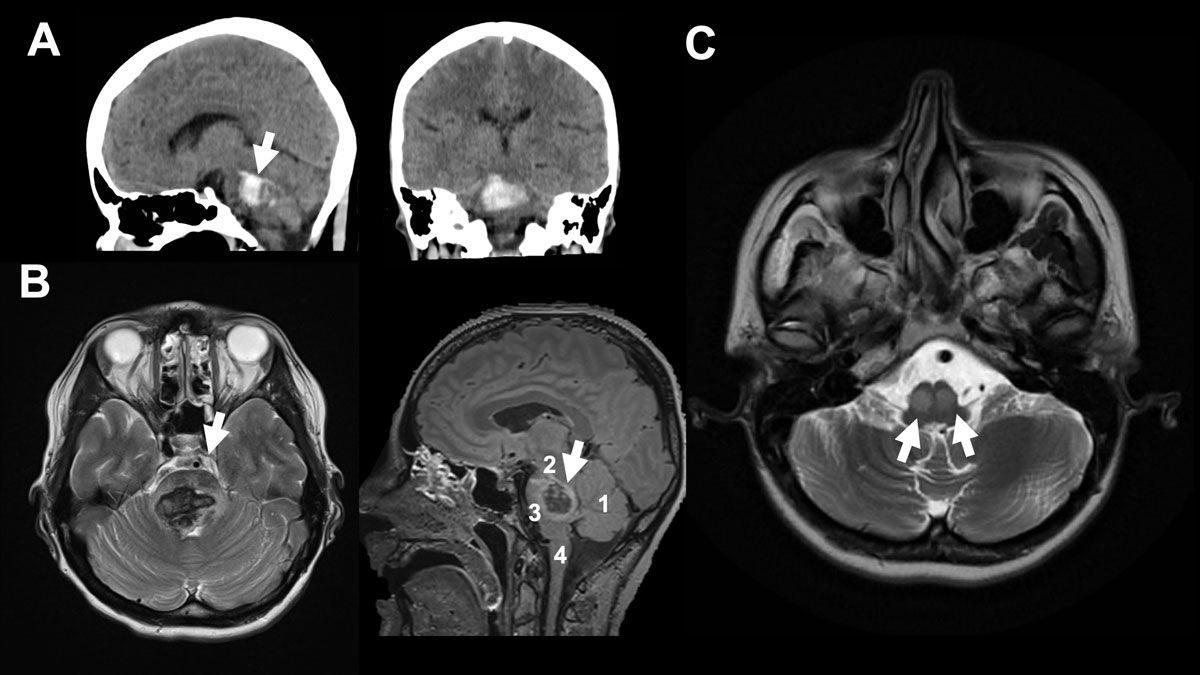
Figure 1
A 37-year-old female patient collapsed after alcohol and drug (cocaine) abuse. The “bilateral tegmental” haematoma was more pronounced on the right, with minimal ventricular extension. The patient’s initial GCS (Glasgow Coma Scale) was 6, with no pupil abnormality, and the patient was intubated in the ED. The patient was a regular cocaine user with a known history of hypertension. On day +4, extubation was unsuccessful due to bulbar palsy. Therefore, tracheotomy was performed on day +6. After decannulation, the patient was discharged to a neurological rehabilitation centre at GCS15 with a horizontal gaze palsy, tetra spasticity, left hypo sensitivity and left facial palsy. A. shows sagittal view and shows axial view of initial CT scan. B. shows initial MRI (axial FLAIR on the left-hand side and sagittal T2 on the right-hand side. Arrow (→) indicates the haemorrhage. 1 indicates cerebellum, 2 mesencephalon, 3 pons and 4 medulla oblongata. C. shows five-month follow-up MRI (axial FLAIR) with hypertrophic olivary degeneration (double arrows →→) caused by the brainstem haemorrhage. The inferior olivaries are part of the dentato-rubro-olivary tract – known as the Triangle of Guillain-Mollaret – connecting the brainstem and the deep cerebellar nuclei. A lesion like hypertrophic olivary degeneration at this triangle may cause modulation problems in spinal cord motor activity and myoclonus.
Vascular malformations, mostly cavernomas and arteriovenous malformations (AVMs), can be secondary causes of brainstem haemorrhages [7]. Brainstem haemorrhages are associated with the highest mortality rate among spontaneous ICHs [8]. Their reported mortality rate varies between 47% and 80% [8, 9].
Clinical presentation
Brainstem haemorrhage is an acute neurological illness with a very sudden onset. It is associated with early primary coma, motor disturbances (e.g. tetraplegia, hemiplegia or extensor posturing), respiratory disturbance, hyperthermia and pupillary abnormalities (e.g. pinpoint pupils, anisocoria) resulting from the destruction of the base or the tegmentum [4]. In the computed tomography (CT) and post-magnetic resonance imaging (MRI) era, even miniscule haemorrhages can be identified, resulting in the possible presentation of a broader spectrum of symptoms. However, in contrast to pontine infarctions, pontine bleedings rarely evolve slowly enough to present initial symptoms that reflect the presence of progressive damage to the cranial nerves and their nuclei [4, 10]. Previous retrospective studies have emphasised that severe initial neurological deficits, especially early coma, the need for mechanical ventilation and hydrocephalus, are associated with poor prognosis [9, 11]. Pupillary abnormalities do not show a significant correlation with outcome [11]. Patients bleeding secondarily into the brainstem due to cavernomas or arteriovenous malformations usually show less severe initial symptoms and have more favourable outcomes [7, 12]. These secondary haemorrhages have different dynamics to primary bleedings as they are more expanding than disruptive. Therefore, we have excluded them from this review.
Radiological parameters and their impact on outcome
Brainstem ICH is a medical emergency, and therefore diagnostics must be acquired quickly. MRI has proven to be more accurate than CT imaging at differentiating between haemorrhage ages and therefore at diagnosing older ICHs. For acute ICHs, they offer the same degree of accuracy [13]. Due to its rapid availability, the CT scan remains the imaging modality of choice for evaluating brainstem haemorrhages. In addition, unstable patients in acute distress represent a challenge where MRI is concerned: MRI is risky for them due to its long duration and the restricted access to the patient. Therefore, previous studies have tried to predict outcome depending on radiological CT findings [14–19]. However, MRI can serve as a follow-up diagnostic tool that is not only helpful for prognostication, but also for identifying the precise extension of the lesion, as well as the long-term effects of brainstem bleeding, e.g. olivary degeneration [20] (see also fig. 1).
Different kinds of classifications have been proposed for brainstem haemorrhages, mostly based on the axial CT features of the exact localisation and anatomical spread [11, 14, 15, 21]. Some classifications include tegmental parts (mesencephalon), and others concentrate solely on pontine haemorrhages (fig. 2). In a study of 62 cases in 1992, Chung et al. classified brainstem haematomas into four types: (1) the “massive” type, which they defined by its bilateral spread into both the basis pontis and the tegmentum; (2) the “bilateral tegmental” type, which occupied the bilateral tegmentum only; (3) the “basal-tegmental” type, which was localised in the junction between the basis pontis and the tegmentum bilaterally; and (4) the “small unilateral tegmental” type, which referred to haematomas located exclusively in the unilateral tegmentum [14]. The survival rates for the types were shown to be 7.1%, 14.3%, 26.1% and 94.1%, respectively [14]. Other groups (Rabinstein et al. and Balci et al.) tried to simplify this classification into three types by combining the basal and bilateral tegmental types in one group [16, 19]. However, a 1999 study by Fong et al. with 39 cases found a survival rate of 30.8% for the bilateral tegmental type and a 100% survival rate for the basal-tegmental type. This suggests that grouping the two locations together may not be justified [17]. In general, previous studies agree that basal-tegmentum and small unilateral tegmentum haemorrhages are associated with more favourable outcomes, while almost all massive haemorrhages and most bilateral tegmental haemorrhage cases are prognostically poor [16–18].
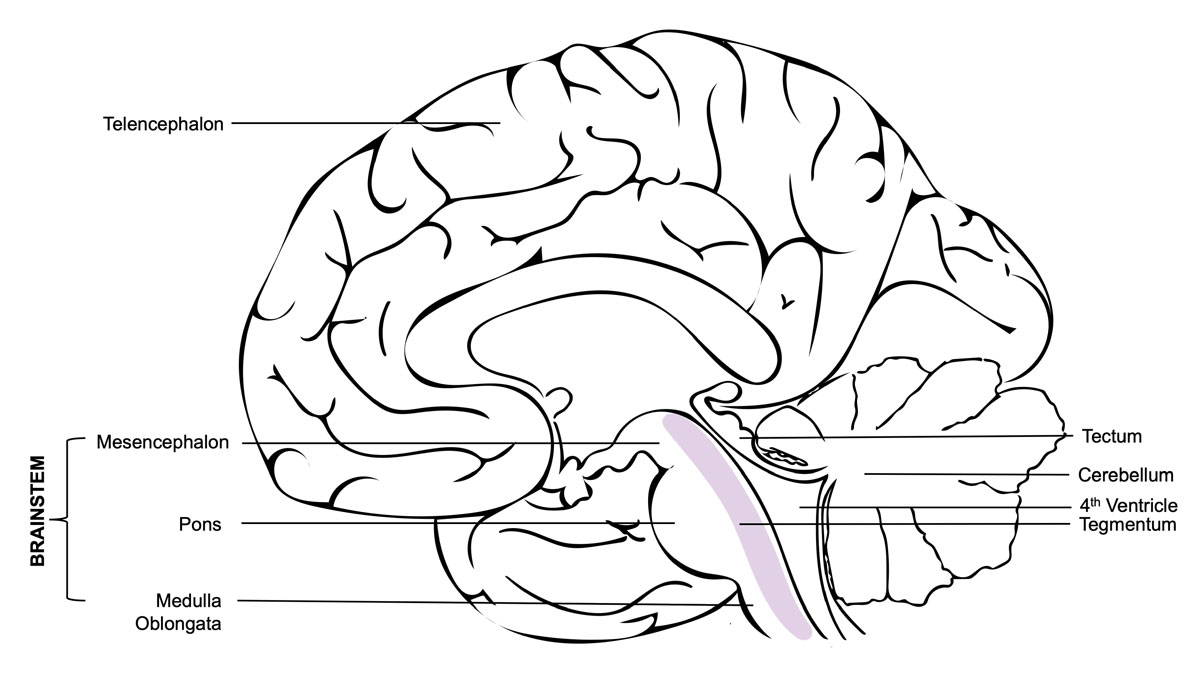
Figure 2 The mesencephalon, pons and medulla oblongata all belong to the functional unit that we call the brainstem. It is situated in the posterior cranial fossa and its ventral surface lies on the clivus. The brainstem is a very compact and dense region packed with cranial nerve nuclei and neurons from the reticular formation. Damage to the brainstem is therefore often life-threatening. Even small lesions can destroy cardiac and respiratory centres, disconnect the forebrain motor areas, or destroy higher centres of consciousness, perception and cognition. The tegmentum (Latin for “covering”) is an anatomical name for the area located between the ventricular system (4th ventricle) and the basal structures of the brainstem. It contains the rostral end of the reticular formation.
Russell et al. proposed a different approach. They divided brainstem haematomas into three types: central, dorsolateral tegmental and tegmentobasilar [21]. Large haematomas resulting from arterial hypertension generally occupied the central pons, leading to a rapid, fatal clinical course due to the involvement of the reticular system. Partial pontine haematomas, mostly occurring due to the rupture of cryptic vascular malformations, were restricted to the lateral half of the pons, sparing the reticular system. These could be either dorsolateral tegmental or tegmentobasilar.
In a study of 19 patients with pontine haemorrhages in 2012, Nishizaki et al. combined the classifications of Russel et al. and Chung et al. with some modifications. The resulting classifications are massive, tegmentobasilar, transverse oval and small unilateral [22]. Nishizaki et al. defined the transverse oval haematoma as an elliptical haematoma with the bilateral involvement of the basis, tegmentum or basal-tegmental junction. This type was similar to one type in Chung’s classification, for which the basal-tegmental junction between the basis pontis and the tegmentum was involved bilaterally. Nishizaki et al. found a mortality rate of 25% in cases with transverse oval or small unilateral haematomas and a mortality rate of 65% in cases with massive or tegmentobasilar haematomas [22].
Wessels et al. reviewed the clinical data of 29 consecutive patients with primary pontine haemorrhages (PPHs) in 2004, and divided PPHs into three new types without mentioning unilateralism or bilateralism: (1) dorsal, (2) ventral and (3) massive [11]. Studies using this classification reported that ventral or massive haematomas were related to high mortality, while dorsal haematomas had a favourable outcome [11, 23, 24]. Jang et al. conducted an outcome study involving 281 patients and using Wessels’ classification. It found 30-day mortality rates of 66.9, 24.7 and 1.5%. The study also investigated 90-day functional recovery and found rates of 3.8%, 14.8% and 14.9% for massive, ventral and dorsal PPHs respectively [25].
In another study of 39 consecutive patients by Dziewas et al., trans-axial pontine haemorrhages (PHs) were divided into three subtypes according to Kase and Caplan: (1) large paramedian PH, (2) unilateral basotegmental PH and (3) lateral tegmental PH. Dziewas et al. reported that paramedian PH was related to a fatal outcome, while lateral tegmental PH was associated with a favourable prognosis [15].
Despite all these variations in anatomical classification systems, the literature agrees on the following points: haematoma size (usually, the threshold values for predicting outcome range between 4 and 5 ml for volume or 20 and 27 mm for trans-axial diameter) and radiological signs of acute hydrocephalus correlate with poor outcomes [11, 15, 21, 23–27]. All studies of brainstem haemorrhages in the past two decades showed that unilateral tegmental haemorrhages had favourable outcomes, whereas bilateral basal bleedings and haemorrhages, including anterior segments (so-called massive), had the worst outcomes [9, 11, 14] (fig. 3). For all patients with radiological findings between these two extremes, survival outcome was very difficult to predict based on CT alone.
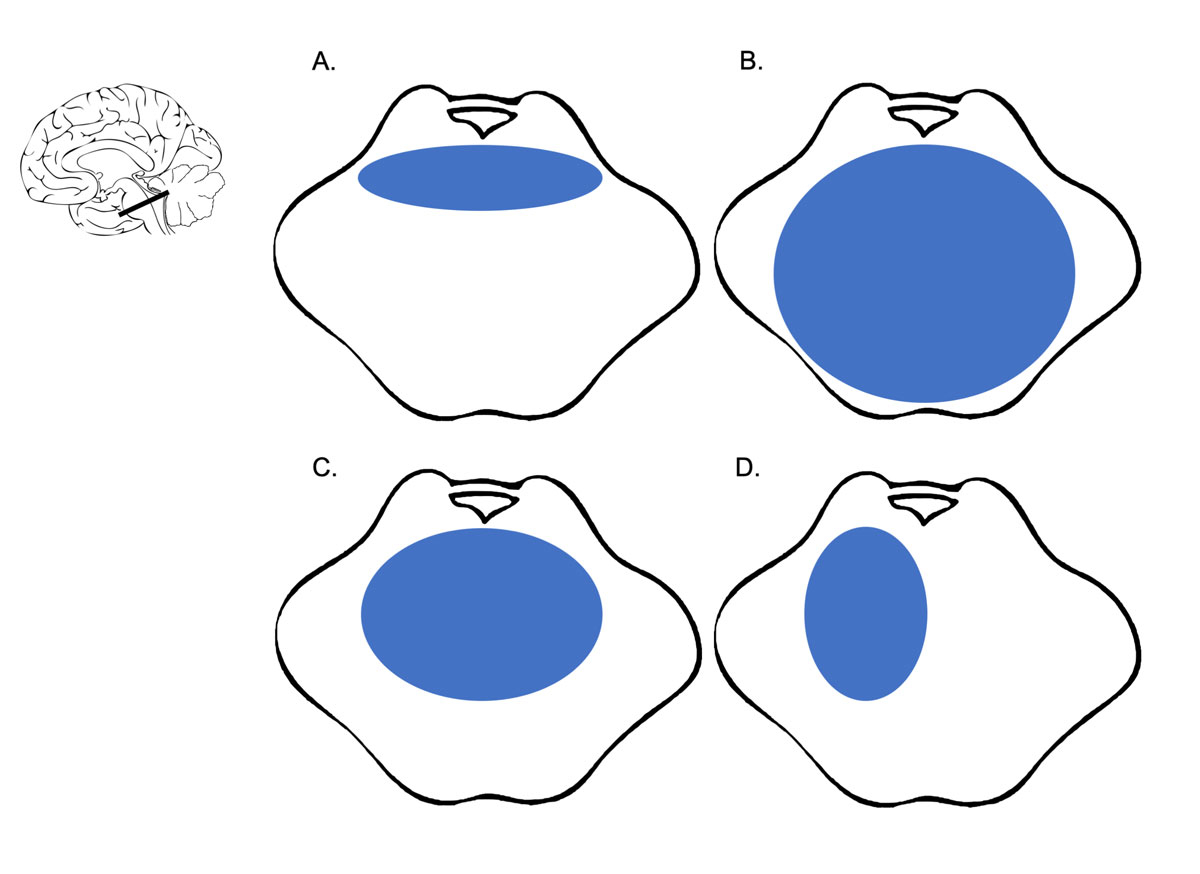
Figure 3 A-D show an axial view of the pontine part of the Brainstem. A. shows a “basal tegmental” haemorrhage, B. a “massive” haemorrhage, C. “bilateral tegmental” haemorrhage and D. “unilateral tegmental” haemorrhage. “Massive” brainstem haemorrhages have the poorest and “unilateral tegmental” the best clinical prognoses according to our literature review.
Electrophysiology
The importance of determining good prediction parameters for functional recovery in this patient group, in which survivors are often described as being in a severely disabled state, is clear [11, 25]. Imaging parameters do not convey any real-time information on the functional integrity of the corticospinal tract and somatosensory pathway. The use of neuromonitoring is widely recognised as an objective tool in terms of predicting post-stroke functional recovery. Little literature is available on the use of neuromonitoring to predict outcome and to guide therapeutic decision-making in patients with brainstem haemorrhages [28, 29].
The role of electroencephalogram (EEG) in brainstem monitoring remains highly disputed. It has been shown that the stimulation of the brainstem (e.g. the reticular formation) evokes changes in the EEG [30]. However, performing an EEG as part of brainstem monitoring, and especially in brainstem death diagnostics, is claimed to be more reassuring for the patients’ relatives than practical enough to guide clinical decision-making, for the obvious reason that recording an EEG from the scalp can hardly test brainstem function [31] (fig. 4 presents an illustrative case).
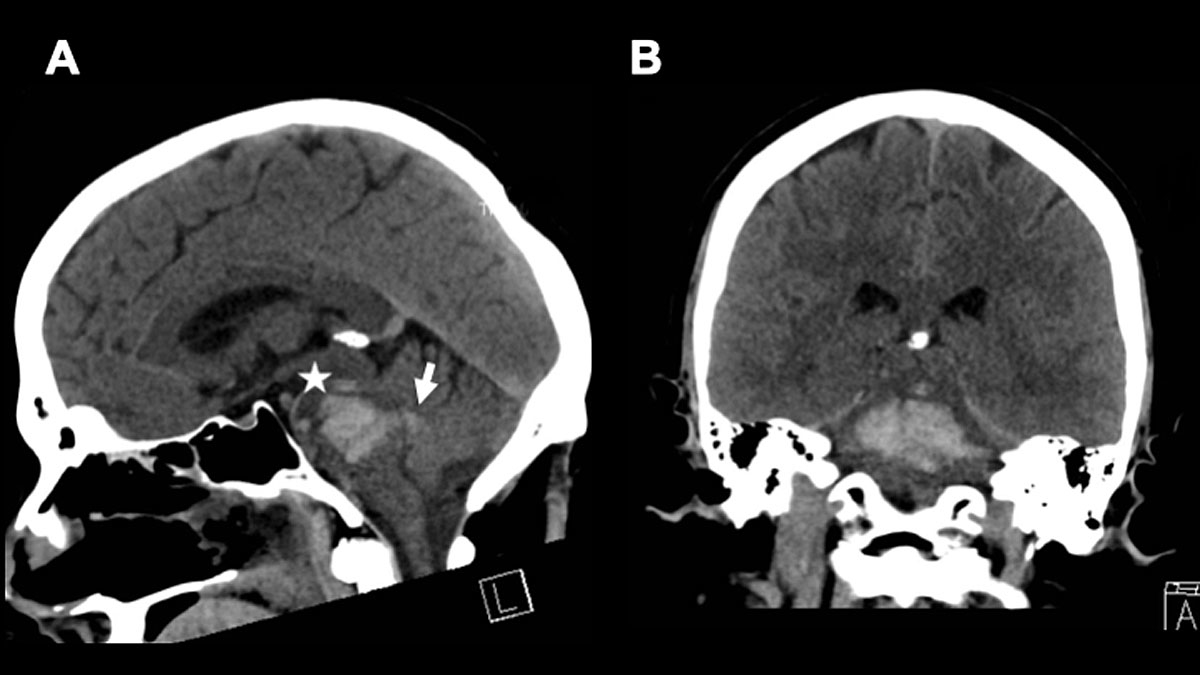
Figure 4 A 47-year-old male patient presented with an initial GSC of 3 at hospital admission. CT diagnostics revealed a “massive” brainstem haemorrhage. Electrophysiology showed highly pathological results (SEP, AEP and EEG). The patient was extubated on day +4 under a palliative care concept. A. shows a sagittal view and B. an axial view of the initial CT scan. * indicates the haemorrhage and → indicates the ventricular extension of the haemorrhage. Ventricular extension may cause hydrocephalus.
Electrophysiological examinations such as somatosensory evoked potentials (SSEPs), brainstem auditory evoked potentials (BAEPs) and motor evoked potentials (MEPs in limb muscles and corticobulbar MEPs (CoMEPs) in cranial nerve innervated muscles) are available to monitor the functional integrity of the motor pathways passing through the brainstem. Before the introduction of MEPs, BAEPs and SSEPs were the only two standard tools in surgical interventions in and around the brainstem. However, it was only possible to monitor approximately 20% of the brainstem with just these two neuromonitoring modalities (BAEPs and SSEPs) [32]. To obtain real-time information on the functional integrity of the motor pathways passing through the brainstem (corticospinal tract, CST), transcranial electrical stimulation (TES) is applied [33]. Multipulse TES allows MEP recording under general anaesthesia [32]. MEP responses are preferentially recorded from the distal muscles of the hand and foot. For CoMEPs, TES is applied to the motor strip (M1) for the cranial motor nerves, and CoMEPs are recorded in the appropriate muscles, i.e. those innervated by the cranial motor nerves [33, 34] (fig. 5). Seong et al. showed that the combined use of SSEPs and MEPs in the Neurocritical Care Unit was a reliable and useful tool for predicting functional recovery in patients with brainstem haemorrhages [35]. Furthermore, Seong et al. insisted that the combination of MEPs and SSEPs was a more powerful tool than measuring either the transverse diameter or the volume using CT. In their study, 14 patients with primary pontine haemorrhages were divided into good-outcome and poor-outcome groups according to the modified Rankin Score (mRS). When the MEP and SEP scores were summed (EP sum), the mRS and functional ambulatory category had the highest value [35].
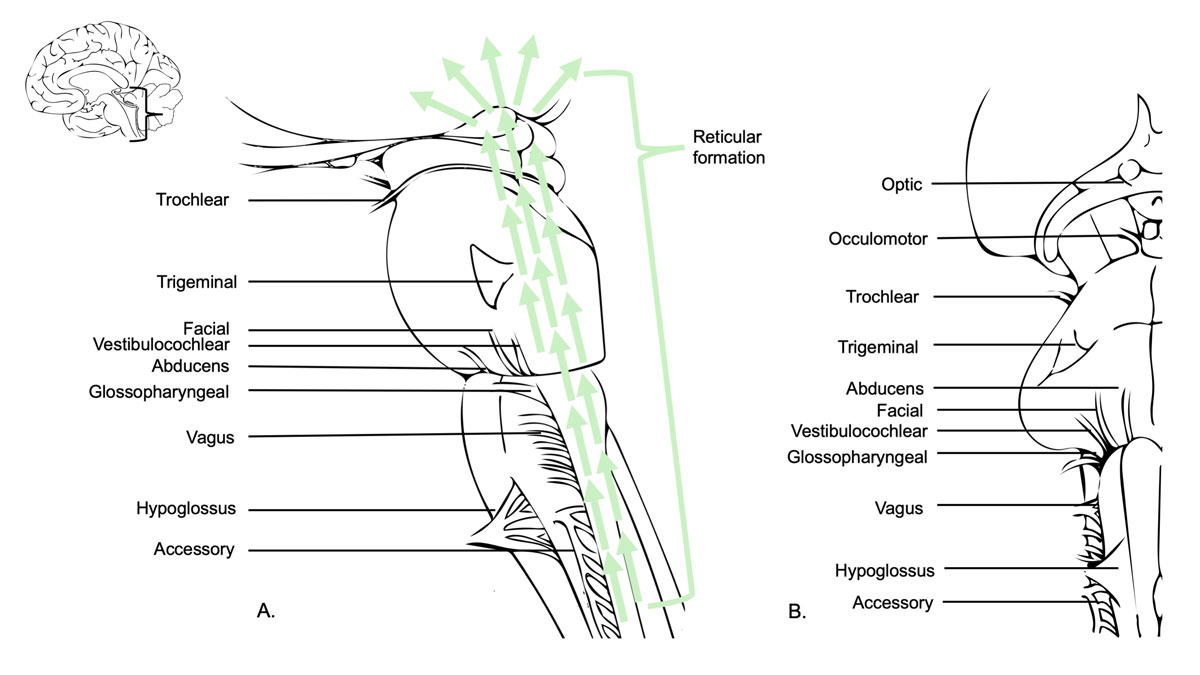
Figure 5 The brainstem contains the nuclei of the cranial nerves III–XII and is therefore responsible for the sensory, motor and autonomic innervation of the head and neck. The vagus nerve also arises from the brainstem and gives rise to other autonomic fibres (not limited to the head and neck). Some nuclei of the reticular formation are vital centres as they regulate cardiac and respiratory activities. Others are essential for cerebral arousal and maintenance of consciousness or are highly involved in the regulation of muscle tone, posture and reflex activities. A. shows sagittal view of the brainstem, the cranial nerves III–XII and the reticular formation. B. shows the ventral view (right side) of the brainstem.
Treatment
Conservative treatment
Prehospital and emergency department management for brainstem ICH includes close monitoring, securing the airway, provision of cardiovascular support and correction of the underlying haemostatic abnormalities. Hyperglycaemia and hypoglycaemia should be avoided. In 2015, Hemphill et al. added a new management recommendation, the treatment of fever, to the well-cited AHA/ASA guidelines for the management of spontaneous. This was because the duration of fever has been associated with poor prognosis and haematoma growth [36–38]. Blood pressure (BP) management in ICH has been extensively discussed recently. The current AHA/ASA guidelines, mostly influenced by the INTERACT2 study, suggest that early intensive lowering of BP to 140 mm Hg is safe and may be effective for patients presenting with a Glasgow Coma Score (GCS) >5 and a 150–220 mm Hg systolic BP. However, ATACH-2, a 2017 meta-analysis that included five studies and 4360 patients, subsequently confirmed that intensive, acute lowering of BP is safe, but found that it has no clinical benefit in terms of mortality or functional outcome [39–41].
There is a lack of clear brainstem haematoma management recommendations regarding when to consider the termination of treatment due to poor prognosis. These decisions can be quite challenging because brainstem haemorrhage patients are often younger than other ICH patients [4]. In our experience, this decision-making requires not only a close overview of a patient’s concomitant disease, radiological findings, initial neurological symptoms and course after treatment, but also the patient’s presumed will and (most often) cultural factors (fig. 6 presents an illustrative case).

Figure 6 A 66-year-old female patient with Down syndrome presented with fluctuating GCS between 7 and 10 and a “unilateral tegmental” brainstem haemorrhage. Neurological status was no pupil abnormalities and positive left Babinski. Due to neurological deterioration, the patient was intubated right after admission and transferred to the Neurosurgical ICU. Radiologically, no source of bleeding (e.g. vascular malformation) was identified. The patient suffered from known hypertension. Follow-up CT on day +1 showed 5 mm of growth in the haematoma with progressive compression of the fourth ventricle and an enlargement of the ventricular space. An external ventricular drain (EVD) procedure was performed the same day. BAEPs and EEG on day +5 were without pathological findings. After removal of the EVD, the patient again deteriorated neurologically. In conclusion with the radiological findings, a VP-shunt would have been needed long-term. With the perspective of a persistent, severely disabled state of the patient, and taking into account the presumed will of the patient, the family opted for comfort-care, which was initiated on day +18. The patient died on day +19. A. shows a sagittal view and an axial view of the initial CT scan. B shows the initial MRI scan and the extent of the haemorrhage (FLAIR left and SWI right). The Arrow (→) indicates the haemorrhage.
Surgical concepts
Neurosurgical interventions like haematoma evacuation remain controversial for ICH because randomised clinical studies have failed to demonstrate a clear benefit to surgical management. Conceptually, the evacuation of the haematoma is aimed not only at decreasing the mass effect and the intracranial pressure (ICP), but also at removing the haemorrhagic products, which cause inflammation and degradation of the brain parenchyma. The brainstem is especially sensitive to these cytotoxic effects [42].
In secondary brainstem haemorrhage due to a cavernoma, if surgical evacuation is indicated it is recommended after a period of about two weeks to allow the patient to stabilise and the haematoma to organise [7]. For primary haematomas in the posterior fossa, however, the AHA/ASA Guidelines recommend considering the surgical removal of cerebellar haemorrhages, but not of brainstem haemorrhages. The guidelines even clearly advise against the surgical evacuation of brainstem haematomas [38].
Ongoing clinical trials plan to deliver new data on hemicraniectomy (SWITCH) and minimal invasive approaches (MISTIE III and MISTICH) to surgical ICH management. However, infratentorial ICH has, without exception, been excluded from all these studies. In 1984, De Pian et al. brought together 50 brainstem haematoma cases from 22 Italian centres, and showed that there was some value to surgical evacuation in chronic cases [43]. Two Japanese groups (Takahama et al. and Hara et al.) proposed stereotactic aspiration. They found a favourable functional outcome in surgically treated patients compared to patients with conservatively managed haematomas [44, 45].
A more commonly performed surgical procedure in the case of brainstem haematoma is the external ventricular drain (EVD) procedure. An EVD is indicated in cases that present with clinical and radiological signs of hydrocephalus. This is often the case when the bleeding extends to the ventricular system [46]. However, as mentioned above, hydrocephalus has been associated with poor outcomes in brainstem haemorrhages, and one can argue that its indication should therefore be thoroughly evaluated. Murata et al. found no significant improvement in outcome when hydrocephalus was treated with ventricular drainage [9]. In a retrospective, observational, single-centre study that focused on the comparison of decompressive craniectomy, medical therapy and EVD placement in patients with haemorrhages in the posterior fossa (focusing on cerebellar haemorrhages with and without brainstem involvement), Luney et al. found a significant increase in hydrocephalus and intraventricular bleeds in patients treated with EVD placement [47].
Conclusion
Primary brainstem haemorrhages remain associated with poor outcomes compared to other forms of spontaneous ICH. Negative prognostic factors are coma on admission, the need for mechanical ventilation, haematoma volume and the ventral extension of the haemorrhage. Many groups have tried to classify brainstem haemorrhages according to radiological parameters such as anatomical spread, haematoma size and signs of hydrocephalus. To summarise their findings, they all agree on the association of poor outcome with so-called “massive” haemorrhages and the association of good prognosis with unilateral tegmental haemorrhages. All cases between these two extremes show large variation in survival and functional recovery rates (see bottom of fig. 7). Therefore, clinical presentation and other surrogate markers must be considered to enable better early prognosis for the patient and their family, and to guide clinical decision-making.
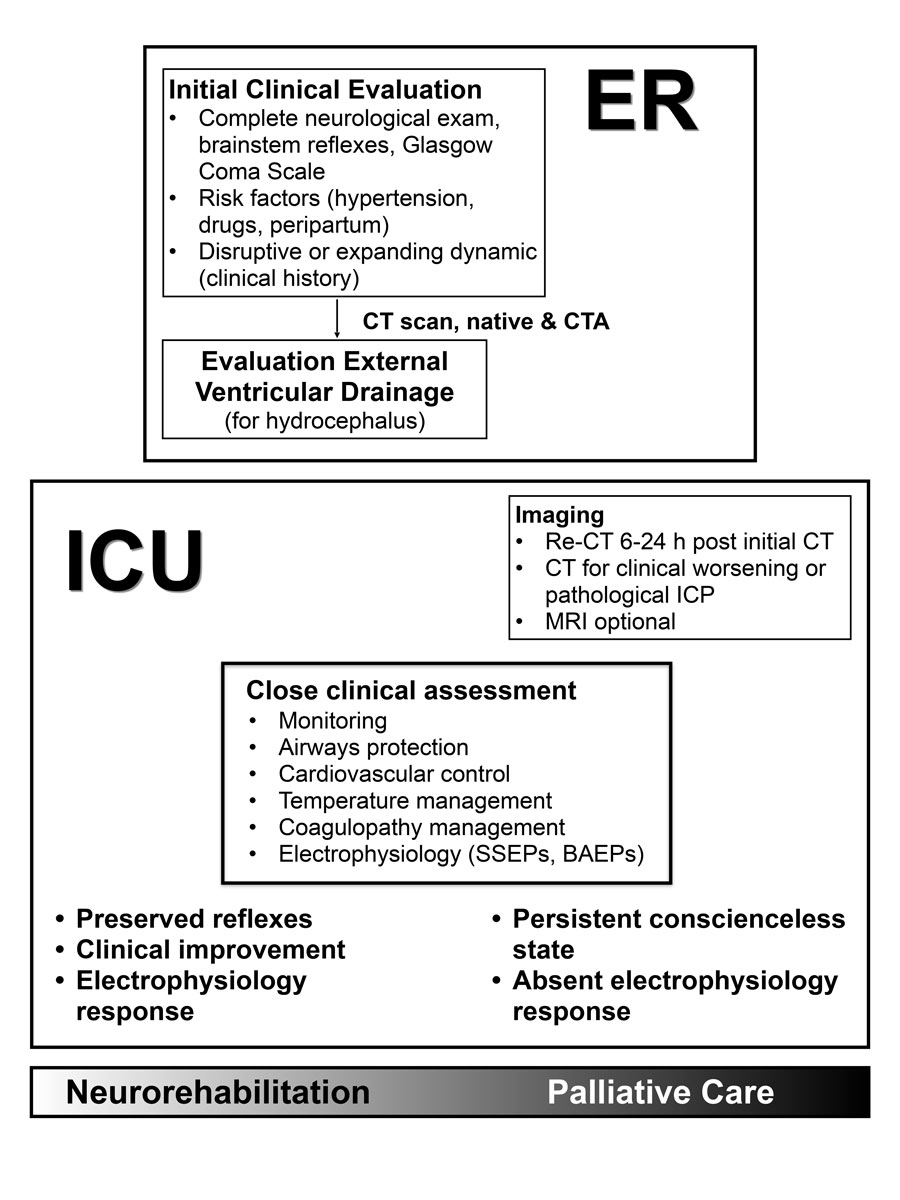
Figure 7 As it is a very heterogeneous disease, clear management guidelines concerning brainstem haemorrhage do not exist. An initial clinical and neuroradiological evaluation must be obtained rapidly in the ER, including thorough neurological exams, the acquisition of the patient’s medical history, risk factors, a cranial CT scan and an angio CT. If there are signs of hydrocephalus, the decision on an EVD must be made after CT. Management should continue in the ICU (Intensive Care Unit) with close clinical assessments, neuroprotective treatment (airway protection, tight control of blood pressure, temperature, glucose and electrolytes, correction of coagulopathy) and electrophysiology. Ultimately, whether to rehabilitate or to initiate palliative care is an individual decision. Clinical assessment and electrophysiological testing can guide the making of this decision.
In our opinion, besides close neurological examinations, the combined use of SSEPs, BAEPs, MEPs and CoMEPs provides the most comprehensive approach to predicting functional recovery in patients with brainstem haemorrhages. In the future, a prospective study on neuromonitoring in patients with brainstem haemorrhages will be necessary to define specific parameters that correlate with good and poor outcomes.
Currently, treatment is still conservative neurocritical care. Well-known guidelines clearly advise against surgical intervention in primary brainstem haemorrhages. However, the dataset supporting this recommendation remains small because ICH in the posterior fossa has been excluded from large ICH surgical intervention trials in the past, as well as from those that are planned for the future. EVD is formally indicated in cases which present with hydrocephalus. However, its benefit for those presenting with brainstem haemorrhages has not been proven yet.
Generally, there have been few studies concerning the neurosurgical management of brainstem haemorrhages, and most of the existing ones were carried out before the present millennium began. Therefore, we see limitations in the adaptation of those results and recommendations to today’s clinical practice. When comparing the numbers, newer studies differ from older studies, showing better survival rates for the same radiological haemorrhage subtypes.
Brainstem haemorrhage management needs re-evaluation and reinvestigation to update the numbers for incidence, mortality, outcome, prognostic factors, standardised management and the indication of surgical procedures. However, the number of cases is limited. Therefore, we recommend a national or international registry that will collect data and shed light on the current state of brainstem haemorrhage treatment. This could be an important step for future clinical trials investigating new treatment options for brainstem haemorrhages.
References
1
Sacco
RL
,
Kasner
SE
,
Broderick
JP
,
Caplan
LR
,
Connors
JJ
,
Culebras
A
, et al.; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–89. doi:.https://doi.org/10.1161/STR.0b013e318296aeca
2
Sacco
S
,
Marini
C
,
Toni
D
,
Olivieri
L
,
Carolei
A
. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. 2009;40(2):394–9. doi:.https://doi.org/10.1161/STROKEAHA.108.523209
3
Sarti
C
,
Rastenyte
D
,
Cepaitis
Z
,
Tuomilehto
J
. International trends in mortality from stroke, 1968 to 1994. Stroke. 2000;31(7):1588–601. doi:.https://doi.org/10.1161/01.STR.31.7.1588
4
Dinsdale
HB
. Spontaneous hemorrhage in the posterior fossa. A study of primary cerebellar and pontine hemorrhages with observations on their pathogenesis. Arch Neurol. 1964;10(2):200–17. doi:.https://doi.org/10.1001/archneur.1964.00460140086011
5
van Asch
CJ
,
Luitse
MJ
,
Rinkel
GJ
,
van der Tweel
I
,
Algra
A
,
Klijn
CJ
. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167–76. doi:.https://doi.org/10.1016/S1474-4422(09)70340-0
6
Martin-Schild
S
,
Albright
KC
,
Hallevi
H
,
Barreto
AD
,
Philip
M
,
Misra
V
, et al.
Intracerebral hemorrhage in cocaine users. Stroke. 2010;41(4):680–4. doi:.https://doi.org/10.1161/STROKEAHA.109.573147
7
Bozinov
O
,
Hatano
T
,
Sarnthein
J
,
Burkhardt
JK
,
Bertalanffy
H
. Current clinical management of brainstem cavernomas. Swiss Med Wkly. 2010;140:w13120.
8
Nilsson
OG
,
Lindgren
A
,
Brandt
L
,
Säveland
H
. Prediction of death in patients with primary intracerebral hemorrhage: a prospective study of a defined population. J Neurosurg. 2002;97(3):531–6. doi:.https://doi.org/10.3171/jns.2002.97.3.0531
9
Murata
Y
,
Yamaguchi
S
,
Kajikawa
H
,
Yamamura
K
,
Sumioka
S
,
Nakamura
S
. Relationship between the clinical manifestations, computed tomographic findings and the outcome in 80 patients with primary pontine hemorrhage. J Neurol Sci. 1999;167(2):107–11. doi:.https://doi.org/10.1016/S0022-510X(99)00150-1
10
Masiyama
S
,
Niizuma
H
,
Suzuki
J
. Pontine haemorrhage: a clinical analysis of 26 cases. J Neurol Neurosurg Psychiatry. 1985;48(7):658–62. doi:.https://doi.org/10.1136/jnnp.48.7.658
11
Wessels
T
,
Möller-Hartmann
W
,
Noth
J
,
Klötzsch
C
. CT findings and clinical features as markers for patient outcome in primary pontine hemorrhage. AJNR Am J Neuroradiol. 2004;25(2):257–60.
12
Choi
JH
,
Mast
H
,
Sciacca
RR
,
Hartmann
A
,
Khaw
AV
,
Mohr
JP
, et al.
Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke. 2006;37(5):1243–7. doi:.https://doi.org/10.1161/01.STR.0000217970.18319.7d
13
Kidwell
CS
,
Chalela
JA
,
Saver
JL
,
Starkman
S
,
Hill
MD
,
Demchuk
AM
, et al.
Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292(15):1823–30. doi:.https://doi.org/10.1001/jama.292.15.1823
14
Chung
CS
,
Park
CH
. Primary pontine hemorrhage: a new CT classification. Neurology. 1992;42(4):830–4. doi:.https://doi.org/10.1212/WNL.42.4.830
15
Dziewas
R
,
Kremer
M
,
Lüdemann
P
,
Nabavi
DG
,
Dräger
B
,
Ringelstein
B
. The prognostic impact of clinical and CT parameters in patients with pontine hemorrhage. Cerebrovasc Dis. 2003;16(3):224–9. doi:.https://doi.org/10.1159/000071120
16
Balci
K
,
Asil
T
,
Kerimoglu
M
,
Celik
Y
,
Utku
U
. Clinical and neuroradiological predictors of mortality in patients with primary pontine hemorrhage. Clin Neurol Neurosurg. 2005;108(1):36–9. doi:.https://doi.org/10.1016/j.clineuro.2005.02.007
17
Fong
C
. Factors affecting the outcome in pontine hemorrhage. Acta Neurol Taiwan. 1999;8:6–11.
18
Wijdicks
EF
,
St Louis
E
. Clinical profiles predictive of outcome in pontine hemorrhage. Neurology. 1997;49(5):1342–6. doi:.https://doi.org/10.1212/WNL.49.5.1342
19
Rabinstein
AA
,
Tisch
SH
,
McClelland
RL
,
Wijdicks
EF
. Cause is the main predictor of outcome in patients with pontine hemorrhage. Cerebrovasc Dis. 2004;17(1):66–71. doi:.https://doi.org/10.1159/000073900
20
Uchino
A
,
Hasuo
K
,
Uchida
K
,
Matsumoto
S
,
Tsukamoto
Y
,
Ohno
M
, et al.
Olivary degeneration after cerebellar or brain stem haemorrhage: MRI. Neuroradiology. 1993;35(5):335–8. doi:.https://doi.org/10.1007/BF00588362
21
Russell
B
,
Rengachary
SS
,
McGregor
D
. Primary pontine hematoma presenting as a cerebellopontine angle mass. Neurosurgery. 1986;19(1):129–33. doi:.https://doi.org/10.1227/00006123-198607000-00023
22
Nishizaki
T
,
Ikeda
N
,
Nakano
S
,
Sakakura
T
,
Abiko
M
,
Okamura
T
. Factors Determining the Outcome of Pontine Hemorrhage in the Absence of Surgical Intervention. Open Journal of Modern Neurosurgery.
2012;02(02):17–20. doi:.https://doi.org/10.4236/ojmn.2012.22004
23
Ye
Z
,
Huang
X
,
Han
Z
,
Shao
B
,
Cheng
J
,
Wang
Z
, et al.
Three-year prognosis of first-ever primary pontine hemorrhage in a hospital-based registry. J Clin Neurosci. 2015;22(7):1133–8. doi:.https://doi.org/10.1016/j.jocn.2014.12.024
24
Matsukawa
H
,
Shinoda
M
,
Fujii
M
,
Takahashi
O
,
Murakata
A
. Risk factors for mortality in patients with non-traumatic pontine hemorrhage. Acta Neurol Scand. 2015;131(4):240–5. doi:.https://doi.org/10.1111/ane.12312
25
Jang
JH
,
Song
YG
,
Kim
YZ
. Predictors of 30-day mortality and 90-day functional recovery after primary pontine hemorrhage. J Korean Med Sci. 2011;26(1):100–7. doi:.https://doi.org/10.3346/jkms.2011.26.1.100
26Behrouz R. Prognostic factors in pontine haemorrhage: A systematic review. European Stroke Journal. 2018???:1-9.
27
Shin
S
,
Lim
DJ
,
Kim
SD
. Primary pontine hemorrhage. an analysis of 35 cases and research in prognostic factors. Kor J Cerebrovasc Surg. 2007;9:41–5.
28
Guérit
JM
. Neurophysiological testing in neurocritical care. Curr Opin Crit Care. 2010;16(2):98–104. doi:.https://doi.org/10.1097/MCC.0b013e328337541a
29
Lee
SY
,
Lim
JY
,
Kang
EK
,
Han
MK
,
Bae
HJ
,
Paik
NJ
. Prediction of good functional recovery after stroke based on combined motor and somatosensory evoked potential findings. J Rehabil Med. 2010;42(1):16–20. doi:.https://doi.org/10.2340/16501977-0475
30
Moruzzi
G
,
Magoun
HW
. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1(4):455–73. doi:.https://doi.org/10.1016/0013-4694(49)90219-9
31
Pallis
C
. ABC of brain stem death. The arguments about the EEG. Br Med J (Clin Res Ed). 1983;286(6361):284–7. doi:.https://doi.org/10.1136/bmj.286.6361.284
32
Deletis
V
,
Sala
F
. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: a review focus on the corticospinal tracts. Clin Neurophysiol. 2008;119(2):248–64. doi:.https://doi.org/10.1016/j.clinph.2007.09.135
33
Deletis
V
,
Fernández-Conejero
I
. Intraoperative monitoring and mapping of the functional integrity of the brainstem. J Clin Neurol. 2016;12(3):262–73. doi:.https://doi.org/10.3988/jcn.2016.12.3.262
34
Slotty
PJ
,
Abdulazim
A
,
Kodama
K
,
Javadi
M
,
Hänggi
D
,
Seifert
V
, et al.
Intraoperative neurophysiological monitoring during resection of infratentorial lesions: the surgeon’s view. J Neurosurg. 2017;126(1):281–8. doi:.https://doi.org/10.3171/2015.11.JNS15991
35
Seong
JW
,
Kim
MH
,
Shin
HK
,
Lee
HD
,
Park
JB
,
Yang
DS
. Usefulness of the combined motor evoked and somatosensory evoked potentials for the predictive index of functional recovery after primary pontine hemorrhage. Ann Rehabil Med. 2014;38(1):13–8. doi:.https://doi.org/10.5535/arm.2014.38.1.13
36
Schwarz
S
,
Häfner
K
,
Aschoff
A
,
Schwab
S
. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology. 2000;54(2):354–61. doi:.https://doi.org/10.1212/WNL.54.2.354
37
Rincon
F
,
Lyden
P
,
Mayer
SA
. Relationship between temperature, hematoma growth, and functional outcome after intracerebral hemorrhage. Neurocrit Care. 2013;18(1):45–53. doi:.https://doi.org/10.1007/s12028-012-9779-9
38
Hemphill
JC, 3rd
,
Greenberg
SM
,
Anderson
CS
,
Becker
K
,
Bendok
BR
,
Cushman
M
, et al.; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–60. doi:.https://doi.org/10.1161/STR.0000000000000069
39
Anderson
CS
,
Heeley
E
,
Huang
Y
,
Wang
J
,
Stapf
C
,
Delcourt
C
, et al.; INTERACT2 Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368(25):2355–65. doi:.https://doi.org/10.1056/NEJMoa1214609
40
Qureshi
AI
,
Palesch
YY
,
Barsan
WG
,
Hanley
DF
,
Hsu
CY
,
Martin
RL
, et al.; ATACH-2 Trial Investigators and the Neurological Emergency Treatment Trials Network. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016;375(11):1033–43. doi:.https://doi.org/10.1056/NEJMoa1603460
41
Boulouis
G
,
Morotti
A
,
Goldstein
JN
,
Charidimou
A
. Intensive blood pressure lowering in patients with acute intracerebral haemorrhage: clinical outcomes and haemorrhage expansion. Systematic review and meta-analysis of randomised trials. J Neurol Neurosurg Psychiatry. 2017;88(4):339–45. doi:.https://doi.org/10.1136/jnnp-2016-315346
42
Broderick
JP
. The STICH trial: what does it tell us and where do we go from here?
Stroke. 2005;36(7):1619–20. doi:.https://doi.org/10.1161/01.STR.0000170714.43167.34
43
Da Pian
R
,
Bazzan
A
,
Pasqualin
A
. Surgical versus medical treatment of spontaneous posterior fossa haematomas: a cooperative study on 205 cases. Neurol Res. 1984;6(3):145–51. doi:.https://doi.org/10.1080/01616412.1984.11739680
44
Takahama
H
,
Morii
K
,
Sato
M
,
Sekiguchi
K
,
Sato
S
. [Stereotactic aspiration in hypertensive pontine hemorrhage: comparative study with conservative therapy]. No Shinkei Geka. 1989;17(8):733–9.
45
Hara
T
,
Nagata
K
,
Kawamoto
S
,
Sashida
J
,
Abe
T
,
Wada
A
, et al.
[Functional outcome of primary pontine hemorrhage: conservative treatment or stereotaxic surgery]. No Shinkei Geka. 2001;29(9):823–9.
46
Nieuwkamp
DJ
,
de Gans
K
,
Rinkel
GJ
,
Algra
A
. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. J Neurol. 2000;247(2):117–21. doi:.https://doi.org/10.1007/PL00007792
47
Luney
MS
,
English
SW
,
Longworth
A
,
Simpson
J
,
Gudibande
S
,
Matta
B
, et al.
Acute posterior cranial fossa hemorrhage-is surgical decompression better than expectant medical management?
Neurocrit Care. 2016;25(3):365–70. doi:.https://doi.org/10.1007/s12028-015-0217-7






