Screening strategy for Chagas disease in a non-endemic country (Switzerland): a prospective evaluation
DOI: https://doi.org/10.4414/smw.2019.20050
Claire
Da Costa-Demaurexa, Maria T.
Cárdenasb, Hernando
Aparicioc, Patrick
Bodenmannb, Blaise
Gentonbd, Valérie
D’Acremontbd
aFaculty of Biology and Medicine, University of Lausanne, Switzerland
bDepartment of Ambulatory Care and Community Medicine, University Hospital of Lausanne, Switzerland
cClinique Bois-Bougy, Nyon, Switzerland
dSwiss Tropical and Public Health Institute, University of Basel, Switzerland
Summary
The WHO recommends screening of Latin American migrants for Chagas disease to reduce morbidity and mortality and increase the likelihood of eradicating the disease. The objective was to assess the feasibility and acceptability of a screening strategy in one Swiss canton. From February 2011 to September 2012, people attending six healthcare centres of different types were offered a rapid diagnostic test if they or their mother were of Latin American origin (or, at the blood donation centre, if they had travelled for ≥1 year in Latin America). In addition, testing was offered during events where Latin Americans gathered. In total, 1,010 people were tested, mainly originating from Brazil (24%), Ecuador (13%) and Chile (10%). 54% were born in Latin America, 15% had a Latin American mother, and 29% were travellers. The prevalence of Chagas disease was 2.3% among migrants (15.5% in the community testing) and 0% among travellers. The prevalence was 18.0%, 0.8%, 0.5% and 0% among Bolivians, Ecuadorians, Brazilians and other countries respectively. Predictors for Chagas disease were: born in Latin America (OR = infinite, p <0.001), Bolivian origin (OR = 95, 95% CI: 19–482, p <0.001), being tested in the community (OR = 56, 95% CI: 14–218, p <0.001), and age >35 years OR = 3.4, 95% CI: 1.1–10.5, p = 0.03). The prevalence of Chagas disease was much higher in people attending social events than healthcare centres, suggesting that observations based only on health facility data underestimate the real prevalence of Chagas disease. Screening in the community was well accepted and should be promoted to reach the population at highest risk.
Introduction
Chagas disease is caused by Trypanosoma cruzi, a tropical protozoan parasite transmitted by the blood-sucking bug Triatoma. It affects around 8 million people worldwide and is no longer restricted to Latin America. Migration and travel have caused the disease to spread out of the continent and reach most countries in the world. This phenomenon has been observed particularly in populations able to transmit the disease in the absence of the vector, such as in pregnant women through vertical transmission [1–4] and blood and organ donors [5–10]. This situation has led the World Health Organization (WHO) to recommend screening migrants of Latin American origin living in non-endemic countries for Chagas disease [11, 12], both to reduce morbidity and mortality, and to work towards eradicating the disease globally. Providing treatment to patients with Chagas disease would not only reduce their risk of developing potentially severe cardiac or intestinal impairments, but would also help to stop transmission in women of childbearing age and through blood transfusions or organ transplants. However, the best screening strategy is not yet known and might not be the same for all settings. Testing of sick patients attending primary healthcare centres has existed for many years in some non-endemic countries, but the systematic screening of asymptomatic people has been introduced more recently. The latter now exists in several cities in Spain, such as Barcelona [13–15], Asturias [16], Elche [17] and Madrid [18], as well as in Italy [19], France [20] and Canada [21]. These studies have enabled us to know the true prevalence of the disease among Latin American migrants, which is essential in order to design an efficient screening strategy. In Geneva, Switzerland, people attending a primary healthcare facility specifically for migrants have been screened [4], revealing that 12.8% of people tested were positive. This high prevalence and the large Latin American population living in Switzerland (estimated to be around 80,000 people [22–24]), highlight the importance of investigating the situation in the nearby canton of Vaud, where testing for Chagas disease had never been offered and the prevalence is thus totally unknown.
In addition to the prevalence of the disease, knowledge of the positive predictors for Chagas disease is important to better target screening for the highest risk population. The few studies which have investigated predictors in asymptomatic migrants have found that Bolivian origin [4], having heard about the disease, knowing someone with Chagas disease, and having lived in adobe houses and rural areas [13] are significantly associated with having the disease.
Another important element in the design of an effective screening strategy is the type of diagnostic test to be used. Rapid diagnostic tests with excellent performance [25, 26] are available for Chagas and are ideal in the context of a mobile population because they allow immediate results. It is also essential to understand the acceptability of and barriers to screening in the migrant Latin American population to have a feasible and durable strategy.
This study aims to assess the feasibility and acceptability of a systematic screening strategy for Chagas disease in the canton of Vaud. It also aims to estimate the number of people needed to test, according to demographic and clinical characteristics, for screening to focus on the population most at risk in the future.
Materials and methods
Population and setting
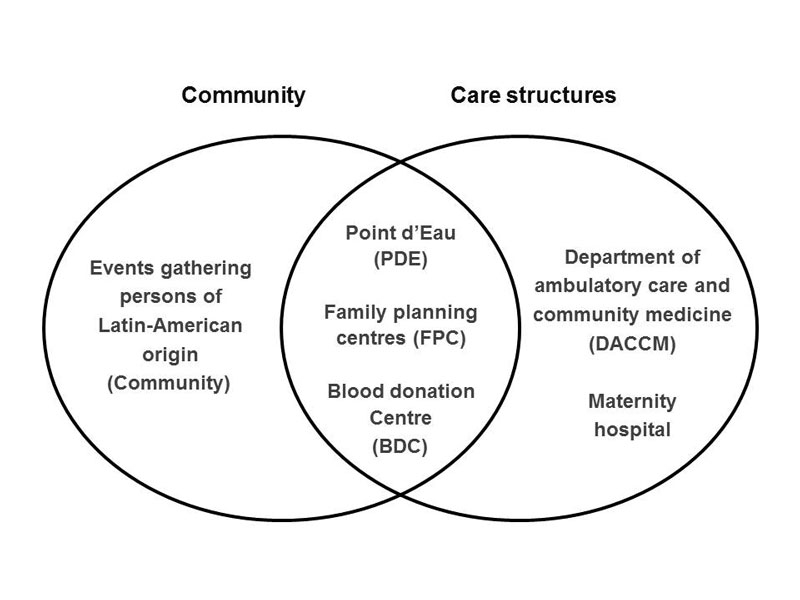
The study took place in six healthcare centres located in Lausanne and the surrounding area. The inclusion criteria were being born (or having spent a considerable part of infancy) in Latin America or having a mother who was born in Latin America (excluding the Caribbean). At the Department of Ambulatory Care and Community Medicine (DACCM), which serves as an outpatient clinic for the University Hospital of Lausanne, the clinician in charge offered a rapid diagnostic test for 20 Swiss francs (22 USD) during routine emergency consultations. At the Point d’Eau (PDE), an urban, low-level healthcare centre attended mainly by undocumented immigrants, the three nurse practitioners offered a rapid test during the usual consultation for 3 Swiss francs. At the maternity hospital, Latin American women attending the obstetric clinic were offered the diagnostic test for 60 Swiss Francs (66 USD). The test was also offered during consultations at two family planning centres (FPC), which provide sexual counselling and related activities, for the price of 22 Swiss francs (24 USD). Systematic screening was implemented at the blood donation centre (BDC), where an additional inclusion criterion was used: having travelled in Latin America for more than one year. Finally, screening was offered free of charge during events (such as cultural celebrations) where people of Latin American origin, Bolivians in particular, gathered (see fig. 1 below).
Study design
From February 2011 to September 2012, Latin Americans attending the six healthcare centres mentioned above were offered screening for Chagas disease. Screening was first offered to patients attending the DACCM and the PDE. The screening was extended to the two FPC in November 2011 and to pregnant women attending the maternity hospital in January 2012. At the blood donation centre, people were tested from the beginning of the study. In May 2012, screening in the community was proposed by a team composed of a Bolivian pharmacist working as cultural mediator, a medical doctor specialising in tropical diseases, a Bolivian medical doctor and a master’s student in medicine working on the topic. Latin American associative organisations were contacted to assist in choosing events which attracted sufficient numbers of people and to help disseminate the information. A leaflet and poster explaining the transmission, symptoms, clinical course, screening and treatment of Chagas disease in French, Spanish and Portuguese, were developed based on a Spanish leaflet from the “Hospital Universitario Ramón y Cajal” of Madrid. These educational materials helped to spread information about the availability of screening at the different healthcare centres, as well as the management and treatment of positive cases.
A rapid diagnostic test (Chagas Stat-Pak, Chembio, USA) was used in all settings except at the blood donation centre, where a serology test was used. At the maternity hospital, the rapid test was performed at the central laboratory at the same time as the other screening tests required during pregnancy. An internal quality control test was performed on each new batch of rapid diagnostic tests. When the test was positive, patients were offered an appointment at the Tropical Diseases Unit of the DACCM for confirmation of the diagnosis by conventional serology (BioElisa Chagas, Biokit®), and management and treatment of Chagas disease.
Data collection and analysis
Age, sex, country of origin or travel, eligibility criteria and test results (the main outcome measure) were collected prospectively. At the DACCM, the PDE and the maternity hospital, nine symptoms and signs (dyspnoea, palpitations, chest pain, dysphagia, odynophagia, constipation, signs or symptoms of cardiac insufficiency, abnormal cardiac sounds and intestinal obstruction) known to be predictive of symptomatic Chagas disease [27] were assessed by the clinician in charge. Data were collected prospectively and anonymously, and entered into an Excel database which was then analysed in Stata version 10.1. Simple proportions and crude odds ratios (a simple stratification was used to adjust for Bolivian origin) were calculated, as were 95% confidence intervals (95% CI) using the exact test and p-values (two-sided) using Pearson χ2 statistics.
Ethical considerations
The study, including the use of verbal consent, was approved by the ‘Commission cantonale d’éthique de la recherche sur l’être humain’, Lausanne, Switzerland. Verbal consent testified by the written signature of the health worker in charge of the diagnostic testing was obtained for each participant prospectively. Written consent could not be obtained because most of the participants had an illegal status in Switzerland and were afraid that writing their name could lead to denunciation.
Results
Population tested
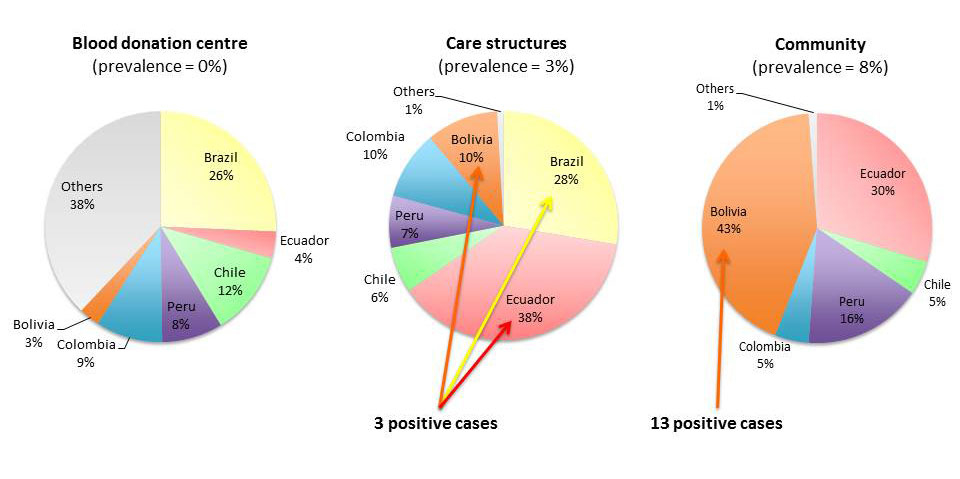
In total, 1010 adults were tested for Chagas disease: 107 (11%) at the DACCM, 83 (8%) at the PDE, 32 (3%) at the two FPC, 11 (1%) at the maternity hospital, 693 (69%) at the BDC and 84 (8%) in the community. 593 (59%) were women (0.2% unknown). The median age was 34 years (IQR 25–46, range 7–76). The most frequent countries of origin (or travel) were: 238 (24%) Brazil, 133 (13%) Ecuador, 100 (10%) Chile, 90 (9%) Colombia, 89 (9%) Peru and 78 (8%) Bolivia. Table 1 details the distribution of country of origin for each testing centre. 546 (54%) were born in Latin America, 152 (15%) had a Latin American mother and 291 (29%) were travellers (2% unknown). The travellers had a mean duration of stay in Latin America of four years (IQR 1.2-10.0, range 0.25-34). Sex, median age, screening criteria and positive results are presented by country in table 2 and figure 1. Among the 231 patients who were asked about symptoms and signs predictive of Chagas disease, 28 (12%) presented with one or more symptoms/signs, the most frequent being dyspnoea and precordialgia, which were found in eight participants each, and constipation, found in five people.
Table 1 Number and characteristics of persons tested by country and by screening location.
| |
Screening location
|
Total
|
|
Community
|
Blood donation centre
|
Maternity hospital
|
Department of ambulatory care and community medicine
|
Point d’Eau
|
Family planning centres
|
|
Female (n, %) |
60 (71%) |
352 (51%) |
11 (100%) |
69 (64%) |
71 (86%) |
30 (94%) |
593 (59%) |
|
Age in years (median, IQR) |
41 (32–49) |
33 (24–46) |
31 (26–35) |
33 (25–41) |
42 (32–51) |
31 (28–39) |
34 (25–46) |
|
Country
|
|
|
|
n |
|
|
n (%) |
| Brazil |
0 |
178 |
3 |
32 (1 pos)
|
19 |
6 |
238 (24%) |
| Ecuador |
25 |
26 |
3 |
34 (1 pos)
|
30 |
15 |
133 (13%) |
| Chile |
4 |
82 |
2 |
7 |
5 |
0 |
100 (10%) |
| Colombia |
4 |
65 |
0 |
12 |
3 |
6 |
90 (9%) |
| Peru |
14 |
59 |
2 |
6 |
8 |
6 |
89 (9%) |
| Bolivia |
36 (13 pos)
|
20 |
1 |
10 (1 pos)
|
7 |
4 |
78 (8%) |
| Mexico |
0 |
49 |
0 |
1 |
0 |
1 |
51 (5%) |
| Argentina |
0 |
44 |
0 |
2 |
0 |
0 |
46 (5%) |
| Venezuela |
1 |
38 |
0 |
2 |
1 |
0 |
42 (4%) |
| Uruguay |
0 |
16 |
0 |
0 |
1 |
0 |
17 (2%) |
| Guyana |
0 |
12 |
0 |
0 |
0 |
0 |
12 (1%) |
| Guatemala |
0 |
10 |
0 |
0 |
2 |
0 |
12 (1%) |
| Nicaragua |
0 |
9 |
0 |
0 |
0 |
0 |
9 (1%) |
| Costa Rica |
0 |
8 |
0 |
0 |
0 |
0 |
8 (1%) |
| Paraguay |
0 |
1 |
0 |
0 |
7 |
0 |
8 (1%) |
| El Salvador |
0 |
8 |
0 |
0 |
0 |
0 |
8 (1%) |
| Honduras |
0 |
6 |
0 |
0 |
0 |
0 |
6 (1%) |
| Panama |
0 |
4 |
0 |
0 |
0 |
0 |
4 (0%) |
| Not recorded |
0 |
58 |
0 |
1 |
0 |
0 |
59 (6%) |
|
Total n (%) |
84 (8%)
(15% pos)
|
693 (69%) |
11 (1%) |
107 (11%)
(3% pos)
|
83 (8%) |
32 (3%) |
1010 (100%)
(1.6% pos)
|
Table 2 Number of persons tested, proportion of women, distribution of screening criteria, median age and number of positive cases, by country.
|
Country
|
Number of persons tested
n (%)
|
Proportion of women
%
|
Screening criteria
B/M/T, %*
|
Age in years
median (IQR)
|
Number of positive cases
n (%)
|
| Brazil |
238 (24%) |
59% |
56/17/26 |
35 (25-47) |
1 (0.4%) |
| Ecuador |
133 (13%) |
63% |
89/4/7 |
35 (29-42) |
1 (0.8%) |
| Chile |
100 (10%) |
56% |
53/23/22 |
32 (24-49) |
0 |
| Colombia |
90 (9%) |
66% |
59/11/29 |
32 (24-44) |
0 |
| Peru |
89 (9%) |
58% |
53/20/25 |
35 (24-48) |
0 |
| Bolivia |
78 (8%) |
69% |
78/5/17 |
36 (29-48) |
14 (17.9%) |
| Mexico |
51 (5%) |
61% |
24/14/63 |
27 (22-47) |
0 |
| Argentina |
46 (5%) |
46% |
41/24/33 |
32 (22-47) |
0 |
| Venezuela |
42 (4%) |
38% |
43/10/48 |
44 (33-52) |
0 |
| Uruguay |
17 (2%) |
47% |
35/35/29 |
33 (27-48) |
0 |
| Guatemala |
12 (1%) |
58% |
33/8/58 |
33 (28-40) |
0 |
| Guyana |
12 (1%) |
67% |
8/25/67 |
32 (20-55) |
0 |
| Nicaragua |
9 (1%) |
33% |
22/0/78 |
37 (33-53) |
0 |
| Costa Rica |
8 (1%) |
63% |
25/25/50 |
28 (26-36) |
0 |
| Paraguay |
8 (1%) |
88% |
88/0/13 |
35 (26-41) |
0 |
| El Salvador |
8 (1%) |
63% |
38/50/13 |
22 (20-24) |
0 |
| Honduras |
6 (1%) |
50% |
17/33/50 |
33 (21-46) |
0 |
| Panama |
4 (0.4%) |
75% |
25/25/50 |
45 (32-56) |
0 |
| Not recorded |
59 (6%) |
53% |
8/19/56 |
37 (26-52) |
0 |
|
Total n (%) |
1,010 (100%) |
59% |
54/15/29 |
34 (25-46) |
16 (1.6%) |
Positive cases for Chagas disease
The overall prevalence was 1.6% (16/1010, 95% CI: 0.9–2.6%) and therefore the number needed to test was 63. Among migrants (excluding travellers), the prevalence was 2.3% (16/698, 95% CI: 1.3–3.7%). None of the travellers who presented at the BDC were affected. The prevalence in the community was 15.5% (13/84, 95% CI: 8.5–25.0%), at the DACCM it was 2.8% (3/107, 95% CI: 0.6–8.0%), and at the other testing sites it was 0% (fig. 2). The prevalence was 18.0% (14/78, 95% CI: 10.2–28.3%) among Bolivians, 0.4% (1/238, 95% CI: 0–2.3%) among Brazilians, 0.8% (1/133, 95% CI: 0–4.1%) among Ecuadorans and 0% among persons originating from other countries. The median age of the 16 positive Chagas cases was 45 years (IQR 36–52, range 30–56), compared to a median age of 34 years for negative persons (p = 0.03). Interestingly, only 1 of the 42 (2%) Bolivians tested at the healthcare clinics was positive, while 13 of the 36 (36%) Bolivians tested in the community were positive (table 2). All positive cases were born in Latin America. The three positive cases who were asked about the symptoms and signs predictive of Chagas disease all had at least one of them.
Predictors for positivity
Regarding the predictors of positivity (table 3), being born in Latin America (n = 546) was the strongest factor (OR = infinite, p <0.001), followed by Bolivian origin (n = 78, OR = 95, 95% CI: 19-484, p <0.001) and being tested in the community (n = 84, OR = 56, 95% CI: 14–219, p <0.001). The latter factor remained significant even after adjusting for Bolivian origin (OR = 23, 95% CI: 2–243, p <0.001). Symptoms of Chagas disease were significantly associated with positivity, although the number of people for whom the information was available was very small. Finally, age >35 years (n = 478, OR = 3.4, 95% CI: 1.1–10.5, p = 0.03) was predictive of Chagas disease, while sex was not significant (p = 0.76).
Table 3 Predictors of being positive for Chagas disease.
|
Predictor
|
Total cases
n/N (%)
|
Positive cases
n/N (%)
|
Negative cases
n/N (%)
|
Crude OR
(95% CI)
|
p-value
|
| Born in Latin America |
546/992 (55%) |
16/16 (100%) |
530/976 (54%) |
∞ |
<0.001 |
| Symptoms of Chagas disease |
28/233 (12%) |
3/3 (100%) |
25/230 (11%) |
∞ |
<0.001 |
| Bolivian origin |
78/951 (8%) |
14/16 (88%) |
64/935 (7%) |
95 (19-484) |
<0.001 |
| Tested in the community |
84/1010 (8%) |
13/16 (81%) |
71/994 (7%) |
56 (14-219) |
<0.001 |
| Tested in the community (when being Bolivian) |
36/78 (46%) |
13/14 (93%) |
23/64 (36%) |
23 (2-243) |
<0.001 |
| Age >35 years |
478/1006 (48%) |
12/16 (75%) |
466/990 (47%) |
3.4 (1.1-10.5) |
0.03 |
| Female sex |
593/1008 (59%) |
10/16 (63%) |
583/992 (59%) |
1.2 (0.4 -3.2) |
0.76 |
Discussion
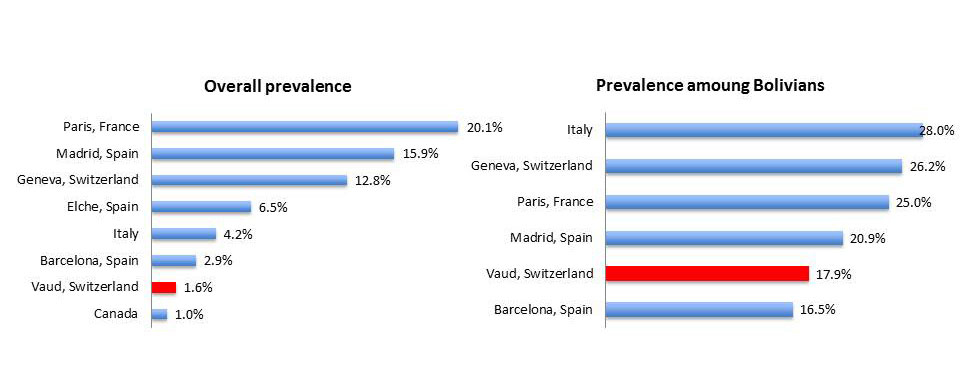
A prevalence of 2.3% was found for Chagas disease in Latin American migrants living in the canton of Vaud, Switzerland. This rate is similar to that found in migrants attending a healthcare centre in Barcelona over the past 14 years (2.9%) [13]. It is, however, lower than the rates found in other European cities such as Elche (6.5%) [17], Madrid (15.9%) [18] and Paris (20.1%) [20]. In Italy, systematic screening found an overall prevalence (including travellers, expatriates, adopted children, blood donors and HIV positive patients) of 4.2% [19], and a prevalence of 11.3% among migrants. Only Canada found a lower prevalence (1.0%) among refugees [21] (fig. 3). The prevalence of Chagas disease in our study was much higher in people attending social events than healthcare centres. This suggests that observations based mainly or exclusively on healthcare facility data tend to underestimate the true prevalence of Chagas disease. This testing at social events could be performed using rapid, point-of-care diagnostic tests, as few people would be missed due to the high sensitivity of the test (in our study only 0.5 people would have been falsely negative if the test had 96% sensitivity).
Prevalence according to country of origin
In Geneva, the canton to the east of Vaud, 12.8% of the 1,012 Latin American migrants screened were positive for Chagas disease [7]. This high prevalence compared to our study can probably be explained mainly by the high proportion of Bolivians: 48% in Geneva compared to 8% in Vaud. In support of previous studies, we found that the majority of affected people originated from Bolivia. The 17.9% prevalence among Bolivians (or 21.5% if we exclude blood donors) is close to that documented in other studies: Barcelona (16.5%), Madrid (20.9%), Paris (25.0%) and Geneva (26.2%). Excluding the BDC, where testing is compulsory, the number needed to test was low for Bolivians - only four people need to be tested to find a positive case – but high for migrants originating from other countries (130). Special attention should therefore be paid to Bolivians when designing a strategy.
Prevalence according to place of screening
In addition to the country of origin, the high variation in prevalence between studies can be explained by the different populations screened and the different screening locations. In our study, the prevalence was much higher in people tested in the community than at the healthcare clinics, regardless of their country of origin. A possible explanation is that people tested in the community were of lower social status and thus originating from the rural regions harbouring the highest levels of endemicity of Chagas disease. Indeed, people without a resident permit tend to avoid contact with the health system as much as possible, even though the low-level health facility in Lausanne targets precisely this population, and even though there is a system in place in the other healthcare facilities for migrants to improve their attendance.
Prevalence in specific populations
None of the 11 pregnant women (of whom only one originated from Bolivia) screened at the maternity hospital were positive. In other studies, the prevalence among pregnant women was 1.3% in Elche [1], 1.4% in Italy [19], 9.7% in Geneva [4], 3.4% in Barcelona [2] and 4.7% in Valencia [3]. In this study, only a very small proportion (11%) of the pregnant Latin American women attending the maternity hospital were tested, and none were positive. Since the end of the study, the testing rate has improved, however, and several pregnant women have tested positive in Vaud. There were also no positive cases detected at the BDC. In the literature, the prevalence at BDCs varies between 0% in Porto and Italy [10, 19], 0.003% in France [6], 0.007% in United States [8] and 0.6% in Catalonia [5]. In Catalonia, donors who have stayed in an endemic country for more than one month are screened, while in France and the United States, travellers with a stay of any duration are screened.
Clinical predictors
Although clinical features could only be evaluated in the context of medical consultations (and not in counselling sessions), having symptoms or signs predictive of chronic Chagas disease was frequent among positive cases (this evaluation was performed by the physician prior to knowing the test result). This might be because transmission of Chagas has decreased substantially during the past 20 years, meaning that most cases were infected a long time ago and are therefore more likely to be at the symptomatic stage of the disease. This hypothesis is corroborated by the fact that older age was a predictor of positivity in our study, as well as in a study in Geneva [7]. These symptoms and signs were not the reason for the consultation and would not necessarily have been assessed by the clinician in charge if not required by the study. Theoretically, to decrease the overall number of people needed to test, the presence of one of these clinical features could be used as a screening criterion in populations with a very low pre-test probability, such as non-Bolivian Latin Americans. However, a screening strategy also aims to detect people during the asymptomatic phase, when treatment has the highest potential impact. Moreover, a medical examination is not pragmatic outside of formal healthcare facilities.
Screening at community level
When the difficulty of reaching those at risk through a healthcare facility became apparent, direct screening in the community was offered, targeting particularly people of Bolivian origin. However, attendance at Latin American social events requires strong commitment from health workers, as these often take place outside of normal working hours. Moreover, it was not always possible to offer screening for Chagas disease during such events, as organisers were sometimes afraid that this activity could impact on the celebration. In contrast, we observed that even when organisers were sceptical, participants at the celebration were pleased to have the opportunity to become more informed and, in particular, to be directly screened. The cultural mediator was a key person in the process of approaching and convincing the community leaders of the importance of testing for Chagas disease. We reached the conclusion that due to the availability of rapid diagnostic tests for Chagas disease, testing at a community level is feasible, effective and well accepted by the community.
Feasibility of the screening strategy
The proportion of participants who consented to be tested was high in all places, except at the maternity hospital. At the latter, nurses highlighted that patients were declining the test because of the high price of the test. Indeed, Chagas is not currently part of the screening tests recommended by the Swiss Society of Gynaecology and Obstetrics and thus is not reimbursed by healthcare insurance. At the other facilities, where the price of the test was much lower, the uptake on the screening strategy depended mainly on the method used by the facility to identify Latin Americans. At the outpatient clinic, for example, the receptionist added a reminder for clinicians on the patient’s medical file if they originated from Latin America, encouraging a high uptake. In health facilities where the clinician in charge had to remember to offer the test, the uptake was much lower, especially when the reason for attendance had nothing to do with Chagas disease. An unexpected difficulty encountered was convincing people who had tested positive to seek treatment. They reported a number of fears, including i) being denounced to legal authorities (as the vast majority of positive cases were undocumented migrants) despite the assurance of total separation between health and security services in Switzerland, ii) losing their jobs due to non-attendance relating to the numerous medical consultations, iii) earning less money (as their salary is often hour-based), and iv) experiencing adverse drug reactions (ADRs) from the medication used to treat Chagas disease. In 2008 in Geneva, nifurtimox was used to treat Chagas disease, and rumours about serious ADRs spread in the community. These fears highlight the importance of an active presence in the community. Providing information through leaflets and posters, as we did, is important but evidently not sufficient to tackle these specific problems. Possible options to improve confidence in healthcare could be to create an association of people affected by Chagas disease or to form an expert patient group.
Conclusion
As migration from Latin America to Switzerland is increasing, it is time an efficient strategy for the systematic screening and treatment of Chagas disease was set up to stop transmission in non-endemic countries and to decrease morbidity. According to our findings, which are supported by those of other studies, the Bolivian population in particular should be targeted. For people originating from other Latin American countries, where the prevalence is much lower, adding criteria that are predictive of Chagas disease could be a good method to reduce the number who need testing. Further research on the most efficient combination of criteria for screening is needed, with the consideration that the profile of the migrant population may change rapidly, and screening strategies will have to be adapted accordingly. Screening for Chagas disease directly in the community, where the positivity rate is much higher, rather than only at healthcare facilities, is essential, and allows problems arising from misinformation and (sometimes justified) fears to be tackled. The warm welcome from the Latin American community when screening was offered to them in a more convenient way, and their realisation that this neglected and serious disease could be appropriately managed, should strengthen the willingness of non-endemic countries to continue to work actively to achieve the WHO goal of eradicating Chagas disease globally.
Acknowledgments
We thank the receptionists, nurses and doctors who selected, counselled and tested people for Chagas disease at the different screening locations. In particular, we thank Danielle Rose Kohler, Fanny Tribolet, Isabel Sangra Bron working at the Point d’Eau; Giorgia Canellini, in charge of the blood donation centre; Cate Esson and Adeline Quach working at the family planning centres of Profa; Saira-Christine Renteria and Françoise Monbaron based at the maternity hospital of Lausanne. We also thank Sophia Bennani and all those who helped with the creation of the information leaflet. We finally thank Eric Masserey from the Health Office of the Canton de Vaud for his constant support in the control of transmissible diseases in migrants, and in this project in particular.
References
1
Ramos
JM
,
Milla
A
,
Rodríguez
JC
,
López-Chejade
P
,
Flóres
M
,
Rodríguez
JM
, et al.
Chagas disease in Latin American pregnant immigrants: experience in a non-endemic country. Arch Gynecol Obstet. 2012;285(4):919–23. doi:.https://doi.org/10.1007/s00404-011-2081-9
2
Muñoz
J
,
Coll
O
,
Juncosa
T
,
Vergés
M
,
del Pino
M
,
Fumado
V
, et al.
Prevalence and vertical transmission of Trypanosoma cruzi infection among pregnant Latin American women attending 2 maternity clinics in Barcelona, Spain. Clin Infect Dis. 2009;48(12):1736–40.
3
Paricio-Talayero
JM
,
Benlloch-Muncharaz
MJ
,
Collar-del-Castillo
JI
,
Rubio-Soriano
A
,
Serrat-Pérez
C
,
Magraner-Egea
J
, et al.
Vigilancia epidemiológica de la transmisión vertical de la enfermedad de Chagas en tres maternidades de la Comunidad Valenciana [Epidemiological surveillance of vertically-transmitted Chagas disease at three maternity hospitals in the Valencian Community]. Enferm Infecc Microbiol Clin. 2008;26(10):609–13. Article in Spanish.
4
Jackson
Y
,
Myers
C
,
Diana
A
,
Marti
HP
,
Wolff
H
,
Chappuis
F
, et al.
Congenital transmission of Chagas disease in Latin American immigrants in Switzerland. Emerg Infect Dis. 2009;15(4):601–3.
5
Piron
M
,
Vergés
M
,
Muñoz
J
,
Casamitjana
N
,
Sanz
S
,
Maymó
RM
, et al.
Seroprevalence of Trypanosoma cruzi infection in at-risk blood donors in Catalonia (Spain). Transfusion. 2008;48(9):1862–8.
6
Assal
A
,
Corbi
C
. [Chagas disease and blood transfusion: an emerging issue in non-endemic countries]. Transfus Clin Biol. 2011;18(2):286–91. Article in French.
7
Jackson
Y
,
Gétaz
L
,
Wolff
H
,
Holst
M
,
Mauris
A
,
Tardin
A
, et al.
Prevalence, clinical staging and risk for blood-borne transmission of Chagas disease among Latin American migrants in Geneva, Switzerland. PLoS Negl Trop Dis. 2010;4(2):e592.
8
Custer
B
,
Agapova
M
,
Bruhn
R
,
Cusick
R
,
Kamel
H
,
Tomasulo
P
, et al.
Epidemiologic and laboratory findings from 3 years of testing United States blood donors for Trypanosoma cruzi. Transfusion. 2012;52(9):1901–11.
9
Kessler
DA
,
Shi
PA
,
Avecilla
ST
,
Shaz
BH
. Results of lookback for Chagas disease since the inception of donor screening at New York Blood Center. Transfusion. 2013;53(5):1083–7.
10
Queiros
L
,
Neto
S
,
Leal
J
,
Carvalho
C
,
Paula
A
,
Couto
MJ
, et al.
Estudo Epidemiologico da doença de Chagas em dadores de sangue. ABO. 2010;41:29–34.
Article in Portuguese.
11World Health Organization (WHO). Statement – Chagas disease in Europe. Geneva: WHO; 2009. [Accessed: 8 Feb 2015]. Available from: http://www.who.int/neglected_diseases/integrated_media_chagas_statement/en/
12World Health Organization (WHO). Control and prevention of Chagas disease in Europe. Report of a WHO Informal Consultation. Geneva: WHO; 2009. [Accessed: 8 Feb 2015]. Available from: http://www.fac.org.ar/1/comites/chagas/Chagas_WHO_Technical%20Report_16_06_10.pdf.
13
Roca
C
,
Pinazo
MJ
,
López-Chejade
P
,
Bayó
J
,
Posada
E
,
López-Solana
J
, et al.; Chagas-Clot Research Group. Chagas disease among the Latin American adult population attending in a primary care center in Barcelona, Spain. PLoS Negl Trop Dis. 2011;5(4):e1135. doi:.https://doi.org/10.1371/journal.pntd.0001135
14
Soriano Arandes
A
,
Muñoz Gutierrez
J
,
Vergés Navarro
M
,
Castells Doménech
C
,
Portús Vinyeta
M
,
Gascón Brustenga
J
. Prevalence of Chagas disease in the Latin American immigrant population in a primary health centre in Barcelona (Spain). Acta Trop. 2009;112(2):228–30. doi:.https://doi.org/10.1016/j.actatropica.2009.07.014
15
Muñoz
J
,
Gómez i Prat
J
,
Gállego
M
,
Gimeno
F
,
Treviño
B
,
López-Chejade
P
, et al.
Clinical profile of Trypanosoma cruzi infection in a non-endemic setting: immigration and Chagas disease in Barcelona (Spain). Acta Trop. 2009;111(1):51–5. doi:.https://doi.org/10.1016/j.actatropica.2009.02.005
16
Rodríguez-Guardado
A
,
Rodríguez
M
,
Alonso
P
,
Seco
C
,
Flores-Chavez
M
,
Mejuto
P
, et al.
Serological screening of Chagas disease in an immigrant population in Asturias, Spain proceeding from Chagas-endemic areas. Scand J Infect Dis. 2009;41(10):774–6.
17
Ramos
JM
,
Ponce
Y
,
Gallegos
I
,
Flóres-Chávez
M
,
Cañavate
C
,
Gutiérrez
F
. Trypanosoma cruzi infection in Elche (Spain): comparison of the seroprevalence in immigrants from Paraguay and Bolivia. Pathog Glob Health. 2012;106(2):102–6. doi:.https://doi.org/10.1179/2047773212Y.0000000013
18
Navarro
M
,
Perez-Ayala
A
,
Guionnet
A
,
Perez-Molina
JA
,
Navaza
B
,
Estevez
L
, et al.
Targeted screening and health education for Chagas disease tailored to at-risk migrants in Spain, 2007 to 2010. Euro Surveill. 2011;16(38):19973. doi:.https://doi.org/10.2807/ese.16.38.19973-en
19
Angheben
A
,
Anselmi
M
,
Gobbi
F
,
Marocco
S
,
Monteiro
G
,
Buonfrate
D
, et al.
Chagas disease in Italy: breaking an epidemiological silence. Euro Surveill. 2011;16(37):19969.
20
Lescure
F-X
,
Paris
L
,
Elghouzzi
MH
,
Le Loup
G
,
Develoux
M
,
Touafek
F
, et al.
[Experience of targeted screening of Chagas disease in Ile-de-France]. Bull Soc Pathol Exot. 2009;102(5):295–9. Article in French.
21
Steele
LS
,
MacPherson
DW
,
Kim
J
,
Keystone
JS
,
Gushulak
BD
. The sero-prevalence of antibodies to trypanosoma cruzi in Latin American refugees and immigrants to Canada. J Immigr Minor Health. 2007;9(1):43–7. doi:.https://doi.org/10.1007/s10903-006-9014-x
22
Basile
L
,
Jansa
JM
,
Carlier
Y
,
Salamanca
DD
,
Angheben
A
,
Bartoloni
A
, et al.; Working Group on Chagas Disease. Chagas disease in European countries: the challenge of a surveillance system. Euro Surveill. 2011;16(37):19968.
23Canton de vaud. Indicateurs démographiques de la population étrangère dans le canton de Vaud, Etat à la fin décembre 2011, Statistique Vaud 2012. [Accessed: 8 Feb 2015]. French. Available from: http://www.scris.vd.ch/default.aspx?docid=7405.
24Bolzman C, Carbajal M, Mainardi G. La Suisse au rythme latino: dynamiques migratoires des Latino-Américains : logiques d’action, vie quotidienne, pistes d’interventions dans les domaines du social et de la santé. Geneva, Switzerland: Haute école de travail social (Institut d’études sociales) ; 2007. In French.
25
Chappuis
F
,
Mauris
A
,
Holst
M
,
Albajar-Vinas
P
,
Jannin
J
,
Luquetti
AO
, et al.
Validation of a rapid immunochromatographic assay for diagnosis of Trypanosoma cruzi infection among Latin-American Migrants in Geneva, Switzerland. J Clin Microbiol. 2010;48(8):2948–52. doi:.https://doi.org/10.1128/JCM.00774-10
26
Otani
MM
,
Vinelli
E
,
Kirchhoff
LV
,
del Pozo
A
,
Sands
A
,
Vercauteren
G
, et al.
WHO comparative evaluation of serologic assays for Chagas disease. Transfusion. 2009;49(6):1076–82. doi:.https://doi.org/10.1111/j.1537-2995.2009.02107.x
27
Gontijo
ED
,
Rocha
MO
,
Torquato de Oliveira
U
. Perfil clínico-epidemiológico de chagásicos atendidos em ambulatório de referência e proposição de modelo de atenção ao chagásico na perspectiva do sus [Clinico-epidemiologic profile of Chagas disease patients attending an ambulatory referral center and proposal of a model of care for the Chagas patient under the perspective of a comprehensive health care]. Rev Soc Bras Med Trop. 1996;29(2):101–8. Srticle in Portuguese. doi:.https://doi.org/10.1590/S0037-86821996000200002