Updated recommendations for diagnosis and treatment of plasma cell myeloma in Switzerland
DOI: https://doi.org/10.4414/smw.2019.20031
Panagiotis
Samarasa, Mario
Bargetzib, Daniel C.
Betticherc, Christoph
Driessend, Michel A.
Duchosale, Dominik
Heimf, Nicolas
Kettererg, Erika
Lerchh, Thomas
Matthesi, Ulrich
Meyj, Thomas
Pabstk, Adrian
Schmidtl, Christian
Tavernam, Thilo
Zandern, Christoph
Rennera
aOncology Centre Hirslanden and Zurich, Switzerland
bCentre of Oncology/Haematology and Transfusion Medicine, Cantonal Hospital Aarau, Switzerland
cClinics of Medical Oncology, HFR Fribourg Cantonal Hospital, Fribourg, Switzerland
dDivision of Oncology/Haematology, Cantonal Hospital St Gallen, Switzerland
eService and Central Laboratory of Haematology, University Hospital of Lausanne, Switzerland
fDepartment of Haematology, University Hospital Basel, Switzerland
gClinic Bois-Cerf, Onco-haematology, Lausanne, Switzerland
hOncology Institute of Southern Switzerland (IOSI), Ospedale San Giovanni, Bellinzona, Switzerland
iDivision of Haematology, University Hospital Geneva, Switzerland
jMedical Oncology and Haematology, Cantonal Hospital Graubuenden, Chur, Switzerland
kDepartment of Medical Oncology, University Hospital of Bern, Switzerland
lClinic of Medical Oncology and Haematology, City Hospital Triemli, Zurich, Switzerland
mOncology/Haematology, Cantonal Hospital Münsterlingen, Switzerland
nDepartment of Medical Oncology, Cantonal Hospital Lucerne, Switzerland
Summary
This update on plasma cell myeloma has been elaborated by a Swiss expert panel as a result of the plethora of new data on the treatment of plasma cell myeloma reported recently. It adds new insights to the more extensive review that was published 3 years ago and may help clinicians on decision making for their patients. The new recommendations for distinguishing plasma cell myeloma from smouldering myeloma are briefly presented, including a section on contemporary imaging studies with this respect. Former panel recommendations that remain unchanged by new results will not be discussed in detail as the major focus of this review is on treatment-relevant new developments.
Introduction
This update on plasma cell myeloma has been elaborated by a Swiss expert panel as a result of the plethora of new data on the treatment of plasma cell myeloma reported recently. It adds new insights to the more extensive review that was published three years ago and may help clinicians on decision making for their patients [1]. The new recommendations for distinction of plasma cell myeloma from smouldering myeloma are briefly presented, including a section on contemporary imaging studies with this respect. Former panel recommendations that remain unchanged by new results will not be discussed in detail as the major focus of this review is on treatment-relevant new developments.
Updated diagnostic criteria
Monoclonal gammopathy of undetermined significance
The definition of monoclonal gammopathy of undetermined significance (MGUS) has not changed. Patients need to have less than 30 g/l serum M-protein, less than 10% clonal plasma cells in the bone marrow, and no end-organ damage for this diagnosis. Patients with immunoglobulin M (IgM) MGUS and non-IgM MGUS differ in terms of progression risk. With the two laboratory parameters M-protein (<15 g/l versus ≥15 g/l) and serum free light chain (FLC) ratio (normal versus pathological), three risk categories can be distinguished for IgM and non-IgM MGUS enabling a risk adapted patient monitoring [2]. As of today, no data are available supporting treatment of patients with MGUS.
Smouldering myeloma or plasma cell myeloma?
The diagnosis of a myeloma demands the presence of a serum M-protein of ≥30 g/l and/or ≥10% of clonal plasma cells in the bone marrow. Asymptomatic patients without myeloma-defining events have a so-called smouldering myeloma, which may progress to a symptomatic plasma cell myeloma over time. The risk of progression can be estimated, as for MGUS, by the presence of various risk factors, which facilitate an individualised patient monitoring [3, 4]. A few years ago, a randomised trial evaluated the impact of early treatment in patients with high-risk smouldering myeloma with lenalidomide and dexamethasone, as compared with observation, and showed improved progression-free survival (PFS) and overall survival (OS) with early treatment [5]. However, it was a rather small study, with 125 treated patients, and the specified risk factors are not used commonly for risk stratification. Therefore, this early treatment approach cannot be generally recommended until additional data are available to further support it.
The presence of end organ damages, primarily the so-called CRAB-criteria (hypercalcaemia, renal failure, anaemia, bone disease), define an underlying plasma cell myeloma in need of therapy. In the most recent update of the criteria for diagnosis of plasma cell myeloma, three additional myeloma-defining events have been introduced to discriminate symptomatic myeloma without evidence of classical end-organ damage from smouldering myeloma: clonal plasma cells of 60% or greater in the bone marrow, a serum FLC ratio of 100 or greater, or more than one focal lesion larger than 5 mm on magnetic resonance imaging (MRI) [6]. The reason for incorporation of these three new biomarkers of malignancy is that each one was found to constitute an “ultrahigh” risk of progression to symptomatic disease within two years of greater than 70–80%. To address these additional myeloma defining events, the term “SLiM-CRAB” (SLiM: S=sixty; Li=light chain; M=MRI) was coined soon after publication of the updated criteria. The new definitions of smouldering and symptomatic plasma cell myeloma are shown in table 1.
Table 1 Adapted from Rajkumar et al. [6].
|
Definition of plasma cell myeloma
|
Clonal plasma cells in the bone marrow ≥ 10% or biopsy proven osseous or extramedullary plasmacytoma
and ≥1 of the following SLiM-CRAB-criteria
• Ultrahigh risk biomarker for malignancy
o (S) sixty % or greater clonal plasma cells in the bone marrow
o (Li) light chain ratio of involved to not involved FLC ≥100 (involved free light chain must be at least 100mg/l)
o (M) more than 1 focal lesion in MRI study (≥5 mm)
• End organ damage, attributed to the underlying disease
o (C) hypercalcaemia >0.25 mmol/l above the upper limit of normal or >2.75 mmol/l
o (R) renal insufficiency: creatinine clearance <40 ml/min or serum creatinine >177 μmol/l
o (A) anaemia: Hb >20g/l below the lower limit of normal or <100 g/l
o (B) bone lesions: ≥1 osteolytic lesion on radiography, CT or PET-CT |
|
Definition of smouldering myeloma
|
The following criteria must be fulfilled
• Serum M-protein (IgG or IgA) ≥30 g/l or M-protein in urine ≥500 mg/24h and/or clonal plasma cells in the bone marrow of 10–60%
• no myeloma defining events or amyloidosis present |
Imaging
Imaging has become an increasingly important tool for the precise staging of the patients. The use of “native” whole-body computed tomography of the bone can be considered standard of care in daily practice. Importantly, this technique has sufficient sensitivity for the detection of small bone defects and the use of a contrast agent can be avoided in most cases. As mentioned above, whole-body MRI needs to be considered in asymptomatic patients with newly diagnosed myeloma without other underlying myeloma defining events to search for occult focal lesions. An 18fluorodeoxyglucose (18F-FDG) positron emission tomography combined with a CT (PET/CT) may be performed optionally for specific indications. It has high sensitivity and specificity for the detection of extramedullary disease, and in patients with suspected solitary plasmacytoma it is a mandatory alternative to whole body MRI to confirm this diagnosis or to detect underlying occult lesions. In addition, it may develop into a helpful tool to assess response to treatment and minimal residual disease (MRD), but this scope of application cannot be considered standard yet [7].
Risk stratification
A few years ago, the International Staging System (ISS), a simple prognostic stratification tool using albumin and beta-2 microglobulin concentrations in serum, which had been introduced more than a decade ago, was revised by the IMWG [8]. The new revised ISS (R-ISS) includes two additional parameters, the presence of chromosomal high risk abnormalities, and an elevated serum lactate dehydrogenase (LDH) value, which both have been identified as risk factors in a pooled analysis of almost 4500 patients enrolled into 11 international trials. By using this new stratification tool patients with newly diagnosed plasma cell myeloma may be assessed reliably in terms of the relative risk for their survival (table 2) [9].
Table 2 The revised international staging system (R-ISS) [9].
|
Stage I (28%)
|
Stage II (62%)
|
Stage III (10%)
|
ISS I and
no high risk cytogenetics* and
normal LDH |
Neither Stage I nor Stage III |
ISS III plus either
High risk cytogenetics* or
elevated LDH |
5 -year OS 82%
5-year PFS 55% |
5 -year OS 62%
5-year PFS 36% |
5 -year OS 40%
5-year PFS 24% |
First-line treatment
Patients need to be evaluated for their eligibility to undergo high dose therapy with melphalan followed by autologous stem cell transplantation (HDT/ASCT), which is still considered standard of care for the younger patients.
Transplant-eligible patients
A few years ago, a randomised trial confirmed the value of HDT/ASCT even in the era of new myeloma drugs [10]. However, this study used lenalidomide and dexamethasone (RD) for induction, which is nowadays considered less efficacious than triple regimens commonly used in clinical practice today. In this trial consolidation consisted of a double autologous transplant, and lenalidomide maintenance was given until progression. In the recently published French IFM2009 trial patients received bortezomib, lenalidomide and dexamethasone (VRD) as first line treatment, and they received either an intensification with high-dose chemotherapy with melphalan and autologous stem cell support, followed by two additional cycles of VRD as further consolidation, or they received eight cycles of VRD alone. All patients were assigned to receive lenalidomide as maintenance for one year. The administration of high-dose melphalan resulted in a significantly improved progression-free survival compared with the conventional treatment with bortezomib, lenalidomide and dexamethasone alone (50 vs 36 months; hazard ratio [HR] 0.65, p <0.001). However, no benefit in OS was observed so far and the authors concluded that high-dose chemotherapy may well be postponed to later salvage treatment to secure long-term survival [11]. A further trial, SWOG S0777, also evaluated VRD as first line treatment in patients not intended to undergo immediate high-dose chemotherapy. After eight cycles of VRD, patients continued with lenalidomide and dexamethasone as maintenance treatment until progression. The VRD triple therapy was compared with lenalidomide and dexamethasone alone and resulted in a significant increase of both PFS and OS [12].
Very recently, the large, international EMN02/HOVON95 trial assessed the value of one or two HDT/ASCTs as intensification after induction with the triple combination bortezomib, cyclophosphamide and dexamethasone (VCD) compared with a conventionally dosed treatment with bortezomib, melphalan and prednisone (VMP) after VCD induction. In addition, the study also assessed the efficacy of a short consolidation with two cycles of VRD. The treatment arm with HDT/ASCT and the use of VRD as a short consolidation resulted in an improved PFS compared with the conventional treatment arm with VCD, followed by VMP [13, 14]. Furthermore, in a more recent analysis, the use of a tandem HDT/ASCT improved PFS and OS in the overall patient population and particularly in patients with high risk disease [15].
These results are in contrast to a study evaluating the role of consolidation treatment after HDT/ASCT that has recently been published (StaMINA trial). In this US trial, after induction treatment whose duration and composition were left to the investigator, and a subsequent HDT/ASCT, patients were randomised to receive a second HDT/ASCT, four cycles of VRD, or no consolidation at all. All patients went on to receive lenalidomide maintenance until progression. After a follow-up of 38 months, no differences in PFS or OS were seen in this study. Although not predefined, most patients (> 50%) received four to ten cycles of VRD as a long induction, suggesting that a highly efficacious upfront treatment may render further consolidative treatment options unnecessary in unselected patients. In addition, the length of induction treatment prior to HDT/ASCT was not defined and sometimes even extended up to 12 months, making it very difficult to compare this study with other trials that were more structured and concise regarding number of induction cycles [16].
Based on all these data, the combination of bortezomib, lenalidomide and dexamethasone should be considered the preferred first-line treatment for transplant-eligible patients. By administering VRD upfront in transplant-eligible patients, the need for further consolidation strategies after HDT/ASCT may be diminished, especially in standard risk patient. More intense treatment with further consolidation or tandem transplantation, based on the underlying risk factors, should be considered especially in high-risk patients.
As depth of response indicated by minimal residual disease (MRD), and time to next treatment (TTNT) are reliable indicators for the prognosis of the patients, HDT/ASCT should remain part of the first-line treatment strategy for younger and/or fit patients, although the lack of OS benefit may suggest that deferral until relapse may achieve comparable long term survival. Standardised assessment of MRD may help to select patients for whom immediate HDT/ASCT is not necessary in the near future, but this technology has not yet been established for daily practice [17].
With respect to maintenance treatment after HDT/ASCT, the data of three randomised studies (CALGB 100104, GIMEMA RV-MM-PI-209, IFM 2005-02) have been included in a meta-analysis published recently, which confirmed an OS benefit for the treatment with lenalidomide after a longer follow-up [18]. It has to be taken into account that the OS benefit observed was not significant in the subgroups of patients with elevated LDH and renal failure.
The UK Myeloma XI trial of a response- and immunomodulatory drug (IMID)-based induction with the addition of bortezomib in the event of an inadequate response to cyclophosphamide, thalidomide, dexamethasone (CTD) or cyclophosphamide, lenalidomide, dexamethasone (CRD), demonstrated the ability of lenalidomide to improve PFS and OS across all subgroups of patients when given as maintenance, even in patients with poor risk cytogenetics [19]. The optimal duration of the maintenance treatment needs further elaboration. The German group suggests, based on their GMMG-MM5 trial, that in the upfront autologous transplant setting the administration of lenalidomide for two years has a superior outcome in terms of OS compared with a treatment until complete remission, whereas PFS is not significantly prolonged [20]. Therefore, based on the available data, lenalidomide maintenance after HDT/ASCT for at least two years is nowadays considered standard of care for the majority of patients. The question of the duration of lenalidomide maintenance may hopefully be answered definitely by the still ongoing Determination trial, which is using lenalidomide maintenance until progression.
Two randomised phase III studies have investigated the efficacy of a proteasome inhibitor for maintenance treatment. In the HOVON-65/GMMG-HD4 trial patients were treated with thalidomide or bortezomib maintenance for two years, and in the TOURMALINE-MM3 study patients received ixazomib or placebo for two years. In both trials, patients with high risk cytogenetics seem to have benefitted most from the proteasome inhibitor, which may thus be considered as a reasonable alternative to lenalidomide for selected patients [21, 22].
Transplant-ineligible patients
Based on the results of the aforementioned study SWOG 0777, in which HDT/ASCT was not a mandatory part of the protocol, VRD can also be considered a treatment option for patients not eligible for transplantation but fit enough to tolerate multi-agent regimens. As higher rates of adverse events have to be anticipated with VRD than with RD alone, older patients and patients with comorbidities need to be selected carefully for this treatment regimen. From a practical point of view, the triple combination may be better tolerated by elderly patients if appropriate dose modifications are made (“VRD-lite”) [23]. The “classic” combination of bortezomib, melphalan and prednisone (VMP) can still be considered a treatment option with a predictable risk profile, particularly if IMIDs are contraindicated or not tolerated. The addition of the monoclonal CD38 antibody daratumumab to VMP as standard first-line treatment in the phase III ALCYONE trial resulted in a dramatic improvement of PFS and response rates, suggesting that this antibody-containing quadruple combination may be an emerging treatment option for elderly patients not eligible for transplantation [24]. Very recently, the addition of daratumumab to RD has also been shown to improve PFS markedly compared with RD alone in the randomised phase III MAIA study, making this an additional future first-line treatment option for transplant-ineligible patients [25]. However, both combinations are not yet approved in Switzerland and since the anti-CD38 antibody also targets normal plasma cells, the rates of infectious complications may be of concern in this vulnerable population.
For older and frail patients, the RD combination can be considered a valuable alternative first-line option, but duration of treatment remains a matter of debate. In the updated survival analysis of the FIRST trial, the improvements of PFS and OS were confirmed for lenalidomide and dexamethasone given continuously compared with the old standard combination of melphalan, prednisone and thalidomide (MPT). In patients achieving complete or very good partial responses, continuous RD achieved a 30-month longer median time to next treatment compared with Rd for 18 months (69.5 vs 39.9 months). Based on these data, RD treatment may be preferably continued until progression, particularly in patients who tolerate it well and who achieve a deep remission [26, 27]. Furthermore, the combination of bortezomib and dexamethasone may also be considered a valuable alternative for this patient group, particularly in frail patients or patients with renal impairment [28]. Unfortunately, we do not have trials comparing VMP with RD as first-line treatment of elderly patients and since both regimens are approved, choice of treatment is based on patient preference, comorbidities and fitness. A recent post hoc comparison of two studies showed that transplant-ineligible patients with high risk cytogenetics and patients aged <75 years may derive more benefit from bortezomib-based induction treatment than with lenalidomide-based treatment [29].
Later line treatment
Patients with progression of their plasma cell disease may need further effective treatment at some point. Disease progression alone may not necessarily demand immediate retreatment. When to start a new treatment is dependent on various factors and needs to be addressed individually. The European Myeloma Network provided criteria for relapse and retreatment, which are helpful for daily practice [30]. Briefly, patients with new or worsening SLiM-CRAB criteria and patients with highly aggressive disease at diagnosis or a short treatment interval should start with a new treatment immediately. Patients with a sole biochemical relapse may be treated in the event of a short doubling time of the serum M-protein or a significant and continuous increase of M-protein or serum free light chains within a time period of up to two months. Significant progress in the treatment of second or later lines has been made during recent years, with the approval of six new antimyeloma agents in the US and Europe, and in part also in Switzerland. Two next generation proteasome inhibitors with a different toxicity profile from bortezomib were made available. Carfilzomib (K) and ixazomib (I) when combined with lenalidomide and dexamethasone (KRD or IRD, respectively) resulted in improved PFS compared with the standard double therapy [31, 32]. Carfilzomib is the first of the next generation agents that has been shown to improve OS compared with the respective comparator arms in the relapsed setting after appropriate follow up [33, 34]. Carfilzomib is administered twice weekly intravenously (at a dose of 27 mg/m2) combined with lenalidomide and dexamethasone. Carfilzomib was also approved in combination with dexamethasone alone at a higher dose of 56 mg/m2 (KD), which resulted in improved OS compared with bortezomib and dexamethasone (VD) in the relapsed setting. Recently, concerns have been raised about the cardiac safety of higher doses of carfilzomib. In general, the drug should preferentially be administered to patients with normal cardiac function, with no underlying severe cardiac comorbidities and only in the case of an optimal control of arterial blood pressure. In contrast to carfilzomib, ixazomib is taken orally once per week, allowing an all-oral combination with lenalidomide and dexamethasone (IRD). Of note, the TOURMALINE-MM1 trial is the only placebo-controlled phase III trial in this setting. The main toxicities are rash and gastrointestinal disturbances. The carfilzomib combinations, either KRD or KD, and IRD are approved in Switzerland and readily available for patients with relapsed disease.
Two monoclonal antibodies have been approved in recent years. Elotuzumab targets the surface antigen SLAMF7 (CD319), which is expressed on clonal plasma cells, inducing an antibody-dependent cellular cytotoxicity. In addition, natural killer cells also express this antigen and may be activated by antibody binding. These modes of action result in an improvement of PFS and, at the 4-year update, also of OS (48 vs 40 months), when elotuzumab is combined with RD after at least one previous treatment line in patients not refractory to lenalidomide. The Eloquent-2 trial has not revealed any particular safety concerns [35]. Infusion reactions are rare and the infusion duration much more convenient than for the second available antibody, daratumumab, which targets the CD38 antigen on plasma cells. Like elotuzumab, daratumumab has an additional, immunological mode of action. The target antigen CD38 is also expressed on normal plasma cells and regulatory T cells, which may be depleted during daratumumab therapy. About half of the patients have mild to moderate reactions during the first daratumumab infusion. Since CD38 is also expressed on the smooth muscle cells of the airways, patients with a severe chronic obstructive pulmonary disease or severe asthma might not qualify for this antibody. Daratumumab monotherapy achieves response rates of over 30% in heavily pretreated patients and has been approved in this setting [36]. Recently, the results of two large randomised twin-studies (CASTOR and POLLUX) have been published, in which daratumumab was combined with either bortezomib and dexamethasone (CASTOR) or lenalidomide and dexamethasone (POLLUX). Both double regimens served as comparators in the standard arms of the two studies. PFS was markedly improved by the addition of the monoclonal antibody and the PFS2 curves of both trials were shown to favour the investigational arm [37, 38]. Daratumumab recently obtained approval by the US Food and Drug Administration in combination with pomalidomide and dexamethasone for the treatment in the relapsed-refractory setting [39].
The third-generation IMID pomalidomide, in combination with dexamethasone, was approved four years ago after at least two previous treatment lines, including a proteasome inhibitor and an IMID, as it offers clinically meaningful benefit in terms of response and survival rates [40]. Recently, the addition of elotuzumab to pomalidomide and dexamethasone resulted in a significantly improved PFS with a strong preliminary signal also for an OS benefit, making this a future standard treatment for patients who are double refractory to an IMID and a proteasome inhibitor [41].
The histone deacetylase (HDAC) inhibitor panobinostat received a first-in-class approval in combination with bortezomib and dexamethasone for treatment of patients after at least two previous regimens, including a proteasome inhibitor and an IMID, who are not refractory to bortezomib [42]. However, panobinostat is not frequently used for myeloma treatment in Switzerland.
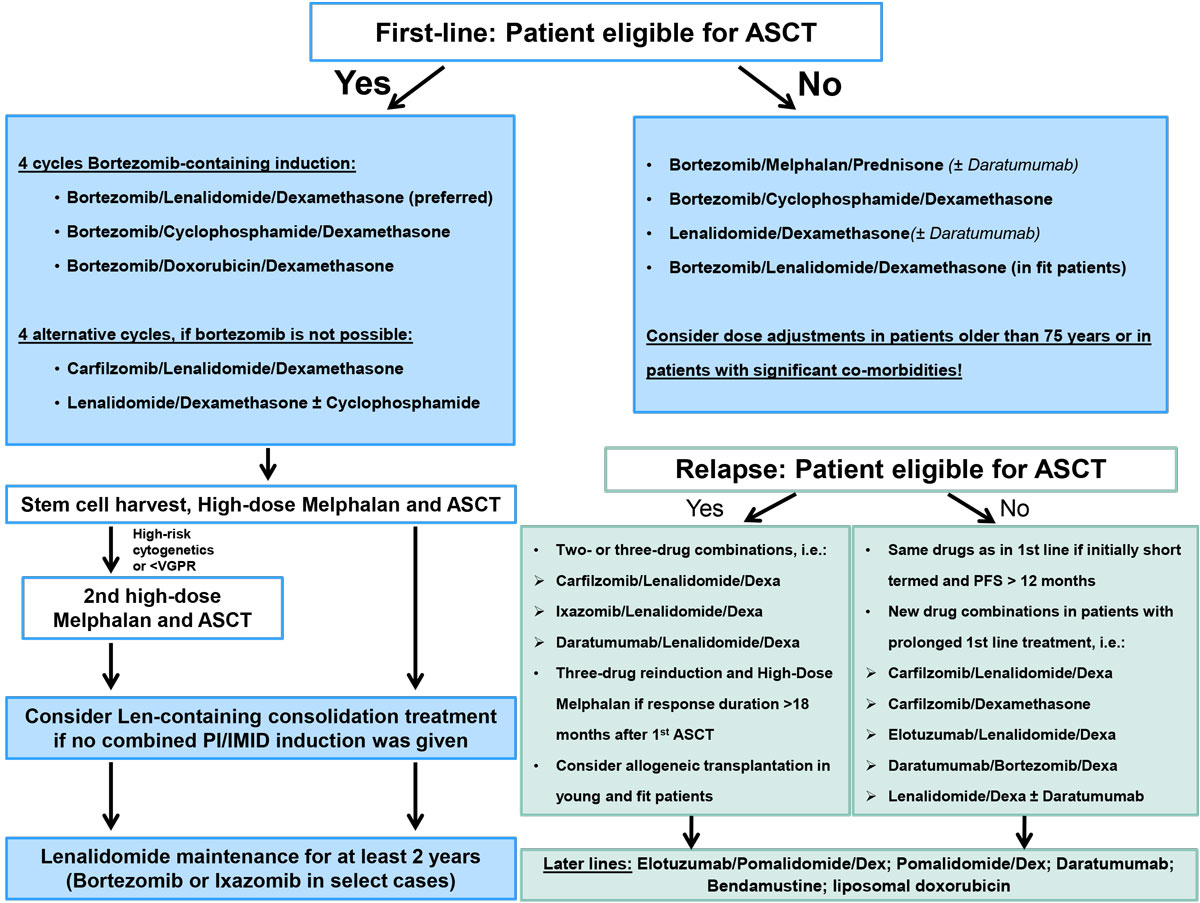
Based on the results discussed here, various new treatment options are available for patients with relapsed multiple myeloma. The choice of treatment depends on disease- and patient-related factors: the number and composition of previous treatment regimens, the tolerability of and response to previous treatments, underlying comorbidities, age and, last but not least, the preferences of the patient. Patients with a long-lasting response of more than 12–18 months may be retreated with the same regimen as in first line. However, patients with a shorter duration of response should receive alternative regimens. In addition, transplant-eligible patients may also undergo salvage-ASCT in the event of a remission duration of more than 18–24 months, based on the data available today. Allogeneic stem cell transplantation may be considered only in young high-risk patients who relapsed shortly after primary treatment with an effective induction treatment and subsequent ASCT, and should be ideally carried out in the context of a clinical trial [43]. A possible algorithm for first and later line treatment is depicted in figure 1.
Additional treatment
All patients with newly diagnosed plasma cell myeloma should receive additional antiresorptive therapy with zoledronic acid, since rates of skeletal events and survival may be favourably affected [44]. A recently published noninferiority trial compared the administration of zoledronic acid every 12 weeks with the standard 4-week administration and found no differences in terms of time to next skeletal event between the two arms. The 12-weekly administration of zoledronic acid may thus be an alternative to the monthly administration routine [45]. The monoclonal antibody denosumab is a receptor activator of NF-κB (RANK)-ligand inhibitor and approved for treatment of patients who have osteoporosis or bone metastases of solid tumours and are receiving chemotherapy. Based on newly available positive data from a placebo-controlled noninferiority trial comparing 120 mg denosumab subcutaneously with 4 mg zoledronic acid intravenously, both given every 4 weeks, this drug might be an option, especially for patients with renal failure, underlying intolerance of or contraindications to zoledronic acid. Denosumab received FDA approval on 5 January 2018, but as it is not yet approved in Switzerland, reimbursement of the treatment costs by the health insurance company needs to be assured beforehand [46].
Bullet points
- High dose chemotherapy (HDT) with subsequent autologous stem cell transplantation (ASCT) can still be considered standard of care for eligible patients in the first-line setting as it achieves deeper responses, although study data suggest that deferral until relapse may achieve comparable long-term survival
- The combination of bortezomib, lenalidomide and dexamethasone (VRD) can be considered the preferred regimen for first-line treatment in transplant-eligible patients, and is an option in fit, transplant-ineligible patients.
- Maintenance treatment with lenalidomide for at least two years may be considered standard of care for the majority of patients after HDT/ASCT.
- Additional options such as consolidation treatment (e.g., two VRD cycles after HDT/ASCT) or second (tandem) transplantation should be based on the patient´s individual risk profile and response to the first HDT/ASCT. Patients with high-risk cytogenetics should be considered for tandem transplantation.
- The combinations of the monoclonal anti-CD38 antibody daratumumab with bortezomib, melphalan and prednisone (D-VMP) or with lenalidomide and dexamethasone (DRD) may evolve as new first-line treatment options for older, transplant-ineligible patients able to undergo multi-agent therapy.
- Lenalidomide and dexamethasone (RD) improves progression-free (PFS) and overall survival (OS) in elderly patients with newly diagnosed plasma cell myeloma and should be considered standard of care.
- Various new treatment options have become available for second or later lines, and the choice of the best regimen needs to be based upon previous treatment regimens, toxicity profile, comorbidities and preference of each patient.
- The daratumumab in combination with dexamethasone and either lenalidomide (DRD) or bortezomib (DVD) results in substantial improvement of PFS in patients with relapsed multiple myeloma.
- Carfilzomib, ixazomib, or the monoclonal anti-SLAMF7 antibody elotuzumab in combination with lenalidomide and dexamethasone are additional effective second line treatment options. Panobinostat may be considered in combination with bortezomib and dexamethasone but is less frequently used.
- Patients treated with at least two previous lines, including an immunomodulatory drug (IMID) and a proteasome inhibitor, may be treated with the triple combination of elotuzumab, pomalidomide and dexamethasone as third line treatment.
- Daratumumab monotherapy may achieve response rates of over 30% in patients pretreated with at least two previous lines and is currently being used as third- or fourth-line treatment.
- The RANK-ligand inhibitor denosumab can be considered an equally effective alternative to zoledronic acid as antiresorptive treatment in patients with newly diagnosed plasma cell myeloma.
References
1
Samaras
P
,
Bargetzi
M
,
Betticher
DC
,
Duchosal
MA
,
Heim
D
,
Hess
U
, et al.
Current status and updated recommendations for diagnosis and treatment of plasma cell myeloma in Switzerland. Swiss Med Wkly. 2015;145:w14100. doi:.https://doi.org/10.4414/smw.2015.14100
2
Kyle
RA
,
Larson
DR
,
Therneau
TM
,
Dispenzieri
A
,
Kumar
S
,
Cerhan
JR
, et al.
Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. N Engl J Med. 2018;378(3):241–9. doi:.https://doi.org/10.1056/NEJMoa1709974
3
Kyle
RA
,
Durie
BGM
,
Rajkumar
SV
,
Landgren
O
,
Blade
J
,
Merlini
G
, et al.; International Myeloma Working Group. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24(6):1121–7. doi:.https://doi.org/10.1038/leu.2010.60
4
Dispenzieri
A
,
Kyle
RA
,
Katzmann
JA
,
Therneau
TM
,
Larson
D
,
Benson
J
, et al.
Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111(2):785–9. doi:.https://doi.org/10.1182/blood-2007-08-108357
5
Mateos
M-V
,
Hernández
M-T
,
Giraldo
P
,
de la Rubia
J
,
de Arriba
F
,
Corral
LL
, et al.
Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(8):1127–36. doi:.https://doi.org/10.1016/S1470-2045(16)30124-3
6
Rajkumar
SV
,
Dimopoulos
MA
,
Palumbo
A
,
Blade
J
,
Merlini
G
,
Mateos
M-V
, et al.
International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–48. doi:.https://doi.org/10.1016/S1470-2045(14)70442-5
7
Cavo
M
,
Terpos
E
,
Nanni
C
,
Moreau
P
,
Lentzsch
S
,
Zweegman
S
, et al.
Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017;18(4):e206–17. doi:.https://doi.org/10.1016/S1470-2045(17)30189-4
8
Greipp
PR
,
San Miguel
J
,
Durie
BGM
,
Crowley
JJ
,
Barlogie
B
,
Bladé
J
, et al.
International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–20. doi:.https://doi.org/10.1200/JCO.2005.04.242
9
Palumbo
A
,
Avet-Loiseau
H
,
Oliva
S
,
Lokhorst
HM
,
Goldschmidt
H
,
Rosinol
L
, et al.
Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol. 2015;33(26):2863–9. doi:.https://doi.org/10.1200/JCO.2015.61.2267
10
Palumbo
A
,
Cavallo
F
,
Gay
F
,
Di Raimondo
F
,
Ben Yehuda
D
,
Petrucci
MT
, et al.
Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371(10):895–905. doi:.https://doi.org/10.1056/NEJMoa1402888
11
Attal
M
,
Lauwers-Cances
V
,
Hulin
C
,
Leleu
X
,
Caillot
D
,
Escoffre
M
, et al.; IFM 2009 Study. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med. 2017;376(14):1311–20. doi:.https://doi.org/10.1056/NEJMoa1611750
12
Durie
BGM
,
Hoering
A
,
Abidi
MH
,
Rajkumar
SV
,
Epstein
J
,
Kahanic
SP
, et al.
Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519–27. doi:.https://doi.org/10.1016/S0140-6736(16)31594-X
13
Cavo
M
,
Beksac
M
,
Dimopoulos
M
,
Pantani
L
,
Gay
F
,
Hájek
R
, et al.
Intensification Therapy with Bortezomib-Melphalan-Prednisone Versus Autologous Stem Cell Transplantation for Newly Diagnosed Multiple Myeloma: An Intergroup, Multicenter, Phase III Study of the European Myeloma Network (EMN02/HO95 MM Trial). Blood. 2016;128(22):673.
14
Sonneveld
P
,
Beksac
M
,
van der Holt
B
,
Dimopoulos
MA
,
Carella
AM
,
Ludwig
H
, et al.
Consolidation Followed By Maintenance Therapy Versus Maintenance Alone in Newly Diagnosed, Transplant Eligible Patients with Multiple Myeloma (MM): A Randomized Phase 3 Study of the European Myeloma Network (EMN02/HO95 MM Trial). Blood. 2016;128(22):242.
15Cavo M, Gay F, Patriarca F, Zamagni E, Montefusco V, Dozza L, et al. Double autologous stem cell transplantation significantly prolongs progression-free survival and overall survival in comparison with single autotransplantation in newly diagnosed multiple myeloma: an analysis of phase 3 EMN02/H095 study. ASH 2017. Abstract 401.
16
Stadtmauer
EA
,
Pasquini
MC
,
Blackwell
B
,
Knust
K
,
Bashey
A
,
Devine
SM
, et al.
Comparison of autologous hematopoietic cell transplant (autoHCT), bortezomib, lenalidomide (len) and dexamethasone (RVD) consolidation with len maintenance (ACM), tandem autohct with len Maintenance (TAM) and autohct with len maintenance (AM) for up-front treatment of patients with multiple myeloma (MM): primary results from the randomized phase III trial of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0702 – StaMINA Trial). Blood. 2016;128:LBA-1.
17
Avet-Loiseau
H
,
Lauwers-Cances
V
,
Corre
J
,
Moreau
P
,
Attal
M
,
Munshi
N
. Minimal Residual Disease in Multiple Myeloma: Final Analysis of the IFM2009 Trial. Blood. 2018;130(Suppl 1):435.
18
McCarthy
PL
,
Holstein
SA
,
Petrucci
MT
,
Richardson
PG
,
Hulin
C
,
Tosi
P
, et al.
Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J Clin Oncol. 2017;35(29):3279–89. doi:.https://doi.org/10.1200/JCO.2017.72.6679
19
Jackson
G
,
Davies
FE
,
Pawlyn
C
,
Cairns
D
,
Striha
A
,
Hockaday
A
, et al.
Lenalidomide Maintenance Significantly Improves Outcomes Compared to Observation Irrespective of Cytogenetic Risk: Results of the Myeloma XI Trial. Blood. 2018;130(Suppl 1):436.
20Goldschmidt H, Mai EK, Dürig J, Scheid C, Weisel KC, Kunz C, et al. Response-Adapted Lenalidomide Maintenance in Newly Diagnosed, Transplant-Eligible Multiple Myeloma: Results from the Multicenter Phase III GMMG-MM5 Trial. ASH 2017. Abstract 400.
21Dimopoulos M, Gay F, Schjesvold FH, Beksac M, Hajek R, Weisel K, et al. Maintenance Therapy with the Oral Proteasome Inhibitor (PI) Ixazomib Significantly Prolongs Progression-Free Survival (PFS) Following Autologous Stem Cell Transplantation (ASCT) in Patients with Newly Diagnosed Multiple Myeloma (NDMM): Phase 3 Tourmaline-MM3 Trial. ASH 2018. Abstract 301.
22
Goldschmidt
H
,
Lokhorst
HM
,
Mai
EK
,
van der Holt
B
,
Blau
IW
,
Zweegman
S
, et al.
Bortezomib before and after high-dose therapy in myeloma: long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia. 2018;32(2):383–90. doi:.https://doi.org/10.1038/leu.2017.211
23
O’Donnell
EK
,
Laubach
JP
,
Yee
AJ
,
Chen
T
,
Huff
CA
,
Basile
FG
, et al.
A phase 2 study of modified lenalidomide, bortezomib and dexamethasone in transplant-ineligible multiple myeloma. Br J Haematol. 2018;182(2):222–30. doi:.https://doi.org/10.1111/bjh.15261
24
Mateos
M-V
,
Dimopoulos
MA
,
Cavo
M
,
Suzuki
K
,
Jakubowiak
A
,
Knop
S
, et al.; ALCYONE Trial Investigators. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N Engl J Med. 2018;378(6):518–28. doi:.https://doi.org/10.1056/NEJMoa1714678
25Facon T, Kumar SK, Plesner T, Orlowski RZ, Moreau P, Bahlis NJ, et al. Phase 3 Randomized Study of Daratumumab Plus Lenalidomide and Dexamethasone (D-Rd) Versus Lenalidomide and Dexamethasone (Rd) in Patients with Newly Diagnosed Multiple Myeloma (NDMM) Ineligible for Transplant (MAIA). ASH 2018. Abstract LBA-2.
26
Facon
T
,
Dimopoulos
MA
,
Dispenzieri
A
,
Catalano
JV
,
Belch
A
,
Cavo
M
, et al.
Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood. 2018;131(3):301–10. doi:.https://doi.org/10.1182/blood-2017-07-795047
27
Hulin
C
,
Belch
A
,
Shustik
C
,
Petrucci
MT
,
Dührsen
U
,
Lu
J
, et al.
Updated Outcomes and Impact of Age With Lenalidomide and Low-Dose Dexamethasone or Melphalan, Prednisone, and Thalidomide in the Randomized, Phase III FIRST Trial. J Clin Oncol. 2016;34(30):3609–17. doi:.https://doi.org/10.1200/JCO.2016.66.7295
28
Li
J
,
Zhou
D-B
,
Jiao
L
,
Duan
MH
,
Zhang
W
,
Zhao
YQ
, et al.
Bortezomib and dexamethasone therapy for newly diagnosed patients with multiple myeloma complicated by renal impairment. Clin Lymphoma Myeloma. 2009;9(5):394–8. doi:.https://doi.org/10.3816/CLM.2009.n.077
29Larocca A, et al. Impact of Bortezomib- or Lenalidomide-Based Induction Treatment on High Risk Cytogenetic Transplant-Ineligible Patients with Newly Diagnosed Multiple Myeloma Enrolled in the Gimema-MM-03-05 and EMN01 Trials. ASH 2017. Abstract 744.
30
Ludwig
H
,
Sonneveld
P
,
Davies
F
,
Bladé
J
,
Boccadoro
M
,
Cavo
M
, et al.
European perspective on multiple myeloma treatment strategies in 2014. Oncologist. 2014;19(8):829–44. doi:.https://doi.org/10.1634/theoncologist.2014-0042
31
Stewart
AK
,
Rajkumar
SV
,
Dimopoulos
MA
,
Masszi
T
,
Špička
I
,
Oriol
A
, et al.; ASPIRE Investigators. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–52. doi:.https://doi.org/10.1056/NEJMoa1411321
32
Moreau
P
,
Masszi
T
,
Grzasko
N
,
Bahlis
NJ
,
Hansson
M
,
Pour
L
, et al.; TOURMALINE-MM1 Study Group. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;374(17):1621–34. doi:.https://doi.org/10.1056/NEJMoa1516282
33
Dimopoulos
MA
,
Goldschmidt
H
,
Niesvizky
R
,
Joshua
D
,
Chng
W-J
,
Oriol
A
, et al.
Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(10):1327–37. doi:.https://doi.org/10.1016/S1470-2045(17)30578-8
34
Siegel
DS
,
Dimopoulos
MA
,
Ludwig
H
,
Facon
T
,
Goldschmidt
H
,
Jakubowiak
A
, et al.
Improvement in Overall Survival With Carfilzomib, Lenalidomide, and Dexamethasone in Patients With Relapsed or Refractory Multiple Myeloma. J Clin Oncol. 2018;36(8):728–34. doi:.https://doi.org/10.1200/JCO.2017.76.5032
35
Lonial
S
,
Dimopoulos
M
,
Palumbo
A
,
White
D
,
Grosicki
S
,
Spicka
I
, et al.; ELOQUENT-2 Investigators. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2015;373(7):621–31. doi:.https://doi.org/10.1056/NEJMoa1505654
36
Usmani
SZ
,
Weiss
BM
,
Plesner
T
,
Bahlis
NJ
,
Belch
A
,
Lonial
S
, et al.
Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128(1):37–44. doi:.https://doi.org/10.1182/blood-2016-03-705210
37
Palumbo
A
,
Chanan-Khan
A
,
Weisel
K
,
Nooka
AK
,
Masszi
T
,
Beksac
M
, et al.; CASTOR Investigators. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;375(8):754–66. doi:.https://doi.org/10.1056/NEJMoa1606038
38
Dimopoulos
MA
,
Oriol
A
,
Nahi
H
,
San-Miguel
J
,
Bahlis
NJ
,
Usmani
SZ
, et al.; POLLUX Investigators. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;375(14):1319–31. doi:.https://doi.org/10.1056/NEJMoa1607751
39
Chari
A
,
Suvannasankha
A
,
Fay
JW
,
Arnulf
B
,
Kaufman
JL
,
Ifthikharuddin
JJ
, et al.
Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974–81. doi:.https://doi.org/10.1182/blood-2017-05-785246
40
Dimopoulos
MA
,
Palumbo
A
,
Corradini
P
,
Cavo
M
,
Delforge
M
,
Di Raimondo
F
, et al.
Safety and efficacy of pomalidomide plus low-dose dexamethasone in STRATUS (MM-010): a phase 3b study in refractory multiple myeloma. Blood. 2016;128(4):497–503. doi:.https://doi.org/10.1182/blood-2016-02-700872
41
Dimopoulos
MA
,
Dytfeld
D
,
Grosicki
S
,
Moreau
P
,
Takezako
N
,
Hori
M
, et al.
Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N Engl J Med. 2018;379(19):1811–22. doi:.https://doi.org/10.1056/NEJMoa1805762
42
San-Miguel
JF
,
Hungria
VTM
,
Yoon
S-S
,
Beksac
M
,
Dimopoulos
MA
,
Elghandour
A
, et al.
Overall survival of patients with relapsed multiple myeloma treated with panobinostat or placebo plus bortezomib and dexamethasone (the PANORAMA 1 trial): a randomised, placebo-controlled, phase 3 trial. Lancet Haematol. 2016;3(11):e506–15. doi:.https://doi.org/10.1016/S2352-3026(16)30147-8
43
Giralt
S
,
Garderet
L
,
Durie
B
,
Cook
G
,
Gahrton
G
,
Bruno
B
, et al.
American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group Consensus Conference on Salvage Hematopoietic Cell Transplantation in Patients with Relapsed Multiple Myeloma. Biol Blood Marrow Transplant. 2015;21(12):2039–51. doi:.https://doi.org/10.1016/j.bbmt.2015.09.016
44
Morgan
GJ
,
Davies
FE
,
Gregory
WM
,
Bell
SE
,
Szubert
AJ
,
Cook
G
, et al.
Long-term follow-up of MRC Myeloma IX trial: Survival outcomes with bisphosphonate and thalidomide treatment. Clin Cancer Res. 2013;19(21):6030–8. doi:.https://doi.org/10.1158/1078-0432.CCR-12-3211
45
Himelstein
AL
,
Foster
JC
,
Khatcheressian
JL
,
Roberts
JD
,
Seisler
DK
,
Novotny
PJ
, et al.
Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients With Bone Metastases: A Randomized Clinical Trial. JAMA. 2017;317(1):48–58. doi:.https://doi.org/10.1001/jama.2016.19425
46
Raje
N
,
Terpos
E
,
Willenbacher
W
,
Shimizu
K
,
García-Sanz
R
,
Durie
B
, et al.
Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19(3):370–81. doi:.https://doi.org/10.1016/S1470-2045(18)30072-X