
Figure 1 Economic model outcomes for direct costs and quality adjusted life-years, by scenario, 2016–2031.
DOI: https://doi.org/10.4414/smw.2019.20026
Swiss francs
direct-acting antiviral agent
decompensated cirrhosis
gross domestic product
Federal Office of Public Health
hepatocellular carcinoma
hepatitis C virus
incremental cost-effectiveness ratio
liver-related death
polymerase chain reaction
quality-adjusted life years
sustained virological response
uncertainty interval
World Health Organization
Although the exact prevalence of the hepatitis C virus (HCV) in Switzerland is unknown, recent efforts by the Federal Office of Public Health (FOPH) suggest that in 2016 there were an estimated 39,500 (36,000–43,000) chronic infections nationwide [1]. Switzerland is a world leader in harm reduction efforts which minimize ongoing transmission among people who inject drugs [2]. However, previous research showed that HCV-related morbidity and mortality are projected to increase by 2030, even as viraemic prevalence continues to decline [3]. In the absence of other interventions, healthcare costs will increase as the infected population ages because late-stage liver disease often requires costly healthcare procedures such as liver transplantation [3]. An analysis of HCV-related healthcare costs in Switzerland found that the annual healthcare costs of untreated HCV (excluding antiviral treatment) could increase from 86 (42–182) million Swiss francs (CHF) in 2013 to 112 (42–267) million CHF by 2030 [3]. This could place further strain on the limited resources of the healthcare system, as the economic costs incurred increase as infection progresses.
In 2014, experts from across the country collaborated to propose a hepatitis elimination strategy, with the aim of curtailing new infections and addressing the harmful and costly impact of HCV as the prevalent population ages [4]. In previous modelling work, multiple scenarios with changes to sustained virological response (SVR) rates, medical eligibility, treatment uptake, diagnosis rates and the number of patients treated were developed and compared [3]. This analysis builds on those efforts by assessing the forecasted impact and direct costs of intervention strategies, i.e., screening, diagnostic, staging and treatment costs, over the next 15 years. In particular, this analysis seeks to evaluate the clinical impact and associated cost of expanding treatment to patients in all fibrosis stages (≥F0) compared with the status quo until October 2017 (treatment of ≥F2 patients), and to calculate a treatment price that is cost-saving (and cost-effective) by 2031. This analysis was not undertaken to recommend any one therapy over another; rather, it was conducted to estimate the impact of growing access to direct-acting antivirals (DAAs) on the HCV disease burden and associated costs.
We developed a disease burden and economic impact model, considering a formal Swiss healthcare sector perspective, to evaluate direct costs, health effects measured in quality-adjusted life years (QALYs), and the incremental cost-effectiveness ratio (ICER). Two HCV treatment scenarios, an intervention and a comparator scenario, were developed, considering the entire HCV RNA-positive population in Switzerland, and the outcomes were measured over a 15-year time horizon from 2016 to 2031. Additionally, a net-zero cost scenario was developed in which the treatment price associated with the intervention scenario was calculated to achieve break-even by 2031.
Future costs and health effects were discounted at an annual rate of 3%. A scenario was considered cost-effective when the ICER (calculated as net cost per QALY gained) was lower than the 2015 Swiss gross domestic product (GDP) per capita (78,000 CHF) [5].
Under the comparator, the “historical” base case, all individuals aged 15 years and older with F2 or greater fibrosis (on the METAVIR scale) were eligible for treatment starting in 2016 (table 1). Under this base case, 2,000 ≥F2 patients were treated in 2016 at a price of 51,400 CHF. A trend to reduce treatment numbers annually after 2016 was applied to achieve a 50% reduction in the number of patients treated by 2020. This was intended to model the expected depletion of the pool of diagnosed and cared for patients which has been seen in the first few years of DAA treatment across Western countries.
Table 1 Comparator (historical base case) and intervention (scenarios 1 and 2) scenario input parameters.
| Historical base case | 2016 | 2017 | 2018 | 2019 | 2020–2030 |
|---|---|---|---|---|---|
| Treated | 2,000 | 1,800 | 1,600 | 1,400 | 1,200 |
| Newly diagnosed | 1,100 | 1,100 | 1,100 | 1,100 | 1,100 |
| Treatment-eligible fibrosis stages | ≥ F2 | ≥ F2 | ≥ F2 | ≥ F2 | ≥ F2 |
| New infections | 700 | 700 | 700 | 700 | 700 |
| Treatment-eligible ages | 15+ | 15+ | 15+ | 15+ | 15+ |
| SVR | 95% | 95% | 95% | 95% | 95% |
| Scenarios 1 and 2 | 2016 | 2017 | 2018 | 2019 | 2020–2030 |
| Treated | 2,000 | 3,000 | 4,300 | 4,300 | 4,300 |
| Newly diagnosed | 1,100 | 1,100 | 1,100 | 1,100 | 1,100 |
| Treatment-eligible fibrosis stages | ≥ F2 | ≥ F0 | ≥ F0 | ≥ F0 | ≥ F0 |
| New infections | 700 | 700 | 700 | 700 | 700 |
| Treatment-eligible ages | 15+ | 15+ | 15+ | 15+ | 15+ |
| SVR | 95% | 95% | 95% | 95% | 95% |
The intervention scenario, Scenario 1, followed the same course as the historical base case through 2016 (table 1). In October 2017, reimbursement restrictions for all DAAs were lifted in Switzerland and the total number of patients treated for the year increased to 3,000. Beginning in 2018, 4,250 ≥F0 patients were modelled as being treated annually, at a price of 31,000 CHF per patient [6]. Under this intervention scenario, no changes were made to the number of diagnosed patients. A net-zero cost scenario, Scenario 2, was developed using the treatment paradigm of Scenario 1 but at a treatment price that would achieve zero net cost by 2031.
The HCV disease progression model used in this analysis has been described previously [7] and detailed model structures, including model validation, are included in Appendix Section 1. Briefly, Swiss population, mortality and HCV epidemiology data were used to populate and calibrate a Markov model that quantified the current and future disease burden of HCV infection in Switzerland. The disease burden was measured through forecasted outcomes of prevalence, mortality, and end-stage outcomes (including hepatocellular carcinoma (HCC) and decompensated cirrhosis (DC)), considering the impact of current and future interventions.
Calculations for the economic model are provided in Appendix Section 3 and briefly described here. To assess cost-effectiveness, QALYs were calculated. QALYs were based on time spent in various health states, using a health state utility value between 0 and 1 [13, 28-31]. The incremental cost-effectiveness ratio (ICER) was calculated as the net cost (the difference in direct costs between the scenario and the base case) divided by the net benefit (the difference in QALYs between the scenario and the base case). Achieving a net cost of zero was defined as the break-even point.
Disease burden outcomes were evaluated through to 2030, in line with the Global Health Sector Strategy targets for the elimination of HCV [8]. However, the economic impact was considered through to 2031 to ensure all costs associated with implementing the intervention scenario were fully realized. Medical costs were denominated in 2016 Swiss francs, using the exchange rate on December 31, 2016.
Input parameters were obtained from peer-reviewed literature or extracted from Swiss-specific sources. The HCV disease progression model inputs for Switzerland have been published previously [3]. In many instances, however, more recent data (number treated, number diagnosed, etc.) were available. All updated and novel parameters are described below and in table 2.
Table 2 HCV disease burden and economic impact module parameters.
| Category | Item | Source | Year | Base Case | Sensitivity (run only where ranges are present) | |
|---|---|---|---|---|---|---|
| Disease burden model parameters | Anti-HCV prevalence | [1] | 2016 | 0.7 | 0.3–1.6% | Beta-PERT |
| Viraemic rate | [9] | 79.7% | - | |||
| Viraemic diagnosed | Calculated using data from [10, 11] | 1988–2012 | 27,300 | 25,500–29,100 | Beta-PERT | |
| 2013–2015 | Refer to original source for annual data | - | ||||
| 2016 | 1,116 | - | ||||
| Treated | IMS Health [11] Expert Input (Prof. Franco Negro) |
2004–2014 | 10,670 | - | ||
| 2015 | 1,280 ≥F3 2,300 ≥F2 |
- | ||||
| 2016 | 2,100 ≥F2 | - | ||||
| Liver transplants | Swiss Transplant | 2003–2016 | Refer to original source for annual data | - | ||
| Liver transplants due to HCV | Personal communication | 21.2% | 18.2–49.2% | Beta-PERT | ||
| Disease burden model validation | Incident liver cancer cases | National Institute for Cancer Epidemiology and Registration | 1988–2012 | Refer to original source for annual data | - | |
| HCC etiology | Geneva Tumour Registry | 1990–2013 | Refer to original source for annual data | 25–90% | Beta-PERT | |
| HCC due to HCV | [12] | 1998–2012 | 44.5% | 43.3–53.3% | Beta-PERT | |
| Economic modelling parameters | Multiplier for private costs | 1.5 | - | |||
| QALY health state utilities | [13] | See study | Beta-PERT | |||
| GDP per capita | 2015 | 78,000 | - | |||
| Discounting | 3% | 0%, 3% | Binomial | |||
| Screening costs (CHF) (public) | Anti-HCV | [14, 15] | 2016 | 25 | 21–29 | Beta-PERT |
| RNA test / PCR | [14, 15] | 2016 | 180 | 153–207 | Beta-PERT | |
| Genotyping | [14, 15] | 2016 | 180 | 153–207 | Beta-PERT | |
| Staging / liver biopsy / fibroscan | [14, 16] | 2016 | 256 | 218–294 | Beta-PERT | |
| Health state costs (CHF) (public) | [14] | See study | Beta-PERT | |||
| Treatment cost (CHF) (public) | Average total cost | [6] | 2015 | 59,100 | 49,100–66,400 | Beta-PERT |
| 2016 | 51,400 | 42,700–61,300 | Beta-PERT | |||
| [6] | 2017 | 31,000 | 26,350–35,650 | Beta-PERT | ||
A full list of the input parameters required for the HCV disease progression model is provided in Appendix Section 1. In summary, there were approximately 39,500 chronic (HCV RNA-positive) cases in Switzerland in 2016 [1]. The percentage diagnosed in 2016 was estimated from Swiss FOPH notification data were considered as follows. In 2013, there were an estimated 30,200 diagnosed viraemic infections in Switzerland (after accounting for mortality) [17]. In 2013–2015, there were an average of 1,600 new HCV notifications to the Swiss FOPH [10]. Assuming a viraemic rate of 79.7% [9], this suggests an average of 1,300 new viraemic diagnoses annually. Removing cured HCV patients over the 2004–2015 period results in a range of 25,400–29,100 total diagnosed viraemic cases alive in 2015 (table 2). In 2016, there were an additional 1,400 newly notified cases, corresponding to approximately 1,116 newly diagnosed viraemic cases [10]. Thus, out of the estimated 39,500 persons living with HCV in 2016, about 67–77% had been diagnosed.
The number of patients treated annually was derived from pharmaceutical sales data, and reimbursement restrictions on the basis of fibrosis stage were confirmed through discussions with the FOPH and providers in Switzerland. In 2015, an estimated 2,300 (2,000–2,500) patients were treated in total. Approximately 1,280 of these were ≥F3 and the remainder were F2 [11]. In 2016, the number of treated patients dropped to 2,100 ≥F2 (table 2). As of October 2017, fibrosis restrictions were lifted, making all patients, regardless of their fibrosis stage, eligible for treatment. In 2017, it was estimated that 3,000 patients were treated (based on extrapolations of data provided by the Swiss Pharmacist Cooperative (OFAC), Swiss National Pharmacy Service (Mediservice) and IMS Health), an increase of 43% compared to 2016 as previously ineligible F0 and F1 patients received treatment.
All input parameters for the economic modelling are listed in table 2. QALY utilities were obtained from a 2016 cost-effectiveness model of US patients and were applied to this model by disease stage [13]. Data on the cost of healthcare for HCV patients by disease stage [3, 14] and diagnostic cost data, including all treatment-related tests such as polymerase chain reaction (PCR) testing, genotyping and fibrosis staging [15], were obtained from previous publications. As treatment regimens and outcomes improve, the number and types of treatment-related tests needed are expected to change. The timeline for these changes was divided into three waves, present (prior to 2018), near future (2018–2021) and pan-genotypic (after 2021).
The FOPH specialties list provided treatment costs by therapy type in 2016, when treatment was limited to ≥F2 patients, while October 2017 updates to the specialties list (corresponding to the change in reimbursement restrictions) were used to inform treatment costs for scenarios where ≥F0 patients were treated [6]. Discussions with the FOPH suggest that treatment costs decreased by 15% when treatment was expanded to F2 patients, so the treatment price of 59,100 CHF in 2015 (when treatment was restricted to ≥F3 patients) was assumed to be 15% higher than the baseline price in 2016 (51,400 CHF).
Sensitivity analyses were carried out using Crystal Ball, an Excel add-in by Oracle. Beta-PERT distributions were used to model uncertainty in all study parameters, unless noted otherwise in table 2. A one-way sensitivity analysis was conducted to identify the variables that most heavily influenced the ICER. Additionally, a Monte Carlo simulation with 1,000 trials was used to determine the 95% uncertainty intervals (UIs).
The historical base case, with approximately 39,500 cases in 2016 and no change to the 2016 treatment paradigm, shows a decrease in the economic and disease burdens of HCV by 2031. The number of infected individuals declines by 35% while rates of DC, HCC and liver-related deaths (LRD) decrease 50–55% by 2031 (Appendix Section 2). In comparison, in the intervention scenario (Scenario 1), an 85% decline in the projected number of viraemic cases is expected between 2016 and 2031, with approximately 560, 710 and 1,000 incident cases of DC, HCC and LRD respectively averted over this period (Appendix Section 2).
Under the historical base case, annual direct costs are projected to decrease from 150 million (95% UI: 132–170 million) CHF in 2016 to 90 million (95% UI: 65–111 million) CHF in 2031 (figure 1a). Cumulative direct costs, however, are projected to reach 1.7 billion (95% UI: 1.2–2.0 billion) CHF by 2031 (figure 1b, table 3). In Scenario 1, annual direct costs first increased to 175 million CHF in 2018, before declining to 44 million CHF by 2031 (figure 1a). Cumulative direct costs in this scenario are projected to reach 1.8 billion CHF by 2031 (figure 1b, Table 3). Considering direct costs only, Scenario 1 does not achieve break-even by 2031 (figure 1b).

Figure 1 Economic model outcomes for direct costs and quality adjusted life-years, by scenario, 2016–2031.
Table 3 Economic model outcomes for quality adjusted life-years gained, cumulative direct costs and ICER, by scenario, 2016–2031
| Outcomes for the 2016–2031 period | QALYs gained | Cumulative direct costs (CHF Millions) | ICER (CHF/QALY) |
|---|---|---|---|
| Historical base case | - | 1,654 | - |
| Scenario 1 | 58,327 | 1,783 | 2,210 |
| Scenario 2 | 58,327 | 1,654 | 0 |
QALY, quality-adjusted life year; CHF, Swiss franc; ICER, incremental cost-effectiveness ratio
Considering only direct costs, a treatment price of 27,900 CHF per patient was found to break even (yield zero net cost relative to the historical base case) in 2031 (figure 1b). The 27,900 CHF price was thus used to define Scenario 2. The impact of this was a reduction in annual direct costs compared with Scenario 1, with 163 million CHF incurred in 2018, dropping to 40 million CHF by 2031 (figure 1a). Cumulative direct costs reach 1.7 billion CHF by 2031, to match the historical base case (figure 1b, Table 3).
Treatment and laboratory costs in the historical base case were projected to decrease from 105 million (95% UI: 105 million–106 million) CHF annually to 61 million (95% UI: 53–69 million) CHF in 2031, a 43% decrease (figure 1c). In Scenario 1, the treatment and laboratory costs would increase from 110 million to 141 million CHF in 2018 before falling to 37 million CHF annually in 2031, a 65% decrease compared to 2016 (figure 1d). In Scenario 2, due to the lower treatment costs, the annual treatment and laboratory costs would peak at 128 million CHF from 2018 to 2021 before falling to 33 million CHF by 2031, a 70% decrease compared to 2016 (figure 1e).
In this model, more than 60% of viraemic cases were estimated to have been diagnosed as of 2016, with 1,120 new viraemic cases diagnosed annually. Assuming no change to screening strategies, annual costs will rise to just over 5 million CHF annually by 2021 (figures 1c, 1d and 1e). After 2021, these costs decline rapidly, and by 2023 annual costs are less than 125,000 CHF for all three scenarios.
Because Scenarios 1 and 2 treat the same number of patients, they gain the same number of QALYs, with 58,300 QALYs gained cumulatively by 2031 (figure 1f, table 3). The cost per QALY gained for both Scenarios 1 and 2 decreases over time as patients are cured. Additionally, the lifting of fibrosis restrictions means that fewer cases are progressing to the costlier advanced stages of disease. Under both scenarios, the cost per QALY gained drops below the cost-effectiveness threshold of 78,000 CHF in 2018 (figure 1g). Over the 15-year span, the cost per QALY gained was determined to be 2,200 CHF for Scenario 1 (and zero for Scenario 2) (table 3). This 15-year ICER of Scenario 1 indicates that it is highly cost-effective compared to the historical base case given a cost-effectiveness acceptability threshold of one GDP per capita of Switzerland, as seen in figure 1g. Scenario 2 was, by design, cost-saving (and highly cost-effective) relative to the base case.
In a sensitivity analysis, ten variables accounted for more than 99% of the uncertainty in the cost per QALY gained (15-year ICER) (figure 2) under Scenario 1. The rate of spontaneous clearance, rate of mild to moderate fibrosis progression, 2017 treatment price, HCV prevalence in 2016, healthcare costs for compensated cirrhosis and the standardized mortality ratio (SMR) for blood transfusion recipients accounted for more than 90% of the uncertainty. All the variables considered in the analysis are listed in table 2, and the impact on the 15-year ICER is presented in figure 2.
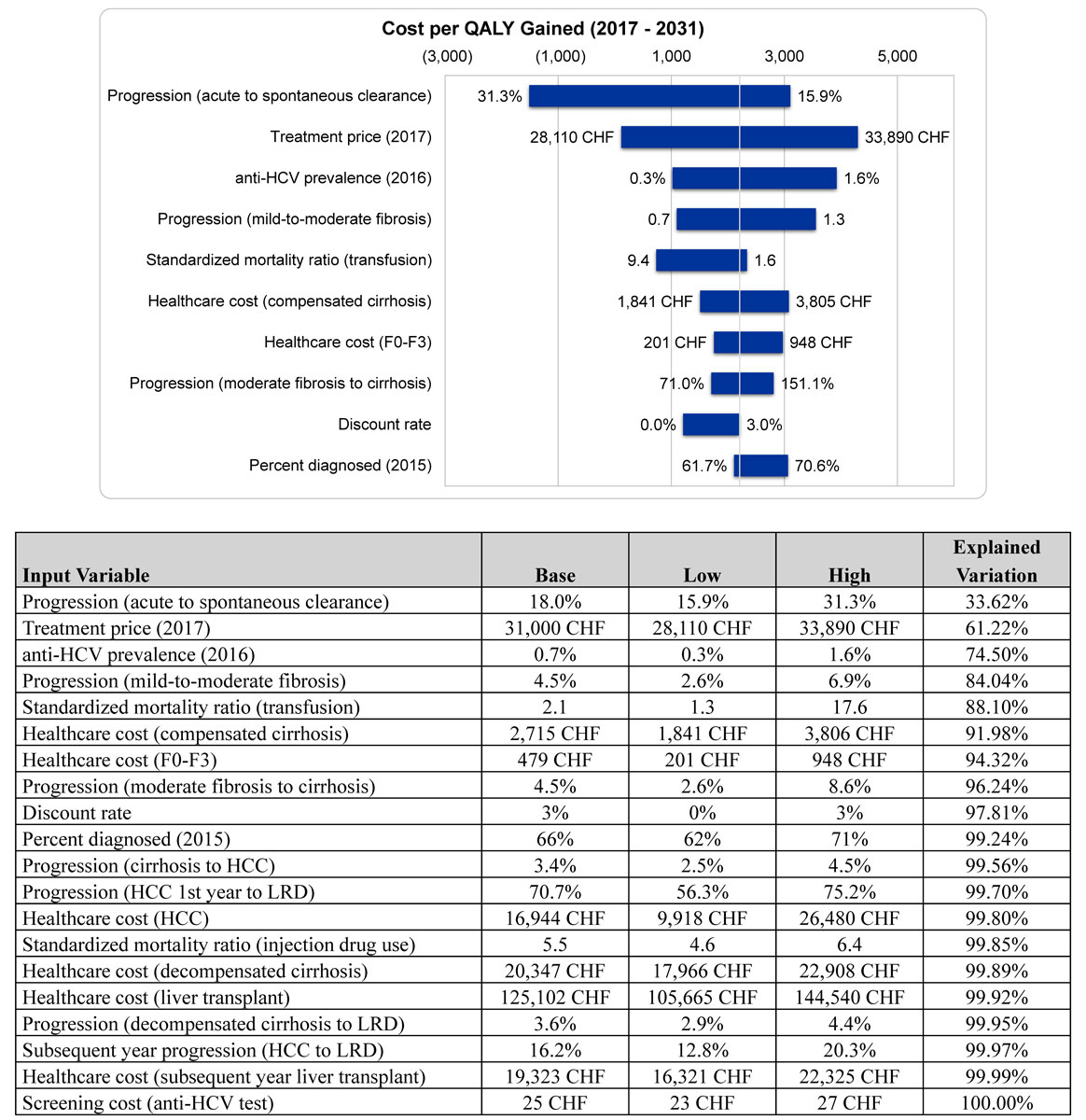
Figure 2 Sensitivity analysis around the average cost per QALY gained and explained variation by input variable, 2017–2031.
The complex and costly-to-manage end-stage outcomes of chronic HCV infection, combined with high DAA costs, have previously led some health systems to require evidence of advanced liver fibrosis before authorizing new therapies [18–21]. This analysis sought to compare the impact of an intervention strategy of treating more patients in earlier fibrosis stages to the more restrictive base case. This was done by assessing the disease burden impact of this strategy, as well as the impact on direct costs and health effects (i.e. costs of reduced quality of life) over the next 15 years. Furthermore, the analysis sought to determine a drug price necessary for break-even by 2031.
Significant decreases in disease burden are estimated by 2031 for both the base case and the intervention scenario, with the intervention scenario seeing a larger impact, as expected. The decrease in late-stage liver disease in the base case scenario, however, is in contrast to previous analyses which reported a reduction in viraemic cases, but 50–85% increases in cases of DC, HCC and LRD by 2030 [3]. The current decrease in disease burden is likely due to increasing efforts in Switzerland to expand access to HCV diagnosis and treatment. Estimates from the FOPH suggest that 1,300 new viraemic cases were diagnosed annually between 2012 and 2015, and up to 2,000 ≥F2 patients were treated with DAAs in 2016, compared to 1,050 newly diagnosed and 1,100 treated patients, projected for the same years from prior studies [3, 10, 11, 17].
Direct costs were largely driven by treatment expansion. In the base case, annual direct costs decline over the 2017–2020 period due to the decreasing number of individuals already diagnosed and available to be treated. After 2020, the base case assumes no expansion of screening or diagnostic efforts and no increase to the number of patients treated, resulting in an equilibration of direct costs (figure 1). By comparison, treatment and laboratory staging costs in the intervention scenarios remain greater than 130 million CHF annually until 2025, at which point they drop drastically as the limits of diagnosis and treatment are reached in Switzerland. The impact of the expanded access to treatment and cure in the intervention scenarios is also seen in the steady decline in healthcare costs, as cases of DC and HCC, as well as of liver transplantations, are prevented. By 2031, healthcare costs in the base case are four times higher than in either intervention scenario. The intervention’s higher upfront costs are therefore offset by reductions in future healthcare costs. In all three scenarios, a major change in screening costs occurs between 2021 and 2023, falling from more than 5 million CHF annually to less than 125,000 CHF annually. These costs are estimated by assuming that the current number of patients diagnosed annually is maintained, and uses formulas to estimate the number of screenings needed from the proportion of prevalent and diagnosed cases [15].
Expanding treatment access to all patients aged 15 years and over, regardless of fibrosis stage, over the next 15 years, was found to be cost-effective by 2018 at the 31,000 CHF price. While this is not sufficient to break even by 2031, it lends further credence to other analyses which have shown that early treatment of HCV is not only beneficial to disease burden, but also cost-effective [13, 22, 23]. Net-zero cost analysis found that a 27,900 CHF treatment price could achieve break-even in direct costs by 2031.
Finally, this analysis has some limitations which should be considered when evaluating the results. The high diagnosis coverage (66%) does not necessarily translate to patients linked to care, meaning that some work may still be required to identify patients for treatment. In addition, the analysis assumed no risk of reinfection after cure, an assumption which could lead to an overestimation of cost-effectiveness. Another limitation of this analysis is that the results do not consider extrahepatic manifestations of HCV, associated lost productivity, or other aspects of societal return on investment. Recent studies suggest that extrahepatic manifestations contribute substantially to the economic burden of HCV [24, 25]. Achieving SVR, especially at an early stage of disease, can attenuate the associated disease and economic burdens of these extrahepatic manifestations [26, 27]. Including this in the analysis would likely show even greater cost savings. Similarly, if we had included the loss in productivity and decreased societal engagement (due to stigma, strained relationships, etc.) associated with ongoing HCV infection (and subsequently improved by achieving SVR), the cost savings associated with treating HCV would be expected to improve. Lastly, the analysis does not incorporate any future changes in price associated with the introduction of generic drugs. Beginning in 2026, treatment prices are estimated to drop to 70% of the initial 2016 prices due to patent expiration and the use of generics. This would make the key outcomes of the economic impact analysis more favourable. However, it is possible that new therapies will be introduced before the patent expiration, which would counterbalance the effect of generic price reductions.
This analysis shows baseline HCV disease burden in Switzerland declining over the next 15 years, even with treatment restricted to ≥F2 patients. Expanding treatment to ≥F0 patients and increasing the number of treated patients (considering a price of 27,900–31,000 CHF per treatment) will result in a larger impact on disease burden and is cost-effective or cost-saving when considering a variety of measures. This confirms that further reductions in treatment prices improve cost-effectiveness and create conditions suitable for positive returns on the investment. To supplement this cost-effectiveness analysis, a comprehensive budget impact analysis would be essential to determine the financial consequences and affordability of implementing such strategies.
We are grateful to Dr. Jörg Indermitte from the Federal Office of Public Health and Ivane Gamkrelidze from the Center for Disease Analysis Foundation (CDAF) for their contributions to the development of this analysis. Additionally, we would like to thank Helen Nde and Sarah Robbins from CDAF for their help drafting and reviewing the manuscript. Finally, we appreciate the input of Prof. Beat Müllhaupt, Dr. Florian Bihl, Prof. Daniel Lavanchy and Dr. David Semela, who provided data and input for the disease burden analysis.
This work was funded by the Swiss Federal Office of Public Health
S. Blach and H. Razavi are employees of the Center for Disease Analysis Foundation (CDAF) and have not received any remuneration from pharmaceutical companies. H. Razavi has been a member of advisory boards for Gilead, AbbVie and Jansson pharmaceuticals; all proceeds went to the CDAF. He is the managing director of Center for Disease Analysis (CDA). The CDAF has received grants from CDC Foundation, John C Martin Foundation, The Association of State and Territorial Health Officials (ASTHO), Zeshan Foundation, Vaccine Impact Modelling Consortium, WHO WPRO, WHO Geneva, Swiss Federal Office of Public Health, Brazil MoH, Center for Disease Analysis and private donors. The CDA has received research funding from Gilead Sciences, AbbVie and Intercept Pharma. P. Bruggmann is a consultant/advisor and has received research/travel grants from AbbVie, Gilead Sciences and Merck/MSD. F. Negro has received research funding from Gilead Sciences and is a consultant/advisor for Gilead, AbbVie and MSD.
1Zahnd C, Brezzi M, Bertisch B, Giudici F, Keiser O. Situation Analysis on hepatitis B and C in Switzerland [Online]. Federal Office of Public Health; 2017 [Available from: www.bag.admin.ch/situationsanalyse-hepatitis.
2 Uchtenhagen A . Heroin-assisted treatment in Switzerland: a case study in policy change. Addiction. 2010;105(1):29–37. doi:.https://doi.org/10.1111/j.1360-0443.2009.02741.x
3 Müllhaupt B , Bruggmann P , Bihl F , Blach S , Lavanchy D , Razavi H , et al. Modeling the Health and Economic Burden of Hepatitis C Virus in Switzerland. PLoS One. 2015;10(6):e0125214. doi:.https://doi.org/10.1371/journal.pone.0125214
4Swiss Hepatitis Strategy 2014-2030. Time to Act Now! Process Paper - A Living Document. http://www.hepatitis-schweiz.ch/files/Dokumente/Process_Paper_2nd_version_final.pdf. 2015.
5World Bank. GDP per capita (current US$) Washington, D.C.2015 [Available from: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD.
6 Leblebicioglu H , Arends JE , Ozaras R , Corti G , Santos L , Boesecke C , et al.; ESGHV (part of ESCMID). Availability of hepatitis C diagnostics and therapeutics in European and Eurasia countries. Antiviral Res. 2018;150:9–14. doi:.https://doi.org/10.1016/j.antiviral.2017.12.001
7 Blach S , Zeuzem S , Manns M , Altraif I , Duberg A-S , Muljono DH , et al.; Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–76. doi:.https://doi.org/10.1016/S2468-1253(16)30181-9
8WHO. Global Health Sector Strategy on Viral Hepatitis, 2016–2021 Towards Ending Viral Hepatitis. World Health Organization; 2016.
9 Armstrong GL , Wasley A , Simard EP , McQuillan GM , Kuhnert WL , Alter MJ . The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–14. doi:.https://doi.org/10.7326/0003-4819-144-10-200605160-00004
10Federal Office of Public Health. Swiss mandatory notifications for hepatitis C per canton, FOPH/CD/EPI/RIC 29.08.16. 2016.
11Health IMS. IMS Health MIDAS Data 2013 [updated 1/1/2013. Available from: http://www.imshealth.com/.
12 Vitali GC , Laurent A , Terraz S , Majno P , Buchs NC , Rubbia-Brandt L , et al. Minimally invasive surgery versus percutaneous radio frequency ablation for the treatment of single small (≤3 cm) hepatocellular carcinoma: a case-control study. Surg Endosc. 2016;30(6):2301–7. doi:.https://doi.org/10.1007/s00464-015-4295-6
13 Chahal HS , Marseille EA , Tice JA , Pearson SD , Ollendorf DA , Fox RK , et al. Cost-effectiveness of Early Treatment of Hepatitis C Virus Genotype 1 by Stage of Liver Fibrosis in a US Treatment-Naive Population. JAMA Intern Med. 2016;176(1):65–73. doi:.https://doi.org/10.1001/jamainternmed.2015.6011
14 Pfeil AM , Reich O , Guerra IM , Cure S , Negro F , Müllhaupt B , et al. Cost-effectiveness analysis of sofosbuvir compared to current standard treatment in Swiss patients with chronic hepatitis C. PLoS One. 2015;10(5):e0126984. doi:.https://doi.org/10.1371/journal.pone.0126984
15 Bruggmann P , Negro F , Bihl F , Blach S , Lavanchy D , Müllhaupt B , et al. Birth cohort distribution and screening for viraemic hepatitis C virus infections in Switzerland. Swiss Med Wkly. 2015;145:w14221. doi:.https://doi.org/10.4414/smw.2015.14221
16 Bruggmann P , Grebely J . Prevention, treatment and care of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26(Suppl 1):S22–6. doi:.https://doi.org/10.1016/j.drugpo.2014.08.014
17Federal Office of Public Health. Number of hepatitis C cases reported in Switzerland between 1988 and 2012 by year of birth (mandatory notification of laboratory confirmed cases): FOPH/ID/EPI/RIC. 2013.
18Committee on a National Strategy for the Elimination of Hepatitis B, Board on Population H, Public Health P, Health, Medicine D, National Academies of Sciences E, et al. In: Buckley GJ, Strom BL, editors. Eliminating the Public Health Problem of Hepatitis B and C in the United States: Phase One Report. Washington (DC): National Academies Press; 2016.
19 Brennan T , Shrank W . New expensive treatments for hepatitis C infection. JAMA. 2014;312(6):593–4. doi:.https://doi.org/10.1001/jama.2014.8897
20 Saag MS . Quantum leaps, microeconomics, and the treatment of patients with hepatitis C and HIV coinfection. JAMA. 2014;312(4):347–8. doi:.https://doi.org/10.1001/jama.2014.7735
21 Steinbrook R , Redberg RF . The high price of the new hepatitis C virus drugs. JAMA Intern Med. 2014;174(7):1172. doi:.https://doi.org/10.1001/jamainternmed.2014.2135
22 Buti M , Domínguez-Hernández R , Oyagüez I , Casado MA , Esteban R . Cost-effectiveness analysis of ledipasvir/sofosbuvir in patients with chronic hepatitis C: Treatment of patients with absence or mild fibrosis compared to patients with advanced fibrosis. J Viral Hepat. 2017;24(9):750–8. doi:.https://doi.org/10.1111/jvh.12704
23 Kondili LA , Romano F , Rolli FR , Ruggeri M , Rosato S , Brunetto MR , et al.; PITER Collaborating Group. Modeling cost-effectiveness and health gains of a “universal” versus “prioritized” hepatitis C virus treatment policy in a real-life cohort. Hepatology. 2017;66(6):1814–25. doi:.https://doi.org/10.1002/hep.29399
24 Cacoub P , Buggisch P , Carrión JA , Cooke GS , Zignego AL , Beckerman R , et al. Direct medical costs associated with the extrahepatic manifestations of hepatitis C infection in Europe. J Viral Hepat. 2018;25(7):811–7. doi:.https://doi.org/10.1111/jvh.12881
25 Cacoub P , Vautier M , Desbois AC , Saadoun D , Younossi Z . Direct medical costs associated with the extrahepatic manifestations of hepatitis C virus infection in France. Aliment Pharmacol Ther. 2018;47(1):123–8. doi:.https://doi.org/10.1111/apt.14382
26 Cacoub P , Desbois AC , Comarmond C , Saadoun D . Impact of sustained virological response on the extrahepatic manifestations of chronic hepatitis C: a meta-analysis. Gut. 2018;67(11):2025–34. doi:.https://doi.org/10.1136/gutjnl-2018-316234
27 Mahale P , Engels EA , Li R , Torres HA , Hwang LY , Brown EL , et al. The effect of sustained virological response on the risk of extrahepatic manifestations of hepatitis C virus infection. Gut. 2018;67(3):553–61. doi:.https://doi.org/10.1136/gutjnl-2017-313983
28 Salomon JA , Weinstein MC , Hammitt JK , Goldie SJ . Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA. 2003;290(2):228–37. doi:.https://doi.org/10.1001/jama.290.2.228
29 Thein HH , Krahn M , Kaldor JM , Dore GJ . Estimation of utilities for chronic hepatitis C from SF-36 scores. Am J Gastroenterol. 2005;100(3):643–51. doi:.https://doi.org/10.1111/j.1572-0241.2005.40976.x
30 Liu S , Cipriano LE , Holodniy M , Owens DK , Goldhaber-Fiebert JD . New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156(4):279–90. doi:.https://doi.org/10.7326/0003-4819-156-4-201202210-00005
31 Hagan LM , Yang Z , Ehteshami M , Schinazi RF . All-oral, interferon-free treatment for chronic hepatitis C: cost-effectiveness analyses. J Viral Hepat. 2013;20(12):847–57. doi:.https://doi.org/10.1111/jvh.12111
This work was funded by the Swiss Federal Office of Public Health
S. Blach and H. Razavi are employees of the Center for Disease Analysis Foundation (CDAF) and have not received any remuneration from pharmaceutical companies. H. Razavi has been a member of advisory boards for Gilead, AbbVie and Jansson pharmaceuticals; all proceeds went to the CDAF. He is the managing director of Center for Disease Analysis (CDA). The CDAF has received grants from CDC Foundation, John C Martin Foundation, The Association of State and Territorial Health Officials (ASTHO), Zeshan Foundation, Vaccine Impact Modelling Consortium, WHO WPRO, WHO Geneva, Swiss Federal Office of Public Health, Brazil MoH, Center for Disease Analysis and private donors. The CDA has received research funding from Gilead Sciences, AbbVie and Intercept Pharma. P. Bruggmann is a consultant/advisor and has received research/travel grants from AbbVie, Gilead Sciences and Merck/MSD. F. Negro has received research funding from Gilead Sciences and is a consultant/advisor for Gilead, AbbVie and MSD.