Interventional treatment of mitral valve regurgitation: an alternative to surgery?
DOI: https://doi.org/10.4414/smw.2019.20023
Fabien
Praza, Nicolas
Bruggera, Mohammad
Kassara, Lukas
Hunzikera, Aris
Moschovitisa, Stefan
Storteckya, David
Reinekeb, Thomas
Pilgrima, Stephan
Windeckera
aDepartment of Cardiology, Bern University Hospital, Bern, Switzerland
bDepartment of Cardiac Surgery, Bern University Hospital, Bern, Switzerland
Summary
Mitral regurgitation (MR) is a highly prevalent condition among elderly patients, affecting almost 10% of the general population aged 75 and older. Left untreated, severe MR results in high mortality and frequent hospitalisation for treatment of heart failure. Surgical treatment remains the first-line therapy for symptomatic, severe MR, especially for patients presenting with a primary aetiology. However, a high proportion of patients with MR are turned down for open-heart surgery, mainly due to advanced age, diminished left ventricular function and comorbidities. Thus, percutaneous treatment options have been recently developed as an alternative. In this article, we will review transcatheter interventional techniques at the level of the mitral valve, including implantation technique, indications and clinical results.
Introduction
Mitral regurgitation (MR) is a highly prevalent condition among elderly patients, affecting almost 10% of the general population aged 75 and older [1]. From a mechanistic point of view, MR is usually classified into two different categories: primary (degenerative) and secondary (functional) MR. The latter condition is further subdivided into ischaemic and non-ischaemic aetiology. Degenerative mitral valve (MV) disease is the most frequent mechanism of MR (60–70%) [2] and is related to the structural modification of the valve leaflet tissue, including prolapse, flail leaflet and annular calcification, and the supporting apparatus. Secondary MR affects patients with left ventricular dysfunction. Ventricular dilation exerts tension on the valve leaflets via the papillary muscles and chordal apparatus, resulting in leaflet tethering and subsequent loss of coaptation.
Left untreated, severe MR results in chronic volume overload of the left ventricle accompanied by increased left atrial and pulmonary artery pressure, ultimately leading to pulmonary oedema, right ventricular dysfunction and secondary tricuspid regurgitation. The mortality associated with untreated severe MR is as high as 50% within five years, with 90% of surviving patients experiencing symptoms of heart failure, atrial fibrillation or an indication for MV repair or replacement [3].
In this article, we will review transcatheter interventional techniques at the level of the MV, including implantation technique, indications and clinical results.
Diagnosis and grading of mitral regurgitation
Although transthoracic echocardiography (TTE) is the preferred diagnostic tool to detect MR, transoesophageal echocardiography (TEE) achieves more precise grading, as well a better assessment of the underlying mechanism. Evaluation of the suitability for transcatheter MV interventions usually requires the acquisition of three-dimensional sequences used to localise the lesion, appreciate valve anatomy and measure the 3D valve area. Table 1 summarises the semi-quantitative and quantitative criteria considered for grading of MR according to the most recent guidelines of the European Society of Cardiology [4]. The integrative implementation of these different parameters is expected to improve diagnostic reliability.
Table 1 Semi-quantitative and quantitative criteria for the diagnosis of severe MR.
|
Semi-quantitative criteria
|
|
| Vena contracta (mm) |
≥7 (>8 for biplane measurements*) |
| Pulmonary veins |
Systolic flow reversal |
| Mitral inflow |
Dominant E-wave ≥1.5 m/s |
| MR velocity (CW Doppler) |
TVI mitral/TVI aortic >1.4 |
|
Quantitative criteria
|
Primary
|
Secondary
|
| EROA (mm2) |
≥40 |
≥20 |
| Regurgitant volume (ml) |
≥60 |
≥30 |
Surgical mitral valve repair/replacement
Surgical treatment remains the first-line therapy of symptomatic, severe MR, especially for patients presenting with primary MR. In asymptomatic patients, the presence of atrial fibrillation, diminished left ventricular function (≤60%) and pulmonary hypertension are predictors of worse outcomes, and thus support early correction. Surgical and clinical results are largely dependent on the underlying aetiology, as well as on valve repairability. In patients with primary valvulopathy, surgical repair is associated with a low recurrence rate (90% of patients alive after 20 years of follow-up are free of severe MR) [5], and observational studies suggest improved clinical outcomes compared to MV replacement [6].
The benefit of surgical correction of secondary MR is not well established [7, 8], and perioperative mortality remains high despite improvements in surgical technique. Recurrent MR, which has been directly linked to impaired clinical outcomes [9], is as high as 30% at six months [10] and 60% within two years [11]. Comparing MV repair and replacement for severe ischemic MR, a recently published randomised trial reported no significant differences in mortality (19.0 vs 23.2%; p = 0.39) and left ventricular reverse remodelling at two years. Of note is that the recurrence of moderate to severe MR was 58.8% in the repair group compared to 3.8% in the replacement group, resulting in a significantly higher rate of rehospitalisation for heart failure in the repair group [12].
Rationale for the development of transcatheter valve solutions
In the past, the structural treatment of severe MR has exclusively been based on surgical MV repair or replacement. However, approximately 50% of patients presenting with severe symptomatic MR are not referred for open-heart surgery due to high operative risk (mainly related to advanced age and impaired left ventricular function) [13]. In-hospital mortality after MV surgery in octogenarians is as high as 13% after MV replacement and 6% after MV repair [14]. Similarly, patients with diminished left ventricular function (<40%) have a post-procedural mortality of 10% following valve replacement and 6% after valve repair [15]. These data support the ongoing development of less invasive procedures which are better adapted to elderly patients with frequent comorbidities.
During the last few years, two methods have been developed for the percutaneous treatment of the MV: transcatheter MV repair and transcatheter MV replacement. Table 2 summarises the principal advantages of each method.
Table 2 Advantages of transcatheter mitral valve repair and replacement.
|
Transcatheter mitral valve repair
|
Transcatheter mitral valve replacement
|
| High safety |
Standardised strategy and short procedure duration |
| Conservation of the native anatomy |
Predictable outcome in terms of MR reduction |
| Low risk of thrombosis |
One system fits all anatomies (?) |
| Low risk of interaction with LVOT and subvalvular apparatus |
Durability (?) |
The Heart Team, consisting of experienced cardiac surgeons, interventional cardiologists, imaging guiding specialists and heart failure physicians, plays a crucial role in the decision-making process. The individual risk constellation is established based on medical history and comorbidities.
Transcatheter mitral valve repair
Contemporary transcatheter MV reconstruction systems are categorised into three groups according to their mechanism of action: leaflet repair, direct, and indirect annuloplasty. While annuloplasty techniques are exclusively applied among patients with sectional MR, leaflet repair can be used for the treatment of both MR categories.
Percutaneous leaflet repair: technical aspects
Edge-to-edge repair replicates a simplified surgical reconstruction method, the Alfieri technique, that consists of connecting the central part of the anterior and posterior MV leaflets (A2-P2) together, thereby creating a double orifice MV. In surgical observational studies, this method has been shown to effectively reduce MR [16], although it is routinely combined with annuloplasty.
Two percutaneous devices have been developed to enable percutaneous leaflet repair.
1. The Abbott MitraClip system
The MitraClip system (Abbott, Abbott Park, Illinois) was first implanted in a human in 2003. Five years later, in 2008, it was the first percutaneous MV therapy to receive CE approval. FDA approval for patients with primary MR followed in 2013. In the meantime, more than 70,000 patients have been treated worldwide.
The 24 French delivery system is introduced into the left atrium using a transvenous, transseptal approach (fig. 1A,B). The assembly consists of an orientable guiding catheter and a steerable clip delivery catheter (CDS). After localisation of the target lesion, usually using 3D TEE, the clip is orientated above it (fig. 1C,D). The device is then introduced into the left ventricle where grasping of the leaflet is attempted. Once both leaflets are placed on the clip arms, the clip is closed, creating a double orifice MV (fig. 1E,F). Leaflet insertion, residual MR, transvalvular gradient and 3D MV area are systematically assessed before the decision to reposition or implant the device is made.
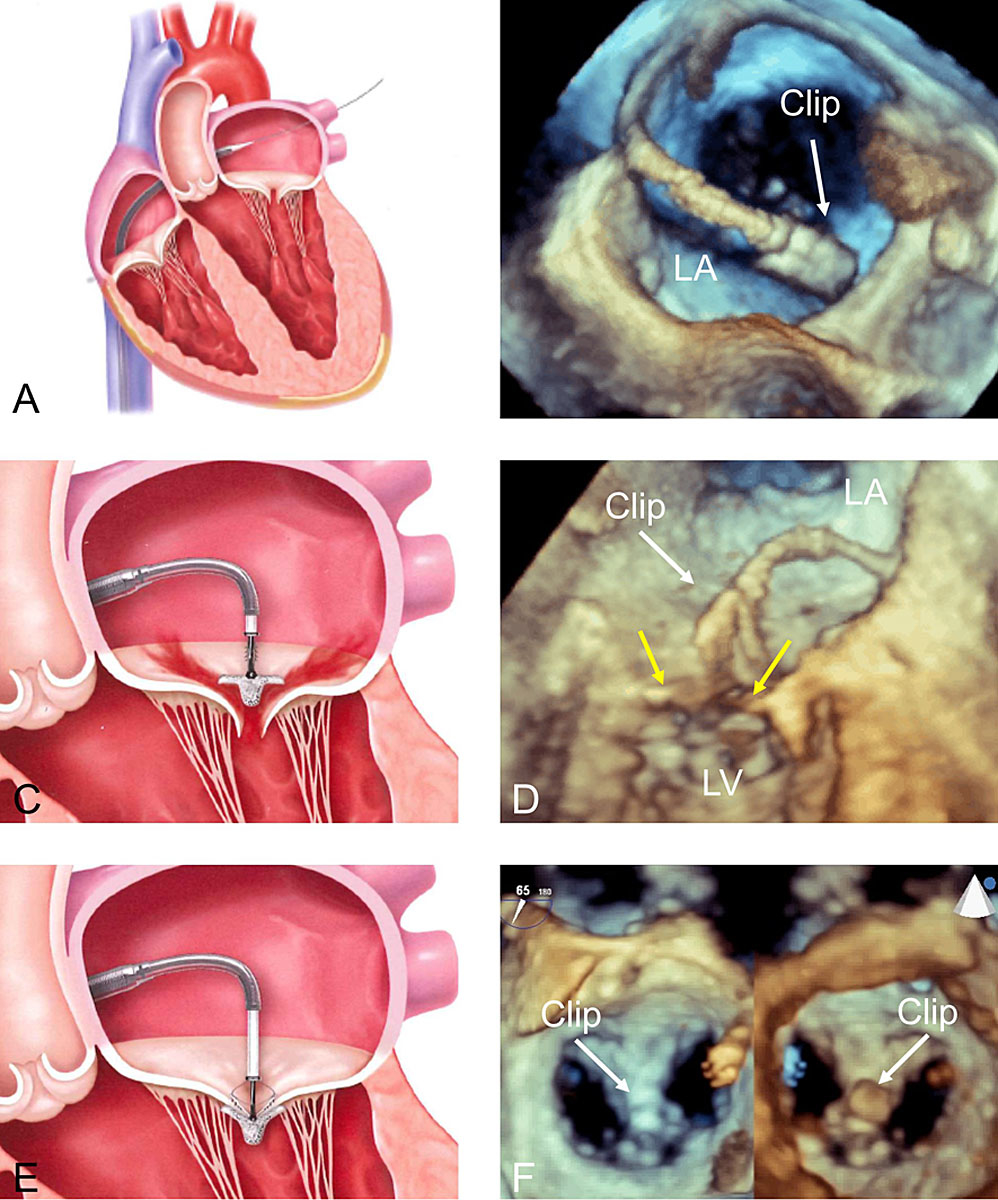
Figure 1 General principles of the Abbott MitraClip system. A: transseptal puncture in superior posterior position; B: 3D TEE view from the top of the left atrium showing introduction of the clip delivery system into the left atrium; C-D: positioning of the steerable catheter above the defect at the origin of the MR jet under 3D echocardiographic guidance (yellow arrows = mitral valve leaflets); E: grasping of the valve leaflets; F: final result shown by 3D TEE with the clip in the middle of the valve (A2-P2), creating a double orifice (left panel view from the left atrium, right panel from the left ventricle). LA: left atrium; LV: left ventricle; 3D: three-dimensional; TEE: transoesophageal echocardiography. The material used for designing panels A, C and E has been used with courtesy of Abbott.
The principal advantages of this intervention include the minimal invasive approach, enabling rapid patient recovery, along with a high safety profile. In the largest registry to date, which investigated the outcomes of 3697 patients treated in the USA, stroke was reported in 0.5% of patients, bleeding complications in 3.8%, and in-hospital mortality in 2.3% [17].
A new iteration of the MitraClip (XTR) featuring longer arms became available in early 2018 and is intended to facilitate leaflet retention.
2. The Edwards PASCAL Transcatheter mitral valve repair system
The novel Edwards PASCAL MV repair system has been designed to address some of the technical limitations observed with the MitraClip system. The device is introduced via transseptal access using a 22 French guide sheath (fig. 2A,B), and incorporates a spacer within the MV regurgitant orifice. It is attached to the valve leaflets by two wide paddles designed to avoid concentrated stress on the leaflets (fig. 2C,D). The system is intended to simplify the navigation in the left atrium, improve MR reduction through implementation of a central spacer and allow for independent leaflet grasping with each individual arm of the device. The latter may be important in complex anatomical situations, e.g. in patients with calcification, retraction or tethering of the posterior leaflet, as well as in the presence of a large prolapse gap (>1 cm). Preliminary results collected in a compassionate use cohort of 23 patients were encouraging, with acute MR ≤2+ in 97% of the patients at discharge, without elevated gradient despite the relatively larger size of the device [18]. The device recently obtained CE approval based on the data of the CLASP study.
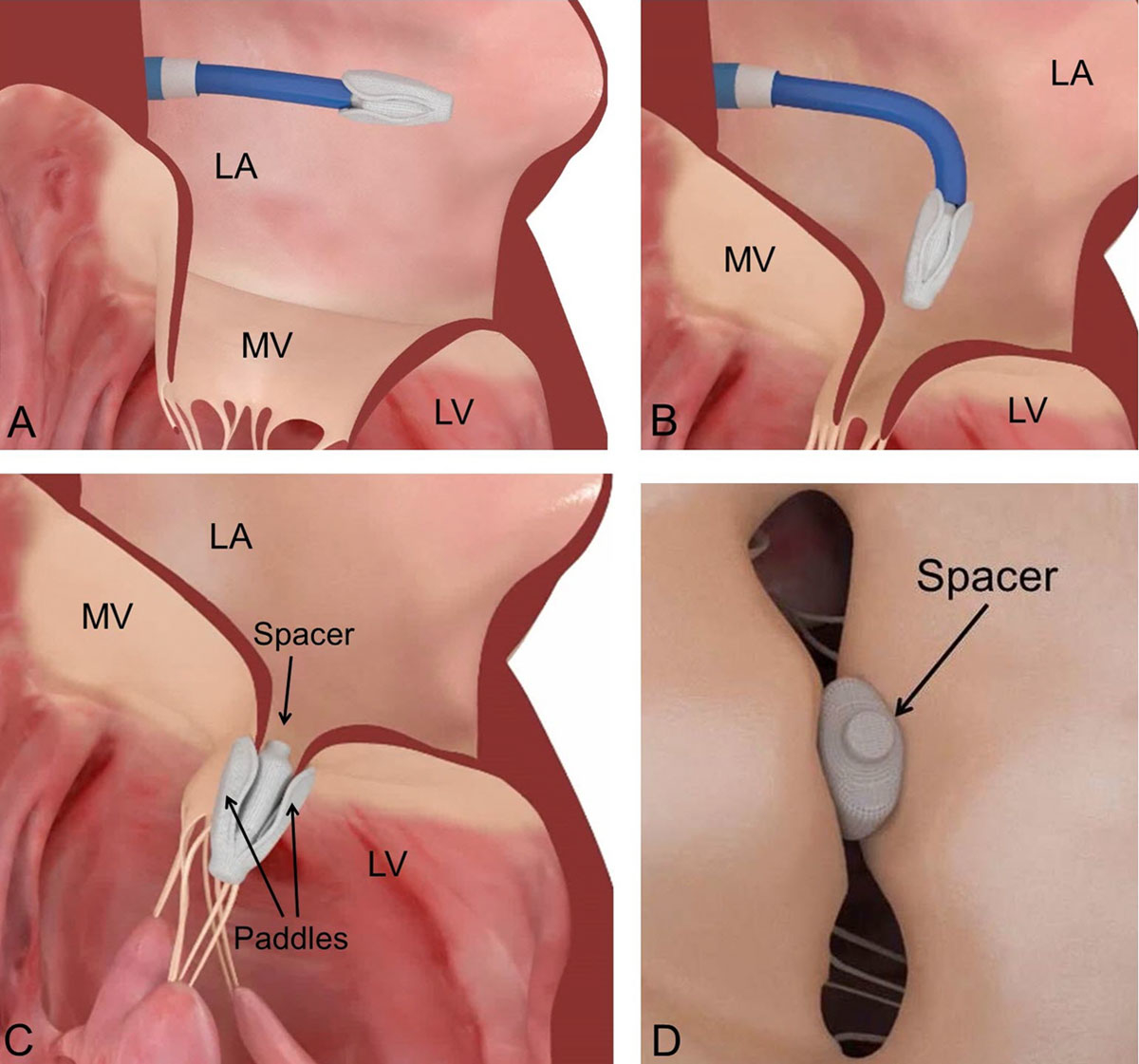
Figure 2 Procedural steps for the implantation of the Edwards PASCAL transcatheter mitral valve repair system. A: Introduction of the implant into the left atrium. B: Flexion of the steerable catheter and orientation of the device towards the mitral valve. C: Attachment of the PASCAL device to the mitral valve leaflets and deployment of the device. D: Spacer fills the regurgitant orifice area and minimises the tension exerted on the valve leaflets. MV: mitral valve; LA: left atrium; LV: left ventricle. The material used for designing the figure has been used with courtesy of Edwards Lifesciences.
Percutaneous edge-to-edge repair: the evidence
The efficacy of the MitraClip system was explored in the first randomised trial, the EVEREST II trial, which reported similar mortality throughout five years of follow-up among patients treated with the Mitraclip compared to patients treated by standard surgical mitral intervention (20.8 vs 26.8%; p = 0.4). In terms of efficacy, surgery was superior to the MitraClip in primary MR (freedom from death, MV surgery, reintervention and moderate to severe MR at five years in 45.5 vs 76.2% of patients; p <0.001), whereas there was no significant difference between patients treated for secondary MR (freedom from the same combined endpoint in 40.5% in the MitraClip group versus 28.6% in the surgical group; p = 0.43). Overall, patients enrolled in the MitraClip group were more likely to have relevant residual MR requiring valve surgery during the first six months, although this was no longer observed in a landmark analysis beyond six months. These results confirm the durability of the acute MR reduction obtained with the MitraClip system. Importantly, in this trial only 27% of patients had secondary MR, a category of patients that has been specifically investigated in two recently published randomised trials, MITRA-FR and COAPT.
In the French MITRA-FR study [19] 307 patients with left ventricular dysfunction and severe secondary MR (effective regurgitant orifice area >20 mm2 or regurgitant volume >30 ml/beat) were randomly assigned to either transcatheter mitral edge-to-edge repair with the MitraClip system combined with medical treatment, or medical treatment alone. MR grade ≤2+ was achieved in 83% of patients at one year. The study found no difference concerning the primary endpoint (all-cause death and unplanned re-hospitalisation for heart failure at one year), which occurred in 83 patients (55%) in the intervention group and 78 patients (52%) in the control group (OR, 1.16; 95% confidence interval [CI], 0.73 to 1.84; p = 0.53). Similarly, the mortality rate was 24.3% in the intervention group vs. 22.4% in the control group (HR in the intervention group, 1.11; 95% CI, 0.69 to 1.77) at 12 months. Unplanned hospitalisation for heart failure occurred in 48.7% of patients in the intervention group vs 47.4% in the control group (HR, 1.13; 95% CI, 0.81 to 1.56). Adverse events like strokes, renal replacement therapy and bleedings were more frequent in the intervention group.
The recently published COAPT trial [20] randomised 614 patients with symptomatic heart failure and moderate to severe or severe secondary MR (semi-quantitative Grade 3+ or 4+ according to integrative assessment as defined by the American Society of Echocardiography) to either guideline-directed medical treatment alone or transcatheter MV repair using the MitraClip system combined with medical therapy. The primary endpoint was the number of all hospitalisations for heart failure at 24 months. Principal powered secondary endpoints included all-cause mortality at two years, as well as change in quality of life, six minute-walk test and left ventricular end-diastolic volume, all at 12 months. MR reduction to grade 2+ or less at 12 months was obtained in 95% of patients. After 24 months, there were 160 hospitalisations for heart failure in 92 patients in the device group and 283 hospitalisations in 151 patients in the control group, corresponding to annualised hospitalisation rates of 35.8% and 67.9% per patient-year respectively. The number of patients needed to be treated to prevent one hospitalisation was 3.1 (95% CI 1.9–7.9). In addition, mortality at two years of follow-up was significantly lower following MitraClip implantation (29.1 vs 46.1%; hazard ratio, 0.62; 95% CI 0.46–0.82; p <0.001) corresponding to a relative risk reduction of 37% and a number needed to be treated to prevent one death of 5.9 (95% CI 3.9–11.7). Significant improvement was also observed for each of the nine additional pre-specified secondary endpoints.
There are important differences between both studies which may have contributed to these divergent findings:
- ‒ Inclusion of patients with less severe MR but more severe cardiomyopathy in MITRA-FR due to the adoption of different definitions concerning the severity of MR, related to the observance of the European guidelines in MITRA-FR and the American guidelines in COAPT. The mean effective regurgitant orifice area was 0.31±0.10cm
- ‒ Less strict optimisation of the medical therapy at time of inclusion, as well as a lack of treatment monitoring in MITRA-FR may have failed to capture symptom-driven therapy intensification in favour of the control group. This may explain the improvements (including MR reduction) seen in the medical group that were not observed in COAPT, which did require persistent heart failure symptoms despite optimised medical therapy.
- ‒ Differences in procedural results and complication rates (14.6% in MITRA-FR versus 8.5% in COAPT), as well as less frequent multiple clip implantations in MITRA-FR (54% versus 62% in COAPT).
- ‒ Lack of power for functional and echocardiographic secondary endpoints in MITRA-FR due to loss of follow-up, which may have masked differences between the groups.
- ‒ Shorter follow-up in MITRA-FR (difference in mortality emerged only after 12 months in COAPT)
The results of both studies provide valuable guidance regarding patient selection for transcatheter MV repair. Each study outlines a different category of patients with different responses to interventional treatment.
The COAPT trial represents an important therapeutic advancement for selected patients with heart failure who have a particularly poor prognosis under medical treatment alone. In addition, these findings profoundly modify the traditional pathophysiological understanding of heart failure with reduced left ventricular function. Indeed, in view of these results, volume overload due to severe MR should no longer be considered a simple bystander of concomitant left ventricular disease, but rather an important mediator of deleterious outcomes, requiring dedicated attention and treatment.
Identification of appropriate candidates in a timely manner implies screening for relevant MR at an early stage of left ventricular dysfunction and periodic echocardiographic surveillance following ischaemic events.
In patients with more advanced disease, the use of transcatheter MV repair in this category of patients should be evaluated on a case-by-case basis, based on drug tolerance and efficacy to maintain cardiac compensation.
Percutaneous direct annuloplasty
Percutaneous direct annuloplasty has been developed to replicate surgical ring implantation and subsequent annular reduction. It has been developed for the treatment of patients with secondary MR. Direct annuloplasty devices are implanted into the annulus itself under echocardiographic guidance.
The Edwards Cardioband mitral reconstruction system
The Cardioband system (Edwards Lifescience, Irvine, CA) received CE mark approval in 2015 for the treatment of patients with secondary MR. It consists of a percutaneous annular reduction device that is implanted via transseptal access into the posterior annulus of the MV (fig. 3A). After delivery, the length of the incomplete ring is contracted in order to reduce the septolateral dimensions and subsequently improve MR (fig. 3B). Results at 12 months in 60 patients showed the feasibility of the implantation with high technical success (97%). In terms of safety, no procedural death occurred. Life-threatening complications occurred in four patients (7%; one stroke, one myocardial infarction and one cardiac arrest, both due to circumflex artery occlusion, one tamponade). Anchor disengagement was observed in 10 patients (17%) leading to device inefficacy in five patients. Immediate MR reduction by at least one grade was obtained in 52 patients (87%). In the 12 months following the procedure, seven patients (12%) required a second intervention due to recurrent MR (six transcatheter and one surgical). Considering the whole cohort (including the patients with re-intervention), 61% of patients had moderate or less MR [21]. The secondary implantation of an edge-to-edge repair system upon the recurrence of MR is feasible [22].
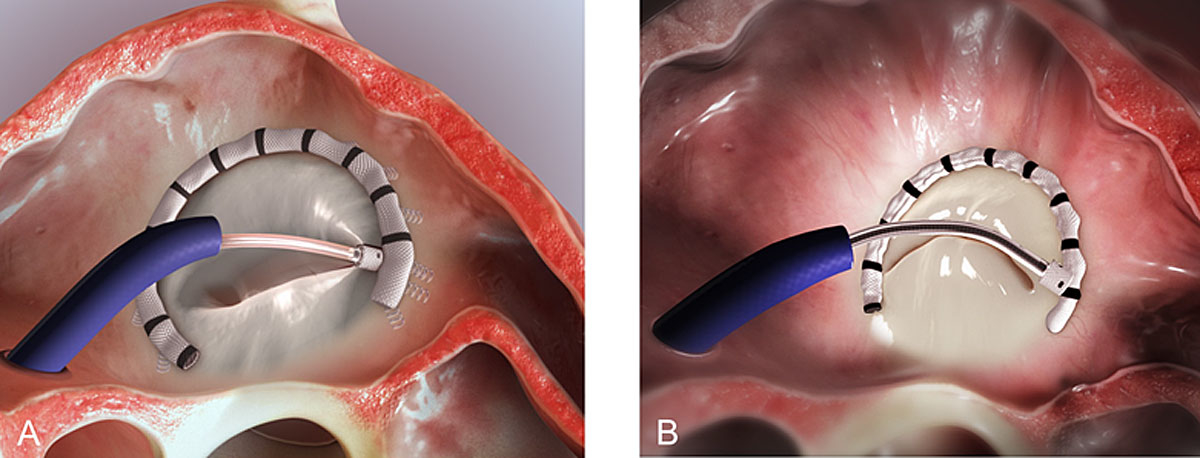
Figure 3 Principles of the Edwards Cardioband system. A: The Edwards Cardioband annular reduction device is introduced into the left atrium via the transseptal access and implanted into the posterior mitral valve annulus using 12-16 anchors; B: The length of the adjustable implant is contracted to decrease the septolateral diameter and reduce mitral regurgitation. The material used for designing the figure has been used with courtesy of Edwards Lifesciences.
The Millipede IRIS complete annuloplasty ring
The Millipede IRIS transcatheter system (Boston Scientific, Marlborough, MA, USA) is a semi-rigid, complete annuloplasty ring (fig. 4A) placed via a transfemoral/transspetal venous approach (fig. 4B). The ring is anchored into the mitral annulus by rotating helical anchors, and can repositioned and retrieved. Actuation of the device results in the controlled reduction of the septo-lateral diameter (fig. 4C). Preliminary results in 11 patients implanted via the transfemoral/transspetal access showed good safety and MR reduction (80% of the patients had MR grade 0-1+ at 1 year). A reduction of the septo-lateral diameter of more than 30% was observed. There was no mortality at 12 months. One patient in whom the Millipede device was implanted surgically had recurrent MR 15 months after the procedure and was treated with the implantation of a single MitraClip into the ring [23].
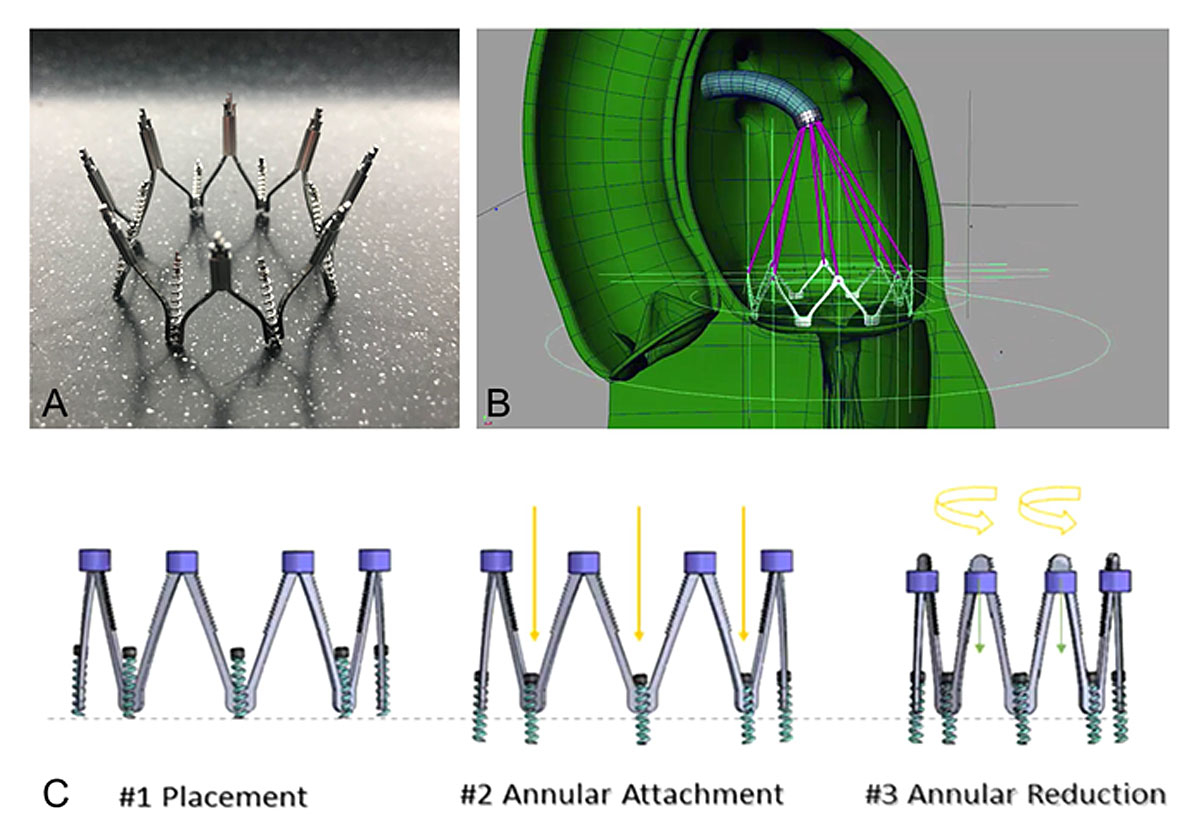
Figure 4 The Millipede IRIS complete annuluploasty ring. A: The Millipede complete annuloplasty ring consists of a semi-rigid, annular ring with a nitinol frame; B: The system is introduced via the transseptal access and secured with six screws; C: actuation of the device reduces the mitral annular dimensions. Each screw can be steered separately allowing for differential cinching according to individual anatomy. The material used for designing the figure has been provided by Boston Scientific.
Although encouraging, these preliminary results need to be confirmed in a larger population of patients.
Percutaneous indirect annuloplasty
Percutaneous indirect annuloplasty devices are designed to exert forces on the annulus from the outside. Most of them are implanted into the coronary sinus.
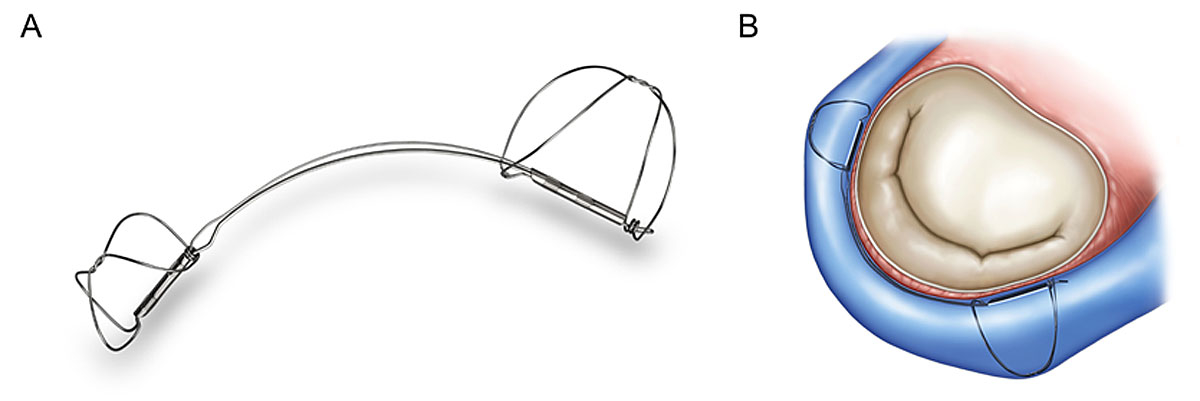
The Carillon Mitral Contour System
The Carillon Mitral Contour System (Cardiac Dimensions, Inc., Kirkland, WA) is implanted into the coronary sinus via the jugular access. It consists of two helical anchors connected by a nitinol arc, which enables self-orientation during deployment (fig. 5). The implant aims to reduce MR through the application of external circumferential pressure around the annulus. Manual traction on the delivery system pulls the proximal anchor towards the coronary sinus ostium, plicating the periannular tissue. Advantages of this system include the short duration of the procedure, which can be performed under local anaesthesia and without echocardiographic guidance, as well as the possibility of it being easily combined with other therapy modalities (e.g. MitraClip or even surgical or transcatheter MV replacement).
The Carillon system has received CE approval for the treatment of patients with symptomatic, severe secondary MR and has been implanted in more than 850 patients worldwide. The TITAN study showed reduction of MR by 30-50% at 12 months, with high peri-procedural safety and improvement of symptoms in the majority of patients [24].
In the randomised REDUCE-FMR trial, 87 patients were treated with the Carillon device, while 33 underwent a sham procedure. After 12 months there was no difference in the cumulative rates of major adverse events of both groups, confirming the high safety of the procedure. In the patients with the Carillon device, a limited reduction in MR (22% of the regurgitant volume) was observed, compared to a 8% increase in the sham group (p = 0.03).
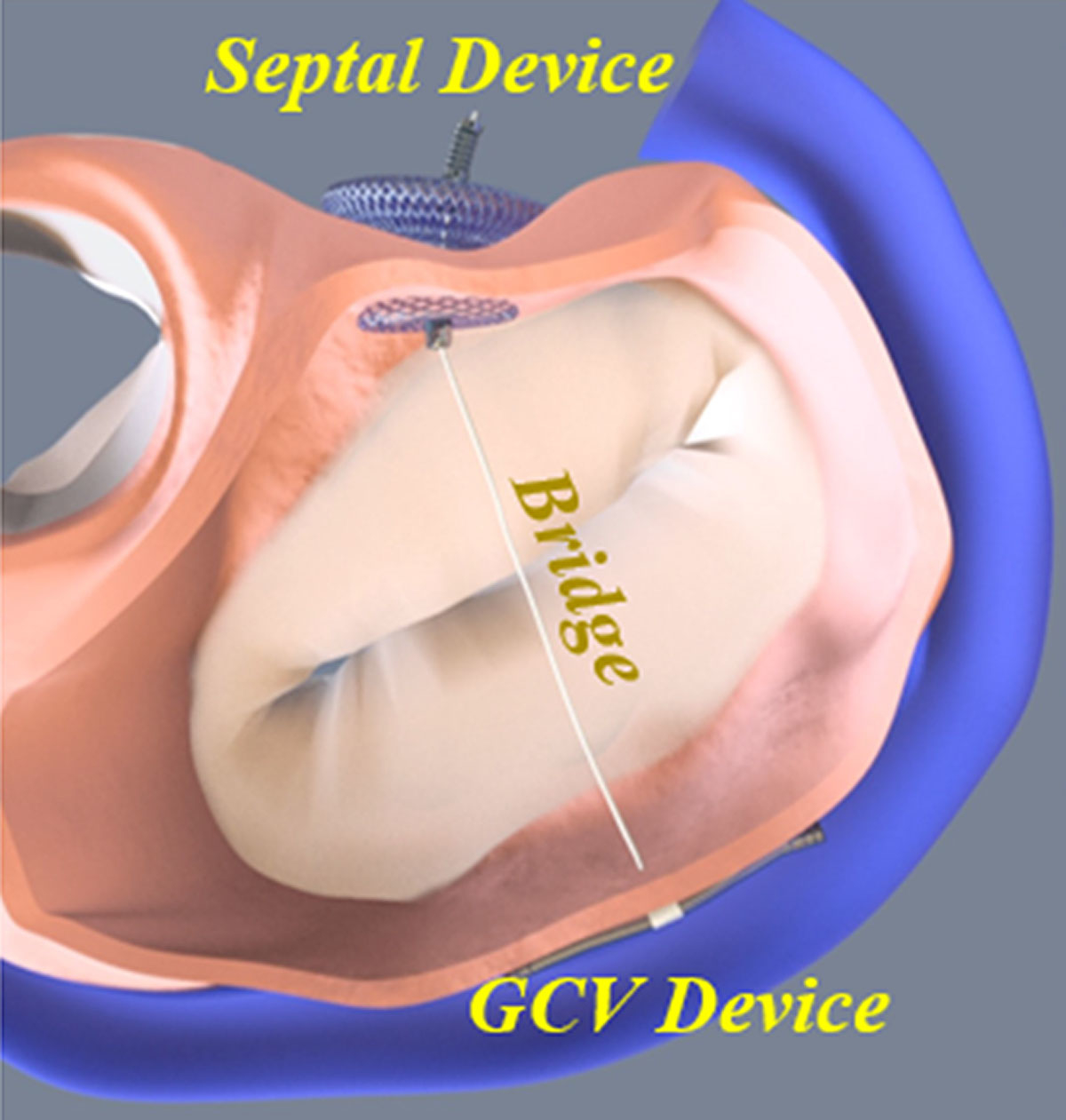
The MVRx ARTO System
The aim of the MVRx ARTO system (MVRx, Inc., San Mateo, CA) is to improve leaflet coaptation by shortening the antero-posterior diameter of the mitral annulus. To achieve this, the coronary sinus is cannulated from the right internal jugular vein and a magnetic catheter is positioned behind the posterior leaflet. Using a standard transseptal puncture from the right femoral vein, a second magnetic catheter is introduced into the left atrium. Both catheters are linked magnetically, allowing a thin crossing wire to be pushed from the coronary sinus into the transseptal catheter. After externalisation of the wire, a loop from the right internal jugular vein to the right femoral vein is created. Over this loop, a 3cm coronary sinus anchor, linked to a septal anchor by a catheter of adjustable length, is deployed, resulting in the reduction of the mitral annulus and MR (fig. 6).
The latest report from the MAVERIC efficacy and safety study [25] showed MR≤2+ in 92% of the 44 included patients at 12 months according to core lab analysis. Death occurred in 11.4%, while 11.7% of the patients were rehospitalised due to heart failure symptoms. Only one patient had a reintervention during the first year. Importantly, the MAVERIC trial enrolled a high proportion of patients (31%) with moderate MR (2+) at baseline, with the same proportion being in NYHA class II. This certainly corresponds to a population of patients with less advanced disease compared to those treated in other, previously mentioned feasibility studies. These findings need to be confirmed in a larger population of patients.
Transcatheter mitral valve replacement
Two different techniques are used for transcatheter MV replacement in experienced centres: the implantation of a TAVI valve, initially designed for the aortic position, and the use of dedicated systems for the mitral position.
Off-label use of TAVI devices in the mitral position
Off-label use of transcatheter heart valves, initially designed to be implanted in the aortic position, has emerged as a valuable alternative to open-heart surgery in selected patients. This includes the percutaneous delivery of a transcatheter bioprosthesis into a degenerated bioprosthesis (fig. 7A), a surgical annuloplasty ring (fig. 7B), or directly into a concentric, calcified native mitral annulus (fig. 7C). Early data show haemodynamic and clinical efficacy of these interventions using either the transapical or, preferably, the transfemoral/transseptal access [26]. The second approach has the advantage of being less invasive, since no surgical access is required. Valve-in-valve implantation has the highest device success and the lowest mortality rate at 30 days (5.7%), whereas valve-in-ring implantation is more frequently associated with residual MR (19.4 vs 6.8%; p = 0.003), as well as second valve implantation (11.1 vs 2.8%; p = 0.008) [27]. The periprocedural risk is highest during valve-in-native-annulus implantation (valve-in-MAC), mainly due to the risk of left ventricular outflow tract (LVOT) obstruction (almost 10%), as well as acute or delayed valve embolisation, usually into the left atrium [28]. The increased risk of valve thrombosis observed in the mitral position renders oral anticoagulation mandatory in all patients, irrespective of the intervention type.
Transcatheter mitral valve implantation (TMVI)
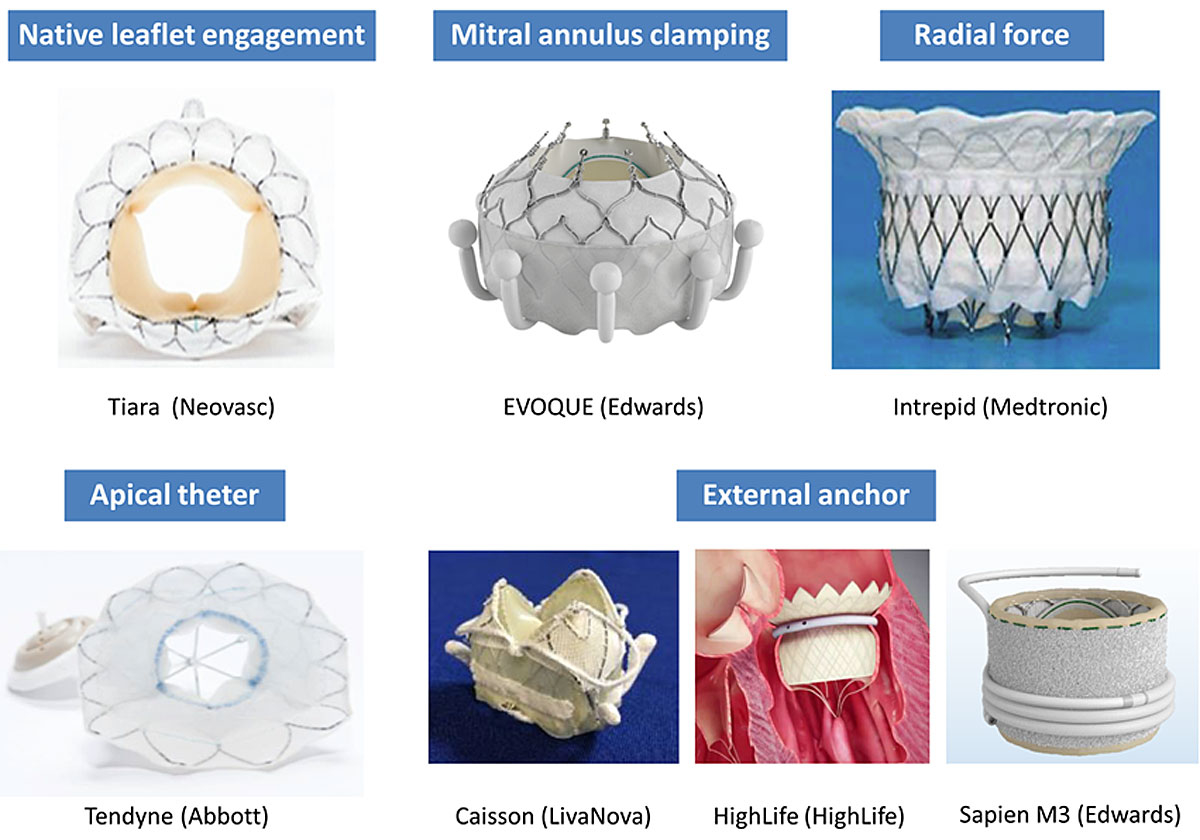
Although no dedicated transcatheter MV replacement system has been approved for commercial use yet, numerous devices are currently under preclinical or clinical investigation. They differ mainly in anchoring mechanisms and access (fig. 8). Although few devices rely exclusively on intrinsic radial force, the majority of devices deploys anchors that grasp the leaflets, the subvalvular apparatus or the trigone itself. In addition, some devices (Caisson and Tiara) have the ability to immobilise the anterior leaflet in order to avoid its displacement into the LVOT, and thus prevent LVOT obstruction.
After the initial development of systems using the transapical access, efforts are currently being made to miniaturise and modify them for implantation via the less invasive transfemoral/transseptal approach. This may be particularly relevant in patients with secondary MR, in whom the transapical access has been shown to result in increased bleeding complications and mortality.
Table 3 summarises the early results of the most frequently implanted systems. In contrast to transcatheter valve repair, MV replacement usually results in the complete resolution of MR. However, several specific safety aspects require further investigations, including the most appropriate antithrombotic management to prevent valve thrombosis, adequate patient selection based on LVOT anatomy, interaction with a previously implanted aortic prosthesis and feasibility in patients with annular calcifications.
Table 3 Early results of the most important transcatheter mitral valve replacement systems
|
TIARA
|
CARDIAQ
|
INTREPID
|
FORTIS*
|
TENDYNE
|
Caisson
|
HighLife
|
Sapien M3
|
|
N
|
58
|
13
|
50
|
13
|
100
|
19
|
15
|
10
|
|
Access
|
transapical |
transseptal |
transapical |
transapical |
transapical |
transseptal |
transapical |
transseptal |
|
Mean age, years
|
73 |
NR |
73 |
71 |
75 |
81 (median) |
69 |
74 |
|
FMR/MMR, %
|
90 |
69 |
72 |
92 |
90 |
57 |
73 |
NR |
|
LVEF, %
|
36±9 |
40 |
43±12 |
34±9 |
47±10 |
46±11 |
38 |
38 |
|
STS-PROM, %
|
8.6±7.2 |
NR |
6.4±5.5 |
7.2±3.6 |
7.9±5.7 |
8.3±3.5 |
NR |
4.9 |
|
Successful implantation, n (%)
|
55 (95) |
12 (92) |
48 (98) |
10 (77) |
97 (97) |
15 (79) |
14 (93) |
10 (100) |
|
30-day mortality, %
|
NR |
NR |
14 |
38 |
6 |
13 |
21 |
0 |
|
Residual MR more than mild, n (%)
|
0 |
NR |
0 |
0 |
0 |
0 |
0 |
2 (20) |
Imaging guidance for transcatheter mitral valve interventions
TEE is generally sufficient for evaluation of the suitability for edge-to-edge repair. Table 4 summarises the main MV anatomical criteria used for this purpose. Patients not fulfilling the suitability criteria may be eligible for treatment depending on centre experience and device used.
Table 4 Principal MV anatomical criteria used for evaluating feasibility of edge-to-edge repair
|
Suitable
|
Unsuitable
|
|
Valve disease
|
Prolapse or flail width <15 mm or gap <10 mm |
Leaflet perforation or cleft |
|
Calcifications
|
No or mild leaflet calcification outside of the grasping zone; annular calcification |
Severe calcification in the grasping zone |
|
Mitral valve area
|
≥3-4 cm2
|
Mitral valve area <3 cm2 or baseline mean gradient ≥5 mmHg |
|
Leaflet mobility
|
Mobile length of the posterior leaflet ≥7 mm |
Mobile length of the posterior leaflet <5 mm; rheumatic valve disease |
For other (technically more complex) repair techniques and for TMVI, ECG-gated, contrast-enhanced, cardiac computed tomography has emerged as a complementary imaging technique for the evaluation of patients considered for transcatheter MV interventions. Its high spatial resolution enables precise delineation of the mitral annulus, assessment of leaflets and papillary muscles anatomy, and localisation and quantification of the extent of valve calcifications [29]. In addition, the identification of the circumflex artery and the measurement of its distance related to the mitral annulus is critical before implantation of an annuloplasty device.
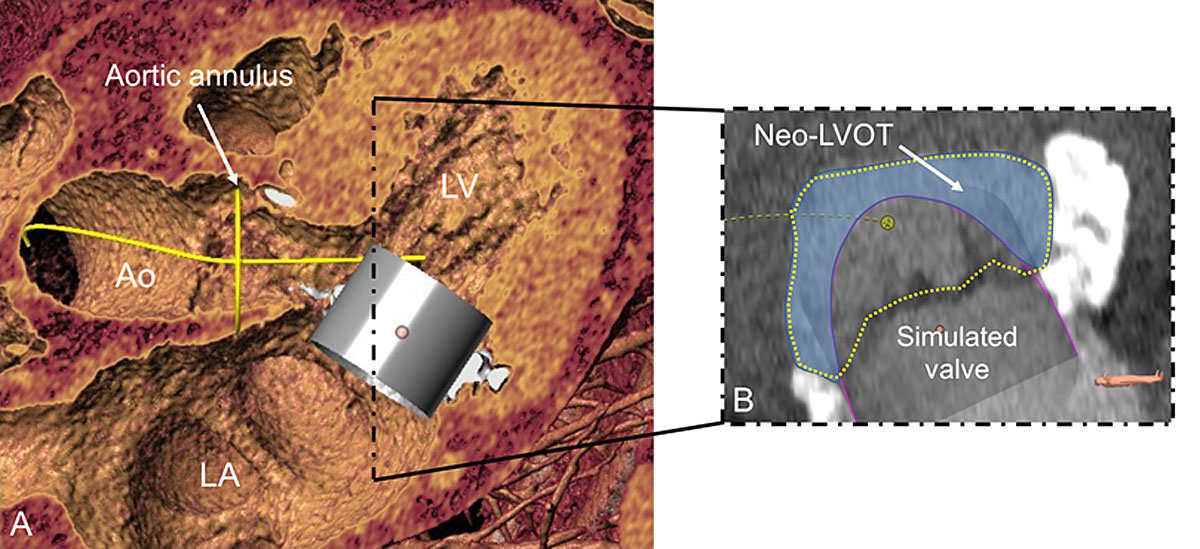
Recent progress allows for the simulation of devices inserted into the individual anatomy (fig. 9) in order to determine appropriate sizing, appreciate the anatomical impact of valve intervention, and anticipate possible complications.
Several potential predictors of LVOT obstruction have been identified [29–31]. They include both device-related and anatomical factors:
- ‒ Size and mobility of the anterior mitral leaflet
- ‒ Dimensions of the left ventricle; bulging of the interventricular septum
- ‒ Aorto-mitral angle (highest risk for patients with an aorto-mitral angle <90°)
- ‒ Device protrusion and device flaring
These factors combine to form the “neo-LVOT” [30], which may differ significantly from the native one with respect to shape, area and perimeter. Whereas an insignificant increase of the blood velocity in the LVOT is frequently recorded after percutaneous MV replacement, insufficient post-procedural LVOT dimensions may result in significant obstruction requiring urgent resolution by means of alcohol ablation, implantation of a TAVI prosthesis into the LVOT, or surgical correction. Careful analysis of the pre-procedural images may be able to prevent the occurrence of such complications.
Conclusions
- A high proportion of elderly patients suffer from symptomatic, severe mitral regurgitation that is not amenable to surgical treatment.
- Transcatheter MV repair represents a safe and effective treatment option for inoperable or surgical high-risk patients. Different methods focus on distinct pathology and can be combined, allowing for individually tailored solutions.
- Recently published results of randomised controlled trials comparing percutaneous edge-to-edge repair using the Mitraclip with medical treatment among patients with secondary MR provide valuable guidance regarding patient selection. Heart failure patients with severe MR, moderate ventricular dilation and persisting symptoms despite optimised medical management (including CRT) should be offered MV repair with the MitraClip system.
- Transcatheter MV replacement has the potential to enable reproducible and effective treatment of MR. However, specific aspects regarding patient selection, as well as the risk of valve thrombosis and LVOT obstruction, require further investigation.
References
1
Nkomo
VT
,
Gardin
JM
,
Skelton
TN
,
Gottdiener
JS
,
Scott
CG
,
Enriquez-Sarano
M
. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–11. doi:.https://doi.org/10.1016/S0140-6736(06)69208-8
2
Enriquez-Sarano
M
,
Akins
CW
,
Vahanian
A
. Mitral regurgitation. Lancet. 2009;373(9672):1382–94. doi:.https://doi.org/10.1016/S0140-6736(09)60692-9
3
Goel
SS
,
Bajaj
N
,
Aggarwal
B
,
Gupta
S
,
Poddar
KL
,
Ige
M
, et al.
Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol. 2014;63(2):185–6. doi:.https://doi.org/10.1016/j.jacc.2013.08.723
4
Baumgartner
H
,
Falk
V
,
Bax
JJ
,
De Bonis
M
,
Hamm
C
,
Holm
PJ
, et al.; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739–91. doi:.https://doi.org/10.1093/eurheartj/ehx391
5
David
TE
,
Armstrong
S
,
McCrindle
BW
,
Manlhiot
C
. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation. 2013;127(14):1485–92. doi:.https://doi.org/10.1161/CIRCULATIONAHA.112.000699
6
Enriquez-Sarano
M
,
Schaff
HV
,
Orszulak
TA
,
Tajik
AJ
,
Bailey
KR
,
Frye
RL
. Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. Circulation. 1995;91(4):1022–8. doi:.https://doi.org/10.1161/01.CIR.91.4.1022
7
Samad
Z
,
Shaw
LK
,
Phelan
M
,
Ersboll
M
,
Risum
N
,
Al-Khalidi
HR
, et al.
Management and outcomes in patients with moderate or severe functional mitral regurgitation and severe left ventricular dysfunction. Eur Heart J. 2015;36(40):2733–41. doi:.https://doi.org/10.1093/eurheartj/ehv343
[REMOVED IF= FIELD]8
Acker
MA
,
Bolling
S
,
Shemin
R
,
Kirklin
J
,
Oh
JK
,
Mann
DL
, et al. Mitral valve surgery in heart failure: insights from the Acorn Clinical Trial. J Thorac Cardiovasc Surg. 2006;132(3):568-77, 77 e1-4.
9
Serri
K
,
Bouchard
D
,
Demers
P
,
Coutu
M
,
Pellerin
M
,
Carrier
M
, et al. Is a good perioperative echocardiographic result predictive of durability in ischemic mitral valve repair? J Thorac Cardiovasc Surg. 2006;131(3):565-73 e2.
10
McGee
EC, Jr
,
Gillinov
AM
,
Blackstone
EH
,
Rajeswaran
J
,
Cohen
G
,
Najam
F
, et al.
Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2004;128(6):916–24. doi:.https://doi.org/10.1016/j.jtcvs.2004.07.037
11
Magne
J
,
Sénéchal
M
,
Dumesnil
JG
,
Pibarot
P
. Ischemic mitral regurgitation: a complex multifaceted disease. Cardiology. 2009;112(4):244–59. doi:.https://doi.org/10.1159/000151693
12
Goldstein
D
,
Moskowitz
AJ
,
Gelijns
AC
,
Ailawadi
G
,
Parides
MK
,
Perrault
LP
, et al.; CTSN. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N Engl J Med. 2016;374(4):344–53. doi:.https://doi.org/10.1056/NEJMoa1512913
13
Mirabel
M
,
Iung
B
,
Baron
G
,
Messika-Zeitoun
D
,
Détaint
D
,
Vanoverschelde
JL
, et al.
What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery?
Eur Heart J. 2007;28(11):1358–65. doi:.https://doi.org/10.1093/eurheartj/ehm001
14
Andalib
A
,
Mamane
S
,
Schiller
I
,
Zakem
A
,
Mylotte
D
,
Martucci
G
, et al. A systematic review and meta-analysis of surgical outcomes following mitral valve surgery in octogenarians: implications for transcatheter mitral valve interventions. EuroIntervention. 2014;9(10):1225-34.
15
Andalib
A
,
Chetrit
M
,
Eberg
M
,
Filion
KB
,
Thériault-Lauzier
P
,
Lange
R
, et al.
A Systematic Review and Meta-Analysis of Outcomes Following Mitral Valve Surgery in Patients with Significant Functional Mitral Regurgitation and Left Ventricular Dysfunction. J Heart Valve Dis. 2016;25(6):696–707.
16
Maisano
F
,
Caldarola
A
,
Blasio
A
,
De Bonis
M
,
La Canna
G
,
Alfieri
O
. Midterm results of edge-to-edge mitral valve repair without annuloplasty. J Thorac Cardiovasc Surg. 2003;126(6):1987–97. doi:.https://doi.org/10.1016/S0022-5223(03)01291-1
17
Grover
FL
,
Vemulapalli
S
,
Carroll
JD
,
Edwards
FH
,
Mack
MJ
,
Thourani
VH
, et al.; STS/ACC TVT Registry. 2016 Annual Report of The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol. 2017;69(10):1215–30. doi:.https://doi.org/10.1016/j.jacc.2016.11.033
18
Praz
F
,
Spargias
K
,
Chrissoheris
M
,
Büllesfeld
L
,
Nickenig
G
,
Deuschl
F
, et al.
Compassionate use of the PASCAL transcatheter mitral valve repair system for patients with severe mitral regurgitation: a multicentre, prospective, observational, first-in-man study. Lancet. 2017;390(10096):773–80. doi:.https://doi.org/10.1016/S0140-6736(17)31600-8
19
Obadia
JF
,
Messika-Zeitoun
D
,
Leurent
G
,
Iung
B
,
Bonnet
G
,
Piriou
N
, et al.; MITRA-FR Investigators. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med. 2018;379(24):2297–306. doi:.https://doi.org/10.1056/NEJMoa1805374
20
Stone
GW
,
Lindenfeld
J
,
Abraham
WT
,
Kar
S
,
Lim
DS
,
Mishell
JM
, et al.; COAPT Investigators. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med. 2018;379(24):2307–18. doi:.https://doi.org/10.1056/NEJMoa1806640
21
Messika-Zeitoun
D
,
Nickenig
G
,
Latib
A
,
Kuck
KH
,
Baldus
S
,
Schueler
R
, et al.
Transcatheter mitral valve repair for functional mitral regurgitation using the Cardioband system: 1 year outcomes. Eur Heart J. 2019;40(5):466–72. doi:.https://doi.org/10.1093/eurheartj/ehy424
22
Ristalli
F
,
Meucci
F
,
Stolcova
M
,
Valente
S
,
Landi
D
,
Di Mario
C
. MitraClip Implantation to Treat Early Recurrence of Mitral Regurgitation After Percutaneous Direct Annuloplasty With Cardioband. JACC Cardiovasc Interv. 2018;11(14):1416–7. doi:.https://doi.org/10.1016/j.jcin.2018.04.049
23
Rogers
JH
,
Boyd
WD
,
Smith
TWR
,
Ebner
AA
,
Bolling
SF
. Combined MitraClip Edge-to-Edge Repair With Millipede IRIS Mitral Annuloplasty. JACC Cardiovasc Interv. 2018;11(3):323–4. doi:.https://doi.org/10.1016/j.jcin.2017.11.007
24
Siminiak
T
,
Wu
JC
,
Haude
M
,
Hoppe
UC
,
Sadowski
J
,
Lipiecki
J
, et al.
Treatment of functional mitral regurgitation by percutaneous annuloplasty: results of the TITAN Trial. Eur J Heart Fail. 2012;14(8):931–8. doi:.https://doi.org/10.1093/eurjhf/hfs076
25
Rogers
JH
,
Thomas
M
,
Morice
MC
,
Narbute
I
,
Zabunova
M
,
Hovasse
T
, et al.
Treatment of Heart Failure With Associated Functional Mitral Regurgitation Using the ARTO System: Initial Results of the First-in-Human MAVERIC Trial (Mitral Valve Repair Clinical Trial). JACC Cardiovasc Interv. 2015;8(8):1095–104. doi:.https://doi.org/10.1016/j.jcin.2015.04.012
26
Praz
F
,
Windecker
S
,
Huber
C
,
Carrel
T
,
Wenaweser
P
. Expanding Indications of Transcatheter Heart Valve Interventions. JACC Cardiovasc Interv. 2015;8(14):1777–96. doi:.https://doi.org/10.1016/j.jcin.2015.08.015
27
Yoon
SH
,
Whisenant
BK
,
Bleiziffer
S
,
Delgado
V
,
Schofer
N
,
Eschenbach
L
, et al.
Transcatheter Mitral Valve Replacement for Degenerated Bioprosthetic Valves and Failed Annuloplasty Rings. J Am Coll Cardiol. 2017;70(9):1121–31. doi:.https://doi.org/10.1016/j.jacc.2017.07.714
28
Guerrero
M
,
Dvir
D
,
Himbert
D
,
Urena
M
,
Eleid
M
,
Wang
DD
, et al.
Transcatheter Mitral Valve Replacement in Native Mitral Valve Disease With Severe Mitral Annular Calcification: Results From the First Multicenter Global Registry. JACC Cardiovasc Interv. 2016;9(13):1361–71. doi:.https://doi.org/10.1016/j.jcin.2016.04.022
29
Blanke
P
,
Naoum
C
,
Webb
J
,
Dvir
D
,
Hahn
RT
,
Grayburn
P
, et al.
Multimodality Imaging in the Context of Transcatheter Mitral Valve Replacement: Establishing Consensus Among Modalities and Disciplines. JACC Cardiovasc Imaging. 2015;8(10):1191–208. doi:.https://doi.org/10.1016/j.jcmg.2015.08.004
30
Blanke
P
,
Naoum
C
,
Dvir
D
,
Bapat
V
,
Ong
K
,
Muller
D
, et al.
Predicting LVOT Obstruction in Transcatheter Mitral Valve Implantation: Concept of the Neo-LVOT. JACC Cardiovasc Imaging. 2017;10(4):482–5. doi:.https://doi.org/10.1016/j.jcmg.2016.01.005
31
Theriault-Lauzier
PP
,
Dorfmeister
M
,
Mylotte
D
,
Andalib
A
,
Spaziano
M
,
Blanke
P
, et al. Quantitative multi-slice computed tomography assessment of the mitral valvular complex for transcatheter mitral valve interventions part 2: geometrical measurements in patients with functional mitral regurgitation. EuroIntervention. 2015;11(7).