Molecular imaging for neuroendocrine tumours
DOI: https://doi.org/10.4414/smw.2019.20017
Kwadwo
Antwia, Guillaume
Nicolasab, Damian
Wildab, Emanuel
Christbc
aDivision of Nuclear Medicine, University Hospital Basel, Basel, Switzerland
bCentre for Neuroendocrine and Endocrine Tumours, University Hospital Basel, Basel, Switzerland
cDivision of Endocrinology, Diabetology and Metabolism, University Hospital Basel, Basel, Switzerland
This article was corrected and republished online on April 3, 2019. Please see Erratum (Swiss Med Wkly. 2019;149:w20076)
Summary
Molecular imaging has found numerous applications in oncology as many tumours express or activate tumour specific target molecules or pathways. This relatively new imaging technique results in a better localisation of tumours and improved tumour staging, especially in the setting of hybrid imaging that is in combination with morphological imaging such as computed tomography. In well differentiated neuroendocrine tumours, somatostatin receptor imaging, as one of the first examples of receptor targeted imaging in humans, plays an important role in the diagnostic work-up of these patients. In poorly differentiated neuroendocrine tumours or medullary thyroid carcinoma, 18F-fluorodeoxyglucose PET/CT and dihydroxyphenylalanine PET/CT play an important role due to the limitations of the somatostatin receptor imaging in these tumour entities. These limitations prompted the development of innovations such as radiolabelled somatostatin receptor antagonists for imaging all types of NET and glucagon-like peptide-1 receptor agonists for the imaging of insulinomas. The current review summarises the actual state of knowledge in the field.
Introduction
Molecular imaging is a relatively new field that has emerged at the intersection of molecular biology and in vivo biomedical imaging. It has found numerous applications in oncology as many tumours express or activate tumour specific target molecules or pathways. Importantly, besides the basic research which defines the specific molecular target of a specific disease (i.e., a receptor, metabolite), the competence of the radiopharmacy in designing, developing and producing the molecule that includes the radioisotope, and the nuclear medicine specialists in administrating the radioisotope-labelled molecule as well as evaluating the images, are essential for this approach. In addition, by changing the radioisotope, molecular imaging can be used beyond diagnostic purposes to targeted radionuclide therapy. The thyroid gland, as the only tissue able to organify iodine, is an example for a well-established molecular imaging application with 123iodine scintigraphy (gamma emitter) as a diagnostic tool, and 131iodine as a therapeutic agent (combined gamma and beta emitter) for ablation of remnant thyroid tissue in patients with thyroid cancer. Newer developments include positron-emitting radioisotopes such as fluorine-18 (18F) or gallium-68 (68Ga), coupled with specific biomolecules as diagnostic agents. The advantage of positron emission tomographic imaging (PET) is the higher spatial resolution of the images and the shorter half-life of the common radioisotopes used. This leads to high quality imaging results, lower radiation exposure and the possibility of obtaining imaging results a few hours after the injection of the radiopharmaceuticals, in contrast to 24 to up to 72 hours with many gamma-emitting radioisotopes (e.g., indium-111 (111In) and technetium-99m (99mTc) based imaging).
Neuroendocrine tumours (NET) are heterogeneous with respect to site of origin and metastatic behaviour. About 25% of the NET secrete hormones leading to specific clinical signs and symptoms, while the remaining approximate 75% are so-called non-secreting NET, which are often diagnosed incidentally, and 40–50% are already in a metastatic tumour stage [1]. NET exhibit a unique biologic feature: they show an overexpression of specific peptide hormone receptors at the surface of the tumours, which are homogeneously distributed [2]. These receptors belong to the class of G protein-coupled membrane receptors [2]. They are targeted by small peptides and have different regulatory functions in the gastrointestinal tract, the endocrine system and the peripheral and central nervous system [3]. In tumours, they mainly control hormone secretion and cell proliferation and represent, therefore, important molecular targets for clinical applications [2].
In vitro studies using autoradiography (123I-labelled somatostatin analogue; fig. 1) have shown that somatostatin receptors are overexpressed in 80-90% of the NETs. In addition, they are homogenously distributed on the surface of the NETs and thus are an ideal target for molecular imaging and therapy [2]. Importantly, the radioisotope has to be linked to the molecule, which targets the specific receptors. The principal composition of the administered molecule for diagnostic purposes in vivo is shown in figure 2.
The clinical significance of the most important peptide receptor for molecular imaging and therapy in NET will be reviewed here. In addition, new developments will be discussed.
Somatostatin receptors
Somatostatin receptors (SSTR) represent one of the first examples of receptor targeting in humans. Five subtypes (SSTR1-5) are known, with subtype 2 being the most important since it is homogenously expressed at high density at the surface of NET cells in 80-90% of cases [2]. The only exceptions are insulin secreting NET (insulinomas) and medullary thyroid cancer where other receptors such as GLP-1 and CCK-2 play an important role (see the corresponding section below).
It was believed that SSTRs are mainly expressed in well differentiated NET (G1–G2) corresponding to a proliferation index (ki-67) of 1–20%. However, recent in vitro analysis of a large number of different gastrointestinal and bronchopulmonary NET showed that SSTRs are expressed independently of NET differentiation [4], indicating that somatostatin receptor targeting for diagnostic or therapeutic use can sometimes also be offered to some patients with G3 NET tumours (Ki-67 >20%).
Somatostatin receptor-regulated functional activities of NET
The most important effect of somatostatin and its analogues with agonistic properties is inhibitory, both physiologically and in tumours. In NET, somatostatin receptor agonists inhibit hormonal secretion, which is well established in secreting gastroenteropancreatic neuroendocrine tumours (GEP-NET) like carcinoids, gastrinomas, VIP-oma and glucagonoma, but is less effective in insulinomas [5].
Furthermore, randomised controlled trials have shown that somatostatin analogues have an antitumour effect by significantly extending the progression-free survival in secreting [6] and non-secreting NET [7] by binding to the somatostatin receptors [6–8], however, without affecting overall survival. Although the anti-proliferative effect of somatostatin analogues is not completely elucidated, they exert an anti-angiogenic effect by inhibiting endothelial cell proliferation and the development of new tumour-associated blood vessels [9]. This may contribute to tumour size reduction [10].
In vitro somatostatin receptor expression in human tissues
For the in vitro somatostatin receptor assessment, two reliable methods are established. (1) Autoradiography using 125I-Tyr3-octreotide is excellent in determining SSTR in tumour tissues in terms of sensitivity and specificity. Furthermore, it shows a good correlation with in vivo somatostatin receptor imaging using 111In-DTPA-octreotide (OctreoScan®; SRS) [11] and 68Ga-DOTATOC. It can, therefore, be helpful to determine whether a patient is a candidate for in vivo targeting of SSTR. (2) Immunohistochemistry for the detection of SSTR is now established after intensive searching for a reliable antibody. In experienced hands, immunohistochemistry with the anti-SSTR antibody UMB-1 (Abcam) is comparable to 125I-Tyr3-octreotide autoradiography in quantifying SSTR in NET [12].
In vivo somatostatin receptor imaging for diagnosis of NET in humans
PET imaging with 68Ga-labelled somatostatin agonists is superior to somatostatin receptor scintigraphy [13, 14] using 111In-DTPA-octreotide or 99mTc-HYNIC-TOC and offers numerous advantages (fig. 3). It allows high-quality imaging with improved sensitivity as early as 45 minutes after injection of the radiotracer, provides higher spatial resolution and enables better absolute quantification of tracer uptake (determination of the standardised uptake value (SUV)). In addition, the radiation dose is less than one-half of that of 111In-DTPA-octreotide [15]. Therefore, the 2017 European Neuroendocrine Tumour Society guidelines recommend imaging of GEP-NET with SSTR PET/CT (e.g., 68Ga-DOTATOC or 68Ga-DOTATATE) whenever available [16].
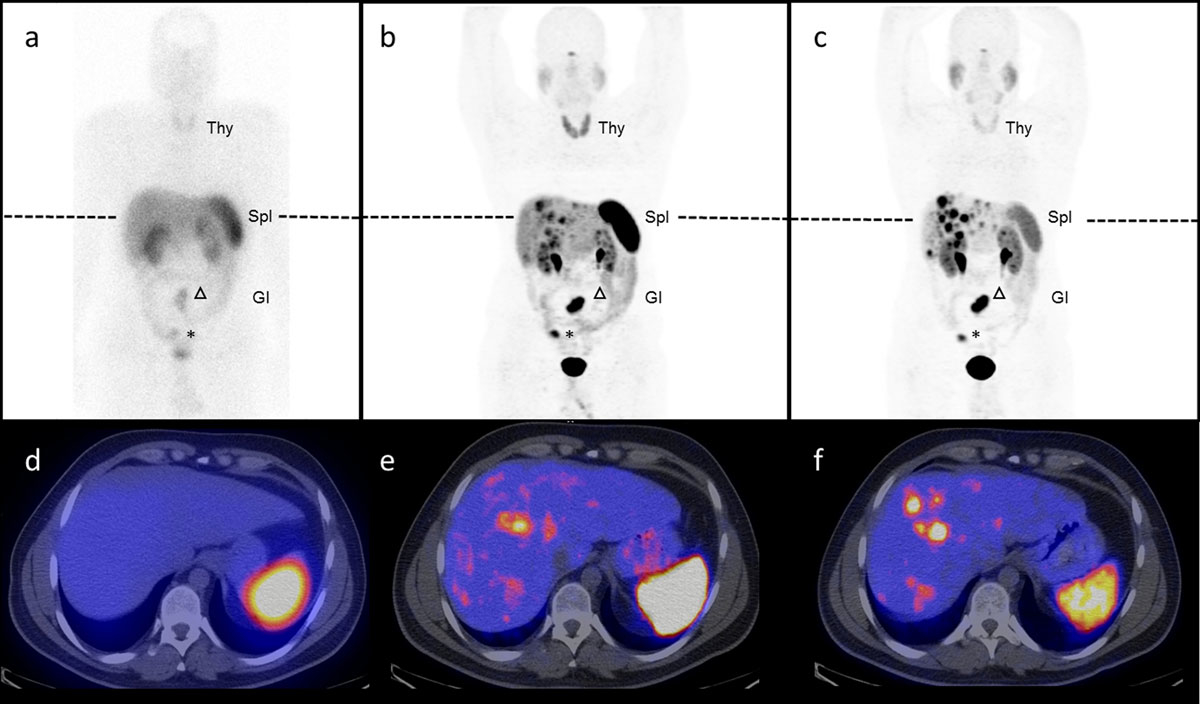
Figure 3
Somatostatin-receptor targeting using 111In-DTPA-octreotide, 68Ga-DOTA-octerotide and 68Ga-DOTA-SSTR-antagonist. Head-to-head comparison of 111In-DTPA-octreotide scintigraphy and SPECT/CT (a,d) versus 68Ga-DOTA-TOC PET/CT (b,e), 2 SSTR agonists and 68Ga-OPS202 PET/CT (c,f), a novel SSTR2 antagonist, in a 32 year-old patient with well differentiated ileal neuroendocrine tumour G2. The primary tumour is in situ (asterisk) and there are a mesenterial lymph (arrow head) and multiple liver metastases. Note, in this otherwise stable disease, the increased number of liver metastases visible on the antagonist scans (c, f) due to an improved signal-to-noise ratio compared to the agonist (a and b) as well as the decreased liver, spleen (Spl), thyroid (T) and gastro-intestinal (GI) uptake.
Indication, advantage and side effects
SSTR imaging is indicated in the following clinical situations: (1) as a diagnostic tool in order to find a primary NET (i.e., ileal NET) which can be very small; (2) for staging of the disease in a patient with histologically proven NET; and (3) pre-therapeutically in situations where a peptide receptor radionuclide therapy (PRRT) is considered. Although the indication for SSTR imaging is established for staging, the usefulness of SSTR imaging needs to be proven as a valid tool in the follow-up of patients with a NET.
The advantage of this molecular imaging is two-fold: (1) confirmation of the presence of SSTR in vivo and (2) a high sensitivity and specificity for NET in the case of an unclear lesion in conventional imaging. Side effects of the application are rare and include nausea and only very rarely vomitus.
Other molecular imaging modalities in NET 18F-Fluorodeoxyglucose (18F-FDG) PET/CT
Glucose metabolism is the main energy source for most cells. Therefore, 18F-Fluorodeoxyglucose (18F-FDG) is able to visualise intracellular increased glucose turnover in patients with neoplastic disease. As GEP-NETs are well differentiated, they usually do not have a high glucose turnover rate and the sensitivity of 18F-FDG PET/CT is consequently low, especially in well differentiated NET (G1 and G2). As such, 18F-FDG is not routinely used for diagnostic purposes. However, high glucose metabolism can be documented in poorly differentiated NETs (G3) and is usually associated with a poor prognosis. On the other hand, negative 18F-FDG PET/CT scans are predictive for low aggressiveness and a high survival rate [17].
18F-Dihydroxyphenylalanine (18F-DOPA) PET/CT
NETs take up dihydroxyphenylalanine (DOPA) and the labelled metabolite (18F-DOPA) via a specific cell membrane bound transporter. Within the cell, it is decarboxylated by the aromatic acid decarboxylase (AADC) to 18F-dopamine. Compared with SRS, 18F-DOPA PET/CT has superior performance in imaging GEP-NETs [18]. Compared with SSTR PET/CT, 18F-DOPA PET/CT has an inferior performance in patients with GEP-NETs [19]. However, 18F-DOPA PET has shown relatively high sensitivity in congenital hyperinsulinism and adult nesidioblastosis. In addition, there are data suggesting the usefulness of this modality to localise medullary thyroid carcinoma, benign insulinomas, paragangliomas and phaeochromocytomas.
Importantly, 18F-FDG and 18F-DOPA PET/CT can be useful for the localisation of NET, guiding biopsy and planning of a surgical or medical intervention. In contrast, a positive result of a SSTR imaging procedure can have immediate therapeutic consequences. In such patients, peptide receptor radionuclide therapy (PRRT) can be administered with the appropriate beta-emitter (e.g., 177Lu) [20].
Glucagon-like peptide-1 receptor (GLP-1R)
The clinical success of somatostatin receptor targeting of neuroendocrine tumours has stimulated the search for other peptide receptors suitable for similar applications. A candidate with growing clinical interest is the glucagon-like peptide 1 (GLP-1) receptor (GLP-1R). This receptor is also a member of the G-protein-coupled receptor family [21] and its expression has been characterised in normal human tissues. It has been found in the pancreatic islets and acini, stomach, small and large intestinal myenteric plexus, lung and kidney vasculature, breast parenchyma, heart, brainstem, hypothalamus, neurohypophysis and meninges. GLP-1 receptor activation upon agonist binding stimulates adenylate cyclase and phospholipase C, with subsequent activation of protein kinase A and C, respectively [22]. Besides the well-known effect as an insulin secretagogue, it promotes resistance to apoptosis and enhanced β-cell survival in human islets [23].
GLP-1 receptor-regulated functional activities in the gastrointestinal tract
The glucagon-like peptide (GLP-1) is an incretin hormone mainly produced in the enteroendocrine L cells of the ileum and colon in response to nutrient intake and is one of the most important glucose-dependent insulin secretagogues. In addition to the glucose-dependent increase in insulin secretion, GLP-1 inhibits glucagon secretion, gastric emptying and food ingestion, and promotes enhanced glucose disposal through neural mechanisms [22]. However, the natural ligand GLP-1 (30 amino acids peptide) has unfavourable characteristics for successful in vivo targeted imaging as it is degraded in the human blood by dipeptidyl-peptidase-4 [24] and therefore has a plasma half-life of less than two minutes.
In search of a more favourable analogue, exendin-4 was identified. This peptide is metabolically resistant to dipeptidyl-peptidase-4 degradation [25]. It was identified in the saliva of the Gila monster lizard (Heloderma suspectum) and it shares 53% homology with the human GLP-1 [26]. Consequently, Exenatide, a synthetic exendin-4 was developed, which is primarily an FDA-approved medication established for the treatment of type 2 diabetes mellitus.
In vitro GLP-1R imaging for diagnosis of benign insulinomas
Benign insulinomas are usually located in the pancreas. They are the most common cause of endogenous hyperinsulinaemic hypoglycaemia in adults [27]. At present, surgery remains the only curative treatment. Pancreas-preserving surgery such as limited segmental resection or enucleation is considered the treatment of choice [28–30]. Therefore, the exact preoperative localisation of insulinomas is critical in order to plan surgical strategy and improve postoperative outcomes. The small size of insulinomas (usually ≤2 cm [31]) challenges the detectability by conventional imaging techniques such as contrast-enhanced computed tomography (CT), contrast-enhanced magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS).
Reubi et. al tested the incidence and mean density of 13 peptide receptors in different (neuro)endocrine tumours using autoradiography [32]. GLP-1 receptors were overexpressed in nearly 100% of all tested benign insulin-secreting neuroendocrine tumours. The density of GLP-1R expression in insulinoma was approximately a five-fold increase compared to a normal pancreatic B-cell ([13]; fig. 1). This is of particular importance since SSTR density is comparatively low in benign insulinomas. Consequently, in vivo SSTR imaging for insulinomas is often negative. The high GLP-1 receptor density makes it an ideal target for molecular imaging, fulfilling the critical prerequisite for successful in vivo targeting [2, 33].
In vivo GLP-1R imaging for diagnosis of insulinomas in humans
In 2008, the first two patients who underwent GLP-1 receptor scintigraphy were published as a letter in the New England Journal of Medicine. Both patients suffered from severe endogenous hyperinsulinaemic hypoglycaemia and previous conventional imaging or selective arterial stimulation and venous sampling was negative or inconclusive. In both patients, GLP-1R detected a focal lesion which was surgically resected, and histologic analysis of surgically removed tissues confirmed the diagnosis of a benign insulinoma containing a high GLP-1R density [34].
In a proof of principle study, 111In-DOTA-exendin-4 was prospectively administered to six patients [35], all of whom presented with neuroglycopenic symptoms due to endogenous hyperinsulinaemic hypoglycaemia. GLP-1R scintigraphy correctly detected the insulinoma in all six consecutive patients. In vitro autoradiography of GLP-1R showed a density of GLP-1R in the range as previously described [2], whereas somatostatin receptor status revealed low density of somatostatin type 1 receptors [35].
The first prospective multicentre study included 30 patients with proven endogenous hyperinsulinaemic hypoglycaemia who underwent 111In-DTPA-exendin-4 imaging GLP-1R scanning. Whole-body planar images and SPECT/CT of the abdomen were performed and in some patients between 72 and 96 and up to 168 hours post injection, the most important time point being the scan 24 hours after injection. In 25 patients who underwent surgery (with histological analysis), 111In-DTPA-exendin-4 SPECT/CT correctly detected 19 insulinomas and four additional positive lesions (two islet-cell hyperplasia and two uncharacterised lesions), resulting in a positive predictive value of 83%. For the 23 assessable patients, 111In-DTPA-exendin-4 SPECT/CT had a higher sensitivity (95%) than did CT/MRI (47%). Seven patients were operated because GLP-1R imaging was the only method that showed a suspicious lesion in the pancreas. Five of these patients had a confirmed insulinoma with normalisation of blood sugar levels after surgery, supporting the clinical value of GLP-1R imaging.
Since PET has several advantages over SPECT, 68Ga-DOTA-exendin-4 and 111In-exendin-4 SPECT were tested in a randomised cross-over order in five patients with endogenous hyperinsulinaemic hypoglycaemia and negative or controversial findings on conventional imaging [36]. The results of this proof of principle study showed a better performance of 68Ga-DOTA-exendin-4 PET compared with 111In-DOTA-exendin-4 SPECT.
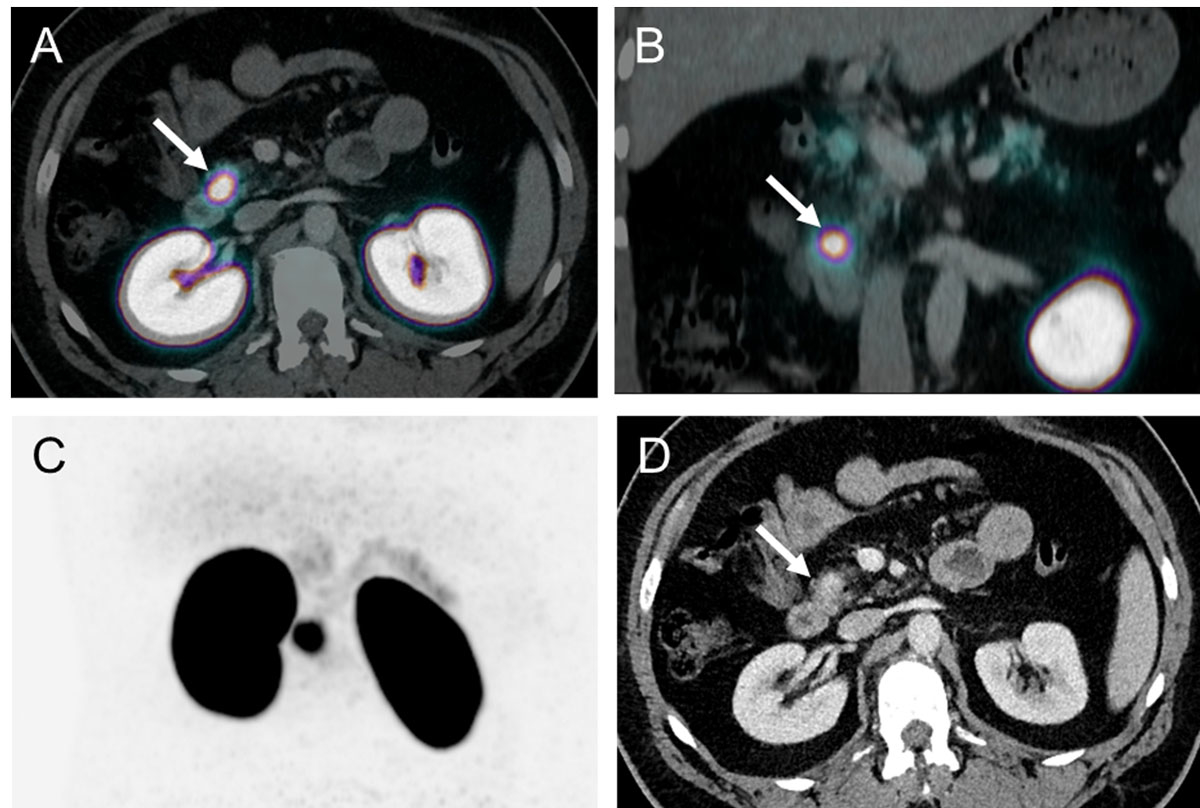
Based on these promising data, a prospective study including 52 consecutive patients was conducted at the University Hospital of Basel. The aim was to compare the diagnostic accuracy and clinical impact of 68Ga-DOTA-exendin-4 PET/CT in comparison with 111In-DOTA-exendin-4 SPECT/CT and 3-Tesla-MRI. The data from this study showed the superiority of 68Ga-DOTA-exendin-4 PET/CT (fig. 4) in comparison with 111In-DOTA-exendin-4 SPECT/CT and 3-Tesla-MRI [37].
Indication, advantage and side effects
GLP-1R imaging with PET/CT or SPECT/CT is most likely to be helpful in patients with a high suspicion for the presence of an insulinoma. This requires the assessment of blood glucose, C-peptide and insulin levels in a standardised fasting test. If an endogenous hyperinsulinaemic hypoglycaemia is confirmed, a GLP-1R molecular imaging is indicated.
Nausea and occasional vomiting are known side effects of DOTA-Exendin-4 Tracers. These side effects are usually confined to the first hour after injection. During the first five hours after the injection of a DOTA-Exendin-4 Tracer, a decrease in blood glucose levels can occur. Therefore, the continued infusion of intravenous glucose administration is recommended during the procedure.
Costs for molecular imaging in NET
The cost of molecular imaging with PET/CT ranges between 2885 and 3400 CHF. In comparison, conventional imaging with SPECT, for example, OctreoScan®, costs around 1800 CHF.
Outlook
Until recently, all somatostatin-based radiotracers used in the clinic for diagnostic (SPECT, PET) or therapeutic (PRRT) procedures in patients with NET were agonists. It was thought that the internalisation of the tracer is essential for a high accumulation and long-lasting retention of the radiotracer into the tumour cells. The paradigm shifted when in vitro and in vivo data showed that a higher tumour uptake and longer tumour retention could be obtained with radiolabelled SSTR antagonists without documented internalisation [38]. Based on in vitro studies, it is likely that radiolabelled SSTR antagonists exhibit a higher number of potential binding sites on the receptor, thereby increasing the tumour uptake [38, 39]. This was confirmed with autoradiographic studies on human tumour sections [40].
First-in-human studies in five patients with NET demonstrated the high potential of radiolabelled antagonist (111In-DOTA-BASS) for imaging and also PRRT [41] (fig. 3). Because of these promising results, PET tracers were synthesised with high affinity for SSTR2 [42]. A first antagonist (68Ga-OPS202, also known as 68Ga-NODAGA-JR11) for PET/CT has been successfully evaluated in a phase I/II clinical trial [43, 44], which shows the superior sensitivity of the antagonist, compared to the well-established agonist 68Ga-DOTA-TOC in a head-to-head intra-patient comparative study. The respective therapeutic radiotracer 177Lu-OPS201 (also known as 177Lu-DOTA-JR11) has shown 1.7 to 10.6 times higher tumour doses than 177Lu-DOTA-TATE (agonist) in four patients with progressive and metastasised NETs [45, 46] (fig. 3). Importantly, the amount of antagonist peptide administered may further enhance the therapeutic index. In fact, it was shown that increasing peptide mass decreases the background dose for a constant tumour dose [46].
Medullary thyroid carcinoma (MTC) belongs to the NET entity. A characteristic feature of these tumours is the high incidence and expression of CCK receptors and the relatively low incidence and density of SSTR [2]. The only curative therapeutic option is surgery. However, the disease is already in a metastatic state in 40-50% of patients and the available systemic therapy stabilises but does not cure the disease and has significant toxic side effects [47]. There is, therefore, an unmet need for a better diagnostic and therapeutic tool. By labelling a minigastrin (ligand for CCK-receptors) with 177Lu, the first proof of principle study with six patients with metastatic MTC was performed with encouraging results [48].
The glucose-dependent insulinotropic polypeptide receptor (GIPR) is overexpressed on the surface of many well differentiated NETs (G1 and G2) [12, 49]. This may be of clincial relevance since 5-15% of NET do not express SSTR or GLP-1R. In vitro and in vivo evaluations of a cocktail of SSTR, GLP-1R and GIPR radioligands would allow detection of virtually all NETs [50]. No clinical study in humans has yet been performed.
Summary and conclusion
Considerable progress has been made over the last decades in the diagnosis of NET with new tracers (GLP-1R agonists, SSTR-antagonists). In addition, the successful administration of these molecules with radioisotopes, which have positron emitting properties, has been extensively and successfully explored. In particular, targeting GLP-1R with 68Ga-DOTA-exendin-4 offers a very effective approach that permits the successful localisation of small benign insulinomas. Since virtually all benign insulinomas express GLP-1Rs, this should be the imaging modality of choice in cases where conventional morphological imaging fails.
Based on the encouraging results of the new imaging tools, the first therapeutic trials with SSTR antagonists (in GEP-NET, bronchopulmonary NET and pheochromocytoma/paraganglioma patients) and minigastrin (in patients with MTC) have been started (NCT0259270 and NCT02088645).
References
1
Dasari
A
,
Shen
C
,
Halperin
D
,
Zhao
B
,
Zhou
S
,
Xu
Y
, et al.
Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3(10):1335–42. doi:.https://doi.org/10.1001/jamaoncol.2017.0589
2
Reubi
JC
,
Waser
B
. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30(5):781–93. doi:.https://doi.org/10.1007/s00259-003-1184-3
3
Körner
M
. Specific biology of neuroendocrine tumors: Peptide receptors as molecular targets. Best Pract Res Clin Endocrinol Metab. 2016;30(1):19–31. doi:.https://doi.org/10.1016/j.beem.2016.01.001
4
Deroose
CM
,
Hindié
E
,
Kebebew
E
,
Goichot
B
,
Pacak
K
,
Taïeb
D
, et al.
Molecular Imaging of Gastroenteropancreatic Neuroendocrine Tumors: Current Status and Future Directions. J Nucl Med. 2016;57(12):1949–56. doi:.https://doi.org/10.2967/jnumed.116.179234
5
Öberg
KE
,
Reubi
JC
,
Kwekkeboom
DJ
,
Krenning
EP
. Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy. Gastroenterology. 2010;139(3):742–53.e1. doi:.https://doi.org/10.1053/j.gastro.2010.07.002
6
Rinke
A
,
Müller
H-H
,
Schade-Brittinger
C
,
Klose
KJ
,
Barth
P
,
Wied
M
, et al.; PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–63. doi:.https://doi.org/10.1200/JCO.2009.22.8510
7
Caplin
ME
,
Pavel
M
,
Ćwikła
JB
,
Phan
AT
,
Raderer
M
,
Sedláčková
E
, et al.; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–33. doi:.https://doi.org/10.1056/NEJMoa1316158
8
Susini
C
,
Buscail
L
. Rationale for the use of somatostatin analogs as antitumor agents. Ann Oncol. 2006;17(12):1733–42. doi:.https://doi.org/10.1093/annonc/mdl105
9
Woltering
EA
. Development of targeted somatostatin-based antiangiogenic therapy: a review and future perspectives. Cancer Biother Radiopharm. 2003;18(4):601–9. doi:.https://doi.org/10.1089/108497803322287691
10
Feelders
RA
,
de Herder
WW
,
Neggers
SJ
,
van der Lely
AJ
,
Hofland
LJ
. Pasireotide, a multi-somatostatin receptor ligand with potential efficacy for treatment of pituitary and neuroendocrine tumors. Drugs Today (Barc). 2013;49(2):89–103. doi:.https://doi.org/10.1358/dot.2013.49.2.1915142
11
de Jong
M
,
Breeman
WA
,
Bernard
BF
,
Rolleman
EJ
,
Hoflande
LJ
,
Visser
TJ
, et al.
Evaluation in vitro and in rats of 161Tb-DTPA-octreotide, a somatostatin analogue with potential for intraoperative scanning and radiotherapy. Eur J Nucl Med. 1995;22(7):608–16. doi:.https://doi.org/10.1007/BF01254561
12
Körner
M
,
Waser
B
,
Schonbrunn
A
,
Perren
A
,
Reubi
JC
. Somatostatin receptor subtype 2A immunohistochemistry using a new monoclonal antibody selects tumors suitable for in vivo somatostatin receptor targeting. Am J Surg Pathol. 2012;36(2):242–52. doi:.https://doi.org/10.1097/PAS.0b013e31823d07f3
13
Gabriel
M
,
Decristoforo
C
,
Kendler
D
,
Dobrozemsky
G
,
Heute
D
,
Uprimny
C
, et al.
68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48(4):508–18. doi:.https://doi.org/10.2967/jnumed.106.035667
14
Etchebehere
EC
,
de Oliveira Santos
A
,
Gumz
B
,
Vicente
A
,
Hoff
PG
,
Corradi
G
, et al.
68Ga-DOTATATE PET/CT, 99mTc-HYNIC-octreotide SPECT/CT, and whole-body MR imaging in detection of neuroendocrine tumors: a prospective trial. J Nucl Med. 2014;55(10):1598–604. doi:.https://doi.org/10.2967/jnumed.114.144543
15
Pettinato
C
,
Sarnelli
A
,
Di Donna
M
,
Civollani
S
,
Nanni
C
,
Montini
G
, et al.
68Ga-DOTANOC: biodistribution and dosimetry in patients affected by neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2008;35(1):72–9. doi:.https://doi.org/10.1007/s00259-007-0587-y
16
Delle Fave
G
,
Kwekkeboom
DJ
,
Van Cutsem
E
,
Rindi
G
,
Kos-Kudla
B
,
Knigge
U
, et al.; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with gastroduodenal neoplasms. Neuroendocrinology. 2012;95(2):74–87. doi:.https://doi.org/10.1159/000335595
17
Garin
E
,
Le Jeune
F
,
Devillers
A
,
Cuggia
M
,
de Lajarte-Thirouard
AS
,
Bouriel
C
, et al.
Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J Nucl Med. 2009;50(6):858–64. doi:.https://doi.org/10.2967/jnumed.108.057505
18
Koopmans
KP
,
Neels
OC
,
Kema
IP
,
Elsinga
PH
,
Sluiter
WJ
,
Vanghillewe
K
, et al.
Improved staging of patients with carcinoid and islet cell tumors with 18F-dihydroxy-phenyl-alanine and 11C-5-hydroxy-tryptophan positron emission tomography. J Clin Oncol. 2008;26(9):1489–95. doi:.https://doi.org/10.1200/JCO.2007.15.1126
19
Ambrosini
V
,
Tomassetti
P
,
Castellucci
P
,
Campana
D
,
Montini
G
,
Rubello
D
, et al.
Comparison between 68Ga-DOTA-NOC and 18F-DOPA PET for the detection of gastro-entero-pancreatic and lung neuro-endocrine tumours. Eur J Nucl Med Mol Imaging. 2008;35(8):1431–8. doi:.https://doi.org/10.1007/s00259-008-0769-2
20
Strosberg
J
,
El-Haddad
G
,
Wolin
E
,
Hendifar
A
,
Yao
J
,
Chasen
B
, et al.; NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376(2):125–35. doi:.https://doi.org/10.1056/NEJMoa1607427
21
Brubaker
PL
,
Drucker
DJ
. Structure-function of the glucagon receptor family of G protein-coupled receptors: the glucagon, GIP, GLP-1, and GLP-2 receptors. Receptors Channels. 2002;8(3-4):179–88. doi:.https://doi.org/10.3109/10606820213687
22
Burcelin
R
,
Da Costa
A
,
Drucker
D
,
Thorens
B
. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes. 2001;50(8):1720–8. doi:.https://doi.org/10.2337/diabetes.50.8.1720
23
Farilla
L
,
Bulotta
A
,
Hirshberg
B
,
Li Calzi
S
,
Khoury
N
,
Noushmehr
H
, et al.
Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144(12):5149–58. doi:.https://doi.org/10.1210/en.2003-0323
24
Baggio
LL
,
Drucker
DJ
. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–57. doi:.https://doi.org/10.1053/j.gastro.2007.03.054
25
Nauck
MA
. Unraveling the science of incretin biology. Eur J Intern Med. 2009;20(Suppl 2):S303–8. doi:.https://doi.org/10.1016/j.ejim.2009.05.012
26
Eng
J
,
Kleinman
WA
,
Singh
L
,
Singh
G
,
Raufman
JP
. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267(11):7402–5.
27
Grant
CS
. Insulinoma. Best Pract Res Clin Gastroenterol. 2005;19(5):783–98. doi:.https://doi.org/10.1016/j.bpg.2005.05.008
28
Grant
CS
. Insulinoma. Best Pract Res Clin Gastroenterol. 2005;19(5):783–98. doi:.https://doi.org/10.1016/j.bpg.2005.05.008
29
Hackert
T
,
Hinz
U
,
Fritz
S
,
Strobel
O
,
Schneider
L
,
Hartwig
W
, et al.
Enucleation in pancreatic surgery: indications, technique, and outcome compared to standard pancreatic resections. Langenbecks Arch Surg. 2011;396(8):1197–203. doi:.https://doi.org/10.1007/s00423-011-0801-z
30
Wenning
AS
,
Kirchner
P
,
Antwi
K
,
Fani
M
,
Wild
D
,
Christ
E
, et al.
Preoperative Glucagon-like peptide-1 receptor imaging reduces surgical trauma and pancreatic tissue loss in insulinoma patients: a report of three cases. Patient Saf Surg. 2015;9(1):23. doi:.https://doi.org/10.1186/s13037-015-0064-7
31
Liu
H
,
Peng
C
,
Zhang
S
,
Wu
Y
,
Fang
H
,
Sheng
H
, et al.
Strategy for the surgical management of insulinomas: analysis of 52 cases. Dig Surg. 2007;24(6):463–70. doi:.https://doi.org/10.1159/000111822
32
Reubi
JC
,
Waser
B
. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30(5):781–93. doi:.https://doi.org/10.1007/s00259-003-1184-3
33
Reubi
JC
,
Körner
M
,
Waser
B
,
Mazzucchelli
L
,
Guillou
L
. High expression of peptide receptors as a novel target in gastrointestinal stromal tumours. Eur J Nucl Med Mol Imaging. 2004;31(6):803–10. doi:.https://doi.org/10.1007/s00259-004-1476-2
34
Wild
D
,
Mäcke
H
,
Christ
E
,
Gloor
B
,
Reubi
JC
. Glucagon-like peptide 1-receptor scans to localize occult insulinomas. N Engl J Med. 2008;359(7):766–8. doi:.https://doi.org/10.1056/NEJMc0802045
35
Christ
E
,
Wild
D
,
Forrer
F
,
Brändle
M
,
Sahli
R
,
Clerici
T
, et al.
Glucagon-like peptide-1 receptor imaging for localization of insulinomas. J Clin Endocrinol Metab. 2009;94(11):4398–405. doi:.https://doi.org/10.1210/jc.2009-1082
36
Antwi
K
,
Fani
M
,
Nicolas
G
,
Rottenburger
C
,
Heye
T
,
Reubi
JC
, et al.
Localization of Hidden Insulinomas with 68Ga-DOTA-Exendin-4 PET/CT: A Pilot Study. J Nucl Med. 2015;56(7):1075–8. doi:.https://doi.org/10.2967/jnumed.115.157768
37
Antwi
K
,
Fani
M
,
Heye
T
,
Nicolas
G
,
Rottenburger
C
,
Kaul
F
, et al.
Comparison of glucagon-like peptide-1 receptor (GLP-1R) PET/CT, SPECT/CT and 3T MRI for the localisation of occult insulinomas: evaluation of diagnostic accuracy in a prospective crossover imaging study. Eur J Nucl Med Mol Imaging. 2018;45(13):2318–27. doi:.https://doi.org/10.1007/s00259-018-4101-5
38
Ginj
M
,
Zhang
H
,
Waser
B
,
Cescato
R
,
Wild
D
,
Wang
X
, et al.
Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc Natl Acad Sci USA. 2006;103(44):16436–41. doi:.https://doi.org/10.1073/pnas.0607761103
39
Wadas
TJ
,
Eiblmaier
M
,
Zheleznyak
A
,
Sherman
CD
,
Ferdani
R
,
Liang
K
, et al.
Preparation and biological evaluation of 64Cu-CB-TE2A-sst2-ANT, a somatostatin antagonist for PET imaging of somatostatin receptor-positive tumors. J Nucl Med. 2008;49(11):1819–27. doi:.https://doi.org/10.2967/jnumed.108.054502
40
Cescato
R
,
Waser
B
,
Fani
M
,
Reubi
JC
. Evaluation of 177Lu-DOTA-sst2 antagonist versus 177Lu-DOTA-sst2 agonist binding in human cancers in vitro. J Nucl Med. 2011;52(12):1886–90. doi:.https://doi.org/10.2967/jnumed.111.095778
41
Wild
D
,
Fani
M
,
Behe
M
,
Brink
I
,
Rivier
JE
,
Reubi
JC
, et al.
First clinical evidence that imaging with somatostatin receptor antagonists is feasible. J Nucl Med. 2011;52(9):1412–7. doi:.https://doi.org/10.2967/jnumed.111.088922
42
Cescato
R
,
Erchegyi
J
,
Waser
B
,
Piccand
V
,
Maecke
HR
,
Rivier
JE
, et al.
Design and in vitro characterization of highly sst2-selective somatostatin antagonists suitable for radiotargeting. J Med Chem. 2008;51(13):4030–7. doi:.https://doi.org/10.1021/jm701618q
43
Nicolas
GP
,
Beykan
S
,
Bouterfa
H
,
Kaufmann
J
,
Bauman
A
,
Lassmann
M
, et al.
Safety, Biodistribution, and Radiation Dosimetry of 68Ga-OPS202 in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Prospective Phase I Imaging Study. J Nucl Med. 2018;59(6):909–14. doi:.https://doi.org/10.2967/jnumed.117.199737
44
Vera
P
,
Thureau
S
,
Chaumet-Riffaud
P
,
Modzelewski
R
,
Bohn
P
,
Vermandel
M
, et al.
Phase II Study of a Radiotherapy Total Dose Increase in Hypoxic Lesions Identified by 18F-Misonidazole PET/CT in Patients with Non-Small Cell Lung Carcinoma (RTEP5 Study). J Nucl Med. 2017;58(7):1045–53. doi:.https://doi.org/10.2967/jnumed.116.188367
45
Wild
D
,
Fani
M
,
Fischer
R
,
Del Pozzo
L
,
Kaul
F
,
Krebs
S
, et al.
Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: a pilot study. J Nucl Med. 2014;55(8):1248–52. doi:.https://doi.org/10.2967/jnumed.114.138834
46
Nicolas
GP
,
Mansi
R
,
McDougall
L
,
Kaufmann
J
,
Bouterfa
H
,
Wild
D
, et al.
Biodistribution, Pharmacokinetics, and Dosimetry of 177Lu-, 90Y-, and 111In-Labeled Somatostatin Receptor Antagonist OPS201 in Comparison to the Agonist 177Lu-DOTATATE: The Mass Effect. J Nucl Med. 2017;58(9):1435–41. doi:.https://doi.org/10.2967/jnumed.117.191684
47
Wells
SA, Jr
,
Asa
SL
,
Dralle
H
,
Elisei
R
,
Evans
DB
,
Gagel
RF
, et al.; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. doi:.https://doi.org/10.1089/thy.2014.0335
48Rottenburger C, Nicolas G, McDougall L, Kaul F, Christ E, Schibli R, et al. Imaging of Advanced Medullary Thyroid Carcinoma with the CCK-2 Receptor Agonist 177Lu-PP-F11N – Preliminary Proof of the Principle within the “Lumed” Study. 15th Annu Eur Neuroendocr Tumor Soc Conf. 2018.
49
Körner
M
,
Waser
B
,
Reubi
JC
. Does somatostatin or gastric inhibitory peptide receptor expression correlate with tumor grade and stage in gut neuroendocrine tumors?
Neuroendocrinology. 2015;101(1):45–57. doi:.https://doi.org/10.1159/000371804
50
Gourni
E
,
Waser
B
,
Clerc
P
,
Fourmy
D
,
Reubi
JC
,
Maecke
HR
. The glucose-dependent insulinotropic polypeptide receptor: a novel target for neuroendocrine tumor imaging—first preclinical studies. J Nucl Med. 2014;55(6):976–82. doi:.https://doi.org/10.2967/jnumed.113.133744