Clinical outcomes in patients with systemic lupus erythematosus treated with belimumab in clinical practice settings: a retrospective analysis of results from the OBSErve study in Switzerland
DOI: https://doi.org/10.4414/smw.2019.20022
Johannes
von Kempisa, Sabine
Duetschb, Nicola
Reuschlingc, Rahel
Villigerd, Peter M
Villigerd, Florence
Valleliane, Dominik J
Schaere, Ruediger B
Muelleraf
aDivision of Rheumatology and Immunology, Department of Internal Medicine, Kantonsspital St. Gallen, St. Gallen, Switzerland
bGlaxoSmithKline AG, Münchenbuchsee, Switzerland
cKlinik Birshof, Münchenstein, Switzerland
dDepartment of Rheumatology, Immunology and Allergology, University Hospital and University of Bern, Bern, Switzerland
eDivision of Internal Medicine, University Hospital of Zurich, Zurich, Switzerland
fDivision of Rheumatology and Clinical Immunology, Department of Internal Medicine IV, Ludwig Maximilian University of Munich, Munich, Germany
Summary
AIMS OF THE STUDY
To describe patterns of systemic lupus erythematosus (SLE) care and the clinical effectiveness of belimumab plus standard of care therapy in a real-world clinical setting in Switzerland.
METHODS
This multicentre, observational, retrospective cohort study included adults with SLE who initiated belimumab as part of their usual care at least six months before data analysis. The primary outcome was the overall clinical response, assessed by a physician on a Physician’s Global Assessment-like scale, to six months’ treatment with belimumab. Secondary outcomes included improvement in disease activity, SLE manifestations and changes in corticosteroid use.
RESULTS
53 patients (81% female) from three hospitals were included. At index (belimumab initiation), 23 patients (43%) had mild, 23 (43%) had moderate, and 7 (13%) had severe SLE. Overall improvement in disease activity in patients receiving belimumab was: ≥80% in 6 patients (11%), ≥50% in 12 (23%), ≥20% in 31 (58%), <20% in 13 (25%), and no improvement in 9 (17%). Mean Safety of Estrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index score decreased from 8.0 at index to 3.6 at six months post index in the 27 patients assessed. In addition, a ≥50% improvement in arthritis, fatigue, rash, low complement (C3, C4 or total haemolytic complement activity), and anti-double-stranded deoxyribonucleic acid antibody levels was experienced six months post index by 10 (38%), 3 (16%), 6 (38%), 2 (12%) and 4 (16%) patients who presented the manifestations at index respectively. At index, 41 patients (77%) received oral corticosteroids at a mean dose of 11.6 mg/day, which decreased to 5.9 mg/day at six months post index. Of the 31 patients receiving a high dose of corticosteroids (≥7.5 mg/day) at index, 18 required <7.5 mg/day and a further two discontinued corticosteroids at six months post index.
CONCLUSIONS
This study provides real-world insight into belimumab use in clinical practice in Switzerland. In line with findings from other countries, Swiss patients with SLE who received belimumab demonstrated clinical and serological improvements in SLE and a reduction in corticosteroid use after six months of treatment.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with a heterogeneous presentation. SLE affects a variety of organ systems, including the skin, heart, joints, kidneys and central nervous system [1]. The estimated incidence of SLE in western European countries ranges from 2.2 to 5.0/100,000, with a prevalence of 25.4–91/100,000 [2]. The majority of patients with SLE require lifelong medication to manage disease activity. Such medications include oral corticosteroids, antimalarials, non-steroidal anti-inflammatory drugs (NSAIDs), cytotoxic agents and conventional synthetic and biological disease-modifying anti-rheumatic drugs [3]. Prolonged treatment with these therapies, especially high-dose corticosteroids and immunomodulatory therapies, is associated with significant morbidity [4, 5].
SLE is characterised by elevated circulating levels of B lymphocyte stimulator (BLyS), also known as B cell-activating factor (BAFF), a member of the tumour necrosis factor ligand superfamily that promotes abnormal B cell activation and differentiation [6, 7]. Belimumab is a recombinant immunoglobulin G1λ monoclonal antibody that binds soluble BLyS and neutralises its biological activity [8]. In Switzerland, belimumab is indicated as add-on therapy in adult patients with active, autoantibody-positive SLE [9], and is reimbursed for patients with a high degree of serological activity (e.g., positive anti-double-stranded deoxyribonucleic acid (dsDNA) antibodies and low complement levels). While the efficacy and safety of intravenous (IV) belimumab plus standard of care (SoC) therapy in patients with active SLE has been demonstrated in three large Phase III trials [10–12], data on its clinical benefit in everyday clinical practice are scarce. OBSErve (evaluation Of use of Belimumab in clinical practice SEttings) is a multinational study programme comprising observational studies designed to assess treatment utilisation and clinical outcomes of belimumab therapy in real-world settings. The OBSErve studies were conducted to provide a more realistic reflection of the overall patterns of SLE care compared with clinical trials. The first OBSErve study was conducted in the United States. Additional studies have now been completed in Germany, Canada, Spain and Argentina [13–17]. In these real-world practice settings, patients with SLE showed overall clinical improvements in SLE and a reduction in corticosteroid use and healthcare resource utilisation.
This study aims to investigate treatment patterns in patients with SLE and the clinical effectiveness of six months of belimumab therapy in patients who received belimumab plus SoC therapy as part of their usual care in clinical practice in Switzerland.
Materials and methods
Study design
This was a multicentre, observational cohort study (GSK study number 201232) designed to retrospectively analyse real-world information on the short-term outcomes of belimumab use in patients with SLE from patient medical records. As the study was retrospective, the protocol had no influence on treatment decisions or the collection of patient data. Data were collected at three time points: six months prior to belimumab initiation, at belimumab initiation (index date, between September 2012 and March 2016), and six months after index (or at time of discontinuation). The study period included a treatment history period (pre index) of six months and a post index follow-up of six months. This allowed for an observation period of approximately 12 months for patients who did not discontinue treatment. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [18].
Study sites and physicians
Physicians (rheumatologists or internal medicine physicians) who at the time of recruitment managed/treated at least 10 patients with SLE, had ≥5 years’ experience in treating patients with SLE, had ≥6 months’ experience in belimumab use, had treated at least two patients with belimumab plus SoC and who were currently treating at least one patient with belimumab plus SoC participated in the study. Physicians completed a physician practice profile form (PPPF), which captured information regarding their medical practices and approaches to the management of SLE, such as routine use of laboratory tests and disease activity assessment tools. Six of the leading academic centres with specialised SLE clinics in the German-speaking parts of the country were contacted, and three agreed to participate in the study. Treating physicians at the three sites (site 1: three physicians; site 2: three physicians; and site 3: two physicians) received continuous training for the diagnosis and treatment of patients with SLE during the study period.
Patient inclusion and exclusion criteria
Physicians enrolled all patients from their practices who fulfilled the following inclusion criteria: adults aged ≥18 years with a confirmed SLE diagnosis who had initiated belimumab plus SoC at least six months before inclusion, for whom reasons for belimumab initiation and discontinuation could be identified, whose medical history was available for up to six months pre index, and whose treatment outcomes were available at six months post index or at discontinuation. Patients already enrolled in an SLE-related clinical trial, or who started belimumab as part of a clinical trial in an intervention arm, were excluded. All patient data were anonymised. To avoid selection bias, all patients in whom belimumab was initiated as part of their usual care six months before documentation, and who met the inclusion criteria (including patients who had discontinued belimumab in the past), were considered for inclusion.
Physicians extracted data from patient medical records onto the patient case report form (CRF). CRFs contained patient information on demographics and clinical characteristics, SLE disease characteristics at index, treatment and clinical outcomes.
Ethical considerations
The study was conducted according to the Guidelines for Good Pharmacoepidemiology Practices and the Guideline for Good Clinical Practice [19, 20]. The study and all study documentation were approved by the lead ethics committee in St. Gallen and subsequently approved by ethics committees in Zurich and Bern (approval number: EKSG 14/128/L). Written informed consent was obtained from all participating patients.
Objectives
The primary objective was to describe the pattern of SLE care and outcomes among patients receiving belimumab for six months, in clinical practice in Switzerland. Secondary objectives were to describe characteristics of the patients, reasons for initiation and discontinuation of belimumab, changes in disease activity as assessed by the Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA) SLE Disease Activity Index (SLEDAI), and use of concomitant medications for patients treated with belimumab.
Endpoints
The primary endpoint was the overall clinical response to six months of belimumab treatment, reported as a percentage of patients with specific levels of clinical improvement since the index date, as assessed by the physician on a Physician’s Global Assessment (PGA)-like scale, and categorised as worse, no improvement, improvement of <20%, 20–49%, 50–79%, or ≥80%. The endpoints to assess the pattern of SLE care were the use of different disease activity instruments and laboratory measures as part of regular SLE management. Endpoints to assess patient outcomes included: clinical response of specific SLE manifestations/organ specific improvement (based on the physician’s judgement, analogous to the assessment of the overall clinical response); change in disease activity (SELENA-SLEDAI score or other disease activity indices, calculated retrospectively by the physician during data collection); treatment patterns of concomitant medication, particularly corticosteroids: dose reduction and switching from high doses (≥7.5 mg/day) to low doses (<7.5 mg/day); and rate of discontinuation of belimumab. The SELENA-SLEDAI analyses 24 items relating to nine organ systems, with scores ranging from 0 to 105 [21]. An increase in SELENA-SLEDAI of >3 points has been suggested as indicative of a flare, and a reduction of >3 points as an improvement [22, 23]. Therefore, a reduction of more than 3 points was chosen to represent a clinically meaningful difference in disease activity.
Although safety assessment was not an objective of this study, adverse events (AEs) were reported to the local/national regulatory authorities or to the study sponsor, as appropriate.
Statistical analyses
Owing to the largely exploratory nature of the study, no formal sample size calculations were performed to determine the number of physicians and patients to be included in the study. Based on feasibility, the target sample size was 40 patients. Analyses were conducted on the full analysis set (FAS), defined as all valid cases (patients who fulfilled the inclusion criteria, and for whom completed CRFs were available) from eligible sites (sites that fulfilled the physician inclusion criteria, and completed the PPPF). Descriptive statistics were used to analyse the categorical and quantitative data (IBM SPSS Statistics 22 software was used). No analyses of loss to follow-up, incomplete case documentation or deviations from the target study population were undertaken. No imputation for missing data was used.
Results
Participating sites and physicians
Three hospital sites participated in the study. The mean (standard deviation [SD]) physician experience in treatment of patients with SLE was 8.7 (4.62) years (range 6–14 years), and the mean number of patients with SLE under the care of each physician/site was 60 (range 30–100). Of these, a mean of 21 patients per physician (range 12–31) had been treated with belimumab plus SoC at some point, a mean of 19 patients were under active treatment at the time of data collection, and 11 had been treated with belimumab for at least six months.
The use of disease assessment tools varied between sites, with only one site routinely using a PGA scale, a SLEDAI or SELENA-SLEDAI, or a Fatigue Severity Scale in their SLE management. In all three study sites, laboratory analyses such as complete blood counts, measurements of the erythrocyte sedimentation rate, C-reactive protein and serum creatinine, urinalysis with examination of urinary sediment, and complement C3/C4 level tests, were routinely performed. Anti-nuclear antibody titres, serum albumin, anti-dsDNA antibody titres and spot (untimed) urine protein and creatinine were each measured by two of the three sites.
Patient characteristics
The FAS consisted of 53 patients; 26 patients (49%) from site 1, 16 (30%) from site 2 and 11 (21%) from site 3. Mean age was 46.7 years and 43 patients (81%) were female. At index, 23 patients (43%) had mild, 23 (43%) had moderate, and 7 (13%) had severe SLE, based on the physician’s clinical judgement (table 1). Physicians reported persistent SLE activity in 31 patients (58%), a flare in 12 (23%), and remission/low disease activity in 10 (19%).
Table 1 Patient characteristics (n = 53).
|
Patient characteristics
|
At index (n = 53)
|
| Female, n (%) |
43 (81.1) |
| Age (years), mean (SD; range) |
46.7 (13.6; 24–82) |
| BMI (kg/m2), mean (SD; range) |
25.4 (5.1; 18.6–41.3) |
| Co-morbidities and manifestations of SLE (most frequent), n (%) |
None |
10 (18.9) |
| Lupus nephritis |
10 (18.9) |
| Hypertension |
10 (18.9) |
| Other (ICD-10, Chapter III – haematologic) |
8 (15.1) |
| Liver disease/problems |
7 (13.2) |
| Other (ICD-10, Chapter XIII – musculoskeletal) |
7 (13.2) |
| Time since SLE diagnosis, n (%) |
<1 year |
1 (1.9) |
| 1–5 years |
23 (43.4) |
| 6–10 years |
18 (34.0) |
| >10 years |
10 (18.9) |
| Unknown |
1 (1.9) |
| SLE severity (physician assessed) at index, n (%) |
Mild |
23 (43.4) |
| Moderate |
23 (43.4) |
| Severe |
7 (13.2) |
| Laboratory values at index (most frequent), n (%) |
High anti-dsDNA levels |
30 (56.6) |
| Low C3 (<lower limit of normal) |
27 (50.9) |
| Low C4 (<lower limit of normal) |
20 (37.7) |
| Proteinuria (>upper limit of normal) |
7 (13.2) |
| Leukopenia |
6 (11.4) |
| Number of SLE clinical manifestationsa at index, n (%) |
1 |
9 (17.0) |
| 2 |
14 (26.4) |
| 3 |
8 (15.1) |
| 4 |
15 (28.3) |
| ≥5 |
7 (13.2) |
Number of SLE medications (physician recorded) prior to index,
n (%) |
1–3 |
36 (67.9) |
| 4–5 |
13 (24.5) |
| >5 |
4 (7.5) |
| Reasons for initiation of belimumab therapy (multiple reasons were permitted), n (%) |
Previous treatment regimen not effective |
35 (66.0) |
| Decrease use of corticosteroids |
25 (47.2) |
| Patient condition worsening |
15 (28.3) |
| Previous treatment not well tolerated |
3 (5.7) |
| Previous treatment regimen inconvenient |
1 (1.9) |
| Patient request |
1 (1.9) |
All patients presented with at least one clinical manifestation of SLE at index, with the majority (n = 44, 83%) experiencing multiple manifestations. The most frequently reported co-morbidities were hypertension and lupus nephritis (also an SLE manifestation), each experienced by 10 patients (19%). Haematological co-morbidities, liver disease/problems and musculoskeletal co-morbidities were documented for 8 (15%), 7 (13%) and 7 (13%) patients respectively. Ten patients (19%) did not have any co-morbid conditions (table 1).
Reasons for belimumab initiation included ineffective previous treatment, reported for 35 patients (66%), the intent to reduce the corticosteroid dose (n = 25, 47%), and a worsening condition (n = 15, 28%). Previous treatment not tolerated, or inconvenient, and a patient request were reported for 3 (6%), 1 (2%) and 1 (2%) patients respectively (table 1).
All patients received the recommended dose of monthly belimumab, 10 mg/kg IV, after the three induction infusions on days 0, 14 and 28. None of the patients discontinued belimumab during the six months post index. After this period, 41 patients (77%) continued belimumab and 12 (23%) discontinued. The reasons for discontinuation were: ineffective medication (n = 9, 75%), adverse drug reaction/AE (prostate carcinoma) combined with lack of response (n = 1, 8%), and patient request (n = 2, 17%).
Overall clinical response
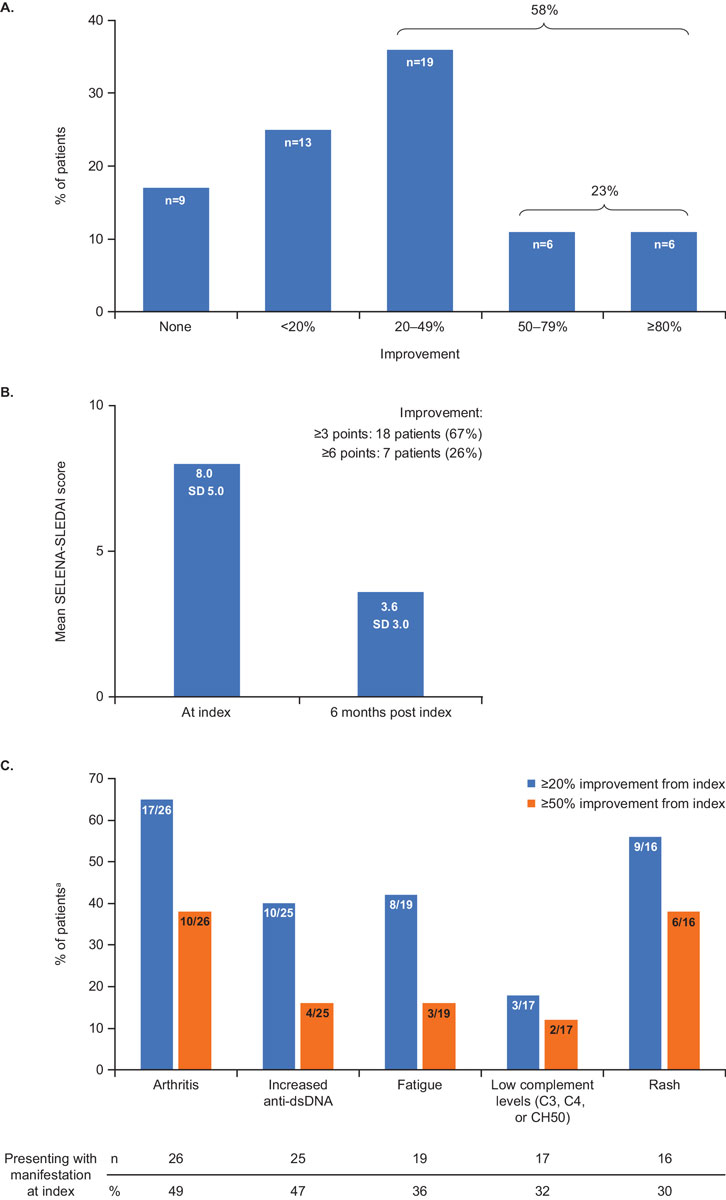
At six months post index, the majority of patients (n = 44, 83%) showed an overall clinical improvement based on the physician’s evaluations on a PGA-like scale. An improvement of <20% was observed in 13 patients (25%), while 31 (58%) had an improvement of at least 20%, 12 (23%) of at least 50% and 6 (11%) of at least 80%. Nine patients (17%) had no improvement, and none experienced worsening (fig. 1A).
Disease activity assessment: SELENA-SLEDAI
SELENA-SLEDAI scores at index and six months post index were available for a subgroup of 27 patients from two study sites. In these patients, there was a decrease in mean SELENA-SLEDAI score from 8.0 at index to 3.6 at six months post index. The majority of these patients (n = 18, 67%) showed a reduction of ≥3.0 points, and 7 (26%) of ≥6.0 points (fig. 1B).
Improvement from index in the most frequent clinical and serological manifestations
At index, the five most frequent clinical and serological manifestations were arthritis (n = 26, 49%), positive anti-dsDNA antibody test (n = 25, 47%), fatigue (n = 19, 36%), low complement (C3, C4, or haemolytic complement activity [CH50]; n = 17, 32%), and rash (n = 16, 30%) (fig. 1C). Among patients presenting arthritis, fatigue and rash at index, a ≥20% improvement was reported six months post index for 17 (65%), 8 (42%) and 9 (56%) patients respectively, with 10 (38%), 3 (16%) and 6 (38%) patients experiencing a ≥50% improvement. At six months post index, 10 (40%) and 3 (18%) patients had a ≥20% improvement in anti-dsDNA antibody levels and C3, C4, or CH50 levels respectively, and 4 (16%) and 2 (12%) patients had a ≥50% improvement (fig. 1C).
Concomitant medications
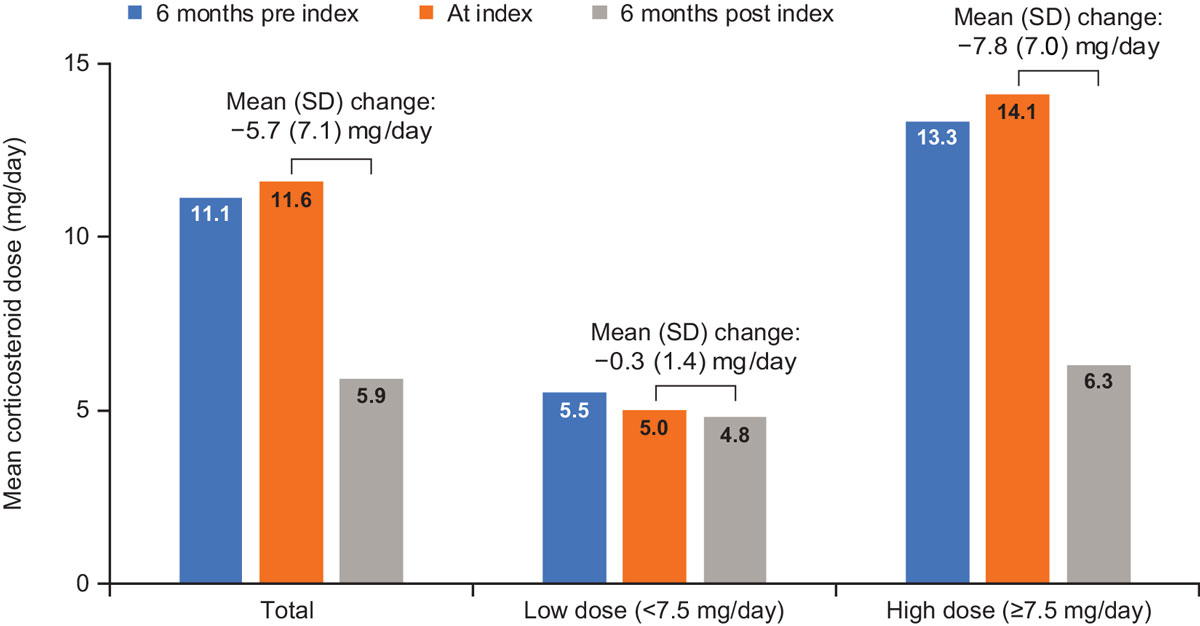
Data on the overall use of corticosteroids were available for 53 patients and data on the corticosteroid dose at index and six months post index were available for 42 patients. At index, 41 patients (77%, n = 53) received oral corticosteroids; mean dose was 11.6 mg/day (n = 42; fig. 2). During the six months post index, patients experienced a mean dose reduction of 5.7 mg/day to a mean dose of 5.9 mg/day (fig. 2). Overall, corticosteroid dose remained stable in nine patients (21%), increased in two (5%), and decreased in 28 (67%) patients during the six months post index. One patient (2%) initiated corticosteroids (mean dose 5 mg/day), and two (5%) discontinued. In contrast, during the six months pre index, corticosteroid dose remained stable in 21 patients (50%), increased in 10 (24%) and decreased in 9 (21%), with only one patient (2%) achieving a dose of <7.5 mg/kg.
Among the 31 patients who required a high dose (≥7.5 mg/day) of corticosteroids at index, 65% had dose reductions to low or no corticosteroids at six months post index (n = 18 to <7.5 mg/day and two discontinued use). The dose of patients who were still receiving ≥7.5 mg/day corticosteroids at six months post index was also reduced. Overall, patients receiving a high dose of corticosteroids at index had a mean dose reduction of 7.8 mg/day at six months post index.
Other concomitant SLE-related medications received at index included antimalarials (n = 44, 83%), azathioprine (n = 22, 42%), cyclosporine (n = 1, 2%), methotrexate (n = 9, 17%), mycophenolate mofetil (n = 7, 13%) and NSAIDs (n = 1, 2%) (appendix 1, table S1). The use of these medications remained stable throughout the study. At six months pre index, the most common non-SLE therapies were osteoporosis and antihypertensive medications and dietary supplements, received by 38 (72%), 22 (42%) and 11 (21%) patients respectively. The medication pattern did not change during belimumab therapy, except for dietary supplements, for which intake decreased to 5 (9%) patients at six months post index.
Safety
Two serious AEs were reported during the study period. Mammary carcinoma was considered by the investigator to be possibly related to belimumab treatment, and prostate carcinoma was considered unrelated to belimumab treatment. No deaths were reported.
Discussion
This observational study investigated the role of belimumab in the management of patients with SLE in clinical practice in Switzerland. Belimumab in addition to SoC is approved to reduce disease activity of adult patients with active, autoantibody-positive SLE, and is reimbursed in patients with a high disease activity (e.g. positive anti-dsDNA and low complement levels) [9, 24] despite SoC therapy. The data show that over a six-month treatment period, patients had an overall improvement in disease activity, with a decrease in SELENA-SLEDAI score, improvements in the most frequent clinical and serological manifestations of SLE and, importantly, a dose reduction of concomitant corticosteroid medication.
A key finding of this study was the reduction in corticosteroid use observed, with 65% of patients in the high-dose group having their dose reduced to below 7.5 mg/day and two patients discontinuing steroids. Reducing the use of corticosteroids is important in the treatment of SLE, as prolonged use of high-dose corticosteroids, predominantly prescribed to patients with high disease activity, is associated with significant toxicity and organ damage, and many patients with more severe SLE are children and adolescents [4, 25–27]. This steroid-sparing effect of belimumab was observed in post hoc analyses of the belimumab clinical trials and the long-term extension study [28, 29].
The effectiveness of belimumab demonstrated in this study supports similar findings in the other OBSErve studies, in which the average SELENA-SLEDAI score also decreased markedly, with parallel reductions in corticosteroid use [13–17]. Interestingly, the real-world observations in the United States, Canada, Germany and Spain saw greater clinical improvements and steroid sparing effects. For example, in the United States, Germany and Canada, an improvement of more than 50% was observed in 48.7%, 42% and 57.7% of patients, compared with in 23% of patients in Switzerland [14–17]. The greater clinical improvements and steroid sparing effects observed in these studies may have been due to a higher disease severity at index, based on physician’s clinical judgement, and a higher baseline SELENA-SLEDAI score, compared with this study, resulting in a more pronounced response to belimumab treatment, which was also observed in post hoc analyses of the belimumab Phase III trials [28].
Belimumab was well tolerated; no patients discontinued belimumab during the six months of therapy, which is in line with belimumab treatment recommendations (treatment for a minimum of six months before belimumab discontinuation is considered) [9].
There is no standard SLE assessment tool recommended by organisations of rheumatology professionals such as the European League against Rheumatism or the American College of Rheumatology. In this study, we found that the use of SELENA-SLEDAI varied between sites and was used for approximately half of all patients, with the majority of physicians not using any disease assessment tools in their routine SLE management. The lack of a consistent use of measures of disease activity demonstrates a gap in the real-world care of patients with SLE in Switzerland. This gap was also highlighted by the OBSErve studies conducted in the United States and Canada [14, 17]. A standard assessment practice would prove helpful in monitoring the disease course and the effect of changes in therapy for a single patient, as already highlighted by Strand et al. nearly two decades ago [30]. Furthermore, it would also enable comparisons to be made between clinical sites throughout Switzerland. To this day, no general recommendations for the optimal measurement of clinical outcomes in SLE have been developed by, for example, the OMERACT (Outcome Measures in Rheumatology) group (www.omeract.org). In 2014, van Vollenhoven et al. further emphasised that clear definitions for assessing disease activity should be developed [31]. Furthermore, disease scores such as SELENA-SLEDAI are difficult to use for retrospective chart review analyses, as physicians often fail to document patient outcomes that they do not regard as clinically important.
The study has some limitations. Firstly, the analyses were conducted with relatively low patient numbers, particularly for the SELENA-SLEDAI assessments, which were performed only in approximately 50% of patients. Although there may be variation in the SLE care experience between different sites, the three hospitals which participated in this study represent 50% of all clinical sites specialising in treating patients with SLE in Switzerland. These sites were selected from the German-speaking part of Switzerland only. A second limitation is that the primary endpoint was based on the physician’s individual clinical judgement. Furthermore, the information provided in the CRFs was an interpretation of the patients’ medical charts, and under-reporting may have occurred if not all events were recorded. Thirdly, the study did not include a control group to compare belimumab treatment with other SoC therapies. The fourth possible bias of this study is the relatively short follow-up of six months. However, because the efficacy measure (SLE responder index) in the BLISS-52 and BLISS-76 studies [10, 11] was found to plateau after six months, and the primary endpoint of the present study was the efficacy/effectiveness of belimumab, we considered the chosen length of follow-up to be adequate. This is further supported by the Swiss and the European product information, which recommend considering discontinuation of belimumab if no improvement is observed after six months [9, 24]. The length of follow-up may be different for studies assessing belimumab safety and the incidence of adverse events.
The incomplete documentation for changes in and/or occurrence of anti-nuclear antibody and anti-dsDNA antibody titres could be considered a further limitation.
Considering the limitations mentioned above, we think that observational studies performed in different national cohorts, such as the OBSErve study in Switzerland, may greatly improve our understanding of how new therapies are used in an everyday clinical practice, and how effective they are in a national setting. Such observational data on national treatment patterns may be the only source of such information, but they are a very important source, because large, multinational Phase III/IV clinical trials often do not analyse specific differences between participating countries. The findings described here may prove useful in designing future treatment strategies for patients with SLE in Switzerland.
In conclusion, this is the first observational study of treatment patterns in patients with SLE and the clinical effectiveness of belimumab for patients with SLE receiving belimumab as part of their usual care in Switzerland. The data provide further evidence to support the previously demonstrated steroid-sparing effect of belimumab, which has important clinical implications, particularly for young patients with high disease activity. Variability in the use of disease assessment tools highlights a care gap in the treatment of SLE in the real-world setting.
Appendix 1 Supplementary tables
Table S1 Number of patients receiving concomitant SLE medications other than corticosteroids (n = 53).
|
6 months
pre index
|
At index
|
6 months post index
|
| Antimalarials (e.g. hydroxychloroquine/chloroquine), n (%) |
44 (83) |
44 (83) |
44 (83) |
| Azathioprine, n (%) |
24 (45) |
22 (42) |
22 (42) |
| Cyclosporine, n (%) |
1 (2) |
1 (2) |
0 (0) |
| Methotrexate, n (%) |
10 (19) |
9 (17) |
8 (15) |
| Mycophenolate mofetil, n (%) |
9 (17) |
7 (13) |
7 (13) |
| NSAIDs, n (%) |
2 (4) |
1 (2) |
1 (2) |
| Rituximab/MabThera, n (%) |
2 (4) |
0 (0) |
0 (0) |
Table S2 Pre-defined clinical manifestations of SLE listed in the Patient Case Report Form.
|
Musculoskeletal
|
Arthritis |
| Myositis |
|
Central nervous system
|
Cerebrovascular accident |
| Organic brain syndrome |
| Cranial nerve neuropathy |
| Seizure |
| Psychosis |
| Headache |
|
Cardiopulmonary
|
Pericarditis |
| Pleurisy |
| Myocarditis |
|
Mucocutaneous
|
Rash |
| Mucosal ulcers |
| Alopecia |
|
Immunologic
|
Increased DNA bindinga
|
| Low complement (C3, C4, or CH50)b
|
|
Renal
|
Proteinuriac
|
| Pyuriad
|
|
Constitutional
|
Inability to taper steroids |
| Fatigue |
| Fever |
| Weight loss |
|
Haematologic
|
Thrombocytopeniae
|
| Leucopeniaf
|
| Haemolytic anaemia |
|
Circulatory
|
Vasculitis |
|
Visual/ocular
|
|
|
Gastrointestinal
|
|
|
Renal/genitourinary
|
Urinary casts |
| Haematuria |
Acknowledgements
OBSErve Switzerland was conducted by Kantar Health GmbH, Munich, Germany (including study coordination, statistical analyses and medical writing assistance), funded by GSK. Medical writing assistance for this manuscript was provided by Gosia Carless, PhD, of Fishawack Indicia Ltd, UK, funded by GSK.
References
1
D’Cruz
DP
,
Khamashta
MA
,
Hughes
GRV
. Systemic lupus erythematosus. Lancet. 2007;369(9561):587–96. doi:.https://doi.org/10.1016/S0140-6736(07)60279-7
2
Danchenko
N
,
Satia
JA
,
Anthony
MS
. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15(5):308–18. doi:.https://doi.org/10.1191/0961203306lu2305xx
3
Bertsias
G
,
Ioannidis
JPA
,
Boletis
J
,
Bombardieri
S
,
Cervera
R
,
Dostal
C
, et al.; Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67(2):195–205. doi:.https://doi.org/10.1136/ard.2007.070367
4
Gladman
DD
,
Urowitz
MB
,
Rahman
P
,
Ibañez
D
,
Tam
L-S
. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol. 2003;30(9):1955–9.
5
Oglesby
A
,
Shaul
AJ
,
Pokora
T
,
Paramore
C
,
Cragin
L
,
Dennis
G
, et al.
Adverse event burden, resource use, and costs associated with immunosuppressant medications for the treatment of systemic lupus erythematosus: a systematic literature review. Int J Rheumatol. 2013;2013:347520. doi:.https://doi.org/10.1155/2013/347520
6
Petri
M
,
Stohl
W
,
Chatham
W
,
McCune
WJ
,
Chevrier
M
,
Ryel
J
, et al.
Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. 2008;58(8):2453–9. doi:.https://doi.org/10.1002/art.23678
7
Zhang
J
,
Roschke
V
,
Baker
KP
,
Wang
Z
,
Alarcón
GS
,
Fessler
BJ
, et al.
Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166(1):6–10. doi:.https://doi.org/10.4049/jimmunol.166.1.6
8
Baker
KP
,
Edwards
BM
,
Main
SH
,
Choi
GH
,
Wager
RE
,
Halpern
WG
, et al.
Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003;48(11):3253–65. doi:.https://doi.org/10.1002/art.11299
9Fachinformation Benlysta. 2017. Accessed April 2018.http://www.swissmedicinfo.ch/.
10
Furie
R
,
Petri
M
,
Zamani
O
,
Cervera
R
,
Wallace
DJ
,
Tegzová
D
, et al.; BLISS-76 Study Group. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63(12):3918–30. doi:.https://doi.org/10.1002/art.30613
11
Navarra
SV
,
Guzmán
RM
,
Gallacher
AE
,
Hall
S
,
Levy
RA
,
Jimenez
RE
, et al.; BLISS-52 Study Group. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9767):721–31. doi:.https://doi.org/10.1016/S0140-6736(10)61354-2
12
Zhang
F
,
Bae
S-C
,
Bass
D
,
Chu
M
,
Egginton
S
,
Gordon
D
, et al.
A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis. 2018;77(3):355–63. doi:.https://doi.org/10.1136/annrheumdis-2017-211631
13
Babini
AGM
,
Barreira
JC
,
Pons-Estel
B
,
Iglesias
M
,
Streger
G
. Evaluation of use of belimumab in clinical practice settings: Results in Argentina. Arthritis Rheum. 2016;68(Suppl 10).
14
Collins
CE
,
Dall’Era
M
,
Kan
H
,
Macahilig
C
,
Molta
C
,
Koscielny
V
, et al.
Response to belimumab among patients with systemic lupus erythematosus in clinical practice settings: 24-month results from the OBSErve study in the USA. Lupus Sci Med. 2016;3(1):e000118. doi:.https://doi.org/10.1136/lupus-2015-000118
15
Cortes
JMC
,
Andreu
J
,
Calvo-Alen
J
,
Garcia-Aparicio
A-M
,
Alvarez
ED
, et al.
Evolution of patients with systemic lupus erythematous treated with belimumab in clinical practice settings. Arthritis Rheumatol. 2014;66(Suppl 11):S291.
16
Schwarting
A
,
Schroeder
JO
,
Alexander
T
,
Schmalzing
M
,
Fiehn
C
,
Specker
C
, et al.
First real-world insights into belimumab use and outcomes in routine clinical care of systemic lupus erythematosus in Germany: Results from the OBSErve Germany study. Rheumatol Ther. 2016;3(2):271–90. doi:.https://doi.org/10.1007/s40744-016-0047-x
17
Touma
Z
,
Sayani
A
,
Pineau
CA
,
Fortin
I
,
Matsos
M
,
Ecker
GA
, et al.
Belimumab use, clinical outcomes and glucocorticoid reduction in patients with systemic lupus erythematosus receiving belimumab in clinical practice settings: results from the OBSErve Canada Study. Rheumatol Int. 2017;37(6):865–73. doi:.https://doi.org/10.1007/s00296-017-3682-9
18
von Elm
E
,
Altman
DG
,
Egger
M
,
Pocock
SJ
,
Gøtzsche
PC
,
Vandenbroucke
JP
; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. doi:.https://doi.org/10.1016/S0140-6736(07)61602-X
19ICH. Guideline for good clinical practice 2016. Accessed March 2018.https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4_2016_1109.pdf.
20ISPE. Guidelines for Good Pharmacoepidemiology Practices 2015. Accessed March 2018.https://www.pharmacoepi.org/resources/policies/guidelines-08027/.
21
Bombardier
C
,
Gladman
DD
,
Urowitz
MB
,
Caron
D
,
Chang
CH
,
Austin
A
, et al.; The Committee on Prognosis Studies in SLE. Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheum. 1992;35(6):630–40. doi:.https://doi.org/10.1002/art.1780350606
22
Gladman
DD
,
Urowitz
MB
,
Kagal
A
,
Hallett
D
. Accurately describing changes in disease activity in Systemic Lupus Erythematosus. J Rheumatol. 2000;27(2):377–9.
23
Petri
M
,
Genovese
M
,
Engle
E
,
Hochberg
M
. Definition, incidence, and clinical description of flare in systemic lupus erythematosus. A prospective cohort study. Arthritis Rheum. 1991;34(8):937–44. doi:.https://doi.org/10.1002/art.1780340802
24EMA. Benlysta Summary of Product Characteristics (SmPC). Accessed March 2017;http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002015/WC500110150.pdf.
25
Ruiz-Arruza
I
,
Ugarte
A
,
Cabezas-Rodriguez
I
,
Medina
JA
,
Moran
MA
,
Ruiz-Irastorza
G
. Glucocorticoids and irreversible damage in patients with systemic lupus erythematosus. Rheumatology (Oxford). 2014;53(8):1470–6. doi:.https://doi.org/10.1093/rheumatology/keu148
26
Ruiz-Arruza
I
,
Lozano
J
,
Cabezas-Rodriguez
I
,
Medina
JA
,
Ugarte
A
,
Erdozain
JG
, et al.
restrictive use of oral glucocorticoids in systemic lupus erythematosus and prevention of damage without worsening long-term disease control: An observational study. Arthritis Care Res (Hoboken). 2018;70(4):582–91. doi:.https://doi.org/10.1002/acr.23322
27
Gutiérrez-Suárez
R
,
Ruperto
N
,
Gastaldi
R
,
Pistorio
A
,
Felici
E
,
Burgos-Vargas
R
, et al.
A proposal for a pediatric version of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index based on the analysis of 1,015 patients with juvenile-onset systemic lupus erythematosus. Arthritis Rheum. 2006;54(9):2989–96. doi:.https://doi.org/10.1002/art.22048
28
van Vollenhoven
RF
,
Petri
MA
,
Cervera
R
,
Roth
DA
,
Ji
BN
,
Kleoudis
CS
, et al.
Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis. 2012;71(8):1343–9. doi:.https://doi.org/10.1136/annrheumdis-2011-200937
29
Furie
RA
,
Wallace
DJ
,
Aranow
C
,
Fettiplace
J
,
Wilson
B
,
Mistry
P
, et al.
Long-Term Safety and Efficacy of Belimumab in Patients With Systemic Lupus Erythematosus: A Continuation of a Seventy-Six-Week Phase III Parent Study in the United States. Arthritis Rheumatol. 2018;70(6):868–77. doi:.https://doi.org/10.1002/art.40439
30
Strand
V
,
Gladman
D
,
Isenberg
D
,
Petri
M
,
Smolen
J
,
Tugwell
P
. Outcome measures to be used in clinical trials in systemic lupus erythematosus. J Rheumatol. 1999;26(2):490–7.
31
van Vollenhoven
RF
,
Mosca
M
,
Bertsias
G
,
Isenberg
D
,
Kuhn
A
,
Lerstrøm
K
, et al.
Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis. 2014;73(6):958–67. doi:.https://doi.org/10.1136/annrheumdis-2013-205139