aDepartment of Consultation-Liaison-Psychiatry and Psychosomatic Medicine, University Hospital Zurich, Switzerland
bDivision of Pulmonology, University Hospital Zurich, Switzerland
Summary
BACKGROUND
Lung transplantation (LTx) provides a viable option for the survival of end-stage lung diseases. Besides survival as a clinical outcome measure, health-related quality of life (HRQoL) and psychological distress have become important outcomes in studies investigating the effectiveness of LTx in the short- and long-term.
OBJECTIVE
To assess and compare HRQoL trajectories of patients after LTx prior to and over a follow-up period of three years post-transplant, and to identify differences regarding distress, HRQoL and patient-related outcomes.
METHODS
In this longitudinal study, 27 lung transplant recipients were prospectively examined for psychological distress (Symptom Checklist short version-9; SCL-K-9), health-related quality of life (EuroQOL five dimensions questionnaire; EQ-5D), depression (HADS-Depression scale), and socio-demographic and medical outcomes at two weeks, three months, six months and three years following LTx. Additionally, potential outcome-related predictors for LTx-outcomes at three years post-transplant were assessed. Data were collected in accordance with guidelines set by the STROBE (strengthening the reporting of observational studies in epidemiology) statement.
RESULTS
Lung transplant recipients showed the most pronounced improvements in HRQoL and reduction in psychological distress between two weeks and three months post-transplant, with relative stable HRQoL and distress trajectories thereafter. The most important predictors of poor somatic health trajectories over time were the pre-transplant disease severity score and the pre-transplant HADS-Depression score. In addition, idiopathic pulmonary fibrosis (IPF) and pre-transplant extracorporeal membrane oxygenation (ECMO)-use predicted poorer survival, while cystic fibrosis was associated with better survival three years post-transplant.
COMCLUSION
Lung transplantation yields significant survival and HRQoL benefits, with its peak improvement at three months post-transplant. The majority of patients can preserve these health changes in the long-term. Patients with a worse HRQoL and higher psychological distress at six months post-transplant tended to have a poorer survival post-transplant. Other risk factors for poorer survival included IPF, pre-transplant ECMO-use, pre-transplant symptoms of depression, high pre-transplant disease severity and worse somatic disease severity trajectories. The majority of LTx-recipients were unable to work due to illness-related reasons.
Introduction
Lung transplantation is an established therapeutic option for various forms of end-stage lung disease when alternative treatment options are no longer effective. Lung transplantation leads to successful outcomes in carefully selected patients, characterised by reduced disability, extended survival and improved health-related quality of life (HRQoL) [1, 2]. More than 50,000 lung transplantations have been performed worldwide, with increasing survival rates over time [3]. Over the past two decades, there has been a remarkable improvement in the short- and long-term survival rates of lung transplant (LTx) patients. In Switzerland, one-, three- and five-year survival rates of 87%, 75% and 69% have been achieved, which are comparable to the results of international programs [4, 5].
Besides survival, HRQoL and psychological distress have become important clinical outcome measures in studies investigating the effectiveness of lung transplantation in the short- and long-term [6]. Furthermore, psychological factors such as psychological distress, depression and poor quality of life have emerged as independent risk factors for post-transplant survival [7, 8]. Numerous studies have conclusively demonstrated a clear improvement in HRQoL after lung transplantation [2, 6, 9–13]. Specifically, results from longitudinal studies reported a significant improvement in the HRQoL in LTx-recipients, especially within the first six months post-transplant, with a relatively stable HRQoL thereafter [14, 15]. Nonetheless, the individual trajectories of HRQoL over time are less clear in LTx-recipients, as their health is often hampered by numerous post-operative complications and the side effects of immunosuppressive therapy [16, 17]. Indeed, few studies have examined distinct HRQoL trajectories within the same patients over time. In a study of our own, we found distinctive post-operative psychological distress and HRQoL trajectories. More precisely, almost a quarter of the investigated LTx-recipients suffered from elevated distress and substantially impaired HRQoL, with no improvement over the first six months post-transplant. Strong predictors for poor post-transplant outcomes were underlying diagnosis, older age, disease severity and length of hospital stay [18].
The purposes of this study were to assess and compare the development of HRQoL trajectories in patients after lung transplantation over a follow-up period of three years, and to identify differences regarding distress and HRQoL, as well as the socio-demographic and medical characteristics, between living and deceased LTx-recipients.
Methods
Study population
Between January 2012 and April 2014, 72 lung transplants were performed at the Transplant Centre of the University Hospital of Zurich, Switzerland. Forty-three of these lung transplant recipients met the following inclusion criteria: (1) older than 18 years of age, (2) able and/or willing to provide informed consent, and (3) able and/or willing to complete questionnaires in German. Out of these 43 patients, three withdrew from participation for medical or personal reasons during the first six months after transplantation (n = 40). Within the three-year post-transplant period, 10 of the 40 participants died. In addition, during data collection for T4, three patients withdrew from participation for medical or personal reasons. The final study sample included 27 lung transplant recipients who survived the three-year follow-up period and 10 LTx-recipients who died before the last assessment time-point. The response rate was 90% (40/43 LTx-patients).
Design and procedures
In this prospective, longitudinal follow-up study, 27 lung transplant recipients were assessed at five different measurement time-points (T0: pre-transplant; T1: two weeks; T2: three months; T3: six months; and T4: three years after transplantation). Following approval from the Ethics Committee of the Canton Zurich, Switzerland, all patients provided written informed consent prior to their participation. Eligible patients were asked to fill in a set of questionnaires. The first two assessments, at T0 and T1, were conducted at bedside during hospitalisation. The follow-up assessments at T2 and T3 were performed during regular transplant clinic outpatient visits at the University Hospital Zurich, and T4 was collected via questionnaires sent by mail. Data was collected in accordance with guidelines set by the STROBE (strengthening the reporting of observational studies in epidemiology) statement [19].
Measures
EuroQuol (EQ-5D)
The EQ-5D, a self-administered psychometric instrument, was used to assess health-related quality of life. The questionnaire evaluates five dimensions of health: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension could be rated on a three-point Likert scale of no problems, some problems or severe problems. A single global score (range 0–100) was generated using the sum model as described by Hinz et al. [20]. In the current study, the EQ-5D showed good internal consistency over the four measurement time points, ranging between 0.64 and 0.77 (Cronbach’s alpha).
The Symptom Checklist (SCL-K-9)
The German version of the Symptom Checklist short version-9 (SCL-K-9 [21]) form was used to assess overall psychological distress. The SCL-K-9 is comprised of nine items, rated on a 5-point Likert scale (0 = not at all to 4 = very strong). The SCL-K-9 is highly correlated with the original SCL-90-R [22], and presents similarly good psychometric properties and sensitivity to change, with a Cronbach’s alpha of 0.87. In this study, internal consistency over the three measurement time points was good, with a Cronbach’s alpha ranging from 0.75 to 0.84.
The patients’ medical records were used to retrieve all the socio-demographic and medical information. In order to generate a pre-transplant baseline score for quality of life, the pre-transplant EQ-5D of the Swiss Transplant Cohort study (STCS) was used. More details on the design of the STCS can be found elsewhere [23, 24].
Somatic Disease Severity Score (SDSS)
In addition, a somatic disease severity score (SDSS) was calculated by the treating transplant pulmonologist (MMS) to assess the individual disease trajectories over time, including the pre-transplant health status, the health status at six months, and at three years post-transplant. The scores reached from 1 = favourable, 2 = regular to 3 = poor.
The eight-item SDSS for the somatic evolution of LTx-recipients over time was calculated based on the transplant pulmonologist’s assessment of the number of complications in the assessment period compared to a predefined expected evolution. The assessment parameters included the evolution of (1) lung function, (2) renal function (eGFR), (3) weight and (4) C-reactive protein (CRP). The number of infectious complications requiring (5) additional antibiotic treatment (oral or intravenous) and (6) other additional anti-infectious treatments, i.e. antifungal or antiviral treatments, as well as (7) steroid augmentation (oral or intravenous), were also evaluated, as was hospitalisation (8) for any complication, including infections, allograft dysfunction or gastrointestinal problems.
All parameters were categorised as “better than expected” (cat. 1), “expected” (cat. 2) and “worse than expected” (cat. 3). For the overall somatic assessment, the most frequent category of the eight parameter evaluations (mode) was taken as the final result of the somatic evolution in the time frame studied.
The expected evolution was an increase in or stability of lung function (for all patients) and an increase in or stability of weight for cachectic (BMI <18.5 kg/m2) and normal weight patients prior to transplant, or a decrease in or stability of weight for pre-transplant overweight (BMI >25 kg/m2) patients. Weight changes of 5% were considered relevant. Stability of renal function was expected. One oral or one intravenous antibiotic treatment episode was expected for each six-month period. One additional anti-infectious therapy episode with antiviral or antifungal medication was expected for every six-month follow-up period, despite use of regular antiviral and antifungal prophylaxis for all patients. Either one modification of immunosuppression medication (switch of drug or steroid augmentation) or one hospitalisation was expected per six-month follow-up period.
The whole six-month period prior to the assessment time point was considered, but to get an idea of the long-term evolution at three years, the whole one-year period prior to the assessment time point was briefly assessed (focusing on CRP and pulmonary function) to put the last six months into the context of that year.
Changes in parameters of 10% or more were considered relevant unless specified otherwise. For CRP, values <5 (considered the normal reference in our setting) were considered low. CRP values of 5-19 were considered moderate (intermittent elevation to this level may be expected), and values of 20 or higher were relevant elevations in these highly immunosuppressed patients taking multiple medications.
Additionally, the pre-transplant co-morbidity index developed by Barrios et al. [25], which includes ten extra-pulmonary comorbidities that may negatively impact short- and long-term post-operative trajectories, was used. This comorbidity index comprises the following factors: (1) body mass index >30 kg/m2, (2) osteoporosis, (3) insulin-dependent diabetes, (4) arterial hypertension, (5) cardiac disease, (6) chronic liver disease, (7) diverticulosis, (8) gastroesophageal reflux, (9) history of malignancy, and (10) psychiatric disorders. The comorbidity index was computed by summing up the number of comorbidities for each patient. Values between 0 and 10 were possible.
Statistical analysis
For the sociodemographic and medical data at T1 we used Excel and reported in either counts and percentages or means and ranges. We compared the results between the living patients (n = 27) and the patients (n = 10) who died after measurement time point T3. For the remaining statistical analyses, the Statistical Package for the Social Sciences (IBM SPSS, version 25) was used. Descriptive statistics are shown as means and standard deviations. A multivariate test, a general linear model with repeated measures and repeated contrasts, was conducted for the dependent variables SCL-K-9 and EQ-5D, with time as the within-subject factor. Assumptions for all analyses with this model were checked in terms of Mauchly’s test of sphericity. Assumptions were met, with the exception of a significant Mauchly’s test for the global severity index (GSI) of the SCL-K-9, which was considered with a Greenhouse-Geisser correction. A p-value smaller than 0.05 was considered statistically significant.
Due to the small sample size, we did not carry out analysis of the statistical significance between the two groups (living and deceased lung transplant recipients) regarding HRQoL and psychological distress over the three-year follow-up period.
Results
Out of the initial sample of 40 LTx-recipients for the six-month follow-up study, 27 (68%) could be included in the three-year follow-up. Ten (25%) patients died within the three-year follow-up period, and three patients (8%) withdrew from the study due to personal and medical reasons.
Detailed socio-demographics and clinical characteristics of the LTx-recipients are summarised in table 1. Comparison of the living and deceased LTx-recipients at T4 revealed some differences. In particular, patients who suffered from idiopathic pulmonary fibrosis were more likely to die. In contrast, patients with cystic fibrosis had a better survival rate after lung transplantation relative to patients with other lung diseases. In addition, the LTx-recipients who died over the three-year follow-up period more frequently had a poor pre-transplant disease severity score (20% vs. 11%), more frequently suffered from bronchiolitis obliterans syndrome (BOS) (70% vs. 17%), and had more frequently used extracorporeal membrane oxygenation (ECMO) directly prior to transplantation (60% vs. 22%).
Table 1 Socio-demographic and medical characteristics.
| Alive | Dead | |||
|---|---|---|---|---|
| n = 27 (73%) | n = 10 (27%) | |||
| Sex (n, %) | ||||
| ‒ Male | 15 | 56% | 6 | 60% |
| ‒ Female | 11 | 41% | 4 | 40% |
| Age (years; mean, range) | 48.63 | 19‒66 | 46.40 | 19‒66 |
| Married (n, %) | 13 | 48% | 4 | 40% |
| Education level (n, %) | ||||
| ‒ Elementary | 6 | 22% | 1 | 10% |
| ‒ Secondary | 13 | 48% | 6 | 60% |
| ‒ Tertiary | 8 | 30% | 3 | 30% |
| Underlying diagnosis (n, %) | ||||
| ‒ CF | 11 | 41% | 2 | 20% |
| ‒ COPD | 11 | 41% | 4 | 40% |
| ‒ IPF | 4 | 15% | 3 | 30% |
| ‒ Other | 4 | 15% | 1 | 10% |
| SDSS T4 (n, %) | ||||
| ‒ Favourable | 10 | 37% | 0 | 0% |
| ‒ Regular | 13 | 48% | 0 | 0% |
| ‒ Poor | 4 | 15% | 0 | 0% |
| T0 HADS-D (mean, range) | 4.7 | 1‒12 | 5.8 | 1‒11 |
| Comorbidities (mean, range) | 1.44 | 0‒4 | 2.60 | 1‒4 |
| Nr. of days on waiting list (mean, range) | 289.81 | 10‒992 | 171.00 | 16‒439 |
| ECMO use (n, %) | 6 | 22% | 6 | 60% |
| Nr. of days in ICU pLTx (mean, range) | 6.59 | 2‒29 | 8.30 | 2‒24 |
| Length of stay (weeks; mean, range) | 5.52 | 3‒14 | 6.30 | 3‒12 |
| Nr. of hospitalizations pLTx (mean, range) | 0.59 | 0‒2 | 1.30 | 0‒4 |
| Nr. of infections pLTx (mean, range) | 0.44 | 0‒2 | 1.10 | 0‒3 |
| Nr. of rejections pLTX (mean, range) | 0.04 | 0‒1 | 0.30 | 0‒2 |
| Use of antidepressants pLTx (n, %) | 6 | 22% | 4 | 40% |
| Working ability pLTx (n, %) | 6 | 22% | 0 | 0 |
Abbreviations. CF = cystic fibrosis; COPD = chronic obstructive pulmonary disease; IPF = idiopathic pulmonary fibrosis; BOS = bronchiolitis obliterans syndrome; SDSS = somatic disease severity score; T0 HADS-D = Hospital Anxiety and Depression Scale - Depression; ECMO = extracorporeal membrane oxygenation; ICU = intensive care unit; pLTx = post lung transplantation. ‒ Note. Somatic disease severity refers to the post-transplant health status at T4 and was assessed by the treating transplant pneumonologist (MMS) by use of a somatic disease severity score resulting in one of three categories (1 = favourable; 2 = regular; 3 = poor).
Comparing the pre-transplant HRQoL data provided by the STCS with the post-transplant HRQoL data of this study (table 2) revealed a moderate impairment of HRQoL before transplantation (M = 70.43; SD = 17.70). In contrast, long-term analysis of our data showed a significant improvement in both, HRQoL (p < 0.001) and psychological stress (p = 0.003) between T1 and T2, specifically between two weeks and three months post-transplant (table 2). Furthermore, after an initial reduction in psychological distress between two weeks and three months, the SCL-K-9 outcomes for the remaining measurement points were stable, reaching a value of 0.43 at T4, almost a community-based standard value (fig. 1). Similarly, after an initial improvement in HRQoL between two weeks and three months post-transplant, HRQoL remained stable during the three-year follow-up period (fig. 2).
Table 2 Pre- and post-transplant quality of life and psychological distress in lung transplant recipients.
| T0 | T1 | T2 | T3 | T4 | Contrasts | ||||
|---|---|---|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | Wilks' Lambda | p-values | p-values | ||
| T0 vs. T1 | 0.06 | ||||||||
| EQ-5D | 70.43 (17.70) | 62.17 (25.75) | 85.22 (15.04) | 87.39 (13.22) | 89.57 (12.24) | 0.29 | <0.001 | T1 vs. T2 | <0.001 |
| n = 23 | T2 vs. T3 | 0.26 | |||||||
| T3 vs. T4 | 0.37 | ||||||||
| T1 vs. T2 | <0.001 | ||||||||
| SCL-K-9 | - | 1.05 (0.73) | 0.53 (0.38) | 0.45 (0.38) | 0.43 (0.34) | 0.57 | 0.003 | T2 vs. T3 | 0.17 |
| n = 27 | T3 vs. T4 | 0.78 | |||||||
M = mean; SD = standard deviation; EQ-5D = EuroQOL five dimensions questionnaire; SCL-K-9 = Symptom Checklist short version-9. T0 = pre-transplant; T1 = two weeks post-transplant; T2 = three months post-transplant; T3 = six months post-transplant; T4 = three years post-transplant.
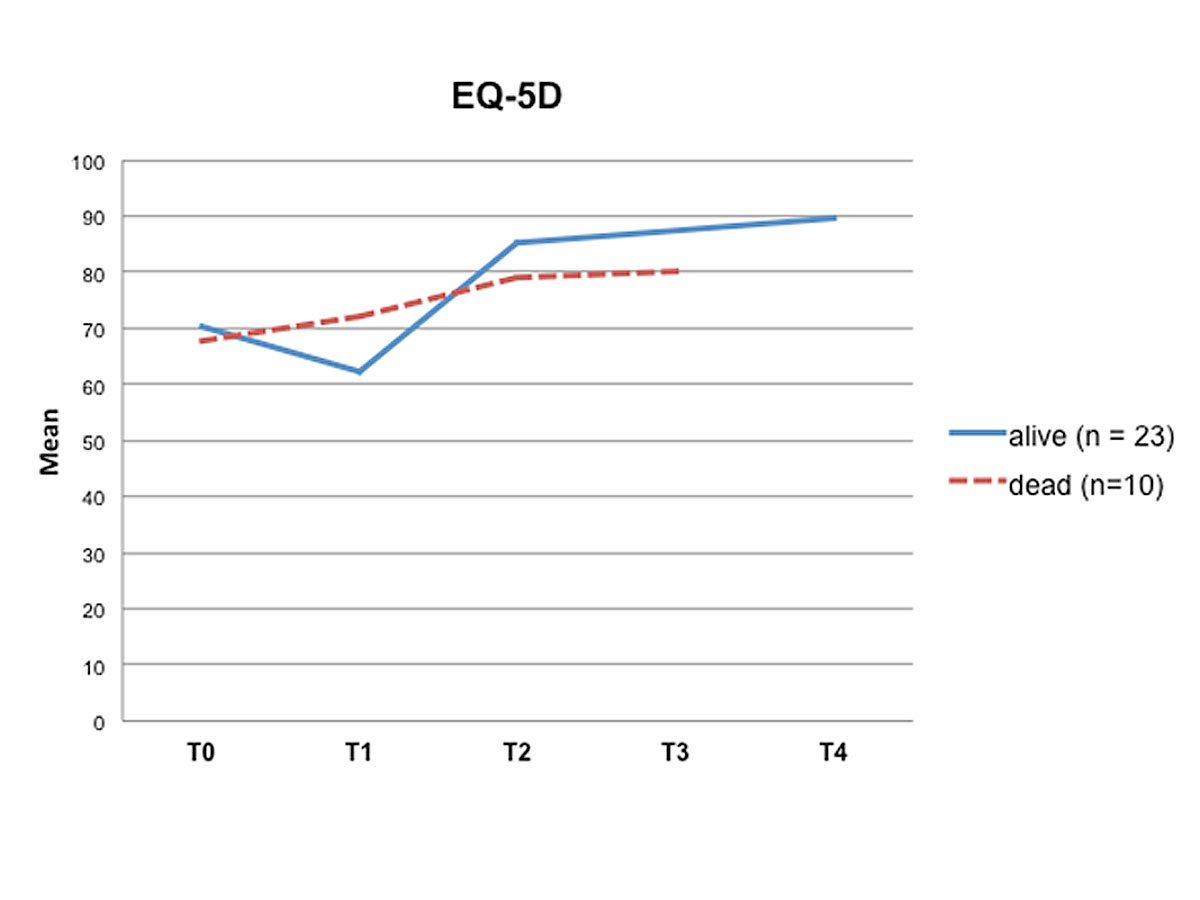
Figure 1 Health-related quality of life over time (T0 – T4). T0 = pre-transplant; T1 = two weeks post-transplant; T2 = three months post-transplant, T3 = six months post-transplant; T4 = three years post-transplant
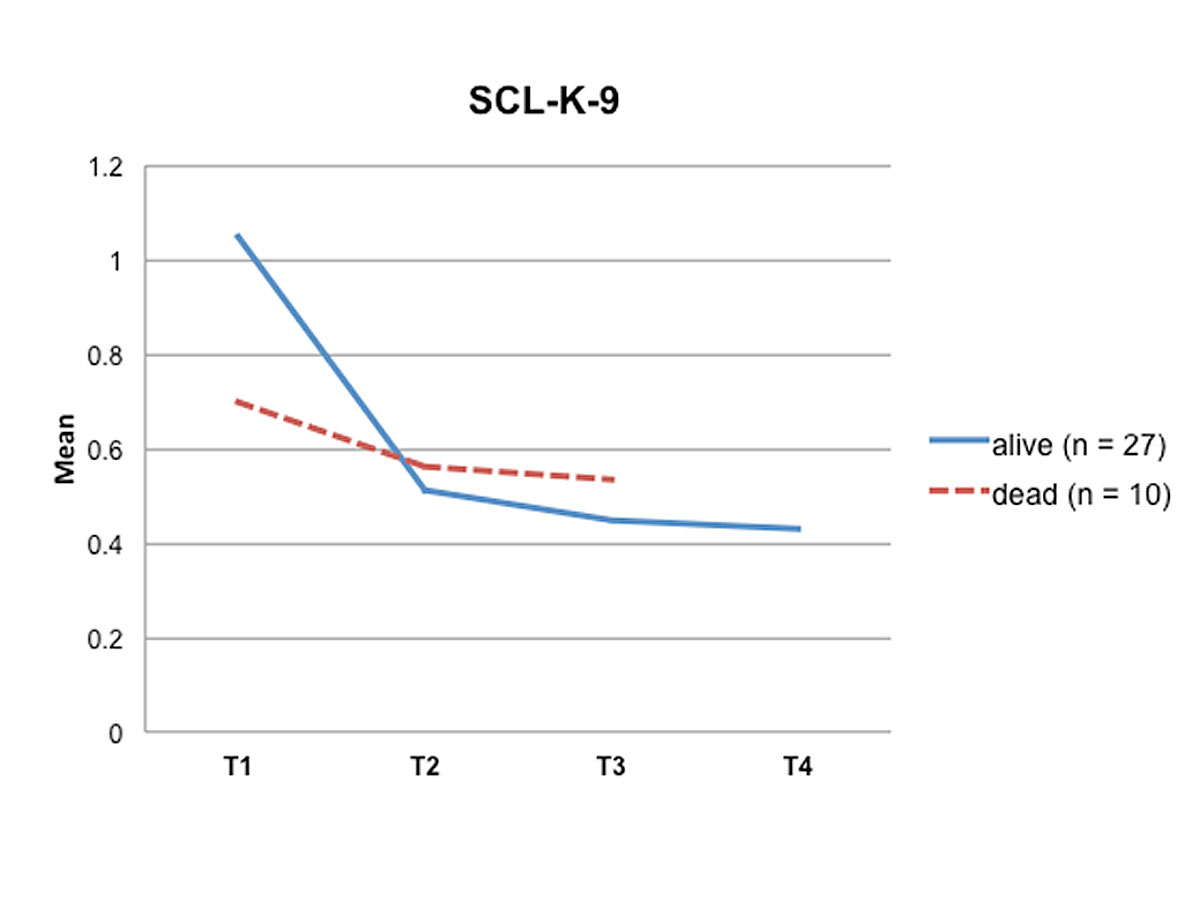
Figure 2 Psychological distress over time (T1 – T4). T0 = pre-transplant; T1 = two weeks post-transplant; T2 = three months post-transplant, T3 = six months post-transplant; T4 = three years post-transplant
Interestingly, patients who died within the three-year follow-up period reported better quality of life and less psychological distress immediately after transplantation, but had impaired HRQoL and increased stress levels six months post-transplant relative to the LTx-recipients who were still alive at the three-year follow-up (figs 1 and 2 ).
Correlation analyses (table 3) revealed statistically significant positive correlations between the T0 pre-transplant disease severity score and both the somatic disease severity score for poor somatic health trajectories (r = 0.432; p < 0.01) and the psychological distress at T3 (r = 0.391; p < 0.05). There were statistically significant positive correlations between the pre-transplant depression score and the T0 pre-transplant disease severity score (r = 0.403; p < 0.01), the psychological distress at T3 (r = 0.668; p < 0.001) and at T4 (r = 0.501; p < 0.01), as well as an inverse correlation for quality of life at T3 (r = 0.425 p < 0.01). There were also statistically significant inverse correlations between the T0 pre-transplant disease severity score and quality of life at T0 (r = -0.376; p <0.05) and at T3 (r = -0.424; p <0.01). The relationship described above was less clear at T4. Furthermore, the somatic disease severity score for poor somatic health trajectories at T3 was significantly positively correlated with the somatic disease severity score for poor somatic health trajectories at T4 (r = 0.566; p <0.01).
Table 3 Bivariate correlations between pre-transplant disease severity score, somatic disease severity score and mental health outcomes.
| Pre-Tx DSS | T3 SDSS | T4 SDSS | HADS-D T0 | T0 EQ-5D | T3 EQ-5D | T4 EQ-5D | T3 SCL-K-9 | T4 SCL-K-9 | |
|---|---|---|---|---|---|---|---|---|---|
| Pre-Tx DSS | 1 | 0.450** | 0.214 | 0.403* | ‒0.376* | ‒0.424** | 0.228 | 0.391* | 0.312 |
| T3 SDSS | 0.450** | 1 | 0.445* | 0.333 | ‒0.433* | ‒0.218 | ‒0.137 | 0.417* | 0.157 |
| T4 SDSS | 0.214 | 0.445** | 1 | 0.119 | ‒0.074 | ‒0.189 | ‒0.363 | 0.093 | 0.416* |
| T0 HADS-D | 0.403 | 0.333 | 0.119 | 1 | ‒0.535** | ‒0.425* | ‒0.106 | 0.668* | 0.501* |
| T0 EQ5D | ‒0.376* | ‒0.433* | ‒0.074 | ‒0.535** | 1 | 0.520** | 0.378 | ‒0.386* | ‒0.094 |
| T3 EQ5D | ‒0.424** | ‒0.218 | ‒0.189 | ‒0.425* | 0.520** | 1 | 0.684** | ‒0.571** | ‒0.219 |
| T4 EQ5D | ‒0.228 | ‒0.137 | ‒0.363 | ‒0.106 | 0.378 | 0.684** | 1 | ‒0.188 | ‒0.424* |
| T3 SCL-K | 0.391* | 0.417* | 0.093 | 0.668* | ‒0.386* | ‒0.571** | ‒0.188 | 1 | 0.502** |
| T4 SCL-K | 0.312 | 0.157 | 0.416* | 0.501* | ‒0.094 | ‒0.219 | ‒0.424* | 0.502** | 1 |
Pre-Tx DSS = pre-transplant disease severity score; HADS-D = Hospital Anxiety Depression Score-Depression; SDSS = somatic disease severity score, EQ-5D = EuroQOL five dimensions questionnaire; SCL-K-9 = Symptom Checklist short version-9. * p < 0.05, ** p < 0.01
Discussion
This longitudinal three-year follow-up study assessed and compared the development of the HRQoL and distress trajectories of 27 LTx-recipients. Our results indicate a clear benefit from lung transplantation, with its peak improvement occurring three months post-transplant. In line with previous studies [6, 26–28], the majority of patients in this cohort reported a deterioration in HRQoL prior to lung transplantation, followed by a major impairment in HRQoL and increase in psychological distress two weeks after transplantation. Three months post-transplant, most of the LTx-recipients had experienced a dramatic improvement in HRQoL and a significant reduction of psychological distress. The post-transplant improvement in HRQoL and reduction of psychological distress remained relatively stable over the three-year follow-up period. The stability of the improvement in HRQoL and the reduction of psychological distress over the entire three-year follow-up period may be attributed to advances in surgical techniques, improved post-transplant management concepts and long-term patient follow-up by the transplant pulmonologists [4].
In a second analytic step, we analysed predictors of poor transplant outcomes.
Patients with idiopathic pulmonary fibrosis (IPF) and patients who required the use of ECMO pre-transplant were clearly more likely to die, whereas patients with cystic fibrosis (CF) showed a better survival rate during the three-year follow-up period. Furthermore, pre-transplant depression was associated with poorer HRQoL and higher levels of psychological distress post-transplant, demonstrating that patients with pre-transplant depression represent a risk population for post-transplant complications. These results are consistent with previous results in the lung transplant literature [18, 29–31].
The most important predictor of poor post-transplant trajectories was the pre-transplant disease severity score, highlighting the importance of integrating this evaluation tool into clinical practice. Due to the shortage of donor organs, it is critical that the patients selected are those who are most likely to benefit from lung transplantation. Assessing a disease severity score prior to lung transplantation could present a promising tool to improve the allocation of donor lungs and the survival of patients undergoing lung transplantation. However, the disease severity score used in this study has not yet been validated or standardised, and therefore the predictive value of this tool remains uncertain. Despite this limitation, the pre-transplant severity score appeared to correlate with the outcomes of our studied cohort. Larger long-term studies are needed to determine whether a disease severity score can improve the overall allocation and survival of eligible LTx-patients.
Interestingly, the periodical collection of a somatic disease severity score seems to be a stable predictor of somatic health trajectories over the post-transplant time. These study results require confirmation in a larger clinical sample.
Professional reintegration was found to be an important predictor of HRQoL following transplantation [32], although few studies have examined employment following lung transplantation. Similar to other studies focusing on work ability after lung transplantation [32, 33], the percentage of LTx-recipients in our study returning to work was relatively low, at 22% within the time frame studied. All of them were patients who survived the three-year follow-up period. Unfortunately, the number of LTx-recipients remaining in an employed position decreased over time due to illness-related reasons, and early retirement or disability compensation was the consequence.
While most other studies described a sample of living patients, we used the information about deaths occurring in the six months post-transplant to identify differences regarding HRQoL and psychological distress, as well as sociodemographic and medical information, between living and deceased LTx-recipients. Interestingly, we found that patients who died during the three-year follow-up period reported better HRQoL and less psychological distress at T1 relative to LTx-recipients who were alive at T4, but did not experience an improvement in HRQoL and a reduction of psychological distress between T2 and T3. Thus, patients with poorer HRQoL and higher psychological distress at six months post-transplant tended to have a poorer survival post-transplant.
The long-term care of LTx-recipients is focused on the prevention and management of complications, optimisation of immunosuppression, treatment of medical comorbidities, and returning to independent daily living [34]. Due to the inter-individual variability of post-operative HRQoL trajectories over time, interventional strategies, including psychiatric and psychosocial counselling, should be offered pre-, peri- and post-operatively throughout the patient’s post-transplant recovery and adjustment.
Strengths and limitations
The present study has some limitations. Firstly, our data were based on a small sample size from a single centre study. Thus, the generalisability of the results may be limited, and they may not be applicable to all transplant programs or allocation systems. Furthermore, the present findings need to be replicated by larger cohorts. Secondly, our non-somatic data were based solely on patients’ self-reporting, representing the subjective perspective of the study participants at certain time points. Another weakness is that the EQ-5D is a generic, preference-based measure. A disease-specific measure could be more sensitive to health changes than a generic one [2]. The EQ-5D was chosen as the outcome measure due to its being easy to complete and its previous use in lung transplant recipients [1].
Counterbalancing these limitations are the notable strengths of our approach. By quantifying HRQoL prior to transplantation as well as repeatedly following transplantation patients for up to three years, we explicitly attempted to account for selection and survivorship bias, which is an important limitation in the existing literature [2].
Conclusion
Lung transplantation results in significant HRQoL and survival benefits in the majority of LTx-recipients in the short- as well as in the long-term. Overall, HRQoL increased and psychological distress decreased significantly between two weeks and three months post-transplant, and remained relatively stable for up to three years post-transplant. We found that poorer HRQoL and higher psychological distress at six months post-transplant were associated with subsequent mortality. Other risk factors for poorer survival included IPF, pre-transplant ECMO-use, higher disease severity and BOS. The majority of LTx-recipients were unable to work because of illness-related reasons. The integration of a pre-transplant disease severity index in clinical practice may present a promising tool to estimate the overall success of lung transplantation and to improve the allocation of donor lungs and the survival of patients undergoing lung transplantation. Future research on LTx-related outcome predictors is necessary for the development of tailored psychosocial interventions.
Members of the Psychosocial Interest Group
Sabina De Geest, Kris Denhaerynck, Remon Helmy, Lynn Leppla, Oliver Mauthner, Marian Struker (University of Basel); Annette Boehler, Sabine Gerull, Michael Koller, Lut Berben (University Hospital Basel); Uyen Huynh-Do (University Hospital Inselspital Bern); Emmanuelle Catana (University Hospital Lausanne); Karine Hadaya (University Hospital Geneva); Annina Seiler, Richard Klaghofer (University Hospital Zurich); Isabelle Binet (Cantonal Hospital St. Gallen); Patrizia Künzler-Heule (University of Basel, Cantonal Hospital St. Gallen); Hanna Burkhalter (Sleepmed Hirslanden), Sonja Beckmann (University of Basel, University Hospital Zurich).
Members of the Swiss Transplant Cohort Study
Patrizia Amico, John-David Aubert, Vanessa Banz, Guido Beldi, Christian Benden, Christoph Berger, Isabelle Binet, Pierre-Yves Bochud, Elsa Boëly, Sanda Branca, Heiner Bucher, Thierry Carell, Emmanuelle Catana, Yves Chalandon, Sabina de Geest, Olivier de Rougemont, Michael Dickenmann, Michel Duchosal, Laure Elkrief, Thomas Fehr, Sylvie Ferrari-Lacraz, Christian Garzoni, Paola Gasche Soccal, Christophe Gaudet, Emiliano Giostra, Déla Golshayan, Karine Hadaya, Jörg Halter, Dominik Heim, Christoph Hess, Sven Hillinger, Hans H. Hirsch, Günther Hofbauer, Uyen Huynh-Do, Franz Immer, Richard Klaghofer, Michael Koller (Head of the data center), Bettina Laesser, Roger Lehmann, Christian Lovis, Pietro Majno; Oriol Manuel, Hans-Peter Marti, Pierre Yves Martin, Pascal Meylan, (Head, Biological samples management group), Paul Mohacsi, Philippe Morel, Ulrike Mueller, Nicolas J Mueller (Chairman Scientific Committee), Helen Mueller-McKenna (Head of local data management), Antonia Müller, Thomas Müller, Beat Müllhaupt, Manuel Pascual (Executive office), Jakob Passweg, Klara Posfay-Barbe, Juliane Rick, Eddy Roosnek, Anne Rosselet, Silvia Rothlin, Frank Ruschitzka, Urs Schanz, Stefan Schaub, Aurelia Schnyder, Christian Seiler, Jan Sprachta; Susanne Stampf, Jürg Steiger (Head, Executive Office), Guido Stirnimann, Christian Toso, Christian Van Delden (Executive office), Jean-Pierre Venetz, Jean Villard, Madeleine Wick (STCS coordinator), Markus Wilhelm, Patrick Yerly.
Notes
This study has been conducted in the framework of the Swiss Transplant Cohort Study, supported by the Swiss National Science Foundation and the Swiss University Hospitals (G15) and transplant centers. Further funding was received through the Olga Mayenfisch Foundation, the Hartmann-Müller Foundation for Medical Research and the Lunge Zürich.
The authors do not have any conflict of interests to declare.
References
1 Singer LG , Chowdhury NA , Faughnan ME , Granton J , Keshavjee S , Marras TK , et al. Effects of Recipient Age and Diagnosis on Health-related Quality-of-Life Benefit of Lung Transplantation. Am J Respir Crit Care Med. 2015;192(8):965–73 .https://doi.org/10.1164/rccm.201501-0126OC
2 Seiler A , Klaghofer R , Ture M , Komossa K , Martin-Soelch C , Jenewein J . A systematic review of health-related quality of life and psychological outcomes after lung transplantation. J Heart Lung Transplant. 2016;35(2):195–202.
3 Thabut G , Mal H . Outcomes after lung transplantation. J Thorac Dis. 2017;9(8):2684–91 .https://doi.org/10.21037/jtd.2017.07.85
4 Inci I , Schuurmans MM , Boehler A , Weder W . Zurich University Hospital lung transplantation programme: update 2012. Swiss Med Wkly. 2013;143:w13836 .https://doi.org/10.4414/smw.2013.13836
5Swiss Transplant Cohort Study (STCS). Swiss Transplant Cohort Study Report, May 2008 - December 2016. p. 27. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwjyiqup8rjeAhUszoUKHdTIDL4QFjAAegQICRAC&url=http%3A%2F%2Fwww.stcs.ch%2Finternal%2Freports%2Fstcs_annual_report_june_2017.pdf&usg=AOvVaw0w4aFfWAt_xiPP0Fjw5nbx.
6 Singer JP , Katz PP , Soong A , Shrestha P , Huang D , Ho J , et al. Effect of Lung Transplantation on Health-Related Quality of Life in the Era of the Lung Allocation Score: A U.S. Prospective Cohort Study. Am J Transplant. 2017;17(5):1334–45 .https://doi.org/10.1111/ajt.14081
7 Smith PJ , Blumenthal JA , Snyder LD , Mathew JP , Durheim MT , Hoffman BM , et al. Depressive symptoms and early mortality following lung transplantation: A pilot study. Clin Transplant. 2017;31(2):e12874 .https://doi.org/10.1111/ctr.12874
8 Baranyi A , Krauseneck T , Rothenhäusler HB . Overall mental distress and health-related quality of life after solid-organ transplantation: results from a retrospective follow-up study. Health Qual Life Outcomes. 2013;11(1):15 .https://doi.org/10.1186/1477-7525-11-15
9 Singer JP , Singer LG . Quality of life in lung transplantation. Semin Respir Crit Care Med. 2013;34(3):421–30 .https://doi.org/10.1055/s-0033-1348470
10 Kugler C , Fischer S , Gottlieb J , Welte T , Simon A , Haverich A , et al. Health-related quality of life in two hundred-eighty lung transplant recipients. J Heart Lung Transplant. 2005;24(12):2262–8 .https://doi.org/10.1016/j.healun.2005.07.005
11 Santana MJ , Feeny D , Ghosh S , Lien DC . Patient-reported outcome 2 years after lung transplantation: does the underlying diagnosis matter? Patient Relat Outcome Meas. 2012;3:79–84 .https://doi.org/10.2147/PROM.S32399
12 Vermuelen KM , van der Bij W , Erasmus ME , TenVergert EM . Long-term health-related quality of life after lung transplantation: different predictors for different dimensions. J Heart Lung Transplant. 2007;26(2):188–93 .https://doi.org/10.1016/j.healun.2006.11.006
13 Rodrigue JR , Baz MA , Kanasky WF, Jr , MacNaughton KL . Does lung transplantation improve health-related quality of life? The University of Florida experience. J Heart Lung Transplant. 2005;24(6):755–63 .https://doi.org/10.1016/j.healun.2004.04.012
14 Myaskovsky L , Dew MA , McNulty ML , Switzer GE , DiMartini AF , Kormos RL , et al. Trajectories of change in quality of life in 12-month survivors of lung or heart transplant. Am J Transplant. 2006;6(8):1939–47 .https://doi.org/10.1111/j.1600-6143.2006.01395.x
15 Kugler C , Strueber M , Tegtbur U , Niedermeyer J , Haverich A . Quality of life 1 year after lung transplantation. Prog Transplant. 2004;14(4):331–6 .https://doi.org/10.1177/152692480401400408
16 Ricotti S , Martinelli V , Caspani P , Monteleone S , Petrucci L , Dalla Toffola E , et al. Changes in quality of life and functional capacity after lung transplantation: A single-center experience. Monaldi Arch Chest Dis. 2017;87(3):831 .https://doi.org/10.4081/monaldi.2017.831
17 Rutherford RM , Fisher AJ , Hilton C , Forty J , Hasan A , Gould FK , et al. Functional status and quality of life in patients surviving 10 years after lung transplantation. Am J Transplant. 2005;5(5):1099–104 .https://doi.org/10.1111/j.1600-6143.2004.00803.x
18 Seiler A , Jenewein J , Martin-Soelch C , Goetzmann L , Inci I , Weder W , et al. Post-transplant outcome-clusters of psychological distress and health-related quality of life in lung transplant recipients. Swiss Med Wkly. 2015;145:w14236. doi:.https://doi.org/10.4414/smw.2015.14236
19Statement STROBE. Strengthening the reporting of observational studies in epidemiology. 2009, Institute of Social and Preventive Medicine (ISPM): Bern.
20 Hinz A , Klaiberg A , Brähler E , König HH . [The Quality of Life Questionnaire EQ-5D: modelling and norm values for the general population]. Psychother Psychosom Med Psychol. 2006;56(2):42–8 .https://doi.org/10.1055/s-2005-867061
21Klaghofer R, Braehler E. Construction and validation of a shor form of the SCL-90-R. 2001: Zeitschrift für Klinische Psychologie, Psychiatrie und Psychotherapie. p. 115-124.
22Franke GH. SCL-90-R. Die Symtpomcheckliste von Derogatis - Deutsche Version. 1995, Beltz Test, Göttingen.
23 De Geest S , Burkhalter H , Berben L , Bogert LJ , Denhaerynck K , Glass TR , et al.; Psychosocial Interest Group, Swiss Transplant Cohort Study. The Swiss Transplant Cohort Study’s framework for assessing lifelong psychosocial factors in solid-organ transplants. Prog Transplant. 2013;23(3):235–46 .https://doi.org/10.7182/pit2013250
24 Koller MT , van Delden C , Müller NJ , Baumann P , Lovis C , Marti HP , et al. Design and methodology of the Swiss Transplant Cohort Study (STCS): a comprehensive prospective nationwide long-term follow-up cohort. Eur J Epidemiol. 2013;28(4):347–55 .https://doi.org/10.1007/s10654-012-9754-y
25 Vaquero Barrios JM , Redel Montero J , Santos Luna F . Comorbidities impacting on prognosis after lung transplant. Arch Bronconeumol. 2014;50(1):25–33 .https://doi.org/10.1016/j.arbr.2014.01.001
26 Kugler C , Tegtbur U , Gottlieb J , Bara C , Malehsa D , Dierich M , et al. Health-related quality of life in long-term survivors after heart and lung transplantation: a prospective cohort study. Transplantation. 2010;90(4):451–7 .https://doi.org/10.1097/TP.0b013e3181e72863
27 Finlen Copeland CA , Vock DM , Pieper K , Mark DB , Palmer SM . Impact of lung transplantation on recipient quality of life: a serial, prospective, multicenter analysis through the first posttransplant year. Chest. 2013;143(3):744–50 .https://doi.org/10.1378/chest.12-0971
28 Shahabeddin Parizi A , Krabbe PFM , Verschuuren EAM , Hoek RAS , Kwakkel-van Erp JM , Erasmus ME , et al. Patient-reported health outcomes in long-term lung transplantation survivors: A prospective cohort study. Am J Transplant. 2018;18(3):684–95 .https://doi.org/10.1111/ajt.14492
29 Trudzinski FC , Kaestner F , Schäfers HJ , Fähndrich S , Seiler F , Böhmer P , et al. Outcome of Patients with Interstitial Lung Disease Treated with Extracorporeal Membrane Oxygenation for Acute Respiratory Failure. Am J Respir Crit Care Med. 2016;193(5):527–33 .https://doi.org/10.1164/rccm.201508-1701OC
30 Kumar A , Kapnadak SG , Girgis RE , Raghu G . Lung transplantation in idiopathic pulmonary fibrosis. Expert Rev Respir Med. 2018;12(5):375–85 .https://doi.org/10.1080/17476348.2018.1462704
31 Smith PJ , Blumenthal JA , Trulock EP , Freedland KE , Carney RM , Davis RD , et al. Psychosocial Predictors of Mortality Following Lung Transplantation. Am J Transplant. 2016;16(1):271–7 .https://doi.org/10.1111/ajt.13447
32 Suhling H , Knuth C , Haverich A , Lingner H , Welte T , Gottlieb J . Employment after lung transplantation--a single-center cross-sectional study. Dtsch Arztebl Int. 2015;112(13):213–9.
33 Nau M , Shrider EA , Tobias JD , Hayes D, Jr , Tumin D . High local unemployment rates limit work after lung transplantation. J Heart Lung Transplant. 2016;35(10):1212–9 .https://doi.org/10.1016/j.healun.2016.05.002
34 Adegunsoye A , Strek ME , Garrity E , Guzy R , Bag R . Comprehensive Care of the Lung Transplant Patient. Chest. 2017;152(1):150–64 .https://doi.org/10.1016/j.chest.2016.10.001
Notes
These authors contributed equally to this work.
This study has been conducted in the framework of the Swiss Transplant Cohort Study, supported by the Swiss National Science Foundation and the Swiss University Hospitals (G15) and transplant centers. Further funding was received through the Olga Mayenfisch Foundation, the Hartmann-Müller Foundation for Medical Research and the Lunge Zürich.
The authors do not have any conflict of interests to declare.