Risk stratification in coronary artery disease: a patient-tailored approach over the ischaemic cascade
DOI: https://doi.org/10.4414/smw.2019.20014
Cardiology Department, University Hospital Basel, University of Basel, Switzerland
Summary
Patient tailored diagnosis and risk stratification in patients with suspected or known coronary artery disease (CAD) are pivotal. At present, cardiac imaging modalities provide the possibility to evaluate the whole ischaemic cascade noninvasively. In asymptomatic patients, the evaluation of the calcium score may be beneficial and also guide the individual preventive strategy. Furthermore, the calcium score provides complimentary information to the information as assessed by functional testing. Coronary computed tomographic angiography (CCTA) is an excellent tool to exclude CAD, having a negative predictive value of 97–99%. Comparably, a normal functional cardiac imaging test (e.g., positron emission tomography (PET); myocardial perfusion SPECT (MPS); cardiac magnetic resonance (CMR); and stress echocardiography) is consistent with a good prognosis and in general an annual cardiac death rate <1%. If a patient has an abnormal imaging test, it is important for risk stratification to evaluate the severity and extent of the abnormality (e.g., the extent and severity of the perfusion defect, or of the wall motion abnormality, which is consistent with the extent of myocardial scar and ischaemia). The patient’s symptoms and the extent of ischaemia, scar and decrease of ejection fraction will guide the strategy, either to an optimal medical therapy or to a further invasive evaluation. If more than 10% of the myocardium are ischaemic, it is very likely that patients will benefit from revascularisation.
The current guidelines leave a lot of room as to which test to choose for noninvasive CAD evaluation and risk stratification. The selection of the particular modality is, in part, led by the pretest probability of CAD and local availability, expertise and preference. However, whenever possible, an imaging-based test rather than a “stand-alone” stress ECG should be used. Cardiac imaging has higher sensitivities and specificities to diagnose or exclude CAD compared with stress testing alone. Using a hybrid approach, integrating complimentary information to that given by functional testing (e.g., PET/CT) provides the highest noninvasive diagnostic and prognostic accuracies in CAD evaluation available so far.
Introduction
A patient tailored risk stratification approach in coronary artery disease (CAD) may help in our preventive efforts, and certainly in the decision-making process, to come up with an individual diagnostic and therapeutic plan for our patients. At present, modern imaging modalities allow us to assess the whole continuum of the ischaemic cascade. This review aims to summarise the potential of noninvasive cardiac imaging in the risk stratification process of patients with suspected and known CAD. However, in daily practice, every evaluation has to start with the assessment of the probability that CAD is present in a particular patient (pretest probability of CAD or ischaemia [1, 2]). This initial assessment is generally based on patients’ sex, age and symptoms and results in a low, intermediate or high pretest probability of CAD [3]. Of course, the overall risk estimates can be refined by applying frequently used scores which also incorporate cardiovascular risk factors (e.g., the AGLA score for Switzerland). In addition, new algorithms may provide far more powerful evaluation of the pretest probability (also, in part, using artificial intelligence approaches) [2, 4]. Patients with a low probability of disease (<15%) should not undergo further coronary testing. However, in specific cases (e.g., a broad cardiovascular risk profile and/or high risk as assessed by a prognostic score) patients may benefit from further risk factor modification (potentially guided by the assessment of coronary calcification). Patients with an intermediate probability (15–85%) of CAD should undergo further noninvasive testing. In patients with a high probability of disease (>85%), noninvasive testing does not add much with respect to CAD diagnosis but may help to provide a better idea of the individual patient’s risk [3]. Patients with a high pretest probability of CAD therefore may directly undergo invasive coronary angiography, also with the possibility of treatment in the same procedure.
The ischaemic cascade
The ischaemic cascade starts with changes in the coronary artery (i.e., noncalcified and calcified changes of the vessel wall). As long as the luminal narrowing of the artery does not exceed 50%, no limitation of flow is expected. When the narrowing of the artery exceeds 50%, reduced myocardial blood flow with decreased myocardial perfusion can occur. The decrease of myocardial perfusion results in diastolic and systolic dysfunction of the left ventricle. Furthermore, after this stage, ECG changes and chest pain can often be observed. This process is depicted in figure 1. Patients without a flow-limiting coronary pathology generally are asymptomatic and may benefit from risk stratification with the goal to get optimised preventive and, in some cases, medical therapy. In symptomatic patients, it is pivotal to evaluate if the symptoms are due to CAD and if so, if patients may be treated with optimal medical therapy or invasive evaluation and revascularisation.
Coronary artery calcification in asymptomatic individuals and stable CAD patients
Calcium is a common component of atherosclerotic plaques and is not present in the normal, “healthy” vessel wall. Coronary calcifications are quickly and easily evaluated by computed tomography (CT) with low radiation exposure and no need for contrast agents [6]. The Agatston score is the most widely used calcium score for the reporting of calcium burden in coronary arteries and is used in most diagnostic and prognostic studies [7]. The presence of calcium and its extent is consistent with the individual, integrated lifetime effect of all cardiovascular risk factors on the coronary arteries. Measures of coronary artery calcium are related to survival and can be used to estimate an individual’s “biological age” [8]. Coronary artery calcification, carotid intima-media thickness, ankle-brachial index, brachial flow-mediated dilation and high-sensitivity C-reactive protein (CRP) have been reported to improve on the Framingham risk score for the prediction of CAD. However, the calcium score provides superior discrimination and risk reclassification compared with the aforementioned other risk markers [9]. The absence of coronary calcium (an Agatston score of zero) in asymptomatic patients is associated with an excellent prognosis. The extent of coronary calcifications has accurately predicted the 15-year overall mortality in a large cohort (n = 9715) of asymptomatic patients, with an absolute mortality rate of 3% in individuals with zero calcium [10]. Figure 2 summarises the overall 15-year mortality rates in relation to an increasing calcium score (adapted from [10]).
Zero calcium is also consistent with the absence of noncalcified plaques and relevant coronary stenosis in more than 87% and 99% of patients, respectively [11]. Among 2730 patients with stable CAD symptoms, a zero calcium score was seen in 1426 (52.2%), of whom 17 (1.2%) had moderate stenosis and 7 (0.5%) had severe stenosis on CCTA. The negative predictive value of a zero calcium score for excluding severe stenosis (on CCTA) was 99.5% [12]. Absent coronary calcium therefore has an excellent negative predictive value for CAD and is a very important cornerstone of CAD risk stratification, either as a standalone result or in combination with functional testing.
Simplified calcium scores of 0, 1 to 100, 101 to 400, and greater than 400 represent no, mild, moderate, and severe coronary calcification, respectively [13]. However, more accurately, coronary calcifications should be interpreted taking into account the age and gender of the individual or patient (e.g., using percentiles) [13, 14]. In patients with a calcium score >400, an abnormal myocardial perfusion SPECT is likely in 31–46% of patients [15–17]. Interestingly, even patients with a calcium score >1,000 may have a normal perfusion (no ischaemia) as assessed by myocardial perfusion SPECT. It has been reported that 51–80% of these patients still do not have a perfusion defect (no ischaemia) [18]. In these patients, invasive coronary angiography still should be considered. Other results (e.g., ischaemic ECG findings during stress testing, transient ischaemic dilation or persistent angina) may be a sign of the presence of balanced ischaemia.
The calcium score may also help to decide who could benefit from statin therapy, since significant heterogeneity exists among those eligible for statins according to the cholesterol management guidelines [19]. Approximately one-half of potential candidates have no coronary calcium in their coronary arteries, and, as a result, they have a much lower observed 10-year risk and a higher estimated number needed to treat (NNT) to prevent an event. Clinicians should consider the role of coronary calcium testing in shared decision-making processes to facilitate informed choices for flexible treatment goals [19]. Since little is known about the temporal dynamics of the calcium score, the value of repeat calcium score testing is still unclear.
The symptomatic patient
In a study by Patel et al., only 38% of patients without prior CAD who underwent elective coronary angiography had obstructive CAD [20]. Improved strategies for risk stratification are needed to increase the diagnostic yield of coronary angiography in daily clinical practice. Noninvasive risk stratification could help to reach this goal. In a widely available clinical setting, noninvasive nuclear cardiology techniques (e.g., myocardial perfusion SPECT) had the ability to substantially increase the diagnostic yield of elective coronary angiography. Furthermore, myocardial perfusion SPECT provided incremental value over risk factors and symptoms in predicting CAD findings, thus emphasising its importance in the decision-making process that lead to the use of coronary angiography [21].
There is a vigorous debate as to whether anatomic or functional testing should be used in the assessment of CAD. However, even after the publication of the PROspective Multicentre Imaging Study for Evaluation of Chest Pain (PROMISE) trial [22], there is still no agreement if anatomical or functional testing provides better results when it comes to the evaluation of patients with suspected CAD. In patients with known CAD, functional rather than anatomical testing should be used in the evaluation and risk stratification process, especially taking into account that patients with stents and prior coronary artery bypass grafts are not optimal candidates to undergo noninvasive anatomic testing by CCTA.
The objective of PROMISE was to compare the outcomes in 10,003 patients who presented with new symptoms suggestive of CAD that required further evaluation and who were randomly assigned to an initial strategy of anatomical testing with the use of CCTA or to functional testing. Over a median follow-up of two years, there were similar outcomes in the CCTA and functional testing groups of patients [22]. More patients in the CCTA than in the functional group underwent coronary angiography early after testing (12.2 vs 8.1%) [22].
The current European Society of Cardiology (ESC) guidelines [3] leave a lot of room as to which test to choose if a patient needs noninvasive CAD evaluation (ergometry/treadmill stress ECG without imaging; myocardial perfusion SPECT; positron emission tomography (PET); stress cardiac magnetic resonance; stress echocardiography; or CCTA). The selection process is, in part, led by the pretest probability of CAD, comorbidities of the patient (e.g., kidney dysfunction, devices such as pacemakers and internal cardioverter defibrillators, contrast agent allergy, arrhythmia, etc.) and local availability, expertise and preference. However, whenever possible, an imaging-based test rather than a “stand-alone” stress test should be used [3] because of the much higher sensitivities and specificities integrating cardiac imaging results. Diagnostic sensitivities and specificities for the available noninvasive tests are summarised in table 1. The ESC discourages the use of stress ECG as the primary tool to evaluate patients with stable CAD, especially in the higher intermediate pretest probability range. Importantly, pharmacological stress testing can only be used in combination with cardiac imaging and not with stand-alone stress ECG.
Table 1 Diagnostic accuracies of noninvasive tests (based on [23–37]).
|
Diagnosis of coronary artery disease
|
|
Sensitivity (%)
|
Specificity (%)
|
| Stress ECG |
45–50 |
85–90 |
| Exercise stress echocardiography |
80–85 |
80–88 |
| Exercise stress SPECT |
73–92 |
63–87 |
| Dobutamine stress echocardiography |
79–83 |
82–86 |
| Dobutamine stress CMR |
79–88 |
81–91 |
| Vasodilator stress SPECT |
90–91 |
75–84 |
| Vasodilator stress CMR |
67–94 |
61–85 |
| Coronary computed tomographic angiography |
95–99 |
64–83 |
| Vasodilator stress PET |
81–97 |
74–91 |
Anatomical testing
The traditional ‘‘gold standard’’ to diagnose or exclude CAD still is invasive coronary angiography, using the percentage of diameter stenosis cut-off values of 50% or 70% to define significant obstructive CAD. Over the last two decades, CCTA has gained significance with respect to diagnosing or excluding CAD (also incorporating information about coronary calcification). At least three prospective multicentre trials have evaluated the diagnostic value of CCTA, demonstrating that CCTA has a 94% to 99% sensitivity and a 64% to 83% specificity for the identification of coronary stenosis [23, 38, 39]. The 97% to 99% negative predictive value of CCTA means that a CT-based approach can effectively and safely rule out anatomic CAD [23, 38, 39]. Whereas stress testing is very effective for predicting risk, it is unable to exclude CAD, including severe CAD.
The prognostic value of CCTA has been documented in large patient populations. A systematic review of 18 studies that evaluated 9592 patients demonstrated an annualised event rate for obstructive (i.e., any vessel with 50% luminal stenosis) versus normal CCTA of 8.8% versus 0.17% per year for major adverse cardiac events (p <0.05) and 3.2% versus 0.15% for death or myocardial infarction (MI) (p <0.05) [40].
In their 2016 guidelines, the UK National Institute for Health and Care Excellence (NICE) expanded the role of CCTA to be the first-line investigation for all patients with typical or atypical chest pain [41] but without prior CAD. This could be an interesting approach, especially by using a stepwise approach of calcium score evaluation and then taking the decision if CCTA should be carried out or not (this consideration is not part of the NICE protocol). Whether to proceed with a CCTA in the presence of extensive coronary calcification remains controversial. Accordingly, some centres do not proceed with a CCTA in the presence of a coronary calcium score (Agatston) that exceeds 400–1000 [3, 42].
Patients undergoing CCTA experience radiation exposure. However, the radiation dose has decreased impressively over the last few years with effective doses of around 1 mSv [39, 43]. To obtain good quality scans (CCTA), patients should be in sinus rhythm with a heart rate <65 beats per minute and should have good breath holding and collaboration capabilities. In patients with renal failure, CCTA may not be an appropriate choice because of the contrast medium that is needed.
Functional (stress) testing
In contrast to anatomic testing in which coronary arteries, coronary calcifications and coronary artery stenoses are directly visualised, functional imaging is focused on the consequences of potentially impaired blood flow to the myocardium. Variables that are used to evaluate coronary blood supply to the myocardium are coronary flow, coronary flow reserve, qualitative and quantitative myocardial perfusion, and stress induced wall motion abnormalities (figure 1). Again, functional tests cannot exclude CAD but they can exclude hemodynamically relevant CAD.
A vast number of papers have been published with respect to diagnosis and prognosis of stable CAD. Prognosis is primarily related to the degree of left ventricular dysfunction and the extent and severity of myocardial ischaemia [44].
Overall, methods evaluating perfusion (e.g., PET, myocardial perfusion SPECT, vasodilator perfusion CMR) are more sensitive to detect ischaemia than methods evaluating wall motion abnormalities during stress (stress echocardiography, dobutamine stress CMR) [3]. On the other hand, methods evaluating wall motion abnormalities to detect ischaemia are more specific than methods evaluating perfusion during stress. Information about diagnostic accuracies is summarised in table 1 [23–37].
A normal imaging stress test or functional test is consistent with a good prognosis and thus a low event rate, irrespective of anatomic information. However, the warranty period of a functional test is shorter than for a normal anatomic test, at 1–2 years [45] and 5–15 years [10, 46], respectively. Knowing these warranty periods is important in the context of serial testing.
The low event rate and good prognosis holds true for all imaging modalities when the results are normal, even though the data are most robust for nuclear cardiology. In a meta-analysis of nearly 30,000 patients, it has been demonstrated that the annualised cardiac death rate of patients with a normal myocardial perfusion SPECT result was 0.6% per year with a survival rate of 99.5% (95% CI 99.3–99.7%), irrespective of the isotope that was used [47]. For CMR, a systematic review and meta-analysis evaluating 11,636 patients showed that stress CMR provided prognostic stratification of patients with suspected or known CAD: patients with a stress CMR without evidence of ischaemia had an annual cardiac death or myocardial infarction rate <1%. In contrast, patients with ischaemia on stress CMR had a 5% annual cardiac death or myocardial infarction rate [48].
If a test result is abnormal, it is essential to consider the extent and severity of scar and inducible ischaemia. More than 20 years ago, a 20-segment model [49], followed a few years later by a 17-segment model, were introduced in the imaging field [50]. The standardised segmentation helped to better describe, report and assess the extent and severity of perfusion abnormalities, also resulting in different risk categories (the endpoints of the risk assessment process which were most widely used in this context were mortality, cardiac death or myocardial infarction, and (early) revascularisation) [49]. These 20- and 17-segment models have been extensively used mainly in nuclear cardiology but later also in CMR to better evaluate the prognosis of various patient populations (e.g., patients without prior CAD [51] (also with silent CAD [52]), patients with known CAD, male and female patients [53, 54], elderly patients [53], obese patients [55], revascularised patients [56, 57], diabetic patients [58–61], and patients with end-stage kidney disease). The incremental prognostic value of using the perfusion information of nuclear cardiology has been demonstrated in these patient groups.
CMR provides the unique opportunity of tissue characterisation which is extremely useful when it comes to the assessment of the myocardium and the question of prior myocardial infarction and myocardial viability, especially in patients with decreased left ventricular ejection fraction.
Furthermore, based on late gadolinium patterns, it is possible to differentiate myocardial infarction scars from other aetiologies of fibrosis (e.g., myocarditis) [62]. Patients (with suspected or known CAD) with late gadolinium enhancement (i.e., evidence of fibrosis in the myocardium) had significantly worse outcomes than patients without late gadolinium enhancement (4.6% versus 1.4% annual event rate of cardiac death or myocardial infarction; p <0.03) [48]. In patients with diabetes but no evidence of myocardial infarction, the prevalence of silent myocardial infarction as detected by CMR is high (28%). The presence of late gadolinium enhancement is associated with a significant risk of adverse cardiac events, including death [63].
Stress echocardiography is unique in its near universal availability. Combined with a physical stress test, it can be carried out with minimal equipment and no radiation exposure. Even though the use of contrast may clarify echocardiographic images, image quality may vary considerably and is highly operator dependent [64].
Hybrid imaging
Hybrid imaging is becoming increasingly available, most often through the combination of computed tomography and nuclear cardiology imaging (PET or SPECT), and thus offers information about anatomy and function. Providing these results, PET/CT hybrid imaging is close to a “one-stop shop” approach when it comes to CAD evaluation. Hybrid imaging using CCTA and PET is only available in tertiary centres because considerable logistic and technical issues have to be taken into account. In contrast to calcium score evaluation which is fairly simple, CCTA acquisition, interpretation and integration with functional information needs far more expertise.
The future outlook for a “one stop-shop” using computed tomography techniques is promising given the complimentary information of CCTA, the functional information of fractional flow reserve, and stress perfusion imaging by CT. We still need to learn how to more effectively incorporate all of the pieces of information gained into the diagnostic and decision-making process (e.g., PET/CT provides information about coronary calcification, coronary artery stenosis, qualitative and quantitative myocardial perfusion, left ventricular function at rest and during stress in less than 45 minutes and with acceptable low radiation exposure); see the example in figure 3. Now that rubidium is available for PET imaging, a cyclotron is no longer necessary to perform cardiac PET because rubidium is a generator-based isotope [65].
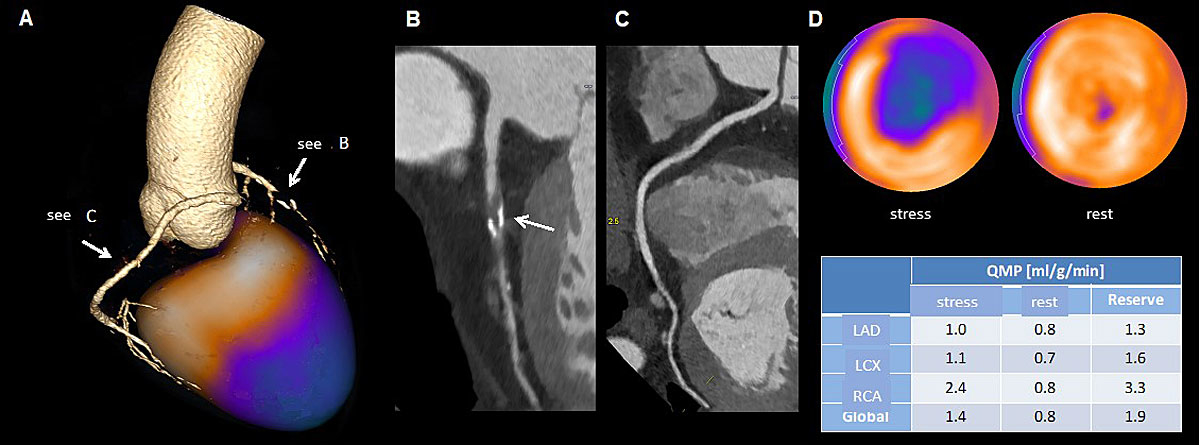
Figure 3 Example of hybrid imaging using coronary computed tomographic angiography anatomical and rubidium positron emission tomography perfusion information (a). In (b) and (c), the anatomic information is depicted, demonstrating a high grade left anterior descending artery (LAD) stenosis with non-calcified and calcified parts (b) and demonstrating the right coronary artery (RCA) (c). Figure 3d demonstrates the perfusion defect during stress (ischaemia), the normal perfusion at rest and the decreased flow rates, especially in the LAD territory.
A possible evidence-based patient tailored imaging approach using the opportunity of PET/CT is suggested in figure 4. Patients with intermediate pretest probability of CAD/ischaemia could be evaluated according to the suggested pathway if no prior CAD is known. Patients with known CAD or a high calcium score (>400) could undergo PET perfusion imaging directly. Patients with a calcium score between 100 and 400 may benefit from the complimentary information regarding coronary stenoses and the evaluation of their corresponding hemodynamic relevance. The suggested pathway is partly based on the NICE approach where CT is used as the first-line diagnostic modality. Combining the high negative predictive value of anatomic information by CCTA [23, 38, 39] and the most sophisticated functional information by PET [65, 66] that is published in the literature provides an overall assessment of the most important components of CAD. However, the functional testing part could also be carried out by other imaging modalities (e.g., standalone PET, myocardial perfusion SPECT, CMR or stress echocardiography), with the drawback that anatomic and functional assessment cannot be accomplished in one session with the same machine and with less of the aforementioned diagnostic elements provided by PET/CT.
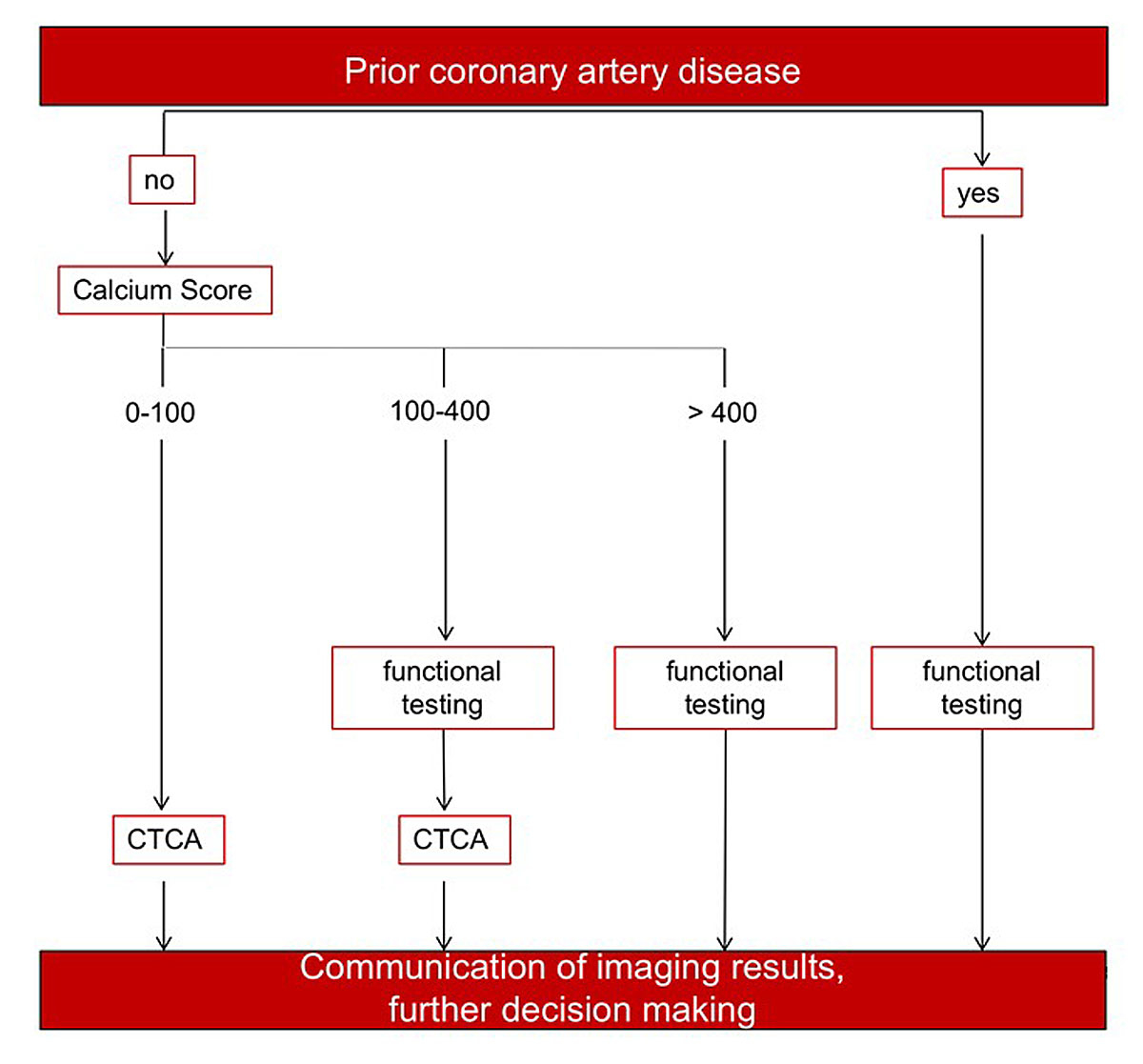
Figure 4 Suggestion of an efficient patient tailored approach for coronary artery disease (CAD) risk stratification using hybrid techniques. In patients with an intermediate pretest probability of disease, the CAD evaluation could be tailored according to this flowchart.
In a hybrid study using myocardial perfusion SPECT and CCTA, early revascularisation of CAD patients with matched findings (a matched hybrid imaging finding was defined as a reversible perfusion defect on SPECT supplied by a coronary artery with CAD on CCTA) was independently associated with an improved outcome when compared with medical therapy alone. In contrast, patients with unmatched findings did not benefit from early revascularisation, irrespective of the presence or absence of high-risk CAD [67, 68].
Cost effectiveness
There is strong evidence that an initial noninvasive approach followed by a selective invasive approach in patients with documented ischaemia is as safe as a directly invasive approach. Furthermore, it is cost effective [69], in part because patients undergoing direct invasive evaluation more often undergo revascularisation.
From CAD diagnosis and risk stratification to decision making and therapy
In contrast to acute CAD where reperfusion/revascularisation is the state-of-the-art therapy, the question of revascularisation in stable CAD patients is still under debate. Overall, the Clinical Outcomes Utilising Revascularisation and Aggressive Drug Evaluation (COURAGE) trial demonstrated that percutaneous coronary intervention did not reduce the risk of death, myocardial infarction or other major cardiovascular events when added to optimal medical therapy in stable CAD patients [70]. However, from a substudy of selected COURAGE patients who underwent serial myocardial perfusion SPECT imaging, adding percutaneous coronary intervention to optimal medical therapy resulted in a greater reduction in inducible ischaemia compared with optimal medical treatment alone, and the benefit was greatest among patients with more severe baseline ischaemia [71]. The exploratory analysis of clinical outcomes revealed that regardless of treatment assignment, the magnitude of residual ischaemia on follow-up myocardial perfusion SPECT was proportionate to the risk for death or MI, and a 5% reduction in ischaemia was associated with a significant reduction in risk [71].
Very similar results were published in relation to diabetic patients in a subgroup analysis of The Bypass Angioplasty Revascularisation Investigation 2 Diabetes (BARI 2D) trial [72, 73]. BARI 2D trial results revealed that revascularisation was associated with reductions in myocardial ischaemia when compared to medical therapy. A one-year post-therapeutic intervention myocardial perfusion SPECT provided important information regarding an intermediate outcome of the extent of residual ischaemia in stable CAD patients with diabetes [73].
These results of the COURAGE and BARI-2D substudies corroborated the findings of an observational landmark study in which revascularisation reduced the absolute and relative risk of cardiac death more than medical therapy in patients with moderate to large amounts of inducible ischaemia by stress myocardial perfusion SPECT [74]. In patients with 10% myocardium ischaemic, revascularisation was associated with a 50% risk-adjusted reduction in cardiac death [74] when compared to medical therapy alone. These findings underscore the importance of not only knowing if a patient suffers from CAD, but also of knowing the extent and severity of ischaemia, which can be evaluated by cardiac imaging.
Conclusions
At present, cardiac imaging provides impressive tools to evaluate the whole continuum of the ischaemic cascade. The diagnostic and prognostic yield of cardiac imaging techniques is high. Using a patient tailored approach, most diagnostic and prognostic questions regarding stable CAD can be answered by noninvasive imaging techniques, even if a patient would instead benefit from revascularisation or optimal medical therapy. Integrating anatomic and functional information into the decision-making process yields the highest value for our patients. It answers the question as to whether a patient suffers from subclinical coronary calcifications or coronary artery stenosis, and if there is ischaemia due to hemodynamically relevant CAD.
References
1
Diamond
GA
,
Forrester
JS
. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300(24):1350–8. doi:.https://doi.org/10.1056/NEJM197906143002402
2
Genders
TS
,
Steyerberg
EW
,
Hunink
MG
,
Nieman
K
,
Galema
TW
,
Mollet
NR
, et al.
Prediction model to estimate presence of coronary artery disease: retrospective pooled analysis of existing cohorts. BMJ. 2012;344(jun12 1):e3485. doi:.https://doi.org/10.1136/bmj.e3485
3
Montalescot
G
,
Sechtem
U
,
Achenbach
S
,
Andreotti
F
,
Arden
C
,
Budaj
A
, et al., Document Reviewers. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. doi:.https://doi.org/10.1093/eurheartj/eht296
4
Zellweger
MJ
,
Tsirkin
A
,
Vasilchenko
V
,
Failer
M
,
Dressel
A
,
Kleber
ME
, et al.
A new non-invasive diagnostic tool in coronary artery disease: artificial intelligence as an essential element of predictive, preventive, and personalized medicine. EPMA J. 2018;9(3):235–47. doi:.https://doi.org/10.1007/s13167-018-0142-x
5
Stary
HC
,
Chandler
AB
,
Dinsmore
RE
,
Fuster
V
,
Glagov
S
,
Insull
W, Jr
, et al.
A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92(5):1355–74. doi:.https://doi.org/10.1161/01.CIR.92.5.1355
6
Gräni
C
,
Vontobel
J
,
Benz
DC
,
Bacanovic
S
,
Giannopoulos
AA
,
Messerli
M
, et al.
Ultra-low-dose coronary artery calcium scoring using novel scoring thresholds for low tube voltage protocols-a pilot study. Eur Heart J Cardiovasc Imaging. 2018;19(12):1362–71. doi:.https://doi.org/10.1093/ehjci/jey019
7
Agatston
AS
,
Janowitz
WR
,
Hildner
FJ
,
Zusmer
NR
,
Viamonte
M, Jr
,
Detrano
R
. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. doi:.https://doi.org/10.1016/0735-1097(90)90282-T
8
Shaw
LJ
,
Raggi
P
,
Berman
DS
,
Callister
TQ
. Coronary artery calcium as a measure of biologic age. Atherosclerosis. 2006;188(1):112–9. doi:.https://doi.org/10.1016/j.atherosclerosis.2005.10.010
9
Yeboah
J
,
McClelland
RL
,
Polonsky
TS
,
Burke
GL
,
Sibley
CT
,
O’Leary
D
, et al.
Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308(8):788–95. doi:.https://doi.org/10.1001/jama.2012.9624
10
Shaw
LJ
,
Giambrone
AE
,
Blaha
MJ
,
Knapper
JT
,
Berman
DS
,
Bellam
N
, et al.
Long-Term Prognosis After Coronary Artery Calcification Testing in Asymptomatic Patients: A Cohort Study. Ann Intern Med. 2015;163(1):14–21. doi:.https://doi.org/10.7326/M14-0612
11
Iwasaki
K
,
Matsumoto
T
,
Aono
H
,
Furukawa
H
,
Samukawa
M
. Prevalence of non-calcified coronary plaque on 64-slice computed tomography in asymptomatic patients with zero and low coronary artery calcium. Can J Cardiol. 2010;26(7):377–80. doi:.https://doi.org/10.1016/S0828-282X(10)70419-0
12
Mittal
TK
,
Pottle
A
,
Nicol
E
,
Barbir
M
,
Ariff
B
,
Mirsadraee
S
, et al.
Prevalence of obstructive coronary artery disease and prognosis in patients with stable symptoms and a zero-coronary calcium score. Eur Heart J Cardiovasc Imaging. 2017;18(8):922–9. doi:.https://doi.org/10.1093/ehjci/jex037
13
Yerramasu
A
,
Lahiri
A
,
Venuraju
S
,
Dumo
A
,
Lipkin
D
,
Underwood
SR
, et al.
Diagnostic role of coronary calcium scoring in the rapid access chest pain clinic: prospective evaluation of NICE guidance. Eur Heart J Cardiovasc Imaging. 2014;15(8):886–92. doi:.https://doi.org/10.1093/ehjci/jeu011
14
Hoff
JA
,
Chomka
EV
,
Krainik
AJ
,
Daviglus
M
,
Rich
S
,
Kondos
GT
. Age and gender distributions of coronary artery calcium detected by electron beam tomography in 35,246 adults. Am J Cardiol. 2001;87(12):1335–9. doi:.https://doi.org/10.1016/S0002-9149(01)01548-X
15
Engbers
EM
,
Timmer
JR
,
Ottervanger
JP
,
Mouden
M
,
Knollema
S
,
Jager
PL
. Prognostic Value of Coronary Artery Calcium Scoring in Addition to Single-Photon Emission Computed Tomographic Myocardial Perfusion Imaging in Symptomatic Patients. Circ Cardiovasc Imaging. 2016;9(5):e003966. doi:.https://doi.org/10.1161/CIRCIMAGING.115.003966
16
Chang
SM
,
Nabi
F
,
Xu
J
,
Peterson
LE
,
Achari
A
,
Pratt
CM
, et al.
The coronary artery calcium score and stress myocardial perfusion imaging provide independent and complementary prediction of cardiac risk. J Am Coll Cardiol. 2009;54(20):1872–82. doi:.https://doi.org/10.1016/j.jacc.2009.05.071
17
He
ZX
,
Hedrick
TD
,
Pratt
CM
,
Verani
MS
,
Aquino
V
,
Roberts
R
, et al.
Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation. 2000;101(3):244–51. doi:.https://doi.org/10.1161/01.CIR.101.3.244
18
Mouden
M
,
Ottervanger
JP
,
Timmer
JR
,
Reiffers
S
,
Oostdijk
AH
,
Knollema
S
, et al.
Myocardial perfusion imaging in stable symptomatic patients with extensive coronary atherosclerosis. Eur J Nucl Med Mol Imaging. 2014;41(1):136–43. doi:.https://doi.org/10.1007/s00259-013-2539-z
19
Michos
ED
,
Blaha
MJ
,
Blumenthal
RS
. Use of the Coronary Artery Calcium Score in Discussion of Initiation of Statin Therapy in Primary Prevention. Mayo Clin Proc. 2017;92(12):1831–41. doi:.https://doi.org/10.1016/j.mayocp.2017.10.001
20
Patel
MR
,
Peterson
ED
,
Dai
D
,
Brennan
JM
,
Redberg
RF
,
Anderson
HV
, et al.
Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362(10):886–95. doi:.https://doi.org/10.1056/NEJMoa0907272
21
Buechel
RR
,
Kaufmann
BA
,
Tobler
D
,
Wild
D
,
Zellweger
MJ
. Non-invasive nuclear myocardial perfusion imaging improves the diagnostic yield of invasive coronary angiography. Eur Heart J Cardiovasc Imaging. 2015;16(8):842–7. doi:.https://doi.org/10.1093/ehjci/jev095
22
Douglas
PS
,
Hoffmann
U
,
Patel
MR
,
Mark
DB
,
Al-Khalidi
HR
,
Cavanaugh
B
, et al.; PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291–300. doi:.https://doi.org/10.1056/NEJMoa1415516
23
Budoff
MJ
,
Dowe
D
,
Jollis
JG
,
Gitter
M
,
Sutherland
J
,
Halamert
E
, et al.
Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52(21):1724–32. doi:.https://doi.org/10.1016/j.jacc.2008.07.031
24
de Jong
MC
,
Genders
TS
,
van Geuns
RJ
,
Moelker
A
,
Hunink
MG
. Diagnostic performance of stress myocardial perfusion imaging for coronary artery disease: a systematic review and meta-analysis. Eur Radiol. 2012;22(9):1881–95. doi:.https://doi.org/10.1007/s00330-012-2434-1
25
Diamond
GA
,
Kaul
S
. Gone fishing!: on the “real-world” accuracy of computed tomographic coronary angiography: Comment on the “Ontario multidetector computed tomographic coronary angiography study”. Arch Intern Med. 2011;171(11):1029–31. doi:.https://doi.org/10.1001/archinternmed.2011.75
26
Froelicher
VF
,
Lehmann
KG
,
Thomas
R
,
Goldman
S
,
Morrison
D
,
Edson
R
, et al.
The electrocardiographic exercise test in a population with reduced workup bias: diagnostic performance, computerized interpretation, and multivariable prediction. Veterans Affairs Cooperative Study in Health Services #016 (QUEXTA) Study Group. Quantitative Exercise Testing and Angiography. Ann Intern Med. 1998;128(12 Pt 1):965–74. doi:.https://doi.org/10.7326/0003-4819-128-12_Part_1-199806150-00001
27
Gibbons
RJ
,
Abrams
J
,
Chatterjee
K
,
Daley
J
,
Deedwania
PC
,
Douglas
JS
, et al.; American College of Cardiology; American Heart Association Task Force on Practice Guidelines. Committee on the Management of Patients With Chronic Stable Angina. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina). Circulation. 2003;107(1):149–58. doi:.https://doi.org/10.1161/01.CIR.0000047041.66447.29
28
Hamon
M
,
Fau
G
,
Née
G
,
Ehtisham
J
,
Morello
R
,
Hamon
M
. Meta-analysis of the diagnostic performance of stress perfusion cardiovascular magnetic resonance for detection of coronary artery disease. J Cardiovasc Magn Reson. 2010;12(1):29. doi:.https://doi.org/10.1186/1532-429X-12-29
29
Heijenbrok-Kal
MH
,
Fleischmann
KE
,
Hunink
MG
. Stress echocardiography, stress single-photon-emission computed tomography and electron beam computed tomography for the assessment of coronary artery disease: a meta-analysis of diagnostic performance. Am Heart J. 2007;154(3):415–23. doi:.https://doi.org/10.1016/j.ahj.2007.04.061
30
Higgins
JP
,
Williams
G
,
Nagel
JS
,
Higgins
JA
. Left bundle-branch block artifact on single photon emission computed tomography with technetium Tc 99m (Tc-99m) agents: mechanisms and a method to decrease false-positive interpretations. Am Heart J. 2006;152(4):619–26. doi:.https://doi.org/10.1016/j.ahj.2006.06.009
31
Jaarsma
C
,
Leiner
T
,
Bekkers
SC
,
Crijns
HJ
,
Wildberger
JE
,
Nagel
E
, et al.
Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2012;59(19):1719–28. doi:.https://doi.org/10.1016/j.jacc.2011.12.040
32
Mark
DB
,
Hlatky
MA
,
Harrell
FE, Jr
,
Lee
KL
,
Califf
RM
,
Pryor
DB
. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med. 1987;106(6):793–800. doi:.https://doi.org/10.7326/0003-4819-106-6-793
33
Mc Ardle
BA
,
Dowsley
TF
,
deKemp
RA
,
Wells
GA
,
Beanlands
RS
. Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease?: A systematic review and meta-analysis. J Am Coll Cardiol. 2012;60(18):1828–37. doi:.https://doi.org/10.1016/j.jacc.2012.07.038
34
Miller
JM
,
Rochitte
CE
,
Dewey
M
,
Arbab-Zadeh
A
,
Niinuma
H
,
Gottlieb
I
, et al.
Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359(22):2324–36. doi:.https://doi.org/10.1056/NEJMoa0806576
35
Morise
AP
,
Diamond
GA
. Comparison of the sensitivity and specificity of exercise electrocardiography in biased and unbiased populations of men and women. Am Heart J. 1995;130(4):741–7. doi:.https://doi.org/10.1016/0002-8703(95)90072-1
36
Nandalur
KR
,
Dwamena
BA
,
Choudhri
AF
,
Nandalur
MR
,
Carlos
RC
. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2007;50(14):1343–53. doi:.https://doi.org/10.1016/j.jacc.2007.06.030
37
Schwitter
J
,
Wacker
CM
,
Wilke
N
,
Al-Saadi
N
,
Sauer
E
,
Huettle
K
, et al.; MR-IMPACT Investigators. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J. 2013;34(10):775–81. doi:.https://doi.org/10.1093/eurheartj/ehs022
38
Marwan
M
,
Hausleiter
J
,
Abbara
S
,
Hoffmann
U
,
Becker
C
,
Ovrehus
K
, et al.
Multicenter Evaluation Of Coronary Dual-Source CT angiography in patients with intermediate Risk of Coronary Artery Stenoses (MEDIC): study design and rationale. J Cardiovasc Comput Tomogr. 2014;8(3):183–8. doi:.https://doi.org/10.1016/j.jcct.2014.04.006
39
Meijboom
WB
,
Meijs
MF
,
Schuijf
JD
,
Cramer
MJ
,
Mollet
NR
,
van Mieghem
CA
, et al.
Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52(25):2135–44. doi:.https://doi.org/10.1016/j.jacc.2008.08.058
40
Hulten
EA
,
Carbonaro
S
,
Petrillo
SP
,
Mitchell
JD
,
Villines
TC
. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;57(10):1237–47. doi:.https://doi.org/10.1016/j.jacc.2010.10.011
41Assessing and diagnosing suspected stable angina (NICE pathways). http://pathwaysniceorguk/pathways/chest-pain 2018.
42
Abbara
S
,
Arbab-Zadeh
A
,
Callister
TQ
,
Desai
MY
,
Mamuya
W
,
Thomson
L
, et al.
SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2009;3(3):190–204. doi:.https://doi.org/10.1016/j.jcct.2009.03.004
43
Schmermund
A
,
Marwan
M
,
Hausleiter
J
,
Barth
S
,
Bruder
O
,
Kerber
S
, et al.
Declining radiation dose of coronary computed tomography angiography: German cardiac CT registry experience 2009-2014. Clin Res Cardiol. 2017;106(11):905–12. doi:.https://doi.org/10.1007/s00392-017-1136-8
44
Bourque
JM
,
Beller
GA
. Stress myocardial perfusion imaging for assessing prognosis: an update. JACC Cardiovasc Imaging. 2011;4(12):1305–19. doi:.https://doi.org/10.1016/j.jcmg.2011.10.003
45
Hachamovitch
R
,
Hayes
S
,
Friedman
JD
,
Cohen
I
,
Shaw
LJ
,
Germano
G
, et al.
Determinants of risk and its temporal variation in patients with normal stress myocardial perfusion scans: what is the warranty period of a normal scan?
J Am Coll Cardiol. 2003;41(8):1329–40. doi:.https://doi.org/10.1016/S0735-1097(03)00125-6
46
Detrano
R
,
Guerci
AD
,
Carr
JJ
,
Bild
DE
,
Burke
G
,
Folsom
AR
, et al.
Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–45. doi:.https://doi.org/10.1056/NEJMoa072100
47
Shaw
LJ
,
Hendel
R
,
Borges-Neto
S
,
Lauer
MS
,
Alazraki
N
,
Burnette
J
, et al.; Myoview Multicenter Registry. Prognostic value of normal exercise and adenosine (99m)Tc-tetrofosmin SPECT imaging: results from the multicenter registry of 4,728 patients. J Nucl Med. 2003;44(2):134–9.
48
Lipinski
MJ
,
McVey
CM
,
Berger
JS
,
Kramer
CM
,
Salerno
M
. Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62(9):826–38. doi:.https://doi.org/10.1016/j.jacc.2013.03.080
49
Berman
DS
,
Kiat
H
,
Friedman
JD
,
Wang
FP
,
van Train
K
,
Matzer
L
, et al.
Separate acquisition rest thallium-201/stress technetium-99m sestamibi dual-isotope myocardial perfusion single-photon emission computed tomography: a clinical validation study. J Am Coll Cardiol. 1993;22(5):1455–64. doi:.https://doi.org/10.1016/0735-1097(93)90557-H
50
Cerqueira
MD
,
Weissman
NJ
,
Dilsizian
V
,
Jacobs
AK
,
Kaul
S
,
Laskey
WK
, et al.; American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–42. doi:.https://doi.org/10.1161/hc0402.102975
51
Hachamovitch
R
,
Berman
DS
,
Kiat
H
,
Cohen
I
,
Cabico
JA
,
Friedman
J
, et al.
Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996;93(5):905–14. doi:.https://doi.org/10.1161/01.CIR.93.5.905
52
Zellweger
MJ
,
Hachamovitch
R
,
Kang
X
,
Hayes
SW
,
Friedman
JD
,
Germano
G
, et al.
Threshold, incidence, and predictors of prognostically high-risk silent ischemia in asymptomatic patients without prior diagnosis of coronary artery disease. J Nucl Cardiol. 2009;16(2):193–200. doi:.https://doi.org/10.1007/s12350-008-9016-2
53
Amanullah
AM
,
Kiat
H
,
Hachamovitch
R
,
Cabico
JA
,
Friedman
JD
,
Berman
DS
. Impact of myocardial perfusion single-photon emission computed tomography on referral to catheterization of the very elderly. Is there evidence of gender-related referral bias?
J Am Coll Cardiol. 1996;28(3):680–6. doi:.https://doi.org/10.1016/0735-1097(96)00200-8
54
Amanullah
AM
,
Kiat
H
,
Friedman
JD
,
Berman
DS
. Adenosine technetium-99m sestamibi myocardial perfusion SPECT in women: diagnostic efficacy in detection of coronary artery disease. J Am Coll Cardiol. 1996;27(4):803–9. doi:.https://doi.org/10.1016/0735-1097(95)00550-1
55
Chow
BJ
,
Dorbala
S
,
Di Carli
MF
,
Merhige
ME
,
Williams
BA
,
Veledar
E
, et al.
Prognostic value of PET myocardial perfusion imaging in obese patients. JACC Cardiovasc Imaging. 2014;7(3):278–87. doi:.https://doi.org/10.1016/j.jcmg.2013.12.008
56
Zellweger
MJ
,
Lewin
HC
,
Lai
S
,
Dubois
EA
,
Friedman
JD
,
Germano
G
, et al.
When to stress patients after coronary artery bypass surgery? Risk stratification in patients early and late post-CABG using stress myocardial perfusion SPECT: implications of appropriate clinical strategies. J Am Coll Cardiol. 2001;37(1):144–52. doi:.https://doi.org/10.1016/S0735-1097(00)01104-9
57
Zellweger
MJ
,
Fahrni
G
,
Ritter
M
,
Jeger
RV
,
Wild
D
,
Buser
P
, et al.; BASKET Investigators. Prognostic value of “routine” cardiac stress imaging 5 years after percutaneous coronary intervention: the prospective long-term observational BASKET (Basel Stent Kosteneffektivitäts Trial) LATE IMAGING study. JACC Cardiovasc Interv. 2014;7(6):615–21. doi:.https://doi.org/10.1016/j.jcin.2014.01.161
58
Wackers
FJ
,
Young
LH
,
Inzucchi
SE
,
Chyun
DA
,
Davey
JA
,
Barrett
EJ
, et al.; Detection of Ischemia in Asymptomatic Diabetics Investigators. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27(8):1954–61. doi:.https://doi.org/10.2337/diacare.27.8.1954
59
Young
LH
,
Wackers
FJ
,
Chyun
DA
,
Davey
JA
,
Barrett
EJ
,
Taillefer
R
, et al.; DIAD Investigators. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301(15):1547–55. doi:.https://doi.org/10.1001/jama.2009.476
60
Zellweger
MJ
,
Maraun
M
,
Osterhues
HH
,
Keller
U
,
Müller-Brand
J
,
Jeger
R
, et al.
Progression to overt or silent CAD in asymptomatic patients with diabetes mellitus at high coronary risk: main findings of the prospective multicenter BARDOT trial with a pilot randomized treatment substudy. JACC Cardiovasc Imaging. 2014;7(10):1001–10. doi:.https://doi.org/10.1016/j.jcmg.2014.07.010
61
Zellweger
MJ
,
Haaf
P
,
Maraun
M
,
Osterhues
HH
,
Keller
U
,
Müller-Brand
J
, et al.; BARDOT Investigators. Predictors and prognostic impact of silent coronary artery disease in asymptomatic high-risk patients with diabetes mellitus. Int J Cardiol. 2017;244:37–42. doi:.https://doi.org/10.1016/j.ijcard.2017.05.069
62
Mahrholdt
H
,
Wagner
A
,
Judd
RM
,
Sechtem
U
,
Kim
RJ
. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26(15):1461–74. doi:.https://doi.org/10.1093/eurheartj/ehi258
63
Kwong
RY
,
Sattar
H
,
Wu
H
,
Vorobiof
G
,
Gandla
V
,
Steel
K
, et al.
Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation. 2008;118(10):1011–20. doi:.https://doi.org/10.1161/CIRCULATIONAHA.107.727826
64
Tweet
MS
,
Arruda-Olson
AM
,
Anavekar
NS
,
Pellikka
PA
. Stress echocardiography: what is new and how does it compare with myocardial perfusion imaging and other modalities?
Curr Cardiol Rep. 2015;17(6):43. doi:.https://doi.org/10.1007/s11886-015-0600-1
65
Arumugam
P
,
Tout
D
,
Tonge
C
. Myocardial perfusion scintigraphy using rubidium-82 positron emission tomography. Br Med Bull. 2013;107(1):87–100. doi:.https://doi.org/10.1093/bmb/ldt026
66
Dorbala
S
,
Di Carli
MF
,
Beanlands
RS
,
Merhige
ME
,
Williams
BA
,
Veledar
E
, et al.
Prognostic value of stress myocardial perfusion positron emission tomography: results from a multicenter observational registry. J Am Coll Cardiol. 2013;61(2):176–84. doi:.https://doi.org/10.1016/j.jacc.2012.09.043
67
Benz
DC
,
Gaemperli
L
,
Gräni
C
,
von Felten
E
,
Giannopoulos
AA
,
Messerli
M
, et al.
Impact of cardiac hybrid imaging-guided patient management on clinical long-term outcome. Int J Cardiol. 2018;261:218–22. doi:.https://doi.org/10.1016/j.ijcard.2018.01.118
68
Pazhenkottil
AP
,
Benz
DC
,
Gräni
C
,
Madsen
MA
,
Mikulicic
F
,
von Felten
E
, et al.
Hybrid SPECT Perfusion Imaging and Coronary CT Angiography: Long-term Prognostic Value for Cardiovascular Outcomes. Radiology. 2018;288(3):694–702. doi:.https://doi.org/10.1148/radiol.2018171303
69
Shaw
LJ
,
Hachamovitch
R
,
Berman
DS
,
Marwick
TH
,
Lauer
MS
,
Heller
GV
, et al.; Economics of Noninvasive Diagnosis (END) Multicenter Study Group. The economic consequences of available diagnostic and prognostic strategies for the evaluation of stable angina patients: an observational assessment of the value of precatheterization ischemia. J Am Coll Cardiol. 1999;33(3):661–9. doi:.https://doi.org/10.1016/S0735-1097(98)00606-8
70
Boden
WE
,
O’Rourke
RA
,
Teo
KK
,
Hartigan
PM
,
Maron
DJ
,
Kostuk
WJ
, et al.; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–16. doi:.https://doi.org/10.1056/NEJMoa070829
71
Shaw
LJ
,
Berman
DS
,
Maron
DJ
,
Mancini
GB
,
Hayes
SW
,
Hartigan
PM
, et al.; COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117(10):1283–91. doi:.https://doi.org/10.1161/CIRCULATIONAHA.107.743963
72
Frye
RL
,
August
P
,
Brooks
MM
,
Hardison
RM
,
Kelsey
SF
,
MacGregor
JM
, et al., BARI 2D Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360(24):2503–15. doi:.https://doi.org/10.1056/NEJMoa0805796
73
Shaw
LJ
,
Cerqueira
MD
,
Brooks
MM
,
Althouse
AD
,
Sansing
VV
,
Beller
GA
, et al.
Impact of left ventricular function and the extent of ischemia and scar by stress myocardial perfusion imaging on prognosis and therapeutic risk reduction in diabetic patients with coronary artery disease: results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. J Nucl Cardiol. 2012;19(4):658–69. doi:.https://doi.org/10.1007/s12350-012-9548-3
74
Hachamovitch
R
,
Hayes
SW
,
Friedman
JD
,
Cohen
I
,
Berman
DS
. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107(23):2900–7. doi:.https://doi.org/10.1161/01.CIR.0000072790.23090.41



