The value of volume substitution in patients with septic and haemorrhagic shock with respect to the microcirculation
DOI: https://doi.org/10.4414/smw.2019.20007
Martin
Siegemund, Alexa
Hollinger, Eva C.
Gebhard, Jonas D.
Scheuzger, Daniel
Bolliger
Departement für Anästhesie, Operative Intensivbehandlung, präklinische Notfallmedizin und Schmerztherapie, Universitätsspital Basel, Departement klinische
Forschung, Universität Basel, Switzerland
Summary
After decades of ordinary scientific interest, fluid resuscitation of patients with septic and haemorrhagic shock took centre stage in intensive care research at the turn of the millennium. By that time, resuscitation fluids were the mainstay of haemodynamic stabilisation, avoidance of vasopressors and treatment of hypovolaemia in patients in shock, but were accompanied by adverse events such as excessive tissue oedema. With the spread of early goal-directed therapy research intensified and it was realised that type, volume and timing of resuscitation fluids might affect the course and outcome of critically ill patients. At the same time, the importance of microvascular blood flow as target of resuscitation was accepted.
Today, once-forbidden albumin is the recommended colloid in severe sepsis and septic shock, and the European Medical Agency is considering the removal of starch solutions from the European market because of an increased incidence of acute kidney injury and mortality. This is unprecedented, especially because the administration of low-molecular-weight starches seems to have advantages in indications other than sepsis, and because practices in fluid resuscitation have changed fundamentally since the negative starch studies. Crystalloids are still the mainstay of hypovolaemia treatment in critically ill patients, but awareness is increasing that electrolyte composition, strong ion gap, tonicity and the bicarbonate-substituting anion may have an effect on adverse effects and outcome. In haemorrhagic shock, the utilisation of crystalloids and colloids is retreating, and plasma and erythrocyte concentrates are gaining more importance in the resuscitation of the patient with acute bleeding. However, there are still influential voices warning against the liberal usage of plasma concentrates and erythrocytes in trauma and haemorrhagic shock.
This review describes the evidence relating to fluid resuscitation in sepsis, septic shock and massive haemorrhage. Beside the scientific evidence based on clinical trials, possible effects on the microcirculation and, therefore, organ function will be illustrated and areas of future research highlighted. The critical appraisal of the existing evidence should enable the reader to choose the optimal volume substitution for an individual patient.
Introduction
Hypovolaemia is an essential part of virtually all types of shock, sometimes even in cardiogenic shock. In haemorrhagic and hypovolaemic shock, an absolute volume deficit exists owing to absolute blood or fluid loss, whereas in septic and anaphylactic shock vasodilatation and extravasation of fluid cause relative hypovolaemia. Both relative and absolute hypovolaemia lead to decreased vascular filling and wall tension, and cardiac output [1]. The reduced cardiac output attenuates microvascular organ blood-flow, resulting in tissue hypoxia, anaerobic metabolism and acidosis [2]. Rapid fluid resuscitation is, therefore, a mainstay of the treatment of this common pathway of shock. In this respect, an ideal resuscitation fluid should normalise intravascular and cardiac filling and improve microvascular blood flow without propagating capillary permeability and impairing endothelial integrity [3].
Erythrocyte and plasma concentrates, colloids and crystalloids all normalise intravascular and cardiac filling to a greater or lesser extent and according to the timing of administration [4]. The effects of resuscitation fluid on the microcirculation of organs are critical for the volume expanding effect under different pathological conditions [3]. Under physiological conditions, fluid shift out of capillaries is a tightly regulated mechanism that depends upon endothelial cells, endothelial cell junctions and the endothelial glycocalyx [5]. The endothelial glycocalyx is a luminal network of membranous glycoproteins and proteoglycans of vessel-proportional thickness [6]. Under physiological conditions, the space between endothelial glycocalyx and endothelial cells is free of proteins and blood cells, preventing the escape of large amounts of plasma protein-containing fluid. Organ-specific gaps within the endothelial cell junctions constitute the pathways for trans-vascular fluid and protein exchange [7]. The classic Starling principle assumes that hydrostatic pressure promotes leakage whereas oncotic pressure promotes the reabsorption of fluid along the length of microvascular blood vessels. The so-called revised Starling equation additionally considers the endothelial glycocalyx, stating that an intact glycocalyx prevents protein extravasation into the interstitial space independent of capillary pressure, and that the low oncotic pressure between glycocalyx and endothelial cells determines fluid exchange [7]. This implies that an increased plasma oncotic pressure will not promote reabsorption of interstitial fluid but rather counteract fluid extravasation, and that lymphatic vessels clear all interstitial fluids back into the venous system via the thoracic duct.
High venous pressure, therefore, increases oedema in two ways; on one hand, high venous outflow pressure transmits to intracapillary pressure promoting fluid extravasation and on the other side, a high central venous pressure hinders thoracic duct lymphatic drainage.
The microcirculatory blood flow provides oxygenation and nutrition to, and removes metabolic products from, the organs. In sepsis and haemorrhagic shock, the integrity of the microcirculation is severely abnormal due to the existing hypovolaemia, dysfunctional vascular tone, activated coagulation, increased leucocyte adherence and activation [8], and shedding of the glycocalyx. In 2002, de Backer and colleagues showed that the microcirculation in septic patients is severely disturbed and that nonsurvivors had less perfused small vessels than survivors [9]. The same group showed in a subsequent study that the microcirculation in nonsurvivors of septic shock did not improve, despite resolution of the clinical signs of shock [10]. The administration of fluid in shock states improves microvascular perfusion and increases capillary pressure (fig. 1). Unfortunately, the endothelial glycocalyx is disrupted by lipopolysaccharides [11] and other inflammatory conditions such as trauma [12] and ischaemia reperfusion [13], as a result of oxidative stress [4]. At the same time, sepsis increases endothelial permeability by a disassembling of intercellular junctions, promoting the extravasation of smaller proteins such as albumin [14, 15]. Finally, the inadequate administration of bolus fluid and hence hypervolaemia increase the level of atrial natriuretic peptide, causing enhanced shedding of the glycocalyx and alteration of the microcirculation [16]. This leaves a narrow path in fluid resuscitation of septic and haemorrhagic shock, with the recovery of microvascular flow by optimal capillary filling on the one hand and the exacerbation of fluid extravasation and tissue oedema by volume overloading on the other hand. Fluid extravasation and oedema alone may lead to pulmonary oedema [17], or abdominal compartment syndrome [18], and contribute to acute kidney injury (AKI) [19].
The objective of this review article is to discuss commonly used resuscitation fluids, to present the scientific evidence related to volume resuscitation in septic and haemorrhagic shock and to classify the different findings with respect to impact on the microcirculation.
Fluid replacement in septic patients
In 2001, the landmark paper of Rivers et al. [20] started a new approach to volume resuscitation in septic patients and intensive care medicine in general. In this single-centre randomised controlled trial, early goal-directed therapy entailing fluid resuscitation (according to central venous and mean arterial pressure), transfusions and inotropic support during the first 6 hours of therapy improved 30-day survival by 16%. Surprisingly, not much weight was given to the 24% increase in the numbers of patients receiving blood transfusions to improve central venous saturation with early goal-directed therapy, although the total amount of fluid was equal after 3 days. Because in this study the types of fluid used for early goal-directed therapy were not specified and there was a sceptical attitude to erythrocyte transfusions in intensive care in general [21], a decade of intense research to find the optimal volume of replacement fluid began. Although the concept of early goal-directed therapy has since been abandoned because its mortality benefit could not be confirmed in three large randomised trials [22–24] and two meta-analysis [25, 26], the search for the best type, volume and time of resuscitation fluid is still ongoing (table 1).
Table 1 Advantages and risks of preparations for volume resuscitation in septic and haemorrhagic shock.
|
Molecular weight
(kDa)
|
Osmolarity (mosm/l)
|
Chloride (mmol/l)
|
Advantages
|
Risks
|
Volume effect
|
| Albumin |
66 |
291 |
140 |
Human protein
Transport capacities for proteins and drugs
Antioxidant potential |
Hyperoncotic preparations may increase risk for AKI
Increased bleeding and mortality in haemorrhagic shock |
Rapid microcirculatory plasma escape rate in septic shock |
| Modern semi-synthetic colloids |
130 |
297-308 |
110–118 |
Improve micro-vascular blood flow
Anti-inflammatory |
In high doses increased risk of mortality
Increased risk of AKI and renal replacement therapy
Itching and rash |
Volume effect longer than duration of infusion |
| Gelatines |
30 |
284 |
103–120 |
|
Allergic reactions
Increased risk of AKI and renal replacement therapy
Insufficient data for mortality risk |
Rapid degradation and temporary volume effect |
| Sodium chloride (“normal saline”) |
|
308 |
154 |
High osmolarity |
Hyperchloraemic acidosis
Decreased renal blood flow
Rapid emergence of tissue oedema |
Volume effect only during infusion |
| Balanced crystalloid solutions |
|
273–294 |
98–112 |
Inert
Comparable electrolyte composition to plasma |
Rapid emergence of tissue oedema |
Volume effect only during infusion |
| Pooled fresh frozen plasma |
50–340 |
322 |
73–79 |
Coagulation factors
Human proteins |
Transfusion associated lung injury (very low risk with pooled preparations)
Transfusion associated circulatory overload
Low risk of infection |
Good volume expanding effect and long intravascular stay |
Recommendations for the application of resuscitation fluids in different types of shock are summarised in table 2.
Table 2 Recommendations for the application of resuscitation fluids in different types of shock.
|
Type of shock
|
First Choice
|
Alternative
|
Comments
|
| Hypovolaemic |
|
Balanced crystalloids |
Albumin |
In patients with a high chloride loss (e.g., hyperemesis) NaCl 0.9% may be indicated. |
| Haemorrhagic |
With apparent severe bleeding |
Erythrocyte concentrates
Plasma
Thrombocytes |
Balanced crystalloids |
Albumin and all synthetic colloid solutions have the potential to increase bleeding, especially with large wound areas. |
| Without apparent bleeding |
Balanced crystalloids |
Erythrocyte concentrates and plasma
Albumin
HES (130/0.4) or gelatines |
Here applies the same as mentioned above. |
| With traumatic brain injury |
NaCl 0.9% |
HES (130/0.4) or gelatines
Erythrocyte concentrates and plasma |
Hypo-oncotic albumin solutions may increase bleeding and mortality. |
| Septic |
|
Balanced crystalloids |
Albumin |
Consider erythrocytes and plasma at low haematocrit and disturbed coagulation parameters.
Synthetic colloids compromise kidney function and may increase mortality. |
| Anaphylactic |
|
Balanced crystalloids |
Albumin
HES (130/0.4) |
Fluid resuscitation only for correction of anaphylaxis-related relative hypovolaemia (usually 1–2 L crystalloids). |
| Cardiogenic |
|
Balanced crystalloids |
Albumin |
In cardiogenic shock, small volume boluses may improve cardiac output due to correction of relative hypovolaemia. Especially in cases with preserved ejection fraction. |
| HES = hydroxylethyl starch |
Albumin
At a first glance, albumin seems to be the ideal reference colloid. This small endogenous protein, synthesised in the liver, has antioxidant properties, transports electrolytes, drugs and hormones/cytokines, provides 80% of the plasma oncotic pressure and interacts with the endothelial glycocalyx [27]. Albumin continuously leaks into the interstitial space, where 40% of total body albumin is located, and is brought back to the circulation by lymphatic return. The normal plasma escape rate of albumin is 5% per hour, increases by at least 200% in patients with septic shock and returns to baseline after 2 days [28]. This is an obvious disadvantage of albumin administration in the very early phase of septic shock when capillary leak is substantial. However, the latest guidelines from the Surviving Sepsis Campaign recommend albumin for fluid resuscitation in sepsis and septic shock patients who require substantial amounts of crystalloids (weak recommendation) [29]. The American College of Critical Care Medicine recommended 20 ml/kg fluid boluses of either isotonic crystalloids or 5% albumin (level 1C) for early fluid resuscitation in children and neonates with septic shock [30].
Following a Cochrane Review conducted in 1998 that suggested increased mortality associated with albumin use in critically ill patients [31], albumin infusion underwent a period of nearly total avoidance in intensive care. The albumin tide was turning in 2006 when a large blinded randomised controlled trial in almost 7000 patients compared 4% albumin with 0.9% sodium chloride for fluid resuscitation in intensive care units (SAFE trial) [32]. The two heterogeneous groups of critically ill patients showed no difference in 28-day mortality, days on mechanical ventilation and renal replacement therapy (RRT), and length of intensive care and hospital stay. On the contrary, in the predefined subgroup of severely septic patients with complete baseline data (919/1218; 75.5%), the adjusted odds ratio for death with albumin versus saline was 0.71 (95% confidence interval [CI] 0.52–0.97) without increased renal or other organ impairment [33]. Despite a significantly higher mortality rate in the subgroup of traumatic brain injury patients resuscitated with hypo-oncotic albumin [34] and a relatively small fluid saving effect, the results of the SAFE trial corroborated the belief in the beneficial effects of colloids for resuscitation in severe sepsis and septic shock.
A prospective, multicentre open-label trial tested the potential of albumin replacement in severe sepsis and septic shock in 1818 patients randomised to receive either albumin and crystalloids or crystalloids alone [35]. This study targeted a serum albumin concentration of 30 g/l with the infusion of 3 × 100 ml of hyperoncotic albumin 20% per day. During the early phase of volume resuscitation, fluid administration in both groups was according to early goal-directed therapy guidelines. There was no difference in 28- and 90-day mortality or in the appearance and severity of organ dysfunction; however, a post hoc subgroup analysis showed a decreased mortality with albumin in patients with shock at enrolment (relative risk [RR] 0.87; 95% CI 0.77–0.99) [35]. The total fluid volume infused after 7 days was not different, although albumin administration resulted in faster haemodynamic stabilisation. A third randomised open-label multicentre study compared 3 ×100 ml albumin 20% daily with saline boluses in patients with septic shock [36]. This French trial (abstract only available) also showed faster circulatory stabilisation and no difference in mortality between the two groups.
Representative of the urgent wish for a safe and effective colloid are not fewer than eight meta-analyses for the use of albumin in patients with sepsis published between 2011 and 2014, based mainly on the results from the three studies described above [37]. The largest meta-analysis, by Patel et al. [38], included 16 prospective randomised clinical trials published between 1982 and 2014 with patients having sepsis of any severity who received albumin for volume resuscitation compared with a control fluid (crystalloid or colloid; n = 4190). Presenting an overall moderate quality of evidence, the analysis showed a similar risk of death for albumin groups and control fluid groups (RR 0.94; 95% CI 0.87–1.01). There was no indication of harm from albumin in patients with sepsis of any severity. Strong evidence of no difference was found in 3878 patients when albumin was compared with crystalloids only (RR 0.93; 95% CI 0.86–1.01). In contrast, Xu and colleagues found a decreased 90-day mortality with albumin compared with crystalloids for resuscitation in septic shock patients [39]. Overall, this meta-analysis of five studies comparing the effects of albumin with those of crystalloid or saline therapy on mortality exclusively in patients with severe sepsis (n = 3658) and septic shock (n = 2180) observed a trend toward reduced 90-day mortality in the albumin group.
In summary, it can be said that the application of human albumin for resuscitation in severe sepsis and septic shock is an option for supporting haemodynamic stabilisation in patients with high fluid requirements, without negative effects on survival. The significance of albumin in the very early phase of fluid resuscitation with marked capillary leakage and the impact on oedema generation needs further evaluation.
Semisynthetic colloid solutions
After the results of the Rivers study [20] and the almost significant improved survival of septic patients treated with albumin in the SAFE trial [32], semisynthetic colloid solutions (hydroxyethyl starch [HES], gelatines) were adopted as ideal resuscitation fluids for early goal-directed therapy. The allegedly reduced amount of resuscitation fluid with colloid solutions was thought to reduce the interstitial oedema accompanying high volume fluid therapy and lacked the high costs of albumin [40]. Initial concerns about the use of HES for volume resuscitation in severe sepsis appeared after two studies with HES solutions of higher molecular weight and grade of substitution (200 kD / 0.5–0.66). Both studies showed an increased risk of AKI and RRT but no difference in mortality compared with either gelatine 3% or Ringer’s lactate [41, 42]. Specifically, the Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) trial was criticised because a substantial proportion of patients received more than the maximum recommended amount of hyperoncotic HES [42]. At about the same time as this unsettling result, new iso-oncotic HES solutions with a lower molecular weight and degree of substitution (130 / 0.4–0.42) were developed [4].
In 2012, three studies investigating these new HES solutions in a general intensive care population and in severe sepsis and septic shock were published. The multicentre double-blind Scandinavian 6S trial compared HES with Ringer’s acetate for the therapy of severe sepsis and septic shock patients [43]. This study found a 17% increased relative risk of 90-day mortality (95% CI 1.01–1.36) for HES, without a difference in fluid volume administered. Interestingly, the mortality rates at 28 days and at 6 and 12 months were not significantly different between HES 130/0.4 and Ringer’s acetate [44]. Despite a significant difference in short-term RRT, without an increased RRT-related mortality, the same number of patients had RRT for >5 days and an adjustment for AKI stage as covariate eliminated the significance of 90-day mortality [45]. Crystalloid versus Hydroxyethyl Starch Trial (CHEST), a randomised controlled double-blind trial (n = 7000, 1937 patients with sepsis) also found an increased risk for RRT with HES (130/0.4) as compared with sodium chloride 0.9% (RR 1.21, 95% CI 1.00–1.45) [46]. Ninety-day mortality did not differ between the groups and the patients receiving HES showed a decreased incidence of AKI assessed with the RIFLE criteria. The HES group received significantly less fluid during the first 4 days. A smaller French study compared HES 130/04 with sodium chloride for early fluid resuscitation and found that a significantly lower amount of HES was needed to achieve haemodynamic stability [47]. This study found no difference in mortality and renal failure in 196 patients with severe sepsis. The open-label, randomised controlled Colloids Versus Crystalloids for the Resuscitation of the Critically ill (CRISTAL) trial randomised 2857 patients in shock to receive either colloid or crystalloid for the initial treatment of shock [48]. In this trial, the majority of patients received HES 130/0.4 as colloid or sodium chloride as crystalloid, and the colloid group required significant less resuscitation fluid. The primary end-point of 28-day mortality was not different, although there was a small mortality benefit in favour of colloids after 90 days. No difference in the number of patients receiving RRT was observed [48].
A meta-analysis comparing only HES 140/03 with either crystalloids or albumin in sepsis found no overall increased mortality in 3546 patients. In the studies with low risk of bias (3016 patients), the relative risk of death with the use of HES was 1.11 (1.00–1.23 trial sequential analysis adjusted 95% CI 0.95–1.29) [49]. Patients receiving HES needed more RRT and showed a nonsignificant increase in the risk of AKI. Another meta-analysis comparing HES of different molecular weights, substitution and oncotic preparations with other resuscitation fluids in critically ill patients found an increased relative risk of mortality (1.09, 95% CI 1.02–1.17) and an increased risk for AKI (RR 1.27, 95% CI 1.09–1.47) and RRT (RR 1.32, 95% CI 1.15–1.50) [50]. Finally, a network meta-analysis examining the effect of different resuscitation fluids on mortality in sepsis showed with high confidence higher mortality with starches than with crystalloids [51]. Balanced crystalloids revealed with moderate confidence a lower mortality than low- and high molecular weight HES preparations.
Although gelatines are widely used semisynthetic colloids, there is a paucity of high-quality randomised controlled trials [40, 52]. In a prospective sequential fluid resuscitation analysis, gelatine 4% was an independent risk factor of AKI (OR 1.85, 95% CI 1.31–2.62) [53]. A recent meta-analysis of three studies comparing gelatine with crystalloids or albumin in 212 patients showed a 15% increased relative risk for mortality and a 35% increased risk for AKI in patients receiving gelatines [54]. Although these findings were not significant, they point to avoidance of gelatine use in septic patients.
Semisynthetic colloids, especially starches, should not be administered to patients with severe sepsis and septic shock because there is an increased risk for mortality and AKI when they are given in large amounts for volume resuscitation in critically ill septic patients. However, particularly the large, randomised controlled trials used high amounts of HES for more than 2 days partly in patients already fluid-resuscitated [42, 43, 46]. A recent meta-regression analysis of studies using HES 130/0.4 in septic patients suggested that the inappropriately high daily delta fluid balance is an important source of heterogeneity and was associated with mortality in these trials [55]. Studies using HES in the very early phase of sepsis resuscitation showed a decreased volume of fluid required in the HES group without an increased risk for mortality and AKI [47, 48]. The increased number blood products received, interpreted as to the detriment of HES [43, 46], could also be viewed as a sign of effectiveness, because haematocrit is more affected with a better plasma expanding capacity [56]. In the light of the increased mortality with higher amounts of fluid in patients with septic shock [57, 58], semisynthetic colloids and their influence on microvascular flow [59] in the initial phase of septic shock therapy deserve further investigation.
Crystalloid solutions
Crystalloids are the mainstay of volume resuscitation and maintenance in critically ill patients [29]. Crystalloids are classified as “balanced” and “unbalanced”. Balanced crystalloids are characterised by a plasma-like strong ion gap (about 24 mmol/l) [4]. Sodium chloride (saline, NaCl), the most commonly used crystalloid worldwide, has a strong ion difference of zero and is therefore called “unbalanced” [40]. Administration of large amounts of saline results in a hyperchloraemic metabolic acidosis [1]. High plasma chloride concentrations increase inflammatory markers, induce coagulopathy and decrease renal blood flow [60, 61]. In sepsis studies, crystalloid solutions are mainly used as comparator fluids for semisynthetic colloids and albumin. Direct comparisons between different balanced crystalloids are rare. No trials have evaluated the differences between different buffered crystalloids or their side effects in critically ill patients, although there are theoretical reasons for preferring acetate- to lactate-buffered solutions. In fact, nearly every human cell type is able to metabolise acetate, whereas lactate increases with declining liver function, hampering the interpretation of serum lactate levels.
A prospective, open-label, sequential period study in a single intensive care unit in Australia compared sodium chloride with balanced electrolytes [62]. In this before and after study, patients who received almost entirely balanced infusions showed a significant decrease in the incidence of AKI (adjusted OR 0.52, CI 95% 0.37–0.75) and RRT (adjusted OR 0.53, CI 95% 0.33–0.81) compared with patients treated with normal saline during an earlier period. Two retrospective studies including patients with sepsis (n = 53,448) [63] and systemic inflammatory response syndrome (n = 109,836) [64] showed a reduced in-hospital mortality with balanced infusions, a finding that was proportional to the chloride load. Patients in the sepsis cohort showed no difference in AKI. Another retrospective study in septic patients found an increased mortality in patients with hyperchloraemia on admission, independent of base deficit, cumulative fluid balance and AKI [65]. A retrospective cohort study in more than 60,000 septic shock patients compared four groups of fluid resuscitation [66]. Patients receiving saline together with balanced electrolytes had a significantly lower mortality (17.7%) than saline 0.9% alone (20.2%), saline and colloids (24.2%), and all three categories together (19.2%). In contrast to this retrospective cohort studies, two prospective, cluster randomised, crossover trials (SALT and SPLIT) comparing more than 3000 intensive care patients to either crystalloids or sodium chloride 0.9% found no difference in mortality, AKI and RRT [67, 68]. In both studies, the amount of fluid infused was rather low and the group of sepsis patients small, so that a possible negative effect on kidney function was obscured. In the SALT trial, patients administered larger volumes of saline showed a significant increase in the composite of death, AKI and RRT [68]. A retrospective study of large volume resuscitation (60 ml/kg per day) showed a significantly increased crude rate of hyperchloraemic acidosis, AKI and hospital mortality with increased chloride load [69]. After adjustment for disease severity, total fluids and age, only mortality remained significant with a 5.5% increased risk of death for each 100 mmol of administered chloride, or 11% for 2 L of sodium chloride 0.9%. Finally a recent cluster-randomised, multiple cross-over trial in 15,802 critically ill adults showed that primarily the administration of balanced fluids resulted in a lower rate of a composite outcome of death, AKI and initiation of RRT [70]. The separate analysis of the three outcomes did not reach significance. In the relatively small group of septic patients (n = 2936), the composite endpoint and the 30-day mortality were lower with balanced infusions [70].
Up-to-date randomised, controlled, double-blind trials comparing saline 0.9% with balanced crystalloids, or different crystalloid preparations are lacking. Nevertheless, the existing data seem to indicate an increased mortality risk with saline 0.9%, especially when administered in high amounts. Hyperchloraemic acidosis seems to outweigh the possible adverse effects of the slightly hypotonic balanced crystalloid solutions. The only exception may be traumatic brain injury, where hypotonic balanced solutions may increase brain oedema more than slightly hypertonic saline 0.9%.
Volume replacement in massive haemorrhage
Fluid resuscitation in uncontrolled haemorrhage aims to maintain adequate organ perfusion and tissue oxygen delivery in a system compromised by the physiological consequences of traumatic injury. However, fluid therapy is only one component of a complex haemodynamic resuscitation strategy in massively injured patients [71]. Additional therapeutic options, including the use of vasopressors, early surgical exploration with emphasis on damage control, angiographic embolisation of bleeding arteries and deliberate hypotension, are comparably important, especially in penetrating trauma [40, 72, 73]. For the latter, patients with traumatic brain injury or patients with or at risk of coronary artery or cerebrovascular disease are important exceptions.
Fluid resuscitation of trauma patients is an ongoing challenge with various recommendations regarding the use of crystalloids, colloids, blood products and clotting factor concentrates [72–77]. No ideal resuscitation fluid for trauma patients exists in clinical practice [40], and the debate about this is highly emotional [76, 77]. The choice of type of resuscitation fluid is mainly based on institutional practice and clinician’s preferences, and rarely on physiological principles and study data. Further, regional variations, varying institutional protocols, availability and legal restrictions might relevantly influence the choice [74, 76].
However, there is accumulating evidence that the amount and type of fluid resuscitation might affect outcome in patients with massive haemorrhage similarly to patients with severe sepsis. Generally, it is suggested to restrict fluid administration in the prehospital period and in the emergency department [72]. Over-resuscitation with overzealous crystalloid administration leading to dilutional coagulopathy, hypothermia and potentially acidosis might further aggravate bleeding and coagulopathy [6, 16, 78].
Although their positive effect and advantage is not fully proven, crystalloids have an established role as inexpensive and widely available first-line resuscitation fluids in trauma [40]. Albeit the use of semisynthetic colloids in trauma patients is often perceived to be associated with improved haemodynamic stability, but with increased risk of coagulopathy and renal failure, a large meta-analysis could not show that one solution is relevantly safer or associated with improved survival [79, 80]. Similarly, the use of human albumin for fluid resuscitation did not improve outcome compared with crystalloid in a randomised study including nearly 7000 patients [32]. In a subgroup of patients with traumatic brain injury, the use of hypo-oncotic albumin 4% actually increased mortality [34].
The administration of colloids over a prolonged period of time and in dosages beyond any recommendations must be avoided. However, colloids might be better than crystalloids for replacing plasma deficits and avoiding acute intravascular hypovolaemia or for preventing tissue oedema [6, 76, 81]. Potentially, limited amounts of colloids in the initial resuscitation phase of haemodynamically unstable patients (fig. 2) might be beneficial, but should be continued with a “crystalloid” maintenance phase. The value of hyperosmolar saline solution is unclear [82], and the solution has been abandoned.
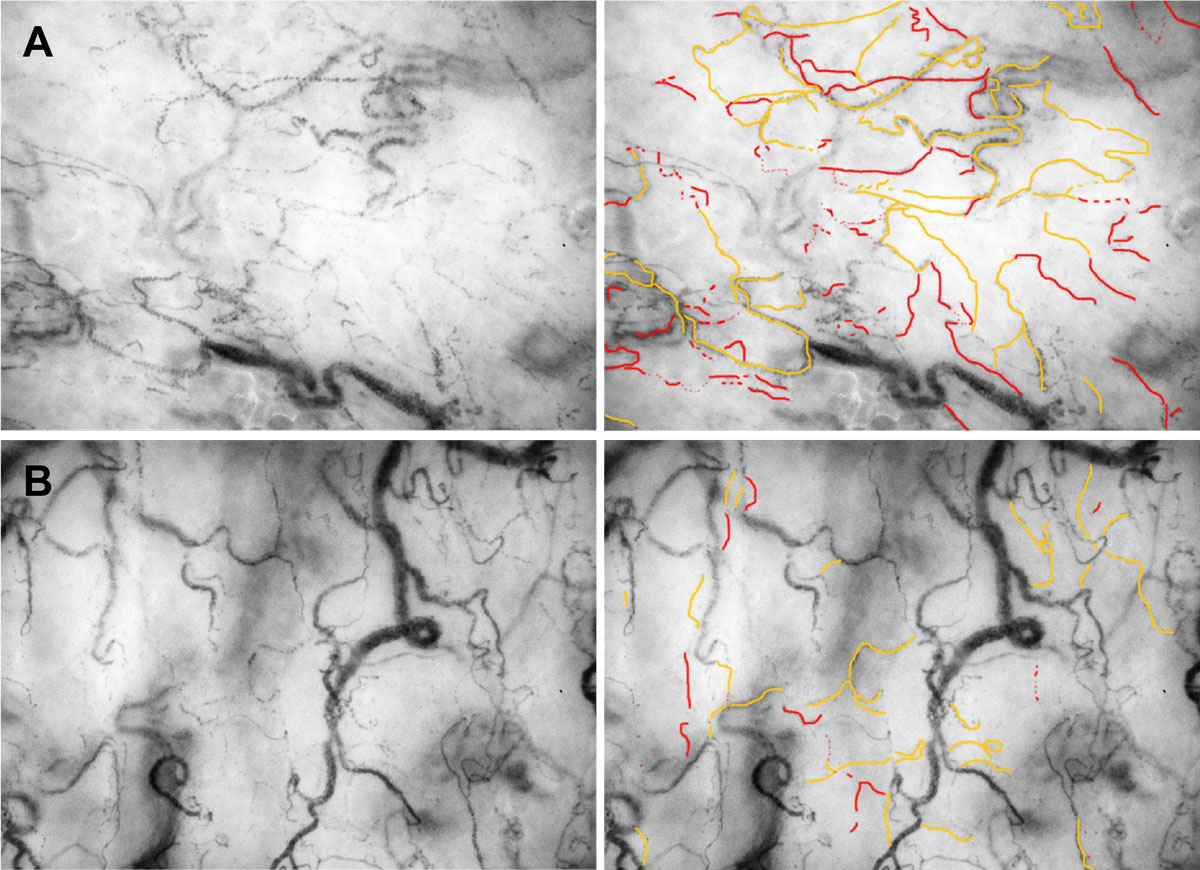
Figure 2 Influence of fluid resuscitation on the sublingual microcirculation. Panel A shows the microcirculation of a patient in haemorrhagic shock. Most of the visible microvessels show are intermittently perfused (orange lines) or show no flow (red lines). Panel B shows the microcirculation of the same patient after resuscitation with on unit packed red cells. The amount of microvessels with intermittent flow differs markedly. Only very view vessel parts show a no flow phenomenon.
Use of blood products
During massive haemorrhage, there is a rapid loss of red blood cells and coagulation factors, in combination with hyperfibrinolysis resulting in acute trauma coagulopathy. In the case of massive haemorrhagic shock, the transfusion of red blood cells is often unavoidable to preserve a minimal haematocrit level. However, the infusion of crystalloids and colloids will lead to further dilutional anaemia and coagulopathy.
Plasma might be an ideal resuscitation fluid as it is protein-rich and contains coagulation factors. The endothelial glycocalyx might be better preserved with less fluid loss in the interstitium and potentially lead to less additional coagulopathic deterioration by administration of plasma products [16, 83]. However, this concept has only been proven in a rodent model of haemorrhagic shock [83]. Recent German guidelines stated that plasma is contraindicated for primary volume resuscitation (recommendation grade 1C) [84]. The clinical effectiveness of fresh frozen plasma to preserve or restore coagulation deficits has been questioned by a large meta-analysis [85]. Nonetheless, despite the questionable indication and clinical benefit of plasma, and although its use had not been studied in non-trauma settings and in septic shock, the use of plasma as a “super-colloid” has been advocated by some experts [4].
In the event of haemorrhagic shock, early transfusion of plasma and platelets together with red blood cell concentrates has been advocated [72] as part of a massive transfusion protocol (MTP) aiming to transfuse “whole blood”. MTPs are also thought to prevent rather than reverse or treat trauma coagulopathy. MTPs with an approximate 1:1:1 ratio of blood components might be especially helpful in the early resuscitation phase before bleeding is controlled surgically. However, the value of MTPs has been evaluated only in retrospective analyses, most of which are associated with a relevant survivorship bias [86]. Further, even an MTP leads to relevant dilution of coagulation factors [87] and might be able to only partially limit rather than prevent further coagulopathy.
Finally, a recent study from Austria suggested that the early use of coagulation factor concentrates instead of the administration of plasma is feasible in the trauma setting and associated with less use of allogeneic blood products [88]. However, it is unclear whether such a regimen is associated with better outcome and lower morbidity and mortality.
Discussion
After 15 years of intense research, no fluid resuscitation panacea has been found and the optimal type and amount of fluid for the early volume replacement should be individualised for each patient and type of shock (table 1). A very recent Cochrane review stated with moderate evidence that fluid resuscitation with starches, albumin or fresh frozen plasma shows little or no difference in mortality compared with crystalloids in critically ill patients [89]. In current surviving sepsis guidelines [29], five out of six recommendations for fluid therapy are based on moderate- or low-quality evidence. The only strong recommendation with high-quality evidence is against the use of HES. Even this recommendation should be considered with caution, because the studies comparing HES 130/0.4 with crystalloids used rather high volumes of resuscitation fluid in patients already volume resuscitated [43, 46, 56]. The two studies testing starches in the very early course of shock did not find an increased mortality or a higher risk of AKI [47, 48]. Studies with an early administration of starch also found a volume-sparing effect in a ratio of 1.2 to 1.5 in favour of colloids, which is far below the theoretical expected value of 3, and an effect that is not so obvious in studies using high amounts of fluid over >2 days [56]. A possible explanation for this weak volume expanding effect could be that the fluid requirement is context sensitive [3]. This means that after initial resuscitation of the microcirculation, further volume replacement increases tissue oedema disproportionately. The reason for this could be a direct transmission of the increased mean arterial pressure through the dilated resistance vessels to the capillaries, where the thinned out glycocalyx together with the increased number of endothelial gaps are unable to retain volume intravascularly. Ospina-Tascone and colleagues showed that infusion of either 1 L of Ringer’s lactate or 400 ml of albumin 4% only increased the proportion of perfused small microcirculatory vessels during the first 24 hours of severe sepsis but not 48 hours after the diagnosis [59]. No relation to mean arterial pressure or cardiac index was found. The study also found a direct relationship between improved microvascular perfusion and decreased serum lactate [59]. Earliest evidence for the relevance of the administered volume came from a study of patients with acute respiratory distress syndrome, predominantly from pneumonia and sepsis, where a conservative fluid therapy improved lung function and decreased duration of mechanical ventilation [17]. In 2006, a European multicentre, observational cohort study in 1177 septic patients found a positive cumulative fluid balance as the only modifiable risk factor for death [90]. In the meantime, several retrospective cohort and prospective observational studies showed an association between higher positive fluid balances from 24 hours to 8 days and mortality [91–93]. In two very recent retrospective analyses of patients with severe sepsis or septic shock, the administration of more than 5 litres of resuscitation fluid on day one or a higher cumulative fluid balance on day three was associated with an increased risk of death [58, 94]. A retrospective review of fluid use in the prospective, randomised, blinded Vasopressin in Septic Shock Trial (VASST) [95] showed that a more positive fluid balance after 12 hours and 4 days was associated with a higher risk of mortality [57]. A central venous pressure of more than 12 mm Hg in the first 12 hours of resuscitation was also associated with increased mortality. Increased central venous pressure increases microvascular outflow and capillary pressure, which also increases the extravasation of fluid to the interstitium [3]. The possible positive impact of modulating microvascular in- and outflow pressure to reduce capillary pressure and thereby fluid extravasation was shown by two studies. Jansen et al. used a treatment algorithm according to which they applied vasodilators to correct microcirculatory derangement to foster decreased blood lactate concentrations [96]. Together with a minimal increased fluid volume during the first 8 hours, this modulation of microvascular outflow pressure decreased ICU and in-hospital mortality. An increase of microvascular inflow pressure with a fixed dose of vasopressin (0.03 U/min) in addition to vasopressors decreased the relative risk of 90-day mortality in patients with less severe sepsis [95]. Norepinephrine increased cardiac preload and reduced preload dependency in septic shock patients [97]. The current surviving sepsis campaign guidelines recommend 30 ml/kg body weight administered during the first 3 hours of sepsis therapy, norepinephrine as the first line vasopressor and vasopressin (0.03 U/min) as the second line to increase mean arterial pressure.
Conclusion
Fifteen years of intensive research have not found the optimal resuscitation fluid, as relative uncertainty remains about type and amount of fluid. Balanced crystalloids seem to be more advantageous than normal saline. Iso-oncotic albumin should be used as a colloid since the liberal use of starches over days has shown an increased incidence of AKI, RRT and mortality. The role of plasma and red blood cell concentrates is still controversial. The classic haemodynamic targets, including mean arterial pressure, cardiac output, lactate and even dynamic tests (e.g., passive leg raising), alone may not be sufficient to guide fluid administration in patients with haemorrhagic and septic shock. Direct or indirect monitoring of microcirculation including skin mottling and turgor, central venous pressure and peripheral oedema, should be considered as well, as the microcirculation it the primary target of fluid therapy.
Acknowledgment
We thank Allison Dwilewski for proofreading the manuscript.
References
1
Semler
MW
,
Rice
TW
. Sepsis Resuscitation: Fluid Choice and Dose. Clin Chest Med. 2016;37(2):241–50. doi:.https://doi.org/10.1016/j.ccm.2016.01.007
2
Siegemund
M
,
van Bommel
J
,
Ince
C
. Assessment of regional tissue oxygenation. Intensive Care Med. 1999;25(10):1044–60. doi:.https://doi.org/10.1007/s001340051011
3
Tatara
T
. Context-sensitive fluid therapy in critical illness. J Intensive Care. 2016;4(1):20. doi:.https://doi.org/10.1186/s40560-016-0150-7
4
Chang
R
,
Holcomb
JB
. Choice of Fluid Therapy in the Initial Management of Sepsis, Severe Sepsis, and Septic Shock. Shock. 2016;46(1):17–26. doi:.https://doi.org/10.1097/SHK.0000000000000577
5
Ince
C
,
Mayeux
PR
,
Nguyen
T
,
Gomez
H
,
Kellum
JA
,
Ospina-Tascón
GA
, et al.; ADQI XIV Workgroup. The Endothelium in Sepsis. Shock. 2016;45(3):259–70. doi:.https://doi.org/10.1097/SHK.0000000000000473
6
Chappell
D
,
Jacob
M
,
Hofmann-Kiefer
K
,
Conzen
P
,
Rehm
M
. A rational approach to perioperative fluid management. Anesthesiology. 2008;109(4):723–40. doi:.https://doi.org/10.1097/ALN.0b013e3181863117
7
Woodcock
TE
,
Woodcock
TM
. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108(3):384–94. doi:.https://doi.org/10.1093/bja/aer515
8
Stiel
L
,
Meziani
F
,
Helms
J
. Neutrophil Activation During Septic Shock. Shock. 2018;49(4):371–84. doi:.https://doi.org/10.1097/SHK.0000000000000980
9
De Backer
D
,
Creteur
J
,
Preiser
JC
,
Dubois
MJ
,
Vincent
JL
. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166(1):98–104. doi:.https://doi.org/10.1164/rccm.200109-016OC
10
Sakr
Y
,
Dubois
MJ
,
De Backer
D
,
Creteur
J
,
Vincent
JL
. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32(9):1825–31. doi:.https://doi.org/10.1097/01.CCM.0000138558.16257.3F
11
Marechal
X
,
Favory
R
,
Joulin
O
,
Montaigne
D
,
Hassoun
S
,
Decoster
B
, et al.
Endothelial glycocalyx damage during endotoxemia coincides with microcirculatory dysfunction and vascular oxidative stress. Shock. 2008;29(5):572–6.
12
Rahbar
E
,
Cardenas
JC
,
Baimukanova
G
,
Usadi
B
,
Bruhn
R
,
Pati
S
, et al.
Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med. 2015;13(1):117. doi:.https://doi.org/10.1186/s12967-015-0481-5
13
Rehm
M
,
Bruegger
D
,
Christ
F
,
Conzen
P
,
Thiel
M
,
Jacob
M
, et al.
Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116(17):1896–906. doi:.https://doi.org/10.1161/CIRCULATIONAHA.106.684852
14
Lee
WL
,
Slutsky
AS
. Sepsis and endothelial permeability. N Engl J Med. 2010;363(7):689–91. doi:.https://doi.org/10.1056/NEJMcibr1007320
15
Liang
Y
,
Li
X
,
Zhang
X
,
Li
Z
,
Wang
L
,
Sun
Y
, et al.
Elevated levels of plasma TNF-α are associated with microvascular endothelial dysfunction in patients with sepsis through activating the NF-κB and p38 mitogen-activated protein kinase in endothelial cells. Shock. 2014;41(4):275–81. doi:.https://doi.org/10.1097/SHK.0000000000000116
16
Chappell
D
,
Bruegger
D
,
Potzel
J
,
Jacob
M
,
Brettner
F
,
Vogeser
M
, et al.
Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care. 2014;18(5):538. doi:.https://doi.org/10.1186/s13054-014-0538-5
17
Wiedemann
HP
,
Wheeler
AP
,
Bernard
GR
,
Thompson
BT
,
Hayden
D
,
deBoisblanc
B
, et al., National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–75. doi:.https://doi.org/10.1056/NEJMoa062200
18
Balogh
Z
,
McKinley
BA
,
Cocanour
CS
,
Kozar
RA
,
Valdivia
A
,
Sailors
RM
, et al.
Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg. 2003;138(6):637–42, discussion 642–3. doi:.https://doi.org/10.1001/archsurg.138.6.637
19
Kellum
JA
,
Prowle
JR
. Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol. 2018;14(4):217–30. doi:.https://doi.org/10.1038/nrneph.2017.184
20
Rivers
E
,
Nguyen
B
,
Havstad
S
,
Ressler
J
,
Muzzin
A
,
Knoblich
B
, et al.; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77. doi:.https://doi.org/10.1056/NEJMoa010307
21
Hébert
PC
,
Wells
G
,
Blajchman
MA
,
Marshall
J
,
Martin
C
,
Pagliarello
G
, et al.
A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–17. doi:.https://doi.org/10.1056/NEJM199902113400601
22
Peake
SL
,
Delaney
A
,
Bailey
M
,
Bellomo
R
,
Cameron
PA
,
Cooper
DJ
, et al., ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–506. doi:.https://doi.org/10.1056/NEJMoa1404380
23
Yealy
DM
,
Kellum
JA
,
Huang
DT
,
Barnato
AE
,
Weissfeld
LA
,
Pike
F
, et al., ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–93. doi:.https://doi.org/10.1056/NEJMoa1401602
24
Mouncey
PR
,
Osborn
TM
,
Power
GS
,
Harrison
DA
,
Sadique
MZ
,
Grieve
RD
, et al.; ProMISe Trial Investigators. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301–11. doi:.https://doi.org/10.1056/NEJMoa1500896
25
Angus
DC
,
Barnato
AE
,
Bell
D
,
Bellomo
R
,
Chong
CR
,
Coats
TJ
, et al.
A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med. 2015;41(9):1549–60. doi:.https://doi.org/10.1007/s00134-015-3822-1
26
Rowan
KM
,
Angus
DC
,
Bailey
M
,
Barnato
AE
,
Bellomo
R
,
Canter
RR
, et al., PRISM Investigators. Early, Goal-Directed Therapy for Septic Shock - A Patient-Level Meta-Analysis. N Engl J Med. 2017;376(23):2223–34. doi:.https://doi.org/10.1056/NEJMoa1701380
27
Quinlan
GJ
,
Martin
GS
,
Evans
TW
. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41(6):1211–9. doi:.https://doi.org/10.1002/hep.20720
28
Fleck
A
,
Hawker
F
,
Wallace
PI
,
Raines
G
,
Trotter
J
,
Ledingham
IM
, et al.
Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;325(8432):781–4. doi:.https://doi.org/10.1016/S0140-6736(85)91447-3
29
Rhodes
A
,
Evans
LE
,
Alhazzani
W
,
Levy
MM
,
Antonelli
M
,
Ferrer
R
, et al.
Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–77. doi:.https://doi.org/10.1007/s00134-017-4683-6
30
Davis
AL
,
Carcillo
JA
,
Aneja
RK
,
Deymann
AJ
,
Lin
JC
,
Nguyen
TC
, et al.
American College of Critical Care Medicine Clinical Practice Parameters for Hemodynamic Support of Pediatric and Neonatal Septic Shock. Crit Care Med. 2017;45(6):1061–93. doi:.https://doi.org/10.1097/CCM.0000000000002425
31
Reviewers
CIGA
,
Reviewers
CIGA
; Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ. 1998;317(7153):235–40. doi:.https://doi.org/10.1136/bmj.317.7153.235
32
Finfer
S
,
Bellomo
R
,
Boyce
N
,
French
J
,
Myburgh
J
,
Norton
R
; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–56. doi:.https://doi.org/10.1056/NEJMoa040232
33
Finfer
S
,
McEvoy
S
,
Bellomo
R
,
McArthur
C
,
Myburgh
J
,
Norton
R
; SAFE Study Investigators. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med. 2011;37(1):86–96. doi:.https://doi.org/10.1007/s00134-010-2039-6
34
Myburgh
J
,
Cooper
DJ
,
Finfer
S
,
Bellomo
R
,
Norton
R
,
Bishop
N
, et al., George Institute for International Health. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357(9):874–84. doi:.https://doi.org/10.1056/NEJMoa067514
35
Caironi
P
,
Tognoni
G
,
Masson
S
,
Fumagalli
R
,
Pesenti
A
,
Romero
M
, et al.; ALBIOS Study Investigators. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370(15):1412–21. doi:.https://doi.org/10.1056/NEJMoa1305727
36
Charpentier
J
,
Mira
J-P
. Efficacy and tolerance of hyperoncotic albumin administration in septic shock patients: the EARSS study. Intensive Care Med. 2011;37(Suppl 1):S115
. [abstract 0438].
37
Caironi
P
,
Langer
T
,
Gattinoni
L
. Albumin in critically ill patients: the ideal colloid?
Curr Opin Crit Care. 2015;21(4):302–8. doi:.https://doi.org/10.1097/MCC.0000000000000223
38
Patel
A
,
Laffan
MA
,
Waheed
U
,
Brett
SJ
. Randomised trials of human albumin for adults with sepsis: systematic review and meta-analysis with trial sequential analysis of all-cause mortality. BMJ. 2014;349(jul22 10):g4561. doi:.. Correction in: BMJ. 2014;349:g4850. doi:https://doi.org/10.1136/bmj.g4561
39
Xu
JY
,
Chen
QH
,
Xie
JF
,
Pan
C
,
Liu
SQ
,
Huang
LW
, et al.
Comparison of the effects of albumin and crystalloid on mortality in adult patients with severe sepsis and septic shock: a meta-analysis of randomized clinical trials. Crit Care. 2014;18(6):702. doi:.https://doi.org/10.1186/s13054-014-0702-y
40
Myburgh
JA
,
Mythen
MG
. Resuscitation fluids. N Engl J Med. 2013;369(13):1243–51. doi:.https://doi.org/10.1056/NEJMra1208627
41
Schortgen
F
,
Lacherade
JC
,
Bruneel
F
,
Cattaneo
I
,
Hemery
F
,
Lemaire
F
, et al.
Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet. 2001;357(9260):911–6. doi:.https://doi.org/10.1016/S0140-6736(00)04211-2
42
Brunkhorst
FM
,
Engel
C
,
Bloos
F
,
Meier-Hellmann
A
,
Ragaller
M
,
Weiler
N
, et al.; German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–39. doi:.https://doi.org/10.1056/NEJMoa070716
43
Perner
A
,
Haase
N
,
Guttormsen
AB
,
Tenhunen
J
,
Klemenzson
G
,
Åneman
A
, et al.; 6S Trial Group; Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367(2):124–34. doi:.https://doi.org/10.1056/NEJMoa1204242
44
Perner
A
,
Haase
N
,
Winkel
P
,
Guttormsen
AB
,
Tenhunen
J
,
Klemenzson
G
, et al.
Long-term outcomes in patients with severe sepsis randomised to resuscitation with hydroxyethyl starch 130/0.42 or Ringer’s acetate. Intensive Care Med. 2014;40(7):927–34. doi:.https://doi.org/10.1007/s00134-014-3311-y
45
Müller
RB
,
Haase
N
,
Lange
T
,
Wetterslev
J
,
Perner
A
. Acute kidney injury with hydroxyethyl starch 130/0.42 in severe sepsis. Acta Anaesthesiol Scand. 2015;59(3):329–36. doi:.https://doi.org/10.1111/aas.12453
46
Myburgh
JA
,
Finfer
S
,
Bellomo
R
,
Billot
L
,
Cass
A
,
Gattas
D
, et al.; CHEST Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901–11. doi:.https://doi.org/10.1056/NEJMoa1209759
47
Guidet
B
,
Martinet
O
,
Boulain
T
,
Philippart
F
,
Poussel
JF
,
Maizel
J
, et al.
Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care. 2012;16(3):R94. doi:.https://doi.org/10.1186/cc11358
48
Annane
D
,
Siami
S
,
Jaber
S
,
Martin
C
,
Elatrous
S
,
Declère
AD
, et al.; CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013;310(17):1809–17. doi:.https://doi.org/10.1001/jama.2013.280502
49
Haase
N
,
Perner
A
,
Hennings
LI
,
Siegemund
M
,
Lauridsen
B
,
Wetterslev
M
, et al.
Hydroxyethyl starch 130/0.38-0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta-analysis and trial sequential analysis. BMJ. 2013;346(feb15 1):f839. doi:.https://doi.org/10.1136/bmj.f839
50
Zarychanski
R
,
Abou-Setta
AM
,
Turgeon
AF
,
Houston
BL
,
McIntyre
L
,
Marshall
JC
, et al.
Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013;309(7):678–88. doi:.https://doi.org/10.1001/jama.2013.430
51
Rochwerg
B
,
Alhazzani
W
,
Sindi
A
,
Heels-Ansdell
D
,
Thabane
L
,
Fox-Robichaud
A
, et al.; Fluids in Sepsis and Septic Shock Group. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med. 2014;161(5):347–55. doi:.https://doi.org/10.7326/M14-0178
52
Perner
A
,
Prowle
J
,
Joannidis
M
,
Young
P
,
Hjortrup
PB
,
Pettilä
V
. Fluid management in acute kidney injury. Intensive Care Med. 2017;43(6):807–15. doi:.https://doi.org/10.1007/s00134-017-4817-x
53
Bayer
O
,
Reinhart
K
,
Kohl
M
,
Kabisch
B
,
Marshall
J
,
Sakr
Y
, et al.
Effects of fluid resuscitation with synthetic colloids or crystalloids alone on shock reversal, fluid balance, and patient outcomes in patients with severe sepsis: a prospective sequential analysis. Crit Care Med. 2012;40(9):2543–51. doi:.https://doi.org/10.1097/CCM.0b013e318258fee7
54
Moeller
C
,
Fleischmann
C
,
Thomas-Rueddel
D
,
Vlasakov
V
,
Rochwerg
B
,
Theurer
P
, et al.
How safe is gelatin? A systematic review and meta-analysis of gelatin-containing plasma expanders vs crystalloids and albumin. J Crit Care. 2016;35:75–83. doi:.https://doi.org/10.1016/j.jcrc.2016.04.011
55
Ma
PL
,
Peng
XX
,
Du
B
,
Hu
XL
,
Gong
YC
,
Wang
Y
, et al.
Sources of Heterogeneity in Trials Reporting Hydroxyethyl Starch 130/0.4 or 0.42 Associated Excess Mortality in Septic Patients: A Systematic Review and Meta-regression. Chin Med J (Engl). 2015;128(17):2374–82. doi:.https://doi.org/10.4103/0366-6999.163387
56
Orbegozo Cortés
D
,
Gamarano Barros
T
,
Njimi
H
,
Vincent
JL
. Crystalloids versus colloids: exploring differences in fluid requirements by systematic review and meta-regression. Anesth Analg. 2015;120(2):389–402. doi:.https://doi.org/10.1213/ANE.0000000000000564
57
Boyd
JH
,
Forbes
J
,
Nakada
TA
,
Walley
KR
,
Russell
JA
. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259–65. doi:.https://doi.org/10.1097/CCM.0b013e3181feeb15
58
Marik
PE
,
Linde-Zwirble
WT
,
Bittner
EA
,
Sahatjian
J
,
Hansell
D
. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med. 2017;43(5):625–32. doi:.https://doi.org/10.1007/s00134-016-4675-y
59
Ospina-Tascon
G
,
Neves
AP
,
Occhipinti
G
,
Donadello
K
,
Büchele
G
,
Simion
D
, et al.
Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010;36(6):949–55. doi:.https://doi.org/10.1007/s00134-010-1843-3
60
Chowdhury
AH
,
Cox
EF
,
Francis
ST
,
Lobo
DN
. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256(1):18–24. doi:.https://doi.org/10.1097/SLA.0b013e318256be72
61
Reid
F
,
Lobo
DN
,
Williams
RN
,
Rowlands
BJ
,
Allison
SP
. (Ab)normal saline and physiological Hartmann’s solution: a randomized double-blind crossover study. Clin Sci (Lond). 2003;104(1):17–24.
62
Yunos
NM
,
Bellomo
R
,
Hegarty
C
,
Story
D
,
Ho
L
,
Bailey
M
. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566–72. doi:.https://doi.org/10.1001/jama.2012.13356
63
Raghunathan
K
,
Shaw
A
,
Nathanson
B
,
Stürmer
T
,
Brookhart
A
,
Stefan
MS
, et al.
Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis*. Crit Care Med. 2014;42(7):1585–91. doi:.https://doi.org/10.1097/CCM.0000000000000305
64
Shaw
AD
,
Raghunathan
K
,
Peyerl
FW
,
Munson
SH
,
Paluszkiewicz
SM
,
Schermer
CR
. Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive Care Med. 2014;40(12):1897–905. doi:.https://doi.org/10.1007/s00134-014-3505-3
65
Neyra
JA
,
Canepa-Escaro
F
,
Li
X
,
Manllo
J
,
Adams-Huet
B
,
Yee
J
, et al.; Acute Kidney Injury in Critical Illness Study Group. Association of Hyperchloremia With Hospital Mortality in Critically Ill Septic Patients. Crit Care Med. 2015;43(9):1938–44. doi:.https://doi.org/10.1097/CCM.0000000000001161
66
Raghunathan
K
,
Bonavia
A
,
Nathanson
BH
,
Beadles
CA
,
Shaw
AD
,
Brookhart
MA
, et al.
Association between Initial Fluid Choice and Subsequent In-hospital Mortality during the Resuscitation of Adults with Septic Shock. Anesthesiology. 2015;123(6):1385–93. doi:.https://doi.org/10.1097/ALN.0000000000000861
67
Young
P
,
Bailey
M
,
Beasley
R
,
Henderson
S
,
Mackle
D
,
McArthur
C
, et al.; SPLIT Investigators; ANZICS CTG. Effect of a Buffered Crystalloid Solution vs Saline on Acute Kidney Injury Among Patients in the Intensive Care Unit: The SPLIT Randomized Clinical Trial. JAMA. 2015;314(16):1701–10. doi:.https://doi.org/10.1001/jama.2015.12334
68
Semler
MW
,
Wanderer
JP
,
Ehrenfeld
JM
,
Stollings
JL
,
Self
WH
,
Siew
ED
, et al.; SALT Investigators * and the Pragmatic Critical Care Research Group; SALT Investigators. Balanced Crystalloids versus Saline in the Intensive Care Unit. The SALT Randomized Trial. Am J Respir Crit Care Med. 2017;195(10):1362–72. doi:.https://doi.org/10.1164/rccm.201607-1345OC
69
Sen
A
,
Keener
CM
,
Sileanu
FE
,
Foldes
E
,
Clermont
G
,
Murugan
R
, et al.
Chloride Content of Fluids Used for Large-Volume Resuscitation Is Associated With Reduced Survival. Crit Care Med. 2017;45(2):e146–53. doi:.https://doi.org/10.1097/CCM.0000000000002063
70
Semler
MW
,
Self
WH
,
Wanderer
JP
,
Ehrenfeld
JM
,
Wang
L
,
Byrne
DW
, et al.; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med. 2018;378(9):829–39. doi:.https://doi.org/10.1056/NEJMoa1711584
71
Cannon
JW
. Hemorrhagic Shock. N Engl J Med. 2018;378(4):370–9. doi:.https://doi.org/10.1056/NEJMra1705649
72
Dutton
RP
. Management of traumatic haemorrhage--the US perspective. Anaesthesia. 2015;70(Suppl 1):108–11, e38. doi:.https://doi.org/10.1111/anae.12894
73
Wise
R
,
Faurie
M
,
Malbrain
MLNG
,
Hodgson
E
. Strategies for Intravenous Fluid Resuscitation in Trauma Patients. World J Surg. 2017;41(5):1170–83. doi:.https://doi.org/10.1007/s00268-016-3865-7
74
Jabaley
C
,
Dudaryk
R
. Fluid resuscitation for trauma patients: crystalloids versus colloids. Curr Anesthesiol Rep. 2014;4(3):216–24. doi:.https://doi.org/10.1007/s40140-014-0067-4
75
Schöchl
H
,
Voelckel
W
,
Schlimp
CJ
. Management of traumatic haemorrhage--the European perspective. Anaesthesia. 2015;70(Suppl 1):102–7, e35–7. doi:.https://doi.org/10.1111/anae.12901
76
Chappell
D
,
Jacob
M
. Hydroxyethyl starch - the importance of being earnest. Scand J Trauma Resusc Emerg Med. 2013;21(1):61. doi:.https://doi.org/10.1186/1757-7241-21-61
77
Haase
N
,
Perner
A
. Hydroxyethyl starch for resuscitation. Curr Opin Crit Care. 2013;19(4):321–5. doi:.https://doi.org/10.1097/MCC.0b013e3283632de6
78
Bickell
WH
. Are victims of injury sometimes victimized by attempts at fluid resuscitation?
Ann Emerg Med. 1993;22(2):225–6. doi:.https://doi.org/10.1016/S0196-0644(05)80208-2
79
Bunn
F
,
Trivedi
D
. Colloid solutions for fluid resuscitation. Cochrane Database Syst Rev. 2012;(7):CD001319.
80
Perel
P
,
Roberts
I
,
Ker
K
. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2013;(2):CD000567. doi:.https://doi.org/10.1002/14651858.CD000567.pub6
81
Rehm
M
,
Haller
M
,
Brechtelsbauer
H
,
Akbulut
C
,
Finsterer
U
. Extra protein loss not caused by surgical bleeding in patients with ovarian cancer. Acta Anaesthesiol Scand. 1998;42(1):39–46. doi:.https://doi.org/10.1111/j.1399-6576.1998.tb05078.x
82
de Crescenzo
C
,
Gorouhi
F
,
Salcedo
ES
,
Galante
JM
. Prehospital hypertonic fluid resuscitation for trauma patients: A systematic review and meta-analysis. J Trauma Acute Care Surg. 2017;82(5):956–62. doi:.https://doi.org/10.1097/TA.0000000000001409
83
Kozar
RA
,
Peng
Z
,
Zhang
R
,
Holcomb
JB
,
Pati
S
,
Park
P
, et al.
Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112(6):1289–95. doi:.https://doi.org/10.1213/ANE.0b013e318210385c
84Bundesärztekammer. Querschnitts-Leitlinien zur Therapie mit Blutkomponenten und Plasmaderivaten. Bundesärztekammer, editor: Deutscher Ärzte Verlag; 2015.
85
Kozek-Langenecker
S
,
Sørensen
B
,
Hess
JR
,
Spahn
DR
. Clinical effectiveness of fresh frozen plasma compared with fibrinogen concentrate: a systematic review. Crit Care. 2011;15(5):R239. doi:.https://doi.org/10.1186/cc10488
86
Ho
AM
,
Dion
PW
,
Yeung
JH
,
Holcomb
JB
,
Critchley
LA
,
Ng
CS
, et al.
Prevalence of survivor bias in observational studies on fresh frozen plasma:erythrocyte ratios in trauma requiring massive transfusion. Anesthesiology. 2012;116(3):716–28. doi:.https://doi.org/10.1097/ALN.0b013e318245c47b
87
Armand
R
,
Hess
JR
. Treating coagulopathy in trauma patients. Transfus Med Rev. 2003;17(3):223–31. doi:.https://doi.org/10.1016/S0887-7963(03)00022-1
88
Innerhofer
P
,
Fries
D
,
Mittermayr
M
,
Innerhofer
N
,
von Langen
D
,
Hell
T
, et al.
Reversal of trauma-induced coagulopathy using first-line coagulation factor concentrates or fresh frozen plasma (RETIC): a single-centre, parallel-group, open-label, randomised trial. Lancet Haematol. 2017;4(6):e258–71. doi:.https://doi.org/10.1016/S2352-3026(17)30077-7
89
Lewis
SR
,
Pritchard
MW
,
Evans
DJ
,
Butler
AR
,
Alderson
P
,
Smith
AF
, et al.
Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 2018;8:CD000567. doi:.https://doi.org/10.1002/14651858.CD000567.pub7
90
Vincent
JL
,
Sakr
Y
,
Sprung
CL
,
Ranieri
VM
,
Reinhart
K
,
Gerlach
H
, et al.; Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–53. doi:.https://doi.org/10.1097/01.CCM.0000194725.48928.3A
91
Micek
ST
,
McEvoy
C
,
McKenzie
M
,
Hampton
N
,
Doherty
JA
,
Kollef
MH
. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care. 2013;17(5):R246. doi:.https://doi.org/10.1186/cc13072
92
Kelm
DJ
,
Perrin
JT
,
Cartin-Ceba
R
,
Gajic
O
,
Schenck
L
,
Kennedy
CC
. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock. 2015;43(1):68–73. doi:.https://doi.org/10.1097/SHK.0000000000000268
93
Besen
BA
,
Taniguchi
LU
. Negative Fluid Balance in Sepsis: When and How?
Shock. 2017;47(1S, Suppl 1):35–40. doi:.https://doi.org/10.1097/SHK.0000000000000701
94
Sakr
Y
,
Rubatto Birri
PN
,
Kotfis
K
,
Nanchal
R
,
Shah
B
,
Kluge
S
, et al.; Intensive Care Over Nations Investigators. Higher Fluid Balance Increases the Risk of Death From Sepsis: Results From a Large International Audit. Crit Care Med. 2017;45(3):386–94. doi:.https://doi.org/10.1097/CCM.0000000000002189
95
Russell
JA
,
Walley
KR
,
Singer
J
,
Gordon
AC
,
Hébert
PC
,
Cooper
DJ
, et al.; VASST Investigators. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877–87. doi:.https://doi.org/10.1056/NEJMoa067373
96
Jansen
TC
,
van Bommel
J
,
Schoonderbeek
FJ
,
Sleeswijk Visser
SJ
,
van der Klooster
JM
,
Lima
AP
, et al.; LACTATE study group. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752–61. doi:.https://doi.org/10.1164/rccm.200912-1918OC
97
Monnet
X
,
Jabot
J
,
Maizel
J
,
Richard
C
,
Teboul
JL
. Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients. Crit Care Med. 2011;39(4):689–94. doi:.https://doi.org/10.1097/CCM.0b013e318206d2a3