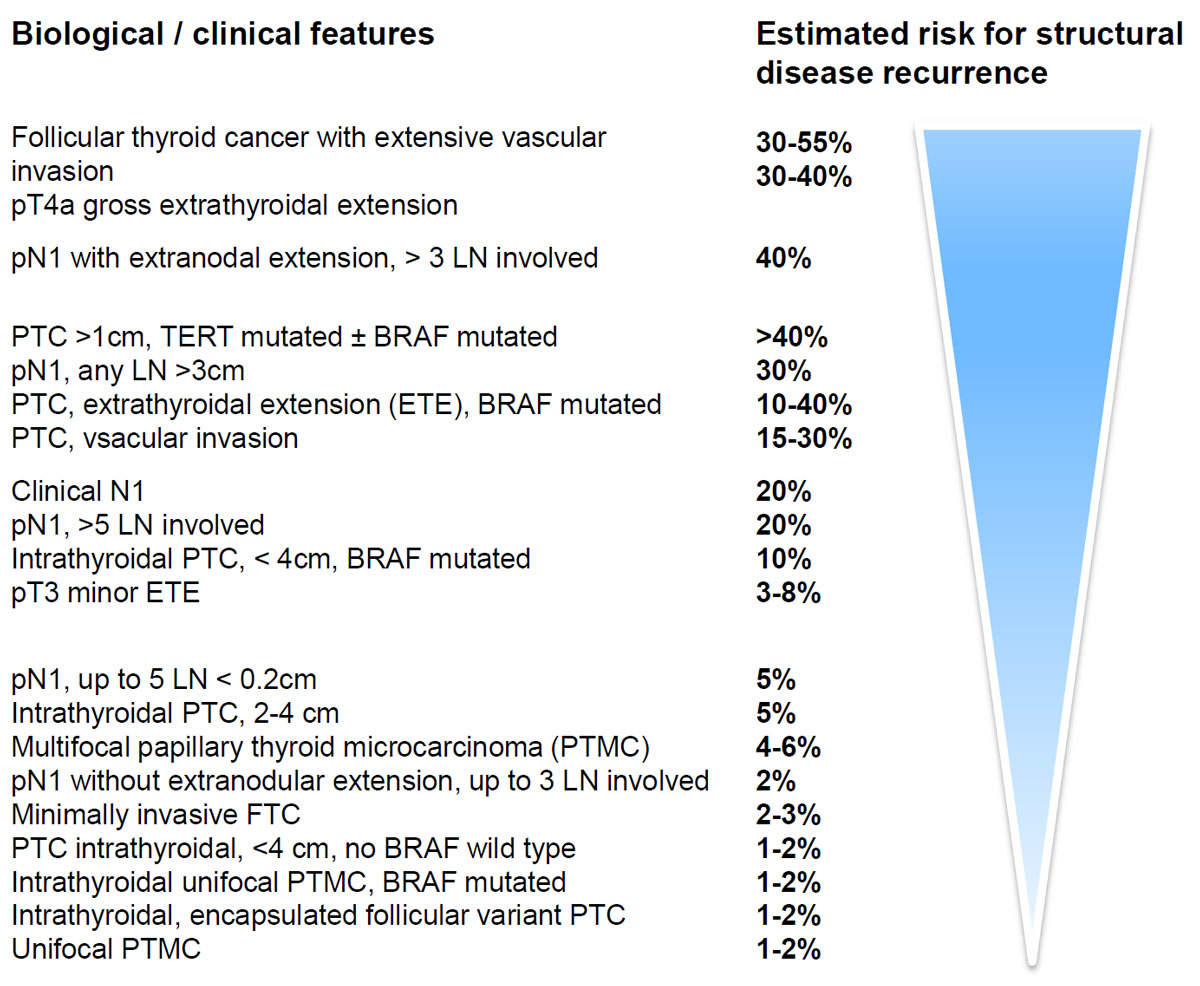
Figure 1 Description of the risk for structural disease recurrence as a continuum in differentiated thyroid cancer (adapted from [1]) in patients without structurally identifiable disease after first initial therapy.
DOI: https://doi.org/10.4414/smw.2019.14700
After the update of the American Thyroid Association (ATA) guidelines on diagnosis and treatment of patients with thyroid cancer in 2015 [1], it became evident that, for patients with low-risk thyroid cancer, these recommendations presented views on the requirement for adjuvant radioiodine therapy different from the recommendations of the European Thyroid Association (ETA) [2] and daily practice in Switzerland. In order to avoid offering differing treatment options to patients and thus potential uncertainties, it is important that physicians who take care of such patients have similar views on the matter. With the intention to find a common view on the management of patients with low-risk papillary thyroid cancer (PTC), a multidisciplinary working group was initiated by the Swiss Society for Endocrinology and Diabetes and endorsed by the Swiss Society for Nuclear Medicine and the working group of Swiss Endocrine Surgeons. The working group thus included the endocrinologists, nuclear medicine physicians, pathologists and endocrine surgeons who are authors of this report. The discussions started with a meeting at the Triemlispital in Zurich on 11 May 2016 and were followed by various written exchanges of opinion during the preparation of a manuscript. The report presented herein is a summary of the meeting and the post-meeting discussions.
The care of patients with thyroid cancer is a wide area that covers presurgical management of thyroid nodules, the choice of the appropriate surgery and postsurgical care including radioiodine treatment. Our discussions focused primarily on the identification of patients with low-risk PTC and their initial treatment options.
Obtaining high-level evidence on patients with low-risk PTC is difficult because the disease progresses very slowly and controlled long-term studies lasting many years in thousands of patients are needed in order to acquire the necessary data. These trials are very difficult to perform. It is, therefore, not surprising that a substantial part of the evidence is based on retrospective and/or observational data. The need for long-term intervention and the actual quality of the data result in different opinions/strategies of the various involved stakeholders, which are also summarised in this article.
The incidence of differentiated thyroid cancer (DTC) has increased in the past 20 years, which is primarily the result of the more frequent use of ultrasound imaging in the management of thyroid disorders. The higher prevalence is almost entirely due to the discovery of small PTCs of less than 2 cm. This observation had already been reported 10 years ago in the USA [3]. The most convincing association between increased use of thyroid ultrasound and increased incidence of small PTCs was reported recently from South Korea [4]. Here, the addition of routine thyroid ultrasound in patients who were screened primarily for other malignancies such as breast cancer led to a more than ten-fold increase in thyroid cancer incidence that was almost entirely due to the accidental discovery of small PTCs [4]. In Switzerland also, the incidence of thyroid cancer increased, although to lesser extent than in the USA, Italy or South Korea [5, 6].
For many years, patients with low-risk DTC were treated with total thyroidectomy, radioiodine treatment and thyroid stimulating hormone-suppressive thyroxine therapy. Various observational studies examined the impact of adjuvant radioiodine therapy in low-risk patients and found no convincing benefit [7–11]. Indeed, the disease-specific survival was excellent and similar with and without radioiodine treatment in these studies, with 99% disease-specific survival after 20 years [6]. Similarly, the tumour recurrence rate was comparable and was estimated to be 1 to 4% after 7 to 20 years of follow-up [11–13]. Although these were retrospective observational studies and different risk scores were used to define low-risk, their results were quite consistent. Concerns were raised regarding the long-term safety of radioiodine treatment in patients with low-risk DTC, and everybody can agree that unnecessary exposure to radiation should be avoided.
The increased incidence of mainly low-risk DTC, the lack of evidence for the therapeutic benefit of total thyroidectomy and of radioiodine therapy in these patients [7–11], as well as the safety concerns mentioned above thus prompted a reassessment of the management of patients with low-risk PTC.
This led to the new comprehensive recommendations released by the ATA in 2015 [1]. The definition of low-risk thyroid cancer according to the new ATA guidelines is given in table 1. As compared with previous guidelines (of the ATA and ETA), the new guidelines additionally describe the risk of structural disease recurrence in patients without structurally identifiable disease after initial therapy as a continuum based on various features including the number of microscopic lymph node metastases, vascular invasion and biological features of the tumour, but without defining a size limit for the primary PTC (fig.1).
Table 1 Characteristics of a low-risk thyroid cancer according to the new ATA 2015 guidelines (adapted from [1]).
| Papillary thyroid cancer with all of the following: | All macroscopic tumour has been resected |
| Absence of local of distant metastases | |
| Without tumour invasion of loco-regional tissues | |
| No aggressive feature on histology (e.g., tall cell, hobnail variant, columnar cell carcinoma | |
| If 131I is given, no evidence for radioiodine-avid foci outside the thyroid bed on first post-treatment whole-body radioiodine scan | |
| No vascular invasion | |
| No clinical evidence for lymph node metastases or LN1 with up to five micrometastases (<0.2 cm in largest dimension) | |
| Intrathyroidal, encapsulated follicular variant of PTC | |
| Intrathyroidal, well differentiated follicular thyroid cancer with capsular invasion and no or minimal (<4 foci) vascular invasion | |
| Intrathyroidal, papillary microcarcinoma, unifocal or multifocal, including BRAF mutation | |

Figure 1 Description of the risk for structural disease recurrence as a continuum in differentiated thyroid cancer (adapted from [1]) in patients without structurally identifiable disease after first initial therapy.
In view of the relatively low aggressiveness of differentiated PTC with excellent prognosis, surgical procedures may have to be weighed against the risk for surgical complications. The invasiveness of the thyroid surgery should thus match the presumed aggressiveness of the tumour.
Numerous studies have shown that in patients with isolated PTC, less invasive surgical procedures such as lobectomy had similar outcomes as total thyroidectomy regarding survival, but with significantly reduced complication rates, namely, reduced frequency of recurrent nerve palsy and reduced rate of permanent hypoparathyroidism [14–16].
The risk for local and distant metastases increases with increasing size of the PTC, especially if the tumour size exceeds 2 cm, as has been described in an observational study involving 366 patients with PTC [17]. This is the reason why it was decided at the thyroid cancer board meeting to limit the low-risk definition to a size of 2 cm or less, in contrast to the ATA guidelines where no formal size limit was set in the definition of low risk. The ATA guidelines also included in their risk assessment for PTC microscopic lymph node involvement and one could argue that without collection of lymph nodes use of such a criterion for proper risk estimation of PTC would be impossible.
In contrast to the ATA guidelines, we decided to limit the low-risk definition to the size of 20 mm. With a PTC of 20 mm or less, the risk for clinically relevant lymph node involvement is very low [17]. It is for this reason that we also do not recommend routine ipsilateral prophylactic clearance of lymph nodes. This recommendation does not, however, exclude exceptional cases such as familial forms of PTC or postradiation PTC, where a different approach, including total thyroidectomy and/or prophylactic clearance of lymph nodes, could be justified. Given the higher risk of complications, such procedures should be limited to very experienced surgeons [18–22]. However, as there are no proven benefits concerning long-term outcome [21, 23–25] and most thyroid procedures in Switzerland are not performed by subspecialised surgeons, prophylactic lymph node dissection cannot be recommended as a standard procedure in patients with small a PTC without macroscopic evidence of lymph node metastases.
The planning of the initial thyroid procedure should therefore be risk oriented, based on the available preoperative data, especially ultrasound studies to detect potential macroscopic lymph node metastases. In the absence of macroscopic lymph node metastases and the presence of a uninodular PTC of 20 mm or less, a lobectomy could be sufficient and should be discussed with patient.
For a long time, routine radioiodine ablation was an established standard of care in differentiated thyroid cancer (DTC) patients, an exception being the PTC microcarcinoma (less than 10 mm. In these cases, there is an overall agreement to refrain from radioiodine treatment if total thyroidectomy was performed, and from completion surgery if lobectomy was the initial treatment and the diagnosis of the microcarcinoma an incidental finding.
According to the new ATA guidelines, radioiodine ablation on a routine basis in low-risk DTC patients is not recommended. This recommendation, however, was made in spite of the presence of conflicting observational data. In absence of 131I administration, the adjuvant and a fortiori therapeutic goals of radioiodine will be lost in patients with unsuspected persistent metastatic disease foci after surgery. Only 2 out of 101 ATA 2015 recommendations were supported by a high level of evidence and the ATA guidelines were challenged by many other scientific societies owing to the observational character of the studies that were the basis for this recommendation and because such an approach had not been tested in prospective studies.
For example, a reduction of recurrences and better outcome are expected in patients with low-risk PTCs larger than 20 mm when radioiodine ablation is part of their treatment, and these differences are relevant for clinical decision making. Indeed, low-risk radioiodine ablated DTC patients with an excellent response can be safely considered cured. A minimal follow-up (clinical examination and thyroglobulin measurement) should be arranged every 1 to 2 years for such patients, thus increasing their comfort and saving resources.
On the other hand, the occurrence of non-thyroidal primary malignancies and the potential association with radioiodine treatment are important concerns in patients with DTC.
An increased incidence of second cancer has been reported after radioiodine treatment for thyroid cancer, but data were conflicting and a causal relationship may be difficult to establish [23–25]. An increased risk of second malignancies is definitely proved when cumulative exposures exceed 37 GBq (1000 mCi). It should be noted, however, that these levels are reached only in patients with advanced disease and a high risk of disease-related death. Additionally, the absolute risk for single patients remains low even in these cases, with standardised incidence ratios about 1.3 to 1.5 [26]. Notably, significantly lower activities (0.8–3.7 GBq [20–100 mCi]) are currently employed to ablate DTC in low-risk patients and recent studies concluded that the risk of a second primary malignancy is not increased in patients receiving these levels of radioiodine compared with those who did not receive radioiodine [27].
Notwithstanding, the risks and benefits of any treatment must be weighted and administration of radioiodine must be justified and optimised according to the ALARA (as low as reasonably achievable) concept. This a specific task of nuclear medicine physicians, as stated in national and international regulations.
In conclusion, since the literature cannot provide a basis for advice for or against current guidelines, further long-term follow-up studies are needed before recommendations to change effective clinical practice are accepted as standard of care.
Prospective randomised trials in Europe (ESTIMABL2, IoN) and South Korea (Clinical trial.gov identifier NCT01837745, NCT01398085, and NCT02418247) to assess the potential benefit of radioiodine ablation in low-risk patients are underway and first results will be available in ∼5 years from now. As long as the results of such studies are still pending, it would be prudent to refrain from a strong position against 131I as thus far the course of the disease with 131I therapy has been so good.
A critical element in the classification of PTC as low risk is its biology. For proper decision making, it is therefore important that the pathology results report all histological features of the cancer associated with its biology, as well as all information related to the Union Internationale Contre le Cancer (UICC) TNM cancer staging system. This should include the histological type of the primary tumour, whether it is encapsulated or not, the presence or absence of capsular and vascular invasion, and the number and size of lymph node metastases (if lymph nodes were removed). It is also important to note that the lesions formerly classified as the encapsulated follicular variant of PTC have been recently reclassified as so-called noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) in order to reduce overtreatment of indolent lesions [28]. The presence or absence of features associated with an adverse outcome such as tall cells, the columnar cell variant or the hobnail variant, and lymph or haemangio-invasion should be noted. These features are summarised and updated in synoptic reporting systems such as the one used by the College of American Pathologists (CAP). Such a system can be readily implemented in the Swiss situation and was recently introduced into the department of pathology of the University of Bern (see table 2).
Table 2 Proposed standardised reporting for differentiated thyroid cancer.
| Diagnosis according to the TNM system (e.g., pT2,pNx, L1, V0, R0) |
| Histological type: e.g., classical papillary thyroid cancer |
| Size of the tumour |
| Extrathyroidal extension |
| Complete resection? |
| Multifocal: yes or no |
| Tall cell variant: present, if yes how many cells (10% or more)? |
| Invasion in blood vessels and lymphatic vessels |
| Metastases in lymph nodes, if yes, how many are macro metastases and how may micro metastases (micro <0.2 cm)? |
Molecular testing of thyroid carcinomas for prediction of patient outcome is evolving rapidly; however, to date no single gold standard molecular marker has been identified. BRAF mutation testing is not routinely recommended because prognostic information is controversial in many different studies [29–31]. BRAF mutation testing may have a role in conjunction with molecular testing of TP53, AKT1 or PIK3CA via a targeted NGS (next generation sequencing) approach, since these double mutant PTCs are more aggressive [32]. TERT promoter mutations, on the other hand, can well be used as a prognostic marker – the drawback is that they are only found in a subset of about 7 to 8% of PTCs [33].
The current practice of total thyroidectomy followed by radioiodine therapy in patients with DTC started a long time before the advent of evidence-based medicine, in 1943 [34], and was used thereafter with lifesaving effects for many patients with severe metastatic disease. The landmark publication by Mazzaferri and Kloos on the treatment of patients with DTC, with 40 years of follow-up [35], was an observational cohort study summarising data from more than 1500 patients and describing the beneficial effect of radioiodine therapy in patients diagnosed before availability of computed tomography or ultrasound. Similarly, the disputed studies that challenged the benefit of radioiodine therapy in patients with low-risk PTC have a very comparable level of evidence; they also were observational and retrospective.
All participants agreed on the general concept of risk-adapted treatment of patients with low-risk PTC. Many, however, felt very uneasy with the suggested definition of low-risk PTC in the new ATA guidelines, which did not include a size limit of the primary tumour (table 1), citing the association between tumour size and the risk for local and distant metastases, which showed that the risk for local extension of PTC increased significantly when the primary tumour exceeded 20 mm [17].
Therefore, we agreed to limit the definition of low risk to those PTCs up to 20 mm in size, while acknowledging the general concept for the definition of low risk as outlined by the ATA. Thus, patients with a PTC up to 20 mm and without local extension of the tumour and without macroscopic evidence for lymph node metastases or other negative histological feature (such as hobnail or tall cell cancer, vascular invasion) can be considered as having low-risk PTC.
We may have to take into account that today patients, as well as their primary care physicians, are more informed than some years ago and that the ATA guidelines are widely published and discussed. Therefore we may have to discuss these guidelines and explain why our recommendations differ from those proposed by the ATA.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Haugen BR , Alexander EK , Bible KC , Doherty GM , Mandel SJ , Nikiforov YE , et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. doi:.https://doi.org/10.1089/thy.2015.0020
2 Pacini F , Schlumberger M , Dralle H , Elisei R , Smit JWA , Wiersinga W ; European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154(6):787–803. doi:.https://doi.org/10.1530/eje.1.02158
3 Davies L , Welch HG . Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295(18):2164–7. doi:.https://doi.org/10.1001/jama.295.18.2164
4 Ahn HS , Kim HJ , Welch HG . Korea’s thyroid-cancer “epidemic”--screening and overdiagnosis. N Engl J Med. 2014;371(19):1765–7. doi:.https://doi.org/10.1056/NEJMp1409841
5Bundesamt für Statistik. Krebs in der Schweiz. Stand und Entwicklung von 1983 bis 2007. Neuchâtel: Bundesamt für Statistik; 2011. Available at: https://www.bfs.admin.ch/bfs/de/home/aktuell/neue-veroeffentlichungen.assetdetail.347610.html.
6 Brito JP , Hay ID , Morris JC . Low risk papillary thyroid cancer. BMJ. 2014;348:g3045. doi:.https://doi.org/10.1136/bmj.g3045
7 Hay ID . Selective use of radioactive iodine in the postoperative management of patients with papillary and follicular thyroid carcinoma. J Surg Oncol. 2006;94(8):692–700. doi:.https://doi.org/10.1002/jso.20696
8 Hay ID , Hutchinson ME , Gonzalez-Losada T , McIver B , Reinalda ME , Grant CS , et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144(6):980–7, discussion 987–8. doi:.https://doi.org/10.1016/j.surg.2008.08.035
9 Sawka AM , Thephamongkhol K , Brouwers M , Thabane L , Browman G , Gerstein HC . Clinical review 170: A systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2004;89(8):3668–76. doi:.https://doi.org/10.1210/jc.2003-031167
10 Sacks W , Fung CH , Chang JT , Waxman A , Braunstein GD . The effectiveness of radioactive iodine for treatment of low-risk thyroid cancer: a systematic analysis of the peer-reviewed literature from 1966 to April 2008. Thyroid. 2010;20(11):1235–45. doi:.https://doi.org/10.1089/thy.2009.0455
11 Schvartz C , Bonnetain F , Dabakuyo S , Gauthier M , Cueff A , Fieffé S , et al. Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. J Clin Endocrinol Metab. 2012;97(5):1526–35. doi:.https://doi.org/10.1210/jc.2011-2512
12 Hay ID , McConahey WM , Goellner JR . Managing patients with papillary thyroid carcinoma: insights gained from the Mayo Clinic’s experience of treating 2,512 consecutive patients during 1940 through 2000. Trans Am Clin Climatol Assoc. 2002;113:241–60.
13 Tuttle RM , Tala H , Shah J , Leboeuf R , Ghossein R , Gonen M , et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20(12):1341–9. doi:.https://doi.org/10.1089/thy.2010.0178
14 Matsuzu K , Sugino K , Masudo K , Nagahama M , Kitagawa W , Shibuya H , et al. Thyroid lobectomy for papillary thyroid cancer: long-term follow-up study of 1,088 cases. World J Surg. 2014;38(1):68–79. doi:.https://doi.org/10.1007/s00268-013-2224-1
15 Adam MA , Pura J , Goffredo P , Dinan MA , Hyslop T , Reed SD , et al. Impact of extent of surgery on survival for papillary thyroid cancer patients younger than 45 years. J Clin Endocrinol Metab. 2015;100(1):115–21. doi:.https://doi.org/10.1210/jc.2014-3039
16 Adam MA , Pura J , Gu L , Dinan MA , Tyler DS , Reed SD , et al. Extent of surgery for papillary thyroid cancer is not associated with survival: an analysis of 61,775 patients. Ann Surg. 2014;260(4):601–5, discussion 605–7. doi:.https://doi.org/10.1097/SLA.0000000000000925
17 Machens A , Holzhausen HJ , Dralle H . The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005;103(11):2269–73. doi:.https://doi.org/10.1002/cncr.21055
18 Wang TS , Cheung K , Farrokhyar F , Roman SA , Sosa JA . A meta-analysis of the effect of prophylactic central compartment neck dissection on locoregional recurrence rates in patients with papillary thyroid cancer. Ann Surg Oncol. 2013;20(11):3477–83. doi:.https://doi.org/10.1245/s10434-013-3125-0
19 Lang BH , Ng SH , Lau LL , Cowling BJ , Wong KP , Wan KY . A systematic review and meta-analysis of prophylactic central neck dissection on short-term locoregional recurrence in papillary thyroid carcinoma after total thyroidectomy. Thyroid. 2013;23(9):1087–98. doi:.https://doi.org/10.1089/thy.2012.0608
20 Shan C-X , Zhang W , Jiang D-Z , Zheng X-M , Liu S , Qiu M . Routine central neck dissection in differentiated thyroid carcinoma: a systematic review and meta-analysis. Laryngoscope. 2012;122(4):797–804. doi:.https://doi.org/10.1002/lary.22162
21 Zetoune T , Keutgen X , Buitrago D , Aldailami H , Shao H , Mazumdar M , et al. Prophylactic central neck dissection and local recurrence in papillary thyroid cancer: a meta-analysis. Ann Surg Oncol. 2010;17(12):3287–93. doi:.https://doi.org/10.1245/s10434-010-1137-6
22 Chisholm EJ , Kulinskaya E , Tolley NS . Systematic review and meta-analysis of the adverse effects of thyroidectomy combined with central neck dissection as compared with thyroidectomy alone. Laryngoscope. 2009;119(6):1135–9. doi:.https://doi.org/10.1002/lary.20236
23 Iyer NG , Morris LG , Tuttle RM , Shaha AR , Ganly I . Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117(19):4439–46. doi:.https://doi.org/10.1002/cncr.26070
24 Sawka AM , Thabane L , Parlea L , Ibrahim-Zada I , Tsang RW , Brierley JD , et al. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis. Thyroid. 2009;19(5):451–7. doi:.https://doi.org/10.1089/thy.2008.0392
25 Hakala TT , Sand JA , Jukkola A , Huhtala HS , Metso S , Kellokumpu-Lehtinen PL . Increased risk of certain second primary malignancies in patients treated for well-differentiated thyroid cancer. Int J Clin Oncol. 2016;21(2):231–9. doi:.https://doi.org/10.1007/s10147-015-0904-6
26 Khang AR , Cho SW , Choi HS , Ahn HY , Yoo WS , Kim KW , et al. The risk of second primary malignancy is increased in differentiated thyroid cancer patients with a cumulative (131)I dose over 37 GBq. Clin Endocrinol (Oxf). 2015;83(1):117–23. doi:.https://doi.org/10.1111/cen.12581
27 Hirsch D , Shohat T , Gorshtein A , Robenshtok E , Shimon I , Benbassat C . Incidence of Nonthyroidal Primary Malignancy and the Association with (131)I Treatment in Patients with Differentiated Thyroid Cancer. Thyroid. 2016;26(8):1110–6. doi:.https://doi.org/10.1089/thy.2016.0037
28 Nikiforov YE , Seethala RR , Tallini G , Baloch ZW , Basolo F , Thompson LD , et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016;2(8):1023–9. doi:.https://doi.org/10.1001/jamaoncol.2016.0386
29 Xing M , Alzahrani AS , Carson KA , Shong YK , Kim TY , Viola D , et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33(1):42–50. doi:.https://doi.org/10.1200/JCO.2014.56.8253
30 Barbaro D , Incensati RM , Materazzi G , Boni G , Grosso M , Panicucci E , et al. The BRAF V600E mutation in papillary thyroid cancer with positive or suspected pre-surgical cytological finding is not associated with advanced stages or worse prognosis. Endocrine. 2014;45(3):462–8. doi:.https://doi.org/10.1007/s12020-013-0029-5
31 Sancisi V , Nicoli D , Ragazzi M , Piana S , Ciarrocchi A . BRAFV600E mutation does not mean distant metastasis in thyroid papillary carcinomas. J Clin Endocrinol Metab. 2012;97(9):E1745–9. doi:.https://doi.org/10.1210/jc.2012-1526
32 Charles RP , Silva J , Iezza G , Phillips WA , McMahon M . Activating BRAF and PIK3CA mutations cooperate to promote anaplastic thyroid carcinogenesis. Mol Cancer Res. 2014;12(7):979–86. doi:.https://doi.org/10.1158/1541-7786.MCR-14-0158-T
33 Dettmer MS , Schmitt A , Steinert H , Capper D , Moch H , Komminoth P , et al. Tall cell papillary thyroid carcinoma: new diagnostic criteria and mutations in BRAF and TERT. Endocr Relat Cancer. 2015;22(3):419–29. doi:.https://doi.org/10.1530/ERC-15-0057
34 Seidlin SM , Marinelli LD , Oshry E . Radioactive iodine therapy; effect on functioning metastases of adenocarcinoma of the thyroid. J Am Med Assoc. 1946;132(14):838–47. doi:.https://doi.org/10.1001/jama.1946.02870490016004
35 Mazzaferri EL , Kloos RT . Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86(4):1447–63. doi:.https://doi.org/10.1210/jcem.86.4.7407
No financial support and no other potential conflict of interest relevant to this article was reported.