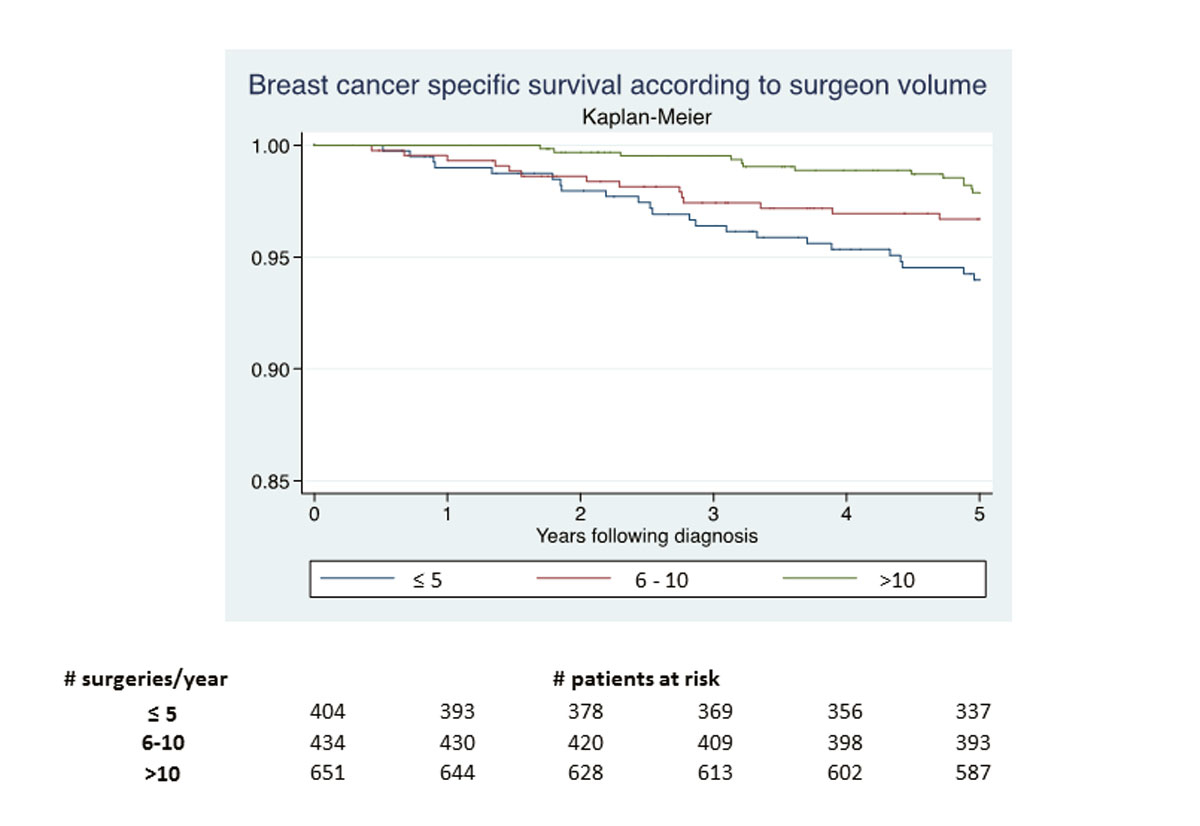
Figure 1 5-year survival following breast cancer diagnosis.
DOI: https://doi.org/10.4414/smw.2019.14704
Despite increasing effectiveness of adjuvant treatments, surgery remains a central component of the treatment for breast cancer. Since the middle of the 1990s, studies started reporting that the surgeon’s experience in breast cancer surgery could influence the prognosis of breast cancer patients [1, 2], and suggested improved survival of patients treated by highly experienced surgeons or in high-volume hospitals [3–18]. A meta-analysis suggested that the surgeon’s experience was a stronger predictor of survival than the hospital volume [19].
However, why the surgeon’s experience could influence breast cancer mortality remains unclear. The authors of two reviews highlighted weaknesses in previously published studies. Most studies were based on administrative data, did not adjust their analyses for differences in the patient characteristics and analysed overall mortality, known to be strongly influenced by patients’ co-morbidities [20, 21]. Finally, they suggested that the observed differences in survival could be explained by differences in the quality of breast cancer management (not limited to the surgery).
Switzerland has one of the most expensive healthcare system worldwide [22, 23] and the canton of Geneva is among those with the highest medical density, with approximately 5 physicians per 1000 inhabitants. In this canton, a large proportion of breast cancer surgery is performed in the private sector, sometimes by breast cancer surgeons who perform fewer than five breast cancer operations per year. However, Geneva provides some of the best quality of care for breast cancer in Switzerland [24] and has breast cancer survival rates that are among the highest in Switzerland and Europe [25–27].
The aim of this population-based retrospective cohort study was to investigate whether the association between surgeon’s experience and breast cancer mortality was also true in this very specific context, even after adjustment for patient and tumour characteristics, and whether this association could be confounded by the quality of care provided.
This study is reported according to the RECORD extension [28] of the STROBE statement [29] for reporting observational studies using routinely collected health data.
Formal ethical approval and patient consent for this study was not required. The Geneva Cancer Registry (GCR) has a general authorisation [30], to collect nominative data and to analyse the anonymised data.
This retrospective cohort study was based on data routinely collected by the GCR, which has recorded all incident cancers occurring in Geneva, Switzerland (approximately 480,000 inhabitants in 2014) since 1970. The data recorded include sociodemographic variables, tumour characteristics coded according to the International Classification of Diseases for Oncology (ICD-O) [31], stage at diagnosis (coded according to the Tumor, Node, Metastasis Classification of Malignant Tumors [32]), and treatment received within 6 months of diagnosis, including the identity of the physician in charge of the first treatment for the patient, for the private sector.
Between 2000 and 2009, 3733 patients were diagnosed with invasive breast cancer (ICDO-3 C50.0-6, C50.8-9, behaviour code/3) of whom 1813 (48.6%) were operated on in the private sector. We excluded 57 patients (3.1%) with previous invasive breast cancer, 151 (8.3%) who did not undergo surgery and 116 (6.4%) who did so after having received neoadjuvant treatment. Eventually, we included 1489 patients with breast cancer who underwent surgery in the private sector.
The primary outcome of this study was the 5-year breast cancer-specific survival of patients according to surgeon’s experience, adjusted for variables known to influence survival, for patient and tumour characteristics that were significantly associated with the surgeon’s experience or patient survival in the present cohort, and for quality-of-care indicators.
To define surgeon experience, we calculated for each surgeon the average number of breast cancer operations performed per year among the resident population. In order to avoid fluctuations due to various reasons (e.g., decreasing activity during the last years of the surgeon’s working life), we considered only the 3 years, between 2000 and 2009, during which the surgeon performed the highest number of breast cancer operations. We then stratified the surgeons into three categories: those performing ≤5, 6–10 or >10 breast cancer operations per year.
The patient characteristics extracted from the GCR database included age (<50, 50–69, 70–79, ≥80 years), period of diagnosis (2000–2002, 2003–2005, 2006–2009), socioeconomic status coded according to the last occupation (high, medium, low, unknown), country of birth (Switzerland, Southern Europe, other), method of breast cancer detection (mammography screening, clinical screening, breast self-examination, other [including symptoms or incidental finding], unknown), and familial risk of breast cancer (high, medium, none, unknown).
The tumour characteristics considered included stage (I, II, III, IV, unknown) [30], lymph node invasion (no, yes, unknown), tumour grade (well, moderately, or poorly differentiated, unknown), tumour histology (ductal, lobular, other), oestrogen and progesterone receptor status (positive if ≥1% expressed, negative, unknown) and human epidermal growth factor receptor 2 (HER2) status (positive, negative, unknown). Information on HER2 has been available only since 2001.
State-of-the-art breast cancer management was defined according to the quality indicators described by the European Society of Breast Cancer Specialists (EUSOMA) [33]. We selected nine indicators for which information was available from the GCR database: (1) reported hormone receptor immune-activity, tumour size, and grading; (2) histological assessment before surgery; (3) a single operation for the primary tumour (excluding reconstruction); (4) sentinel lymph node excision for clinically negative axillae; (5) ≥10 lymph nodes removed when axillary dissection performed; (6) breast-conserving surgery for tumours ≤3 cm; (7) radiotherapy if indicated (after breast-conserving surgery if no metastasis or after mastectomy for pT3 or pT4 or positive margin or ≥pN2a); (8) endocrine therapy for oestrogen-receptor positive tumours; and (9) chemotherapy for oestrogen-receptor negative tumours >1 cm or with a positive lymph node (we also considered an age of ≤35 years as an indication for chemotherapy, according to the 2003 Saint Gallen Consensus [34]). We added two additional criteria, not included in EUSOMA: (10) axillary lymph node dissection if clinical involvement or positive sentinel lymph node biopsy and (11) presence of negative margins after the last surgery. Each indicator was scored 1 when correctly performed or 0 if not correctly performed, and was omitted from the score if not applicable to the patient.
For each patient, we calculated the proportion of pertinent indicators correctly fulfilled, as explained in detail in a previous study [35]. This overall quality-of-care score was categorised as <75%, 75–90% or 90–100% of the items fulfilled.
In order to allow indirect comparison with the public sector, we additionally present the data reported in a previously published study on a similar cohort from a public breast cancer unit [35].
The GCR performs active follow-up yearly, by linking the GCR files with those of the Cantonal Population Office. The cause of death is provided by the Federal Office for Statistics, and coded according to the International Statistical Classification of Diseases and Related Health Problems [29]. The exact cause of death is confirmed by a physician at the GCR after consulting clinical records and/or inquiring of the patient’s physician.
The patient and tumour characteristics, and the 11 individual items included in the quality-of-care score were reported according to the surgeon’s experience as numbers (percentages) or means (95% confidence intervals [CIs]), and compared with a χ2 test or analysis of variance (ANOVA), as appropriate, to identify the variables significantly associated with surgeon experience.
All patients were followed up from the date of confirmation of a breast cancer diagnosis until 31 December 2014, death or the date of loss to follow-up, whichever occurred first. Only deaths from breast cancer were considered. Each variable (patient and tumour characteristics) was included in a univariate Cox regression model to identify those significantly associated with 5-year breast cancer-specific mortality.
The crude association between surgeon experience and 5-year breast cancer-specific survival was examined graphically with Kaplan–Meier curves, and a Cox regression model was constructed to report the hazard ratios (HRs) and 95% CIs for comparison of breast cancer mortality in patients treated by surgeons performing 6–10 and >10 breast cancer operations/year with those treated by surgeons performing ≤5 operations/year (baseline).
We used a multivariate Cox regression model including all variables known to be strongly associated with breast cancer-specific mortality (age, tumour stage, grade, and oestrogen and progesterone receptor status), and the patient or tumour characteristics that were shown to be associated with either 5-year breast cancer-specific survival or with surgeon experience in the univariate analyses. A variable was then dropped from the model if it were not significantly associated with the outcome, did not contribute significantly to the fit of the model to the data, established with a likelihood ratio test comparing the model including the variable with one excluding it (p >0.1), or did not act as a confounder, evident as a change in HR of >10%.
Finally, we quantified the impact of the quality of care by introducing the quality-of-care score into the last multivariate model.
Missing data for different variables were retained in the models as a category labelled “unknown”. We considered differences as statistically significant at p <0.05; all p-values reported are two-sided. The proportional hazard assumption was assessed graphically.
All analyses were performed using STATA 15 (StataCorp, College Station, TX 77845, USA).
During the study period, 88 surgeons operated on 1489 breast cancer patients. Most (n = 67) surgeons were gynaecologists; 18 were thoracic and 3 were plastic surgeons. A total of 651 breast cancer patients (44%) were operated on by 5 surgeons who performed >10 operations/year, 434 (29%) by 12 surgeons who performed 6–10, and 404 (27%) by 71 surgeons who performed ≤5. Among the latter group, 37 (9%) women were operated on by one of the 25 surgeons who performed ≤1 breast cancer intervention per year.
During the study period, the patients recruited by the surgeons performing >10 operations/year increased from 39 to 50%, whereas recruitment by the surgeons performing ≤5 decreased from 31 to 21% (p <0.001). Compared with the patients treated by the latter, those treated by surgeons performing >10 operations/year were more often of a higher socioeconomic status and were less frequently born in Southern Europe (table 1). Although there were differences in the proportion of “unknown data” for some variables, tumour characteristics did not differ significantly across the surgeon groups (table 2). In comparison with previously published data from the public sector [35] patients tended to be younger, from higher socio-economic status and more often born in Switzerland. The tumour characteristics, however, were similar (tables 1 and 2 ).
Table 1 Characteristics of breast cancer patients according to the surgeon's experience (Geneva Cancer Registry 2000–2009).
| Characteristics | Private surgeons’ experiencea ≤5 years (n = 404) | Private surgeons’ experiencea 6–10 years (n = 434) | Private surgeons’ experiencea >10 years (n = 651) | p-valueb | Public BC unitc (n = 752) | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Age (years) mean (95% CI) | 60.6 (59.4–61.7) | 59.9 (58.8–61.0) | 59.2 (58.3–60.1) | 0.188d | 61.8 | ||||
| Age (years) | 0.606 | ||||||||
| <50 | 80 | 19.8% | 85 | 19.6% | 133 | 20.4% | 141 | 18.8% | |
| 50–69 | 231 | 57.2% | 268 | 61.8% | 398 | 61.1% | 394 | 52.4% | |
| 70–79 | 73 | 18.1% | 60 | 13.8% | 93 | 14.3% | 150 | 19.9% | |
| ≥80 | 20 | 5.0% | 21 | 4.8% | 27 | 4.1% | 67 | 8.9% | |
| Period of diagnosis | <0.001 | ||||||||
| 2000–2 | 154 | 38.1% | 146 | 33.6% | 190 | 29.2% | 350 | 46.5% | |
| 2003–5 | 133 | 32.9% | 127 | 29.3% | 182 | 28.0% | 402 | 53.5% | |
| 2006–9 | 117 | 29.0% | 161 | 37.1% | 279 | 42.9% | |||
| Socioeconomic status | 0.026 | ||||||||
| High | 88 | 21.8% | 122 | 28.1% | 201 | 30.9% | 90 | 12.0% | |
| Medium | 268 | 66.3% | 259 | 59.7% | 386 | 59.3% | 427 | 56.8% | |
| Low | 43 | 10.6% | 42 | 9.7% | 50 | 7.7% | 215 | 28.6% | |
| Unknown | 5 | 1.2% | 11 | 2.5% | 14 | 2.2% | 20 | 2.7% | |
| Country of birth | 0.007 | ||||||||
| Swiss | 211 | 52.2% | 240 | 55.3% | 344 | 52.8% | 362 | 48.1% | |
| Southern Europe | 99 | 24.5% | 98 | 22.6% | 114 | 17.5% | 258 | 34.3% | |
| Other | 94 | 23.3% | 96 | 22.1% | 193 | 29.6% | 132 | 17.6% | |
| Method of detection | 0.093 | ||||||||
| Mammography screening | 180 | 44.6% | 212 | 48.8% | 298 | 45.8% | 290 | 38.6% | |
| Clinical screening | 53 | 13.1% | 57 | 13.1% | 64 | 9.8% | 67 | 8.9% | |
| Breast self-examination | 118 | 29.2% | 125 | 28.8% | 201 | 30.9% | 288 | 38.3% | |
| Other | 46 | 11.4% | 35 | 8.1% | 66 | 10.1% | 106 | 14.1% | |
| Unknown | 7 | 1.7% | 5 | 1.2% | 22 | 3.4% | 1 | 0.1% | |
| Familial risk | 0.058 | ||||||||
| None | 253 | 62.6% | 291 | 67.1% | 378 | 58.1% | 505 | 67.2% | |
| Medium | 97 | 24.0% | 80 | 18.4% | 165 | 25.3% | 173 | 23.0% | |
| High | 25 | 6.2% | 25 | 5.8% | 40 | 6.1% | 68 | 9.0% | |
| Unknown | 29 | 7.2% | 38 | 8.8% | 68 | 10.4% | 6 | 0.8% | |
BC = breast cancer; CI = confidence interval a Surgeon's experience: mean annual new primary breast cancer (invasive or in situ) operations during the 3 years with the highest number of breast cancer intervention along the study period. b p-value of a χ2 test c Data from Taban et al. 2013 [35] d p-value for ANOVA test
Table 2 Characteristics of the tumours according to the surgeon's experience (Geneva Cancer Registry 2000–2009).
| Characteristics | Private surgeons’ experience a ≤5 years (n = 404) | Private surgeons’ experience a 6–10 years (n = 434) | Private surgeons’ experience a >10 years (n = 651) | p-value b |
Public BC unit
c
(n = 752) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| Stage | 0.251 | |||||||||
| I | 197 | 48.8% | 224 | 51.6% | 325 | 49.9% | 389 | 51.7% | ||
| II | 149 | 36.9% | 167 | 38.5% | 267 | 41.0% | 301 | 40.0% | ||
| III | 38 | 9.4% | 29 | 6.7% | 45 | 6.9% | 51 | 6.8% | ||
| IV | 7 | 1.7% | 5 | 1.2% | 5 | 0.8% | 4 | 0.5% | ||
| Unknown | 13 | 3.2% | 9 | 2.1% | 9 | 1.4% | 7 | 0.9% | ||
| Lymph node invasion | 0.017 | |||||||||
| No | 255 | 63.1% | 283 | 65.2% | 427 | 65.6% | 517 | 68.8% | ||
| Yes | 129 | 31.9% | 142 | 32.7% | 214 | 32.9% | 231 | 30.7% | ||
| Unknown | 20 | 5.0% | 9 | 2.1% | 10 | 1.5% | 4 | 0.5% | ||
| Grade | 0.258 | |||||||||
| Well differentiated | 132 | 32.7% | 143 | 32.9% | 182 | 28.0% | 250 | 33.2% | ||
| Moderately differentiated | 189 | 46.8% | 182 | 41.9% | 317 | 48.7% | not reported | |||
| Poorly or undifferentiated | 79 | 19.6% | 103 | 23.7% | 146 | 22.4% | not reported | |||
| Unknown | 4 | 1.0% | 6 | 1.4% | 6 | 0.9% | 18 | 2.4% | ||
| Histology | 0.966 | |||||||||
| Ductal | 323 | 80.0% | 340 | 78.3% | 521 | 80.0% | 612 | 81.4% | ||
| Lobular | 63 | 15.6% | 72 | 16.6% | 101 | 15.5% | 109 | 14.5% | ||
| Other | 18 | 4.5% | 22 | 5.1% | 29 | 4.5% | 31 | 4.1% | ||
| Oestrogen receptor status | 0.020 | |||||||||
| Positive | 344 | 85.1% | 383 | 88.2% | 583 | 89.6% | 652 | 86.7% | ||
| Negative | 49 | 12.1% | 46 | 10.6% | 65 | 10.0% | 99 | 13.2% | ||
| Unknown | 11 | 2.7% | 5 | 1.2% | 3 | 0.5% | 1 | 0.1% | ||
| Progesterone receptor status | 0.065 | |||||||||
| Positive | 313 | 77.5% | 344 | 79.3% | 510 | 78.3% | 552 | 73.4% | ||
| Negative | 81 | 20.0% | 85 | 19.6% | 138 | 21.2% | 199 | 26.5% | ||
| Unknown | 10 | 2.5% | 5 | 1.2% | 3 | 0.5% | 1 | 0.1% | ||
| HER2 status d | 0.019 | |||||||||
| Positive | 47 | 11.6% | 50 | 11.5% | 100 | 15.4% | 135 | 18.0% | ||
| Negative | 210 | 52.0% | 235 | 54.1% | 370 | 56.8% | 409 | 54.4% | ||
| Unknown | 147 | 36.4% | 149 | 34.3% | 181 | 27.8% | 208 | 27.7% | ||
BC = breast cancer; CI = confidence interval; HER2 = human epidermal growth factor receptor-2 a Surgeon's experience: mean annual new primary breast cancer (invasive or in situ) operations during the 3 years with the highest number of breast cancer intervention along the study period. b p-value of a χ2 test c Data from Taban et al. 2013 [35] d Available since 2001
Table 3 presents the 11 quality indicators according to surgeon experience. Significant differences across the categories of surgeon experience were observed for histological assessment before surgery, sentinel lymph node procedure (when indicated), and ≥10 lymph nodes removed during axillary dissection. The mean overall quality indicator score was high in all groups (above 82%), but was higher in the women treated by surgeons performing >10 operations/year; 50.5% of their patients benefited from >90% of pertinent items fulfilled. This proportion was 47.2% in patients treated by surgeons performing 6–10 operations/year and 34.7% for those treated by surgeons performing ≤5 (p <0.001). Most of the EUSOMA minimum requirements were reached except for sentinel lymph node excision (if indicated) and the number of lymph nodes removed, which did not reach 90% and 95%, respectively, in any group. Also, the administration of chemotherapy when indicated failed to reach the 80% required by EUSOMA. Interestingly, the public sector also failed to reach these standards (table 3).
Table 3 Quality of diagnosis assessment and treatment according to the surgeon's experience (Geneva Cancer Registry, 2000–2009).
| Indicator of quality | Private surgeon's experience a ≤5 years (n = 404) | Private surgeon's experience a 6–10years (n = 434) | Private surgeon's experience a >10 years (n = 651) | p-value b | Public BC unit c (n = 752) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| Reporting of hormone receptor immune-activity, tumour size, and grading | 0.121 | |||||||||
| EUSOMA d 4b (min: >90%; target: >95%) | Yes | 389 | 96.3% | 423 | 97.5% | 640 | 98.3% | 719 | 95.6% | |
| No | 15 | 3.7% | 11 | 2.5% | 11 | 1.7% | 33 | 4.4% | ||
| Histological assessment before surgery | <0.001 | |||||||||
| EUSOMA 3 (min: 80%; target: 90% | Yes | 323 | 80.0% | 391 | 90.1% | 610 | 93.7% | 652 | 86.7% | |
| No | 81 | 20.0% | 43 | 9.9% | 41 | 6.3% | 100 | 13.3% | ||
| Number of surgeries required | 0.113 | |||||||||
| EUSOMA 9a (min: 80%; target: 90%) | One | 340 | 84.2% | 373 | 85.9% | 529 | 81.3% | 651 | 86.6% | |
| More | 64 | 15.8% | 61 | 14.1% | 122 | 18.7% | 101 | 13.4% | ||
| Surgical margins | 0.549 | |||||||||
| Non-EUSOMA | Negative | 370 | 91.6% | 396 | 91.2% | 606 | 93.1% | 679 | 90.3% | |
| Positive | 31 | 7.7% | 37 | 8.5% | 44 | 6.8% | 68 | 9.0% | ||
| Unknown | 3 | 0.7% | 1 | 0.2% | 1 | 0.2% | 5 | 0.7% | ||
| Sentinel lymph node excision, if indicated | <0.001 | |||||||||
| EUSOMA 9c (min: 90%; target: 95%) | Yes | 165 | 57.5% | 242 | 72.9% | 418 | 82.1% | 368 | 70.5% | |
| No | 122 | 42.5% | 90 | 27.1% | 91 | 17.9% | 160 | 30.7% | ||
| Not pertinent | 117 | – | 102 | – | 142 | – | 224 | |||
| Axillary dissection when indicated | 0.199 | |||||||||
| Non-EUSOMA | Yes | 100 | 89.3% | 107 | 87.0% | 184 | 92.9% | 187 | 85.4% | |
| No | 12 | 10.7% | 16 | 13.0% | 14 | 7.1% | 32 | 14.6% | ||
| Not pertinent | 292 | – | 311 | – | 453 | – | 533 | |||
| Number of lymph nodes removed | 0.002 | |||||||||
| EUSOMA 9d (min: 95%; target: 98% | ≥10 | 137 | 57.8% | 138 | 67.6% | 214 | 72.1% | 281 | 75.9% | |
| <10 | 100 | 42.2% | 66 | 32.4% | 83 | 27.9% | 89 | 24.1% | ||
| Not pertinent | 167 | – | 230 | – | 354 | – | 382 | |||
| Breast-conserving surgery when indicated | 0.536 | |||||||||
| EUSOMA 11a (min: 70%; target: 80%) | Yes | 298 | 86.9% | 339 | 87.6% | 489 | 85.2% | 518 | 79.2% | |
| No | 45 | 13.1% | 48 | 12.4% | 85 | 14.8% | 136 | 20.8% | ||
| Not pertinent | 61 | – | 47 | – | 77 | – | 98 | |||
| Radiotherapy use when indicated | 0.066 | |||||||||
| EUSOMA 10 (min: 90%; target: 95%) | Yes | 304 | 89.7% | 349 | 93.3% | 508 | 93.7% | |||
| No | 35 | 10.3% | 25 | 6.7% | 34 | 6.3% | ||||
| Not pertinent | 65 | – | 60 | – | 109 | – | ||||
| Anti-oestrogen use when indicated | 0.877 | |||||||||
| EUSOMA 12a (min: 80%; target: 90%) | Yes | 295 | 86.3% | 331 | 86.9% | 492 | 85.7% | 611 | 93.7% | |
| No | 47 | 13.7% | 50 | 13.1% | 82 | 14.3% | 41 | 6.3% | ||
| Not pertinent | 62 | – | 53 | – | 77 | – | 100 | |||
| Chemotherapy use when indicated | 0.402 | |||||||||
| EUSOMA 13a (min: 80%; target: 90%) | Yes | 115 | 75.7% | 121 | 74.2% | 172 | 69.9% | 164 | 56.0% | |
| No | 37 | 24.3% | 42 | 25.8% | 74 | 30.1% | 129 | 44.0% | ||
| Not pertinent | 252 | – | 271 | – | 405 | – | 459 | |||
| Quality-of care-score | <0.001 | |||||||||
| Mean (SD) | 82.6% | (16.0%) | 86.8% | (14.3%) | 87.7% | (14.1%) | 0.01e | 85.0 | ||
| <75% | 125 | 30.9% | 94 | 21.7% | 131 | 20.1% | <0.001 | 196 | 27.0% | |
| 75–90% | 139 | 34.4% | 135 | 31.1% | 191 | 29.3% | 291 | 40.1% | ||
| >90% | 140 | 34.7% | 205 | 47.2% | 329 | 50.5% | 265 | 36.6% | ||
BC = breast cancer; CI = confidence interval; SD = standard deviation a Surgeon's experience: mean annual new primary breast cancer (invasive or in situ) operations during 3 years with the highest number of breast cancer intervention along the study period b p-value of the χ2 test leaving non-pertinent out c Data from Taban et al. 2013 [35] d Del Turco et al. 2010 [33] e ANOVA
The 1489 patients represented a total of 7046.9 person-years of follow-up.
Fifty women died of their breast cancer (3.4%; death rate 7.1/1000 person-years). Of these, 13 (2.0%; 4.2/1000 person-years) were treated by surgeons performing >10, 14 (3.2%, 6.8/1000 person-years) by surgeons performing 6–10, and 23 (5.7%, 12.3/1000 person-years) by surgeons performing ≤5 operations/year.
The crude 5-year breast cancer-specific survival rates were high, but differed significantly across groups (>10: 98%, 95%CI 97–99%; 6–10: 96%, 95% CI 95–98%; and <5, 94%, 95% CI 92–96%; p = 0.004 in a log rank test; fig. 1).

Figure 1 5-year survival following breast cancer diagnosis.
Variables significantly associated with 5-year breast cancer-specific survival in the univariate analyses included age, socioeconomic status, method of breast cancer detection, familial risk of breast cancer, stage, lymph node invasion, grade, histology, and oestrogen and progesterone receptor and HER2 status.
The variables retained in the final Cox model were age, socioeconomic status, stage, lymph node invasion, grade, histology, and oestrogen and progesterone receptor status. A second model was constructed that included the quality-of-care score.
In the crude analysis, the patients operated on by surgeons performing >10 operations/year presented with 66% lower breast cancer-specific mortality than those treated by surgeons performing ≤5 (HR 0.34, 95% CI 0.17–0.67; p = 0.002). Adjustment for patient and tumour characteristics reduced the strength of the association (HRadj-1 0.45, 95% CI 0.21–0.94; p = 0.034). In the final model, additional adjustment for quality of care further decreased the strength of the association (HRadj-2 0.51, 95% CI 0.24–1.08; p = 0.078) and failed to reach statistical significance. The crude HR comparing the patients treated by surgeons performing 6–10 operations/year with those treated by surgeons performing ≤5 followed the same trend. (table 4)
Table 4 Effect of the surgeon's experience on breast cancer-specific mortality at 5 years (Geneva Cancer Registry, 2000–2009).
| Surgeon's experience | Crude Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) adjusted for patient and tumour characteristics a | p-value | Hazard ratio (95% CI) adjusted for patient, tumour characteristics and quality of care b | p-value |
|---|---|---|---|---|---|---|
| ≤5 surgeries/year | 1 (reference) | 1 (reference) | 1 (reference) | |||
| 6–10 surgeries/year | 0.55 (0.28-1.06) | 0.074 | 0.63 (0.30-1.32) | 0.223 | 0.67 (0.32-1.40) | 0.285 |
| >10 surgeries/year | 0.34 (0.17-0.67) | 0.002 | 0.45 (0.21-0.94) | 0.034 | 0.51 (0.24-1.08) | 0.078 |
Surgeon's experience: mean annual new primary breast cancer (invasive or in situ) operations during the 3 years with the highest number of breast cancer intervention along the study period. a Adjusted for age, socio-economic status, stage, lymph node invasion, grade, histology, oestrogen and progesterone receptors c Additional adjustment for quality of care
This study confirms previously reported findings, highlights new ones and generates several questions. First, as previously reported, we found a statistically significant crude association between high surgeon experience, and improved breast cancer-specific survival in their patients. Second, this study demonstrates that the strength of this association decreases after adjustment for patient and tumour characteristics, and further decreases after adjustment for measurable indicators of quality of care, suggesting that these factors may, at least partly, explain the previously reported differences in survival. Third, the quality of care provided in the private sector for breast cancer is good and comparable to that of the public breast cancer unit, although some EUSOMA targets were not reached. Fourth, in Geneva, the surgeon’s experience may not impact on the 5-year breast cancer-specific survival of patients operated on in the private sector. Finally, other factors reflecting the quality of care should be investigated as they may further decrease the reported association.
In crude analyses, patients operated on by surgeons performing >10 operations/year had a lower risk of death as a consequence of their breast cancer than patients operated on by surgeons with less experience, which is consistent with the findings of other researchers. Based on 12 of 63 studies published between 1990 and 2010, Gooiker et al. [19] reported that the pooled survival advantage conferred by high-volume surgeons was around 20% (range 10–39%). Other studies have also reported an association between hospital volume and breast cancer survival [19]. In particular, Skinner et al. reported that breast cancer patients operated on by low-volume surgeons in high-volume hospitals had similar outcomes to those of breast cancer patients operated on by high-volume surgeons in low-volume hospitals [4].
Our study also confirms that patients as well as treatments may differ according to surgeon experience [36]. Although better care has generally been observed among breast cancer patients treated by high-volume surgeons, none of the previously published studies have used the EUSOMA criteria to assess the quality of care received. As reported in previous studies, we found that surgeons who performed >10 operations/year more frequently performed a histological assessment before surgery [37, 38], removed sentinel lymph nodes when indicated [39–43], removed an adequate number of axillary lymph nodes when performing axillary clearance [44, 45], and referred their patients for adjuvant radiotherapy when indicated [5, 45, 46].
What our study newly highlights is that the unexplained association between surgeon experience and breast cancer survival may be partly explained by patient and tumour characteristics, but also by the quality of care provided. In fact, taking these variables into account decreases the association between surgeon experience and breast cancer survival. This is an important finding, since our study highlights that better survival after breast cancer may be due to better quality of care, and not to the surgeon’s technical ability.
One of the strengths of our study is that we examined breast cancer-specific mortality and not overall mortality, which is influenced by patients’ comorbidities. A second strength is that we adjusted our final survival model for all patient and tumour characteristics that are known to be associated with survival, or that were associated with surgeon experience or survival in our cohort. Finally, we used well-defined and recognised quality indicators to control for the impact of the quality of care, although we were unable to quantify all of these criteria based on registry data. The main limitation of this study is its observational nature. However, it is unlikely that a randomised clinical trial of this issue will ever be performed for practical and ethical reasons. Furthermore, we cannot exclude residual confounding by unrecorded variables. For example, the GCR collects information on patient characteristics and treatments, but does not collect detailed information regarding the specifics of the surgical procedures used. Also, the GCR records the name of the physician responsible for the first treatment administrated only, and for this reason, patients who had received neoadjuvant chemotherapy (about 6%) had to be excluded from our analyses. The surgeons’ experience was probably underestimated in this study because we considered only the operations performed on breast cancer patients living in Geneva. Resident cancer patients represent 75% of all breast cancer patients treated in Geneva. However, we have no reason to believe that the proportion of nonresidents operated on differed according to surgeon experience, and we are quite confident that our categorisation is robust. The cut-offs used to define surgeon experience were lower than those used in most other studies [19], which reflects the reality of a city such as Geneva with both a high number of health providers in the private sector and a small population. Other studies have used various cut-off values to classify surgeon volume [19, 47] and have shown a positive relationship between surgeon volume and breast cancer survival, independently of the cut-offs used. Also, this study focuses on breast cancer patients treated in the private sector; no extrapolation of our results to the public sector can be made, and we were unable to reproduce similar analyses for the public sector since the identity of the surgeons in university hospitals is unclear. However, indirect comparison with a public breast cancer unit during a similar time period showed comparable quality of care [35]. We did not control for the potential impact of “hospital volume”, but we are quite confident that, since there are only three private hospitals in Geneva, which are very similar in size, in their recruitment of breast cancer patients and in the quality of care they provide, this should not influence our results. Finally, some EUSOMA quality-of-care indicators were unavailable, some have changed in the latest version (i.e., recommendation on the number of lymph nodes to remove), the reasons why some procedures were performed remain unknown, and residual confounding is possible. For example, differences probably exist between surgeons in their access to multidisciplinary care. A multidisciplinary approach, which is now routinely available in specialist breast cancer units, could balance out any effect of the surgeon’s experience on survival [48]. At the time the breast cancer patients were enrolled in our study, a breast cancer network, SONGe (réseau de Sénologie et ONco-gynecologie Genevois), attracted some private professionals with a particular interest in the field of breast cancer care. Breast cancer surgeons affiliated to such a network may be more likely to work in a multidisciplinary context and probably have greater experience in breast cancer surgery than those who are not affiliated. Such a network could affect breast cancer-specific survival and could, once adjusted for, further decrease the strength of the association observed.
This study suggests that the previously reported association between surgeon experience and breast cancer mortality may be at least partly explained by patient selection and measurable indicators of quality of care. Further adjustment for variables reflecting quality of care, such as the degree of involvement in a breast cancer network with multidisciplinary meetings and co-operation, should be explored as they may confirm our findings by further decreasing the strength of the association.
We would like to thank all the patients and professionals associated with the Geneva Breast Cancer Network Research Group for their contributions. We also thank Catherine Lacour for her technical and editorial assistance, and the Geneva Cancer Registry team for providing data and support. The protocol and analysis plan for this study were not registered in an independent institutional registry.
This research was supported by the Swiss Bridge Foundation, Switzerland, which played no role in the design, collection, analysis or interpretation of the study.
The authors declare that they have no direct financial competing interests. FT declares that he belongs to the SONGe network and is one of the surgeons who performed >10 surgeries/year included in the present analysis. The datasets used and analysed are available from the corresponding author on reasonable request.
1 Sainsbury R , Haward R , Round C , Rider L , Johnston C . Influence of clinician workload and patterns of treatment on survival from breast cancer. Lancet. 1995;345(8960):1265–70. doi:.https://doi.org/10.1016/S0140-6736(95)90924-9
2 Gillis CR , Hole DJ . Survival outcome of care by specialist surgeons in breast cancer: a study of 3786 patients in the west of Scotland. BMJ. 1996;312(7024):145–8. doi:.https://doi.org/10.1136/bmj.312.7024.145
3 Stefoski Mikeljevic J , Haward RA , Johnston C , Sainsbury R , Forman D . Surgeon workload and survival from breast cancer. Br J Cancer. 2003;89(3):487–91. doi:.https://doi.org/10.1038/sj.bjc.6601148
4 Skinner KA , Helsper JT , Deapen D , Ye W , Sposto R . Breast cancer: do specialists make a difference? Ann Surg Oncol. 2003;10(6):606–15. doi:.https://doi.org/10.1245/ASO.2003.06.017
5 Allgood PC , Bachmann MO . Effects of specialisation on treatment and outcomes in screen-detected breast cancers in Wales: cohort study. Br J Cancer. 2006;94(1):36–42. doi:.https://doi.org/10.1038/sj.bjc.6602894
6 Bailie K , Dobie I , Kirk S , Donnelly M . Survival after breast cancer treatment: the impact of provider volume. J Eval Clin Pract. 2007;13(5):749–57. doi:.https://doi.org/10.1111/j.1365-2753.2006.00748.x
7 Clayforth C , Fritschi L , McEvoy SP , Byrne MJ , Ingram D , Sterrett G , et al. Five-year survival from breast cancer in Western Australia over a decade. Breast. 2007;16(4):375–81. doi:.https://doi.org/10.1016/j.breast.2007.01.010
8 Nattinger AB , Laud PW , Sparapani RA , Zhang X , Neuner JM , Gilligan MA . Exploring the surgeon volume outcome relationship among women with breast cancer. Arch Intern Med. 2007;167(18):1958–63. doi:.https://doi.org/10.1001/archinte.167.18.1958
9 Chen CS , Liu TC , Lin HC , Lien YC . Does high surgeon and hospital surgical volume raise the five-year survival rate for breast cancer? A population-based study. Breast Cancer Res Treat. 2008;110(2):349–56. doi:.https://doi.org/10.1007/s10549-007-9715-4
10 Guller U , Safford S , Pietrobon R , Heberer M , Oertli D , Jain NB . High hospital volume is associated with better outcomes for breast cancer surgery: analysis of 233,247 patients. World J Surg. 2005;29(8):994–9, discussion 999–1000. doi:.https://doi.org/10.1007/s00268-005-7831-z
11 Hébert-Croteau N , Brisson J , Lemaire J , Latreille J , Pineault R . Investigating the correlation between hospital of primary treatment and the survival of women with breast cancer. Cancer. 2005;104(7):1343–8. doi:.https://doi.org/10.1002/cncr.21336
12 Simunovic M , Rempel E , Thériault ME , Coates A , Whelan T , Holowaty E , et al. Influence of hospital characteristics on operative death and survival of patients after major cancer surgery in Ontario. Can J Surg. 2006;49(4):251–8.
13 Gilligan MA , Neuner J , Zhang X , Sparapani R , Laud PW , Nattinger AB . Relationship between number of breast cancer operations performed and 5-year survival after treatment for early-stage breast cancer. Am J Public Health. 2007;97(3):539–44. doi:.https://doi.org/10.2105/AJPH.2005.075663
14 Gutierrez JC , Hurley JD , Housri N , Perez EA , Byrne MM , Koniaris LG . Are many community hospitals undertreating breast cancer?: lessons from 24,834 patients. Ann Surg. 2008;248(2):154–62. doi:.https://doi.org/10.1097/SLA.0b013e31816c4030
15 Peltoniemi P , Peltola M , Hakulinen T , Häkkinen U , Pylkkänen L , Holli K . The effect of hospital volume on the outcome of breast cancer surgery. Ann Surg Oncol. 2011;18(6):1684–90. doi:.https://doi.org/10.1245/s10434-010-1514-1
16 Roohan PJ , Bickell NA , Baptiste MS , Therriault GD , Ferrara EP , Siu AL . Hospital volume differences and five-year survival from breast cancer. Am J Public Health. 1998;88(3):454–7. doi:.https://doi.org/10.2105/AJPH.88.3.454
17 Jonker FHW , Hagemans JAW , Verhoef C , Burger JWA . The impact of hospital volume on perioperative outcomes of rectal cancer. Eur J Surg Oncol. 2017;43(10):1894–900. doi:.https://doi.org/10.1016/j.ejso.2017.07.009
18 Manchon-Walsh P , Aliste L , Espinàs JA , Prades J , Guarga A , Balart J , et al.; Catalonian Rectal Cancer Group. Improving survival and local control in rectal cancer in Catalonia (Spain) in the context of centralisation: A full cycle audit assessment. Eur J Surg Oncol. 2016;42(12):1873–80. doi:.https://doi.org/10.1016/j.ejso.2016.08.009
19 Gooiker GA , van Gijn W , Post PN , van de Velde CJ , Tollenaar RA , Wouters MW . A systematic review and meta-analysis of the volume-outcome relationship in the surgical treatment of breast cancer. Are breast cancer patients better of with a high volume provider? Eur J Surg Oncol. 2010;36(Suppl 1):S27–35. doi:.https://doi.org/10.1016/j.ejso.2010.06.024
20 Hébert-Croteau N , Brisson J , Pineault R . Review of organizational factors related to care offered to women with breast cancer. Epidemiol Rev. 2000;22(2):228–38. doi:.https://doi.org/10.1093/oxfordjournals.epirev.a018035
21 Hogan AM , Winter DC . Does practice make perfect? Ann Surg Oncol. 2008;15(5):1267–70. doi:.https://doi.org/10.1245/s10434-007-9804-y
22OECD Data. Health spending: https://data.oecd.org/healthres/health-spending.htm (accessed on the 28 September 2018)
23 Reinhardt UE . The Swiss health system: regulated competition without managed care. JAMA. 2004;292(10):1227–31. doi:.https://doi.org/10.1001/jama.292.10.1227
24 Ess S , Savidan A , Frick H , Rageth Ch , Vlastos G , Lütolf U , et al. Geographic variation in breast cancer care in Switzerland. Cancer Epidemiol. 2010;34(2):116–21. doi:.https://doi.org/10.1016/j.canep.2010.01.008
25 Fisch T , Pury P , Probst N , Bordoni A , Bouchardy C , Frick H , et al. Variation in survival after diagnosis of breast cancer in Switzerland. Ann Oncol. 2005;16(12):1882–8. doi:.https://doi.org/10.1093/annonc/mdi404
26Report WC. Lyon: International Agency for Research on Cancer (IARC); 2008.
27 Sant M , Chirlaque Lopez MD , Agresti R , Sánchez Pérez MJ , Holleczek B , Bielska-Lasota M , et al.; EUROCARE-5 Working Group. Survival of women with cancers of breast and genital organs in Europe 1999-2007: Results of the EUROCARE-5 study. Eur J Cancer. 2015;51(15):2191–205. doi:.https://doi.org/10.1016/j.ejca.2015.07.022
28 Benchimol EI , Smeeth L , Guttmann A , Harron K , Moher D , Petersen I , et al.; RECORD Working Committee. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi:.https://doi.org/10.1371/journal.pmed.1001885
29 von Elm E , Altman DG , Egger M , Pocock SJ , Gøtzsche PC , Vandenbroucke JP ; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. doi:.https://doi.org/10.1016/S0140-6736(07)61602-X
30Autorisation de la commission d'experts du secret professionnel en matière de recherche médicale: https://www.unige.ch/medecine/rgt/files/7914/6462/0509/Article321bis_1994_Texte_Commission_Experts.pdf (accessed on the 28 September 2018)
31World Health Organization. ICD-O International classification of diseases for oncology. 3rd ed. Geneva: WHO; 2000. 240 p.
32TNM Classification of Malignant Tumours (UICC). 6 ed. New York: John Wiley & Sons, Inc.; UICC; 2002 2002.
33 Rosselli Del Turco M , Ponti A , Bick U , Biganzoli L , Cserni G , Cutuli B , et al. Quality indicators in breast cancer care. Eur J Cancer. 2010;46(13):2344–56. doi:.https://doi.org/10.1016/j.ejca.2010.06.119
34 Goldhirsch A , Wood WC , Gelber RD , Coates AS , Thürlimann B , Senn HJ . Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol. 2003;21(17):3357–65. doi:.https://doi.org/10.1200/JCO.2003.04.576
35 Taban F , Rapiti E , Fioretta G , Wespi Y , Weintraub D , Hugli A , et al. Breast cancer management and outcome according to surgeon’s affiliation: a population-based comparison adjusted for patient’s selection bias. Ann Oncol. 2013;24(1):116–25. doi:.https://doi.org/10.1093/annonc/mds285
36 Hébert-Croteau N , Roberge D , Brisson J . Provider’s volume and quality of breast cancer detection and treatment. Breast Cancer Res Treat. 2007;105(2):117–32. doi:.https://doi.org/10.1007/s10549-006-9439-x
37 Tamirisa NP , Sheffield KM , Parmar AD , Zimmermann CJ , Adhikari D , Vargas GM , et al. Surgeon and Facility Variation in the Use of Minimally Invasive Breast Biopsy in Texas. Ann Surg. 2015;262(1):171–8. doi:.https://doi.org/10.1097/SLA.0000000000000883
38 Eberth JM , Xu Y , Smith GL , Shen Y , Jiang J , Buchholz TA , et al. Surgeon influence on use of needle biopsy in patients with breast cancer: a national medicare study. J Clin Oncol. 2014;32(21):2206–16. doi:.https://doi.org/10.1200/JCO.2013.52.8257
39 Posther KE , McCall LM , Blumencranz PW , Burak WE, Jr , Beitsch PD , Hansen NM , et al. Sentinel node skills verification and surgeon performance: data from a multicenter clinical trial for early-stage breast cancer. Ann Surg. 2005;242(4):593–9, discussion 599–602.
40 McDermott AM , Wall DM , Waters PS , Cheung S , Sibbering M , Horgan K , et al.; ABS Audit Committee. Surgeon and breast unit volume-outcome relationships in breast cancer surgery and treatment. Ann Surg. 2013;258(5):808–13, discussion 813–4. doi:.https://doi.org/10.1097/SLA.0b013e3182a66eb0
41 Yen TW , Laud PW , Sparapani RA , Nattinger AB . Surgeon specialization and use of sentinel lymph node biopsy for breast cancer. JAMA Surg. 2014;149(2):185–92. doi:.https://doi.org/10.1001/jamasurg.2013.4350
42 Yen TW , Li J , Sparapani RA , Laud PW , Nattinger AB . The interplay between hospital and surgeon factors and the use of sentinel lymph node biopsy for breast cancer. Medicine (Baltimore). 2016;95(31):e4392. doi:.https://doi.org/10.1097/MD.0000000000004392
43 Kong AL , Pezzin LE , Nattinger AB . Identifying patterns of breast cancer care provided at high-volume hospitals: a classification and regression tree analysis. Breast Cancer Res Treat. 2015;153(3):689–98. doi:.https://doi.org/10.1007/s10549-015-3561-6
44 Kingsmore D , Hole D , Gillis C . Why does specialist treatment of breast cancer improve survival? The role of surgical management. Br J Cancer. 2004;90(10):1920–5. doi:.https://doi.org/10.1038/sj.bjc.6601846
45 Ma M , Bell J , Campbell S , Basnett I , Pollock A , Taylor I . Breast cancer management: is volume related to quality? Clinical Advisory Panel. Br J Cancer. 1997;75(11):1652–9. doi:.https://doi.org/10.1038/bjc.1997.281
46 Ingram DM , McEvoy SP , Byrne MJ , Fritschi L , Joseph DJ , Jamrozik K . Surgical caseload and outcomes for women with invasive breast cancer treated in Western Australia. Breast. 2005;14(1):11–7. doi:.https://doi.org/10.1016/j.breast.2004.06.008
47 Morche J , Mathes T , Pieper D . Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5(1):204. doi:.https://doi.org/10.1186/s13643-016-0376-4
48 Kesson EM , Allardice GM , George WD , Burns HJ , Morrison DS . Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ. 2012;344(apr26 1):e2718. doi:.https://doi.org/10.1136/bmj.e2718
FT contributed to the study concept, interpreted the data and revised the different versions of the manuscript; NE contributed to the study design, data cleaning and statistical analyses, interpreted the results and revised the final manuscript; ER contributed to the study concept and design, analysed and interpreted the data and reviewed the different versions of the manuscript; CR contributed to the analysis and interpretation of the results and revised the final manuscript ; GF was responsible for the quality control of data and algorithms, performed the statistical analyses and revised the final manuscript; SB contributed to the analysis and interpretation of the results and revised the final manuscript; EDM contributed to the data analyses and interpretation and to the final manuscript editing and revision; TGL contributed to the interpretation of the results and revised the final manuscript; CB contributed to the study concept and design, interpreted the data, drafted the first manuscript and revised the different versions of the manuscript. All authors have approved the final manuscript.
This research was supported by the Swiss Bridge Foundation, Switzerland, which played no role in the design, collection, analysis or interpretation of the study.
The authors declare that they have no direct financial competing interests. FT declares that he belongs to the SONGe network and is one of the surgeons who performed >10 surgeries/year included in the present analysis. The datasets used and analysed are available from the corresponding author on reasonable request.