Use of coronary computed tomography angiography in clinical practice – single centre experience in Switzerland in light of current recommendations based on pretest probability considerations
DOI: https://doi.org/10.4414/smw.2019.20010
Evelyne
Neurautera, Sebastian
Leschkab, Simon
Wildermuthb, Niklas F.
Ehla, Lucas
Joerga, Hans
Ricklia, Micha T.
Maedera
aDepartment of Cardiology, Kantonsspital St. Gallen, Switzerland
bDepartment of Division of Radiology and Nuclear Medicine, Kantonsspital St. Gallen, Switzerland
Summary
AIMS OF THE STUDY
Coronary computed tomography angiography (CCTA) is recommended as a first-line option for the exclusion of coronary artery disease in patients with low to intermediate (15–50%) pretest probability. We aimed to study the use of CCTA in clinical practice in a single centre in Switzerland in light of this recommendation.
METHODS
In 523 consecutive patients (age 56 ± 13 years, 48% females) undergoing CCTA during a period of 2 years, the pretest probability of coronary artery disease was assessed using the revised Diamond-Forrester model (CAD consortium score). In patients who had invasive coronary angiography following CCTA, angiographic findings and the consequences regarding management are reported.
RESULTS
The majority of patients (n = 316; 60%) had a pretest probability <15%, 188/523 (36%) had a pretest probability between 15 and 50%, and 19/523 (4%) had a pretest probability >50%. The prevalences of coronary artery disease (≥50% lumen diameter reduction) by CCTA in patients with pretest probability <15%, 15–50%, and >50% were 25/316 (8%), 45/188 (24%) and 8/19 (42%), respectively. In 438/523 patients (84%), a CCTA scan showing no coronary artery disease represented the final diagnostic step. In patients undergoing invasive coronary angiography (n = 59, age 58 ± 9 years, 88% with coronary artery disease by CCTA), coronary artery disease was found in 47/59 (80%) patients and 36/59 (61%) patients underwent revascularisation. The prevalences of coronary artery disease by invasive coronary angiography in patients with pretest probability <15%, 15–50%, and >50% were 14/21 (67%), 28/32 (88%) and 5/6 (83%).
CONCLUSIONS
The present data suggest that the currently used pretest probability model is still imperfect and that guideline recommendations regarding pretest probability use for the selection of CCTA candidates are not followed completely. Still, in more than 80% of patients coronary artery disease could be excluded by CCTA, while CCTA also detected a significant number of patients with coronary artery disease in the low pretest probability population. Thus, the data suggest a very judicious use of CCTA as a gatekeeper for invasive coronary angiography in current practice.
Introduction
Coronary artery disease (CAD) is a leading cause of morbidity and mortality in the Western world [1]. The timely diagnosis of coronary artery disease is important for the prevention of cardiovascular events with pharmacological treatment and revascularisation in selected patients [2]. However, the clinical diagnosis of coronary artery disease is difficult, and the selection of patients needing invasive coronary angiography remains challenging. The general principle underlying the diagnostic approach is to first assess a patient’s pretest probability of coronary artery disease based on age, gender and type of symptoms, since the prevalence of coronary artery disease has been shown to strongly depend on these parameters [2–4], and then to perform non-invasive tests in selected patients. It is assumed that in patients with very low ( <15%) or very high (>85%) pretest probability, non-invasive stress tests are not helpful for the diagnosis of coronary artery disease given the imperfect sensitivity and specificity of all these tests [2]. In contrast, for patients with intermediate pretest probability (15-85%), such non-invasive stress tests, including exercise stress tests, myocardial perfusion scintigraphy, stress echocardiography and stress magnetic resonance imaging are helpful, as the results of these tests modify the probability of coronary artery disease in a clinically relevant manner [2], i.e., from an intermediate pretest probability to a low or a high pretest probability.
In recent years, coronary computed tomography angiography (CCTA) has emerged as an alternative and/or addendum to non-invasive stress tests in the diagnostic algorithm [5, 6]. Due to its high negative predictive value, CCTA is a valuable tool to exclude coronary artery disease, although its specificity is limited [7]. The current guidelines of the European Society of Cardiology (ESC) [2] therefore recommend CCTA as a first-line option for the exclusion of coronary artery disease in patients with low to intermediate pretest probability (15-50%), and as a second-line test in selected patients with higher pretest probability and an ambiguous result from a stress test or a stress test that contradicts clinical judgement. It is obvious that CCTA is now frequently performed in daily routine. However, how patients are selected for CCTA in clinical practice is not well known. In the present study, we aimed to describe the use of CCTA in a single centre in Switzerland in light of current ESC guideline recommendations.
Materials and methods
Study design
This was a retrospective analysis of data which have been collected prospectively for clinical purposes and from procedures which are performed in a standardised manner. The local ethics committee approved the study.
Study population
We studied consecutive patients undergoing CCTA at our institution during a two-year period from January 2014 to December 2015. The Kantonsspital St. Gallen represents a referral centre in Eastern Switzerland with approximately 2500 patients undergoing invasive coronary angiography each year. Patients undergoing CCTA in a context other than suspected coronary artery disease (e.g. computed tomography for planning of valve interventions) were excluded. Clinical information was obtained from the letters accompanying the referrals as well as from medical records and the clinical information system for all patients admitted to the hospital. For all patients also undergoing invasive coronary angiography at our institution, detailed information on non-invasive tests and invasive coronary angiography and subsequent management were obtained from medical records and the clinical information system.
Assessment of pretest probability
The revised Diamond-Forrester model of the CAD consortium was used to calculate pretest probability [4]. This model, published in a paper by Genders et al. [4] in 2011 and also in the form of an online calculator, represents the method for pretest probability assessment which is currently recommend by the ESC guidelines [2]. This model has been shown to more accurately predict coronary artery disease than the traditional Diamond-Forrester model [8]. We used the online calculator to calculate pretest probability based on age, gender and type of symptoms, including typical, atypical or unspecific chest pain. Typical chest pain was defined as (i) substernal chest pain or discomfort that is (ii) provoked by exertion or emotional stress and (iii) relieved by rest and/or nitroglycerin. Atypical chest pain was defined as two of the above-mentioned criteria. If one or none of the criteria were present, the patient was classified as having nonspecific chest pain. This information was extracted from the available clinical documents. This model is referred to as the “basic model”. The online calculator also offers two more refined models: (i) a model including detailed information on cardiovascular risk factors (four items: diabetes, hypertension, dyslipidaemia, smoking), and (ii) a model including risk factors + coronary artery calcium score (CAC). For all patients also undergoing invasive coronary angiography, their pretest probability according to these two models was also calculated, since for these patients detailed information on risk factors was also available.
Definition of coronary artery disease
For both CCTA and invasive coronary angiography, coronary artery disease was defined as stenosis ≥50% lumen diameter reduction in at least one vessel.
Coronary calcium score and coronary computed tomography angiography
All patients underwent assessment of CAC and CCTA according to standard institutional scanning and contrast injection protocols, which were individually adapted relative to heart rate and body mass index (BMI). Nitroglycerin was applied in all patients prior to CCTA if there were no contraindications. Beta-blockers were not routinely administered. Each patient first received a non-contrast acquisition for the quantification of coronary calcium, expressed as CAC (which is based on the density and extent of calcium in the coronary tree), followed by a high-resolution, contrast-enhanced acquisition using a 192-slice or 128-slice dual-source CT system (SOMATOM Force; Siemens Healthineers, Forchheim, Germany, Siemens Flash 128 detector-row CT or Siemens Force 192 detector-row CT) equipped with a high resolution detector (Stellar Technology; Siemens Healthineers). The scan parameters were slice acquisition, 2 × 192 × 0.6 mm and 2 × 128 × 0.6 mm; gantry rotation time, 250 msec and 280 msec respectively; and tube potential, 120 kV for non-contrast scans. For CCTA, tube potential was 100 kV if BMI was <30 kg/m2 and 120 kV if BMI was >30 kg/m2. Tube current-time product was set at 80 mAs for non-contrast scans and 320 mAs for contrast-enhanced scans. The computed tomography acquisition was performed with prospective ECG synchronisation at high pitch (3.2) if the heart rate was regular and less than 70 bpm, or in a retrospective manner if the heart rate was irregular or more than 70 bpm (required in one third of patients). The scan length extended from the pulmonary artery bifurcation to the apex of the heart. An average of 60 ml of contrast medium (iobitridol, Xenetix® 350, 350mg iodine/ml, Guerbet, Aulnay-sous-Bois, France) was injected into an antecubital vein, followed by a chaser of 30 ml of diluted contrast medium (20% vol) with a dual-head power injector (Stellant®, Medrad, Inianola, USA) at a flow rate of 5.0–6.0 ml/s. Scan initiation was controlled by bolus tracking with a region of interest in the ascending aorta, using a signal attenuation threshold of 120 Hounsfield units at 120 kVp. Calcifications were quantified with commercial evaluation software (Syngo.via, Siemens Healthineers, Forchheim, Germany). All lesions on more than two contiguous pixels with attenuation values greater than 130 Hounsfield units were marked by a radiologist and the calcium load in each patient was computed by using the Agatston method [9].
Invasive coronary angiography
Coronary angiography was performed by femoral or radial approach using standard techniques by senior invasive cardiologists. The severity of coronary stenosis, as described by % lumen diameter reduction, was assessed visually. The decision about revascularisation and the mode of revascularisation was at the discretion of the invasive cardiologist. This decision usually took into account all the clinical information, coronary anatomy and patient preference. In our institution, fractional flow reserve is routinely measured in patients with ambiguous severity of coronary stenosis to provide a basis on which to decide about revascularisation.
Radiation doses
To get an estimate of the radiation required for CCTA compared to invasive coronary angiography, we assessed the dose length products and dose area products respectively for those patients who underwent both a CCTA and a purely diagnostic invasive coronary angiography. The radiation dose estimate was calculated from the dose length product and a conversion coefficient of 0.017 mSv × mGy-1 × cm-1 (for CCTA) and from the dose area product and a conversion coefficient of 0.02 mSv × mGy-1 × cm-1 (for invasive coronary angiography).
Statistical analysis
Categorical data are presented as numbers and percentages, and continuous variables are presented as mean ± standard deviation or median (interquartile range) as appropriate. Data from patients with pretest probability <15%, 15–50% and >50% were compared using chi-square tests (for categorical data), analysis of variance (for continuous data with a normal distribution), and Kruskal Wallis tests (for continuous data with a skewed distribution). Estimated doses for CCTA and invasive coronary angiography in the same patients were compared using paired t-tests. A p value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 20.0 (SPSS Inc, Chicago, Illinois).
Results
Study population
The study population included 523 patients (mean age 56 ± 13 years, 48% females). According to the basic model, the median (interquartile range) pretest probability in the entire population was 11% (6–20%). The majority of patients (n = 316; 60%) had a pretest probability <15%, 188 (36%) had a pretest probability between 15 and 50% and 19/523 (4%) had a pretest probability >50%.
CCTA findings according to pretest probability
Age increased, and there was a higher prevalence of males, with increasing pretest probability (table 1). The prevalence of coronary artery disease by CCTA in the entire study population was 78/523 (15%). As shown in table 1 and figure 1, the prevalences of coronary artery disease by CCTA in patients with pretest probability <15%, 15–50% and >50% were 8%, 24% and 42%, respectively. Detailed information on CCTA findings according to the three pretest probability categories and further testing is provided in figure 1. There were 25 patients in the low pretest probability population (8%) who had coronary artery disease according to CCTA. These patients with low pretest probability but coronary artery disease (n = 25) had significantly higher CAC than those with low pretest probability but without coronary artery disease (n = 292) [214 (73–318) vs 0 (0–7); p <0.001]. The same was true for patients with intermediate pretest probability [CAD: 651 (139–1029) vs no CAD: 4 (0–67); p <0.001] and high pretest probability [CAD: 648 (190–1126) vs no CAD: 3 (0–251); p = 0.02].
Table 1 Characteristics of the entire study group (n = 523) according to the three pretest probability (PTP) categories.
|
PTP <15%
n = 316
|
PTP 15–50%
n = 188
|
PTP >50%
n = 19
|
p-value
|
| Age (years) |
55 ± 11 |
57 ± 15 |
60 ± 19 |
0.09 |
| Gender (female) |
212 (67%) |
38 (20%) |
2 (11%) |
<0.001 |
| PTP (%) |
8±3 |
25±9 |
60±9 |
<0.001 |
| Agatston score |
0 (0-19) |
30 (0-273) |
205 (0-699) |
<0.001 |
|
CCTA
|
|
|
|
<0.001 |
| Normal coronary arteries |
197 (62%) |
71 (38%) |
6 (32%) |
| Calcifications without plaques |
77 (24%) |
48 (26%) |
4 (21%) |
| Atherosclerosis ( <50% lumen diameter reduction) |
17 (6%) |
24 (13%) |
1 (5%) |
| CAD (≥50% lumen diameter reduction) |
25 (8%) |
45 (24%) |
8 (42%) |

Figure 1 Diagram showing the diagnostic pathway in all patients undergoing coronary computed tomography angiography (CCTA) according to the three pre-test probability (PTP) categories.
CAD: coronary artery disease, ICA: invasive coronary angiography, OMT: optimal medical therapy.
Patient pathways following CCTA
There were 59 patients (age 58 ± 9 years, 32% females) out of the 532 patients undergoing CCTA who subsequently also underwent invasive coronary angiography at our centre. As shown in figure 1, there were a variety of diagnostic pathways following CCTA. The majority of patients with a CCTA scan showing coronary artery disease (52 out of 78; 67%) underwent invasive coronary angiography. However, there were also 26 patients with coronary artery disease by CCTA who did not undergo invasive coronary angiography. Also, there were eight out of 445 patients without coronary artery disease by CCTA who still underwent invasive coronary angiography. Notably, a CCTA scan showing no coronary artery disease represented the final diagnostic step in 438 patients (84% of the entire population).
Characteristics and pretest probability according to different models in patients undergoing both CAC/CCTA and invasive coronary angiography
The characteristics of the subgroup of patients undergoing invasive coronary angiography according to the three pretest probability categories are shown in table 2. There was a broad spectrum of risk factors in all three categories. The use of the basic model (age, gender, type of symptoms) and the basic model + risk factors revealed similar pretest probability values in all three pretest probability groups, with slightly higher values when using the model which also considered risk factors. In contrast, the addition of CAC resulted in significantly higher pretest probability values in all three groups: only nine patients had a pretest probability <15% (basic model: 21 patients), 21 patients had a pretest probability 15–50% (basic model: 32 patients), and 27 patients had a pretest probability >50% (basic model: 6 patients). In two patients, CAC was not measured, and thus the pretest probability integrating CAC could not be assessed. As also shown in table 2, the majority of patients (76%) had had an exercise stress test prior to CCTA, indicating that CCTA was performed not as a first-line, but as a second-line test.
Table 2 Characteristics of the subgroup of patients undergoing both coronary computed tomography angiography (CCTA) and invasive coronary angiography (ICA; n = 59) according to the three pretest probability (PTP) categories.
|
|
PTP <15%
n = 21
|
PTP 15–50%
n = 32
|
PTP >50%
n = 6
|
p-value
|
| Age |
|
57 ± 6 |
60 ± 11 |
64 ± 5 |
0.28 |
| Gender (female) |
|
14 (66%) |
5 (16%) |
0 |
<0.001 |
| PTP (%) |
|
7 ± 4 |
25 ± 9 |
61 ± 8 |
<0.001 |
| + risk factors |
|
8 ± 7 |
26 ± 11 |
64 ± 13 |
<0.001 |
| + risk factors + CAC |
|
20 ± 14 |
51 ± 19 |
80 ± 8 |
<0.001 |
| Risk factors |
Smoking |
9 (43%) |
17 (53%) |
4 (66%) |
0.55 |
| Hypertension |
11 (52%) |
15 (47%) |
4 (66%) |
0.66 |
| Diabetes |
2 (10%) |
3 (9%) |
1 (17%) |
0.86 |
| Family history |
12 (57%) |
14 (44%) |
1 (17%) |
0.20 |
| Total cholesterol (mmol/l) |
5.1 ± 1.3 |
4.8 ± 1.1 |
5.5 ± 1.7 |
0.36 |
| LDL cholesterol (mmol/l) |
3.0 ± 1.1 |
2.9 ± 0.9 |
3.6 ± 1.6 |
0.34 |
| HDL cholesterol (mmol/l) |
1.5 ± 0.4 |
1.4 ± 0.4 |
1.4 ± 0.2 |
0.80 |
| eGFR (ml/min/1.73 m2) |
|
80 ± 11 |
80 ± 12 |
87 ± 5 |
0.36 |
| Left ventricular ejection fraction (%) |
|
60 ± 9 |
61 ± 8 |
61 ± 6 |
0.75 |
| Exercise stress test (n = 45) |
Work rate (% predicted) |
104 ± 34 |
98 ± 30 |
98 ± 14 |
0.84 |
| Rate pressure product (mm Hg*bpm) |
29006 ± 6208 |
27143 ± 6189 |
21719 ± 4191 |
0.05 |
| Angina |
3 (14%) |
5 (16%) |
2 (33%) |
0.66 |
| ST depression |
9 (43%) |
5 (16%) |
1 (17%) |
0.06 |
| Medication |
Aspirin |
4 (19%) |
13 (41%) |
3 (50%) |
0.18 |
| Statin |
8 (38%) |
11 (34%) |
0 |
0.20 |
| Insulin |
0 |
0 |
0 |
|
| Oral antidiabetic drugs |
1 (5%) |
2 (6%) |
0 |
0.81 |
| Beta-blocker |
7 (33%) |
10 (31%) |
2 (33%) |
0.10 |
| Calcium channel blocker |
2 (10%) |
3 (9%) |
2 (33%) |
0.23 |
| ACEI/ARB |
4 (19%) |
8 (25%) |
3 (50%) |
0.31 |
| Nitrate |
1 (5%) |
1 (3%) |
1 (17%) |
|
| Agatston score |
|
225 (67-349) |
577 (116-1023) |
648 (207-907) |
0.1 |
| CAD by CCTA |
|
17 (81%) |
30 (94%) |
5 (83%) |
0.18 |
| CAD by ICA |
|
14 (67%) |
28 (88%) |
5 (83%) |
0.18 |
| Management |
None |
7 (33%) |
4 (13%) |
1 (17%) |
|
| Medical therapy |
5 (24%) |
6 (18%) |
0 |
|
| Percutaneous coronary intervention |
6 (29%) |
18 (56%) |
4 (66%) |
|
| Coronary artery bypass grafting |
3 (14%) |
4 (13%) |
1 (17%) |
|
Findings of invasive coronary angiography and clinical consequences
As shown in table 2 and figure 1, not all patients undergoing invasive coronary angiography had coronary artery disease by CCTA. However, invasive coronary angiography identified coronary artery disease in a high proportion of patients in all pretest probability groups (between 67 and 88%): there were 47 patients with coronary artery disease by invasive coronary angiography, 36 of whom underwent revascularisation. Notably, there were 14 patients with pretest probability <15% in whom invasive coronary angiography demonstrated coronary artery disease and nine out of these 14 patients underwent coronary revascularisation. Detailed findings of CCTA and invasive coronary angiography in the 59 patients who underwent both tests are shown in supplementary table S1 in appendix 1. In figures 2 and 3
, some representative examples of CCTA and invasive coronary angiography are shown.
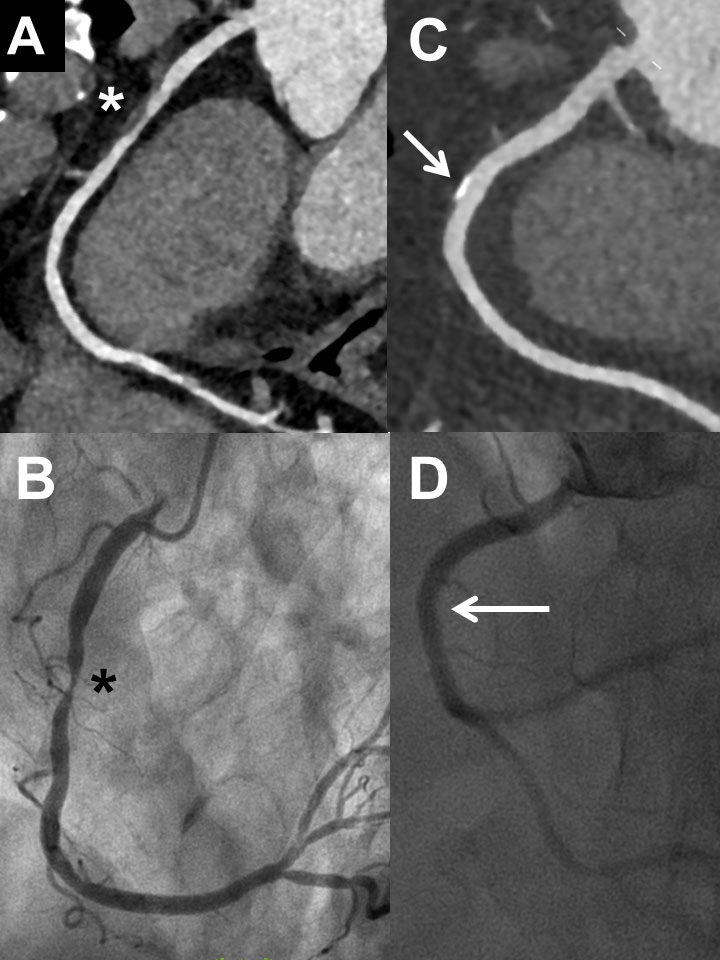
Figure 2 Examples of coronary computed tomography (CCTA) and invasive coronary angiography (ICA) in two patients with excellent correlation of the two methods: First patient (male, 62 years) with severe stenosis in the mid right coronary artery in CCTA (panel A, asterisk) and ICA (panel B, asterisk). Second patient (male, 58 years) with plaque (no relevant stenosis) in the mid right coronary artery in CCTA (panel C, arrow) and ICA (panel D, arrow).
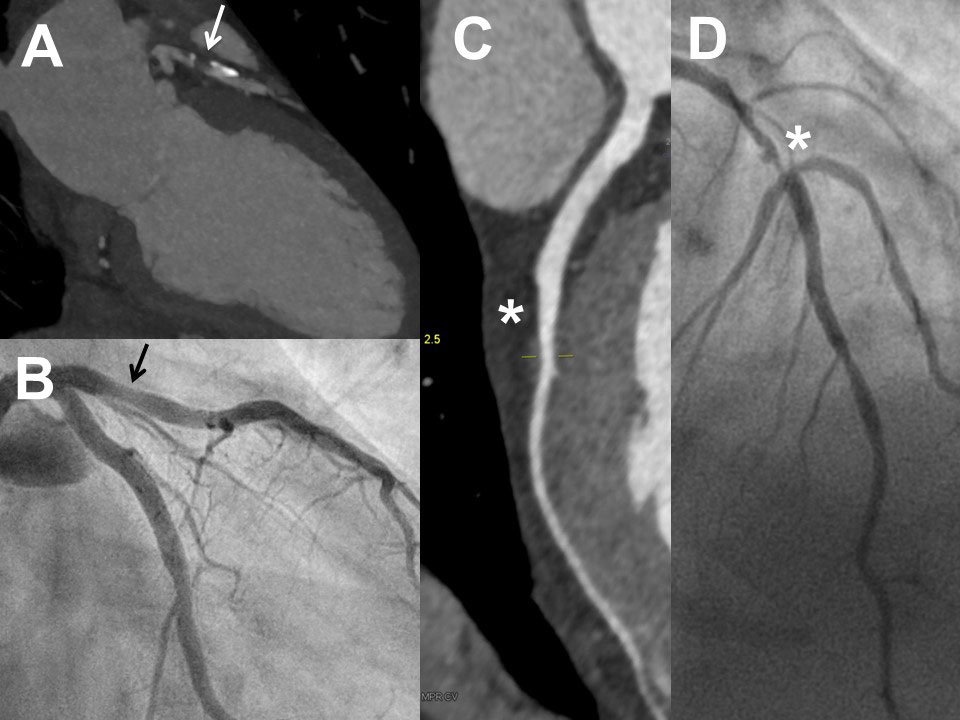
Figure 3 Examples of coronary computed tomography (CCTA) and invasive coronary angiography (ICA) in two patients with non-optimal correlation of the two methods: Frist patient (female, 54 years) with severe stenosis of the proximal left anterior descending artery in CCTA (panel A, arrow) but eccentric plaque without significant stenosis in ICA (panel B, arrow; lesion looking less relevant in other projections, absolutely normal fractional flow reserve). Second patient (female, 61 years) with no significant stenosis of the mid left anterior descending artery in CCTA (panel C, asterisk) but severe stenosis of the mid left anterior descending artery in ICA (panel D, asterisk).
Radiation doses
In the 34 patients who underwent both CCTA and purely diagnostic invasive coronary angiography, estimated doses were lower for CCTA than for invasive coronary angiography (4.5 ± 4.1 vs 9.9±7.5 mSv; p <0.001).
Discussion
The present study revealed important insights into current practice regarding the use of CCTA in Switzerland. At first glance, only one third of patients referred for CCTA are representative of those in whom CCTA is recommended based on pretest probability, while nearly two thirds of patients had a pretest probability <15%. This could be interpreted as a very high number of unnecessary CCTA referrals, with many subjects inappropriately exposed to radiation. However, a more detailed examination of the data revealed an unexpectedly high prevalence, 8%, of stenotic coronary artery disease by CCTA in the low pretest probability population. Although not all patients with an abnormal CCTA underwent invasive coronary angiography (at least not at our centre), and there were some patients with false positive CCTA findings, more than 4% of the low pretest probability population were found to have stenotic coronary artery disease on invasive coronary angiography, and nearly 3% of the low pretest probability population finally underwent coronary revascularisation. On the other hand, CCTA could exclude stenotic coronary artery disease in 84% of patients from the entire population and was therefore the final diagnostic step. In the following sections, we discuss some relevant aspects of the present study.
Pretest probability estimation versus true prevalence of coronary artery disease
Current guidelines [2] reinforce the routine application of the Bayesian model in clinical decision-making regarding the choice of tests for the diagnosis of coronary artery disease. However, the utility of the model very much relies on a correct estimation of the pretest probability. The traditional Diamond-Forrester model [3] includes only age, gender and symptoms. The latter is critical but is subjective to a certain degree as it depends on how a patient’s history is obtained. The Diamond-Forrester model has recently been shown to overestimate the prevalence of coronary artery disease in a contemporary cohort of patients undergoing CCTA [8]. There are other models for the estimation of pretest probability, such as the Duke Clinical Score [10]. This score also requires information about the electrocardiogram and may be somewhat more accurate than the Diamond-Forrester model [11], but also seems to overestimate the prevalence of coronary artery disease [12]. The current ESC guidelines [2] recommend the use of the model published by Genders et al., which has been derived from a multicentre study of 2260 patients undergoing invasive coronary angiography [4]. When using this model (basic model), a pretest probability <15% is attributed to nearly two thirds of our study population. Detailed information on risk factors was not available for all patients, but the analysis of the subgroup of patients undergoing invasive coronary angiography showed that the integration of risk factors into the model did not result in major changes to pretest probability overall. However, the relatively high prevalence of stenotic coronary artery disease in the pretest probability <15% population (8% by CCTA, 4% by invasive coronary angiography, 3% with revascularisation) suggests that this method of pretest probability calculation underestimates the prevalence of coronary artery disease. The availability of the CAC significantly modifies pretest probability, as shown in the invasive coronary angiography subgroup. However, CAC is rarely available for decision-making as CAC alone (i.e., without CCTA) is rarely performed. A recent study in a contemporary cohort with very similar age, gender distribution and median pretest probability according to the basic model as our population showed that the coronary artery disease consortium model more accurately predicts the prevalence of coronary artery disease by CCTA than the Diamond-Forrester model [8]. The present data suggest that the basic model of the coronary artery disease consortium is still imperfect. The inclusion of modifiable risk factors and CAC may further improve the estimation of the coronary artery disease prevalence. The clinical judgment of the referring doctors obviously resulted in a higher suspicion of coronary artery disease than the estimated pretest probability would have suggested, which finally resulted in referral for CCTA. Information not reflected by the pretest probability (basic model, basic model + risk factors) but leading to a decision to perform CCTA may include the chronicity of chest sensations and their impact on quality of life, special risk factors such as family history of premature coronary artery disease and an ambiguous or abnormal result in an exercise stress test. To the best of our knowledge, similar data from other centres in Switzerland are not available. In a large, Danish multicentre registry of patients undergoing CCTA, the vast majority (≈80%) had an intermediate pretest probability [13]. However, pretest probability in this study was calculated according to the original Diamond Forrester model, which results in higher values than the coronary artery disease consortium basic model as mentioned above. Interestingly, the proportion of patients with coronary artery disease by CCTA (same definition as in our study) was similar to in our study (16 vs 15%). Thus, our study suggests that further research for the refinement of pretest probability models is required.
Patient pathways
The present study gave insights into diagnostic pathways, which is a particularly interesting feature since such information is usually not available. A key piece of information is the fact that in 84% of patients, CCTA excluded coronary artery disease and was therefore the final diagnostic step. Studies have shown that a normal CCTA scan is associated with a very low risk of future coronary events [14, 15]. As discussed above, some might argue that given the low pretest probability in many patients, it was not necessary to perform any test at all. However, as also mentioned above, the relatively high prevalence of coronary artery disease in the low pretest probability population contradicts this argument. As expected, many patients with coronary artery disease by CCTA underwent invasive coronary angiography to accurately assess coronary anatomy and to provide a basis for treatment decisions including percutaneous revascularisation or bypass surgery. There were also patients with coronary artery disease by CCTA who did not undergo invasive coronary angiography. There are two possible reasons for this: first, in some patients with moderate stenosis and no limiting symptoms, a decision for medical treatment may sometimes by based on a CCTA finding alone, although the specificity of the test is limited. Second, some patients may have undergone invasive coronary angiography at another institution. A few patients without coronary artery disease by CCTA still underwent invasive coronary angiography, the reason for which remains speculative. Given the high negative predictive value of a normal CCTA [16], it is unlikely that invasive coronary angiography will reveal a different finding. Still, in the presence of symptoms significantly affecting quality of life, patients and/or doctors may want to proceed to invasive coronary angiography to exclude coronary artery disease with an additional method. Most of these patients had high CAC, which may have led to a decision for invasive coronary angiography.
CCTA versus invasive coronary angiography
Studies concur that CCTA has a high sensitivity of 94–99% and a moderate specificity of 64–83% for the diagnosis of coronary artery disease, defined as 50% or more stenosis by invasive coronary angiography [16]. If we calculate the sensitivity and specificity of CCTA for the prediction of coronary artery disease by invasive coronary angiography (any stenosis >50%, no anatomical correlation required) in the subgroup of patients undergoing both CCTA and invasive coronary angiography, we get a sensitivity of 94% and a specificity of 33%. However, given that this was a highly selected subgroup with a higher prevalence of coronary artery disease and higher CAC than both the remainder of the study population and the average patient for whom CCTA is recommended, this does not allow conclusions about the performance of CCTA in general, and therefore the data are not reported in the results section but are provided here for the readers’ information. The referring doctors were obviously aware of the limited specificity of CCTA, since the proportion of patients with high pretest probability in the present cohort was low.
Appropriateness of the use of CCTA
As discussed above, it seems incorrect to classify the majority of CCTA scans as inappropriate based simply on the calculation of pretest probability. In contrast, the data suggest a very judicious use of CCTA in general practice. Although the data for the entire population are not available, the findings from the invasive coronary angiography group indicate that many patients had an exercise stress test prior to CCTA. Although the sensitivity of the exercise test for the prediction of coronary artery disease is very limited [2], the exercise response helps doctors to understand the prognostic relevance [17] of the CCTA findings and aids their decision whether or not to proceed to invasive coronary angiography. The CCTA findings also aid in deciding whether or not a patient will require long-term treatment with aspirin and a statin. In 84% of patients, CCTA excluded coronary artery disease and was not followed by invasive coronary angiography. For patients with normal scans, this allowed them to stop taking drugs such as aspirin and statin, which otherwise may have been administered for years without clear indication. For patients with atherosclerosis but no significant stenosis, CCTA offered an opportunity for aggressive risk factor management. Furthermore, CCTA detected coronary artery disease in a relevant number of subjects in whom the disease may have otherwise remained unknown until an acute coronary event.
Notably, the number of invasive coronary angiographies at our institution has not decreased over the years [18], suggesting that CCTA is not replacing invasive coronary angiography. It is speculated that a population of patients which formerly had been managed based on non-invasive testing (mainly exercise stress tests) and for which invasive coronary angiography is considered too aggressive an approach given the low yield of invasive coronary angiography in not well selected cohorts is now tested by CCTA [19]. Notably, only 7% of the entire population undergoing CCTA underwent revascularisation. This also argues against a significant number of unnecessary revascularisations triggered by CCTA. The radiation dose required for CCTA in clinical practice has significantly decreased over recent years [20]. The present small snapshot regarding estimated doses for CCTA versus invasive coronary angiography confirms low doses for CCTA compared to invasive coronary angiography performed by experienced operators, and thereby further supports the application of the method.
Limitations
The study is primarily limited by its retrospective design and the limited information on patient characteristics. Furthermore, the number of patients was limited, and it is a single centre observation which does not allow firm conclusions regarding other centres in Switzerland. In addition, follow-up information is not available, and the considerations regarding some clinical consequence of CCTA remain speculative. In addition, as mentioned already in the discussion section, we cannot exclude the possibility that some patients who apparently did not undergo invasive coronary angiography following a CCTA scan showing coronary artery disease underwent invasive coronary angiography at another institution. This information is not available. There is however no catheter laboratory closer than 35 km to our institution. With regards to CCTA preparation, we acknowledge that beta-blockers were not routinely administered and that approximately one third of patients required retrospective gating because of heart rates exceeding the 65 bpm limit for prospective gating.
Conclusions
The present data suggest that the currently used pretest probability model is still imperfect, and also that guideline recommendations regarding pretest probability use for the selection of CCTA candidates are not fully followed. Still, coronary artery disease could be excluded by CCTA in more than 80% of patients. CCTA also detected a relevant number of patients with coronary artery disease in the low pretest probability population. Thus, the data suggest a very judicious use of CCTA as a gatekeeper for invasive coronary angiography in current practice.
Appendix 1 Supplementary table
Table S1 Findings from coronary computed tomography angiography (CCTA) and invasive coronary angiography (ICA) in patients undergoing both tests (n = 59)
|
Age
|
Gender
|
Agatston score
|
CCTA
|
Invasive coronary angiography (ICA)
|
CCTA versus ICA*
|
| 1 |
54 |
f |
0 |
No stenosis |
No stenosis |
True negative |
| 2 |
47 |
f |
287 |
No stenosis |
No stenosis |
True negative |
| 3 |
62 |
f |
167 |
30–40% mid LAD |
No stenosis |
True negative |
| 4 |
56 |
m |
256 |
40% proximal LAD |
No stenosis |
True negative |
| 5 |
57 |
f |
310 |
No stenosis |
50% mid LAD, 50% first diagonal branch, 50% RCA |
False negative |
| 6 |
61 |
f |
33 |
No stenosis |
70–90% mid LAD, distal LAD and second diagonal branch |
False negative |
| 7 |
58 |
m |
687 |
40% proximal LAD |
70–90% proximal LAD |
False negative |
| 8 |
54 |
f |
268 |
90–99% proximal LAD |
<50% proximal LAD |
False positive |
| 9 |
59 |
f |
386 |
80% distal LAD |
No stenosis |
False positive |
| 10 |
54 |
f |
125 |
85% mid LAD |
No stenosis |
False positive |
| 11 |
63 |
f |
14 |
70–80% proximal LAD |
No stenosis |
False positive |
| 12 |
56 |
m |
546 |
60–70% distal LAD, 40–50% proximal LAD |
No stenosis |
False positive |
| 13 |
66 |
m |
1109 |
Significant stenoses LAD, LCX, RCA |
No stenosis |
False positive |
| 14 |
73 |
m |
667 |
70–75% proximal LAD |
<50% mid LAD |
False positive |
| 15 |
70 |
m |
141 |
80–85% mid LAD |
<50% mid LAD |
False positive |
| 16 |
34 |
m |
37 |
90–99% mid RCA |
90–99% mid RCA |
True positive |
| 17 |
47 |
f |
65 |
60% proximal RCA, 70–75% mid LAD, 50% first diagonal branch |
50–70% proximal LAD and 70–90% mid LAD, 50–70% first diagonal branch |
True positive |
| 18 |
64 |
f |
77 |
70–75% mid LAD |
70–80% proximal AD, 50–70% first diagonal branch. |
True positive |
| 19 |
66 |
f |
|
70% mid and distal LAD, 30% LCX |
Occlusion mid LAD, 50% proximal LCX, 50–70% mid RCA |
True positive |
| 20 |
67 |
f |
376 |
80% proximal LAD, 50–60% mid LAD |
70–90% proximal and mid LAD, 70–90% first and second diagonal branch |
True positive |
| 21 |
48 |
m |
1791 |
90–99% RCA, 40–50% LAD, 60–70% marginal branch of LCX |
50–70% mid LAD, 70–90% second diagonal branch, 50–70% first marginal branch of LCX, total occlusion proximal ACD |
True positive |
| 22 |
54 |
m |
182 |
95–99% distal LCX, 45–50% PDA of RCA |
50–70% proximal LAD, 50–70% proximal LCX |
True positive |
| 23 |
66 |
f |
340 |
90–99 proximal LAD, 70–80% proximal and mid RCA |
70–90% proximal and mid LAD, 70–90% proximal and mid RCX, 70% PLA |
True positive |
| 24 |
55 |
m |
331 |
70–90% mid RCA and PDA, 70–75% proximal and mid LAD |
50% mid RCA, 70–90% PDA |
True positive |
| 25 |
57 |
m |
68 |
Severe stenoses LAD and intermediate branch |
<50% proximal LAD, 70–80% intermediate branch |
True positive |
| 26 |
56 |
m |
|
70% distal LCX |
60% mid LCX, 50% mid RCA |
True positive |
| 27 |
57 |
m |
|
90% proximal LAD, patent bypass grafts |
50–70% mid LAD, occlusion LCX, 70–90% mid RCA |
True positive |
| 28 |
58 |
m |
1341 |
65–70% proximal RCA, 90% PDA, 40–50% mid LAD |
50–70% first diagonal branch, 50–70% LAD, 70% mid LCX 90–99% second marginal branch of LCX, 70% proximal RCX |
True positive |
| 29 |
51 |
m |
705 |
50–60% ostial RCA, 70% distal RCA, 70% mid LAD |
50% ostial RCA |
True positive |
| 30 |
62 |
m |
39 |
80–85% mid RCA, 50% proximal LAD, 95% first diagonal branch |
70–90% mid RCA, 70% first diagonal branch, 70–90% LCX |
True positive |
| 31 |
62 |
m |
1039 |
80–95% LAD, 90–99% marginal branch of LCX, 90% PDA |
50–70% mid LAD, 50–70% mid LCX, 50–70% PDA, 90–99% PLA |
True positive |
| 32 |
62 |
m |
2485 |
75–80% proximal LAD |
70–90% proximal LAD, 70–90% proximal LCX, 50–70% mid RCA |
True positive |
| 33 |
62 |
m |
2165 |
80% proximal RCA, 70 – 80% mid LAD |
85% mid RCA; 50–70% proximal LAD, 85% mid LAD |
True positive |
| 34 |
63 |
m |
1097 |
Chronic total occlusion RCA, 90–99% LCX and first diagonal branch, |
90–99% first diagonal branch, occlusion LCX and ACD |
True positive |
| 35 |
75 |
f |
607 |
90–99% RCA, 75% proximal LAD, 90% distal LAD; 80% proximal LCX |
90–99% proximal ACD, 50–70% mid RCA, 50–70%, 50–70% distal RCA, 50–70% proximal LCX, 90–99% mid and distal LCX |
True positive |
| 36 |
36 |
m |
185 |
Severe stenosis LAD |
50–70% proximal LAD |
True positive |
| 37 |
35 |
m |
13 |
70% stenosis ACD with thrombus |
70–90% proximal RCA, thrombus, occlusion PLA |
True positive |
| 38 |
55 |
m |
1598 |
85% distal left main |
70% left main, 70% intermediate branch, 50–70% mid RCA |
True positive |
| 39 |
57 |
m |
507 |
Severe stenosis proximal LAD, 70% PDA |
70–90% proximal LAD, 90–99% mid LAD |
True positive |
| 40 |
66 |
m |
866 |
65% proximal RCX, 80% mid RCA |
70–90% proximal and mid LAD, first diagonal branch, 50–70% proximal RAX, distal RCA, PDA, 90–99% PLA |
True positive |
| 41 |
78 |
f |
1025 |
50 – 60% mid RCX, >90% first diagonal branch, 90% intermediate branch, 50% marginal branch LCX |
70–90% first diagonal branch, 70% intermediate branch |
True positive |
| 42 |
59 |
m |
392 |
90% distal RCX, 90–95% mid LAD, 90–95% marginal branch of LCX |
50% mid LAD, 50–70% proximal LCX, 70–90% first marginal branch, 50% distal RCA |
True positive |
| 43 |
59 |
m |
37 |
Occlusion distal LAD |
50–70% mid LAD |
True positive |
| 44 |
60 |
m |
765 |
75% proximal and distal LAD, occlusion proximal RCA, occlusion first diagonal branch and first marginal branch of LCX |
Occlusion RCA, 50% LCX, 70–90% proximal LAD and first diagonal branch |
True positive |
| 45 |
40 |
m |
664 |
80–90% mid and distal LAD |
90–99% mid LAD. |
True positive |
| 46 |
62 |
f |
151 |
50–60% proximal and mid LAD |
<50% mid LAD, 50% first marginal branch |
True positive |
| 47 |
61 |
f |
2288 |
Significant stenoses proximal and mid LAD and RCX |
Severe stenoses LAD, LCX, RCA |
True positive |
| 48 |
72 |
m |
1016 |
90% RCA, 85% LAD, 90% marginal branch of LCX |
70–80% mid RCA |
True positive |
| 49 |
44 |
m |
104 |
Severe stenosis proximal LAD |
70–90% proximal LAD, 90–99% distal LAD, 50% proximal RCA, 70–90% distal RCA, 50% PDA |
True positive |
| 50 |
45 |
m |
27 |
Occlusion RCX |
Occlusion proximal ACD |
True positive |
| 51 |
68 |
f |
633 |
70% LCX ostial, 75% mid RCA, 60% distal RCA |
60% LCX, 70% ostial RCA, 75% 50–65% mid RCA, distal RCA |
True positive |
| 52 |
71 |
m |
344 |
80% marginal branch of LCX, 30–40% LAD and LCX |
70–90% first diagonal branch, 70–90% first marginal branch of LCX |
True positive |
| 53 |
52 |
m |
273 |
70 – 75% mid LAD, 70% distal LAD, 50 – 60% distal LCX |
50% proximal LAD |
True positive |
| 54 |
55 |
m |
61 |
Occlusion mid RCA |
Occlusion proximal RCA |
True positive |
| 55 |
56 |
m |
83 |
Significant stenosis proximal and mid LAD |
Significant stenoses LAD and first and second diagonal branch, 50% RCA |
True positive |
| 56 |
59 |
m |
1126 |
70–75% mid LAD |
70–90% mid RCA |
True positive |
| 57 |
61 |
m |
272 |
90–99% distal RCA |
90–99% distal RCA, 70–90% PDA, 50% PLA |
True positive |
| 58 |
68 |
m |
648 |
60–70% ACD and mid LAD, 70% first diagonal branch, 50% LCX, 90–99% marginal branch of LCX |
50–70% mid LAD, 70–90% first and second diagonal branch, 50–70% mid LCX, 70–90% first marginal branch, 50% second marginal branch, 50% proximal and mid RCA, 70% distal RCA |
True positive |
| 59 |
69 |
m |
|
70–75% mid RCA |
50% proximal RCA, 50–70% mid RCA |
True positive |
Acknowledgement
The administrative support by Irene Schneider is very much appreciated.
References
1
Benjamin
EJ
,
Virani
SS
,
Callaway
CW
,
Chamberlain
AM
,
Chang
AR
,
Cheng
S
, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137(12):e67–492
. [doi:.].https://doi.org/10.1161/CIR.0000000000000558
2
Montalescot
G
,
Sechtem
U
,
Achenbach
S
,
Andreotti
F
,
Arden
C
,
Budaj
A
, et al.
2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003
. [doi:.].https://doi.org/10.1093/eurheartj/eht296
3
Diamond
GA
,
Forrester
JS
. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300(24):1350–8
. [doi:.].https://doi.org/10.1056/NEJM197906143002402
4
Genders
TS
,
Steyerberg
EW
,
Alkadhi
H
,
Leschka
S
,
Desbiolles
L
,
Nieman
K
, et al.
CAD Consortium. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. Eur Heart J. 2011;32(11):1316–30
. [doi:.].https://doi.org/10.1093/eurheartj/ehr014
5
Mordi
IR
,
Badar
AA
,
Irving
RJ
,
Weir-McCall
JR
,
Houston
JG
,
Lang
CC
. Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease. Vasc Health Risk Manag. 2017;13:427–37
. [doi:.].https://doi.org/10.2147/VHRM.S106838
6
Dweck
MR
,
Williams
MC
,
Moss
AJ
,
Newby
DE
,
Fayad
ZA
. Computed Tomography and Cardiac Magnetic Resonance in Ischemic Heart Disease. J Am Coll Cardiol. 2016;68(20):2201–16
. [doi:.].https://doi.org/10.1016/j.jacc.2016.08.047
7
Salavati
A
,
Radmanesh
F
,
Heidari
K
,
Dwamena
BA
,
Kelly
AM
,
Cronin
P
. Dual-source computed tomography angiography for diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. J Cardiovasc Comput Tomogr. 2012;6(2):78–90
. [doi:.].https://doi.org/10.1016/j.jcct.2011.10.018
8
Bittencourt
MS
,
Hulten
E
,
Polonsky
TS
,
Hoffman
U
,
Nasir
K
,
Abbara
S
, et al.
European Society of Cardiology-Recommended Coronary Artery Disease Consortium Pretest Probability Scores More Accurately Predict Obstructive Coronary Disease and Cardiovascular Events Than the Diamond and Forrester Score: The Partners Registry. Circulation. 2016;134(3):201–11
. [doi:.].https://doi.org/10.1161/CIRCULATIONAHA.116.023396
9
Agatston
AS
,
Janowitz
WR
,
Hildner
FJ
,
Zusmer
NR
,
Viamonte
M, Jr
,
Detrano
R
. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32
. [doi:.].https://doi.org/10.1016/0735-1097(90)90282-T
10
Pryor
DB
,
Harrell
FE, Jr
,
Lee
KL
,
Califf
RM
,
Rosati
RA
. Estimating the likelihood of significant coronary artery disease. Am J Med. 1983;75(5):771–80
. [doi:.].https://doi.org/10.1016/0002-9343(83)90406-0
11
Wasfy
MM
,
Brady
TJ
,
Abbara
S
,
Nasir
K
,
Ghoshhajra
BB
,
Truong
QA
, et al.
Comparison of the Diamond-Forrester method and Duke Clinical Score to predict obstructive coronary artery disease by computed tomographic angiography. Am J Cardiol. 2012;109(7):998–1004
. [doi:.].https://doi.org/10.1016/j.amjcard.2011.11.028
12
Kumamaru
KK
,
Arai
T
,
Morita
H
,
Sekine
T
,
Takamura
K
,
Takase
S
, et al.
Overestimation of pretest probability of coronary artery disease by Duke clinical score in patients undergoing coronary CT angiography in a Japanese population. J Cardiovasc Comput Tomogr. 2014;8(3):198–204
. [doi:.].https://doi.org/10.1016/j.jcct.2014.02.002
13
Nielsen
LH
,
Bøtker
HE
,
Sørensen
HT
,
Schmidt
M
,
Pedersen
L
,
Sand
NP
, et al.
Prognostic assessment of stable coronary artery disease as determined by coronary computed tomography angiography: a Danish multicentre cohort study. Eur Heart J. 2017;38(6):413–21.
14
Min
JK
,
Dunning
A
,
Lin
FY
,
Achenbach
S
,
Al-Mallah
M
,
Budoff
MJ
, et al.
CONFIRM Investigators. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58(8):849–60
. [doi:.].https://doi.org/10.1016/j.jacc.2011.02.074
15
Pontone
G
,
Andreini
D
,
Bartorelli
AL
,
Bertella
E
,
Cortinovis
S
,
Mushtaq
S
, et al.
A long-term prognostic value of CT angiography and exercise ECG in patients with suspected CAD. JACC Cardiovasc Imaging. 2013;6(6):641–50
. [doi:.].https://doi.org/10.1016/j.jcmg.2013.01.015
16
Van Mieghem
CAG
. CT as gatekeeper of invasive coronary angiography in patients with suspected CAD. Cardiovasc Diagn Ther. 2017;7(2):189–95
. [doi:.].https://doi.org/10.21037/cdt.2017.04.03
17
Mark
DB
,
Shaw
L
,
Harrell
FE, Jr
,
Hlatky
MA
,
Lee
KL
,
Bengtson
JR
, et al.
Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325(12):849–53
. [doi:.].https://doi.org/10.1056/NEJM199109193251204
18
http://www.ptca.ch/public/reports/reports_english.html
19
Patel
MR
,
Peterson
ED
,
Dai
D
,
Brennan
JM
,
Redberg
RF
,
Anderson
HV
, et al.
Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362(10):886–95
. [doi:.].https://doi.org/10.1056/NEJMoa0907272
20
Stocker
TJ
,
Deseive
S
,
Leipsic
J
,
Hadamitzky
M
,
Chen
MY
,
Rubinshtein
R
, et al.
PROTECTION VI investigators. Reduction in radiation exposure in cardiovascular computed tomography imaging: results from the PROspective multicenter registry on radiaTion dose Estimates of cardiac CT angIOgraphy iN daily practice in 2017 (PROTECTION VI). Eur Heart J. 2018;39(41):3715–23
. [doi:.].https://doi.org/10.1093/eurheartj/ehy546


