Chronic thromboembolic pulmonary hypertension
DOI: https://doi.org/10.4414/smw.2018.14702
Isabelle
Opitz, Silvia
Ulrich
CTEPH Programme University Hospital Zurich, Switzerland
Summary
Chronic thromboembolic pulmonary hypertension (CTEPH) is a potentially fatal disease, which may occur as a rare complication after acute pulmonary embolism, although the exact epidemiology of CTEPH is unknown. The mechanisms involved in nonresolution of thrombotic material and scarring of large and/or small pulmonary arteries are unknown; some risk factors have been identified. To date, CTEPH is still underdiagnosed and undertreated. The cardinal symptom of CTEPH is dyspnoea on exertion, but diagnosis is challenging owing to nonspecific symptoms. Right heart catheterisation is mandatory for the diagnosis of pulmonary hypertension, followed by several imaging methods including besides ventilation/perfusion scan, computed tomography pulmonary angiography and conventional angiography. Operability assessment by a multidisciplinary team is crucial for the management in all CTEPH patients, as pulmonary endarterectomy (PEA) remains the only curative treatment of choice. PEA leads to substantial improvement of haemodynamics, symptoms, and life expectancy enabling many patients to lead unrestricted lives under sole anticoagulation therapy. For inoperable patients or those with disease not amenable to surgery, medical therapy or balloon angioplasty are emerging treatment options. Owing to the complexity of CTEPH, the diagnosis and treatment of CTEPH patients is reserved exclusively to experienced CTEPH centres.
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH), classified in group 4 of pulmonary hypertension according to ESC/ERS 2015 guidelines [1], is defined as symptomatic pulmonary hypertension with persistent pulmonary perfusion defects despite adequate anticoagulation for 3 to 6 months [2]. The exact epidemiology of CTEPH is unknown; it is most probably largely underdiagnosed and therefore undertreated. One of the aims of the present article is to increase awareness of this rare disease and demonstrate how much patients can benefit from modern multimodal treatment concepts in expert centres.
Epidemiology and aetiology
An analysis of CTEPH epidemiology showed that in the USA, Europe and Japan, the crude annual incidence is 3 to 5 cases/100,000 population [2]. The projection model indicated that the incidence of CTEPH will continue to increase over the next decade [2].
Only 50 to 75% of patients new cases diagnosed each year have a history of acute pulmonary embolism, but in over a quarter of the cases there is no documented history of acute pulmonary embolism (data from an international CTEPH registry covering Canada and countries in Europe) [3]. Therefore, other risk factors seem to be involved even in the absence of acute pulmonary embolism.
Identified risk factors for CTEPH are autoimmune and haematological disorders [4]. Ventriculo-atrial shunts, infected pacemaker leads, splenectomy, prior venous thromboembolism (particularly recurrent venous thromboembolism), non-O blood group, presence of lupus anticoagulant / antiphospholipid antibodies, thyroid replacement therapy and a history of malignancy were linked to CTEPH [5]. The increased risk for patients with malignancies results from the same mechanisms as for the increased risk of thromboembolic events: activation of the fibrinolytic and coagulation systems, acute-phase reactions, inflammation and cytokine production [6, 7].
CTEPH incidence after PE
The incidence of CTEPH following an episode of acute pulmonary embolism is highly variable. In eight studies from Europe and the USA [2, 8–15] (fig. 1), it ranged from 0.1 to 9.1%, with a calculated weighted average of 4%. Some studies even calculate an incidence of CTEPH after acute pulmonary embolism of up to 10% [2], whereas the incidence reported in Switzerland was much lower (0.79%) [16]. A systematic literature review revealed an average incidence of around 4% in Europe and the USA, but up to 14% in Japan [17]. This variance may partly be explained by the setting of the studies (tertiary versus primary care) and the detection rate of true CTEPH; however, other influences such as environmental or genetic factors cannot be excluded. Some factors have been identified to be predictors for CTEPH after acute pulmonary embolism, such as large pulmonary emboli at the initial event, which carry a higher risk for developing CTEPH [7, 18, 19]. Young age, multiple episodes of pulmonary embolism and elevated pulmonary artery pressure at the time of pulmonary embolism, especially a right ventricular systolic pressure ≥50mmHg [20], are also risk factors.
Even though there is no clear recommendation, echocardiographic screening after acute pulmonary embolism could be considered up to 2 years after the acute event for patients with high- and intermediate-risk acute pulmonary embolism [21]. Surveillance programmes after acute pulmonary embolism are to be considered for patients with mainly central vessel embolisation, evidence of right ventricle dysfunction, and thrombophilia [22]. They are especially vital for patients who remain symptomatic 3 months after the event.
Pathogenesis
The pathogenesis of pulmonary hypertension in CTEPH is related to multiple mechanisms. Pulmonary vascular alterations may be observed from central pulmonary arteries to segmental, subsegmental and distal vessels to a varying degree in individual patients. Thus, CTEPH may predominantly involve persistent organised thrombi and scars in proximal pulmonary arteries (main, lobar, and segmental) or small-vessel disease or both (fig. 2) [23]. Nonresolution with subsequent organisation and fibrosis of residual thrombotic material impairs blood flow and leads to CTEPH [18, 19]. The following factors have been related to failure of thrombus resolution [7]: inflammation and infection; biological and genetic factors; fibrinogen and fibrinolytic abnormalities; platelet function; impaired angiogenesis; small vessel disease.
Inflammation and infection
An inflammatory component in CTEPH pathogenesis is suspected because of elevated levels of C-reactive protein (CRP) [24], interleukin (IL)-6, IL-8, interferon-γ-induced protein (IP)-10, monokine induced by interferon-γ and macrophage inflammatory protein-1α, which were significantly higher compared with age- and sex-matched healthy controls [25]. Besides this, increased blood-levels of IP-10 (leading to fibroblast migration and activation) and tumour necrosis factor [26] were reported to be correlated with CTEPH. In addition, chronic infection (e.g., Staphylococcus aureus) has been detected in endarterectomy specimens of patients with CTEPH [27].
Biological and genetic risk factors
It has been hypothesised that patients with CTEPH have hypercoagulability. However, protein C, protein S and antithrombin deficiencies, and mutations of factor V and II, the classical hereditary thrombotic risk factors, are not more frequent in patients with CTEPH than in healthy controls [28]. Increased coagulation factors identified in CTEPH were antiphospholipid antibodies and lupus anticoagulant (higher frequency compared with idiopathic pulmonary arterial hypertension), and increased levels of clotting factor VIII [29] and of von Willebrand factor. Molecular profiling revealed >1600 genes that were expressed in pulmonary artery endothelial cells from CTEPH patients differently from normal controls [30].
Fibrinogen and fibrinolytic abnormalities
Abnormal fibrinogen molecules, such as fibrinogen Aα-Thr312Ala, have been found in the blood of CTEPH patients [31–33]. Common to all aberrant fibrin characteristics found in CTEPH patients is their ability to resist physiological thrombolysis [18, 34].
Platelet function
A prothrombotic state with higher platelet turnover has been observed in CTEPH patients, which is supported by thyroid hormone replacement therapy and splenectomy as known risk factors for CTEPH [3, 5].
Impaired angiogenesis
Impaired angiogenesis and recanalisation of the thrombus could be involved in the pathophysiology of CTEPH, as has been indicated by studies in animal models of impaired thrombus resolution [35, 36].
Small-vessel disease in CTEPH
The initial trigger for developing CTEPH is most probably the scarring process of thrombi leading to stenosis, webs and bands completely or nearly completely occluding the lumen of the large vessels [37, 38]. But some patients develop, in addition to mechanical obstruction of proximal arteries, a more or less severe pulmonary microvasculopathy (small vessel disease), first described by Moser and Bloor [39]. Changes similar to those in pulmonary arterial hypertension are observed in the small vessel compartment of CTEPH patients [38]. Abnormal endothelial function, excessive proliferation of smooth muscle cells, migration of fibroblasts and inhibition of apoptosis in vascular smooth muscle cells lead to endothelial dysfunction, vascular remodelling, and micro- thrombosis [39]. As in pulmonary arterial hypertension, a persistent vasoconstrictive state, characterised by high levels of plasma endothelin-1 and overexpression of type B endothelin receptors, may be present in CTEPH patients [21, 40]. The wall of distal muscular pulmonary arteries (0.1−0.5 mm in diameter) is affected by this vascular remodelling, which is explained by redistribution of the pulmonary flow in non-obstructed pulmonary arteries leading to high pressures and shear stress, which results into endothelial dysfunction, increasing pulmonary vascular resistance (PVR) and therefore symptomatic CTEPH [7].
Clinical presentation
The cardinal symptom of CTEPH is dyspnoea on exertion and patients usually present with progressive exercise intolerance, fatigue or depression [41, 42]; in later stages exertional syncope and progressive oedema are prevalent [43]. These symptoms are nonspecific and potentially misleading, especially in the presence of comorbidities such as chronic obstructive lung disease, deconditioning and obesity [43, 44]. Thus, CTEPH diagnosis is often delayed [3, 41, 42] and patients may see several doctors and are confronted with incorrect diagnoses before the pulmonary hypertension is correctly diagnosed and classified [45]. For example, a survey conducted in the UK (n = 488) showed that 44% of patients saw four or more doctors before a correct pulmonary hypertension diagnosis was made and the delay from first consultation to diagnosis was 2 or more years for more than 30% of the patients [45, 46]. This delay reflects a significant challenge in every day practice, the need for proper education of physicians and early referral to expert centres.
On clinical examination, accentuation of the pulmonic component of the second heart sound may be present, as a result of a flow murmur caused by turbulence in blood flow through incompletely obstructed pulmonary arteries. In the later course of the disease exertional syncope and signs of right ventricular failure develop.
CTEPH diagnosis
According to the 2016 Consensus Conference, transthoracic echocardiography is the first tool of assessment after presentation of a patient with the clinical signs described above (fig. 3). However, we also want to mention first-hand tools such as chest radiography and ECG, and the findings typical for CTEPH:
ECG and pulmonary function testing
P-pulmonale, right bundle branch block, abnormalities of the T wave in the chest leads and right-axis deviation are indications of right heart strain [1, 47, 48]. For patients with dyspnoea and normal flow and volumes on pulmonary function testing, but a reduced transfer factor for carbon monoxide, pulmonary vascular disease should be considered [49–51]. Decreased carbon dioxide levels in blood gases or end-tidal measures are a recognised feature of pulmonary arterial hypertension [52]. Those symptoms of hyperventilation and ineffective ventilation are even stronger in CTEPH [53]. Central sleep apnoea and Cheyne-Stokes respiration can be a result of CTEPH and should always be cause for closer examination [43, 54].
Chest x-ray
Besides distinct right heart enlargement, pronounced pulmonary artery dilatation is visible on a postero-anterior chest x-ray (fig. 4). Furthermore, the so-called Hampton (sign of previous infarction) and Westermark (areas of hypoperfusion) signs can be observed.

Figure 4 Chest x-ray. Arrow: prominent pulmonary artery. (Courtesy of Prof. T. Frauenfelder.)
Echocardiography
Echocardiography is a first screening tool, where the transtricuspid pressure gradients can be calculated from peak velocity of the tricuspid valve regurgitation. Further indirect signs of pulmonary hypertension are right atrial and right ventricular dilatation and potential compression-induced D-shaping of the left ventricle, reduced right ventricular contractility, and Doppler flow abnormalities in the right ventricular outflow tract [1, 43].
Cardiopulmonary exercise testing
For functional assessment and classification of patients with pulmonary vascular disease, cardiopulmonary exercise testing (CPET) seems to be a promising additional clinical tool [43]. Hyperventilation in pulmonary arterial hypertension and CTEPH typically appears as ineffective ventilation [53–56]. Patients with ineffective ventilation caused by pulmonary vascular obstruction show elevated alveolar-capillary gradients of oxygen and carbon dioxide [53–56] (fig. 5 and fig. 6).

Figure 5 Cardiopulmonary exercise testing of a patient with chronic thromboembolic pulmonary hypertension showing fields 4, 6 and 9 of the Wasserman panel. (a) Elevated slope of minute ventilation (V′E) / carbon dioxide output (V′CO2) ratio showing hyperventilation (field 4); (b) elevated ventilator equivalents for oxygen (EQO2) and carbon dioxide (EQCO2) showing ineffective ventilation (field 6); (c) low and decreasing end-tidal carbon dioxide tension (PETCO2), elevated alveolar-arterial oxygen tension gradient (PA-aO2) and elevated arterial end-tidal carbon dioxide gradient (Pa-ETCO2) (field 9). Reproduced with permission of the European Respiratory Society. Eur Respir Rev. 2017;26(143):160108 [43]. © ERS 2017. PETO2 = end-tidal oxygen tension; PaO2 = arterial oxygen tension; PaCO2 = arterial carbon dioxide tension; PO2 = oxygen tension; PCO2 = carbon dioxide tension.

Figure 6 Heterogeneous pulmonary perfusion is the hallmark of CTEPH, with progressive CTEPH, nonoccluded arteries tend to narrow (remodelling), this reduced extend of blood flow heterogeneity. Reproduced with permission of the European Respiratory society. Eur Respir J. 2012;39(1):119-124 [53]. © ERS 2012.
Imaging
Ventilation/perfusion scintigraphy
The initial screening tool to distinguish CTEPH from other causes of pulmonary hypertension is a ventilation/perfusion (V/Q) scan, which has the advantages of a limited radiation dose, no need for intravenous contrast agents and relatively low costs. With 96% sensitivity, a negative V/Q is the imaging technique of choice for exclusion of CTEPH. International guidelines still place V/Q as the first-line imaging methodology in CTEPH (fig. 7) [57] to rule out chronic pulmonary embolism as a cause of pulmonary hypertension [58].
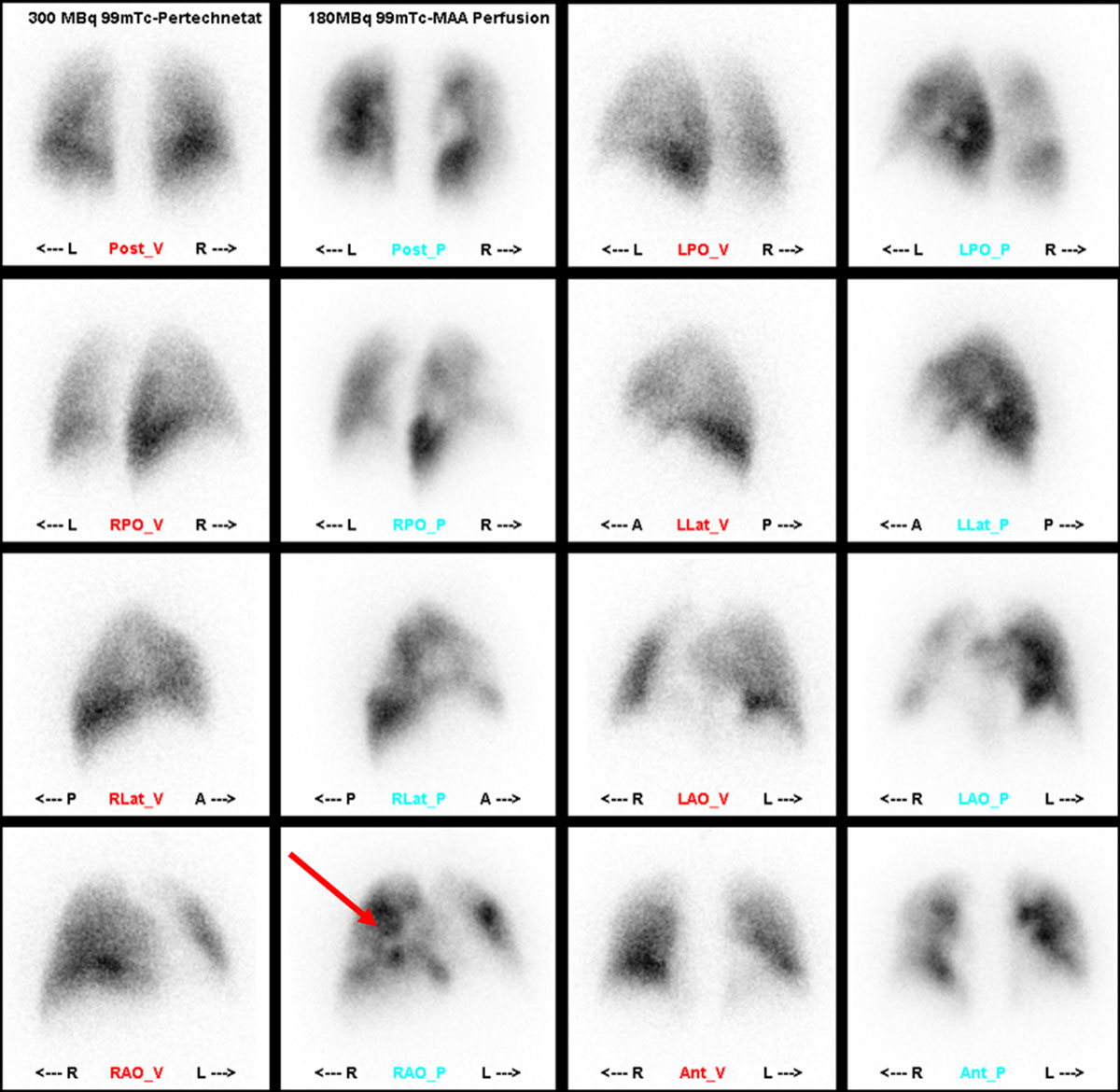
Figure 7 Ventilation/perfusion (V/Q) scan. In the perfusion images patchy perfusion defects (arrow) unmatched in the ventilation images. (Courtesy of Prof. T. Frauenfelder.)
Computed tomography pulmonary angiography
Whereas computed tomography pulmonary angiography (CTPA) is widely accepted as imaging technique of choice for acute pulmonary embolism, its role in CTEPH is less well defined. Perfect imaging delivery relies on technical conditions such as short breath hold (3–5 s) acquisition, thin collimation and thin-slice (≤1 mm) reconstruction and three-dimensional analysis. Owing to its high sensitivity and specificity, CTPA has a good potential in detecting thromboembolic changes at the lobar level (97–100% and 95–100%, respectively) and the segmental level (86–100% and 93–99%, respectively) [43, 59–61]. One of the main advantages of computed tomography (CT) in comparison to digital subtraction angiography is the visualisation of many more details, such as mediastinal area, lung parenchyma, collateralisation, and other features that help to exclude other differential diagnoses.
Typical local vascular characteristics on CT (fig. 8) are:

Figure 8 Local vascular characteristics on computed tomography. (a) Partial filling defects, mostly eccentric; (b) organised embolism; (c) intravascular bands and webs; (d) poststenotic dilatation. (Courtesy of Prof. T. Frauenfelder.)
- Complete obstruction
- Partial filling defects, mostly eccentric (a)
- Organised emboli (b)
- Intravascular bands and webs (c)
- Poststenotic dilatation (d)
- Calcifications
Typical systemic vascular characteristics on CT (fig. 9) are:

Figure 9 Systemic vascular characteristics on computed tomography. (a) Enlargement of central pulmonary vessels; (b) enlargement and hypertrophy of right ventricle; (c) enlargement of bronchial arteries. (Courtesy of Prof. T. Frauenfelder.)
- Enlargement of central pulmonary vessels (a)
- Enlargement and hypertrophy of right ventricle (b)
- Pericardial effusion
- Enlargement of bronchial arteries (c)
- Enlargement of neighbouring vessels
- Intercostal vessels, mammary artery
Furthermore, typical parenchymal characteristics on CT (fig. 10) are:

Figure 10 Parenchymal characteristics on computed tomography. (a) Mosaic pattern; (b) pulmonary infarctions; (c) bronchial dilatation / ipsilateral bronchiectasis. (Courtesy of Prof. T. Frauenfelder.)
- Mosaic pattern (a)
- Pulmonary infarctions (b)
- Scars irregular, linear, wedge like form, near pleura
- Bronchial dilatation / ipsilateral bronchiectasis (c)
- Localisation correlates with position of affected vessels
Dual-energy computed tomography
The introduction of dual-energy CT (DECT) (fig. 11) permits functional data on lung perfusion (iodine distribution maps / lung perfusion blood volume images) in addition to anatomical images [58]. With the processed data, the following images can be generated: conventional grey-scale images, colour-coded overlays that highlight the iodine distribution, and virtual nonenhanced images from post-contrast images by use of iodine-subtraction techniques [43]. These are reader-independent and rapid tools to quantitatively assess regional iodine density, reflecting pulmonary perfusion, and correlate with a V/Q scan or single photon emission computed tomography (SPECT) V/Q scan [62, 63]. Qualitative assessment of less- or under-perfused lung areas is comparable to scintigraphy [43, 64, 65], and might replace V/Q scans in the future.

Figure 11 Dual-energy computed tomography (CT). (a) Contrast-enhanced CT axial reconstruction demonstrating a dilated right atrium, and narrowing of the pulmonary arteries of the right side by thromboembolic material; (b) contrast-enhanced CT coronary reconstruction with mosaic perfusion pattern; (c) dual-energy CT scan showing iodine mapping of unmatched perfusion defects. (Courtesy of Prof. T. Frauenfelder.)
Positron emission tomography
Distinguishing CTEPH from other conditions mimicking the disease such as pulmonary artery sarcoma is the primary role of positron emission tomography (PET). Pulmonary artery sarcoma and CTEPH distinctively differ in their specific uptake value (SUVmax) on PET [43]. SUVmax of pulmonary artery sarcoma is generally approximately three-fold or more higher than that of thrombi [43, 66].
Digital subtraction angiography
Digital subtraction angiography (DSA) was for a long time the gold standard for diagnosis and therapy planning in CTEPH (fig. 12). The morbidity and mortality rates of invasive angiography are 3.5–6% and 0.2–0.5%, respectively [58, 67, 68], but obviously strongly depend on the centre’s experience. The use of CTPA is expanding, and it seems to outperform DSA for detection of CTEPH, as shown in a comparative study of 24 patients [43, 59]. The sensitivity of DSA varied from 66% at the main/lobar level to 76% at segmental level, in contrast to 100% for CTA at comparable levels. Specificity of both techniques was excellent (100%), although DSA had a slight advantage over CTA in depicting subsegmental arteries (DSA 97%; CTA 80%) [43, 59]. One particular advantage is the option for simultaneous pulmonary artery haemodynamic assessment during right heart catheterisation. Furthermore, balloon angioplasty is an emerging technique for inoperable CTEPH, and to this end, traditional angiography has experienced a renaissance. Rotational angiography and cone beam CT are emerging techniques for optimised visualisation of intraluminal webs and bands.
Right heart catheterisation
Assessment of pulmonary haemodynamics by right heart catheterisation is mandatory for the diagnosis of CTEPH, with a mean pulmonary artery pressure (mPAP) ≥25 mm Hg [1] (this threshold will most probably be reduced to ≥20 mmHg as this threshold better reflects the upper limit of normal), and to exclude postcapillary pulmonary hypertension with a pulmonary artery wedge pressure ≤15 mm Hg. Assessing pulmonary haemodynamics during exercise as multipoint pressure-flow slopes may be especially important in chronic thromboembolic pulmonary vascular disease with a still normal resting mPAP [69]. Correct assessment of the cardiac output by thermodilution or the direct Fick method is crucial for correctly calculating the pulmonary vascular resistance (PVR), an important factor in the assessment of prognosis and risks for surgery [70].
Cardiac magnetic resonance imaging
Over the last decade, magnetic resonance imaging (MRI) has undergone significant technical improvement, with faster sequences, shorter acquisition times, larger coverage and ability to perform functional studies, lung perfusion imaging, and high-resolution MR pulmonary angiography (MRPA) [58]. However, the use of MRI is still highly dependent on local practice and is not yet fully integrated in the CTEPH diagnostic algorithm. MRI is suitable for research and for the diagnostic work-up and follow-up of patients with CTEPH, where it enables both an assessment of the pulmonary arterial obstructive disease and detailed evaluation of right ventricular function or alternative causes for pulmonary hypertension [58]. There are only a few comparative studies assessing the accuracy of MRI in CTEPH. Ley et al. [59] compared CTA, ce-MRA, and DSA, showing the sensitivity and specificity of MR angiography for diagnosing disease at the main/lobar level to be 83.1% and 98.6%, respectively, and at the segmental level 87.7% and 98.1%, respectively. Subsegmental arteries were demonstrated in only 75% of cases, compared with 87% by DSA [43]. Another role of MRI could be as a follow-up tool after surgery as it can detect non-invasively changes in parameters reflecting cardiac remodelling and pulmonary clearance [71].
Prognosis
Pathological haemodynamic parameters at the time of CTEPH diagnosis negatively impact survival. A mean pulmonary arterial pressure (mPAP) ≥50 mm Hg is associated with a 2-year mortality of >80% if untreated and a mPAP >30 mm Hg with a 3-year mortality of 90% [72].
Once CTEPH is diagnosed and confirmed at an expert centre, treatment assessment is mandatory. European Society of Cardiology (ESC) / European Respiratory Society (ERS) guidelines have defined the clear recommendations for pulmonary hypertension referral centres (fig. 13) [1].
Treatment guidelines
Surgical therapy
The ERS/ESC guidelines [1] recommend pulmonary endarterectomy (PEA) as the treatment of choice for patients with surgically accessible CTEPH. PEA improves pulmonary hypertension by ameliorating lung ventilation-perfusion mismatch, significantly reducing right ventricular dysfunction and changing pulmonary haemodynamics, restricting retrograde expansion of thromboembolic material, and preventing arteriopathic changes in the remaining patent small pulmonary vessels [73] (fig. 14).
In the past, more than a third of patients diagnosed with CTEPH did not undergo PEA [3], which demonstrates that treatment in an expert centre and patient selection is crucial.
Patient selection for pulmonary endarterectomy
Operability should be evaluated by an interdisciplinary expert team including pulmonologists, radiologists and surgeons [74]. The severity of the patient’s symptoms and the severity of pulmonary hypertension and right heart dysfunction, technical challenges, patients’ comorbidities, as well as the level of expectation for long-term benefits, are all important factors in the decision-making process [73]. The most important selection criteria are sufficient thromboembolic material surgically accessible and a proportional PVR as an indicator of missed extensive distal vasculopathy. However, symptomatic patients should be offered surgery regardless of the severity of pulmonary hypertension or right ventricular dysfunction [73]. Thus, neither the PVR nor the degree of right ventricular dysfunction should exclude a patient from consideration for surgery [73]. Surgically accessible disease is not clearly defined. Disease located proximally in the main, lobar or segmental arteries is usually amenable for PEA, but experienced CTEPH surgical teams can operate successfully on more distal disease with good haemodynamic and functional results [75]. PEA should therefore be considered in all patients who have evidence of thromboembolic disease, including those with more distal disease, as proposed by experienced surgeons [70, 75–78].
Recent years have brought growing confidence to operate not only on patients with more severe pulmonary hypertension and more distal obstruction, but also on patients with chronic thromboembolic pulmonary vascular disease and normal resting haemodynamics, which potentially reveal an inadequate flow-related-pressure increase during exercise [75]. It has been observed that substantial improvements in functional and exercise performance can be achieved and PEA should be considered for these patients [1, 75] in order to improve the significant V/Q mismatch, and eventually prevent chronic changes and development of secondary vasculopathy.
PEA: the surgical technique
PEA requires specialist training and sophisticated intensive care postoperatively [75]. It is performed via a median sternotomy for a bilateral approach. Cardiopulmonary bypass is installed and the body is cooled to 18‒20°C to allow deep hypothermic circulatory arrest (DHCA) for provision of a clear operating field. DHCA is limited to 20-minute intervals. Usually one period for each side is enough to complete dissection [75]. In the PEACOG (PEA and COGnition) trial [79], the impact of DHCA on cognitive function at 3 months and 1 year, compared with cerebral perfusion during PEA, was investigated. With respect to cognitive function and postoperative improvement the two techniques performed equally, indicating that DHCA at 20°C for PEA is safe and well tolerated with careful anaesthetic and cardiopulmonary bypass management, and provides reproducible, excellent results for the lungs and the brain [75]. Longer circulatory arrest times should be avoided, as they may be associated with neurological complications, as suggested by registry results [80].
In order to be able to dissect to the level of segmental and subsegmental branches, the endarterectomy dissection plane should be circumferential. PVR is not reduced by a simple thrombectomy or embolectomy without a true endarterectomy [81]. Adequate removal of thromboembolic material and prevention of perforation of the pulmonary artery can only be achieved by identification of the correct surgical plane [75, 82, 83].
After completion of the endarterectomy, the patient is reperfused and the warming process is started. Depending on the patient’s body mass, rewarming generally takes approximately 90 to 120 minutes. The systemic rewarming period can be conveniently used for other cardiac procedures required, such as coronary artery or mitral or aortic valve surgery (most common are closure of the foramen ovale and coronary bypass surgery) [73]. The outcome is comparable to combined interventions in terms of haemodynamic effects and early mortality. A difference has been reported in length of intubation, number of complications and longer hospitalisation [84, 85]. Tricuspid valve repair is not necessary unless there is an anatomical abnormality, even though tricuspid valve regurgitation is variable in these patients and often moderate to severe, as right ventricular remodelling occurs naturally within a few days, with the return of tricuspid competence [73, 86].
Morbidity and mortality
There are two major well-known complications in the postoperative course of PEA, even with favourable outcomes in most patients: residual pulmonary hypertension and reperfusion pulmonary oedema [74]. Persistently elevated pulmonary artery pressure often occurs in combination with reperfusion lung injury. Both situations can be improved by means of extracorporeal membrane oxygenation (ECMO): in the case of haemodynamic instability, venoarterial ECMO can be used. Cardiac output and gas exchange are improved as a result of right ventricle off-loading and reduction of pulmonary artery pressure. In the case of reperfusion injury alone, conservative therapy or venovenous ECMO may be an option.
Acute mortality depends on multiple factors [75], including chronicity of disease, CTEPH team experience, preoperative PVR, exercise capacity, the patient’s New York heart Association (NYHA) functional class, comorbidities and the distribution of the disease [41, 70, 77, 80]. Higher preoperative PVR may increase mortality [41, 77, 87] (fig. 15). In-hospital mortality is three times higher in patients with PVR >1200 dyn·s·cm–5 at diagnosis compared with PVR 400–800 dyn·s·cm–5, as demonstrated by the international CTEPH registry [41]. Early postoperative morbidity and mortality are most often caused by residual pulmonary hypertension (>500 dyn.s.cm-5) after surgery [73]. At the University of California San Diego Medical Center, mortality rates were 10.3 and 0.9% in patients with and without residual pulmonary hypertension, respectively [77]. The higher the PVR, the higher the gain from PEA surgery, as these patients have the greatest relative improvement and the most potential prognostic benefit. Patients with high PVR >1200 dyn·s·cm–5 with poor right ventricular function and more distal disease on imaging have the highest risk [75].
Haemodynamic, functional, and survival outcomes [75]
Following successful PEA, significant improvement of functional dyspnoea, NYHA class and 6-minute walk test (6MWT), increased oxygen uptake, and improvement of the minute ventilation (V′E) / carbon dioxide output (V′CO2) ratio (VE:VCO2), as well as a decrease in oxygen supply necessity have been documented [88, 89]. Average improvements from PVR 700–800 dyn·s·cm–5 to 250 dyn·s·cm–5 have been experienced [41, 77] following surgery, a fall of ∼65%, mPAP (from 46 to 26 mm Hg) [77] and increase in 6-minute walking distance from 362 to 459 m [41], as well as an improved NYHA functional class and quality of life after PEA [41, 75, 90]. In the medium to long term, survival rates of >90% at 1 year, >80% at 5 years, and >70% at 6–10 years have been reported [91–93]. Recent data from the international CTEPH registry report estimated survival rates of 93% at 1 year, 91% at 2 years, and 89% at 3 years after PEA; a significant improvement to the survival rate of patients who did not have PEA [80].
Persistent pulmonary hypertension after PEA [75]
Even after apparently successful PEA, up to 30% of patients may have persistent (or residual) pulmonary hypertension [94–96]. To date there is no agreed definition because right heart catheterisation is not routinely performed in all patients after PEA [76]. The best thresholds correlating with higher risk of death independent of cause were a mPAP ≥36 mm Hg and a PVR ≥416 dyn·s·cm–5 (as time-varying measures), whereas a mPAP ≥38 mm Hg and a PVR ≥425 dyn·s·cm–5 identified those patients at higher risk of death caused by CTEPH [75].
However, postoperative pulmonary hypertension does not seem to affect medium-term survival [75]. A prospective study from Papworth Hospital (Cambridge, UK) revealed 5-year survival rates that did not differ significantly in patients discharged with a postoperative mPAP <30 mm Hg compared with those with mPAP ≥30 mmHg (90.3 and 89.9%, respectively) [97].
Medical treatment can benefit patients with residual pulmonary hypertension after removal of the mechanical obstruction, as suggested by the recent clinical trials with riociguat in this setting [98–100].
Identification of expert centres for PEA [75]
The first step in the ESC/ERS treatment algorithm is advice to confirm the diagnosis at a CTEPH expert centre [1] (fig. 14). There is a potential concern that centres with limited expertise may offer unlicensed drug treatment or balloon pulmonary angioplasty (BPA) to patients who are eligible for, and would benefit more from, PEA [75]. A CTEPH expert centre should thus have surgeons, experienced BPA interventionists and pulmonologists with broad experience in pulmonary hypertension available [75], in particular because the combination of all techniques is developing as a future direction (table 1). Several approaches have already been successfully used, such as intraoperative planned BPA during PEA [101], acute rescue BPA after failure of PEA [102], or BPA for residual or recurrent pulmonary hypertension months or years after PEA [103].
Following the international recommendation on expert centres, Switzerland as a country with 8.4 million inhabitants [104] should concentrate treatment of CTEPH patients in one or two centres in order to comply with international quality standards. Since 2015, a CTEPH programme fulfilling all international requirements can be offered in Switzerland with the concentration of cases mandatory in order to perform on a high quality level. The University Hospital Zurich fulfils all the conditions required and we have assembled a multidisciplinary team that assesses every CTEPH patient. Since 2015 we have assessed 62 patients, and we operated on 34 in the first 2 years, a resectability rate comparable to international registries (55%), as was the perioperative mortality of 3% [3]. With a short median follow-up period of 13 months (range 0–30 months), we have invasive haemodynamic data on 11 patients with a clear and significant improvement of the mPAP (fig. 16), but also in NYHA classification and 6MWT (unpublished data).
Under the umbrella of the Swiss Society of Pulmonary Hypertension, a national CTEPH board was initiated at the beginning of 2018 as a close collaboration between university hospitals broadcasting the case discussion to every centre interested and offering the opportunity to referring physicians to present their cases live and join the discussion (CTEPH@SGPH.ch; CTEPH@usz.ch).
Balloon pulmonary angioplasty
Since 2001, reports have emerged, mainly from Japan, of innovative BPA for mainly females not eligible for surgery owing to comorbidities [105–108]. Multiple angioplasty procedures in single patients were necessary to achieve a significant reduction of the PVR and the studies were not randomised-controlled. However, haemodynamic improvements reported were impressive and so were improvements in the 6-minute walk distance and NYHA/WHO functional class. In general, lobar and proximal segmental disease is better suited for surgical resection, whereas distal segmental and subsegmental disease is more appropriate for BPA [109] (fig. 17).

Figure 17 Balloon pulmonary angioplasty angiography demonstrating a well-perfused segment of a left sided pulmonary artery segment after dilatation. (Courtesy of Prof. T. Pfammatter.)
Many questions remain unresolved about this new intervention, such as patient selection, risk of bleeding, vessel rupture, restenosis rate and other complications [109]. Data on long-term results are not yet available [109].
Adverse events during balloon pulmonary angioplasty [109]
In recent series, BPA periprocedural mortality ranged from 0 to 10% [106–108, 110–114]. The two most common complications of BPA were reperfusion pulmonary oedema and pulmonary vascular injury, with rare episodes of vessel perforation or rupture [109]. Despite the advances in and improvement of the procedure, reperfusion pulmonary oedema remains a frequent complication of BPA with an incidence as high as 53 to 60% in some studies [107, 108]. Pulmonary artery perforation or rupture is a serious complication of BPA and is reported in 0 to 7% of procedures [109]. Thus, this treatment modality should be reserved for expert centres offering rescue and salvage strategies [109]. As pointed out in the previous paragraphs, the concentration of BPA also to expert centres is mandatory and again should be limited to no more than one or two centres in Switzerland.
Medical therapy
Patients with distal CTEPH that is not surgically accessible or residual pulmonary hypertension after PEA benefit from medical therapy [37, 74]. CTEPH and pulmonary arterial hypertension share many pathogenetic features [39, 98, 100, 115]. Thus, medical therapy as used for pulmonary arterial hypertension (PAH-targeted therapy) can be offered as a therapeutic option for patients with postoperative persistent pulmonary hypertension and patients with inoperable CTEPH. This concept is supported by the following clinical trials: BENEFiT was the first large randomised controlled trial showing that bosentan given for 6 months was associated with an unchanged 6-minute walk distance but a PVR reduced by 24% compared with placebo in patients with inoperable CTEPH [116].
More recently, clinically relevant primary endpoints could be reached in the large CHEST-1 trial with the soluble guanylate cyclase stimulator riociguat, and the CHEST-2 extension trial [98]. After a cautious up-titration schema in order to avoid systemic vasodilatory effects with hypotension, patients under riociguat had an improved 6-minute walk distance by on average 46 m and a reduced PVR by 31%. In the MERIT trial, the PVR was significantly reduced by 16%, with improvements in functional class and walk distance; the improvements were also found in patients pretreated with phosphodiesterase-5 (PDE-5) inhibitors, indicating successful combination therapy also in patients with distal CTEPH [115].
Oral anticoagulation
Lifelong oral anticoagulation (OAC) is a prerequisite for every patient with CTEPH regardless of other therapies, and therapeutic decisions should be considered only after at least 3 month of OAC [1, 57]. Traditionally, vitamin K antagonists have been used, although there is no prospective study of any type of OAC in CTEPH and all data are derived from recommendations for OAC after acute pulmonary embolism [117]. In recent years, non-vitamin K dependent oral anticoagulants that inhibit factor Xa or prothrombin (so-called novel oral anticoagulants, NOACs) have increasingly been shown to be as effective as vitamin K antagonists but with a better safety profile, and are thus more and more preferentially used to prevent recurrence after acute pulmonary embolism [57, 118]. Because of the long experience with vitamin K antagonists, many experts still recommend them as first-line treatment; however, as a result of the equal efficacy in the first high-risk phase after acute pulmonary embolism and the more favourable profile concerning bleeding events, it seems reasonable to assume that the favourable risk-benefit profile would persist in the long term and make these NOAC suitable for life-long treatment in CTEPH after careful patient instruction about the need not to forget any dose. To date, we still treat patients with vitamin K antagonists after pulmonary endarterectomy, but if dose finding is difficult and often off the targeted range of international normalised ratio (INR) 2.5–3.5, we treat patients with NOACs.
Conclusion
CTEPH and chronic thromboembolic pulmonary vascular diseases should be diagnosed and treated in expert centres with multimodal management that has to include evaluation for surgery in every single patient. PEA substantially improves functional and exercise capacity and haemodynamics, as well as life expectancy. Patients with distal, surgically inaccessible disease or residual pulmonary hypertension after surgery may substantially improve with medical therapy. BPA may be a promising option in patients with subsegmental disease alone or in combination with surgery and, as all CTEPH treatment modalities, should only be performed at expert centers. For patient assessment, the Swiss Society of Pulmonary Hypertension promoted a national CTEPH board which referring physicians can connect to for presentation and discussion of their cases CTEPH@usz.ch, CTEPH@sgph.ch).
|
Table 1: Characteristics of an expert centre. Reproduced with permission of the European Respiratory Society©. Eur Respir Rev. 2017;26 (143):160111 [75]. |
| Extensive experience with cardiothoracic surgery, including procedures requiring DHCA |
| Excellent pulmonary and cardiac services |
| Emphasis on pulmonary hypertension |
| Expert diagnostic imaging |
| Experienced multidisciplinary team comprising surgeons, radiologists, anaesthetists, intensivists, nurses, perfusionists, respiratory therapists and interventionalists, including specialists experienced in BPA |
| BPA = balloon pulmonary angioplasty; DHCA = deep hypothermic circulatory arrest |
Acknowledgements
First of all, we thank the CTEPH Team of University Hospital Zurich with PD D. Bettex and the Anesthesiology team and PD R. Schüpbach and the Intensive Care team. We thank Prof. T. Frauenfelder and Prof. T. Pfammatter for critical reading and support in selection of imaging figures. We thank Dr. C. Spichiger for the formatting and editing process of the article.
References
1
Galiè
N
,
Humbert
M
,
Vachiery
JL
,
Gibbs
S
,
Lang
I
,
Torbicki
A
, et al.
2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–75. doi:.https://doi.org/10.1183/13993003.01032-2015
2
Gall
H
,
Hoeper
MM
,
Richter
MJ
,
Cacheris
W
,
Hinzmann
B
,
Mayer
E
. An epidemiological analysis of the burden of chronic thromboembolic pulmonary hypertension in the USA, Europe and Japan. Eur Respir Rev. 2017;26(143):160121. doi:.https://doi.org/10.1183/16000617.0121-2016
3
Pepke-Zaba
J
,
Delcroix
M
,
Lang
I
,
Mayer
E
,
Jansa
P
,
Ambroz
D
, et al.
Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124(18):1973–81. doi:.https://doi.org/10.1161/CIRCULATIONAHA.110.015008
4
Blauwet
LA
,
Edwards
WD
,
Tazelaar
HD
,
McGregor
CG
. Surgical pathology of pulmonary thromboendarterectomy: a study of 54 cases from 1990 to 2001. Hum Pathol. 2003;34(12):1290–8. doi:.https://doi.org/10.1016/j.humpath.2003.07.003
5
Bonderman
D
,
Wilkens
H
,
Wakounig
S
,
Schäfers
HJ
,
Jansa
P
,
Lindner
J
, et al.
Risk factors for chronic thromboembolic pulmonary hypertension. Eur Re777spir J. 2009;33(2):325–31. doi:.https://doi.org/10.1183/09031936.00087608
6
Karimi
M
,
Cohan
N
. Cancer-associated thrombosis. Open Cardiovasc Med J. 2010;4:78–82.
7
Simonneau
G
,
Torbicki
A
,
Dorfmüller
P
,
Kim
N
. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):160112. doi:.https://doi.org/10.1183/16000617.0112-2016
8
Korkmaz
A
,
Ozlu
T
,
Ozsu
S
,
Kazaz
Z
,
Bulbul
Y
. Long-term outcomes in acute pulmonary thromboembolism: the incidence of chronic thromboembolic pulmonary hypertension and associated risk factors. Clin Appl Thromb Hemost. 2012;18(3):281–8. doi:.https://doi.org/10.1177/1076029611431956
9
Otero
R
,
Oribe
M
,
Ballaz
A
,
Jimenez
D
,
Uresandi
F
,
Nauffal
D
, et al.
Echocardiographic assessment of pulmonary arterial pressure in the follow-up of patients with pulmonary embolism. Thromb Res. 2011;127(4):303–8. doi:.https://doi.org/10.1016/j.thromres.2010.12.010
10
Poli
D
,
Grifoni
E
,
Antonucci
E
,
Arcangeli
C
,
Prisco
D
,
Abbate
R
, et al.
Incidence of recurrent venous thromboembolism and of chronic thromboembolic pulmonary hypertension in patients after a first episode of pulmonary embolism. J Thromb Thrombolysis. 2010;30(3):294–9. doi:.https://doi.org/10.1007/s11239-010-0452-x
11
Surie
S
,
Gibson
NS
,
Gerdes
VE
,
Bouma
BJ
,
van Eck-Smit
BL
,
Buller
HR
, et al.
Active search for chronic thromboembolic pulmonary hypertension does not appear indicated after acute pulmonary embolism. Thromb Res. 2010;125(5):e202–5. doi:.https://doi.org/10.1016/j.thromres.2009.12.016
12
Dentali
F
,
Donadini
M
,
Gianni
M
,
Bertolini
A
,
Squizzato
A
,
Venco
A
, et al.
Incidence of chronic pulmonary hypertension in patients with previous pulmonary embolism. Thromb Res. 2009;124(3):256–8. doi:.https://doi.org/10.1016/j.thromres.2009.01.003
13
Martí
D
,
Gómez
V
,
Escobar
C
,
Wagner
C
,
Zamarro
C
,
Sánchez
D
, et al.
Incidencia de hipertensión pulmonar tromboembólica crónica sintomática y asintomática [Incidence of symptomatic and asymptomatic chronic thromboembolic pulmonary hypertension]. Arch Bronconeumol. 2010;46(12):628–33. Article in Spanish. doi:.https://doi.org/10.1016/S1579-2129(10)70137-3
14
Klok
FA
,
van Kralingen
KW
,
van Dijk
AP
,
Heyning
FH
,
Vliegen
HW
,
Huisman
MV
. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica. 2010;95(6):970–5. doi:.https://doi.org/10.3324/haematol.2009.018960
15
Noble
S
,
Pasi
J
. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer. 2010;102(S1, Suppl 1):S2–9. doi:.https://doi.org/10.1038/sj.bjc.6605599
16
Coquoz
N
,
Weilenmann
D
,
Stolz
D
,
Popov
V
,
Azzola
A
,
Fellrath
JM
, et al.
Multicentre observational screening survey for the detection of CTEPH following pulmonary embolism. Eur Respir J. 2018;51(4):1702505. doi:.https://doi.org/10.1183/13993003.02505-2017
17
Tiede
H
,
Hoeper
MM
,
Richter
M
,
Cacheris
W
,
Hinzmann
B
,
Mayer
E
. Global burden of chronic thromboembolic pulmonary hypertension (CTEPH): An epidemiological analysis. Eur Respir J. 2014;44(Suppl 58).
18
Lang
IM
,
Dorfmüller
P
,
Noordegraaf
AV
. The Pathobiology of Chronic Thromboembolic Pulmonary Hypertension. Ann Am Thorac Soc. 2016;13(Suppl 3):S215–21. doi:.https://doi.org/10.1513/AnnalsATS.201509-620AS
19
Banks
DA
,
Pretorius
GV
,
Kerr
KM
,
Manecke
GR
. Pulmonary endarterectomy: part I. Pathophysiology, clinical manifestations, and diagnostic evaluation of chronic thromboembolic pulmonary hypertension. Semin Cardiothorac Vasc Anesth. 2014;18(4):319–30. doi:.https://doi.org/10.1177/1089253214536621
20
Ribeiro
A
,
Lindmarker
P
,
Johnsson
H
,
Juhlin-Dannfelt
A
,
Jorfeldt
L
. Pulmonary embolism: one-year follow-up with echocardiography doppler and five-year survival analysis. Circulation. 1999;99(10):1325–30. doi:.https://doi.org/10.1161/01.CIR.99.10.1325
21
Jaff
MR
,
McMurtry
MS
,
Archer
SL
,
Cushman
M
,
Goldenberg
N
,
Goldhaber
SZ
, et al.; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788–830. doi:.https://doi.org/10.1161/CIR.0b013e318214914f
22
McNeil
K
,
Dunning
J
. Chronic thromboembolic pulmonary hypertension (CTEPH). Heart. 2007;93(9):1152–8. doi:.https://doi.org/10.1136/hrt.2004.053603
23
Kim
NH
. Group 4 Pulmonary Hypertension: Chronic Thromboembolic Pulmonary Hypertension: Epidemiology, Pathophysiology, and Treatment. Cardiol Clin. 2016;34(3):435–41. doi:.https://doi.org/10.1016/j.ccl.2016.04.011
24
Quarck
R
,
Nawrot
T
,
Meyns
B
,
Delcroix
M
. C-reactive protein: a new predictor of adverse outcome in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;53(14):1211–8. doi:.https://doi.org/10.1016/j.jacc.2008.12.038
25
Zabini
D
,
Heinemann
A
,
Foris
V
,
Nagaraj
C
,
Nierlich
P
,
Bálint
Z
, et al.
Comprehensive analysis of inflammatory markers in chronic thromboembolic pulmonary hypertension patients. Eur Respir J. 2014;44(4):951–62. doi:.https://doi.org/10.1183/09031936.00145013
26
Reesink
HJ
,
Meijer
RC
,
Lutter
R
,
Boomsma
F
,
Jansen
HM
,
Kloek
JJ
, et al.
Hemodynamic and clinical correlates of endothelin-1 in chronic thromboembolic pulmonary hypertension. Circ J. 2006;70(8):1058–63. doi:.https://doi.org/10.1253/circj.70.1058
27
Bonderman
D
,
Jakowitsch
J
,
Redwan
B
,
Bergmeister
H
,
Renner
MK
,
Panzenböck
H
, et al.
Role for staphylococci in misguided thrombus resolution of chronic thromboembolic pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2008;28(4):678–84. doi:.https://doi.org/10.1161/ATVBAHA.107.156000
28
Wolf
M
,
Boyer-Neumann
C
,
Parent
F
,
Eschwege
V
,
Jaillet
H
,
Meyer
D
, et al.
Thrombotic risk factors in pulmonary hypertension. Eur Respir J. 2000;15(2):395–9. doi:.https://doi.org/10.1034/j.1399-3003.2000.15b28.x
29
Bonderman
D
,
Turecek
PL
,
Jakowitsch
J
,
Weltermann
A
,
Adlbrecht
C
,
Schneider
B
, et al.
High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb Haemost. 2003;90(3):372–6. doi:.https://doi.org/10.1160/TH03-02-0067
30
Gu
S
,
Su
P
,
Yan
J
,
Zhang
X
,
An
X
,
Gao
J
, et al.
Comparison of gene expression profiles and related pathways in chronic thromboembolic pulmonary hypertension. Int J Mol Med. 2014;33(2):277–300. doi:.https://doi.org/10.3892/ijmm.2013.1582
31
Morris
TA
,
Marsh
JJ
,
Chiles
PG
,
Magaña
MM
,
Liang
NC
,
Soler
X
, et al.
High prevalence of dysfibrinogenemia among patients with chronic thromboembolic pulmonary hypertension. Blood. 2009;114(9):1929–36. doi:.https://doi.org/10.1182/blood-2009-03-208264
32
Le Gal
G
,
Delahousse
B
,
Lacut
K
,
Malaviolle
V
,
Regina
S
,
Blouch
MT
, et al.; Groupe d’Etudes sur la Thrombose des Hôpitaux Universitaires du Grand Ouest. Fibrinogen Aα-Thr312Ala and factor XIII-A Val34Leu polymorphisms in idiopathic venous thromboembolism. Thromb Res. 2007;121(3):333–8. doi:.https://doi.org/10.1016/j.thromres.2007.05.003
33
Suntharalingam
J
,
Goldsmith
K
,
van Marion
V
,
Long
L
,
Treacy
CM
,
Dudbridge
F
, et al.
Fibrinogen Aα Thr312Ala polymorphism is associated with chronic thromboembolic pulmonary hypertension. Eur Respir J. 2008;31(4):736–41. doi:.https://doi.org/10.1183/09031936.00055107
34
Marsh
JJ
,
Chiles
PG
,
Liang
NC
,
Morris
TA
. Chronic thromboembolic pulmonary hypertension-associated dysfibrinogenemias exhibit disorganized fibrin structure. Thromb Res. 2013;132(6):729–34. doi:.https://doi.org/10.1016/j.thromres.2013.09.024
35
Alias
S
,
Redwan
B
,
Panzenböck
A
,
Winter
MP
,
Schubert
U
,
Voswinckel
R
, et al.
Defective angiogenesis delays thrombus resolution: a potential pathogenetic mechanism underlying chronic thromboembolic pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2014;34(4):810–9. doi:.https://doi.org/10.1161/ATVBAHA.113.302991
36
Frey
MK
,
Alias
S
,
Winter
MP
,
Redwan
B
,
Stübiger
G
,
Panzenboeck
A
, et al.
Splenectomy is modifying the vascular remodeling of thrombosis. J Am Heart Assoc. 2014;3(1):e000772. doi:.https://doi.org/10.1161/JAHA.113.000772
37
Mayer
E
. Surgical and post-operative treatment of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2010;19(115):64–7. doi:.https://doi.org/10.1183/09059180.00007409
38
Humbert
M
. Pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: pathophysiology. Eur Respir Rev. 2010;19(115):59–63. doi:.https://doi.org/10.1183/09059180.00007309
39
Moser
KM
,
Bloor
CM
. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest. 1993;103(3):685–92. doi:.https://doi.org/10.1378/chest.103.3.685
40
Bauer
M
,
Wilkens
H
,
Langer
F
,
Schneider
SO
,
Lausberg
H
,
Schäfers
HJ
. Selective upregulation of endothelin B receptor gene expression in severe pulmonary hypertension. Circulation. 2002;105(9):1034–6. doi:.https://doi.org/10.1161/hc0902.105719
41
Mayer
E
,
Jenkins
D
,
Lindner
J
,
D’Armini
A
,
Kloek
J
,
Meyns
B
, et al.
Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141(3):702–10. doi:.https://doi.org/10.1016/j.jtcvs.2010.11.024
42
Held
M
,
Grün
M
,
Holl
R
,
Walter
F
,
Schäfers
HJ
,
Graeter
T
, et al.
Chronisch thromboembolische pulmonale Hypertonie: Latenz bis zur Diagnosesicherung und klinischer Zustand bei Diagnosestellung [Chronic thromboembolic pulmonary hypertension: Time delay from onset of symtoms to diagnosis and clinical condition at diagnosis]. Dtsch Med Wochenschr. 2014;139(33):1647–52. Article in German.
43
Gopalan
D
,
Delcroix
M
,
Held
M
. Diagnosis of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):160108. doi:.https://doi.org/10.1183/16000617.0108-2016
44
Fedullo
P
,
Kerr
KM
,
Kim
NH
,
Auger
WR
. Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2011;183(12):1605–13. doi:.https://doi.org/10.1164/rccm.201011-1854CI
45
Armstrong
I
,
Rochnia
N
,
Harries
C
,
Bundock
S
,
Yorke
J
. The trajectory to diagnosis with pulmonary arterial hypertension: a qualitative study. BMJ Open. 2012;2(2):e000806. doi:.https://doi.org/10.1136/bmjopen-2011-000806
46
Armstrong
I
,
Harries
C
,
Yorke
J
. The imPAHct survey: living with pulmonary arterial hypertension.
Am J Respir Crit Care Med. 2011;183:A6130.
47
Al-Naamani
K
,
Hijal
T
,
Nguyen
V
,
Andrew
S
,
Nguyen
T
,
Huynh
T
. Predictive values of the electrocardiogram in diagnosing pulmonary hypertension. Int J Cardiol. 2008;127(2):214–8. doi:.https://doi.org/10.1016/j.ijcard.2007.06.005
48
Bonderman
D
,
Wexberg
P
,
Martischnig
AM
,
Heinzl
H
,
Lang
MB
,
Sadushi
R
, et al.
A noninvasive algorithm to exclude pre-capillary pulmonary hypertension. Eur Respir J. 2011;37(5):1096–103. doi:.https://doi.org/10.1183/09031936.00089610
49
Sun
XG
,
Hansen
JE
,
Oudiz
RJ
,
Wasserman
K
. Pulmonary function in primary pulmonary hypertension. J Am Coll Cardiol. 2003;41(6):1028–35. doi:.https://doi.org/10.1016/S0735-1097(02)02964-9
50
Trip
P
,
Nossent
EJ
,
de Man
FS
,
van den Berk
IA
,
Boonstra
A
,
Groepenhoff
H
, et al.
Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: patient characteristics and treatment responses. Eur Respir J. 2013;42(6):1575–85. doi:.https://doi.org/10.1183/09031936.00184412
51
Coghlan
JG
,
Denton
CP
,
Grünig
E
,
Bonderman
D
,
Distler
O
,
Khanna
D
, et al.; DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. 2014;73(7):1340–9. doi:.https://doi.org/10.1136/annrheumdis-2013-203301
52
Hoeper
MM
,
Pletz
MW
,
Golpon
H
,
Welte
T
. Prognostic value of blood gas analyses in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2007;29(5):944–50. doi:.https://doi.org/10.1183/09031936.00134506
53
Scheidl
SJ
,
Englisch
C
,
Kovacs
G
,
Reichenberger
F
,
Schulz
R
,
Breithecker
A
, et al.
Diagnosis of CTEPH versus IPAH using capillary to end-tidal carbon dioxide gradients. Eur Respir J. 2012;39(1):119–24. doi:.https://doi.org/10.1183/09031936.00109710
54
Held
M
,
Meintz
S
,
Baron
S
,
Roth
C
,
Wilkens
H
,
Schäfers
HJ
, et al.
Surgical cure of central sleep apnea?
Am J Respir Crit Care Med. 2013;188(3):395–6. doi:.https://doi.org/10.1164/rccm.201210-1944IM
55
Held
M
,
Grün
M
,
Holl
R
,
Hübner
G
,
Kaiser
R
,
Karl
S
, et al.
Cardiopulmonary exercise testing to detect chronic thromboembolic pulmonary hypertension in patients with normal echocardiography. Respiration. 2014;87(5):379–87. doi:.https://doi.org/10.1159/000358565
56
Held
M
,
Linke
M
,
Jany
B
. Echokardiographie und Rechtsherzkatheterisierung bei pulmonaler Hypertonie [Echocardiography and right heart catheterization in pulmonal hypertension]. Dtsch Med Wochenschr. 2014;139(30):1511–7. Article in German.
57
Konstantinides
SV
,
Torbicki
A
,
Agnelli
G
,
Danchin
N
,
Fitzmaurice
D
,
Galiè
N
, et al., Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–73. doi:.https://doi.org/10.1093/eurheartj/ehu283
58
Ruggiero
A
,
Screaton
NJ
. Imaging of acute and chronic thromboembolic disease: state of the art. Clin Radiol. 2017;72(5):375–88. doi:.https://doi.org/10.1016/j.crad.2017.02.011
59
Ley
S
,
Ley-Zaporozhan
J
,
Pitton
MB
,
Schneider
J
,
Wirth
GM
,
Mayer
E
, et al.
Diagnostic performance of state-of-the-art imaging techniques for morphological assessment of vascular abnormalities in patients with chronic thromboembolic pulmonary hypertension (CTEPH). Eur Radiol. 2012;22(3):607–16. doi:.https://doi.org/10.1007/s00330-011-2290-4
60
Reichelt
A
,
Hoeper
MM
,
Galanski
M
,
Keberle
M
. Chronic thromboembolic pulmonary hypertension: evaluation with 64-detector row CT versus digital substraction angiography. Eur J Radiol. 2009;71(1):49–54. doi:.https://doi.org/10.1016/j.ejrad.2008.03.016
61
Sugiura
T
,
Tanabe
N
,
Matsuura
Y
,
Shigeta
A
,
Kawata
N
,
Jujo
T
, et al.
Role of 320-slice CT imaging in the diagnostic workup of patients with chronic thromboembolic pulmonary hypertension. Chest. 2013;143(4):1070–7. doi:.https://doi.org/10.1378/chest.12-0407
62
Thieme
SF
,
Becker
CR
,
Hacker
M
,
Nikolaou
K
,
Reiser
MF
,
Johnson
TR
. Dual energy CT for the assessment of lung perfusion--correlation to scintigraphy. Eur J Radiol. 2008;68(3):369–74. doi:.https://doi.org/10.1016/j.ejrad.2008.07.031
63
Thieme
SF
,
Graute
V
,
Nikolaou
K
,
Maxien
D
,
Reiser
MF
,
Hacker
M
, et al.
Dual Energy CT lung perfusion imaging--correlation with SPECT/CT. Eur J Radiol. 2012;81(2):360–5. doi:.https://doi.org/10.1016/j.ejrad.2010.11.037
64
Dournes
G
,
Verdier
D
,
Montaudon
M
,
Bullier
E
,
Rivière
A
,
Dromer
C
, et al.
Dual-energy CT perfusion and angiography in chronic thromboembolic pulmonary hypertension: diagnostic accuracy and concordance with radionuclide scintigraphy. Eur Radiol. 2014;24(1):42–51. doi:.https://doi.org/10.1007/s00330-013-2975-y
65
Renard
B
,
Remy-Jardin
M
,
Santangelo
T
,
Faivre
JB
,
Tacelli
N
,
Remy
J
, et al.
Dual-energy CT angiography of chronic thromboembolic disease: can it help recognize links between the severity of pulmonary arterial obstruction and perfusion defects?
Eur J Radiol. 2011;79(3):467–72. doi:.https://doi.org/10.1016/j.ejrad.2010.04.018
66
Ito
K
,
Kubota
K
,
Morooka
M
,
Shida
Y
,
Hasuo
K
,
Endo
H
, et al.
Diagnostic usefulness of 18F-FDG PET/CT in the differentiation of pulmonary artery sarcoma and pulmonary embolism. Ann Nucl Med. 2009;23(7):671–6. doi:.https://doi.org/10.1007/s12149-009-0292-y
67
Hudson
ER
,
Smith
TP
,
McDermott
VG
,
Newman
GE
,
Suhocki
PV
,
Payne
CS
, et al.
Pulmonary angiography performed with iopamidol: complications in 1,434 patients. Radiology. 1996;198(1):61–5. doi:.https://doi.org/10.1148/radiology.198.1.8539407
68
Stein
PD
,
Athanasoulis
C
,
Alavi
A
,
Greenspan
RH
,
Hales
CA
,
Saltzman
HA
, et al.
Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation. 1992;85(2):462–8. doi:.https://doi.org/10.1161/01.CIR.85.2.462
69
Kovacs
G
,
Herve
P
,
Barbera
JA
,
Chaouat
A
,
Chemla
D
,
Condliffe
R
, et al.
An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J. 2017;50(5):1700578. doi:.https://doi.org/10.1183/13993003.00578-2017
70
Jenkins
D
,
Mayer
E
,
Screaton
N
,
Madani
M
. State-of-the-art chronic thromboembolic pulmonary hypertension diagnosis and management. Eur Respir Rev. 2012;21(123):32–9. doi:.https://doi.org/10.1183/09059180.00009211
71
Berman
M
,
Gopalan
D
,
Sharples
L
,
Screaton
N
,
Maccan
C
,
Sheares
K
, et al.
Right ventricular reverse remodeling after pulmonary endarterectomy: magnetic resonance imaging and clinical and right heart catheterization assessment. Pulm Circ. 2014;4(1):36–44. doi:.https://doi.org/10.1086/674884
72
Lewczuk
J
,
Piszko
P
,
Jagas
J
,
Porada
A
,
Sobkowicz
B
,
Wrabec
K
, et al.
Prognostic factors in medically treated patients with chronic pulmonary embolism. Chest. 2001;119(3):818–23. doi:.https://doi.org/10.1378/chest.119.3.818
73
Madani
MM
. Surgical Treatment of Chronic Thromboembolic Pulmonary Hypertension: Pulmonary Thromboendarterectomy. Methodist DeBakey Cardiovasc J. 2016;12(4):213–8. doi:.https://doi.org/10.14797/mdcj-12-4-213
74
Kim
NH
,
Delcroix
M
,
Jenkins
DP
,
Channick
R
,
Dartevelle
P
,
Jansa
P
, et al.
Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62(25, Suppl):D92–9. doi:.https://doi.org/10.1016/j.jacc.2013.10.024
75
Jenkins
D
,
Madani
M
,
Fadel
E
,
D’Armini
AM
,
Mayer
E
. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):160111. doi:.https://doi.org/10.1183/16000617.0111-2016
76
Jenkins
D
. Pulmonary endarterectomy: the potentially curative treatment for patients with chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2015;24(136):263–71. doi:.https://doi.org/10.1183/16000617.00000815
77
Madani
MM
,
Auger
WR
,
Pretorius
V
,
Sakakibara
N
,
Kerr
KM
,
Kim
NH
, et al.
Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2,700 patients. Ann Thorac Surg. 2012;94(1):97–103, discussion 103. doi:.https://doi.org/10.1016/j.athoracsur.2012.04.004
78
Ng
C
,
Jenkins
DP
. Surgical management of chronic thromboembolic pulmonary hypertension. Br J Hosp Med (Lond). 2013;74(1):31–5. doi:.https://doi.org/10.12968/hmed.2013.74.1.31
79
Vuylsteke
A
,
Sharples
L
,
Charman
G
,
Kneeshaw
J
,
Tsui
S
,
Dunning
J
, et al.
Circulatory arrest versus cerebral perfusion during pulmonary endarterectomy surgery (PEACOG): a randomised controlled trial. Lancet. 2011;378(9800):1379–87. doi:.https://doi.org/10.1016/S0140-6736(11)61144-6
80
Delcroix
M
,
Lang
I
,
Pepke-Zaba
J
,
Jansa
P
,
D’Armini
AM
,
Snijder
R
, et al.
Long-Term Outcome of Patients With Chronic Thromboembolic Pulmonary Hypertension: Results From an International Prospective Registry. Circulation. 2016;133(9):859–71. doi:.https://doi.org/10.1161/CIRCULATIONAHA.115.016522
81
Opitz
I
,
de Perrot
M
. Technique of Pulmonary Thromboendarterectomy. Oper Tech Thorac Cardiovasc Surg. 2012;17(3):168–80. doi:.https://doi.org/10.1053/j.optechstcvs.2012.07.004
82
Jamieson
SW
,
Kapelanski
DP
. Pulmonary endarterectomy. Curr Probl Surg. 2000;37(3):165–252. doi:.https://doi.org/10.1016/S0011-3840(00)80005-2
83
Madani
M
,
Jamieson
SW
. Pulmonary Endarterectomy for Chronic Thromboembolic Disease. Oper Tech Thorac Cardiovasc Surg. 2006;11(4):264–74. doi:.https://doi.org/10.1053/j.optechstcvs.2006.10.002
84
Lindner
J
,
Ambrož
D
,
Novotný
R
,
Nižňanský
M
,
Šimková
I
,
Boháčeková
M
, et al.
Pulmonary endarterectomy combined with cardiac surgery: A 7-year retrospective analysis. Cor Vasa. 2015;57(2):e115–20. doi:.https://doi.org/10.1016/j.crvasa.2015.02.009
85
Thistlethwaite
PA
,
Auger
WR
,
Madani
MM
,
Pradhan
S
,
Kapelanski
DP
,
Jamieson
SW
. Pulmonary thromboendarterectomy combined with other cardiac operations: indications, surgical approach, and outcome. Ann Thorac Surg. 2001;72(1):13–7, discussion 17–9. doi:.https://doi.org/10.1016/S0003-4975(01)02686-8
86
Raisinghani
A
,
Ben-Yehuda
O
. Echocardiography in chronic thromboembolic pulmonary hypertension. Semin Thorac Cardiovasc Surg. 2006;18(3):230–5. doi:.https://doi.org/10.1053/j.semtcvs.2006.09.006
87
Saouti
N
,
de Man
F
,
Westerhof
N
,
Boonstra
A
,
Twisk
J
,
Postmus
PE
, et al.
Predictors of mortality in inoperable chronic thromboembolic pulmonary hypertension. Respir Med. 2009;103(7):1013–9. doi:.https://doi.org/10.1016/j.rmed.2009.01.017
88
Rahnavardi
M
,
Yan
TD
,
Cao
C
,
Vallely
MP
,
Bannon
PG
,
Wilson
MK
. Pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension : a systematic review. Ann Thorac Cardiovasc Surg. 2011;17(5):435–45. doi:.https://doi.org/10.5761/atcs.oa.10.01653
89
Condliffe
R
,
Kiely
DG
,
Gibbs
JS
,
Corris
PA
,
Peacock
AJ
,
Jenkins
DP
, et al.
Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2008;177(10):1122–7. doi:.https://doi.org/10.1164/rccm.200712-1841OC
90
Taboada
D
,
Pepke-Zaba
J
,
Jenkins
DP
,
Berman
M
,
Treacy
CM
,
Cannon
JE
, et al.
Outcome of pulmonary endarterectomy in symptomatic chronic thromboembolic disease. Eur Respir J. 2014;44(6):1635–45. doi:.https://doi.org/10.1183/09031936.00050114
91
Cannon
JE
,
Su
L
,
Kiely
DG
,
Page
K
,
Toshner
M
,
Swietlik
E
, et al.
Dynamic Risk Stratification of Patient Long-Term Outcome After Pulmonary Endarterectomy: Results From the United Kingdom National Cohort. Circulation. 2016;133(18):1761–71. doi:.https://doi.org/10.1161/CIRCULATIONAHA.115.019470
92
Hoeper
MM
,
Madani
MM
,
Nakanishi
N
,
Meyer
B
,
Cebotari
S
,
Rubin
LJ
. Chronic thromboembolic pulmonary hypertension. Lancet Respir Med. 2014;2(7):573–82. doi:.https://doi.org/10.1016/S2213-2600(14)70089-X
93
Archibald
CJ
,
Auger
WR
,
Fedullo
PF
,
Channick
RN
,
Kerr
KM
,
Jamieson
SW
, et al.
Long-term outcome after pulmonary thromboendarterectomy. Am J Respir Crit Care Med. 1999;160(2):523–8. doi:.https://doi.org/10.1164/ajrccm.160.2.9808109
94
Bonderman
D
,
Skoro-Sajer
N
,
Jakowitsch
J
,
Adlbrecht
C
,
Dunkler
D
,
Taghavi
S
, et al.
Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation. 2007;115(16):2153–8. doi:.https://doi.org/10.1161/CIRCULATIONAHA.106.661041
95
Jamieson
SW
,
Kapelanski
DP
,
Sakakibara
N
,
Manecke
GR
,
Thistlethwaite
PA
,
Kerr
KM
, et al.
Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003;76(5):1457–62, discussion 1462–4. doi:.https://doi.org/10.1016/S0003-4975(03)00828-2
96
Thistlethwaite
PA
,
Madani
MM
,
Kemp
AD
,
Hartley
M
,
Auger
WR
,
Jamieson
SW
. Venovenous extracorporeal life support after pulmonary endarterectomy: indications, techniques, and outcomes. Ann Thorac Surg. 2006;82(6):2139–45. doi:.https://doi.org/10.1016/j.athoracsur.2006.07.020
97
Freed
DH
,
Thomson
BM
,
Berman
M
,
Tsui
SS
,
Dunning
J
,
Sheares
KK
, et al.
Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg. 2011;141(2):383–7. doi:.https://doi.org/10.1016/j.jtcvs.2009.12.056
98
Ghofrani
HA
,
D’Armini
AM
,
Grimminger
F
,
Hoeper
MM
,
Jansa
P
,
Kim
NH
, et al.; CHEST-1 Study Group. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–29. doi:.https://doi.org/10.1056/NEJMoa1209657
99
Simonneau
G
,
D’Armini
AM
,
Ghofrani
HA
,
Grimminger
F
,
Hoeper
MM
,
Jansa
P
, et al.
Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2). Eur Respir J. 2015;45(5):1293–302. doi:.https://doi.org/10.1183/09031936.00087114
100
Simonneau
G
,
D’Armini
AM
,
Ghofrani
HA
,
Grimminger
F
,
Jansa
P
,
Kim
NH
, et al.
Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med. 2016;4(5):372–80. doi:.https://doi.org/10.1016/S2213-2600(16)30022-4
101
Wiedenroth
CB
,
Liebetrau
C
,
Breithecker
A
,
Guth
S
,
Lautze
HJ
,
Ortmann
E
, et al.
Combined pulmonary endarterectomy and balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2016;35(5):591–6. doi:.https://doi.org/10.1016/j.healun.2015.10.030
102
Collaud
S
,
Brenot
P
,
Mercier
O
,
Fadel
E
. Rescue balloon pulmonary angioplasty for early failure of pulmonary endarterectomy: The earlier the better?
Int J Cardiol. 2016;222:39–40. doi:.https://doi.org/10.1016/j.ijcard.2016.07.021
103
Shimura
N
,
Kataoka
M
,
Inami
T
,
Yanagisawa
R
,
Ishiguro
H
,
Kawakami
T
, et al.
Additional percutaneous transluminal pulmonary angioplasty for residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol. 2015;183:138–42. doi:.https://doi.org/10.1016/j.ijcard.2015.01.034
104
Federal Statistical Office S. Population of Switzlerland. last accessed 15.03.2018. https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung.html.
105
Feinstein
JA
,
Goldhaber
SZ
,
Lock
JE
,
Ferndandes
SM
,
Landzberg
MJ
. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001;103(1):10–3. doi:.https://doi.org/10.1161/01.CIR.103.1.10
106
Sugimura
K
,
Fukumoto
Y
,
Satoh
K
,
Nochioka
K
,
Miura
Y
,
Aoki
T
, et al.
Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J. 2012;76(2):485–8. doi:.https://doi.org/10.1253/circj.CJ-11-1217
107
Kataoka
M
,
Inami
T
,
Hayashida
K
,
Shimura
N
,
Ishiguro
H
,
Abe
T
, et al.
Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012;5(6):756–62. doi:.https://doi.org/10.1161/CIRCINTERVENTIONS.112.971390
108
Mizoguchi
H
,
Ogawa
A
,
Munemasa
M
,
Mikouchi
H
,
Ito
H
,
Matsubara
H
. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012;5(6):748–55. doi:.https://doi.org/10.1161/CIRCINTERVENTIONS.112.971077
109
Mahmud
E
,
Behnamfar
O
,
Ang
L
,
Patel
MP
,
Poch
D
,
Kim
NH
. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. Interv Cardiol Clin. 2018;7(1):103–17. doi:.https://doi.org/10.1016/j.iccl.2017.09.003
110
Andreassen
AK
,
Ragnarsson
A
,
Gude
E
,
Geiran
O
,
Andersen
R
. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart. 2013;99(19):1415–20. doi:.https://doi.org/10.1136/heartjnl-2012-303549
111
Inami
T
,
Kataoka
M
,
Ando
M
,
Fukuda
K
,
Yoshino
H
,
Satoh
T
. A new era of therapeutic strategies for chronic thromboembolic pulmonary hypertension by two different interventional therapies; pulmonary endarterectomy and percutaneous transluminal pulmonary angioplasty. PLoS One. 2014;9(4):e94587. doi:.https://doi.org/10.1371/journal.pone.0094587
112
Inami
T
,
Kataoka
M
,
Yanagisawa
R
,
Ishiguro
H
,
Shimura
N
,
Fukuda
K
, et al.
Long-Term Outcomes After Percutaneous Transluminal Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. Circulation. 2016;134(24):2030–2. doi:.https://doi.org/10.1161/CIRCULATIONAHA.116.024201
113
Taniguchi
Y
,
Miyagawa
K
,
Nakayama
K
,
Kinutani
H
,
Shinke
T
,
Okada
K
, et al.
Balloon pulmonary angioplasty: an additional treatment option to improve the prognosis of patients with chronic thromboembolic pulmonary hypertension. EuroIntervention. 2014;10(4):518–25. doi:.https://doi.org/10.4244/EIJV10I4A89
114
Olsson
KM
,
Wiedenroth
CB
,
Kamp
JC
,
Breithecker
A
,
Fuge
J
,
Krombach
GA
, et al.
Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: the initial German experience. Eur Respir J. 2017;49(6):1602409. doi:.https://doi.org/10.1183/13993003.02409-2016
115
Ghofrani
HA
,
Simonneau
G
,
D’Armini
AM
,
Fedullo
P
,
Howard
LS
,
Jaïs
X
, et al.; MERIT study investigators. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med. 2017;5(10):785–94. doi:.https://doi.org/10.1016/S2213-2600(17)30305-3
116
Jaïs
X
,
D’Armini
AM
,
Jansa
P
,
Torbicki
A
,
Delcroix
M
,
Ghofrani
HA
, et al.; Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension Study Group. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52(25):2127–34. doi:.https://doi.org/10.1016/j.jacc.2008.08.059
117
Bauersachs
R
,
Berkowitz
SD
,
Brenner
B
,
Buller
HR
,
Decousus
H
,
Gallus
AS
, et al., EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–510. doi:.https://doi.org/10.1056/NEJMoa1007903
118
Konstantinides
SV
,
Torbicki
A
,
Agnelli
G
,
Danchin
N
,
Fitzmaurice
D
,
Galiè
N
, et al.; Authors/Task Force Members. Corrigendum to: 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2015;36(39):2642. doi:.https://doi.org/10.1093/eurheartj/ehu479
















