Early diagnosis and management of dementia in general practice – how do Swiss GPs meet the challenge?
DOI: https://doi.org/10.4414/smw.2018.14695
Stéphanie
Giezendannera, Andreas U.
Monschb, Reto W.
Kressigc, Yolanda
Muellerd, Sven
Streite, Stefan
Essigf, Andreas
Zellera, Klaus
Ballya
aCentre for Primary Health Care, University of Basel, Basel, Switzerland
bMemory Clinic, University Centre for Medicine of Aging, Felix Platter Hospital Basel; Faculty of Psychology, University of Basel, Switzerland
cUniversity Centre for Medicine of Aging, Felix Platter Hospital Basel; Faculty of Medicine, University of Basel, Switzerland
dUniversity Institute for Family Medicine, Department of Ambulatory Care and Community Medicine, University of Lausanne, Switzerland
eInstitute of Primary Health Care (BIHAM), University of Bern, Switzerland
fInstitute of Primary and Community Care Lucerne, Switzerland
Summary
INTRODUCTION
In general practice, the diagnosis of dementia is often delayed. Therefore, the Swiss National Dementia Strategy 2014 concluded that action was needed to improve patient care. Little is known about GPs’ confidence in and approach to the diagnosis, disclosure and post-diagnostic management of individuals with dementia in Switzerland. The aim of this survey is to assess these elements of dementia care and GPs’ views on the adequacy of health care services regarding dementia.
MATERIALS AND METHODS
Cross-sectional postal survey in Switzerland in 2017 supported by all academic institutes of general practice in Swiss universities. Members of the Swiss Association of General Practitioners (n = 4460) were asked to participate in the survey. In addition to the GPs’ demographic characteristics, the survey addressed the following issues: GPs’ views on the adequacy of health care services, clinical approach and confidence in the management of dementia.
RESULTS
The survey response rate was 21%. The majority of GPs (64%) felt confident diagnosing dementia, but not in patients with a migration background (15%). For neuropsychological testing, three-quarters of GPs collaborated with memory clinics and were satisfied with the access to diagnostic services. At the time of first diagnosis, 62% of GPs diagnosed the majority of their patients with a mild stage of dementia, and 31% with a mild cognitive impairment. The most frequent actions taken by GPs after the diagnosis of mild dementia were giving advice to relatives (71%), testing fitness-to-drive (66%) and minimising cardiovascular risk factors (63%). While 65% of GPs felt confident taking care of patients with dementia, fewer (53%) felt confident in pharmacological treatment, coping with suicidal ideation (44%) or caring for patients with a migration background (16%). Half of GPs preferred to delegate the assessment of fitness-to-drive to an official authority. One in four GPs was not satisfied with the local provision of care and support facilities for patients with dementia.
CONCLUSIONS
Overall, GPs reported confidence in establishing a diagnosis of dementia and sufficient access to diagnostic services. Post-diagnostic management primarily focused on counselling and harm reduction rather than pharmacological treatment. Future educational support for GPs should be developed, concentrating on coping with their patients’ suicidal ideation and caring for patients with a migration background.
Introduction
General practitioners (GPs) typically play a key role in timely dementia diagnosis and on-going care of their patients with dementia. However, there is evidence that the diagnosis of dementia is insufficient in general practice [1], resulting in delayed detection of dementia and disadvantages for patients and their caregivers [2, 3]. In Switzerland, the detection rate of cognitive impairment by GPs in an elderly population is low, ranging from 24 to 42% in out-patients [4]. A European key informant survey indicated that the majority of GPs in Switzerland tried to establish a diagnosis of dementia on their own, and only half of them referred a suspected case of dementia to a secondary care specialist [5]. Evidence suggests that disease disclosure does not routinely occur in general practice, despite the fact that it may help patients with dementia and their caregivers with future planning [6, 7], and that GPs are reluctant to speak openly with their patients about dementia [8, 9].
Many reasons why a timely dementia diagnosis might be challenging for GPs are reported in the literature. Typical barriers include the lack of time during consultations [5], particularly in combination with multiple health issues in older patients [10]. There is evidence that GPs have difficulty conveying the diagnosis when the patient and his/her caregiver are unaware of the symptoms of cognitive decline [10], there is no informant history available [11–13], when GPs lack the training to detect early signs of dementia [14] or when the presenting symptoms are vague [15]. Further obstacles for GPs include their own feelings of uneasiness about carrying out cognitive examinations and difficulties in communicating the diagnosis [16], as well as a lack of patient-centeredness in their diagnostic approach [17]. Moreover, GPs may think that nobody stands to benefit from an early diagnosis because no effective treatment is available [18].
There is also evidence of shortcomings in the health care system and the possibility of support for persons with dementia and their families. In particular, studies have revealed problems with late referral to specialist and social services [19], difficulties accessing and communicating with specialists, GPs’ lack of time, low reimbursement and a lack of interdisciplinary teams [20]. Further constraints include delayed detection of behavioural problems, a lack of proactive management of dementia and an increased reliance on pharmacological rather than psychosocial treatment strategies [20]. Another study found a lack of counselling ability about safety and accident prevention in GPs and a lack of caregiver support and conflict management [21]. It is important that GPs are aware of all local resources to alleviate the burden on the patient and his/her caregivers, particularly in the absence of disease modifying therapies [20, 22].
Improving early detection of dementia was one of the aims in the Swiss National Dementia Strategy 2014–2017, because there are multiple benefits for patients, families and resources [23]. Examining GPs’ approach to the management of dementia is critical for the planning and development of services.
Thus, the aim of this study is to explore GPs’ approach to the diagnosis, disclosure and management of dementia, and their perception of the provision of care and health services for individuals with dementia via a national survey. In particular, this study set out to examine (i) diagnosis and disclosure practice, (ii) frequency of different treatment options in a case vignette of mild dementia, (iii) GPs’ satisfaction with post-diagnostic dementia services and (iv) GPs’ confidence in different areas of dementia management. Since findings may differ regionally due to political, cultural or residential factors, findings on the demand for dementia-specific health care services were assessed across different regional characteristics.
Materials and methods
Study design and setting
The Centre for Primary Health Care of the University of Basel undertook a national study among GPs across Switzerland in 2017 to measure GPs’ attitudes, practice and confidence in caring for patients with dementia, and mild dementia in particular. The survey was part of an observational, cross-sectional study entitled “General practitioners dementia report Switzerland.”
Sampling and data collection
All GPs who are members of “mfe” (Swiss Association of General Practitioners and Paediatricians, n = 4460) were contacted by mail in August 2017 and asked to participate in the survey. Paediatricians (n = 450) were excluded from the survey. The letter included a recommendation from all institutes of general practice in Switzerland encouraging the addressees to take part in the study, study information with a link to the online questionnaire, a questionnaire and a stamped, addressed envelope to return the study documents. Since the survey was anonymous, a reminder was sent to all members by e-mail 1 month later.
Measures
The questionnaire assessed GPs’ practice in caring for patients with dementia and comprised 66 items in total. The initial questionnaire was developed in German. Content validity was pre-tested among a small group of physicians for readability and acceptability (two of them are listed as authors). Two independent translations into French and Italian were made by professional translators. Translations were assessed by members of the study team. GPs were required to specify their age and gender, the location of their practice (rural, urban or agglomeration of a city and the canton), the year in which they started working in their practice, their satisfaction with dementia-specific education, the average hours of work per week (patient contact and administration) and the number of patient consultations per half day. GPs were also asked to estimate the percentage of their patients over the age of 70.
The items were mostly based on previous questionnaires such as the final report of the “Joint Action on Alzheimer Cooperation Valuation in Europe” (ALCOVE) on timely diagnosis of dementia [24], two questionnaires about GPs’ attitudes to dementia [25, 26], or otherwise developed by the authors of the study as indicated below. The items comprised GPs’ use of diagnostic tools [24], diagnostic collaborations with other specialists [24], GPs’ need for care facilities for dementia patients [25] and their caregivers in the practice’s local area, and the stage of dementia at the moment of diagnosis [24]. GPs’ confidence in the diagnosis, treatment and care of patients with dementia, with or without a migration background, was also assessed [25, 26]. From a theoretical perspective, a migration background can be understood as a life situation characterised by one's own migration or the migration experience of close family members. The biographical event of a migration may result in peculiarities in life situation which can, among other effects, be relevant to health, leading to health differences between the native and the migrant populations [27, 28]. Additional questions created by the core study team (KB, SG, AUM) were incorporated, such as on the disclosure process and reasons for the diagnostic assessment. Furthermore, GPs’ treatment strategies were assessed in a case vignette. The vignette comprised the question “What measures would you take if a patient was diagnosed with early stage Alzheimer's disease (MMSE of 24 and needing some assistance in activities of daily living)?”, since that is the disease stage that GPs see most often. GPs were asked to rate the frequency of each item on a 5-point Likert scale (see table S1 in appendix 1 and fig. 2).
Most answers were assessed on a 5-point Likert scale from strongly disagree (1) to partially agree (3) to strongly agree (5) or from always (100%) to half (50%) to never (0%). Multiple answers were possible for certain items, e.g. when GPs had to choose from a set of seven different cognitive assessment tools.
Statistical analyses
All data analyses were conducted using R version 1.3 [29]. Descriptive results were provided across the whole sample using number and percentage (%) for categorical data or mean and standard deviation (SD) for continuous data. Answers on five-point Likert scales such as “agree/strongly agree” and “disagree/fully disagree” were grouped together. We then reported the sum of the two percentages. Additionally, we summarised the Likert scales with their median (Mdn) and interquartile range (IQR) (see appendix 1). Regional patterns in dementia management across Switzerland were displayed using the function “spplot” from the R package “sp”. The percentages or medians of variables were displayed across cantons or municipality. To assess the relationship between regional characteristics and GPs’ provision with diagnostic assessment possibilities or care, Pearson's chi-squared test was used. Regional differences encompassed urban and rural areas (city, agglomeration of a city, countryside), seven major Swiss regions (see table 1) [30] and three language regions (German, French, Italian). Statistical significance was set at the 5% level.
Results
Sample
Of the 4460 initially contacted GPs, 306 were either no longer practicing as a GP or the letter was undeliverable, leaving a sample of 4154 GPs. A total of 882 GPs (21%) returned the questionnaire. We found no strong evidence for a non-responder bias for gender (p = 0.62) but did find a small over-representation of German-speaking GPs (79% in the survey, 75% in the population, p = 0.046). The demographic, regional and professional characteristics of the resulting total sample of 882 GPs are presented in table 1. In terms of responder participation, the survey covered all regions across Switzerland.
Table 1 Demographic, regional and professional characteristics of the respondent GPs (n = 822).
|
Variable
|
n* (%)
|
| Gender (male) |
617 (70.0) |
| Age (years) (mean, SD) |
55.8 (8.86) |
| Language region |
|
| German |
692 (78.5) |
| French |
151 (17.1) |
| Italian |
39 (4.42) |
| Major Swiss regions |
|
| Lake Geneva (GE, VS, VD) |
106 (12.3) |
| Middle Switzerland (BE, SO, FR, NE, JU) |
216 (25.1) |
| Northwestern Switzerland (BS, BL, AG) |
161 (18.7) |
| Eastern Switzerland (SG, TG, AI, AR, GL, SH, GR) |
130 (15.1) |
| Ticino (TI) |
38 (4.41) |
| Central Switzerland (UR, SZ, OW, NW, LU, ZG) |
72 (8.35) |
| Zurich (ZH) |
140 (16.2) |
| Type of area in which practice is located |
|
| City |
297 (34.0) |
| Agglomeration of a city |
274 (31.4) |
| Country side |
302 (34.6) |
| Numbers of years practicing in practice |
20.0 (9.92) |
| Average hours of work per week (patients and administration) (mean, SD) |
46.0 (14.2) |
| No. of consultations per half day (mean, SD) |
12.94 (4.74) |
| Estimate of the percentage of patients over the age of 70 (mean, SD) |
36.3 (17.5) |
GPs’ diagnosis and disclosure practice
More than half of GPs (57%) stated that caring relatives often asked for a diagnostic assessment having noticed cognitive impairment or behavioural changes. Approximately half of respondents reported that cognitive impairment or behavioural changes were frequently noticed by patients (49%) or by GPs themselves (45%). A third of GPs initiated a diagnostic assessment due to an official cause such as a fitness-to-drive assessment or establishing a last will document. Eight percent of GPs initiated a diagnostic assessment in the context of a screening test, meaning that they “proactively” asked older patients at risk of dementia about their memory and offered a screening test (see table S1, appendix 1).
Sixty-four percent of GPs felt confident in the early diagnostic assessment of patients with cognitive impairment (only 10% did not, 26% felt partially confident, see table S2, appendix 1). However, half of the respondent GPs felt unconfident in carrying out an early diagnostic assessment if their patients had a migration background. While 10% of GPs felt unconfident in the early diagnostic assessment of patients with cognitive impairment, up to 52% felt unconfident when patients had a migration background (table S2). The most frequently used tests to assess cognitive impairment and the specialist with whom GPs worked for the diagnostic assessment are reported in table 2.
Table 2 Diagnostic tools and specialist allies to assess cognitive impairment.
|
Variables
|
n
|
%
(of the total n = 882)
|
|
Tests
|
|
|
| Mini-Mental-Status-Examination (MMSE) |
749 |
84.9 |
| Clock Drawing Test |
739 |
83.8 |
| Trail Making Test (A and B) |
386 |
43.8 |
| Montreal Cognitive Assessment (MoCA) |
149 |
16.9 |
| DemTect |
52 |
5.9 |
| BrainCheck (as a case-finding tool) |
52 |
5.9 |
| CERAD-Neuropsychological Battery |
18 |
2.0 |
|
Specialists
|
|
|
| Memory Clinic (outpatient) |
668 |
75.7 |
| Neurology (outpatient) |
334 |
37.9 |
| Neuropsychology (outpatient) |
287 |
32.5 |
| Geriatrics (outpatient) |
161 |
18.3 |
| Psychiatry (outpatient) |
118 |
13.4 |
| Geriatrics (inpatient) |
85 |
9.6 |
| Neurology (inpatient) |
21 |
2.4 |
| Psychiatry (inpatient) |
21 |
2.4 |
Three-quarters of GPs were satisfied with the access to diagnostic services such as memory clinics or referral to specialists. Only 13% of respondents reported having insufficient access (see table S3, appendix 1). There was a significant difference in terms of access to diagnostic services between language regions (χ2 = 79.3, df = 8, p < 0.001) (fig. 1) but not between rural and urban regions (χ2 = 11.6, df = 8, p = 0.167). French-speaking GPs were less satisfied with their access to diagnostic services compared with German-speaking GPs. In particular, GPs of the canton Valais (n = 19) were on average only partially satisfied with the provision of diagnostic assessment possibilities (table 2).

Figure 1 Boxplot (left) and map (right) of satisfaction with the access to diagnostic assessment possibilities for patients with cognitive impairment across language region. The map displays the median agreement across cantons. ++ = strongly agree (satisfied), +/- partially agree, -- strongly disagree (not satisfied)
Three-quarters of respondents reported often disclosing the diagnosis themselves. Only 9% of respondents almost never or never disclosed the diagnosis themselves (table S1). The disclosure usually took place with the patient and their relatives (89.6%), and less often with the patient alone (9.9%). This consultation lasted 28.5 minutes (SD = 11.9) on average. The most frequent stage of dementia at the moment of first diagnosis was mild dementia with an MMSE of 20-30 and difficulties in activities of daily living (62.5%). Thirty-one percent of respondents reported that MCI is the most frequent stage of dementia at diagnosis. Only 6.2% of GPs reported that the stage of dementia at the moment of diagnosis was moderate with a MMSE of 10-19.
GPs’ post-diagnostic strategies and interventions in early dementia
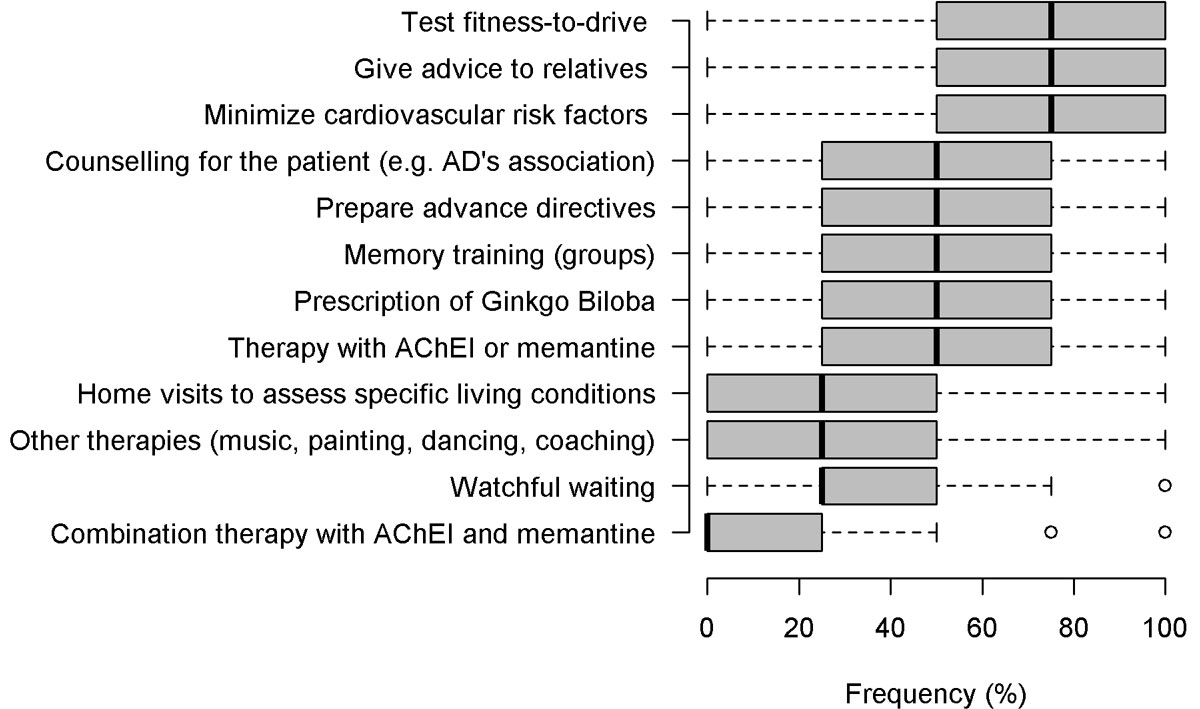
In the case vignette of a patient diagnosed with mild dementia, the most commonly implemented strategies were to counsel relatives (71% frequent and always), to test fitness-to-drive (66% frequent and always) and to minimise cardiovascular risks (63% frequent and always) (fig. 2 and table S4, appendix 1). The least used measures were pharmacological therapy with either acetylcholinesterase inhibitors or memantine (29% frequent and always), a wait-and-see strategy with no intervention (watchful waiting) (21% frequent and always), to send patients to other therapies involving music, painting, dancing or coaching (21% frequent and always) and home visits to assess specific living conditions (12% frequent and always). The use of pharmacological combination therapy with acetylcholinesterase inhibitors and memantine was almost never implemented (3%). GPs planned a follow up visit after an average of 3.7 months (SD = 2.3). While 53% of respondents felt confident in pharmacological therapy, 18% did not feel confident in this domain and the rest felt partially confident (29%). Moreover, almost half of the respondent GPs would like to delegate the assessment of fitness-to-drive of patients with cognitive impairment to an official authority, while 36% did not want to do so.
Care and support of patient with dementia
Two-thirds of GPs felt confident caring for and supporting their patients. Only 7% of GPs felt unconfident caring for and supporting their patients with dementia (table S2, appendix 1). However, in caring for patients with a migration background, only 16% of GPs felt confident and 49% of respondents stated that they did not feel confident with these patients (table S2). Thirty percent of respondents felt unconfident handling suicidal ideation of patients with early dementia (table S2). Half of the respondents considered the local provision of care and support facilities as sufficient. However, there was some evidence for regional differences in the perception of the provision of care facilities across the seven major Swiss regions (χ2 = 37.3, df = 24, p = 0.041), but not across language regions or the urban-rural regions. In particular, GPs in the rural cantons of Jura (n = 7) and Uri (n = 2) reported insufficient provision of care facilities (fig. 3), while the regions of Geneva, North West Switzerland, Tessin and Zürich reported sufficient health care facilities.

Figure 3 Regional differences in the perception of the provision of care and support facilities. The median across all cantons of Switzerland is presented in the spatial plot (left), and across seven major Swiss regions in the boxplot (right). ++ = strongly agree with sufficient provision of care and support facilities, +/- partially agree, -- strongly disagree with sufficient provision of care and support facilities.
GPs stated that memory training (46.4%), vacation offers (43.4%), night care (43.0%) and day care (41.3%) were needed (see table S5, appendix 1). GPs who declared that the provision of care facilities was insufficient (n = 218) most often required day care (58.3) and memory training (58.3) as well as night care (50.9).
Discussion
This survey of GPs in Switzerland has revealed a very positive approach to dementia care, with more than 60% of respondents feeling confident in diagnosing dementia and in caring and supporting patients with dementia. Health services were considered sufficient; three-quarters of respondents stated that there was sufficient access to diagnostic services for referring patients with cognitive impairment, and half of respondents stated that the regional provision of care facilities was sufficient for patients with dementia.
Post-diagnostic management of individuals with mild dementia mostly consisted of counselling relatives, testing fitness-to-drive and minimising cardiovascular risk factors. However, we also detected some gaps in post-diagnostic support services for people with dementia and their carers, as well as in certain aspects of GPs’ confidence in the diagnosis and management of dementia care. In particular, up to one fifth of respondents did not feel confident with the pharmacological treatment of patients with dementia, one third felt insecure in coping with patients’ suicidal ideation and half of respondents did not feel confident managing patients with a migration background. Furthermore, one quarter stated that the provision of care facilities for patients with dementia was insufficient.
One of the core areas of the Swiss National Dementia Strategy [23] and a consensus conference on the diagnosis and treatment of patients with dementia in Switzerland [31] was to improve access to dementia diagnosis and to stress the importance of early detection to help prepare families for future challenges. Screening tests play a crucial role in assessing cognitive impairment in general practice. Currently, the Mini-Mental State Examination (MMSE [32];) is the most commonly used scale for the assessment of cognitive disorders. This is also the case in other European countries [33]. The case-finding ability of the MMSE is best when confirming a suspected diagnosis in specialist settings [34]. However, in general practice, the utility of MMSE in the detection of dementia has been challenged due to its false-positive rate of 86% when adopting the cut-off point of 26. More stringent cut-off points of 24 and 21 lead to some increase in the predictive value in this sample, resulting in false-positive rates of 78% and 59% respectively [35]. Furthermore, there is limited evidence for detecting mild cognitive impairment with the MMSE, with a sensitivity of 78.4% and a specificity of 87.8 [34]. Therefore, the MMSE should not be used in isolation to confirm or exclude dementia [33] or to identify MCI patients who could develop dementia [36]. The current findings showed that the majority of respondents used the MMSE in combination with the Clock Drawing Test to assess cognitive functioning. This procedure shows higher sensitivity and specificity in the detection of mild Alzheimer’s disease than using the MMSE or the Clock Drawing Test alone [37, 38].
For the detection of MCI, the use of both tests (MMSE and Clock Drawing Test) still shows poor clinical utility in general practice [37, 39]. An alternative method to assess cognitive impairment is the Montreal Cognitive Assessment (MoCA [40];) [41]. The MoCA provides additional items measuring executive functions [42]. Results from a meta-analysis indicated that the MoCA was the best tool for the detection of mild cognitive impairment among patients over 60 years of age when compared with the MMSE [41]. For screening of cognitive impairment in general practice, the MoCA threshold of 26 appeared optimal [43].
The current findings indicate that only 17% of the respondent GPs used this test when they had a clinical suspicion of cognitive impairment. One reason for the frequent use of the MMSE rather than another test to assess cognition might be due to the fact that health insurance companies in Switzerland suggest MMSE values to verify assumption of costs for anti-dementia treatment. In addition, MoCA normative data have only very recently become available for German-speaking Europe [44].
In 55% of European countries the diagnosis of dementia frequently occurred at a moderate stage, which is currently considered a late stage of diagnosis, when activities of daily living, relationships, behaviour and quality of life are already significantly challenged by cognitive decline [24, 45]. In contrast, most Swiss GPs reported a mild stage of dementia, with the MMSE ranging from 20-30 and difficulties in activities of daily living, at the moment of first diagnosis. This finding might be related to GPs’ collaboration with specialists such as memory clinics and neurologists for further diagnostic assessments and their access to diagnostic services. In Germany, recent results from a cross-sectional survey indicated that early recognition and dementia care management was highly appreciated by GPs, who considered it feasible or wanted it to be implemented in routine care [46]
A frank disclosure of the diagnosis of dementia helps the patient and his/her caregivers to cope with the situation and to develop a strategy for the future [47]. The diagnostic disclosure requires time, in particular so that the patients’ deficiencies and resources, the social situation and his/her biography can be understood. In this context, the currently reported average of 30 minutes for disclosing a diagnosis seems adequate and necessary. Furthermore, the majority of respondents reported disclosing the diagnosis to their patients, and also informing both their patient and his/her caregiver themselves. On the one hand, Australian GPs also preferred disclosure to the patient with his/her caregiver present due to issues of confidentiality and the importance of offering hope [10]. On the other hand, a German study found that most GPs described difficulties disclosing the diagnosis of dementia, and said that they were more likely to give the diagnosis to family members rather than to their patients, thereby avoiding the words ‘dementia’ or ‘Alzheimer’s’ in discussions [48].Thus, communicating the diagnosis to the patient and his/her caregiver may raise various ethical questions such as how to balance the different communicative needs of patients and their caregivers, clarity versus sensitivity in delivery of the diagnosis, and whether to minimise or expose interactional difficulties and misunderstanding to enrich patient understanding and involvement [49]. Consequently, GPs need training and guidance in delivering a diagnosis and in strategies to optimise patient and caregiver participation [49].
The demographic changes accompanying an aging population will increase the demand for services, while regional circumstances may hinder adequate access to health care services. An Irish study showed that rural GPs felt geographically disadvantaged when accessing diagnostic services, and that both rural and urban GPs experienced considerable time delays accessing specialist diagnostic services [50]. In the current study, we could not find systematic differences in the access to diagnostic services between rural and urban areas, but did find differences between language regions. French-speaking GPs felt significantly disadvantaged in their access to diagnostic services. In particular, rural cantons such as Valais (n = 19), Fribourg (n = 20) and Neuchâtel (n = 20) reported limited access (see fig. 1). Consequently, the access to diagnostic services should be improved in these regions.
We also found regional differences in the provision of care facilities. GPs in the cantons of Jura and Uri reported insufficient provision of care facilities (see fig, 3). These findings are of particular importance since rural areas are expected to experience accentuated aging due to the migration of young adults and the arrival of older people [51]. The major economic burden of dementia was found to be the costs of care [52], which is why certain rural cantons might lack cost-intensive care facilities. Our results suggest that broader structural changes are needed in these areas, including day and night care and memory training to better meet the needs of the elderly with dementia and their families
GPs’ post-diagnostic strategies and interventions in the early stages of dementia revealed a focus on non-pharmacological interventions including assessing fitness-to-drive, caregiver counselling and interventions to minimise cardiovascular risk factors. On the one hand, testing fitness-to-drive at the moment of diagnosis may also be a reverse cause mechanism: cognitive screening is often undertaken at the time of the fitness-to-drive assessment and reveals the dementia. On the other hand, the focus on assessing fitness-to-drive is important since individuals with mild dementia may have limited executive functions, which may affect their driving aptitude [53]. However, mild dementia does not a priori exclude the driving capability, as opposed to the case of moderate dementia, with no further driving capability [53, 54]. Furthermore, testing the driving capability entails certain challenges such as concerns about damaging the patient-physician relationship and uncertainties about the GP's own legal role [55]. In fact, almost half of the physicians in our survey would welcome the option to delegate the assessment of fitness-to-drive to an official authority. Nevertheless, Pentzek and colleagues (2015) [55] recommended raising the issue of driving in patients with dementia, since it is a prerequisite for resource-oriented and patient-centred management.
The current study indicated that almost one fifth of GPs felt unconfident with pharmacological treatment of their patients and that symptomatic pharmacological treatment was among the least frequently implemented post-diagnostic actions in the case of mild dementia diagnosis. The currently reported confidence levels in Swiss GPs align with the confidence levels of German GPs [25]. GPs might be insecure about the indication of and justification for prescribing quite expensive drugs (except Gingko biloba) with a rather low level of clinical effectiveness. In mild to moderate Alzheimer's disease, results have been equivocal and no disease modifying agents are either licensed or can be currently recommended for clinical use [56]. France has very recently stopped reimbursing anti-dementia drugs, which is also in line with a recent systematic review funded by the Agency for Healthcare Research and Quality [57], which does not support pharmacological treatment. Another possible explanation for the current findings of rather low anti-dementia drug prescription by Swiss GPs might be based on the potential negative implications of polypharmacy and the side-effects for patient safety. A recent Danish study indicated that people with dementia were more frequently exposed to polypharmacy, as well as to potentially inappropriate medication [58]. Furthermore, a systematic review of a risk-benefit assessment of dementia medications indicted that cholinergic side effects were clinically significant and could be especially detrimental in the frail elderly population in which the risks of treatment outweigh the benefits [59].
The current study found that GPs felt less confident in managing patients with a migration background. A Dutch study also described obstacles to the diagnosis of dementia in non-western, elderly migrants in memory clinics [60]. The authors indicated that memory clinics were not well prepared for non-western, elderly migrant patients. One problem was that health professionals lacked knowledge about important obstacles in intercultural dementia diagnostics such as language barriers, cultural differences, low level of education and illiteracy, ignorance about dementia, shame, and special care expectations of patients and their families [60]. In Norway, there is evidence that immigrants receive less dementia diagnosis and treatment than Norwegians, indicating a lack of cultural validity of the assessment tools, linguistic barriers and challenges for general practitioners due to the migration background of their patients [61]. Furthermore, studies of help-seeking among various ethnic groups in the US have reported that many do not prioritise dementia as a health issue, given more pressing concerns [62]. Consequently, GP training should consider issues related to migration background as described above.
Almost one third of respondents felt unconfident handling suicidal ideation of patients with early dementia. Despite the fact that risk of suicide in patients with dementia is generally low, suicide is an important issue in dementia. A recent review found that Alzheimer’s dementia was associated with a moderate risk of suicide, even many years after the diagnosis of dementia [63]. Risk factors for suicide are hopelessness, depression, mild cognitive impairment, preserved insight, younger age, male gender, highly educated professional status, limitations in activities of daily living, economic stress, functional decline, and lack of social support and a positive history of prior suicide attempts [63–66]. Consequently, it was suggested that physicians needed to consider the potential for suicide in vulnerable individuals, particularly early in the dementia course [63, 64], and emotional needs, especially in patients with young onset Alzheimer’s disease [67]. According to Swiss law, assisting suicide without any self-interest is legal [68]. Assisted suicide requires an attestation from a specialist physician which attests the ability to judge. Since psychiatrists usually refuse to attest the ability to judge for such purposes, GPs remain alienated as to how to cope with their patients’ suicidal ideation in the case of early dementia.
A limitation of this study was the response rate of 21%. We chose a relatively time- and cost-effective method. In view of this simple strategy, the length of the questionnaire (seven pages and a moderate total respondent burden of 10-15 minutes for the completion of the questionnaire), and other barriers to high response rates [69–71], participation was low. The current response rate is comparable with a randomised study conducted in Western Switzerland and in France, which compared GPs' response rates to a postal versus a web-based survey, and showed a response rate of 22.47% (95% CI 21.07–23.87%) for the postal survey [72]. Although there is evidence that the preferred mode of survey administration is postal [73], response rates are generally low in general practice [74]. Therefore, representative conclusions cannot be made for the current study. Nevertheless, the respondents did not differ from non-respondents in terms of gender and age [75]. However, they did differ in terms of language region, indicating a selection bias towards German-speaking GPs. We cannot exclude the possibility of confounding or alternative explanations for our results, since the survey responses show subjective attitudes and not objective performance. In particular, we cannot exclude the possibility that more GPs participated who were interested in or who were particularly affected by the issue of dementia, and who felt there was a need for such research. Furthermore, social desirability can induce an over-reporting of positive attitudes in reply to questions related to professional standards and behaviour. However, if the confidence in the management of dementia patients with migration background or with suicide ideation is already low in a very motivated sample, we would expect them to be even lower in a sample including less motivated physicians with less interest in the topic. Therefore, despite the low response rate, our findings that GPs lack confidence in coping with suicidal ideation and with patients with a migration background, suggesting a particular need to support less experienced GPs, remains valid. A further limitation is that we only assessed the attitudes of GPs on early dementia recognition, omitting the patients’ and relatives’ views on this topic. However, we believe that GPs play a crucial role in designing and putting into practice health services and health policy to increase the health outcomes of their patients. Understanding and assessing GPs’ knowledge, attitudes, behaviours, practices and their views on solutions to health care issues is vital to improving the quality of health care. Nevertheless, our aim for future research is to determine the attitudes of patients with mild dementia and their relatives towards the disclosure of diagnoses of dementia and to explore their needs and experiences regarding dementia management in general practice. A strength of this study was the quantitative assessment of a wide range of GPs’ practice patterns for the diagnosis, disclosure and post-diagnostic management of dementia.
Our study identified gaps in Swiss GPs’ dementia practice concerning dementia diagnostic assessment. GPs further described difficulties in managing suicidal ideation and dementia patients with a migration background. Moreover, regional variations in health care provision were detected. Our findings may enable the definition of policy priorities to provide training and information, as well as to establish access to diagnostic services and provision of care and support facilities.
Appendix 1 Supplementary tables
Table S1 Frequency of statements about diagnostic assessment and disclosure.
|
Statements
|
Frequent
n (%)
|
50% of cases
n (%)
|
Not frequent
n (%)
|
Mdn
|
IQR
|
| Reasons for early diagnostic assessment of cognitive decline: |
|
|
|
|
|
| Relatives report cognitive impairment or behavioural problems |
490(57) |
205(24) |
172(19) |
75% |
50‒75% |
| Screening test |
64(8) |
105(13) |
635(79) |
25% |
0‒25% |
| Patient complains about cognitive decline |
420(49) |
185(21) |
262(30) |
50% |
25‒100% |
| GP detects cognitive decline |
378(45) |
191(23) |
278(33) |
50% |
25–75% |
| Official occasion (e.g., test fitness-to-drive, establishing the last will, advance directive, etc.) |
276(33) |
123(15) |
440(52) |
25% |
0–50% |
| GP discloses him/herself the dementia diagnosis to the patient |
654(74) |
148(17) |
77(9) |
75% |
50–100% |
Table S2 Agreement with statements about confidence in different situations of managing patients with dementia.
|
Statements
|
Agree
n (%)
|
Partially agree
n (%)
|
Disagree
n (%)
|
Mdn
|
IQR
|
| I feel confident in the provision of care for patients with cognitive impairment |
573 (65) |
245 (28) |
60 (7) |
4 |
3–4 |
| I feel confident in the early diagnostic assessment of patients with cognitive impairment. |
561 (64) |
228 (26) |
91 (10) |
4 |
2‒3 |
| I feel confident in the pharmacological treatment of patients with cognitive impairment. |
467 (53) |
252 (29) |
157 (18) |
4 |
3‒4 |
| I would like to delegate the assessment of fitness-to-drive of patients with cognitive impairment to an official authority. |
421 (48) |
137 (16) |
317 (36) |
3 |
2–5 |
| Suicide ideation of patients with cognitive impairment make me insecure |
264 (30) |
225 (26) |
384 (44) |
3 |
2–4 |
| I feel confident in caring and supporting for patients with dementia with migration background |
135 (16) |
302 (35) |
415 (49) |
3 |
3–4 |
| I feel confident in the early diagnostic assessment of patients with cognitive impairment and migration background |
122 (15) |
285 (33) |
450 (52) |
2 |
2–3 |
Table S3 Agreement with statements about regional provision with health care facilities.
|
Statements
|
Agree
n (%)
|
Part-ially agree
n (%)
|
Disagree
n (%)
|
Mdn
|
IQR
|
| The access to diagnostic services for referring patients with cognitive impairment is sufficient. |
644 (74) |
120 (14) |
112 (13) |
4 |
3–5 |
| In my catchment area, the provision with care facilities is insufficient for patients with cognitive impairment. |
218 (25) |
207 (24) |
449 (51) |
2 |
2–3 |
Table S4 Frequency of strategies/ interventions after a diagnosis of mild dementia.
|
Strategies
|
Frequent
n (%)
|
50% of cases
n (%)
|
Not frequent
n (%)
|
Mdn
|
IQR
|
| Counselling of relatives |
613(71) |
159(18) |
92(11) |
75 |
50–100 |
| Assessment of driving aptitudes |
555(66) |
131(16) |
153(18) |
75 |
50–100 |
| Measures to minimise cardiovascular risks |
528(63) |
156(19) |
149(18) |
75 |
50–100 |
| Prepare advanced directives or to designate a power of trustee |
386(46) |
202(24) |
259(31) |
50 |
25–75 |
| Memory training activities |
351(42) |
166(20) |
321(38) |
50 |
25–75 |
| Prescription of Ginkgo biloba |
308(36) |
163(19) |
381(45) |
50 |
25–75 |
| Refer patient to counselling centre e.g. Alzheimer's association |
296(35) |
226(27) |
325(38) |
50 |
25–75 |
| Pharmacological mono therapy (i.e., acetylcholinesterase inhibitors or memantine) |
235(29) |
209(25) |
388(47) |
50 |
25–75 |
| Other non-pharmacological treatments (involving music-, painting, dancing, coaching strategies or conversations to cope with the situation) |
174(21) |
147(18) |
510(61) |
25 |
0–50 |
| Wait-and-see strategy with no intervention |
161(21) |
167(21) |
464(58) |
25 |
25–50 |
| Home visits to assess specific living conditions |
91(12) |
124(17) |
526(71) |
25 |
0–50 |
| Pharmacological combination therapy (i.e. acetylcholinesterase inhibitors and memantine) |
21(3) |
41(5) |
710(92) |
0 |
0–25 |
Table S5 Most required care facilities for patients with cognitive impairment in the catchment area.
|
Item
|
From total
(n = 882)
|
From GPs not satisfied with care facilities
(n = 218)
|
|
n
|
%
|
n
|
%
|
| Memory or activation therapy |
409 |
46.37 |
127 |
58.26 |
| Holidays for people with dementia |
383 |
43.42 |
100 |
45.87 |
| Night care centre |
379 |
42.97 |
111 |
50.92 |
| Day care centre |
364 |
41.27 |
127 |
58.26 |
| Voluntary aids |
322 |
36.51 |
102 |
46.79 |
| Day and night care centre |
238 |
26.98 |
87 |
39.91 |
Acknowledgments
We wish to thank all participating GPs for their contribution to this study and the collaborating institutes of general practice in Switzerland, in particular Dagmar M. Haller, Christoph Merlo and Thomas Rosemann for their support.
References
1
Iliffe
S
,
Robinson
L
,
Brayne
C
,
Goodman
C
,
Rait
G
,
Manthorpe
J
, et al.; DeNDRoN Primary Care Clinical Studies Group. Primary care and dementia: 1. diagnosis, screening and disclosure. Int J Geriatr Psychiatry. 2009;24(9):895–901. doi:.https://doi.org/10.1002/gps.2204
2
Koch
T
,
Iliffe
S
; EVIDEM-ED project. Rapid appraisal of barriers to the diagnosis and management of patients with dementia in primary care: a systematic review. BMC Fam Pract. 2010;11(1):52. doi:.https://doi.org/10.1186/1471-2296-11-52
3
Lang
L
,
Clifford
A
,
Wei
L
,
Zhang
D
,
Leung
D
,
Augustine
G
, et al.
Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta-analysis. BMJ Open. 2017;7(2):e011146. doi:.https://doi.org/10.1136/bmjopen-2016-011146
4
Joray
S
,
Pertoldi
W
,
Büla
C
. [Detection of cognitive disorders in clinical practice]. Rev Med Suisse Romande. 2000;120(11):847–52. Article in French.
5
Petrazzuoli
F
,
Vinker
S
,
Koskela
TH
,
Frese
T
,
Buono
N
,
Soler
JK
, et al.
Exploring dementia management attitudes in primary care: a key informant survey to primary care physicians in 25 European countries. Int Psychogeriatr. 2017;29(9):1413–23. doi:.https://doi.org/10.1017/S1041610217000552
6
Caruana-Pulpan
O
,
Scerri
C
. Practices in diagnosis, disclosure and pharmacotherapeutic management of dementia by general practitioners--a national survey. Aging Ment Health. 2014;18(2):179–86. doi:.https://doi.org/10.1080/13607863.2013.819833
7
Viret
O
,
Schwarz
J
,
Senn
N
,
Mueller
Y
. [« It’s normal to get old and weak » : should functional decline be addressed with elderly patients in family medicine? ]. Rev Med Suisse. 2018;14(606):971–5. Article in French.
8
Moore
V
,
Cahill
S
. Diagnosis and disclosure of dementia--a comparative qualitative study of Irish and Swedish General Practitioners. Aging Ment Health. 2013;17(1):77–84. doi:.https://doi.org/10.1080/13607863.2012.692763
9
Cantegreil-Kallen
I
,
Turbelin
C
,
Olaya
E
,
Blanchon
T
,
Moulin
F
,
Rigaud
AS
, et al.
Disclosure of diagnosis of Alzheimer’s disease in French general practice. Am J Alzheimers Dis Other Demen. 2005;20(4):228–32. doi:.https://doi.org/10.1177/153331750502000404
10
Phillips
J
,
Pond
CD
,
Paterson
NE
,
Howell
C
,
Shell
A
,
Stocks
NP
, et al.
Difficulties in disclosing the diagnosis of dementia: a qualitative study in general practice. Br J Gen Pract. 2012;62(601):e546–53. doi:.https://doi.org/10.3399/bjgp12X653598
11
Dyer
AH
,
Foley
T
,
O’Shea
B
,
Kennelly
SP
. Cognitive assessment of older adults in general practice: the collateral history. Ir J Med Sci. 2018;187(3):683–7. doi:.https://doi.org/10.1007/s11845-017-1723-8
12
Briggs
R
,
O’Neill
D
. The informant history: a neglected aspect of clinical education and practice. QJM. 2016;109(5):301–2. doi:.https://doi.org/10.1093/qjmed/hcv145
13
Dyer
AH
,
Nabeel
S
,
Briggs
R
,
O’Neill
D
,
Kennelly
SP
. Cognitive assessment of older adults at the acute care interface: the informant history. Postgrad Med J. 2016;92(1087):255–9. doi:.https://doi.org/10.1136/postgradmedj-2015-133768
14
Cahill
S
,
Clark
M
,
Walsh
C
,
O’Connell
H
,
Lawlor
B
. Dementia in primary care: the first survey of Irish general practitioners. Int J Geriatr Psychiatry. 2006;21(4):319–24. doi:.https://doi.org/10.1002/gps.1464
15
Downs
MG
. The role of general practice and the primary care team in dementia diagnosis and management. Int J Geriatr Psychiatry. 1996;11(11):937–42. doi:.https://doi.org/10.1002/(SICI)1099-1166(199611)11:11<937::AID-GPS540>3.0.CO;2-0
16
van Hout
H
,
Vernooij-Dassen
M
,
Bakker
K
,
Blom
M
,
Grol
R
. General practitioners on dementia: tasks, practices and obstacles. Patient Educ Couns. 2000;39(2-3):219–25. doi:.https://doi.org/10.1016/S0738-3991(99)00034-8
17
Pentzek
M
,
Vollmar
HC
,
Wilm
S
,
Leve
V
. Frühwahrnehmung von Demenzen in der Hausarztpraxis [Putting dementia awareness into general practice : The CADIF approach]. Z Gerontol Geriatr. 2017;50(S2, Suppl 2):44–7. Article in German. doi:.https://doi.org/10.1007/s00391-017-1206-6
18
Woods
RT
,
Moniz-Cook
E
,
Iliffe
S
,
Campion
P
,
Vernooij-Dassen
M
,
Zanetti
O
, et al.; INTERDEM (Early Detection and Intervention in Dementia) Group. Dementia: issues in early recognition and intervention in primary care. J R Soc Med. 2003;96(7):320–4. doi:.https://doi.org/10.1177/014107680309600703
19
Iliffe
S
,
Walters
K
,
Rait
G
. Shortcomings in the diagnosis and management of dementia in primary care: towards an educational strategy. Aging Ment Health. 2000;4(4):286–91. doi:.https://doi.org/10.1080/713649957
20
Hinton
L
,
Franz
CE
,
Reddy
G
,
Flores
Y
,
Kravitz
RL
,
Barker
JC
. Practice constraints, behavioral problems, and dementia care: primary care physicians’ perspectives. J Gen Intern Med. 2007;22(11):1487–92. doi:.https://doi.org/10.1007/s11606-007-0317-y
21
Belmin
J
,
Min
L
,
Roth
C
,
Reuben
D
,
Wenger
N
. Assessment and management of patients with cognitive impairment and dementia in primary care. J Nutr Health Aging. 2012;16(5):462–7. doi:.https://doi.org/10.1007/s12603-012-0026-z
22
Turner
S
,
Iliffe
S
,
Downs
M
,
Wilcock
J
,
Bryans
M
,
Levin
E
, et al.
General practitioners’ knowledge, confidence and attitudes in the diagnosis and management of dementia. Age Ageing. 2004;33(5):461–7. doi:.https://doi.org/10.1093/ageing/afh140
23Federal Office of Public Health, Swiss Conference of Cantonal Health Directors. Nationale Demenzstrategie 2014-2017 http://www.bag.admin.ch/themen/gesundheitspolitik/13916/: Bundesamt für Gesundheit; 2013 [03.12.2015].
24ALCOVE Project. The European Joint Action on Dementia. Synthesis Report 2013. ALzheimer COoperative Valuation in Europe (ALCOVE) 2013 [cited 2016 17.11.]. Available from: http://www.alcoveproject.eu/images/pdf/ALCOVE SYNTHESIS REPORTVF.pdf.
25
Pentzek
M
,
Abholz
HH
,
Ostapczuk
M
,
Altiner
A
,
Wollny
A
,
Fuchs
A
. Dementia knowledge among general practitioners: first results and psychometric properties of a new instrument. Int Psychogeriatr. 2009;21(6):1105–15. doi:.https://doi.org/10.1017/S1041610209990500
26
Ahmad
S
,
Orrell
M
,
Iliffe
S
,
Gracie
A
. GPs’ attitudes, awareness, and practice regarding early diagnosis of dementia. Br J Gen Pract. 2010;60(578):e360–5. doi:.https://doi.org/10.3399/bjgp10X515386
27
Schenk
L
,
Bau
A-M
,
Borde
T
,
Butler
J
,
Lampert
T
,
Neuhauser
H
, et al.
Mindestindikatorensatz zur Erfassung des Migrationsstatus. Empfehlungen für die epidemiologische Praxis [A basic set of indicators for mapping migrant status. Recommendations for epidemiological practice]. Bundesgesundheitsblatt. 2006;49(9):853–60. Article in German. doi:.https://doi.org/10.1007/s00103-006-0018-4
28
Schenk
L
. Migration und Gesundheit--Entwicklung eines Erklärungs- und Analysemodells für epidemiologische Studien [Migration and health--developing an explanatory and analytical model for epidemiological studies]. Int J Public Health. 2007;52(2):87–96. Article in German. doi:.https://doi.org/10.1007/s00038-007-6002-4
29R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria2015.
30Federal Statistical Office. Die sieben Grossregionen der Schweiz. Die Schweiz im europäischen Regionalsystem https://www.bfs.admin.ch/bfs/en/home/statistics/catalogues-databases/press-releases.assetdetail.11611.html1999 [cited 2018 14.06.].
31
Monsch
AU
,
Büla
C
,
Hermelink
M
,
Kressig
RW
,
Martensson
B
,
Mosimann
U
, et al.
Konsensus 2012 zur Diagnostik und Therapie von Demenzkranken in der Schweiz. Praxis (Bern). 2012;101(19):1239–49. doi:.https://doi.org/10.1024/1661-8157/a001085
32
Folstein
MF
,
Folstein
SE
,
McHugh
PR
. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi:.https://doi.org/10.1016/0022-3956(75)90026-6
33
Creavin
ST
,
Wisniewski
S
,
Noel-Storr
AH
,
Trevelyan
CM
,
Hampton
T
,
Rayment
D
, et al.
Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst Rev. 2016;(1):CD011145. doi:.https://doi.org/10.1002/14651858.CD011145.pub2
34
Mitchell
AJ
. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43(4):411–31. doi:.https://doi.org/10.1016/j.jpsychires.2008.04.014
35
White
N
,
Scott
A
,
Woods
RT
,
Wenger
GC
,
Keady
JD
,
Devakumar
M
. The limited utility of the Mini-Mental State Examination in screening people over the age of 75 years for dementia in primary care. Br J Gen Pract. 2002;52(485):1002–3.
36
Arevalo-Rodriguez
I
,
Smailagic
N
,
Roqué I Figuls
M
,
Ciapponi
A
,
Sanchez-Perez
E
,
Giannakou
A
, et al.
Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2015;(3):CD010783. doi:.https://doi.org/10.1002/14651858.CD010783.pub2
37
Cacho
J
,
Benito-León
J
,
García-García
R
,
Fernández-Calvo
B
,
Vicente-Villardón
JL
,
Mitchell
AJ
. Does the combination of the MMSE and clock drawing test (mini-clock) improve the detection of mild Alzheimer’s disease and mild cognitive impairment?
J Alzheimers Dis. 2010;22(3):889–96. doi:.https://doi.org/10.3233/JAD-2010-101182
38
Thalmann
B
,
Spiegel
R
,
Stähelin
HB
,
Brubacher
D
,
Ermini-Fünfschilling
D
,
Bläsi
S
, et al.
Dementia screening in general practice: optimised scoring for the clock drawing test. Brain Aging. 2002;2:36–43.
39
Carnero-Pardo
C
,
Cruz-Orduña
I
,
Espejo-Martínez
B
,
Martos-Aparicio
C
,
López-Alcalde
S
,
Olazarán
J
. Utility of the mini-cog for detection of cognitive impairment in primary care: data from two spanish studies. Int J Alzheimers Dis. 2013;2013:285462. doi:.https://doi.org/10.1155/2013/285462
40
Nasreddine
ZS
,
Phillips
NA
,
Bédirian
V
,
Charbonneau
S
,
Whitehead
V
,
Collin
I
, et al.
The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. doi:.https://doi.org/10.1111/j.1532-5415.2005.53221.x
41
Ciesielska
N
,
Sokołowski
R
,
Mazur
E
,
Podhorecka
M
,
Polak-Szabela
A
,
Kędziora-Kornatowska
K
. Czy test Montreal Cognitive Assessment (MoCA) może być skuteczniejszy od powszechnie stosowanego Mini-Mental State Examination (MMSE) w wykrywaniu łagodnych zaburzeń funkcji poznawczych u osób po 60. roku życia? [Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis]. Psychiatr Pol. 2016;50(5):1039–52. Article in English, Polish. doi:.https://doi.org/10.12740/PP/45368
42
Van Heugten
CM
,
Walton
L
,
Hentschel
U
. Can we forget the Mini-Mental State Examination? A systematic review of the validity of cognitive screening instruments within one month after stroke. Clin Rehabil. 2015;29(7):694–704. doi:.https://doi.org/10.1177/0269215514553012
43
Damian
AM
,
Jacobson
SA
,
Hentz
JG
,
Belden
CM
,
Shill
HA
,
Sabbagh
MN
, et al.
The Montreal Cognitive Assessment and the mini-mental state examination as screening instruments for cognitive impairment: item analyses and threshold scores. Dement Geriatr Cogn Disord. 2011;31(2):126–31. doi:.https://doi.org/10.1159/000323867
44
Thomann
AE
,
Goettel
N
,
Monsch
RJ
,
Berres
M
,
Jahn
T
,
Steiner
LA
, et al.
The Montreal Cognitive Assessment: Normative Data from a German-Speaking Cohort and Comparison with International Normative Samples. J Alzheimers Dis. 2018;64(2):643–55. doi:.https://doi.org/10.3233/JAD-180080
45
Brooker
D
,
La Fontaine
J
,
Evans
S
,
Bray
J
,
Saad
K
. Public health guidance to facilitate timely diagnosis of dementia: ALzheimer’s COoperative Valuation in Europe recommendations. Int J Geriatr Psychiatry. 2014;29(7):682–93. doi:.https://doi.org/10.1002/gps.4066
46
Thyrian
JR
,
Eichler
T
,
Pooch
A
,
Albuerne
K
,
Dreier
A
,
Michalowsky
B
, et al.
Systematic, early identification of dementia and dementia care management are highly appreciated by general physicians in primary care - results within a cluster-randomized-controlled trial (DelpHi). J Multidiscip Healthc. 2016;9:183–90. doi:.https://doi.org/10.2147/JMDH.S96055
47
Bopp-Kistler
I
. Diagnoseeröffnung und Begleitung [Disclosing the diagnosis and guidance]. Ther Umsch. 2015;72(4):225–31. Article in German. doi:.https://doi.org/10.1024/0040-5930/a000669
48
Kaduszkiewicz
H
,
Bachmann
C
,
van den Bussche
H
. Telling “the truth” in dementia--do attitude and approach of general practitioners and specialists differ?
Patient Educ Couns. 2008;70(2):220–6. doi:.https://doi.org/10.1016/j.pec.2007.10.010
49
Dooley
J
,
Bailey
C
,
McCabe
R
. Communication in healthcare interactions in dementia: a systematic review of observational studies. Int Psychogeriatr. 2015;27(8):1277–300. doi:.https://doi.org/10.1017/S1041610214002890
50
Cahill
S
,
Clark
M
,
O’Connell
H
,
Lawlor
B
,
Coen
RF
,
Walsh
C
. The attitudes and practices of general practitioners regarding dementia diagnosis in Ireland. Int J Geriatr Psychiatry. 2008;23(7):663–9. doi:.https://doi.org/10.1002/gps.1956
51Federal Statistical Office. Szenarien zur Bevölkerungsentwicklung der Kantone 2015–2045. Neuchâtel, Switzerland: 2016.
52
Kraft
E
,
Marti
M
,
Werner
S
,
Sommer
H
. Cost of dementia in Switzerland. Swiss Med Wkly. 2010;140:w13093. doi:.https://doi.org/10.4414/smw.2010.13093
53
Seeger
R
. Fahreignung bei Demenz-Erkrankungen [Automobile driving capacity in dementia]. Ther Umsch. 2015;72(4):239–45. Article in German. doi:.https://doi.org/10.1024/0040-5930/a000671
54
Mosimann
UP
,
Bächli-Biétry
J
,
Boll
J
,
Bopp-Kistler
I
,
Donati
F
,
Kressig
RW
, et al.
Konsensusempfehlungen zur Beurteilung der medizinischen Mindestanforderungen für Fahreignung bei kognitiver Beeinträchtigung. Praxis (Bern). 2012;101(7):451–64. doi:.https://doi.org/10.1024/1661-8157/a000893
55
Pentzek
M
,
Michel
JV
,
Ufert
M
,
Vollmar
HC
,
Wilm
S
,
Leve
V
. Fahrtauglichkeit bei Demenz – Theoretische Rahmung und Konzept einer Vorgehensempfehlung für die Hausarztpraxis [Fitness to drive in dementia - theoretical framing and design of a recommendation for German general practice]. Z Evid Fortbild Qual Gesundhwes. 2015;109(2):115–23. Article in German. doi:.https://doi.org/10.1016/j.zefq.2015.03.005
56
O’Brien
JT
,
Holmes
C
,
Jones
M
,
Jones
R
,
Livingston
G
,
McKeith
I
, et al.
Clinical practice with anti-dementia drugs: A revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2017;31(2):147–68. doi:.https://doi.org/10.1177/0269881116680924
57
Fink
HA
,
Jutkowitz
E
,
McCarten
JR
,
Hemmy
LS
,
Butler
M
,
Davila
H
, et al.
Pharmacologic Interventions to Prevent Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer-Type Dementia: A Systematic Review. Ann Intern Med. 2018;168(1):39–51. doi:.https://doi.org/10.7326/M17-1529
58
Kristensen
RU
,
Nørgaard
A
,
Jensen-Dahm
C
,
Gasse
C
,
Wimberley
T
,
Waldemar
G
. Polypharmacy and Potentially Inappropriate Medication in People with Dementia: A Nationwide Study. J Alzheimers Dis. 2018;63(1):383–94. doi:.https://doi.org/10.3233/JAD-170905
59
Buckley
JS
,
Salpeter
SR
. A Risk-Benefit Assessment of Dementia Medications: Systematic Review of the Evidence. Drugs Aging. 2015;32(6):453–67. doi:.https://doi.org/10.1007/s40266-015-0266-9
60
Goudsmit
M
,
Parlevliet
JL
,
van Campen
JP
,
Schmand
B
. Dementiediagnostiek bij oudere migranten op de geheugenpolikliniek: obstakels en oplossingen [Diagnosis of dementia in non-western elderly migrants in memory clinics: obstacles and solutions]. Tijdschr Gerontol Geriatr. 2011;42(5):204–14. Article in dutch. doi:.https://doi.org/10.1007/s12439-011-0036-z
61
Diaz
E
,
Kumar
BN
,
Engedal
K
. Immigrant patients with dementia and memory impairment in primary health care in Norway: a national registry study. Dement Geriatr Cogn Disord. 2015;39(5-6):321–31. doi:.https://doi.org/10.1159/000375526
62
Daker-White
G
,
Beattie
AM
,
Gilliard
J
,
Means
R
. Minority ethnic groups in dementia care: a review of service needs, service provision and models of good practice. Aging Ment Health. 2002;6(2):101–8. doi:.https://doi.org/10.1080/13607860220126835
63
Serafini
G
,
Calcagno
P
,
Lester
D
,
Girardi
P
,
Amore
M
,
Pompili
M
. Suicide Risk in Alzheimer’s Disease: A Systematic Review. Curr Alzheimer Res. 2016;13(10):1083–99. doi:.https://doi.org/10.2174/1567205013666160720112608
64
Draper
B
,
Peisah
C
,
Snowdon
J
,
Brodaty
H
. Early dementia diagnosis and the risk of suicide and euthanasia. Alzheimers Dement. 2010;6(1):75–82. doi:.https://doi.org/10.1016/j.jalz.2009.04.1229
65
de Beaufort
ID
,
van de Vathorst
S
. Dementia and assisted suicide and euthanasia. J Neurol. 2016;263(7):1463–7. doi:.https://doi.org/10.1007/s00415-016-8095-2
66
Haw
C
,
Harwood
D
,
Hawton
K
. Dementia and suicidal behavior: a review of the literature. Int Psychogeriatr. 2009;21(3):440–53. doi:.https://doi.org/10.1017/S1041610209009065
67
Baptista
MAT
,
Santos
RL
,
Kimura
N
,
Lacerda
IB
,
Dourado
MCN
. Disease awareness may increase risk of suicide in young onset dementia: A case report. Dement Neuropsychol. 2017;11(3):308–11. doi:.https://doi.org/10.1590/1980-57642016dn11-030015
68
Bosshard
G
,
Jermini
D
,
Eisenhart
D
,
Bär
W
. Assisted suicide bordering on active euthanasia. Int J Legal Med. 2003;117(2):106–8. doi:.https://doi.org/10.1007/s00414-002-0346-3
69
Stocks
N
,
Braunack-Mayer
A
,
Somerset
M
,
Gunnell
D
. Binners, fillers and filers--a qualitative study of GPs who don’t return postal questionnaires. Eur J Gen Pract. 2004;10(4):146–51. doi:.https://doi.org/10.3109/13814780409044302
70
Edwards
P
,
Roberts
I
,
Clarke
M
,
DiGuiseppi
C
,
Pratap
S
,
Wentz
R
, et al.
Increasing response rates to postal questionnaires: systematic review. BMJ. 2002;324(7347):1183. doi:.https://doi.org/10.1136/bmj.324.7347.1183
71
Kaner
EF
,
Haighton
CA
,
McAvoy
BR
. ‘So much post, so busy with practice--so, no time!’: a telephone survey of general practitioners’ reasons for not participating in postal questionnaire surveys. Br J Gen Pract. 1998;48(428):1067–9.
72
Sebo
P
,
Maisonneuve
H
,
Cerutti
B
,
Fournier
JP
,
Senn
N
,
Haller
DM
. Rates, Delays, and Completeness of General Practitioners’ Responses to a Postal Versus Web-Based Survey: A Randomized Trial. J Med Internet Res. 2017;19(3):e83. doi:.https://doi.org/10.2196/jmir.6308
73
Bonevski
B
,
Magin
P
,
Horton
G
,
Foster
M
,
Girgis
A
. Response rates in GP surveys - trialling two recruitment strategies. Aust Fam Physician. 2011;40(6):427–30.
74
Creavin
ST
,
Creavin
AL
,
Mallen
CD
. Do GPs respond to postal questionnaire surveys? A comprehensive review of primary care literature. Fam Pract. 2011;28(4):461–7. doi:.https://doi.org/10.1093/fampra/cmr001
75
Hostettler
S
,
Kraft
E
. FMH-Ärztestatistik 2017 – aktuelle Zahlen. Schweiz Arzteztg. 2018;99(1314):408–13. doi:].https://doi.org/10.4414/saez.2018.06573