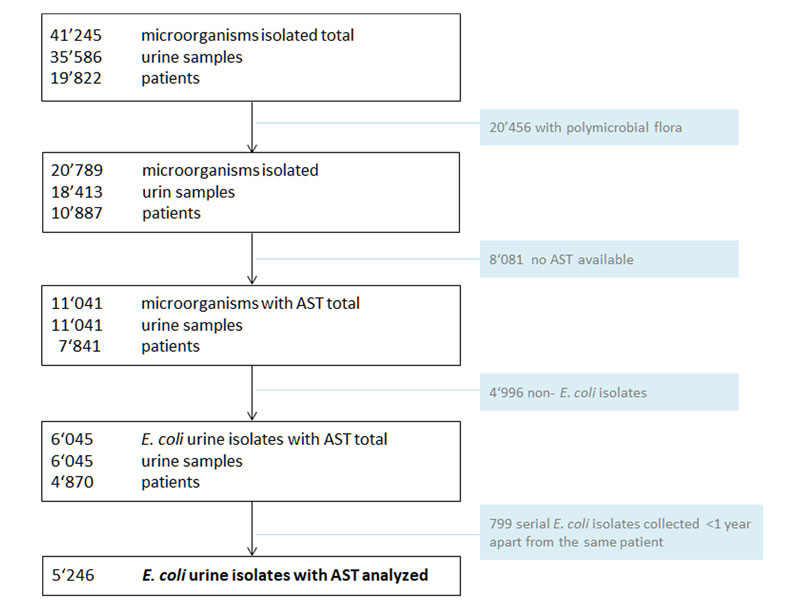
Figure 1 Study overview.
AST = antimicrobial susceptibility testing
Polymicrobial flora: >2 pathogens without a predominant microorganism
Urine samples submitted from the dialysis and hematological outpatient unit were excluded.
DOI: https://doi.org/10.4414/smw.2018.14660
Urinary tract infections (UTIs) are very common both in the community and healthcare settings. The most common pathogen isolated is Escherichia coli: it accounts for 70–90% of uncomplicated and 50–60% of recurrent or complicated infections [1, 2]. Appropriate empirical antibiotic treatment of UTIs is important for successful outcome and preventing complications. However, antimicrobial resistance to antibiotics commonly used against E. coli has emerged worldwide and has converted UTIs into infectious diseases challenging to treat [3–5].
The recently published US and European guidelines recommend empirical treatment of UTIs based on ongoing surveillance of local resistance rates of uropathogens [6]. However, common surveillance networks provide cumulative resistance rates, which give only a rough estimate of the local resistance situation and may not predict E. coli resistance patterns on different wards of an institution or even on an individual level. Only a few studies have investigated the role of demographic and host-related factors in colonisation or infection with resistant urinary tract pathogens, and these have had inconclusive results [3, 7–11].
The aim of our study was to determine bedside-available patient- and institution-related risk factors impacting on antimicrobial resistance of E. coli urine isolates in Switzerland to improve appropriate empirical antibiotic treatment of UTIs.
The study was approved by the local ethics committee as part of the continuous quality improvement programme.
The University Hospital Basel is a 865-bed tertiary academic care centre in Switzerland with an average of 38,000 hospital admissions per year and around 90,000 urine samples processed at the Clinical Microbiology Laboratory in the years 2012 to 2015.
For our study, all consecutive urine samples from in- and outpatients collected at the University Hospital Basel from 1 January 2012 until 31 May 2015 with growth of E. coli (≥103 colony forming units [CFU]/ml) and antimicrobial susceptibility testing were included. Urine samples came from patients with asymptomatic bacteriuria as well as from patients with UTIs.
Serial urine samples from the same patient within 1 year, samples from patients in the dialysis and haematological outpatient unit, from paediatric patients aged <15 years, samples with polymicrobial flora (>2 pathogens without a dominant microorganism) and specimens from external clinics were excluded from the study.
Urine cultures were performed according to standard laboratory procedures [12]. The antimicrobial susceptibility was tested using Vitek 2 automated system (bioMérieux) or Etest (bioMérieux). Non-susceptible, in the following termed “resistant”, was defined as being resistant or intermediate according to EUCAST clinical breakpoints version 2.0-5.0.
An outpatient urine sample was defined as a specimen that was collected in one of the various outpatient clinics (emergency room, or internal medicine, surgical, gynaecological or urological outpatient units) or from hospitalised patients when obtained within the first 2 days after admission. The remaining urine samples were considered as inpatient samples.
Hospital units were grouped as follows: (1) medicine – inpatient wards of internal medicine, geriatrics, neurology, oncology, radiooncology; (2) surgery – inpatient wards of all surgical disciplines except gynaecolgy and urology; (3) gynecology – all inpatient gynecological and obstetric wards and the gynecological outpatient unit; (4) intensive care units – medical and surgical intensive care units and intermediate care unit; (5) haematology – inpatient haematology isolation unit; (6) urology – urology in- and outpatient units; (7) outpatient units – all outpatient units such as the emergency room, internal medicine and surgical outpatient units (except gynaecology and urology outpatient units).
Demographic, clinical and micobiological data were collected from the computerised database of the Microbiology Laboratory and the Division of Infectious Diseases and Hospital Epidemiology. Inhospital antibiotic consumption data were estimated by defined daily doses (DDDs) per 100 patient-days.
The antimicrobial susceptibility results of E. coli were stratified by age, sex, location (in-/outpatient), hospital unit (e.g., medicine, surgery, urology) of urine sampling, and type of urine collection (indwelling catheter, single-use catheter or midstream).
Univariable analysis was performed using the chi-squared test for binary data and Mann-Whitney U-test for continuous variables. Multivariable analysis was performed using logistic regression. The results were reported as odds ratios (ORs) and 95% confidence intervals (CIs). A p-value <0.05 was considered statistically significant. Statistical analysis was done by IBM SPSS Statistics for Windows, Version 21.0 (Armonk, NY, USA).
During the study period, 20,789 microorganisms (polymicrobial flora excluded) were detected in 18,413 urine samples from 10,887 patients (fig. 1). E. coli accounted for 35.4% of these microorganisms. In 5246 urine specimens from 4870 patients with detection of E. coli, antimicrobial susceptibility testing was available and fully analysable (fig. 1).

Figure 1 Study overview.
AST = antimicrobial susceptibility testing
Polymicrobial flora: >2 pathogens without a predominant microorganism
Urine samples submitted from the dialysis and hematological outpatient unit were excluded.
The mean age of the patients was 64.1 years (standard deviation [SD] 21.6). Urine specimens were mainly obtained from female patients (80.0%) and outpatients (78.2%). Specimens were clean-catch midstream urine in 59.4%, from single-use catheterisation in 12.9%, from indwelling catheters in 12.3% and from urine of unknown origin in 15.3% (table 1). Compared with urine samples from female patients, samples from males more frequently came from elderly patients ≥65 years, outpatients, surgical and urological units, and from indwelling catheters (all p <0.05, table 1).
Table 1 Baseline characteristics of all E. coli urine samples with available atimicrobial susceptibility testing resuots (n = 5246).
| Male | Female | All | p-value | ||||
|---|---|---|---|---|---|---|---|
| Total E. coli isolates (n, %) | 1049 | 20.0% | 4197 | 80.0% | 5246 | 100% | p <0.001 |
| Patient age years (mean, SD) | 68.6 | 15.5 | 63.0 | 22.8 | 64.1 | 21.6 | p <0.001† |
| Age ≥65 years (n, %) | 706 | 67.3% | 2407 | 57.4% | 3113 | 59.3% | p <0.001 |
| Type of urine sample (n, %) | |||||||
| Indwelling catheter | 221 | 21.1% | 423 | 10.1% | 644 | 12.3% | p <0.001 |
| Single-use catheter | 96 | 9.2% | 583 | 13.9% | 679 | 12.9% | |
| Midstream urine | 546 | 52.0% | 2572 | 61.3% | 3118 | 59.4% | |
| Unknown origin | 186 | 17.7% | 619 | 14.7% | 805 | 15.3% | |
| Location of urine sampling (n, %) | |||||||
| Outpatients | 847 | 80.7% | 3256 | 77.6% | 4103 | 78.2% | p = 0.026 |
| Inpatients | 202 | 19.3% | 941 | 22.4% | 1143 | 21.8% | |
| Hospital unit of urine sampling* (n, %) | |||||||
| Medicine | 145 | 13.8% | 538 | 12.8% | 683 | 13.0% | p = 0.386 |
| Surgery | 156 | 14.9% | 514 | 12.2% | 670 | 12.8% | p = 0.023 |
| Gynaecology | 4 | 0.4% | 1074 | 25.6% | 1078 | 20.5% | na |
| Intensive care units | 31 | 3.0% | 161 | 3.8% | 192 | 3.7% | p = 0.174 |
| Haematology | 3 | 0.3% | 22 | 0.5% | 25 | 0.5% | p = 0.453 |
| Urology | 278 | 26.5% | 228 | 5.4% | 506 | 9.6% | p <0.0001 |
| Outpatient units | 432 | 41.2% | 1660 | 39.6% | 2092 | 39.9% | p = 0.335 |
| Sample distribution by year | |||||||
| 2012 | 221 | 4.2% | 904 | 17.2% | 1125 | 21.4% | p <0.001 |
| 2013 | 362 | 6.9% | 1338 | 25.5% | 1700 | 32.4% | |
| 2014 | 337 | 6.4% | 1385 | 26.4% | 1722 | 32.8% | |
| 2015 (until 31 May 2015) | 129 | 2.5% | 570 | 10.9% | 699 | 13.3% | |
SD = standard deviation The p-values refer to comparison between male and female; na, not applicable * Medicine: all internal medicine inpatient units inlcuding geriatrics, neurology, oncology, radio-oncology; surgery: all surgical inpatient units except gynaecolgy and urology; gynaecology: all gynecological and obstetric in- and outpatient units; intensive care units: medical and surgcial intensive care units, intermediate care unit; hematology: haematological isolation unit; urology: urological in- and outpatient unit; outpatient units: all outpatient units like emergency department, surgcial and internal medicine outpatient unit, excluded are gynaecological and urological outpatient units † Student t-test
Resistance rates were highest for amoxicillin (43.1%), followed by trimethoprim-sulfamethoxazole (cotrimoxazole, 24.5%) and ciprofloxacin (17.4%), and lowest for meropenem (0.0%), fosfomycin (0.9%) and nitrofurantoin (1.5%) (table 2). Extended spectrum beta-lactamases were found in 5.4% of the E. coli. No carbapenemase-producing E. coli isolates were detected.
Table 2 Percentage of E. coli resistant to different antibiotics cummulative and stratified by gender, age, location, hospital unit, type of urine specimen and quantification.
| Amoxicillin | Amoxicillin-clavulanic acid | Piperacillin-tazobactam | Ceftriaxone | Cefepime | Meropenem | Ciprofloxacin | Cotrimoxazole | Nitrofurantoin | Amikacin | Fosfomycin | All samples (n/%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 5235 | n = 5241 | n = 5171 | n = 5246 | n = 5244 | n = 5246 | n = 5233 | n = 5245 | n = 5231 | n = 5233 | n = 5232 | n = 5246 | |
| All samples | 43.2% | 15.5% | 3.9% | 5.8%* | 4.4% | 0.0% | 17.4% | 24.5% | 1.5% | 1.8% | 0.9% | 5246/100% |
| Gender | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ns | <0.001 | 0.001 | 0.006 | <0.001 | ns | 5246/100% |
| Male | 54.0% | 22.4% | 6.4% | 8.7% | 7.1% | 0.0% | 29.0% | 28.5% | 2.4% | 3.3% | 1.1% | 1049/20.0% |
| Female | 40.6% | 13.8% | 3.3% | 5.0% | 3.7% | 0.0% | 14.5% | 23.5% | 1.2% | 1.4% | 0.9% | 4197/80.0% |
| Age (years) | <0.001 | 0.002 | 0.024 | <0.001 | <0.001 | ns | <0.001 | <0.001 | ns | ns | ns | 5246/100% |
| 15–29 | 45.8% | 14.7% | 3.0% | 3.9% | 3.4% | 0.0% | 10.4% | 27.6% | 0.9% | 1.1% | 0.7% | 536/10.2% |
| 30–39 | 40.7% | 12.8% | 2.6% | 4.1% | 3.7% | 0.0% | 10.0% | 23.3% | 1.1% | 2.2% | 0.9% | 460/8.8% |
| 40–49 | 42.8% | 16.7% | 4.4% | 4.8% | 3.6% | 0.0% | 11.8% | 25.6% | .5% | 1.2% | 1.0% | 414/7.9% |
| 50–59 | 48.9% | 18.7% | 4.1% | 8.8% | 6.2% | 0.0% | 20.0% | 29.5% | 1.3% | 2.8% | 1.1% | 465/8.9% |
| 60–69 | 46.8% | 17.9% | 6.2% | 8.2% | 6.6% | 0.0% | 19.4% | 30.4% | 1.7% | 2.0% | 0.6% | 649/12.4% |
| 70–79 | 45.5% | 17.5% | 4.2% | 7.2% | 5.6% | 0.0% | 21.9% | 24.6% | 2.0% | 1.9% | 1.2% | 1107/21.1% |
| ≥80 | 38.7% | 13.0% | 3.3% | 4.2% | 2.9% | 0.0% | 18.7% | 19.8% | 1.6% | 1.6% | 0.9% | 1615/30.8% |
| Age (years) | 0.012 | ns | ns | ns | ns | ns | <0.001 | <0.001 | 0.029 | ns | ns | 5246/100% |
| <65 | 45.4% | 15.8% | 3.8% | 5.7% | 4.5% | 0.0% | 13.8% | 27.1% | 1.0% | 1.7% | 0.8% | 2133/40.7% |
| ≥65 | 41.8% | 15.3% | 4.0% | 5.8% | 4.3% | 0.0% | 19.9% | 22.8% | 1.8% | 1.8% | 1.0% | 3113/59.3% |
| Location | ns | ns | ns | 0.041 | ns | ns | ns | ns | ns | ns | ns | 5246/100% |
| Outpatients | 43.0% | 15.2% | 3.8% | 5.4% | 4.2% | 0.0% | 17.4% | 24.7% | 1.5% | 1.9% | 1.0% | 4103/78.2% |
| Inpatients | 44.1% | 16.6% | 4.3% | 7.0% | 5.3% | 0.0% | 17.5% | 24.0% | 1.4% | 1.3% | 0.8% | 1143/21.8% |
| Hospital unit | <0.001 | 0.003 | ns | 0.001 | 0.001 | ns | <0.001 | 0.001 | 0.030 | 0.014 | ns | 5246/100% |
| Urology | 53.8% | 20.0% | 4.8% | 9.1% | 7.3% | 0.0% | 32.8% | 30.6% | 2.6% | 3.2% | 0.9% | 506/9.6% |
| All others† | 42.1% | 15.0% | 3.8% | 5.4% | 4.1% | 0.0% | 15.8% | 23.9% | 1.4% | 1.6% | 1.2% | 4740/90.4% |
| Hospital unit | 0.006 | <0.001 | 0.009 | 0.005 | 0.001 | ns | <0.001 | ns | ns | 0.017 | ns | 5246/100% |
| Gynaecology | 39.6% | 12.1% | 2.5% | 4.0% | 2.5% | 0.0% | 12.2% | 23.5% | 1.0% | 0.9% | 1.4% | 1074/20.5% |
| All others‡ | 44.2% | 16.4% | 4.3% | 6.2% | 4.9% | 0.0% | 18.8% | 24.8% | 1.6% | 2.0% | 0.8% | 4161/79.5% |
| Type | 0.005 | ns | ns | 0.05 | ns | ns | <0.001 | 0.034 | ns | ns | ns | 5246/100% |
| Indwelling catheter | 48.4% | 17.9% | 5.2% | 7.4% | 5.6% | 0.0% | 23.6% | 27.9% | 1.5% | 2.2% | 0.9% | 645/12.3% |
| All others§ | 42.6% | 15.2% | 3.7% | 5.5% | 4.2% | 0.0% | 16.6% | 24.1% | 1.4% | 1.7% | 0.9% | 4602/87.7 |
* In total 283 (5.4%) E. coli produced extended spectrum beta-lactamases † Medicine, surgery, gynaecology, intensive care unit, outpatient units ‡ Medicine, surgery, urology, intensive care unit, outpatient units § all others: include midstream urines, single-use catheter urines and urines of unknown origin p-values in italics; ns = not significant (p ≥0.05)
Compared with female patients, Males showed up to 2-fold higher resistance rates for all antibiotics except for fosfomycin and meropenem (table 2 and fig. 2a). The highest resistence rates were documented for ciprofloxacin (OR 1.93, 95% CI 1.73–2.17), followed by amikacin (OR 1.90, 95% CI 1.45–2.47), piperacillin-tazobactam (OR 1.68, 95% CI 1.36–2.06) and cefepime (OR 1.67, 95% CI 1.38–2.03), amoxicillin-clavulanic acid (OR 1.58, 95% CI 1.39–1.79) and ceftriaxone OR 1.56, 95% CI 1.30–1.86) and cotrimoxazole (OR 1.23, 95% CI 1.09–1.38).
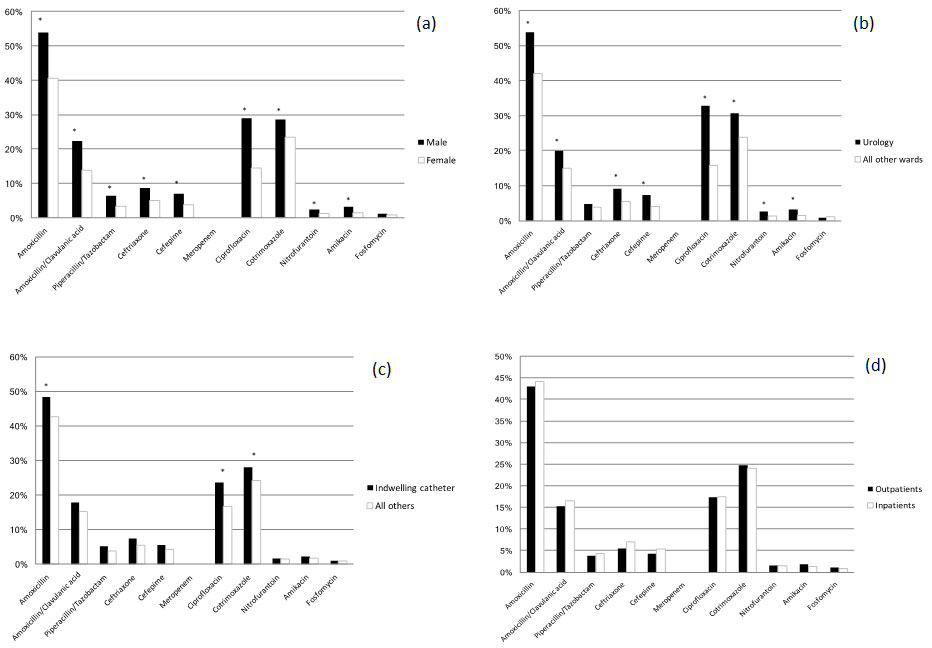
Figure 2 E. coli resistance to various antibiotics stratified for gender, hospital unit, type and location of urine sampling
(a) E. coli resistance to various antibiotics stratified by gender
(b) E. coli resistance to various antibiotics stratified by urological versus other hospital units: urology includes all urine samples from urological in- and outpatients; all other wards include all other nonurological hospital units (medicine, surgery, gynecology, intensive care unit, haematology, outpatient units). Ciprofloxacin had the highest (odds ration [OR] 2.3, 95% confidence interval [CI] 1.95–2.75), followed by cefepime (OR 1.71, 95% CI 1.16–2.22), ceftriaxone (OR 1.64, 95% CI 1.24–2.17) and amoxicillin (OR 1.52, 95% CI 1.52–1.80), all p ≤0.001.
(c) E. coli resistance to various antibiotics stratified by urine samples from indwelling catheters versus all others: all others inlcude midstream urine, single-use catheter urine and urine of unknown origin.
(d) E. coli resistance to various antibiotics stratified by in-/outpatients: no significant difference between in- and outpatients for all antibiotics (p ≥0.05)
y-axis: percentage of resistance
* significant difference with p <0.05
In patients ≥65 years, resistance rates for ciprofloxacin and nitrofurantoin were significantly higher than those in patients <65 years old (OR 1.18, 95% CI 1.12–1.24; p <0.001 and OR 1.21, 95% CI 1.05–1.39; p = 0.03, respectively), whereas resistance rates for amoxicillin (OR 0.93, 95% CI 0.90–0.99; p = 0.012) and cotrimoxazole (OR 0.91, 95% CI 0.86–0.96; p <0.001) were significantly lower. For the other antibiotics, no difference could be found between these age groups.
After results were stratified into 10-year age intervals, antimicrobial resistance to beta-lactam antibiotics, cotrimoxazole and ciprofloxacin was highest between the ages of 50 and 79 years, and significantly decreased in patients aged ≥80 years except for ciprofloxacin (p <0.01). For other antibiotics such as meropenem, amikacin, nitrofurantoin and fosfomycin, no significant differences between these age groups were found (table 2 and fig. 3).
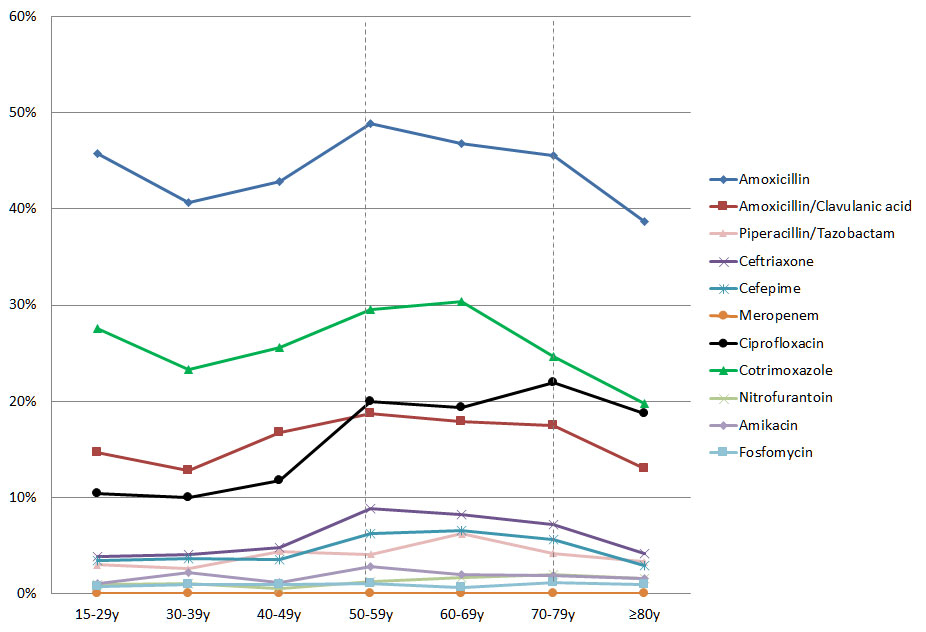
Figure 3 E. coli resistance to various antibiotics stratified by age.
y-axis: percentage of resistance
Resistance of most antibiotics peaked between the ages of 50 and 79 years (area between dotted lines).
The antimicrobial susceptibility profiles differed between hospital units except for piperacillin-tazobactam, nitrofurantoin and fosfomycin (table 2). E. coli from the urology unit showed significantly higher resistance rates for most of the antibiotics compared with isolates from other hospital units, in particular for amoxicillin (53.5% resistant in the urology unit, OR 1.52, 95% CI 1.52–1.80; p <0.001), ciprofloxacin (32.8%, OR 2.31, 95% CI 1.95–2.75; p <0.001) and cotrimoxazole (30.6%, OR 1.36, 95% CI 1.14–1.62; p = 0.001) (table 2 and figure 1b). E. coli from gynaecological in- and outpatients had the lowest resistance rates compared with other hospital units (table 2).
Overall, E. coli isolates in urine collected from indwelling catheters showed generally higher resistance rates compared with those taken after single-use catheterisation, midstream urine specimens and urine of unknown origin together. However, these differences were only significant for amoxicillin, ciprofloxacin and cotrimoxazole (table 2 and fig. 1c).
The antimicrobial susceptibility pattern did not differ significantly between in- and outpatient urine samples, except for ceftriaxone (table 2 and fig. 1d).
Multivariable analysis was performed to identify independent associations with resistance against the commonly used oral antibiotics amoxicillin-clavulanic acid, ciprofloxacin and cotrimoxazole. Male gender, age ≥65years, indwelling catheters and urine samples from the urological unit were independently associated with resistance to ciprofloxacin (table 3). Similarly, resistance to cotrimoxazole was significantly associated with male gender, urine from indwelling catheters and sample collection from the urological unit, but age ≥65years was predictive for lower resistance. For amoxicillin-clavulanic acid only male gender was associated with resistance.
Table 3 Uni- and multivariable analysis of risk factors for E. coli resistance to commonly used antimicrobial agents.
| Crude OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | |
|---|---|---|---|---|
| Ciprofloxacin | ||||
| Male gender | 2.40 (2.05–2.81) | <0.001 | 1.93 (1.630–2.29) | <0.001 |
| Age ≥65 years | 1.54 (1.33–1.80) | <0.001 | 1.42 (1.21–1.67) | <0.001 |
| Indwelling catheter | 1.55 (1.27–1.89) | <0.001 | 1.30 (1.05–1.61) | 0.014 |
| Inpatient | 1.01 (0.85–1.20) | 0.940 | 1.04 (0.86–1.25) | 0.687 |
| Urological ward | 2.60 (2.13–3.18) | <0.001 | 2.04 (1.63–2.54) | <0.001 |
| Cotrimoxazole | ||||
| Male gender | 1.30 (1.11–1.51) | 0.001 | 1.22 (1.22–1.04) | 0.015 |
| Age ≥65 years | 0.79 (0.70–0.90) | <0.001 | 0.76 (0.67–0.86) | <0.001 |
| Indwelling catheter | 1.22 (1.02–1.47) | 0.034 | 1.26 (1.04–1.53) | 0.020 |
| Inpatient | 0.96 (0.83–1.12) | 0.615 | 1.00 (0.85–1.18) | 0.950 |
| Urological ward | 1.41 (1.15–1.72) | 0.001 | 1.33 (1.07–1.64) | 0.010 |
| Amoxicilln-clavulanic acid | ||||
| Male gender | 1.81 (1.53–2.15) | <0.001 | 1.76 (1.47–2.11) | <0.001 |
| Age ≥65 years | 0.97 (0.83–1.13) | 0.682 | 0.90 (0.77–1.05) | 0.194 |
| Indwelling catheter | 1.22 (0.98–1.51) | 0.076 | 1.08 (0.86–1.36) | 0.508 |
| Inpatient | 1.11 (0.93–1.33) | 0.241 | 1.16 (0.96–1.41) | 0.118 |
| Urological ward | 1.42 (1.12–1.79) | 0.003 | 1.18 (0.92–1.51) | 0.196 |
CI = confidence interval; OR = odds ratio
Analyses of this kind may be confounded by changes in the overall antibiotic consumption in our institution during the study period. However, consumption (expressed in DDDs per 100 patient-days) in the urological inpatient unit was similar to that of the whole hospital for all antibiotics (55.2 and 52.0, respectively) and for the fluoroquinolones (4.5 and 4.5); higher for amoxicillin-clavulanic acid (23.2 and 15.7) and for broad-spectrum antibiotics (piperacillin-tazobactam, cefepime, carbapenems: 17.2 and 10.0); and lower for the first to third generation cephalosporins (3.5 and 8.4) and cotrimoxazole (0.2 and 0.7).
Antimicrobial susceptibility patterns of E. coli are influenced by many factors and vary considerably in different parts of the world [3–5, 13]. Cumulative resistance data from national and local antimicrobial surveillance networks are used to develop local prescribing guidelines, but they do not take individual patient factors into account.
Our study did not intend to search for new risk factors by using exhaustive clinical and microbiological data, but to find readily available bedside clinical data to optimise empirical antimicrobial therapy for suspected UTI. In our study we could demonstrate that simple demographic and patient characteristics further helped in predicting antimicrobial resistance of urinary tract E. coli isolates. For most of the analysed antibiotics, male, middle-aged and urological patients showed higher resistance rates, whereas indwelling urinary tract catheters and hospitalisation seemed to have worsening influence on antimicrobial resistance.
We found overall high resistance rates for the commonly used oral antibiotics, such as amoxicillin (43.2%), cotrimoxazole (24.5%), ciprofloxacin (17.4%) and amoxicillin-clavulanic acid (15.5%), whereas resistance remained low for nitrofurantoin and fosfomycin (≤1.5%).
The high rates of resistance to ciprofloxacin (17.4%) and cotrimoxazole (24.5%), two of the oral antibiotics most commonly prescribed to treat UTIs, are of particular concern since they exceeded the IDSA cut-offs of 10 and 20%, respectively [6], above which empirical use of fluoroquinolones and cotrimoxazole in the treatment of UTIs is no longer recommended. For both antibiotics we observed a steady increase of resistant E. coli urine isolates in our institution from 2007 up to the current study period of 2012 to 2015, from 15.9 to 17.4% for ciprofloxacin and 21.3 to 24.5% for cotrimoxazole [9]. The increasing fluoroquinolone resistance in urinary tract E. coli isolates has been described in many reports and is explained by their widespread use [14–16]. Interestingly, at our institution, including the urological unit, fluoroquinolone consumption is rather low and has even decreased from 6.4 DDD/100 patient-days in 2008 [9] to 4.5 DDD/100 patient-days in 2012–2015. On a national level, overall antibiotic use in Switzerland is low compared with other European countries [17]; however, fluoroquinolone prescription, particularly in the outpatient setting including urological patients, is very high at 20.1% [18] of all antibiotics compared with an average of 7.3% in the other European countries [19]. The widespread use of fluoroquinolones in the outpatient setting may explain the comparable resistance rates in in- and outpatients [20, 21] in our study as well as in the Swiss national antimicrobial resistance surveillance database ANRESIS [22] 2012 to 2014.
Various risk factors for antimicrobial resistance of urinary tract E. coli isolates have been described, but remain to some extent controversial. Advanced age, male sex, nosocomial UTIs [20, 21, 23–25], an indwelling urinary tract catheter and specimens from urological patients [26, 27] have been described as associated with resistance mainly to fluroquinolones [3, 7–10] [14] [28, 29].
In our study, male and urological patients, and to a lesser extent patients with indwelling catheters, were independent predictors for ciprofloxacin and cotrimoxazole resistance. Remarkably, >30% of the E. coli from urological patients were resistant to ciprofloxacin and cotrimoxazole. A high resistance rate in urological patients was also described in other studies [26, 27, 30, 31]. Explanations might be the frequent use of fluoroquinolones for “prolonged” antibiotic prophylaxis in transurethral resection of the prostate and other urological procedures, and the treatment of UTIs, particularaly in males who may receive repeated and prolonged fluorochinolone therapy cycles for susupected prostatitis [31]. Inadequate tissue penetration of the antimicrobial agent with subinhibitory minimal inhibitory concentration (MIC) effects and prolonged therapy may predispose to the selection of more resistant microorganisms in male patients [7, 10, 30, 32–34].
Interestingly, advanced age ≥65 year was associated with higher E. coli resistance to ciprofloxacin, whereas for cotrimoxazole resistance rates decreased in eldery patients. These resistance trends for cotrimoxazole and ciprofloxacin were already described at our institution in 2007 [9]. Of note, a reversing resistance trend could also be observed for the beta-lactam antibiotics in patients ≥80 years. No age dependence was found for antibiotics with a very low resistance rate, such as meropenem, amikacin, fosfomycin and nitrofurantoin. Our observation is in contrast to many other studies in which age was associated with higher resistance [14, 28]; however, these studies usually did not evaluate the resistance profile in very eldery patient owing to the decrease sample size with increasing age.
There are limitations of the study that should be mentioned. First, it was a single centre study at a tertiary care hospital in Switzerland. Hospital and laboratory based surveillance data of susceptibility patterns probably overestimate the antibiotic resistance rates, since clinicians may treat uncomplicated UTIs empirically in the outpatient setting without sending a urine sample to the laboratory. Cultures are only performed if the patient fails to respond to treatment, has recurrent episodes of UTI or has complicated UTI [35, 36].
Second, the retrospective design precluded collecting standardised clinical information on previous antibiotic treatment, previous hospitalisations, catheter dwelling time or whether urine samples came from patients with asymptomatic bacteriuria, UTIs or even prostatitis. However, some studies have found that the E. coli antimicrobial susceptibility profile does not seem to differ greatly between patients with UTI and patients with colonisation only [9, 10, 15, 20]. In addition, the definition and clinical diagnosis of a UTI is not clear cut, but microbiology results are unambiguous.
Third, misclassification may have influenced the results, but because of the large sample size of over 5000 consecutive samples may not be sufficient to change the results generated.
Strengths of our study are the large sample size, use of only one isolate per patient per year to avoid selection bias of more resistant E. coli, the very low loss of data from the samples and the use of simple bedside clinical data.
In conclusion, using readily available bedside data from patients and wards can improve the choice of appropriate antimicrobial therapy in patients with suspected UTI, in addition to the annual reports of the microbiology laboratory on antimicrobial resistance of pathogens isolated from urine.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Gupta K , Hooton TM , Stamm WE . Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med. 2001;135(1):41–50. doi:.https://doi.org/10.7326/0003-4819-135-1-200107030-00012
2 Naber KG , Schito G , Botto H , Palou J , Mazzei T . Surveillance study in Europe and Brazil on clinical aspects and Antimicrobial Resistance Epidemiology in Females with Cystitis (ARESC): implications for empiric therapy. Eur Urol. 2008;54(5):1164–78. doi:.https://doi.org/10.1016/j.eururo.2008.05.010
3 Zhanel GG , Hisanaga TL , Laing NM , DeCorby MR , Nichol KA , Palatnick LP , et al., NAUTICA Group. Antibiotic resistance in outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents. 2005;26(5):380–8. doi:.https://doi.org/10.1016/j.ijantimicag.2005.08.003
4 Weist K , Diaz Högberg L . ECDC publishes 2013 surveillance data on antimicrobial resistance and antimicrobial consumption in Europe. Euro Surveill. 2014;19(46):19. doi:.https://doi.org/10.2807/1560-7917.ES2014.19.46.20962
5ECDC. European centre for disease prevention and control. Antimicrobial resistance surveillance in europe 2014. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). 2015.
6 Gupta K , Hooton TM , Naber KG , Wullt B , Colgan R , Miller LG , et al.; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103–20. doi:.https://doi.org/10.1093/cid/ciq257
7 Alós JI , Serrano MG , Gómez-Garcés JL , Perianes J . Antibiotic resistance of Escherichia coli from community-acquired urinary tract infections in relation to demographic and clinical data. Clin Microbiol Infect. 2005;11(3):199–203. doi:.https://doi.org/10.1111/j.1469-0691.2004.01057.x
8 Arslan H , Azap OK , Ergönül O , Timurkaynak F ; Urinary Tract Infection Study Group. Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J Antimicrob Chemother. 2005;56(5):914–8. doi:.https://doi.org/10.1093/jac/dki344
9 Blaettler L , Mertz D , Frei R , Elzi L , Widmer AF , Battegay M , et al. Secular trend and risk factors for antimicrobial resistance in Escherichia coli isolates in Switzerland 1997-2007. Infection. 2009;37(6):534–9. doi:.https://doi.org/10.1007/s15010-009-8457-0
10 Boyd LB , Atmar RL , Randall GL , Hamill RJ , Steffen D , Zechiedrich L . Increased fluoroquinolone resistance with time in Escherichia coli from >17,000 patients at a large county hospital as a function of culture site, age, sex, and location. BMC Infect Dis. 2008;8(1):4. doi:.https://doi.org/10.1186/1471-2334-8-4
11 Brown PD , Freeman A , Foxman B . Prevalence and predictors of trimethoprim-sulfamethoxazole resistance among uropathogenic Escherichia coli isolates in Michigan. Clin Infect Dis. 2002;34(8):1061–6. doi:.https://doi.org/10.1086/339491
12 Johnson JR . Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis. 2004;39(6):873 , author reply 873–4. doi:.https://doi.org/10.1086/423844
13 Dalhoff A . Global fluoroquinolone resistance epidemiology and implictions for clinical use. Interdiscip Perspect Infect Dis. 2012;2012:976273. doi:.https://doi.org/10.1155/2012/976273
14 Ena J , Amador C , Martinez C , Ortiz de la Tabla V . Risk factors for acquisition of urinary tract infections caused by ciprofloxacin resistant Escherichia coli. J Urol. 1995;153(1):117–20. doi:.https://doi.org/10.1097/00005392-199501000-00040
15 Gagliotti C , Nobilio L , Moro ML ; Emilia-Romagna Antibiotic Resistance Study Group. Emergence of ciprofloxacin resistance in Escherichia coli isolates from outpatient urine samples. Clin Microbiol Infect. 2007;13(3):328–31. doi:.https://doi.org/10.1111/j.1469-0691.2006.01615.x
16 Goettsch W , van Pelt W , Nagelkerke N , Hendrix MG , Buiting AG , Petit PL , et al. Increasing resistance to fluoroquinolones in escherichia coli from urinary tract infections in the netherlands. J Antimicrob Chemother. 2000;46(2):223–8. doi:.https://doi.org/10.1093/jac/46.2.223
17Gelband H, Miller-Petrie M, Pant S, et al. The state of the world's antibiotics 2015. In: The Center for Disease Dynamics EPC, ed. Washington, D.C. 2015.
18Helsana. Helsana-arzneimittelreport 2014. In: Helsana, ed. Zürich 2014.
19 Filippini M , Masiero G , Moschetti K . Socioeconomic determinants of regional differences in outpatient antibiotic consumption: evidence from Switzerland. Health Policy. 2006;78(1):77–92. doi:.https://doi.org/10.1016/j.healthpol.2005.09.009
20 Karlowsky JA , Kelly LJ , Thornsberry C , Jones ME , Sahm DF . Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatients in the United States. Antimicrob Agents Chemother. 2002;46(8):2540–5. doi:.https://doi.org/10.1128/AAC.46.8.2540-2545.2002
21 Stelling JM , Travers K , Jones RN , Turner PJ , O’Brien TF , Levy SB . Integrating Escherichia coli antimicrobial susceptibility data from multiple surveillance programs. Emerg Infect Dis. 2005;11(6):873–82. doi:.https://doi.org/10.3201/eid1106.041160
22ANRESIS. Antibiotikaresistenzdaten. Bern. 2016. http://www.anresis.ch/index.php/resistenzdaten-humanmedizin.html
23 Saperston KN , Shapiro DJ , Hersh AL , Copp HL . A comparison of inpatient versus outpatient resistance patterns of pediatric urinary tract infection. J Urol. 2014;191(5, Suppl):1608–13. doi:.https://doi.org/10.1016/j.juro.2013.10.064
24 Dahle KW , Korgenski EK , Hersh AL , Srivastava R , Gesteland PH . Clinical value of an ambulatory-based antibiogram for uropathogens in children. J Pediatric Infect Dis Soc. 2012;1(4):333–6. doi:.https://doi.org/10.1093/jpids/pis055
25 Lutter SA , Currie ML , Mitz LB , Greenbaum LA . Antibiotic resistance patterns in children hospitalized for urinary tract infections. Arch Pediatr Adolesc Med. 2005;159(10):924–8. doi:.https://doi.org/10.1001/archpedi.159.10.924
26 Nicoletti J , Kuster SP , Sulser T , Zbinden R , Ruef C , Ledergerber B , et al. Risk factors for urinary tract infections due to ciprofloxacin-resistant Escherichia coli in a tertiary care urology department in Switzerland. Swiss Med Wkly. 2010;140:w13059.
27 Cullen IM , Manecksha RP , McCullagh E , Ahmad S , O’Kelly F , Flynn RJ , et al. The changing pattern of antimicrobial resistance within 42,033 Escherichia coli isolates from nosocomial, community and urology patient-specific urinary tract infections, Dublin, 1999-2009. BJU Int. 2012;109(8):1198–206. doi:.https://doi.org/10.1111/j.1464-410X.2011.10528.x
28 Falagas ME , Polemis M , Alexiou VG , Marini-Mastrogiannaki A , Kremastinou J , Vatopoulos AC . Antimicrobial resistance of Esherichia coli urinary isolates from primary care patients in Greece. Med Sci Monit. 2008;14(2):CR75–9.
29 Sotto A , De Boever CM , Fabbro-Peray P , Gouby A , Sirot D , Jourdan J . Risk factors for antibiotic-resistant Escherichia coli isolated from hospitalized patients with urinary tract infections: a prospective study. J Clin Microbiol. 2001;39(2):438–44. doi:.https://doi.org/10.1128/JCM.39.2.438-444.2001
30 Wagenlehner FM , Naber KG . Fluoroquinolone antimicrobial agents in the treatment of prostatitis and recurrent urinary tract infections in men. Curr Infect Dis Rep. 2005;7(1):9–16. doi:.https://doi.org/10.1007/s11908-005-0018-9
31 Bonkat G , Müller G , Braissant O , Frei R , Tschudin-Suter S , Rieken M , et al. Increasing prevalence of ciprofloxacin resistance in extended-spectrum-β-lactamase-producing Escherichia coli urinary isolates. World J Urol. 2013;31(6):1427–32. doi:.https://doi.org/10.1007/s00345-013-1031-5
32 Hummers-Pradier E , Koch M , Ohse AM , Heizmann WR , Kochen MM . Antibiotic resistance of urinary pathogens in female general practice patients. Scand J Infect Dis. 2005;37(4):256–61. doi:.https://doi.org/10.1080/00365540410021009
33 Naber CK , Steghafner M , Kinzig-Schippers M , Sauber C , Sörgel F , Stahlberg HJ , et al. Concentrations of gatifloxacin in plasma and urine and penetration into prostatic and seminal fluid, ejaculate, and sperm cells after single oral administrations of 400 milligrams to volunteers. Antimicrob Agents Chemother. 2001;45(1):293–7. doi:.https://doi.org/10.1128/AAC.45.1.293-297.2001
34 Sahm DF , Thornsberry C , Mayfield DC , Jones ME , Karlowsky JA . Multidrug-resistant urinary tract isolates of Escherichia coli: prevalence and patient demographics in the United States in 2000. Antimicrob Agents Chemother. 2001;45(5):1402–6. doi:.https://doi.org/10.1128/AAC.45.5.1402-1406.2001
35 Ti TY , Kumarasinghe G , Taylor MB , Tan SL , Ee A , Chua C , et al. What is true community-acquired urinary tract infection? Comparison of pathogens identified in urine from routine outpatient specimens and from community clinics in a prospective study. Eur J Clin Microbiol Infect Dis. 2003;22(4):242–5.
36 Richards DA , Toop LJ , Chambers ST , Sutherland MG , Harris BH , Ikram RB , et al. Antibiotic resistance in uncomplicated urinary tract infection: problems with interpreting cumulative resistance rates from local community laboratories. N Z Med J. 2002;115(1146):12–4.
No financial support and no other potential conflict of interest relevant to this article was reported.