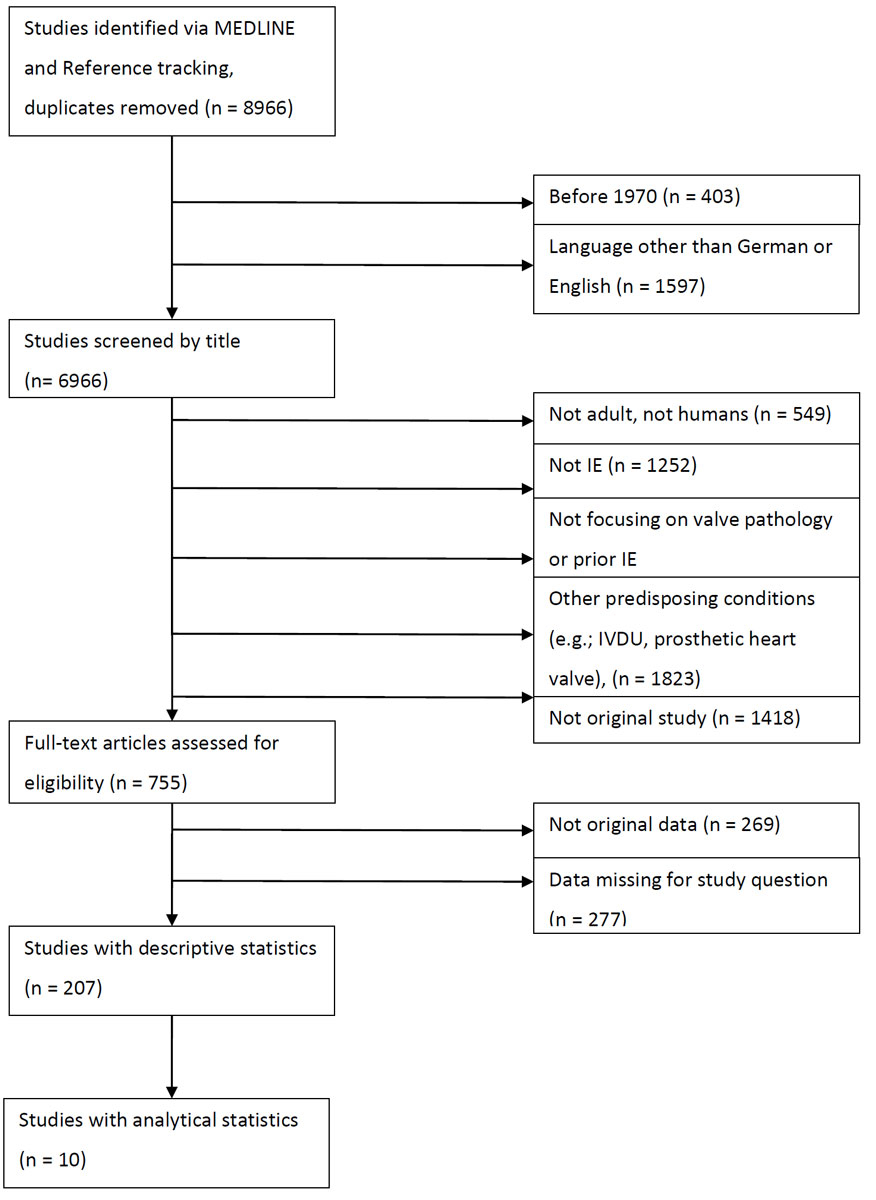
Figure 1 Algorithm used for systematic literature review.
DOI: https://doi.org/10.4414/smw.2018.14675
The incidence of infective endocarditis has not changed over the past three decades, despite improvements in health care [1]. Whereas in the pre-antibiotic era most patients with infective endocarditis had a history of rheumatic heart disease, patients at risk nowadays include elderly people with degenerative valve disease, those with nosocomial disease and those undergoing haemodialysis treatment. Predisposing heart conditions were early on recognised as being associated with infective endocarditis. Several authors identified underlying cardiac lesions in patients with infective endocarditis and came to the conclusion that rheumatic heart disease, mitral valve prolapse, congenital heart disease and degenerative valve lesions predispose to infective endocarditis [2–6]. With current diagnostic methods, the term “degenerative valve lesions” includes a wide spectrum of valvular diseases. Analysing the term “predisposing heart condition” in native valves from today’s perspective is challenging. Over the past decades, the technology has improved significantly. Also, definitions of valvulopathies have changed over the last decades (table S1 in appendix 1). Thus, it is important to match the evidence for a given heart condition with the corresponding imaging technique and definition at the time of the corresponding study.
The term “predisposing heart condition” is used as an indication for antimicrobial prophylaxis to prevent infective endocarditis and as a criterion for diagnosing infective endocarditis according to modified Duke criteria. The term for diagnosing infective endocarditis is not well defined. A recently performed survey with 318 physicians demonstrated considerable uncertainty about this term [7]. Therefore, we performed a systematic review and aimed to stratify the results according to study type, year and number of publications. Thereby, we aimed to identify heart valve conditions for the Duke criterion “predisposing heart condition” in infective endocarditis, and to analyse the term from today’s perspective.
Our objective was to review the literature according to PRISMA [10] criteria on specific heart conditions in native valves predisposing to infective endocarditis. In patients with specific heart conditions, we aimed to review their risk for developing infective endocarditis (studies with analytical statistics). In studies with descriptive statistics including patients with a specific heart condition, we aimed to review the proportion with infective endocarditis. On the basis of the year in which patients were included in a study, we aimed to review the proportion of studies published before and after the release of the modified Duke criteria. Finally, we aimed to align the results from the imaging technique available at that time with past and current definitions of a specific heart valvular disease.
The review protocol was not registered, but has been published elsewhere [11].
Heart valve conditions considered relevant for this study were prior endocarditis, aortic valve stenosis or insufficiency, bicuspid aortic valve, mitral valve stenosis or insufficiency, mitral valve prolapse, pulmonary valve insufficiency or stenosis, and tricuspid valve insufficiency or stenosis. Original articles in English or German published from 1 January 1970 to 31 July 31 2015 were screened for eligibility. Articles published after 1970 were included because von Reyn et al. [9] published criteria on the basis of infective endocarditis cases that were treated between 1970 and 1977. If the article did not concern adult humans or concerned diseases other than endocarditis, it was excluded. Case reports, letters and review articles were excluded.
A comprehensive, systematic search of the Medline database was performed in August 2015.
To identify relevant articles, we used the following keywords: “endocarditis”, “predisposing”, “predisposition”, “risk factor” and “heart condition”. The following search strategy was used: endocarditis AND (predisposing OR predisposition OR risk factor OR heart condition). Articles that were cited by the articles identified in the search were tracked in the reference list of the corresponding article and, if relevant, were also included. The retrieved articles were reviewed and the articles were included or excluded after screening for predefined criteria.
In a first step, all eligible studies were included, with no specific criteria concerning participants, interventions, outcomes or study design. Titles and abstracts were screened for eligibility, then full papers of relevant articles were examined in further detail. In a second step, mitral valve stenosis, pulmonary valve insufficiency or stenosis, and tricuspid valve insufficiency or stenosis were excluded from this review because of unknown or low prevalence in Switzerland. In a third step, results were differentiated between studies with analytical and descriptive statistics.
For relevant articles, the full text article was reviewed to extract data concerning the total number of patients/cases included in the study and the year patient/cases were included, as well as patients/cases with specific heart conditions.
In studies with analytical statistics, the method of risk assessment and the focus of the study question was reviewed. Primary data collection was in Microsoft Excel 2013. Statistical analysis was conducted in GraphPad Prism (GraphPad Software, La Jolla, CA, USA) and R (www.r-project.org).
Empirically, and on the arbitrary basis of 100 desired studies per valve disease, three categories were predefined, namely valve diseases with a high (≥75 included studies), medium (25–74 included studies) and low number of publications (≤24 included studies). The results were differentiated by the inclusion of patients into studies prior to and after 2001, the year of publication of the modified Duke criteria [8].
In a primary analysis of studies with descriptive statistics, Freeman-Tukey and Begg’s funnel plots were applied to assess the contribution to heterogeneity and the influence of each study on the overall results. The results of this primary analyses are published elsewhere [11]. As a result of this primary analysis, and owing to the strong heterogeneity between the studies, the numbers are presented here as both mean and median with interquartile range (IQR). Studies with calculations of odds ratios or hazard ratios and not contributing to a large heterogeneity were considered as good quality.
This theme was reviewed by MH and SZ, is presented elsewhere in detail [11] and used here in the context of the year of publication for each valve disease.
The literature review was performed by one person only (AB) and the study selection process was unblinded (AB and PS).
Of 8966 articles, 207 were identified as being eligible for this systematic review (fig. 1). A detailed list of all included studies is available in appendix 1 and online in reference [11].

Figure 1 Algorithm used for systematic literature review.
Conditions with a high number of publications: mitral valve prolapse (112 studies), prior infective endocarditis (96) and bicuspid aortic valve (78) were the most commonly described heart conditions predisposing for infective endocarditis.
Conditions with a medium number of publications: These included aortic valve stenosis (46 studies), mitral valve insufficiency (41) and aortic valve insufficiency (39).
Conditions with a low number of publications: These included mitral valve stenosis (23 studies), pulmonary valve stenosis or insufficiency (18), and tricuspid valve stenosis or insufficiency (9). The latter group paralleled the exclusion criteria for this systematic review and is not presented here [11].
The vast majority of studies (>95%) reported epidemiological data and descriptive statistics without analytical statistics. Few studies (<5%) presented results from analytical statistics (table 1). Three-quarters of the studies included patients at the time period prior to the publication of the modified Duke criteria [8].
Table 1 Distribution of studies between publications with descriptive and analytical statistics.
| Valve disease | Total no. of studies | Proportion of patients included in studies prior to the publication of modified Duke criteria | No. of studies with descriptive statistics only | Proportion of patients with IE | No. of studies with analytical statistics | No. of studies with analytical statistics and good quality in the context of the review | Risk calculation |
|---|---|---|---|---|---|---|---|
| MVP | 112 | 81.8% | 110* | Mean 8.5% Median 7.7% IQR 4.4–11.4% |
6* | 3 | OR 3.5–8.2 see table 2 |
| Prior IE | 96 | 75.5% | 94‡ | Mean 8.3% Median 7.1%, IQR 4.9–10.2% | 3‡ | 2 | OR 2.2–2.8 |
| BAV | 78 | 74% | 77 | Mean 8.8% Median 5.6%, IQR 3–12% | 1 | 1 | HR 6.3 |
| AS | 46 | 75.6% | 45‡ | Mean 7.3% Median 6.7%, IQR 2.6–9.7% | 2‡ | 1 | HR 4.9 |
| MI | 41 | 78% | 41 | Mean 19.9% Median 16%, IQR 5.2–28.6% |
0 | – | – |
| AI | 39 | 79.5% | 39 | Mean 10.2% Median 8.1%, IQR 3.1–16.6% | 0 | – | – |
AI = aortic valve insufficiency/regurgitation; AS = aortic valve stenosis; BAV = bicuspid aortic valve; HR = hazard ratio; IE = infective endocarditis; IQR = interquartile range; MI = mitral valve insufficiency/ regurgitation; MVP = mitral valve prolapse; OR = odds ratio * Four studies used descriptive and analytical statistics. ‡ One study used descriptive and analytical statistics.
We identified six studies with analytical statistics showing that mitral valve prolapse was associated with a higher risk of infective endocarditis. The study of Zuppiroli et al. [16] is not presented in table 2. The authors followed 275 patients with mitral valve prolapse for 10 to 216 months (mean 98 ± 52 months). One patient developed infective endocarditis. The authors calculated a risk rate of 0.04 (−0.04 to 0.1) per 100 patient-years. Five studies calculated odds ratios, two of them in a special context. First, MacMahon et al. [15] estimated the relative risk of infective endocarditis associated with the presence of a systolic murmur in patients with mitral valve prolapse. Second, Strom et al. [14] aimed to quantitate the risk for infective endocarditis from dental treatment in patients with cardiac abnormalities. In both studies, the involved valve in the overall infective endocarditis population was not specified, and the odds ratios are considered high with a large 95% confidence interval. Both studies contributed considerably to the heterogeneity of data in the preliminary analysis (presented elsewhere [11]). In the other three studies [3, 12, 13], the odds ratios ranged from 3.5 to 8.2. All six studies were prior to the release of the modified Duke criteria [8].
Table 2 Studies of mitral valve prolapse and infective endocarditis that included analytical statistics.
| First author of the study | Years patients included |
Study method | Number of patients with MV IE and number of patients with MVP |
Number of controls and number of controls with MVP |
OR (95% CI) |
|---|---|---|---|---|---|
| Clemens [3] | 1976-1980 | Case-control | 51 patients with MV IE 13 patients with MVP |
153 controls without IE 10 controls with MVP |
8.2 (2.4–28.4) |
| Devereux [12] | 1980–1983 | Case-control | 67 patients with MV IE 11 patients with MVP |
196 controls (population control group) 8 controls with MVP 2146 controls (clinical control group) 84 controls with MVP |
4.6 (2.0–7.2) 6.7 (1.96–22.9)* 4.8 (2.0–7.2) 6.7 (1.96–22.9)* |
| Danchin [13] | 1981–1986 | Case-control | 48 patients with MV IE 9 patients with MVP |
96 controls 6 controls with MVP |
3.5 (1.1 – 10.5) |
| Strom [14] | 1988–1990 | Population-based, case-control† | 273 patients with IE 248 patients with native IE‡ 52 patients with MVP |
273 controls§
6 controls with MVP |
19.4 (6.4–58.4) |
| MacMahon [15] | 1976–1984 | Case-control¶ | 136 patients with IE‡
19 patients with MVP** 17 patients with MVP and murmurs |
144 controls††
57 controls with MVP 27 controls with MVP and murmurs |
13.0 (2.1–79.0)‡‡ |
CI = confidence interval; IE = infective endocarditis; MV = mitral valve; MVP = mitral valve prolapse; OR = odds ratio * Matched-triplets analysis. † Aim of the study was to quantitate the risk of endocarditis from dental treatment and cardiac abnormalities. During the preceding 3 months, dental treatment was similar among case- and control-patients (adjusted OR, 0.8 [95% CI, 0.4 to 1.5]). ‡ The involved valve (site of infection) of IE was not specified. § One control from the community was selected for each case-patient. ¶ Aim of the study was to evaluate the risk of IE in patients with MVP with and without precordial systolic murmurs. **The site of infection was the mitral valve in 18 cases and tricuspid valve in 1 case. †† The control subjects were selected from 144 consecutive patients with echocardiographic MVP. ‡‡ The number reflects the relative risk of IE associated with presence of a systolic murmur in a patient with MVP.
A total of 110 studies were identified that published descriptive statistics on the proportion of patients with mitral valve prolapse in newly diagnosed infective endocarditis cases. The mean number of patients included in the 110 studies was 160 (median 111, IQR 72–202). Of the 110 studies, the mean proportion of patients with mitral valve prolapse was 8.5% (median 7.7%, IQR 4.4–11.4%). Of the 110 studies, 20 (18.2%) enrolled patients after the publication of the modified Duke criteria [8].
The 1998 definition stated that there was no consensus on the two-dimensional echocardiographic criteria for mitral valve prolapse and no single view should be considered diagnostic [17]. Meanwhile, echo technique has improved, and with three-dimensional echocardiography, mitral valve prolapse can be detected more precisely.
Three studies used analytical statistics. Todd et al. [18] described 29 patients with echocardiographically confirmed infective endocarditis and 79 controls (with echocardiograms) from 2002 to 2004 in the UK. The authors reported that a patient with prior infective endocarditis has an odds ratio of 2.2 (95% CI 0.4–10.3, p = 0.383) for developing infective endocarditis. Alagna et al. [19] reported a study of 1874 patients from the ICE Cohort from 2000 to 2006 with a 1-year follow-up. Prior infective endocarditis had a reported odds ratio of 2.8 (95% CI 1.5–5.1) for causing infective endocarditis. Strom et al. [14] reviewed 279 cases of infective endocarditis from 1988 to 1990 from 54 hospitals in Delaware Valley (USA). Compared with that of the controls, the odds ratio for developing infective endocarditis with prior infective endocarditis in these cases was 35.2. In a preliminary analysis of heterogeneity (data shown elsewhere [11]), this study contributed over-proportionately to the heterogeneity.
Ninety-four studies were identified that published descriptive statistics on the proportion of patients with a history of prior infective endocarditis in newly diagnosed infective endocarditis cases. Twenty-three (24.5%) of them included patients only after the publication of the modified Duke criteria [8]. The mean number of patients included in the study was 263 (median 122, IQR 80–239).The mean proportion of patients with infective endocarditis plus a history of previous infective endocarditis was 8.3% (median 7.1%, IQR 4.9–10.2%).
Only one study used analytical statistics. Verheugt et al. [20] described patients from the CONCOR national registry for adults with congenital heart disease from the Netherlands, reporting a hazard ratio of 6.3 (95% CI 3.0–13.4).
Seventy-seven studies were identified that published descriptive statistics on the proportion of patients with a history of a bicuspid aortic valve in newly diagnosed infective endocarditis cases. Of these studies, 20 (26%) included patients after the publication of the modified Duke criteria [8]. The mean number of patients included in the study was 185 (median 134, IQR 79–249.5).The mean proportion of patients with a bicuspid aortic valve was 8.8% (median 5.6%, IQR 3–12%).
In 1974, Nanda et al. [21] had already demonstrated that a noninvasive diagnosis of bicuspid aortic valve is possible.
Forty-six studies mentioned aortic stenosis in association with infective endocarditis, with two that used analytical statistics. In 2011, Verheugt et al. [20] described patients from the CONCOR national registry for adults with congenital heart disease from the Netherlands and reported a hazard ratio of 4.9 (95% CI 2.2–10.5). Gersony et al. [22] reported a study of 462 patients with aortic stenosis from the second natural history study of congenital heart defects conducted in the USA between 1958 and 1965, and a prevalence rate of 21.6 per 10,000 patients (95% CI 0.5–120.6). Follow-up was 8115 person-years, and patients with conservative management had an infective endocarditis incidence rate of 15.7 per 10,000 person-years (95% CI 6.3–32.4). The authors stated that only the severity of the aortic stenosis was related to the occurrence of infective endocarditis.
Of the 45 descriptive studies, 11 (24.4%) included patients only after the publication of the modified Duke criteria [8]. The mean number of patients included in the study was 242 (median 106, IQR 62–212). Of these studies, the mean proportion of patients with a history of aortic stenosis was 7.3% (median 6.7%, IQR 2.6–9.7%).
The most important change concerning the echo criteria was the mean gradient, which defines the severity of aortic stenosis. The guidelines from 2006 defined severe aortic stenosis as a mean gradient of ≥40 mm Hg (instead of ≥50 mm Hg in 1998), and the guidelines from 2014 changed the definition of moderate aortic stenosis to begin at ≥20 mm Hg instead of ≥25 mm Hg. Low-flow, low-gradient aortic stenosis was first defined in 2006, which is important for patients with reduced systolic ejection fraction (table S1 in appendix 1). Developments in echo techniques and quality (e.g., better screen resolution, better transducers) also played an important role in diagnostic improvements.
None of the studies reported analytical statistical methods.
Forty-one studies were found with mitral insufficiency in association with infective endocarditis. Nine (22%) included patients only after the publication of the modified Duke criteria [8]. The mean number of patients included in the study was 288 (median 101, IQR 56–210).The mean proportion of patients with mitral insufficiency was 19.9% (median 16%, IQR 5.2–28.6%).
The definitions of the grades of mitral insufficiency were implemented in the 2006 guidelines [23, 24]. Echo criteria were mentioned previously in the 1998 American Heart Association (AHA) guidelines concerning the time of surgery and left ventricle diameters [17].
None of the studies reported analytical statistical methods.
Thirty-nine publications with aortic insufficiency in association with infective endocarditis were found. Eight (20.5%) included patients only after the publication of the modified Duke criteria [8]. The mean number of patients included in the study was 234 (median 95, IQR 53.5–211). The mean proportion of patients with a history of aortic insufficiency was 10.2% (median 8.1%, IQR 3.1–16.6%).
Prior to 1998, visualisation by cineangiography and “eyeball guessing” of the regurgitant volume was common. In 2003, with recommendations by the American Society of Echocardiography [24], and later in 2006 with implementation in the AHA guidelines [23], the echo criteria were published.
For the diagnosis of definite infective endocarditis, Duke minor criteria play a relevant role when only one major criterion or none of the major criteria are fulfilled. In a study by Rognon et al. [25], 76% of patients with infective endocarditis had a predisposing heart condition as a minor criterion for the diagnosis. The authors stated that in the absence of the minor criterion, 27% of definite cases of infective endocarditis would be relegated to lower diagnostic categories. In a study by Durante-Mangoni et al. [26], the criterion “predisposing native cardiac condition” was fulfilled in 29.7% of younger infective endocarditis patients and in 34.9% of elderly patients. In a study by Habib et al. [27], the criterion “predisposition, heart disease” was fulfilled in 71% of patients. It is commonly accepted that certain heart valve pathologies predispose to infective endocarditis. In clinical practice, however, it is unclear which of the possible heart pathologies should be counted as Duke minor criterion. Thus, in the absence of echocardiographic findings qualifying for a major criterion, definite infective endocarditis may be over- or underdiagnosed.
The vast majority of the studies (>95%) included in our systematic review used descriptive statistics only. Only a few studies investigated a valve pathology as a risk factor for infective endocarditis with analytical statistics. Moreover, at least three quarters of all included studies involved patients who presented with infective endocarditis prior to the publication of the modified Duke criteria [8]. Considering the evolution of echocardiographic techniques and classifications of valve disease severity in the past two decades, it is not surprising that there is substantial confusion on this subject [7].
Here, we reviewed six heart conditions divided into two categories on the basis of the number of publications. The rationale for this methodology was developed after we found only few studies that used analytical statistics, making quality assessment difficult. There were a high number of publications on mitral valve prolapse, prior infective endocarditis and bicuspid aortic valve, but less than 5% used analytical statistics to assess the risk for developing infective endocarditis. In three studies on mitral valve prolapse, the odds ratio was 3.5 to 8.2 [3, 12, 13]. For many years, mitral valve prolapse was diagnosed via auscultation. For this reason, and considering that 81.8% of the studies were published prior to the publication of the modified Duke criteria, the influence of modern echocardiographic techniques on the diagnosis of mitral valve prolapse is likely to be considerable. We assessed the influence of echocardiographic techniques as less relevant for the predisposing conditions “prior infective endocarditis” and “bicuspid aortic valve”. Two studies reported an odds ratio of 2.2 and 2.8 for prior infective endocarditis [18, 19] and one a hazard ratio of 6.3 for presence of a bicuspid aortic valve [20] for developing infective endocarditis.
From today’s perspective, our results on aortic stenosis, aortic insufficiency and mitral insufficiency are not helpful for clinical practice. Apart from two publications, we found no studies that used analytical statistics. We also assessed the influence of modern echocardiographic techniques on the diagnosis of these valve pathologies (present or absent) as being high. It was not possible to judge the influence of the categorisation into mild, moderate or severe valve pathology, because severity was not assessed in most studies. The evolution of these categories over past decades was not considered (table S1 in appendix 1), because three quarters of the studies included patients prior to the publication of the modified Duke criteria.
Our systematic review has several limitations. It is possible that our search strategy failed to find additional relevant publications, as only publications in German and English were considered and only one database was used to identify relevant publications. The search did not include grey literature sources. We did not assess for publication bias. The literature review included studies with considerable heterogeneity, as assessed previously [11]. We included studies with different criteria for infective endocarditis. We tried to counterbalance this by categorising studies as prior to 2001 and after 2001. We thereby focused on the years in which the patients were included in each study and not on the publication year of the corresponding study. Finally, the categorisation by numbers of publications was arbitrary and the number of publications does not necessarily reflect the quality of studies.
In conclusion, this systematic review demonstrates insufficient evidence to identify native valve diseases that predispose to infective endocarditis. Mitral valve prolapse, prior infective endocarditis and bicuspid aortic valve had the highest number of included publications. The numbers of well-described analytical studies focussing on these valve pathologies were 3, 2 and 1, respectively. The reported risks were odds ratios of 3.5 to 8.2 for mitral valve prolapse, ORs of 2.2 and 2.8 for prior infective endocarditis and a hazard ratio 6.3 for bicuspid aortic valve.
In clinical practice, the diagnostic uncertainty about the Duke criterion “predisposing heart condition” may lead to a high suspicion of infective endocarditis in patients with positive results of blood cultures (e.g., non-staphylococcal bacteraemia), but inconclusive imaging results. In the early phase of disease, it may be prudent to over diagnose infective endocarditis and to perform echocardiography. In the longer course of the disease, however, overtreatment of infective endocarditis contributes to the development of resistance of organisms in the microbiome and is associated with adverse effects of antimicrobial agents. This systematic review supports (though with little evidence) the view that it is reasonable to consider mitral valve prolapse, prior infective endocarditis and bicuspid aortic valve as predisposing conditions when infective endocarditis is suspected at first clinical presentation (i.e., possible infective endocarditis). However, over a 2-week period, the clinical course, microbiological criteria and repeated imaging with modern techniques should allow confirmation or rejection of the “definite” diagnosis of infective endocarditis in most cases, irrespective of the presence of valve disease.
The appendices 1 and 2 can be downloaded from https://smw.ch/en/article/doi/smw.2018.14675/
This systematic review is part of a medical thesis published on the website of the University of Bern, Switzerland [11].
No funding was available for this publication.
No potential conflict of interest relevant to this article was reported
1 Pant S , Patel NJ , Deshmukh A , Golwala H , Patel N , Badheka A , et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65(19):2070–6 . [doi:.].https://doi.org/10.1016/j.jacc.2015.03.518
2 Cherubin CE , Neu HC . Infective endocarditis at the Presbyterian Hospital in New York City from 1938-1967. Am J Med. 1971;51(1):83–96 . [doi:.].https://doi.org/10.1016/0002-9343(71)90326-3
3 Clemens JD , Horwitz RI , Jaffe CC , Feinstein AR , Stanton BF . A controlled evaluation of the risk of bacterial endocarditis in persons with mitral-valve prolapse. N Engl J Med. 1982;307(13):776–81 . [doi:.].https://doi.org/10.1056/NEJM198209233071302
4 Beton DC , Brear SG , Edwards JD , Leonard JC . Mitral valve prolapse: an assessment of clinical features, associated conditions and prognosis. Q J Med. 1983;52(206):150–64.
5 McKinsey DS , Ratts TE , Bisno AL . Underlying cardiac lesions in adults with infective endocarditis. The changing spectrum. Am J Med. 1987;82(4):681–8 . [doi:.].https://doi.org/10.1016/0002-9343(87)90001-5
6 Weinberger I , Rotenberg Z , Zacharovitch D , Fuchs J , Davidson E , Agmon J . Native valve infective endocarditis in the 1970s versus the 1980s: underlying cardiac lesions and infecting organisms. Clin Cardiol. 1990;13(2):94–8 . [doi:.].https://doi.org/10.1002/clc.4960130206
7 Büchi AE , Hoffmann M , Zbinden S , Sendi P . Infective Endocarditis: How Do We Currently Interpret the Duke Minor Criterion “Predisposing Heart Condition” in Native Valves? Cardiol Ther. 2017;6(1):121–8 . [doi:.].https://doi.org/10.1007/s40119-016-0074-2
8 Li JS , Sexton DJ , Mick N , Nettles R , Fowler VG, Jr , Ryan T , et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633–8 . [doi:.].https://doi.org/10.1086/313753
9 Von Reyn CF , Levy BS , Arbeit RD , Friedland G , Crumpacker CS . Infective endocarditis: an analysis based on strict case definitions. Ann Intern Med. 1981;94(4 pt 1):505–18 . [doi:.].https://doi.org/10.7326/0003-4819-94-4-505
10 Moher D , Altman DG , Liberati A , Tetzlaff J . PRISMA statement. Epidemiology. 2011;22(1):128 , author reply 128 . [doi:.].https://doi.org/10.1097/EDE.0b013e3181fe7825
11Büchi AE, Hoffmann M. Infective endocarditis: What are predisposing conditions in native valves? https://boris.unibe.ch/105476/: Universität Bern; 2017.
12 Devereux RB , Hawkins I , Kramer-Fox R , Lutas EM , Hammond IW , Spitzer MC , et al. Complications of mitral valve prolapse. Disproportionate occurrence in men and older patients. Am J Med. 1986;81(5):751–8 . [doi:.].https://doi.org/10.1016/0002-9343(86)90339-6
13 Danchin N , Briancon S , Mathieu P , Dureux J-B , Voiriot P , Bairati I , et al. Mitral valve prolapse as a risk factor for infective endocarditis. Lancet. 1989;333(8641):743–5 . [doi:. ].https://doi.org/10.1016/S0140-6736(89)92571-3
14 Strom BL , Abrutyn E , Berlin JA , Kinman JL , Feldman RS , Stolley PD , et al. Dental and cardiac risk factors for infective endocarditis. A population-based, case-control study. Ann Intern Med. 1998;129(10):761–9 . [doi:.].https://doi.org/10.7326/0003-4819-129-10-199811150-00002
15 MacMahon SW , Hickey AJ , Wilcken DE , Wittes JT , Feneley MP , Hickie JB . Risk of infective endocarditis in mitral valve prolapse with and without precordial systolic murmurs. Am J Cardiol. 1987;59(1):105–8 . [doi:.].https://doi.org/10.1016/S0002-9149(87)80080-2
16 Zuppiroli A , Rinaldi M , Kramer-Fox R , Favilli S , Roman MJ , Devereux RB . Natural history of mitral valve prolapse. Am J Cardiol. 1995;75(15):1028–32 . [doi:.].https://doi.org/10.1016/S0002-9149(99)80718-8
17 Bonow RO , Carabello B , de Leon AC , Edmunds LH, Jr , Fedderly BJ , Freed MD , et al. ACC/AHA Guidelines for the Management of Patients With Valvular Heart Disease. Executive Summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Valvular Heart Disease). J Heart Valve Dis. 1998;7(6):672–707.
18 Todd AJ , Leslie SJ , Macdougall M , Denvir MA . Clinical features remain important for the diagnosis of infective endocarditis in the modern era. QJM. 2006;99(1):23–31 . [doi:.].https://doi.org/10.1093/qjmed/hci150
19 Alagna L , Park LP , Nicholson BP , Keiger AJ , Strahilevitz J , Morris A , et al. Repeat endocarditis: analysis of risk factors based on the International Collaboration on Endocarditis - Prospective Cohort Study. Clin Microbiol Infect. 2014;20(6):566–75 . [doi:.].https://doi.org/10.1111/1469-0691.12395
20 Verheugt CL , Uiterwaal CS , van der Velde ET , Meijboom FJ , Pieper PG , Veen G , et al. Turning 18 with congenital heart disease: prediction of infective endocarditis based on a large population. Eur Heart J. 2011;32(15):1926–34 . [doi:.].https://doi.org/10.1093/eurheartj/ehq485
21 Nanda NC , Gramiak R , Manning J , Mahoney EB , Lipchik EO , DeWeese JA . Echocardiographic recognition of the congenital bicuspid aortic valve. Circulation. 1974;49(5):870–5 . [doi:.].https://doi.org/10.1161/01.CIR.49.5.870
22 Gersony WM , Hayes CJ , Driscoll DJ , Keane JF , Kidd L , O’Fallon WM , et al. Bacterial endocarditis in patients with aortic stenosis, pulmonary stenosis, or ventricular septal defect. Circulation. 1993;87(2, Suppl):I121–6.
23 Bonow RO , Carabello BA , Chatterjee K , de Leon AC , Faxon DP , Freed MD , et al., Society of Thoracic Surgeons. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114(5):e84–e231 . [doi:.].https://doi.org/10.1161/CIRCULATIONAHA.106.176857
24 Zoghbi WA , Enriquez-Sarano M , Foster E , Grayburn PA , Kraft CD , Levine RA , et al.; American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777–802 . [doi:.].https://doi.org/10.1016/S0894-7317(03)00335-3
25 Rognon R , Kehtari R , Francioli P . Individual value of each of the Duke criteria for the diagnosis of infective endocarditis. Clin Microbiol Infect. 1999;5(7):396–403 . [doi:.].https://doi.org/10.1111/j.1469-0691.1999.tb00163.x
26 Durante-Mangoni E , Bradley S , Selton-Suty C , Tripodi MF , Barsic B , Bouza E , et al.; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med. 2008;168(19):2095–103 . [doi:.].https://doi.org/10.1001/archinte.168.19.2095
27 Habib G , Derumeaux G , Avierinos JF , Casalta JP , Jamal F , Volot F , et al. Value and limitations of the Duke criteria for the diagnosis of infective endocarditis. J Am Coll Cardiol. 1999;33(7):2023–9 . [doi:.].https://doi.org/10.1016/S0735-1097(99)00116-3
No funding was available for this publication.
No potential conflict of interest relevant to this article was reported