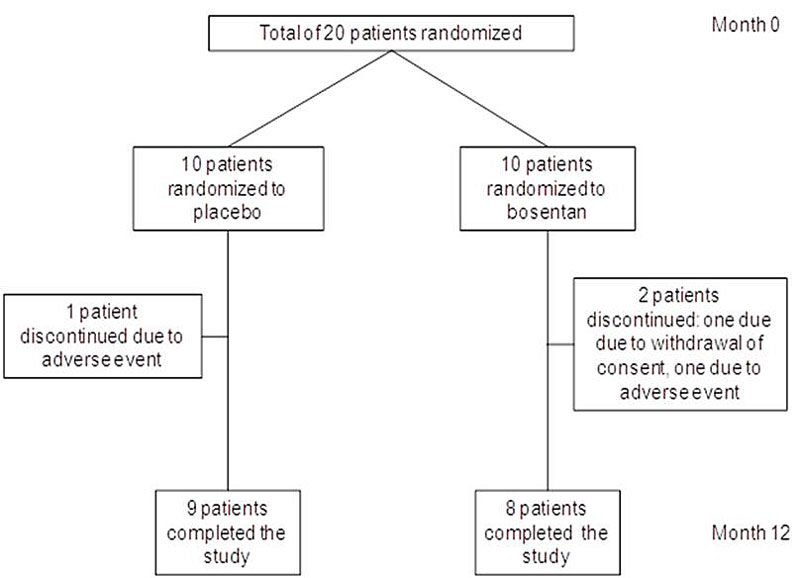
Figure 1 Patient disposition.
DOI: https://doi.org/10.4414/smw.2018.14677
Sarcoidosis is a systemic inflammatory disorder of unknown origin occurring most commonly in young and middle-aged adults. Ninety percent of patients have thoracic involvement, with typically bilateral hilar lymphadenopathy and parenchymal lung involvement [1]. Spontaneous resolution of the disease occurs in nearly 60% of patients [2, 3], but chronic fibrotic disease with functional deficit or organ failure may affect up to 30% of patients [4]. The treatment of pulmonary sarcoidosis is mostly empirical, with corticosteroids as the standard first choice therapy [5, 6]. However, in a minority of patients corticosteroids treatment does not achieve full control of the disease and alternative strategies are required.
Endothelin-1 (ET-1) is a peptide that has several actions such as vaso- and bronchoconstriction [7, 8]. In the normal lung ET-1 is secreted by vascular endothelial cells, airway epithelial cells and mesenchymal cells [9, 10], whereas in fibrotic lung diseases ET-1 is also released by macrophages [11]. ET-1 exerted mitogenic activity on mesenchymal cells [12–14] and elevated levels of ET-1 has been shown in animal models of pulmonary fibrosis [15–17]. Enhanced expression of ET-1 has been demonstrated in the lung tissue and serum derived from patients with idiopathic pulmonary fibrosis, suggesting a role of ET-1 in fibrotic lung diseases [18, 19]. Importantly, in patients with sarcoidosis elevated levels of ET-1 were detected in the bronchoalveolar lavage fluid as well as in the serum [20–22].
ET-1 mediates its effects by two specific receptors, ET-A and ET-B [23]. Bosentan is an orally active ET-A and ET-B receptor antagonist [24] and the pro-fibrotic effects of ET-1 were attenuated by bosentan in an animal model [15]. In a single case report, improvement of lung function was reported in a patient with pulmonary sarcoidosis after bosentan treatment [25] and in patients with sarcoidosis-associated pulmonary hypertension bosentan had a significant beneficial effect on pulmonary haemodynamics [26]. Therefore, we conducted a double-blind, placebo-controlled trial to determine whether bosentan was a valid treatment alternative for patients with corticosteroid-resistant pulmonary sarcoidosis.
Adult patients were eligible if they had histology-proven sarcoidosis, evidence of lung parenchymal disease on high resolution computed tomography (HRCT) scan, an impaired exercise capacity (maximal oxygen uptake [VO2max] <80% predicted) or impaired resting lung function (forced expiratory volume in 1 second [FEV1], forced vital capacity [FVC], diffusion capacity of the lung for carbon monoxide [DLCO] <80% predicted). Patients had to have had persistent impairment of lung function or exercise capacity despite long-term treatment (5 mg/d prednisone or equivalent and/or other immunosuppressive agents for at least 2 months). Concurrent corticosteroids and/or immunosuppressive agents were to remain stable throughout the study. Major exclusion criteria were the presence of a systemic illness other than sarcoidosis requiring immunosuppressive therapy, honey combing of >10% on HRCT scan, marked disturbance of liver enzymes at baseline (>4-fold increase in serum glutamic-oxaloacetic transaminase [SGOT] or serum glutamic-pyruvic transaminase [SGPT]), pregnancy, relevant psychiatric illness or addictive disorder, previous or current treatment with bosentan, and therapy with ciclosporin. In this placebo-controlled phase II study patients were randomly assigned in a 1:1 ratio to either bosentan 62.5 mg twice daily for 4 weeks followed by bosentan 125 mg twice daily for 11 months, or to placebo. A permutated block randomisation procedure was used. The allocation was concealed by means of the following process: randomisation was centralised in a site remote from the trial location and the investigator received only coded numbers of drug containers at the time of patient’s allocation. The total duration of patient follow-up was twelve months. The study agent was provided by Actelion Pharma Schweiz AG.
Twenty patients from two centres in Switzerland were randomised between November 2007 and July 2012. The study was approved by the ethics committees at each site and patients have given their written, informed consent.
Primary endpoints were safety and overall response rate of total lung capacity (TLC), FVC, DLCO, VO2max, 6-minute walking distance (6MWD), and chest HRCT score at month 12. Secondary endpoints included adverse events and quality of life assessed with the Medical Outcomes Study Short Form 36 (SF-36) questionnaire [27]. Pulmonary function tests (PFTs) and 6MWD tests were performed at baseline and at months 3, 6 and 12. Pulmonary function was measured using body plethysmography and carbon monoxide diffusion capacity (Jaeger, Wuerzburg, Germany). All testing was according to the European Respiratory Society or American Thoracic Society standards [28, 29]. Cardiopulmonary exercise testing with measurement of VO2max [30] were performed at baseline and at month 12. The chest HRCT scoring system [31, 32] compared the scans at baseline and at month 12 (maximal score of 18 points). Pulmonary hypertension was excluded by echocardiography at baseline. Blood samples were collected monthly for control of leukocytes, haemoglobin, platelets, SGOT, SGPT, bilirubin, and alkaline phosphatase.
The original per-protocol sample size of 36 patients provided the trial 80% power to detect a significant change of 10% in at least one of the primary endpoints between the treatment and placebo groups. The estimated sample size was calculated based on the expected effect of bosentan on TLC, assuming a baseline TLC of 70% percent predicted (standard deviation [SD] 5%) in both arms and a clinically relevant increase of 10% (SD 3%) TLC in the bosentan arm vs 0% in the placebo arm. The sample size calculation was estimated through simulations, taking into account the within-patient study design. Owing to impeded recruitment the trial had to be terminated before the planned sample sized could be reached. Baseline patient characteristics were reported using descriptive statistics (median and inter-quartile-range [IQR], unless otherwise specified). The changes from baseline of the primary and secondary endpoints were analysed using linear mixed effect models. From the resulting models, p-values were reported and model-based least-squares means were used to test the between-group differences using post-hoc t-tests. All analyses and graphical representations were done using the R statistical software (v. 3.1.0) [33] including the extension packages lme4, multcomp and ggplot2.
A total of 20 patients were randomised: 10 to placebo and 10 to bosentan. Three patients discontinued the study medication prematurely: one patient in the bosentan group withdrew consent; in two patients (one from the placebo group and one from the bosentan group) adverse events occurred. Seventeen patients completed the study (fig. 1). Baseline demographics, PFT, 6MWD, VO2max, HRCT scores and concomitant medication of the 20 patients are shown in table 1. Four patients (two from the placebo group and two from the bosentan group) were receiving corticosteroid monotherapy for sarcoidosis, five patients (one from the placebo group and four from the bosentan group) were on azathioprine monotherapy, three patients (two from the placebo group and one from the bosentan group) were receiving infliximab only, two patients (both from the placebo group) were treated with a combination therapy of corticosteroids and azathioprine, three patients (all from the bosentan group) were on a combination therapy with corticosteroids and infliximab, and three patients (all from the placebo group) were receiving a combination of infliximab and azathioprine.

Figure 1 Patient disposition.
Table 1 Baseline demographics, pulmonary function tests, high resolution computed tomography score and concomitant medication.
| Characteristics |
Placebo
(n = 10) |
Bosentan
(n = 10) |
|---|---|---|
| Age | 42.8 (40.0–56.9) | 47 (44.1–59.6) |
| Male/Female (n/n) | 8 / 2 | 6 / 4 |
| TLC (L) | 5.6 (5.3–6.0) | 5.3 (4.2–6.1) |
| FVC (L) | 3.8 (2.9–4.3) | 3.4 (2.9–4.2) |
| DLCO (mmol/min/kPa) | 7.8 (6.2–9.9) | 6.1 (5–10) |
| 6MWD (m) | 499.5 (412.0–540.0) | 525 (480.0–568.0) |
| VO2max (ml/min) | 1592.0 (1239.0–1866.0) | 1413.5 (1334.0–1733.0) |
| HRCT score | 7 (4–8) | 6 (6–8) |
| Concomitant medication, n (%) | ||
| Corticosteroids only | 2 (20.0) | 2 (22.2) |
| Corticosteroid + immunomodulator | 2 (20.0) | 0 (0.0) |
| Corticosteroid + anti-TNFα therapy | 0 (0.0) | 2 (22.2) |
| Immunomodulator only | 1 (10.0) | 4 (44.4) |
| Anti-TNFα therapy only | 2 (20.0) | 1 (11.1) |
| Anti-TNFα therapy + immunomodulator | 3 (30.0) | 0 (0.0) |
6MWD = 6-minute walking distance; DLCO = diffusion capacity of the lung for carbon monoxide; FVC = forced vital capacity; HRCT = high resolution computed tomography; TLC = total lung capacity; TNF = tumour necrosis factor; VO2max = maximal oxygen uptake Values are presented as median (interquartile range) unless otherwise specified.
Mean absolute values of TLC, FVC, DLCO, 6MWD, and VO2max at baseline, and months 3, 6 and 12 are shown in fig. 2. There was no statistically significant change for any of the primary endpoints (table 2). In addition, no statistically significant between-group differences were found (table 2). Figure 3 depicts the percent change of mean absolute values from baseline to month 12, and figure 4 demonstrates absolute values of TLC, FVC, DLCO, 6MWD and VO2max for all individuals at baseline, and at months 3, 6 and 12. There was no statistically significant change of FVC over time (decrease or increase) in either the bosentan group or the placebo group, with also no significant interaction. Five out of eight patients (63%) treated with bosentan showed an increase of 10% in at least one of the primary endpoints, compared with six out of nine (67%) in the placebo group (p = 1).
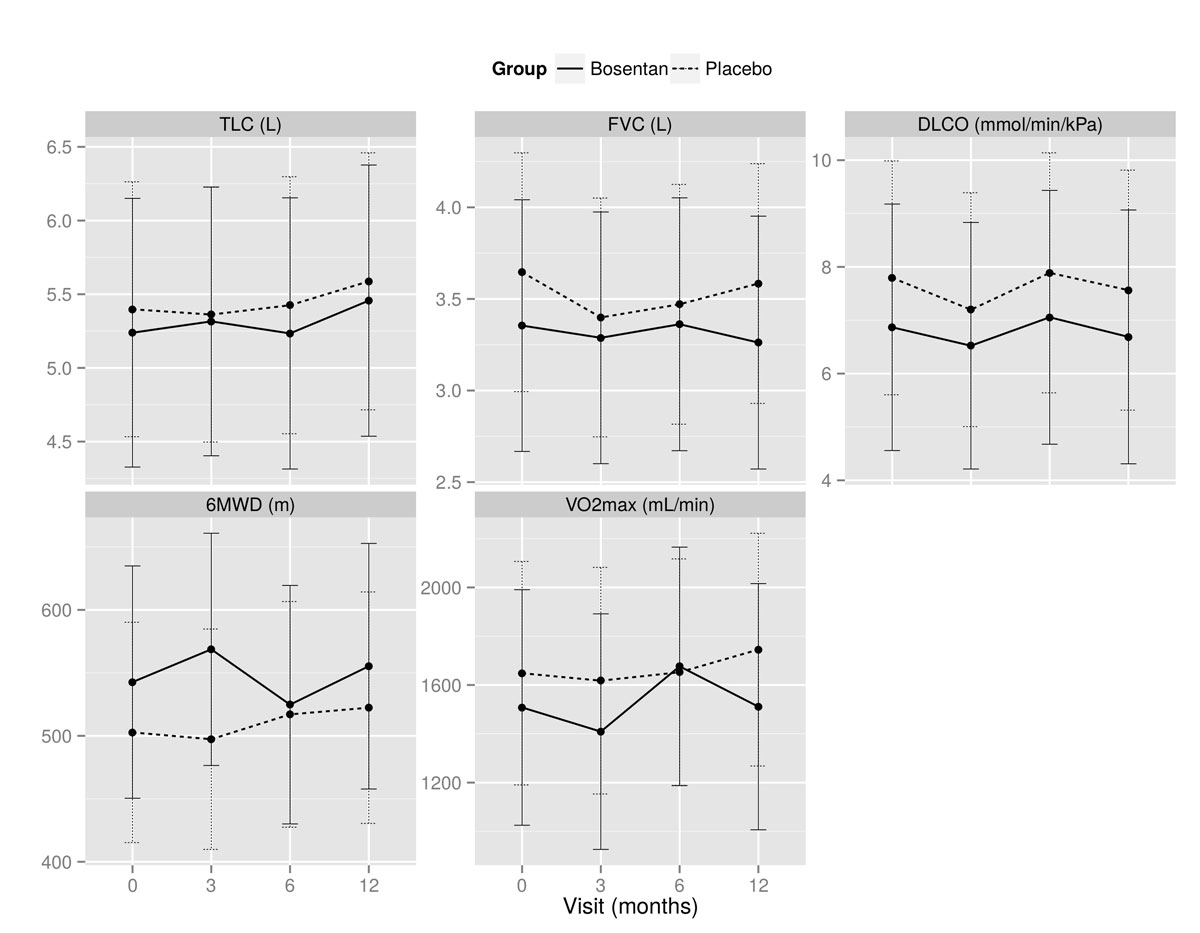
Figure 2 Evolution of mean (± SD) absolute values at baseline and months 3, 6 and 12 for total lung capacity (TLC), forced vital capacity (FVC), diffusion capacity of the lung for carbon monoxide (DLCO), 6-minute walking distance (6MWD), and maximal oxygen uptake (VO2max).
Table 2 Change from baseline to 12 months in mean absolute total lung capacity (TLC), forced vital capacity (FVC), diffusion capacity of the lung for carbon monoxide (DLCO), 6-minute walking distance (6MWD), and maximal oxygen uptake (VO2max).
|
Placebo
(n = 9) |
Bosentan
(n = 8) |
Between-group differences | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | p-value | Baseline | 12 months | p-value | Δ placebo − Δ bosentan | p-value | |
| TLC (L) | 5.4 ± 0.4 | 5.6 ± 0.4 | 0.402 | 5.2 ± 0.4 | 5.4 ± 0.4 | 0.570 | 0.0 ± 0.3 | 0.887 |
| FVC (L) | 3.6 ± 0.3 | 3.6 ± 0.3 | 0.996 | 3.4 ± 0.3 | 3.3 ± 0.3 | 0.594 | 0.1 ± 0.1 | 0.545 |
| DLCO (mmol/min/kPa) | 7.8 ± 0.8 | 7.8 ± 0.8 | 0.997 | 6.9 ± 0.9 | 6.7 ± 0.9 | 0.829 | 0.2 ± 0.5 | 0.725 |
| 6MWD (m) | 502.7 ± 30.2 | 518.0 ± 31.6 | 0.740 | 542.7 ± 31.8 | 558.3 ± 33.6 | 0.758 | −0.4 ± 32.4 | 0.991 |
| VO2max (mL/min) | 1648.4 ± 155.2 | 1731.9 ± 160.2 | 0.449 | 1508.0 ± 163.6 | 1528.8 ± 169.8 | 0.958 | 62.7 ± 108.6 | 0.563 |
6MWD = 6-minute walking distance; DLCO = diffusion capacity of the lung for carbon monoxide; FVC = forced vital capacity; TLC = total lung capacity; VO2max = maximal oxygen uptake Values are mean estimates ± standard error.

Figure 3 Percent change at baseline and months 3, 6 and 12 for total lung capacity (TLC), forced vital capacity (FVC), diffusion capacity of the lung for carbon monoxide (DLCO), 6-minute walking distance (6MWD), and maximal oxygen uptake (VO2max).
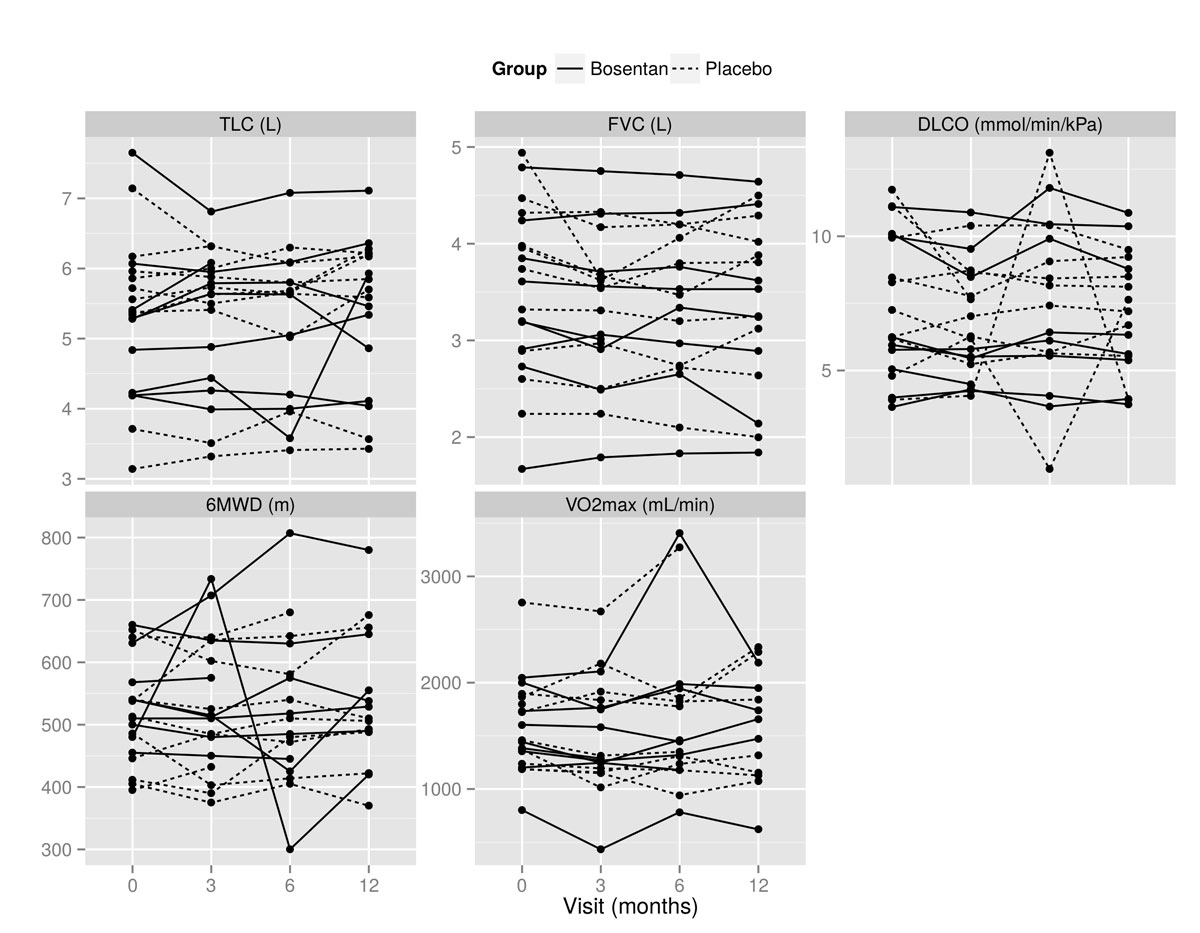
Figure 4 All individual values at baseline and months 3, 6 and 12 for total lung capacity (TLC), forced vital capacity (FVC), diffusion capacity of the lung for carbon monoxide (DLCO), 6-minute walking distance (6MWD), and maximal oxygen uptake (VO2max).
There was no significant difference in the HRCT score between baseline and 12 months for either the bosentan group or the placebo group (table 3). Similarly, there was no significant difference with regard to change from baseline to months 3, 6, 9 and 12 in SF-36 score (table 3). No statistically significant between-group differences were found (table 3).
Table 3 Within-patient change from baseline in high resolution computed tomography (HRCT) score and the Medical Outcomes Study Short Form 36 (SF-36) questionnaire score.
| Placebo (n = 9) | Bosentan (n = 8) | Between-group differences | ||||
|---|---|---|---|---|---|---|
| Change from baseline | p-value | Change from baseline | p-value | Δ placebo − Δ bosentan | p-value | |
| HRCT score at 12 months | −0.24 ± 0.25 | 0.571 | −0.25 ± 0.25 | 0.534 | 0.01 ± 0.35 | 0.968 |
| SF-36 scores | ||||||
| 3-month | −4.70 ± 4.05 | 0.843 | 3.17 ± 4.27 | 0.985 | −7.86 ± 5.88 | 0.480 |
| 6-month | −6.66 ± 4.21 | 0.546 | 2.65 ± 4.46 | 0.996 | −9.31 ± 6.13 | 0.364 |
| 9-month | −3.23 ± 4.21 | 0.982 | −1.90 ± 4.46 | 1.000 | −1.33 ± 6.13 | 0.999 |
| 12-month | 1.44 ± 4.37 | 1.000 | −0.71 ± 4.66 | 1.000 | 2.15 ± 6.39 | 0.992 |
Values are mean estimates ± standard error.
Nine patients from a total of ten receiving bosentan tolerated the 12 months of treatment without any clinically relevant side effects. One patient from the bosentan group discontinued the study agent because of malaise. One patient from the placebo group developed hyperthyroidism, which proved to be Graves’ disease, after 3 months of treatment. In both groups no liver function abnormalities were detected during the 12-month study and no other organ-specific toxicities were noted.
In this prospective trial we found that 12 months of treatment with bosentan did not improve lung function, 6MWD, and exercise capacity in patients with steroid-resistant pulmonary sarcoidosis. Bosentan was well tolerated and no drug-related adverse effects were observed within the study population.
Glucocorticoid therapy has been the standard treatment for many years in patients with pulmonary sarcoidosis, and there are no large, randomised, controlled studies that show the superiority of other agents [34]. The majority of patients improve under corticosteroid treatment; however, long-term use of corticosteroids may cause considerable side effects, and whether or not corticosteroids are able to alter the long-term outcome of patients with sarcoidosis is not clear [25]. Finally, in the case of refractory disease and/or the development of pulmonary fibrosis, there is little evidence to support corticosteroid treatment, and thus alternative agents are warranted.
In the present study we investigated the effect of bosentan for patients with refractory pulmonary sarcoidosis, and we observed no improvement of FVC, TLC, and DLCO in the treatment group. Our findings are in line with data obtained in patients with idiopathic pulmonary fibrosis [35]. In this large randomised controlled trial of bosentan in patients with idiopathic pulmonary fibrosis no significant difference in absolute FVC and DLCO was observed, but the authors considered the criteria applied to assess treatment response in their study as “challenging” [35]. Furthermore, in a double-blind, placebo-controlled trial of 16 weeks bosentan for patients with sarcoidosis-associated pulmonary hypertension, a significant improvement of pulmonary haemodynamics was demonstrated in the bosentan treated group, but no improvement of FVC was observed [26]. The anti-tumour necrosis factor (TNF) agent infliximab has been studied in patients with refractory, steroid-resistant pulmonary sarcoidosis [36, 37]. However results are somewhat contradictory. Whereas one trial showed a statistically significant improvement in percentage predicted FVC after 24 weeks of infliximab therapy [36], in another randomised trial no difference in FVC between placebo and infliximab-treated patients was observed [37]. In our own retrospective analysis of long-term infliximab use in patients with chronic steroid-resistant sarcoidosis, we pointed out that patients with predominantly extrapulmonary sarcoidosis seemed to profit more than patients with predominantly pulmonary disease [38]. In a recent retrospective study, TNF antagonists were efficacious in about two-thirds of patients but their use led to a high rate of adverse events [39]. In a small, open-label study with the humanised monoclonal anti-TNF antibody adalimumab, an improvement in patients with refractory pulmonary sarcoidosis was shown, but only in four out of ten patients FVC improved [40]. Studies with other agents such as thalidomide, etanercept, ustekinumab, golimumab and rituximab failed to show a significant improvement of lung volumes and DLCO [41–44]. Leflunomide was shown to cause a significant gain in FVC in patients with pulmonary sarcoidosis. However, this was a retrospective and uncontrolled trial [45]. A possible reason that the primary endpoint of our study was not met might be that we opted for a change of 10% in FVC or TLC, which is in contrast to the positive infliximab trial where efficacy was demonstrated by a mean increase of FVC of 2.5% only [36]. Equally, an improvement of only 5% in FVC was considered as successful outcome in the adalimumab study [40].
Forced vital capacity is the most commonly used parameter to assess treatment response in patients with pulmonary sarcoidosis [5]. However, as recent data have demonstrated that a considerable number of symptomatic patients with normal DLCO at rest had pulmonary gas exchange impairment during exercise [46], we additionally assessed exercise capacity by 6MWD and cardiopulmonary exercise testing. The precise role of exercise capacity as part of monitoring response to therapy has yet to be defined [47], but it has been demonstrated recently that cardiopulmonary exercise testing variables predict the decline of pulmonary function in patients with pulmonary sarcoidosis [48]. In the present study, we did not see any beneficial effect of bosentan on either 6MWD or VO2max. Our findings are in line with data from the infliximab trial, where no effect on 6MWD was observed [36]. Similarly, no positive effect on 6MWD was found in patients with sarcoidosis-associated pulmonary hypertension when treated with bosentan for 16 weeks, even though pulmonary haemodynamics improved significantly [26]. However, exercise tests need to be interpreted with caution as musculoskeletal factors, cardiac involvement, and deconditioning may influenced their performance [49].
Although HRCT is a sensitive tool for the early diagnosis of diffuse lung disease in sarcoidosis [31], it has not been used to assess treatment response in any clinical sarcoidosis trial yet [5]. We used a HRCT scoring system which has been evaluated before [31, 32] and which has demonstrated a strong association between HRCT abnormalities and functional parameters [32]. In accordance with the lack of benefit in lung function parameters, DLOC and exercise tests we found no beneficial effect of bosentan treatment regarding the HRCT score. This is in agreement with data obtained in the rituximab trial where chest radiographic stages remained unchanged in patients with refractory sarcoidosis [43]. In contrast, the chest radiograph score improved significantly in patients with refractory sarcoidosis treated with infliximab; however, only reticulonodular opacities improved, not fibrosis [36]. An improvement in pulmonary radiographic abnormalities was reported in 12 of 19 patients with advanced pulmonary sarcoidosis treated with high-dose chloroquine; however, previous corticosteroid therapy was not a requirement for study entry [50] and thus the study population was not comparable to ours.
We assessed health-related quality of life using the SF-36 questionnaire, which has been demonstrated earlier to be a sensitive tool for assessing quality of life in patients with interstitial lung diseases [27]. We found no significant change in SF-36 scores, which is in line with the results obtained from patients with chronic sarcoidosis treated with infliximab [36].
We acknowledge that our study is limited by the small population due to the impeded recruitment of patients, as this reduced the power of our trial. However, even though the risk of false negative findings was increased, there is strong evidence that no statistically significant difference between the two groups would have been found with the planned sample size, as no trend in favour of the bosentan group was detected for either primary or secondary study endpoints. Eventually, we are the first to study the effect of bosentan in patients with steroid-resistant pulmonary sarcoidosis and data on larger populations is currently not available,
In conclusion, we found that there is no evidence to support efficacy of bosentan as an antifibrotic treatment for patients with steroid-resistant pulmonary sarcoidosis. Bosentan was well tolerated and no drug-related adverse effects were observed within the study population. It can be speculated that anti-fibrotic drugs such as pirfenidone or nintedanib might represent new therapeutic options for patients with steroid-resistant pulmonary sarcoidosis.
An abstract of his study has been published in the European Respiratory Journal, Vol. 44, Suppl. 58.
This study was supported by Actelion Pharma Schweiz AG.
MHB received a nonconditional grant from Actelion Pharma Schweiz AG. No other potential conflict of interest was reported.
1 Baughman RP . Pulmonary sarcoidosis. Clin Chest Med. 2004;25(3):521–30, vi. doi:.https://doi.org/10.1016/j.ccm.2004.04.006
2 Hillerdal G , Nöu E , Osterman K , Schmekel B . Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130(1):29–32.
3 Hunninghake GW , Gilbert S , Pueringer R , Dayton C , Floerchinger C , Helmers R , et al. Outcome of the treatment for sarcoidosis. Am J Respir Crit Care Med. 1994;149(4):893–8. doi:.https://doi.org/10.1164/ajrccm.149.4.8143052
4 Hunninghake GW , Costabel U , Ando M , Baughman R , Cordier JF , du Bois R , et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16(2):149–73.
5 Baughman RP , Nunes H , Sweiss NJ , Lower EE . Established and experimental medical therapy of pulmonary sarcoidosis. Eur Respir J. 2013;41(6):1424–38. doi:.https://doi.org/10.1183/09031936.00060612
6 Patterson KC , Chen ES . The Pathogenesis of Pulmonary Sarcoidosis and Implications for Treatment. Chest. 2018;153(6):1432–42.
7 Yanagisawa M , Kurihara H , Kimura S , Tomobe Y , Kobayashi M , Mitsui Y , et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–5. doi:.https://doi.org/10.1038/332411a0
8 Inoue A , Yanagisawa M , Kimura S , Kasuya Y , Miyauchi T , Goto K , et al. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci USA. 1989;86(8):2863–7. doi:.https://doi.org/10.1073/pnas.86.8.2863
9 Giaid A , Polak JM , Gaitonde V , Hamid QA , Moscoso G , Legon S , et al. Distribution of endothelin-like immunoreactivity and mRNA in the developing and adult human lung. Am J Respir Cell Mol Biol. 1991;4(1):50–8. doi:.https://doi.org/10.1165/ajrcmb/4.1.50
10 Ehrenreich H , Anderson RW , Fox CH , Rieckmann P , Hoffman GS , Travis WD , et al. Endothelins, peptides with potent vasoactive properties, are produced by human macrophages. J Exp Med. 1990;172(6):1741–8. doi:.https://doi.org/10.1084/jem.172.6.1741
11 Saleh D , Furukawa K , Tsao MS , Maghazachi A , Corrin B , Yanagisawa M , et al. Elevated expression of endothelin-1 and endothelin-converting enzyme-1 in idiopathic pulmonary fibrosis: possible involvement of proinflammatory cytokines. Am J Respir Cell Mol Biol. 1997;16(2):187–93. doi:.https://doi.org/10.1165/ajrcmb.16.2.9032126
12 Hirata Y , Takagi Y , Fukuda Y , Maruno F . Endothelin is a potent mitogen for rat vascular smooth muscle cells. Atherosclerosis. 1989;78(2-3):225–8. doi:.https://doi.org/10.1016/0021-9150(89)90227-X
13 Takuwa N , Takuwa Y , Yanagisawa M , Yamashita K , Masaki T . A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem. 1989;264(14):7856–61.
14 Peacock AJ , Dawes KE , Shock A , Gray AJ , Reeves JT , Laurent GJ . Endothelin-1 and endothelin-3 induce chemotaxis and replication of pulmonary artery fibroblasts. Am J Respir Cell Mol Biol. 1992;7(5):492–9. doi:.https://doi.org/10.1165/ajrcmb/7.5.492
15 Park SH , Saleh D , Giaid A , Michel RP . Increased endothelin-1 in bleomycin-induced pulmonary fibrosis and the effect of an endothelin receptor antagonist. Am J Respir Crit Care Med. 1997;156(2):600–8. doi:.https://doi.org/10.1164/ajrccm.156.2.9607123
16 Mutsaers SE , Foster ML , Chambers RC , Laurent GJ , McAnulty RJ . Increased endothelin-1 and its localization during the development of bleomycin-induced pulmonary fibrosis in rats. Am J Respir Cell Mol Biol. 1998;18(5):611–9. doi:.https://doi.org/10.1165/ajrcmb.18.5.2898
17 Hocher B , Schwarz A , Fagan KA , Thöne-Reineke C , El-Hag K , Kusserow H , et al. Pulmonary fibrosis and chronic lung inflammation in ET-1 transgenic mice. Am J Respir Cell Mol Biol. 2000;23(1):19–26. doi:.https://doi.org/10.1165/ajrcmb.23.1.4030
18 Giaid A , Michel RP , Stewart DJ , Sheppard M , Corrin B , Hamid Q . Expression of endothelin-1 in lungs of patients with cryptogenic fibrosing alveolitis. Lancet. 1993;341(8860):1550–4. doi:.https://doi.org/10.1016/0140-6736(93)90694-C
19 Uguccioni M , Pulsatelli L , Grigolo B , Facchini A , Fasano L , Cinti C , et al. Endothelin-1 in idiopathic pulmonary fibrosis. J Clin Pathol. 1995;48(4):330–4. doi:.https://doi.org/10.1136/jcp.48.4.330
20 Reichenberger F , Schauer J , Kellner K , Sack U , Stiehl P , Winkler J . Different expression of endothelin in the bronchoalveolar lavage in patients with pulmonary diseases. Lung. 2001;179(3):163–74. doi:.https://doi.org/10.1007/s004080000058
21 Terashita K , Kato S , Sata M , Inoue S , Nakamura H , Tomoike H . Increased endothelin-1 levels of BAL fluid in patients with pulmonary sarcoidosis. Respirology. 2006;11(2):145–51. doi:.https://doi.org/10.1111/j.1440-1843.2006.00826.x
22 Letizia C , Danese A , Reale MG , Caliumi C , Delfini E , Subioli S , et al. Plasma levels of endothelin-1 increase in patients with sarcoidosis and fall after disease remission. Panminerva Med. 2001;43(4):257–61.
23 Sakurai T , Yanagisawa M , Takuwat Y , Miyazakit H , Kimura S , Goto K , et al. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348(6303):732–5. doi:.https://doi.org/10.1038/348732a0
24 Clozel M , Breu V , Gray GA , Kalina B , Löffler BM , Burri K , et al. Pharmacological characterization of bosentan, a new potent orally active nonpeptide endothelin receptor antagonist. J Pharmacol Exp Ther. 1994;270(1):228–35.
25 Paramothayan S , Jones PW . Corticosteroid therapy in pulmonary sarcoidosis: a systematic review. JAMA. 2002;287(10):1301–7. doi:.https://doi.org/10.1001/jama.287.10.1301
26 Baughman RP , Culver DA , Cordova FC , Padilla M , Gibson KF , Lower EE , et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest. 2014;145(4):810–7. doi:.https://doi.org/10.1378/chest.13-1766
27 Chang JA , Curtis JR , Patrick DL , Raghu G . Assessment of health-related quality of life in patients with interstitial lung disease. Chest. 1999;116(5):1175–82. doi:.https://doi.org/10.1378/chest.116.5.1175
28 Quanjer PH , Tammeling GJ , Cotes JE , Pedersen OF , Peslin R , Yernault JC ; Official Statement of the European Respiratory Society. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Eur Respir J Suppl. 1993;6(Suppl 16):5–40. doi:.https://doi.org/10.1183/09041950.005s1693
29 ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7. doi:.https://doi.org/10.1164/ajrccm.166.1.at1102
30 American Thoracic Society/American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–77. doi:.https://doi.org/10.1164/rccm.167.2.211
31 Oberstein A , von Zitzewitz H , Schweden F , Müller-Quernheim J . Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14(1):65–72.
32 Drent M , De Vries J , Lenters M , Lamers RJ , Rothkranz-Kos S , Wouters EF , et al. Sarcoidosis: assessment of disease severity using HRCT. Eur Radiol. 2003;13(11):2462–71. doi:.https://doi.org/10.1007/s00330-003-1965-x
33Team RCR. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria URL http://wwwR-projectorg/. 2014.
34 Morgenthau AS , Iannuzzi MC . Recent advances in sarcoidosis. Chest. 2011;139(1):174–82. doi:.https://doi.org/10.1378/chest.10-0188
35 King TE, Jr , Brown KK , Raghu G , du Bois RM , Lynch DA , Martinez F , et al. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(1):92–9. doi:.https://doi.org/10.1164/rccm.201011-1874OC
36 Baughman RP , Drent M , Kavuru M , Judson MA , Costabel U , du Bois R , et al.; Sarcoidosis Investigators. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174(7):795–802. doi:.https://doi.org/10.1164/rccm.200603-402OC
37 Rossman MD , Newman LS , Baughman RP , Teirstein A , Weinberger SE , Miller W, Jr , et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23(3):201–8.
38 Hostettler KE , Studler U , Tamm M , Brutsche MH . Long-term treatment with infliximab in patients with sarcoidosis. Respiration. 2012;83(3):218–24. doi:.https://doi.org/10.1159/000328738
39 Jamilloux Y , Cohen-Aubart F , Chapelon-Abric C , Maucort-Boulch D , Marquet A , Pérard L , et al.; Groupe Sarcoïdose Francophone. Efficacy and safety of tumor necrosis factor antagonists in refractory sarcoidosis: A multicenter study of 132 patients. Semin Arthritis Rheum. 2017;47(2):288–94. doi:.https://doi.org/10.1016/j.semarthrit.2017.03.005
40 Sweiss NJ , Noth I , Mirsaeidi M , Zhang W , Naureckas ET , Hogarth DK , et al. Efficacy Results of a 52-week Trial of Adalimumab in the Treatment of Refractory Sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(1):46–54.
41 Fazzi P , Manni E , Cristofani R , Cei G , Piazza S , Calabrese R , et al. Thalidomide for improving cutaneous and pulmonary sarcoidosis in patients resistant or with contraindications to corticosteroids. Biomed Pharmacother. 2012;66(4):300–7. doi:.https://doi.org/10.1016/j.biopha.2012.03.005
42 Utz JP , Limper AH , Kalra S , Specks U , Scott JP , Vuk-Pavlovic Z , et al. Etanercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest. 2003;124(1):177–85. doi:.https://doi.org/10.1378/chest.124.1.177
43 Sweiss NJ , Lower EE , Mirsaeidi M , Dudek S , Garcia JG , Perkins D , et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J. 2014;43(5):1525–8. doi:.https://doi.org/10.1183/09031936.00224513
44 Judson MA , Baughman RP , Costabel U , Drent M , Gibson KF , Raghu G , et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J. 2014;44(5):1296–307. doi:.https://doi.org/10.1183/09031936.00000914
45 Sahoo DH , Bandyopadhyay D , Xu M , Pearson K , Parambil JG , Lazar CA , et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38(5):1145–50. doi:.https://doi.org/10.1183/09031936.00195010
46 Marcellis RG , Lenssen AF , de Vries GJ , Baughman RP , van der Grinten CP , Verschakelen JA , et al. Is there an added value of cardiopulmonary exercise testing in sarcoidosis patients? Lung. 2013;191(1):43–52. doi:.https://doi.org/10.1007/s00408-012-9432-6
47 Bradley B , Branley HM , Egan JJ , Greaves MS , Hansell DM , Harrison NK , et al., Irish Thoracic Society. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63(Suppl 5):v1–58. doi:.https://doi.org/10.1136/thx.2008.101691
48 Lopes AJ , Menezes SL , Dias CM , Oliveira JF , Mainenti MR , Guimarães FS . Cardiopulmonary exercise testing variables as predictors of long-term outcome in thoracic sarcoidosis. Braz J Med Biol Res. 2012;45(3):256–63. doi:.https://doi.org/10.1590/S0100-879X2012007500018
49 Keir G , Wells AU . Assessing pulmonary disease and response to therapy: which test? Semin Respir Crit Care Med. 2010;31(4):409–18. doi:.https://doi.org/10.1055/s-0030-1262209
50 Baltzan M , Mehta S , Kirkham TH , Cosio MG . Randomized trial of prolonged chloroquine therapy in advanced pulmonary sarcoidosis. Am J Respir Crit Care Med. 1999;160(1):192–7. doi:.https://doi.org/10.1164/ajrccm.160.1.9809024
This study was supported by Actelion Pharma Schweiz AG.
MHB received a nonconditional grant from Actelion Pharma Schweiz AG. No other potential conflict of interest was reported.