
Figure 1 Inclusion and exclusion criteria.
RCT = randomised controlled trials
DOI: https://doi.org/10.4414/smw.2018.14665
It is widely acknowledged that the first 1000 days from pregnancy to a child’s second birthday is “the crucial window of opportunity” for delivery of nutrition to children, potentially impacting on a child’s current and adult health, and the health of subsequent generations ([1] p.1). Exclusive breastfeeding is promoted as the infant’s best first source of nutrition and the World Health Organization recommends that infants are exclusively breastfed for at least 6 months stating “with full confidence that breastfeeding reduces child mortality and has health benefits that extend into adulthood” ([2] para.1).
To promote health in the infant and mother, breastfeeding women are advised to make alterations to their diet, including recommendations about caffeine intake. For example, the European Food Safety Authority suggests that “habitual caffeine consumption at doses of 200 mg per day consumed by lactating women in the general population do not give rise to safety concerns for the breastfed infant” ([3] p.5); the UK National Health Service (NHS) has recently updated its recommendations for breastfeeding women to restrict “intake to less than 200 mg a day” (previously 300 mg/day) as caffeine “can make your baby restless” and “may keep them awake” ([4] sec.6). However, the evidence behind the advice is unclear. Both a major recent systematic review of the safety of caffeine consumption [5] and a recent umbrella review of clinical trials and observational studies of coffee intake and outcomes [6] did not consider the demographic of breastfeeding mothers and their infants. A further recent systematic review on caffeine and sleep [7] highlighted studies that found no significant effects on frequency of infant night waking between infants of 177 mothers who were heavy caffeine consumers (≥300 mg/day) versus infants of mothers who were low consumers during pregnancy and at 3 months postpartum [8], or on the heart rate and sleep time in breastfed infants of mothers ingesting caffeine over five days versus mothers ingesting no caffeine over 5 days [9].
Caffeine is a methylxanthine that acts as a central nervous system (CNS) stimulant, and naturally occurs in several foods. Coffee and tea account for over 90% of caffeine consumption, and in countries where these are consumed, average daily caffeine consumption of adults is approximately 200 mg [10]. The health effects of caffeine are frequently debated, but the potential adverse effects of high intake mean that moderate intake of ≤400 mg/day is recommended for adults [5, 6]. During pregnancy, for example, caffeine has been associated with increased risk of miscarriage [11] and low birth weight [12]. Caffeine consumed by the mother passes into breastmilk, and caffeine has a longer half-life in infancy with even more delayed elimination in breastfed infants [13]. On the other hand, caffeine is not completely contraindicated in infants. The stimulant effects of caffeine are used clinically for the treatment of apnoea of prematurity, which is “cessation of breathing that lasts for more than 15 seconds…accompanied by hypoxia or bradycardia” ([14] p.2113). Trials in preterm, low birthweight infants demonstrate that caffeine is effective and safe with fewer respiratory complications, better neurodevelopmental outcomes in early childhood [15, 16], and no evidence of significant longer term complications (such as functional impairment and abnormalities in sleep architecture) compared with placebo [17, 18]. Side effects, such as tachycardia and increased metabolic rates, may be caused by methylxanthines but caffeine has a better side effect profile, longer half-life, and does not need drug level monitoring compared with other methylxanthines, and is therefore the current drug of choice [19].
In the interests of both child health and provision of evidence-based advice to breastfeeding mothers, this systematic review aimed to examine evidence on the effects of maternal caffeine consumption on the breastfed infant, regarding both primary outcomes – impact on infant sleeping behaviour, feeding behaviour, health, and developmental milestones – and secondary outcomes, namely maternal outcomes impacting on the breastfed infant, and long term health effects in the breastfed infant.
The systematic review protocol was registered on PROSPERO, review registration number: CRD42017078790.
A joint literature search was undertaken alongside a separate review regarding exposure to maternal alcohol consumption. The initial search therefore contained both caffeine- and alcohol-related terms, with no restriction on publication year or language.
Online literature searches to October 2017 were performed in the databases MEDLINE, EMBASE, Web of Science, and CINAHL. Text words and relevant MeSH terms related to caffeine, alcohol, and breastfeeding were combined using Boolean logical operators (appendix 1). The same search procedure was applied to all databases with minor alterations where appropriate.
Retrieved references were imported to reference management and an online systematic review platform (Covidence) where they were deduplicated.
Additional studies and published review articles were searched online in the Cochrane Library (including Cochrane Database of Systematic Reviews), and the catalogue of the British Library, which covers doctoral theses, was also searched (appendices 2 and 3). In the Cochrane Library, terms were searched only in “Title, Abstract, Keywords” and non-human titles were removed. Remaining references were title and abstract screened by two reviewers (appendices 2 and 3 contain summary tables of Cochrane Library and British Library search results).
Hand-searching of the Journal of Human Lactation was performed, but this was not possible for Breastfeeding Review owing to a lack of subscription. Citation searching of included studies was undertaken.
Study selection was performed independently by two reviewers based on criteria detailed in figure 1. Conflicts were resolved by discussion between the two reviewers, with a third reviewer consulted as necessary. Studies excluded at full text review were assigned an exclusion reason by each reviewer, with conflicts again resolved through discussion (appendix 4). Included studies were divided according to topic area, with only studies concerning caffeine intake included in this review. The full texts of the remaining included studies were imported to Covidence for data extraction and quality assessment.

Figure 1 Inclusion and exclusion criteria.
RCT = randomised controlled trials
The main reviewer performed data extraction on included studies using a standardised form (appendix 5). Extracted data were checked by the second reviewer. Information was collected about study, mother and child characteristics (including breastfeeding status), maternal diet, quantity and frequency of caffeine consumed, and effects on the breastfed child. Study outcomes were divided into primary outcomes, where caffeine exposure via breastmilk directly affected the child in the shorter term (impact on infant sleeping behaviour, feeding behaviour, infant health and developmental milestones), and secondary outcomes including longer-term child health effects that did not manifest themselves during breastfeeding, and maternal outcomes, for example, maternal behaviour or health influencing extent of breastfeeding.
Risk of bias was assessed by two reviewers independently using the Newcastle-Ottawa scale for cohort studies [20]. For crossover and N-of-1 studies, quality was assessed using the same scale, plus assessment of the risk of carryover effect, and availability of paired data for assessing the differences within individuals, see appendix 5. Risk of bias assessments were compared and conflicts resolved by agreement between the two reviewers, with input of a third reviewer as necessary.
Results are presented in narrative and tabular form as there was insufficient comparable data to perform meta-analysis. Where data were presented only for individual participants [9], we calculated and presented the summary statistics (means and standard deviations) from the data.
Online searches of databases returned 2547 unique records for title and abstract screening, of which 65 studies were eligible for full text review (fig. 2). Five studies met the study selection criteria and were included in the review.

Figure 2 PRISMA flow chart for literature retrieval and study selection.
Of the five included studies, two were prospective cohort studies, two were crossover studies, and one was an N-of-1 trial. They were conducted between 1981 and 2011. Though undertaken in various locations worldwide, all were published in English. Detailed information about participant characteristics, exposures and outcomes reported is provided in table 1.
Table 1 Characteristics of included studies.
|
Article
Study design |
Participant characteristics | Exposure (maternal caffeine intake during breastfeeding) and Control | Outcome (effect on breastfed infant) and method of measurement |
|---|---|---|---|
| Ryu 1985 [9] Randomised crossover trial |
“Twelve healthy normal white women and their normal infants” from mothers who gave birth at Iowa City Hospital between Jan–May 1983 and were planning to breastfeed. All consumed caffeine on a regular basis (pre-pregnancy intake 54–877 mg/day with voluntary reduction in consumption during pregnancy and lactation). No statement on exclusivity of breastfeeding. At onset of study, mean infant age 47.1 (SD 15.9) days, and mean maternal age 29.2 (SD 3.5) years. |
Exposure: 500 mg caffeine on 5 days, with precise timings of intake measured on days 4 and 5 and heart-rates / sleeping time measured for 24 hours on day 5. Control: No caffeine on 5 days, but precise timings of placebo measured on days 4 and 5 and heart-rates / sleeping time measured for 24 hours on day 5. |
Heart rate over single 24-hour period via automatic monitor. Sleeping time over same 24 hours via counter and mother reporting |
| Santos et al. 2012 [8] Prospective cohort study |
625 breastfed infants drawing from 885 infants in a subsample (born Oct–Dec 2004) of the Pelotas cohort, which included all 4231 children born in city of Pelotas, Brazil, in 2004. Only infants from singleton pregnancies were included in analyses. No statement on exclusivity of breastfeeding. Maternal age: 171 <20 years, 626 20–35 years, 87 >35 years. |
Exposure: Heavy caffeine consumption (≥300 mg/day) from coffee and/or mate at 3 months postpartum Control: <300 mg/day caffeine consumption from coffee and/or mate at 3 months postpartum |
For subsample (n = 885), infant sleeping pattern for the 15 days prior to 3 months postpartum interview, mother reported. Crying and colic at 3 months via mother interview of entire cohort (n = 3985). Frequent night waking at 12 months via mother interview (n = 3907)*. |
| Clifford et al. 2006 [21] Prospective cohort study |
856 mothers (mean age: 29.4 (SD 4.9) years) who gave birth at term to live singletons of birth weight appropriate for gestational age in either of the 2 London (Ontario) hospitals providing obstetrical services were approached between January 15 and September 16 1999 before hospital discharge. The association between maternal caffeine intake and continuation of FBF was explored in a subgroup of 396 infants who were FBF at 1 week. Maternal age of subsample: 4 <20 years, 318 20-34 years, 74 >34 years. |
Exposure: Caffeine at 6 months postpartum Control: No caffeine at 6 months postpartum |
Continuation of FBF at 6 months, via postal survey of mothers. |
| Evans et al. 1981 [22] Randomised crossover trial† |
20 exclusively breastfed infants (12 girls and 8 boys) aged 3–18 weeks (average 7 weeks) presenting with colic initially diagnosed by family doctor or community nurse and confirmed by paediatrician. Onset of colic at median age 3 weeks. All but two of infants born at 39 weeks plus gestation. |
Exposure: Chocolate Control: No chocolate in maternal diet |
One or more colic symptoms via mother reported diary over 12 day period. |
| Uenishi et al. 2011 [23] N-of-1 trial |
92 exclusively breastfed Japanese infants with atopic dermatitis aged 3–8 months (average 4.8 months), suffering from skin lesions for at least 2 months and fulfilling Japanese Dermatology Association criteria for diagnosis of atopic dermatitis. | Exposure: Chocolate or coffee challenge test (eaten at breakfast for 2 days, volume equivalent to that the mothers would have for a meal). Control: Initial exclusion test of chocolate/coffee, and exclusion of chocolate/coffee after positive challenge test. |
Aggravation of atopic dermatitis, observed by the dermatologists “before and after” the exclusion test, and on the day after completion of the challenge test. |
FBF = full breast feeding; SD = standard deviation * Outcomes not reviewed because data unavailable. † This trial investigated the effect of maternal consumption of cow’s milk on colic symptoms using a crossover design; maternal chocolate consumption was not restricted by this design and therefore the study was regarded as an observational cohort study for the purpose of this review.
Sixty studies were excluded at full text review. The majority of the studies were excluded on grounds of wrong exposure, mainly due to maternal consumption of caffeine not being measured during the postpartum period (versus during pregnancy), see figure 2.
The two crossover trials [9, 22] and the N-of-1 trial [23] had small sample sizes (ranging from 12 to 92 mother-infant pairs). The two other studies drew their samples from larger cohorts: Santos et al. [8] examined the effects of maternal caffeine consumption in 625 breastfed infants from a subsample of a population cohort of Pelotas, Brazil; Clifford et al. [21] investigated 395 mothers who were still fully breastfeeding at 1 week postpartum out of a sample of 856 mothers.
Only three of the studies reported intake of caffeine itself [8, 9, 21], and only Ryu [9] and Santos et al. [8] specified a quantity or threshold amount of maternal caffeine consumption during breastfeeding (500 mg/day and ≥300 mg/day respectively). The remaining two studies [22, 23] investigated consumption of specific foods, some of which contain caffeine.
A summary of the risk of bias assessment is presented in figure 3. The three most relevant studies (Ryu [9], Santos et al. [8], and Clifford et al. [21]) were found to be of moderate to poor quality in the risk of bias assessment, whereas Evans et al. [22] and Uenishi et al. [23] had still higher risk of bias for the question of how caffeine affects the breastfed child. Where the effects of exposure to various foods (rather than exposure to caffeine per se) were the focus of the research [22, 23], the risk of bias was assessed with respect to exposure to caffeine-containing foods (chocolate and/or coffee in these studies) and the low scores of these studies reflect this. There is potential risk of bias associated with incomplete outcome reporting in Santos et al. [8], with data not provided for some investigated outcomes (crying and colic at 3 months, and frequent night waking at 12 months) where no significant associations were found. The author was contacted to request data but gave no response. The additional questions assessed for crossover and N-of-1 studies are reported in table 2 which shows a reasonable performance in Ryu [9] but relatively high risk of bias in Uenishi et al. [23] As chocolate exposure in Evans et al. was a self-reported exposure rather than a focus of the crossover trial, the crossover questions were not applicable to the caffeine exposure but reported for completion.
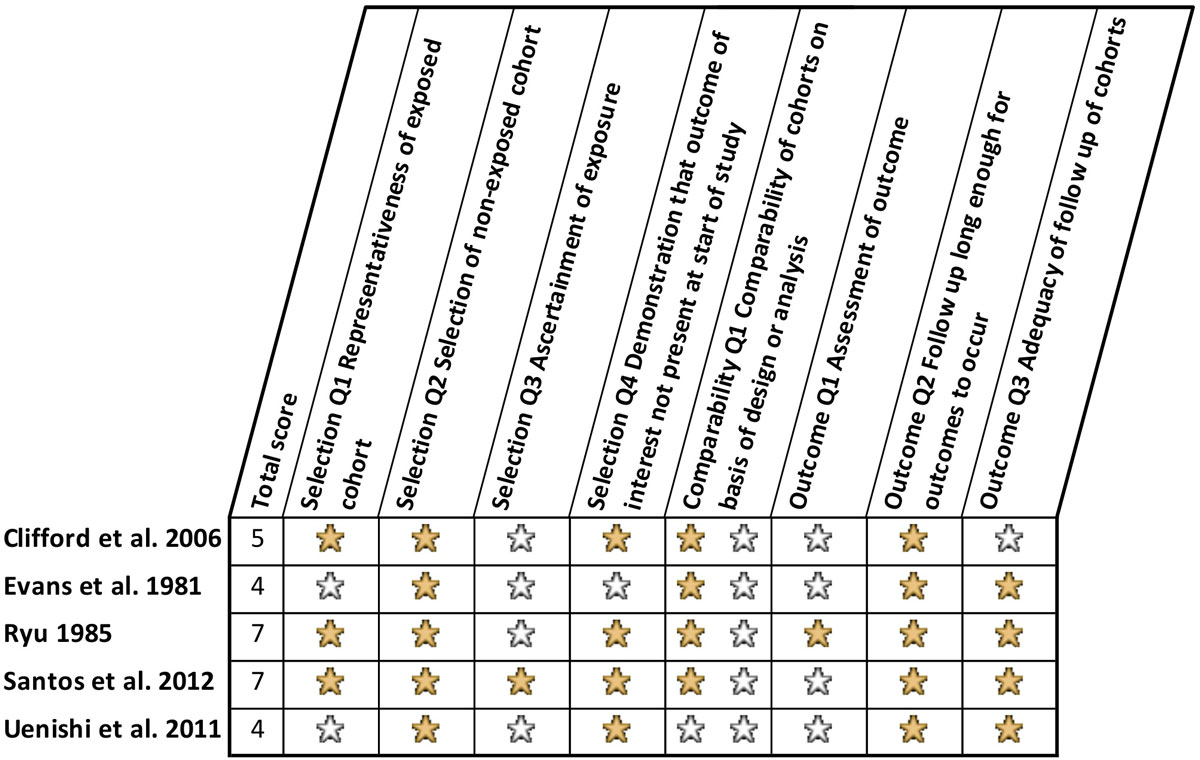
Figure 3 Summary of risk of bias assessment based on Newcastle-Ottawa Quality Assessment Scale for Cohort Studies. A filled yellow star indicates that a star has been awarded, as outlined by Wells et al. [18], and a blank star indicates that no star has been awarded, and the study has been graded as poor quality in that category.
Table 2 Summary of crossover / N-of-1 trials additional risk of bias assessment.
| Study | Q1 Can it be assumed that the trial was not biased from carryover effects? | Q2 Are unbiased data (paired data assessing difference within individual participants) available? |
|---|---|---|
| Ryu 1989 [9] | Yes. Caffeine intake was blinded and the order was randomised. Although there was no washout period between the exposure and non-exposure period for caffeine, testing of the infant took place on days 4 and 9 (of two blocks of 5 days) to protect against carryover effect from pre-study diet and substance consumed during the first 5-day block. | Available but not assessed. |
| Evans et al. 1985 [22] | Not applicable to caffeine-related data: maternal chocolate consumption was not investigated in a crossover design, rather it was a secondary self-reported exposure. (Main exposure studied was cow's milk. Regarding cow’s milk, study design attempted to exclude carryover effect by grouping trial days into 2 day blocks of either cow's or soya milk, randomly assigned, and only assessing data from day 2 of each block.) | Not applicable to caffeine-related data. (Unbiased data also unavailable regarding main exposure studied.) |
| Uenishi et al. 2011 [23] | No. No explicit consideration was given to carryover effect and, apart from the initial 2 week maternal exclusion tests, no information is given about period of time elapsed between challenge tests for different foods and rechallenge tests. Only infants with a positive exclusion test underwent subsequent challenge tests and therefore potential influence of regression to the mean cannot be ruled out. The authors stated that acceptability for carrying out rechallenge tests was low and that in the few rechallenge tests undertaken, the challenge test results were reaffirmed. | Unavailable. |
Two papers investigated the effect of caffeine on sleep behaviour, as well as 24-hour heart rate [9], and crying and colic [8], see table 3. In a small, double-blind crossover trial, Ryu found no significant effects of short-term daily intake of 500 mg caffeine on 24-hour infant heart rate or 24-hour infant sleep time [9]. Santos et al. found an increased (but not statistically significant) prevalence ratio (adjusted ratio 1.49, 95% confidence interval [CI] 0.89–2.51; p = 0.128) of frequent night waking amongst breastfed infants whose mothers were heavy caffeine consumers versus breastfed infants whose mothers were not heavy caffeine consumers [8]. Crying and colic were measured by Santos et al. but the data were unavailable. Clifford et al. [21] found a decreased rate of full breastfeeding at 6 months postpartum amongst infants of mothers who consumed caffeine (29%) versus those who abstained (53%). Evans et al. [22] measured infant colic rates and found significantly increased colic rates on days when mothers consumed chocolate. Uenishi et al. [23] investigated aggravation of atopic dermatitis and found that skin lesions increased following chocolate and coffee challenge tests in 18 instances and 7 instances, respectively, of total 107 maternal challenge tests; however, statistical significance was not reported.
Table 3 Summary of results from included studies.
|
Article
Outcome type |
Results and statistics | Narrative summary of key study findings, strengths and limitations |
|---|---|---|
| Ryu, 1985 [9] Physiological |
For caffeine vs no caffeine periods • Mean 24-hr heart rate: 143.7 bpm (SD 5.1) vs 145.3 bpm (SD 5.4) (n = 11) • Mean 24-hr sleep time as % of mean observation time:* - Counter 60.1% (SD 9.0) vs 60.6% (SD 5.7) - Mother recorded 57.9% (SD 9.2) vs 61.0% (SD 7.3) • No significant difference between caffeine vs non-caffeine periods in mean heart rate (t = 1.49, p = 0.17) and counter sleep time (t = 1.00, p = 0.34) in 9 infants with counting times >21 hrs and in sleep time recorded by 10 mothers† (t = 1.06, p = 0.32). |
No evidence of 24-hour heart rate or sleep time alteration during caffeine period versus no caffeine period, and no evidence of dose-related response. Moderate level of maternal coffee consumption during lactation appears unlikely to affect breast-fed infants after first few weeks of life. Key strengths and limitations: randomised cross-over study with objectively measured outcomes; small sample size; level of caffeine consumption during pregnancy unclear; short periods of exposure and follow-up. |
| Santos et al. 2012 [8] Physiological |
PR of frequent night waking (>3 times/night) among 77 breastfed only infants of mothers with heavy caffeine consumption vs those of mothers with no heavy caffeine consumption (n = 548) at 3 mo postpartum: Crude analysis: PR 1.46 (95% CI 0.88–2.41); p = 0.138 Adjusted analysis: PR 1.49 (95% CI 0.89–2.51); p = 0.128. Controlled for maternal age, skin colour, parity, alcohol consumption, child’s gender, and family income. |
Infants of mothers with heavy caffeine consumption at 3 mo postpartum demonstrated increased PR for frequent night waking, but at a nonsignificant level. Also assessed effect of caffeine on child crying and colic at 3 mo postpartum, and frequent night waking at 12 mo postpartum. Neither crude nor adjusted analysis showed evidence of association.‡ Key strengths and limitations: participants were drawn from a prospective population birth cohort; caffeine content of consumed drinks objectively verified; quantity of drink consumption and infant outcomes self-reported; analysis not controlling for caffeine consumption during pregnancy. Data for some measured outcomes not reported. |
| Clifford et al., 2006 [21] Maternal |
Of 395 mothers who were fully breastfeeding at 1 week postpartum: Maintenance of FBF for maternal caffeine vs no caffeine consumption at 6 mo postpartum: 102/350 (29.1%) vs 24/45 (53.3%); p <0.05, chi-squared test. |
Maternal caffeine consumption at 6 mo postpartum appeared to be associated with decreased rates of FBF at 6 mo postpartum. Key strengths and limitations: prospective cohort study; infant outcomes not examined, self-reported caffeine consumption and outcome (FBF); univariate analysis not adjusting for potential confounders. |
| Evans et al. 1981§ [22] Clinical |
For 20 mother-infant pairs: Infants with colic symptoms on days when exposed to chocolate vs days when not exposed: 80.4% (34/42) vs 63.6% (126/198); p <0.05 |
Significantly increased infant colic rates on days when breastfeeding mother consumed chocolate. Key strengths and limitations: disease-specific cohort (infants with persistent colic); small sample size; association could be attributed to ingredients other than caffeine; self-reported exposure and infant outcome; univariate analysis not adjusting for potential confounders. |
| Uenishi et al. 2011§ [23] Clinical |
Atopic dermatitis lesions: Of 92 mother-infant pairs who undertook a preliminary 2 week exclusion test of all tested foods (both tree-nut related, and fermented foods), 67 infants (73%) showed positive response (great or fair improvement in skin lesions), and underwent challenge tests where each food was reintroduced in succession for two days at a time, and the lesions assessed. The 67 infants responded positively to a total of 107 maternal challenges (tree-nut related and fermented foods), 1–4 positive results each (1.6 average): – severely exacerbated: 43 infants – moderately exacerbated: 64 infants Chocolate challenge test positive in 18/67 infants. Coffee challenge test positive in 7/67 infants. |
Exposure to caffeine via a challenge test with caffeine-containing food. Of all the foods tested, chocolate was a predominant challenge test-positive food: 18 of 107 maternal challenges (third most common after soy sauce[n = 26] and yogurt [n = 19]). Coffee consumption resulted in positive challenge tests in 7 instances. Statistical significance was not reported. Key strengths and limitations: disease-specific cohort (infants with atopic dermatitis); association could be attributed to ingredients other than caffeine; assessment of exposure and outcome not blinded; discrepant challenge tests between chocolate and coffee suggests that caffeine may not be the causal allergen. |
FBF = full breast feeding; PR = prevalence ratio; SD = standard deviation * Counter time was derived from record of electronic heart beat counting device, which estimated sleep time based on heart rate dropping below a heart rate associated with sleep in each infant (predetermined at about 30% lower than the individual’s heart rate when awake and quiet). During the same period, mothers also recorded infant sleep time. † Mother of one infant failed to record sleep time. ‡ Numerical results for these outcomes (crying and colic at 3 mo; frequent night waking at 12 mo) were not reported. § Caffeine per se not investigated, rather maternal intake of certain foods, in some of which caffeine occurs.
The most prominent finding of this review is the extreme paucity of evidence about maternal consumption of a commonplace stimulant during breastfeeding which could affect both child and maternal health. A second significant observation is that the studies found are of limited quality, mostly having very small sample sizes, inadequate control of potential confounders, and risk of bias in measuring exposure and outcomes. Coupling the lack of high quality studies with the inconsistency of the data available makes it difficult to draw any conclusions about the effects of caffeine exposure during breastfeeding.
In Wikoff and colleagues’ 2017 systematic review regarding caffeine safety [5], studies where attempts were made to quantify caffeine exposure, for example by sample analysis, rather than relying on self-report of exposure, were considered to “better inform the body of evidence” ([5] p. 631). Of the five included studies, Ryu [9] and Santos et al. [8] attempted to do this, making these studies potentially more informative than those that were not specific about the quantity of caffeine consumed. Clifford et al. [21] specified caffeine or no caffeine which at least provides a clear threshold for exposure. Evans et al. [22] and Uenishi et al. [23] did not measure caffeine.
The different approaches to measuring caffeine exposure and outcomes also affect the reliability of the outcomes reported. Studies relying on mothers’ self-reports of infant night waking [8, 9] could be influenced by mothers themselves experiencing the CNS stimulant effects of heavy caffeine consumption and being more easily awakened from sleep than mothers drinking less or no caffeine. This issue can be overcome by providing additional, objective measures of the sleep-related outcomes, e.g., by reporting an estimated sleep time based on a heart rate counter as adopted in the Ryu study [9]. However, the increased technical requirement may restrict such studies to small sample sizes, which may limit generalisability.
Study design poses a challenge for investigating effects of maternal caffeine intake on the breastfed infant. A randomised crossover design has the advantage of avoiding problems with case-control parity and therefore overcomes the issue of unmeasured confounding in observational studies. Carryover effect is the major potential threat for studies of such design, although none of the included studies tested for this effect.
The disparity in directness of exposure between the studies presents difficulty in comparing and interpreting their results. Evans et al. [22] and Uenishi et al. [23] did not investigate caffeine per se, but rather the consumption of foods incidentally containing caffeine. These studies were nevertheless included because they documented outcomes in infants relating to foods that contain caffeine. However, the indirect focus reduces the relevance and reliability of the results. It is unclear whether caffeine is the causal ingredient, as the observed effects may be associated with another substance in the food. Indeed, results related to chocolate consumption appear to have been more pronounced in these studies, and the different rate of aggravation of atopic dermatitis in response to coffee and chocolate [23] at least suggests that caffeine was not the sole aggravating substance.
Finally, a crucial issue in assessing the effects of maternal caffeine consumption during breastfeeding on the breastfed child is the control of potential confounders, which are factors independently associated with both maternal caffeine consumption and the infant/maternal outcomes of interest. Failure to take into account these confounders may result in under- or over-estimation of the true effect of maternal caffeine consumption during breastfeeding. Potentially important confounders include various demographic and socioeconomic factors such as maternal age, co-morbidity, education, income, smoking, family support, and so forth. One potential confounder worth highlighting is maternal caffeine consumption during pregnancy. On the one hand, caffeine consumption during pregnancy may be closely associated with caffeine consumption during breastfeeding through common socioeconomic risk factors mentioned above (although they could represent two disparate decisions that the mother could make). On the other hand, regular caffeine exposure in utero could have direct effects on the fetus, potentially leading to an infant developing “tolerance” to caffeine [24] and hence attenuating the effects of maternal consumption of caffeine during breastfeeding. These complicated relationships were not adequately investigated in the studies included in this review and it is important that future studies explore the importance and evaluate the impact of controlling for these confounding variables.
Strengths of this review were the comprehensive search using broadly defined keywords to capture as many potentially relevant references as possible, careful appraisal of the included studies, and an organised and well-documented screening process facilitated by the Covidence software.
Limitations included the inaccessibility of a potentially relevant reference source for hand search (Breastfeeding Review journal, although this journal was covered in the online databases with abstracts) and, on the other hand, the issue of titles and keywords not always being indicative of article relevance when hand-searching elsewhere. Although only studies published in English language were included because of resource constraints, we intentionally did not apply language restrictions in our searches. No non-English language articles that would have otherwise met the inclusion criteria were found. Nevertheless, as we only used English terms when carrying out the searches, there remains a small risk of language bias due to non-identification of articles that have not been indexed in English language. In addition, due to the small number of studies identified and diversity of outcome measures that they reported, we could not carry out meta-analyses and were unable to assess potential publication bias (or small study effects) using funnel plots. A further major limitation of the review relates to the lack of high quality evidence (i.e., from randomised or quasi-randomised parallel group trials) that is currently available, which prevents us from drawing any firm conclusions.
Further research on the effects of maternal consumption of caffeine on the breastfed child is warranted. The included studies appear to be directed more toward investigation of potentially negative health impacts given the existing association between heavy caffeine consumption in pregnancy and negative fetal outcomes [5, 6, 11, 12]; future research should potentially address more diverse outcomes specifically for maternal caffeine consumption during breastfeeding on the breastfed child, considering positive as well as negative implications for the health of both the mother and the infant, and thus reducing the risk of confirmation bias.
On the other hand, although the clinical use of caffeine in neonates appears to have a relatively good safety profile, the potential effects of caffeine on breastfed infants (e.g., on weight gain) related to its diuretic and calcium excretion effects may require further confirmation. Most effectively, research could take the form of large birth cohort studies investigating direct effects on child health and behavioural outcomes, alongside maternal outcomes such as extent of breastfeeding (exclusivity and duration), and potentially other more indirect maternal outcomes such as anxiety and fatigue which may also affect the breastfed infant. Furthermore, given the safety profile of clinical use of caffeine in neonates and lack of serious adverse effects observed in the limited research to date, randomised controlled trials investigating the effect on the breastfed infant of moderate maternal caffeine consumption (for example, within current NHS guidelines for breastfeeding mothers) versus no caffeine consumption could potentially be acceptable and provide the most reliable evidence.
Also of interest are the pronounced effects on colic rates [22] and atopic dermatitis lesions [23] that were found in relation to maternal chocolate consumption, suggesting that further research into maternal chocolate exposure on the breastfed infant could be useful in informing dietary advice for breastfeeding mothers.
To our knowledge, this is the first systematic review of evidence on the effects of maternal caffeine consumption during breastfeeding on the breastfed child. Considering the prevalence of caffeine consumption in our society, it is surprising that there are so few published studies, mostly of small sample sizes and moderate to poor quality. With a view to both child health and providing evidence-based dietary advice to breastfeeding mothers, further research should be conducted into the potential positive and negative effects of moderate maternal caffeine consumption during breastfeeding on the breastfed child.
The appendices are available as separate files for downloading at https://smw.ch/en/article/doi/smw.2018.14665/
The authors are very grateful to Warwick University librarian Samantha Johnson, for assisting with the search strategy, and King’s College London neonatology registrar Dr Kunal Babla, who offered additional clinical perspectives in the planning stage.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors; the authors remain independent of any funding influence. YFC was supported by the UK National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) West Midlands. This paper presents independent research and the views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. All other authors declare no support from any organisation for the submitted work. All authors declare no financial relationships with any organisations that might have an interest in the submitted work in the previous three years and no other relationships or activities that could appear to have influenced the submitted work.
1 Bryce J Coitinho D Darnton-Hill I Pelletier D Pinstrup-Andersen P Maternal and Child Undernutrition Study Group . Maternal and child undernutrition: effective action at national level. Lancet. 2008;371(9611):510–26. doi:.https://doi.org/10.1016/S0140-6736(07)61694-8
2World Health Organization. WHO Breastfeeding [Internet]. 2018. Available from: http://www.who.int/maternal_child_adolescent/topics/child/nutrition/breastfeeding/en/
3 EFSA NDA Panel . (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on the safety of caffeine. EFSA J. 2015;13(5):4102. doi:. [Internet].https://doi.org/10.2903/j.efsa.2015.4102
4Choices NHS. Breastfeeding and diet. 2016; Available from: https://www.nhs.uk/conditions/pregnancy-and-baby/breastfeeding-diet/
5 Wikoff D Welsh BT Henderson R Brorby GP Britt J Myers E Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxicol. 2017;109(Pt 1):585–648. doi:.https://doi.org/10.1016/j.fct.2017.04.002
6 Poole R Kennedy OJ Roderick P Fallowfield JA Hayes PC Parkes J . Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ. 2017;359:j5024. doi:.. Corrected in: BMJ. 2018;360:k194. doi: https://doi.org/10.1136/bmj.j5024
7 Clark I Landolt HP . Coffee, caffeine, and sleep: A systematic review of epidemiological studies and randomized controlled trials. Sleep Med Rev. 2017;31:70–8. doi:.https://doi.org/10.1016/j.smrv.2016.01.006
8 Santos IS Matijasevich A Domingues MR . Maternal caffeine consumption and infant nighttime waking: prospective cohort study. Pediatrics. 2012;129(5):860–8. doi:.https://doi.org/10.1542/peds.2011-1773
9 Ryu JE . Effect of maternal caffeine consumption on heart rate and sleep time of breast-fed infants. Dev Pharmacol Ther. 1985;8(6):355–63. doi:.https://doi.org/10.1159/000457060
10 Fredholm BB Bättig K Holmén J Nehlig A Zvartau EE . Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51(1):83–133. Available at: http://pharmrev.aspetjournals.org/content/51/1/83 . [Internet].
11 Chen L-W Wu Y Neelakantan N Chong MF-F Pan A van Dam RM . Maternal caffeine intake during pregnancy and risk of pregnancy loss: a categorical and dose-response meta-analysis of prospective studies. Public Health Nutr. 2016;19(7):1233–44. doi:.https://doi.org/10.1017/S1368980015002463
12 Chen L-W Wu Y Neelakantan N Chong MF-F Pan A van Dam RM . Maternal caffeine intake during pregnancy is associated with risk of low birth weight: a systematic review and dose-response meta-analysis. BMC Med. 2014;12(1):174. doi:.https://doi.org/10.1186/s12916-014-0174-6
13 Le Guennec J-C Billon B . Delay in caffeine elimination in breast-fed infants. Pediatrics. 1987;79(2):264–8. Available at: http://pediatrics.aappublications.org/content/79/2/264.short . [Internet].
14 Schmidt B Roberts RS Davis P Doyle LW Barrington KJ Ohlsson A Caffeine for Apnea of Prematurity Trial Group . Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112–21. doi:.https://doi.org/10.1056/NEJMoa054065
15 Henderson-Smart DJ De Paoli AG . Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst Rev. 2010;(12):CD000140. doi:.https://doi.org/10.1002/14651858.CD000140.pub2
16 Henderson-Smart DJ Steer PA . Caffeine versus theophylline for apnea in preterm infants. Cochrane Database Syst Rev. 2010;(1):CD000273. doi:.https://doi.org/10.1002/14651858.CD000273.pub2
17 Marcus CL Meltzer LJ Roberts RS Traylor J Dix J D’ilario J Caffeine for Apnea of Prematurity–Sleep Study . Long-term effects of caffeine therapy for apnea of prematurity on sleep at school age. Am J Respir Crit Care Med. 2014;190(7):791–9. doi:.https://doi.org/10.1164/rccm.201406-1092OC
18 Schmidt B Roberts RS Anderson PJ Asztalos EV Costantini L Davis PG Caffeine for Apnea of Prematurity (CAP) Trial Group . Academic Performance, Motor Function, and Behavior 11 Years After Neonatal Caffeine Citrate Therapy for Apnea of Prematurity: An 11-Year Follow-up of the CAP Randomized Clinical Trial. JAMA Pediatr. 2017;171(6):564–72. doi:.https://doi.org/10.1001/jamapediatrics.2017.0238
19 Shrestha B Jawa G . Caffeine citrate - Is it a silver bullet in neonatology? Pediatr Neonatol. 2017;58(5):391–7. doi:.https://doi.org/10.1016/j.pedneo.2016.10.003
20Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. 2018. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
21 Clifford TJ Campbell MK Speechley KN Gorodzinsky F . Factors influencing full breastfeeding in a southwestern ontario community: assessments at 1 week and at 6 months postpartum. J Hum Lact. 2006;22(3):292–304. doi:.https://doi.org/10.1177/0890334406290043
22 Evans RW Allardyce RA Fergusson DM Taylor B . Maternal diet and infantile colic in breast-fed infants. Lancet. 1981;317(8234):1340–2. doi:.https://doi.org/10.1016/S0140-6736(81)92519-8
23 Uenishi T Sugiura H Tanaka T Uehara M . Aggravation of atopic dermatitis in breast-fed infants by tree nut-related foods and fermented foods in breast milk. J Dermatol. 2011;38(2):140–5. doi:.https://doi.org/10.1111/j.1346-8138.2010.00968.x
24 Mulder EJH Tegaldo L Bruschettini P Visser GHA . Foetal response to maternal coffee intake: role of habitual versus non-habitual caffeine consumption. J Psychopharmacol. 2010;24(11):1641–8. doi:.https://doi.org/10.1177/0269881109106310
AM, LJB, and YFC conceived and designed the study. AM and LJB screened the articles and collated the data. AM, YFC and JSS interpreted the data. SB revised the draft with a clinical perspective. All authors drafted and revised critically the manuscript for important intellectual content. All authors gave final approval of the version to be published and have contributed to the manuscript. YFC is the guarantor.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors; the authors remain independent of any funding influence. YFC was supported by the UK National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) West Midlands. This paper presents independent research and the views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. All other authors declare no support from any organisation for the submitted work. All authors declare no financial relationships with any organisations that might have an interest in the submitted work in the previous three years and no other relationships or activities that could appear to have influenced the submitted work.