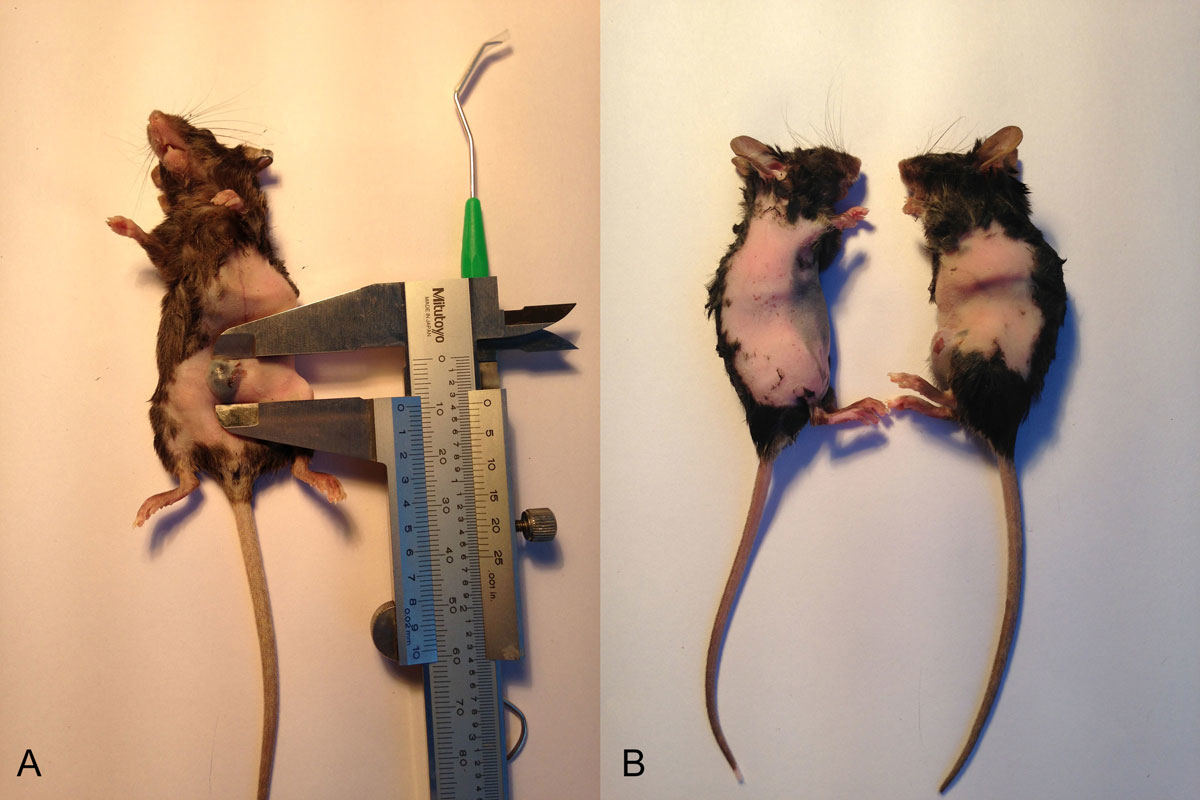
Figure 1 Measure and make a pair.
DOI: https://doi.org/10.4414/smw.2018.14678
Parabiosis is an experimental medical model that reflects well the interaction of blood and body fluids between two conjoined animals. The aim is to achieve mutualism and observe the effects in the conjoined bodies, ranging from alterations in blood to immunological effects. Mice are usually used in parabiosis models and this method has been applied for more than 150 years. There has been relevant parabiosis model research carried out in America, Britain Switzerland and Japan, but only a little in China. The article entitled "Rejuvenation of aged progenitor cells by exposure to a young systemic environment" by Thomas A. Rando and co-workers was published by Nature in 2005 [1]. They established parabiotic pairings (that is, a shared circulatory system) between young and old mice (heterochronic parabioses), thus exposing old mice to factors present in young serum. In 2011, Tony Wyss-Coray found that the aging systemic milieu negatively regulates neurogenesis and cognitive function [2]. In 2013, Loffredo F.S. reported in Cell that growth differentiation factor-11 was a circulating factor that reversed age-related cardiac hypertrophy [3], which immediately attracted extensive attention. Subsequently, more related articles that supported this standpoint appeared in Nature and Science. For example, Nature published articles which evaluated the peritoneal parabiosis model in 2016 [4].
A cancer is composed of malignant cells that have escaped from the body's immune surveillance. The theory of tumour immune editing, by R.D. Schreiber, argues that the immune system not only has the ability to eliminate tumour cells, but also promotes tumour growth [5]. The occurrence and development of cancer cells in the body is a dynamic process of interaction between the immune system and the tumour cells. Hence, whether tumour growth is promoted or suppressed in the parabiosis model is still unclear. Is it true that the parabiosis model can provide new directions for the immunotherapy of cancer, and help to answer the question of whether healthy mice can give immunological support and thus play a role in combating cancer by decreasing the size of a tumour in the other mouse after a successfully established interaction. If this happens, is a result of the influence of both cellular and humoral factors in the healthy mouse on the ill mouse, of the activation of humoral factors from healthy mouse inn the diseased one, or of a vaccination effect, which leads to secretion of a large number of antitumor factors that act against tumour cells? Moreover, how the two parabiosis mice accomplish symbiotic transplantation tolerance and the kind of relationship between the chimaera and the state of the tolerance still need to be resolved.
Our study focused on the further application of the parabiosis model in oncology and the search for factors on cells and in body fluid that can kill and inhibit tumour cells. We researched the these aspects as follows: firstly, we established a parabiosis model; secondly, based on this model, we tested the spleen T cells using flow cytometry, interactions of the two T cells in mice, and the effect of killing tumours; thirdly, we analysing the state of tumour destruction in parabiosis mice by means of morphological comparisons using immunohistochemical technology.
We used the following mouse strains:
The experimental groups were:
C57BL/6 GFP– mice were treated with melanoma B16 tumour cells (1 × 106/100μl). When the tumour diameter was 5 mm, we started the parabiosis operation. Ten tumour-bearing mice paired with GFP+ mice were included in the parabiosis experiment (fig. 1).

Figure 1 Measure and make a pair.
Ultraviolet light was used to disinfect the operating table and high temperatures to sterilise the surgical instruments. Anaesthesia was induced by injecting 2% pentobarbital sodium at a dose of 40 mg/kg. Hair removal and weighing were then carried out while the mice were kept warm. The parabiosis pairs were matched by their weight. The incision was from 4–5 mm distal to the elbow, along the humerus and axilla, to the waistline. The 5-0 lines were sutured continuously, matching the skin and subcutaneous tissue of the animals. The suture was then coved with erythromycin ointment. Because of the mobility of mice, the wounds were prone to tear. As a result, the two mice were fixed together with gauze bound tape (fig. 2).
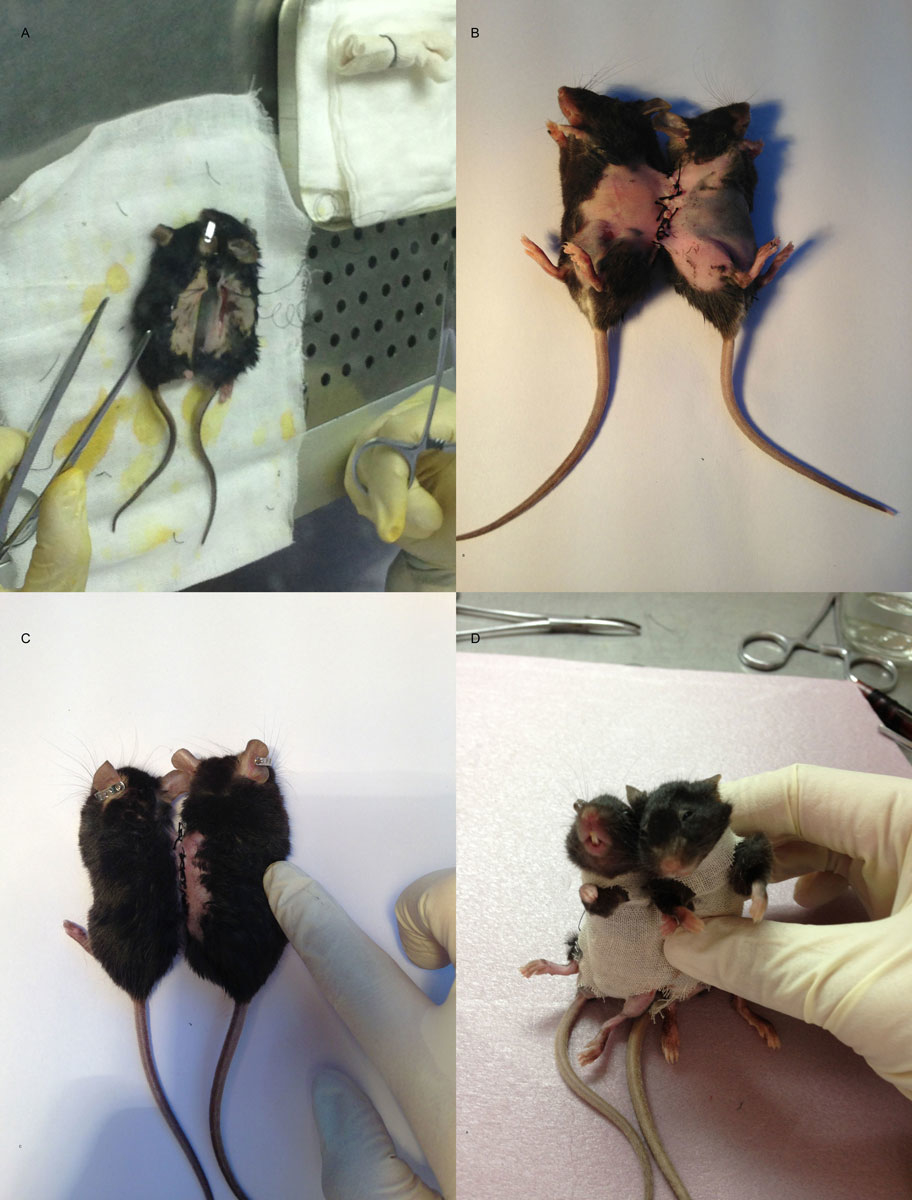
Figure 2 Parabiosis operating procedure.
The parabiosis mice were fed normally, with painkillers in their drinking water to alleviate postoperative pain. Postoperatively, the wound, tumour size, water and food intake and activity were observed and recorded daily. Blood was collected from the eyes of tumour-bearing mice day 3, and GFP+ fluorescence was tested by means of flow cytometry to determine whether the conjoining operation was successful.
After the conjoining operation, 7 of the 10 pairs survived. In order to observe the volume of the tumours, one conjoined pair was killed on days 2, 4, 6, 8, 10, 12 and 14. One pair of mice from positive control group and the negative control group were killed on the same day.
Mice were sacrificed by breaking the neck broken and then immersed in 75% ethanol. The spleen was extracted from the mice in super clean operating area, and T cells from the spleen were extracted by grinding, centrifugation and addition of phorbol-12-myristate-13-acetate. The presence of CD4+ T cells and CD8+ T cells, and expression of interleukins (ILs) 2, 4 and 10 and interferon-gamma (IFN-γ) in spleen cells was detected.
Tumour tissue from the conjoined group and the positive control group mice that were killed on days 8 and 14 was isolated, fixed with 4% paraformaldehyde and stained immunohistochemically. Changes in expression of CD3, CD4, CD8, CD31, IFN-γ and vascular endothelial growth factor (VEGF) were observed and recorded. Microscopic examination and image collection showed that haematoxylin stained the nuclei blue and 3,3′-diaminobenzidine (DAB) staining was brownish yellow (haematoxylin-eosin magnification ×100). IPP 6 image analysis software was used to determine the optical density (OD) of the positive samples of each group, and then the average value was taken as the OD value of the sample.
The experimental data were expressed as mean ± standard deviation (x ± s). SSPS16.0 software was used for statistical processing of all the data. The paired t-test was used in between- and within-group comparisons. With an α-value set at 0.05, a p-value<0.05 was considered to indicate a statistical difference.
Conjoining operations were performed in 10 pairs, with no intraoperative deaths. Conjoined mice all recovered within 20–60 min; however, there was a suture tear happened in one case, and three pairs died at 12 hours, day 2 (the next day) and day 4, respectively, after the conjoined operation. The deaths were all considered to be related to graft-versus-host disease (GVHD). The rest survived. After waking up, the mice showed agitation, excitement, the behaviour of trying to break free from the fixed gauze, and an increasing frequency of standing and circling than before being conjoined. They started to eat in about 10 hours and defecate in about 20 hours after the conjoining operation. Their eating was poor on days 1 and 2, and became better from day 3. The quantity of each meal including eating and drinking returned to preoperative level on day 5.
Tumour volumes in the tumour-bearing parabiosis mice on days 2, 4, 6 and 12 were significantly lower than those in the positive control group (106.1±25.3 vs 132.1± 23.2, 157.9 ± 40.3 vs 210.8 ± 34.4, 228.8 ± 72.4 vs 279.5 ± 62.6, 550.1 ± 201.9 vs 731.9 ± 162.2, respectively; p <0.05) mm3. There was no significant difference in tumour volume between the two groups (p >0.05) on days 8, 10 or 14 (table 1 and fig. 3).
Table 1 Changes in tumour volume (mm3) after the parabiosis operation.
| Day | Parabiosis | Positive control |
|---|---|---|
| D2 | 106.1 ± 25.3 | 132.1 ± 23.2* |
| D4 | 157.9 ± 40.3 | 210.8 ± 34.4* |
| D6 | 228.8 ± 72.4 | 279.5 ± 62.6* |
| D8 | 326.9 ± 99.4 | 381.4 ± 100.6 |
| D10 | 440.4 ± 158.3 | 526.0 ± 110.1 |
| D12 | 550.1 ± 201.9 | 731.9 ± 162.2* |
| D14 | 763.0 ± 277.5 | 1034.2 ± 262.8 |
* p <0.05
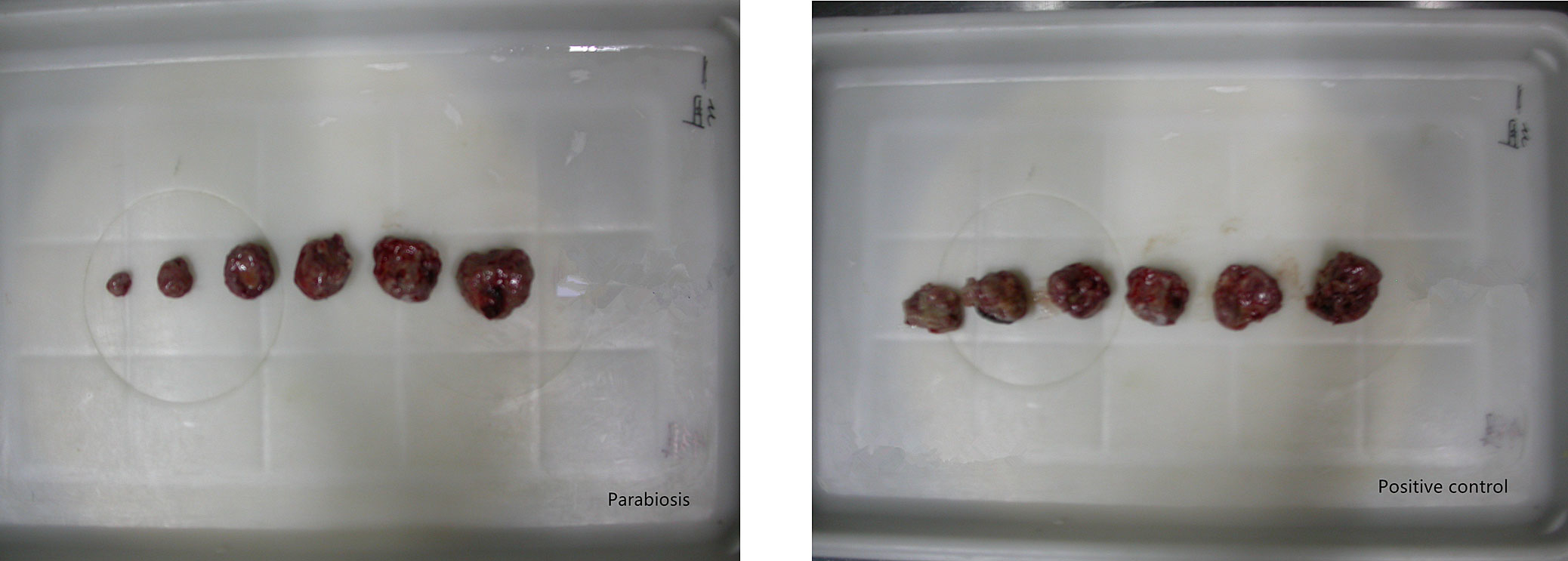
Figure 3 Changes in tumour volume after the parabiosis operation
The wounds were swollen and needed iodine and alcohol for disinfection on day 1. On day 2, the redness of the wound was less than before and the skin became slightly hardened.
On day 3, orbital blood was taken and GFP fluorescence was examined with flow cytometry. Four pairs of the seven showed fluorescence (fig. 4). One of these died on day 4. Thus, the modelling rate was 85.7%.
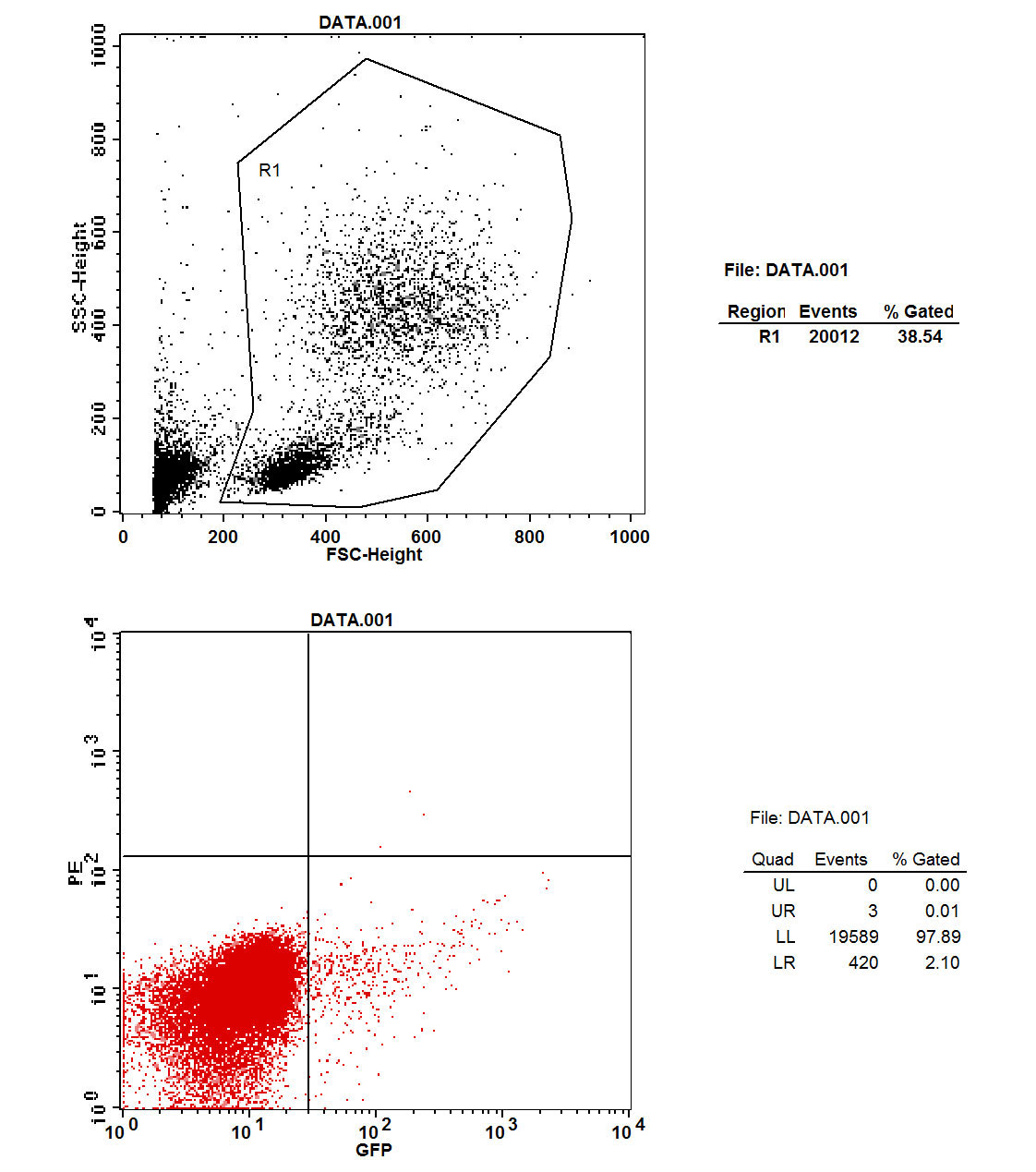
Figure 4 Flow cytometry: green fluorescent protein in parabiosis tumour-bearing mice.
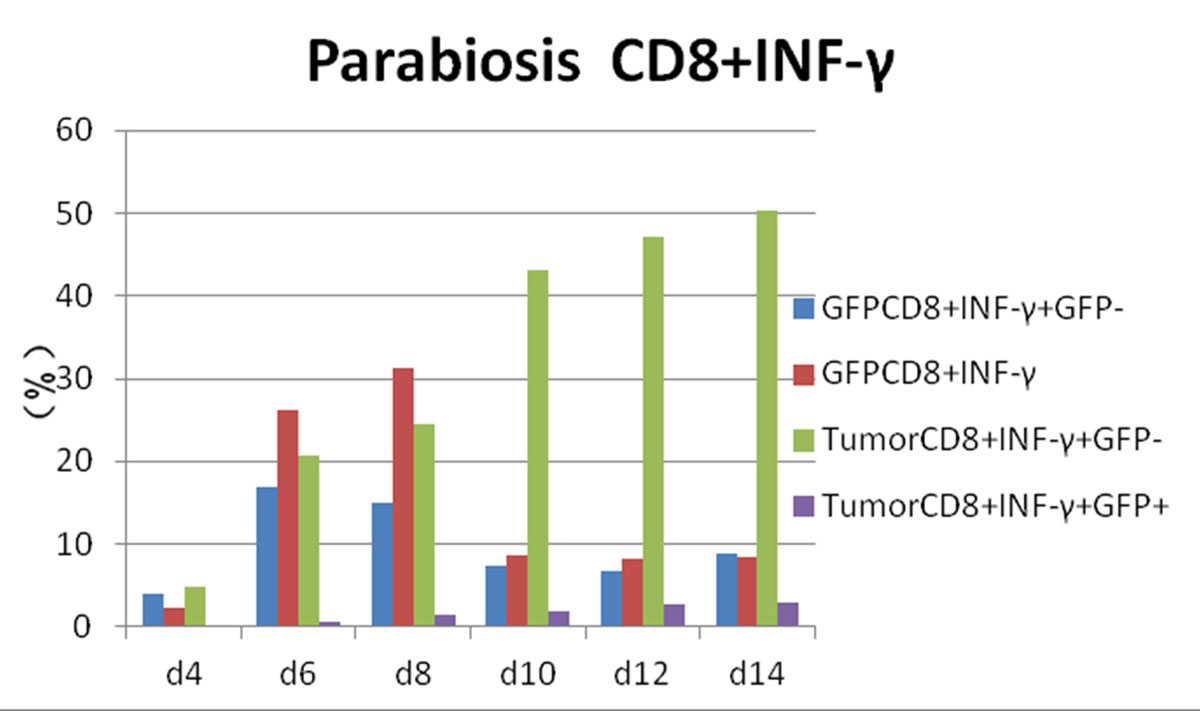
Figure 5 Flow cytometry results: changes in CD8 and interferon-gamma (IFN-γ) expression in each experimental group.
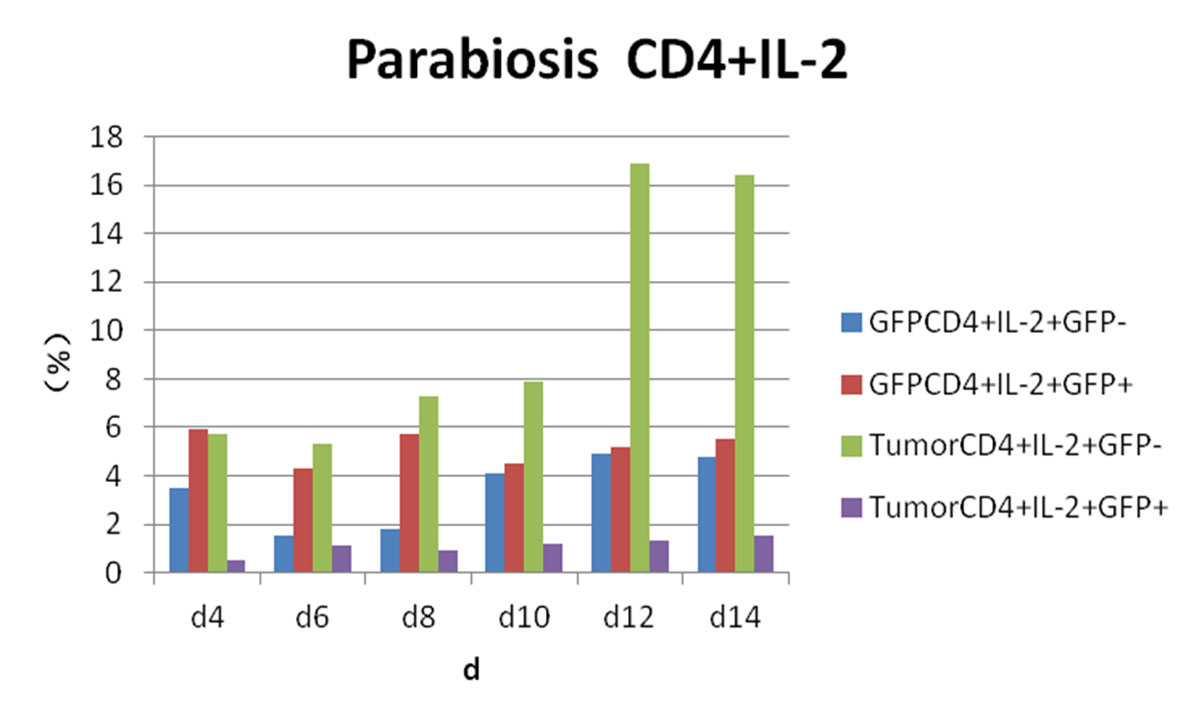
Figure 6 Flow cytometry results: changes in CD4 and interleukin-2 (IL-2) expression in each experimental group.
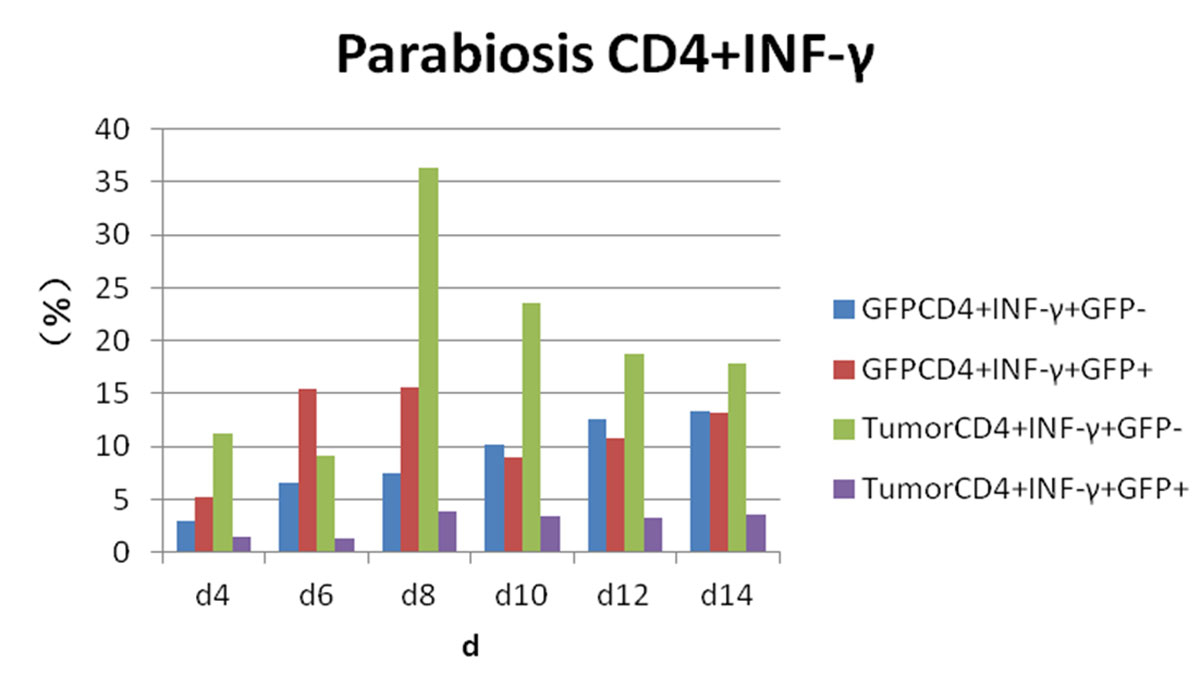
Figure 7 Flow cytometry results: changes in CD4 and interferon-gamma (IFN-γ) expression in each experimental group.
Whereas GFP+ cells appeared in CD4+/IL-4+ tumour-bearing mice and then gradually increased, CD4+/IL-4+/GFP– peaked on day 4 and then gradually decreased. We considered that CD4+/IL-4+ in GFP+ conjoined mice had stimulated the expression of CD4+/IL-4+ in tumour-bearing mice after the blood interaction, as there was a statistically significant difference between the expression of CD4+/IL-4+ in the two groups (p <0.05) (fig. 8). After conjoining surgery, GFP+ cells appeared in the tumour-bearing CD4+/IL-10+ group on day 4, gradually increased, and peaked on day 10. Compared with the positive control group, there was a statistically significant difference (p <0.05) (fig. 9). Specific flow cytograms are shown in figures10, 11 and 12 .
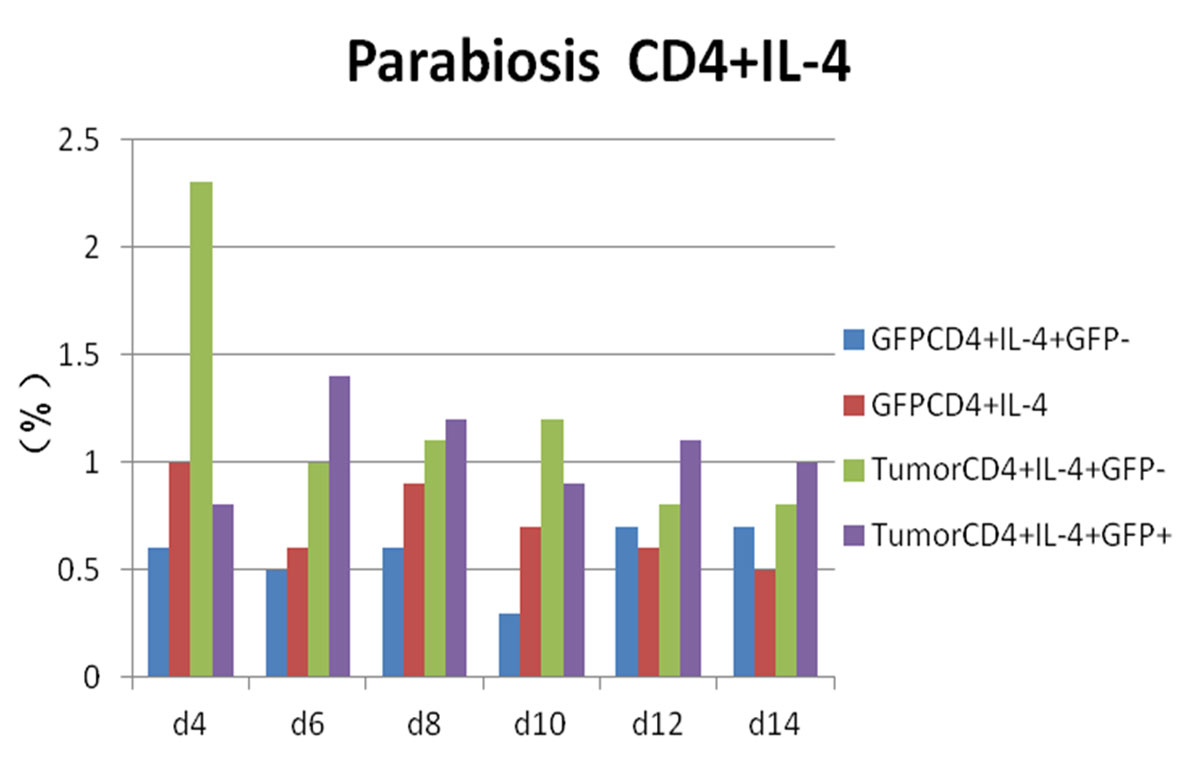
Figure 8 Flow cytometry results: changes in CD4 and interleukin-4 (IL-4) expression in each experimental group.
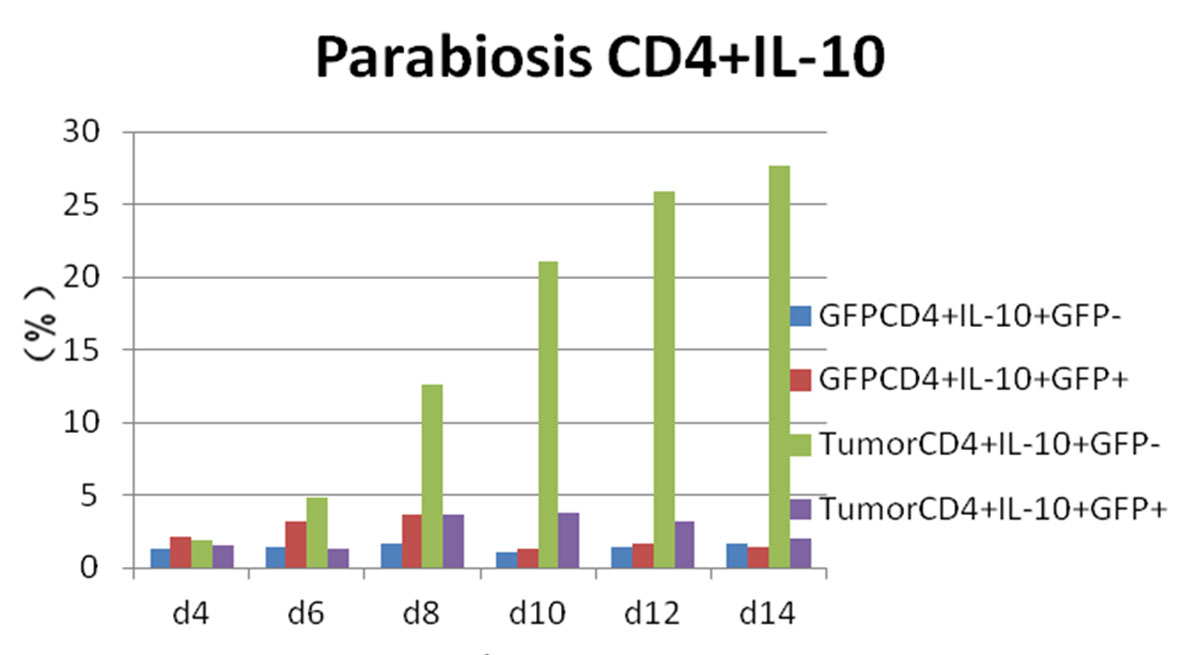
Figure 9 Flow cytometry results: changes in CD4 and interleukin-10 (IL-10) expression in each experimental group.
Postoperative expression of CD8 in GFP+ mice was significantly lower than before the operation (p <0.05). One of the most significant increases, in CD8+/IFN-γ+ double-positivity, peaked on day 8 and accounted for 31.3% of cells. Total CD8+ in conjoined tumour-bearing mice decreased significantly (p <0.05), but the proportion of CD8+/IFN-γ+ double-positive cells and CD8+/IFN-γ+/GFP− cells was not statistically different from the positive control group (p >0.05) (fig. 5). The expression of CD4/IL-2/GFP in GFP+ mice was stable. GFP+ cells appeared in tumour-bearing CD4+/IL-2+ mice on day 4 and gradually increased. The expression of CD4/IL-2 in GFP− mice was significantly higher than that in GFP+ mice (p <0.05) (fig. 6). The expression of CD4/ IFN-γ/ GFP in GFP+ mice was stable. After conjoining surgery, GFP+ cells appeared in tumour-bearing mice that were CD4+/IFN-γ+ on day 4 and gradually increased. Tumour cells that were CD4+/IFN-γ+/GFP− peaked on day 8, and, compared with GFP+ mice, there was a significant difference(p <0.05) (fig. 7). The expression of CD4/ IL-4 in GFP mice and tumour-bearing mice was low.
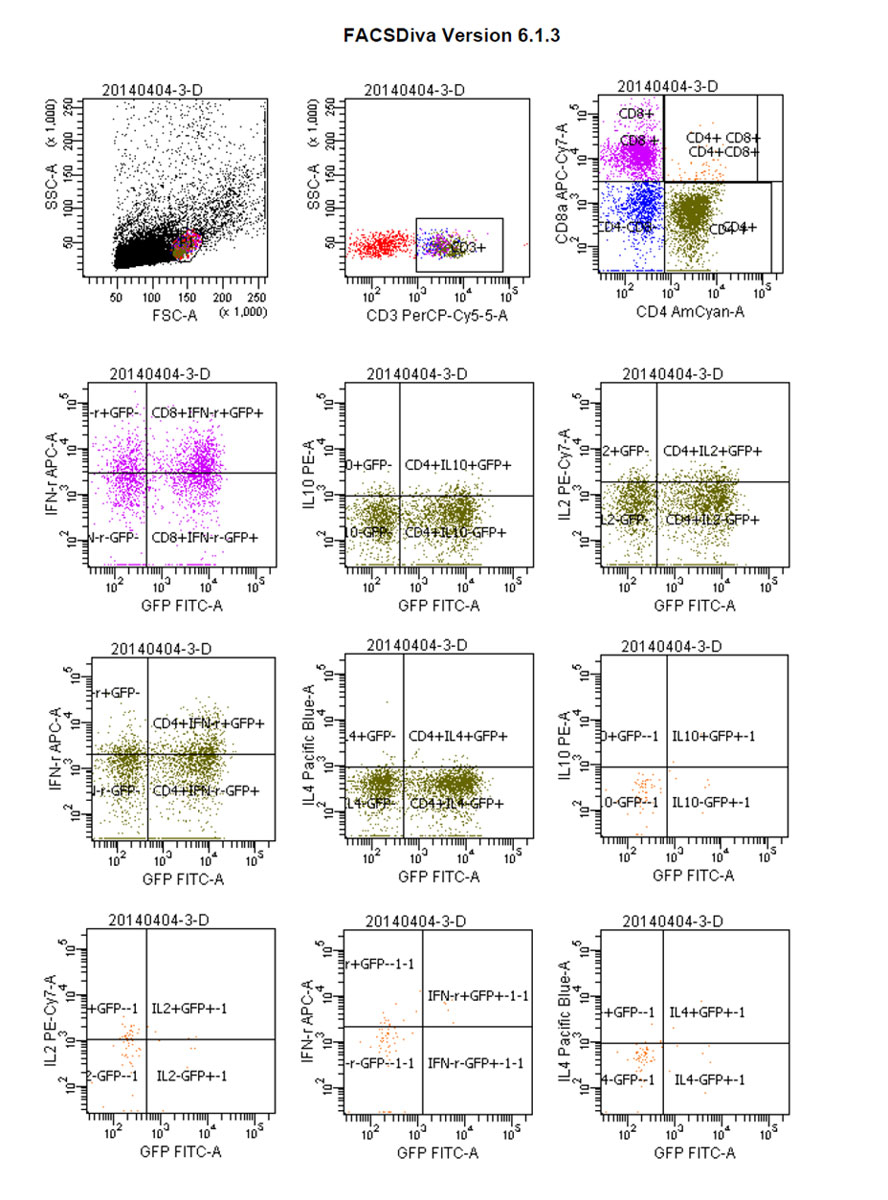
Figure 10 Flow cytograms: green fluorescent protein (GFP)-positive parabiosis mice.
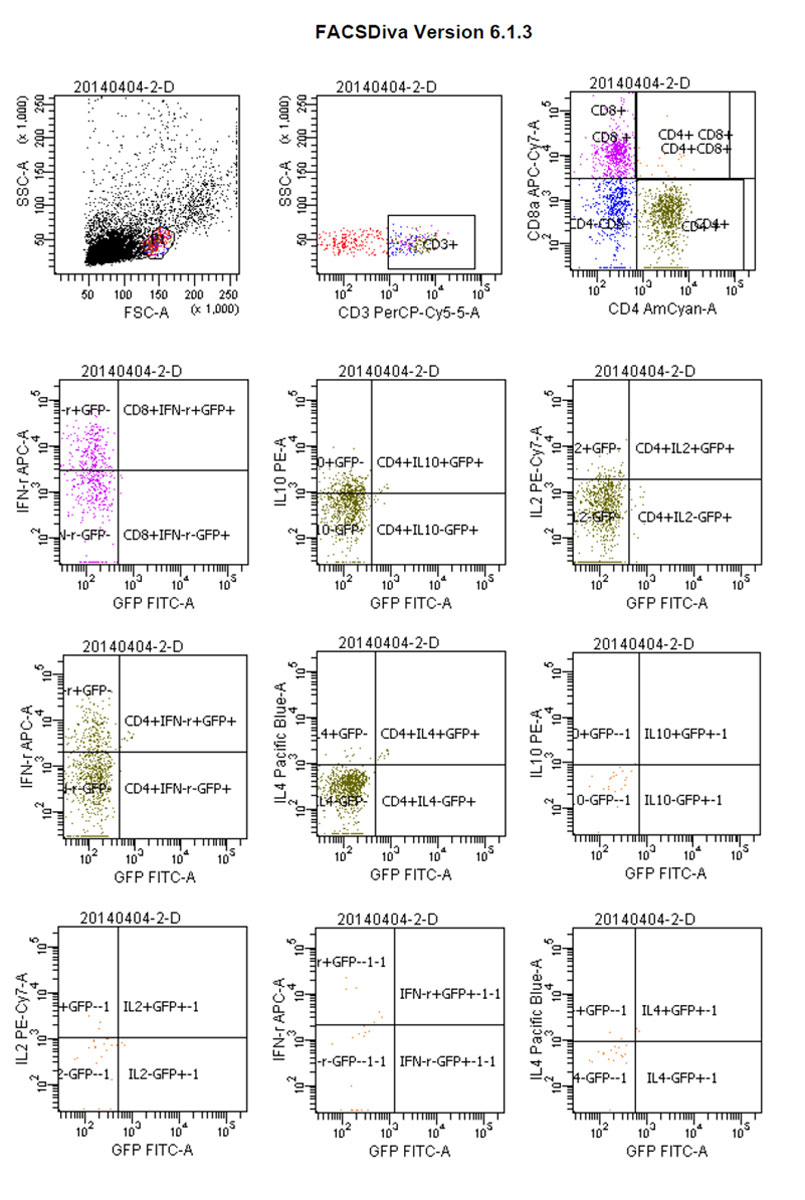
Figure 11 Flow cytograms: parabiosis tumour-bearing mice.
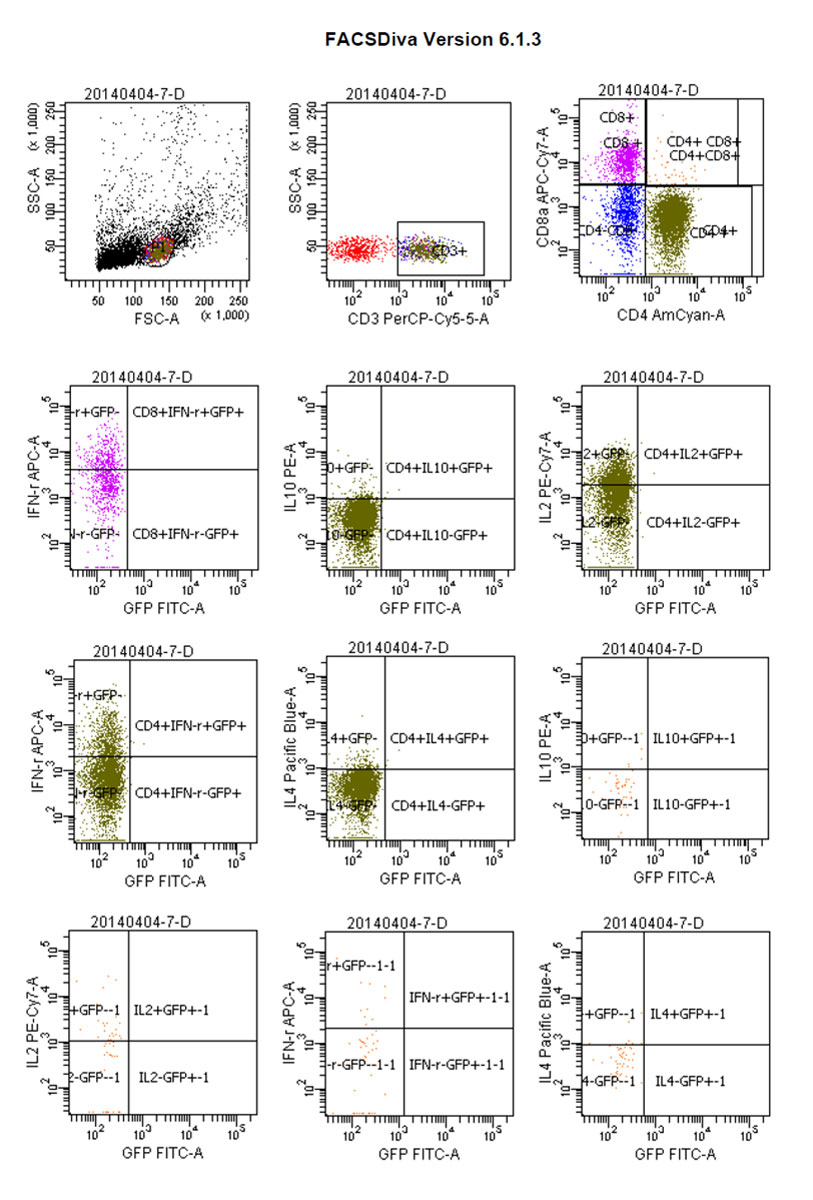
Figure 12 Flow cytograms: positive control tumour-bearing mice.
Tumours were incompletely encapsulated, and the tumour cells featured nested accumulation with large, deeply staining nuclei showing more mitoses, and with more or less necrotic lesions. There were considered positive if the cytoplasm and nucleus stained brownish yellow. Expression (mean ± SD optical density) of CD3, CD4, CD8 and IFN-γ in the tumour-bearing group was 0.32 ± 0.63, 0.33 ± 0.00, 0.31 ± 0.91 and 0.28 ± 0.14, respectively, and there was a statistically significant difference (p <0.05) between these and the positive control group. The expression of CD31 and VEGF was 0.19 ± 0.50 and 0.19 ± 0.21, respectively, with no significant difference between the two groups (p >0.05) (figs 13 and 14 , table 2). For CD31 and VEGF, the values were 0.32 ± 0.35 and 0.29 ± 0.35, respectively, in the positive control group, but there was no significant difference between the two groups (p >0.05). The expression of CD3, CD4, CD8 and INF-γ in the positive control group was 0.22, 0.17, 0.15, 0.16, respectively, on day 8, and there was also no significant difference between the above two groups (p >0.05) (figs 15 and 16 , table 2).
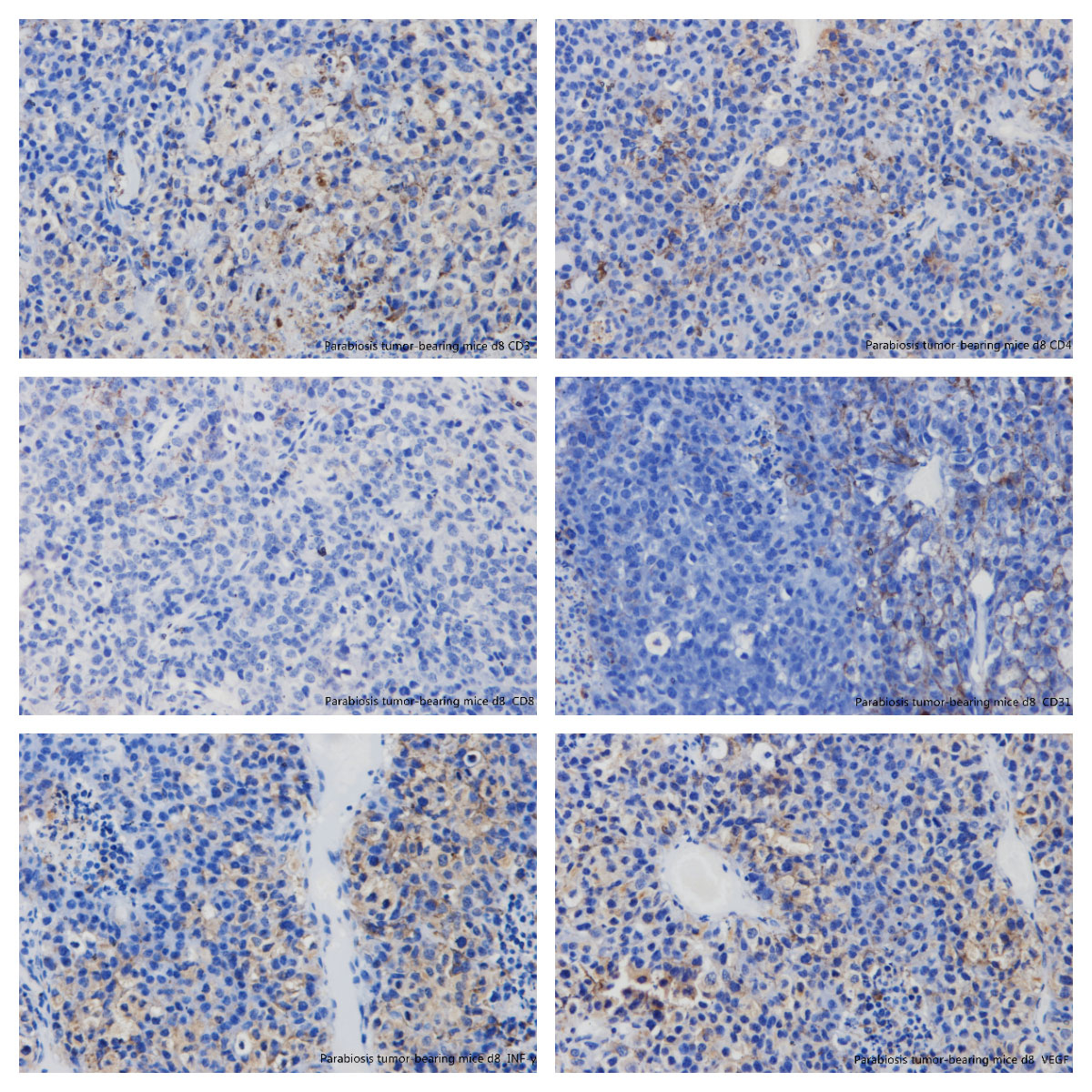
Figure 13 Immunohistochemistry: parabiosis tumour-bearing mice day 8. (A) CD3; (B) CD4; (C) CD8; (D) CD31; (E) interferon-gamma; (F) vascular endothelial growth factor.
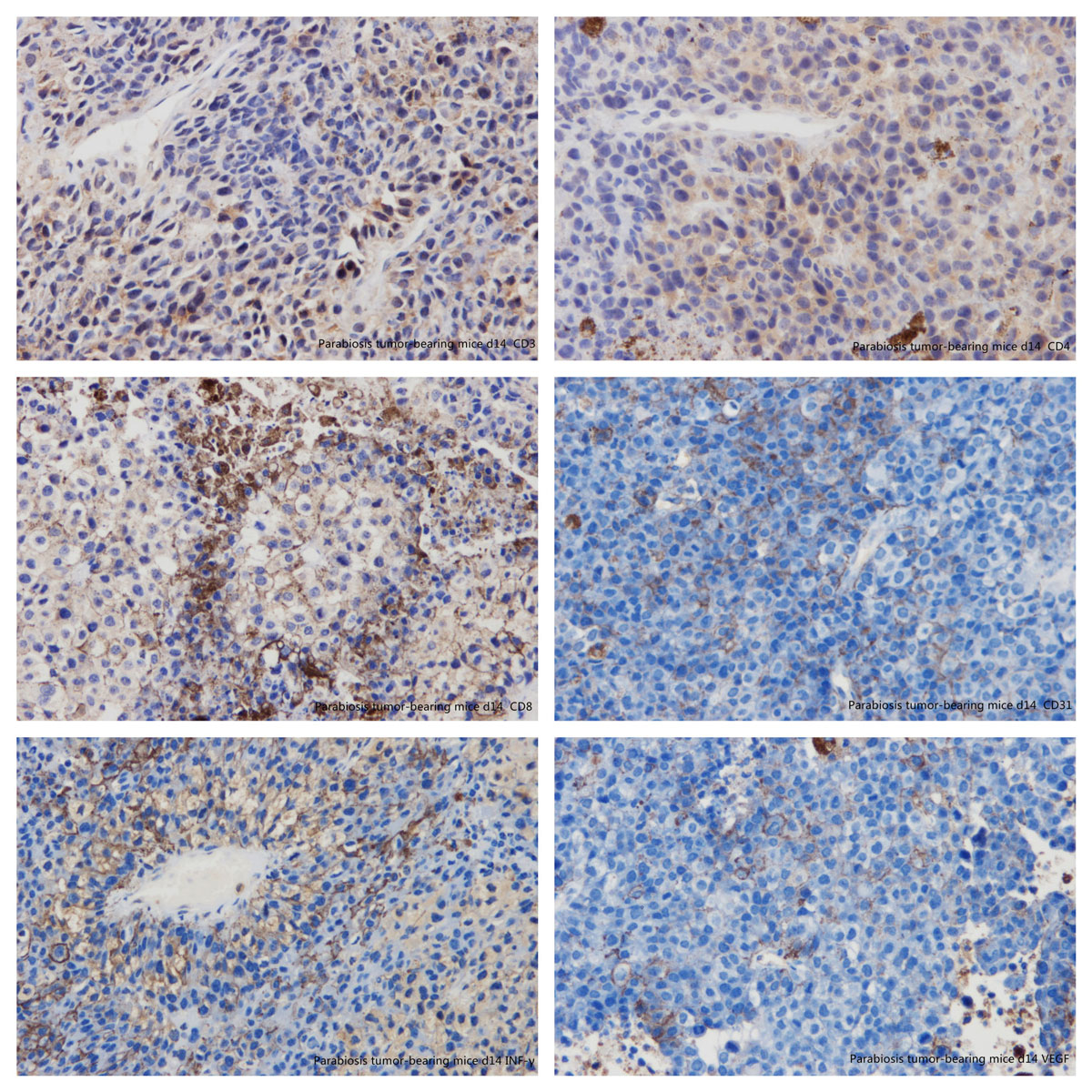
Figure 14 Immunohistochemistry: parabiosis tumour-bearing mice day 14. (A) CD3; (B) CD4; (C) CD8; (D) CD31; (E) interferon-gamma; (F) vascular endothelial growth factor.
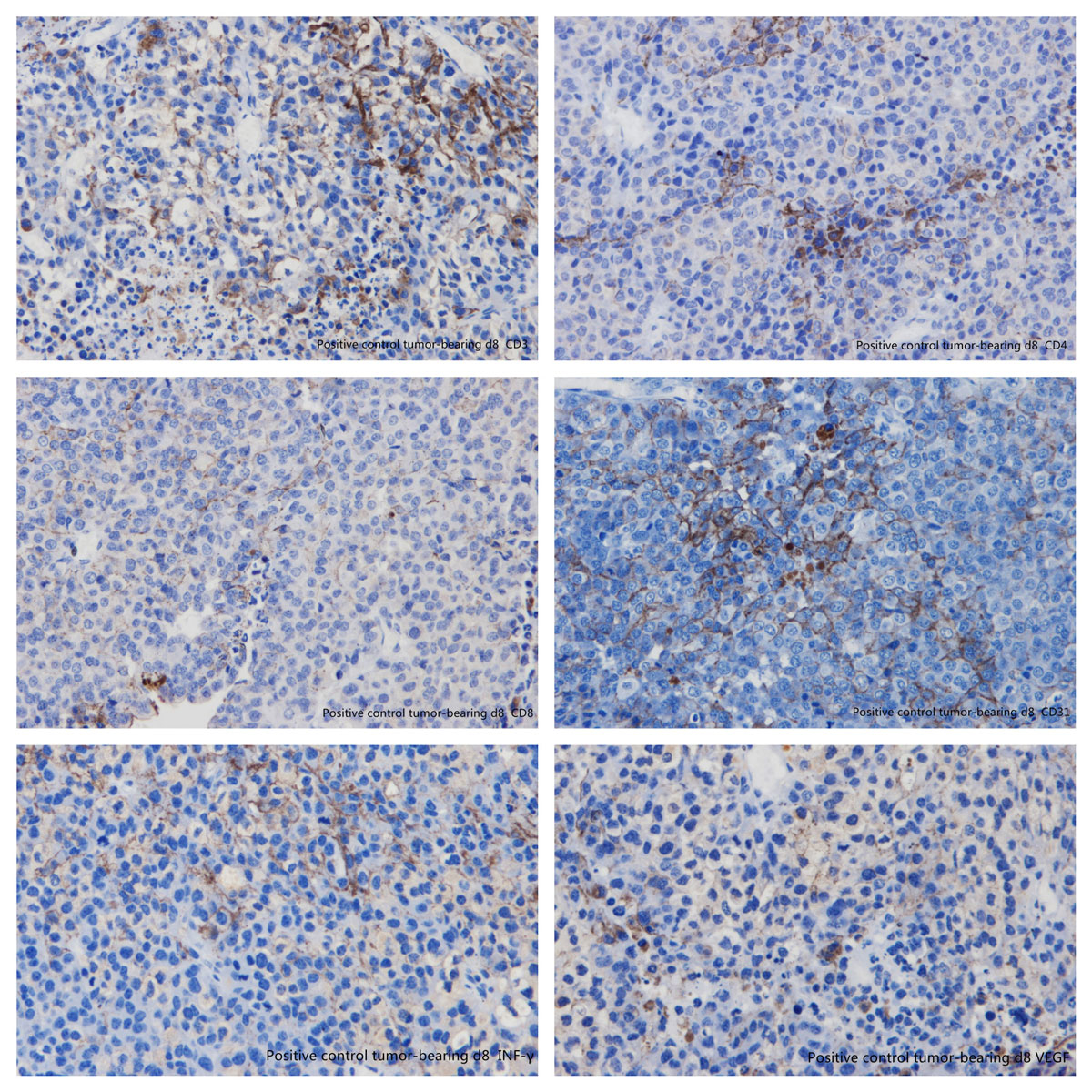
Figure 15 Immunohistochemistry: positive control tumour-bearing mice day 8. (A) CD3; (B) CD4; (C) CD8; (D) CD31; (E) interferon-gamma; (F) vascular endothelial growth factor.
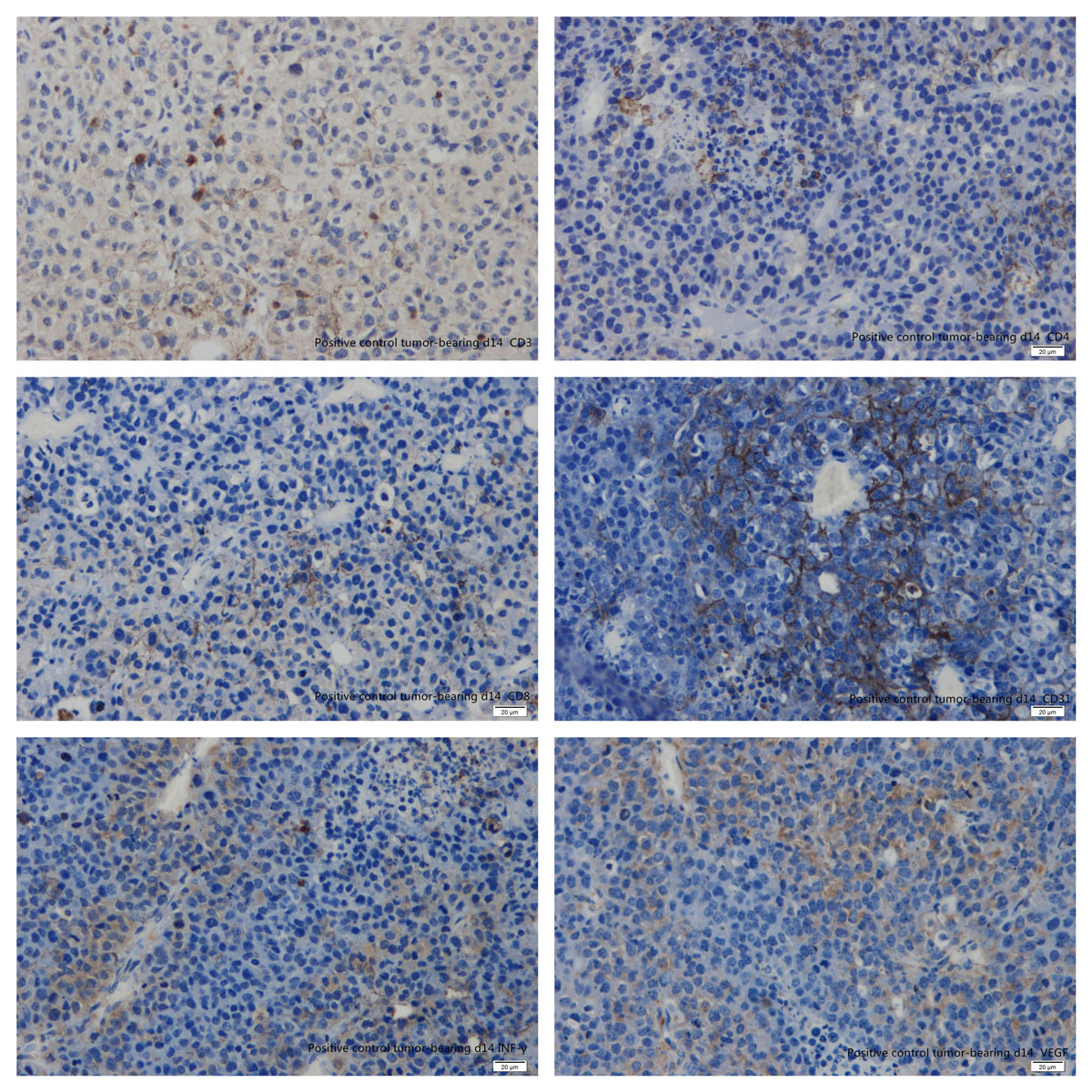
Figure 16 Immunohistochemistry: parabiosis tumour-bearing mice day 14. (A) CD3; (B) CD4; (C) CD8; (D) CD31; (E) interferon-gamma; (F) vascular endothelial growth factor.
A mouse symbiotic model was first used in medical experiments in 1860 by the French physiologist Paul Bert, who used white mice in symbiotic animal models in order to understand the feasibility of future organ transplantation [9]. In the following 100 years of medical research, the creation of the symbiotic animal model basically followed Paul Bert's surgical method, in which the lateral skin of the two mice was longitudinally incised and the incision sutured to form a joint body. Blood mingling could be detected after the capillaries of the two mice had connected [10]. On the basis of his ground-breaking work, Paul Bert won the French Academy of Sciences Experimental Physiology Award in 1866. In 1908, German physicians Sauerbruch and Heyde successfully replicated this animal model [11]. In 2013, Conboy modified Bert's surgical approach by stitching the neighbouring anterior and posterior legs of the two mice to increase their stability [3]. In our experiment, we followed Paul Bert's surgical methods, and B16 melanoma-bearing mice were selected as a joint symbiotic model of experimental animals. This research was approved by the ethics committee (Number/ID of the approval(s):2014129). Anaesthetic drugs were applied before operation and analgesics were used afterwards. The tumour success rate of experimental GFP− C57BL / 6 mice was 85% and the average time for tumours to form was 6.53 ± 1.23 days [12].
Blood circulation interaction could be detected as early as day 3, after the capillaries of the two mice had connected, which was consistent with the literature [13]. The skin sutures of the conjoined models showed different degrees of hardening, which was considered to be related to varying degrees of GVHD. After waking, the experimental models showed agitation and excitement, and tried to break free from the fixed gauze. Their standing and circling frequency increased significantly compared with before surgery. They started to eat at around 10 hours, and defecated at about 20 hours after surgery. Eating and drinking ability had recovered totally on day 5. The rate of model-forming was 85.7% in our experiment.
CD4+ cells are the most important T cells in the body's immune system. A decrease in the number of CD4+ T lymphocytes represents an impaired immune function. In this experiment, there was a significant difference of the CD4+ level between the conjoined group of conjunct GFP+ mice and in vivo tumour-bearing mice, considering that the associated surgery destroyed the immune system. The percentage CD4+ cells in the group of conjoined GFP+ mice on day 14 was 70.6%, while the percentage of CD4+ in the group of conjoined tumour-bearing mice on day 14 was 56.2%. Compared with the positive control group, there was a statistically significant difference (p <0.05). It was considered that GFP+ mice were involved in the immune responses and immune reconstruction of the tumour-bearing mice.
The studies of Rogers and of others have shown that a local high concentration of IL-2 was conducive to the growth of T cell precursors and T cell formation. It also stimulated natural killer (NK) cell proliferation, enhanced NK cytotoxic activity and cytokine production, induced the production of LAK (lymphokine activated killer) cells, and promoted B cell proliferation and antibody secretion. Elevated IL-2 was associated with graft rejection. Wu Z et al. [6] pointed out that virus-specific CD4+ T cells were the key to the control of latent infection and re-infection. In particular, IL-2 could help NK cells against human cytomegalovirus infection. Meehan et al. [9] used a high dose of IL-2 (1000 U/ml) and AIM-V™ culture medium to differentiate haematopoietic progenitor cells in the peripheral blood of patients with multiple myeloma. The expanded cells had a strong ability to kill K562 and RPMI8226 cells.
In our experiment, expression of GFP/CD4/IL-2 in the conjoined group of was statistically significantly higher than in the normal negative control group (p <0.05). It was considered that the associated GFP+/CD4+/IL-2+ group influenced the immune response of the tumour-bearing mice. Also, it might relate to transplant rejection after the conjoining surgery.
IL-4 was found by Howard and is produced by T helper-2 (Th2) cells and mononuclear macrophages. It inhibits Th1 cell activation and secretion of cytokines, and promotes B cell proliferation and differentiation, while inducing IgG1 and IgE production, and enhancing the function of macrophages and cytotoxic t lymphocytes (CTLs) [10]. In the mouse experimental system, IL-4 in most cases positively regulated the differentiation of CTLs and LAK cells, and also inhibited the LAK activity of NK cells induced by IL-2. By decreasing the production of proinflammatory cytokines; IL-4 inhibited the expression of major histocompatibility complex (MHC)-II molecules and the like, and forming synergistic antagonism with IL-2 [16]
In our experiment, CD4+/IL-4+ in the combined group of GFP+ mice was significantly higher than that of the negative control group (p <0.05), and CD4+/IL-4+ in the combined tumour-bearing mice was significantly higher than in the positive control group (p <0.05). It was considered that the increase of CD4+/IL-4+ in GFP+ mice was related to inhibition of the inflammatory reaction and inhibition of the production of IL-2.
IL-10 belongs to the interferon family of cytokines. It is a Th2-derived protein that suppresses Th1 cell function and has a strong anti-inflammatory effect. It inhibits Th1 cells from synthesising IFN-γ, IL-2 and IL-10, and reduces antigen presentation by down-regulating the expression of MHC-II antigens on the surface of monocytes. IL-10 protects body tissues from excessive pathological changes caused by inflammatory cytokines; on the other hand, IL-10 could suppress immune function and thus reduce the capacity to clear pathogenic microorganisms [17].
In this experiment, the increase of CD4+/IL-10+ was most obvious in the parabiosis tumour-bearing mice, and was statistically greater than in the positive control group (p <0.05). The CD4+/IL-10+ level in the conjoined GFP+ mice was significantly higher than that in the negative control group (p <0.05). It was considered that conjoining surgery stimulated the secretion of CD4+/IL-10+ to maintain the body's homeostasis.
IFN-γ, discovered by Younger and Salvin, is a major macrophage activating factor that promotes macrophage phagocytosis and inflammation, directly promotes T and B cell differentiation and CTL maturation, and stimulates B cells to secrete antibodies, thereby enhancing the body's immune function. In the appropriate stimulation of TNF and lipopolysaccharide, IFN-γ could be a suitable inducer of NO production, which is of great significance for inhibiting the proliferation and cardiovascular remodelling of tumour tissue. It also help in the diagnosis of early transplant rejection [17, 18].
In our experiment, the increase of CD4+/IFN-γ+ in the conjoined tumour-bearing mice was most obvious at day 8 postoperatively. However, the CD4+/IFN-γ+ of GFP+ mice in the conjoined group was significantly lower than that of the negative control group (p <0.05). It was considered that the CD4+/IFN-γ+ in GFP+ mice was related to the surgical injury and tumourigenicity in tumour-bearing mice.
CTL cells are also known as CD8+ cells. CD8+ T cells recognise endogenous antigenic peptides presented by MHC-I molecules. After activation, CTL cells mainly differentiate into cytotoxic T cells, and generally do not secrete lymphokines. CTL cells play an important role in monitoring body cells, virus-infected cells, tumour cells and foreign allograft tissue cells [19]. In our study, the rate of CD8+ in the tumour-bearing mice in the positive control group was 19.80 ± 4.27%, began to decrease after reaching a peak of 25.8% at day 8 and decreased to 15.7% at day 14, which was the lowest level among the four groups of mice. It was considered to be related to tumour destruction by the immune system in C57 mice. However, there was no significant difference between the CD8+ of the tumour-bearing mice and the positive control group (p >0.05).
IFN-γ is an important negative regulator of growth. Its function includes blocking viral replication, inhibiting tumour cell proliferation, regulating the function of the lymphatic system, and enhancing immunity [20]. Stern et al. pointed out that CD4+ and CD8+ T cells can reject new tumours; CD8 + T cells could even reject established tumours. Besides, IFN-γ was involved in the antitumor activity of T cells [21].
The conjoining surgery had a significant effect on the expression of CD/ IFN-γ. After the operation, CD8+/IFN-γ increased gradually in the conjoined tumour-bearing mice and reached 53.2% at day 14. CD8+ IFN-γ+ of the positive control group at day 14 was 30.3%. It was considered that the increase of CD8+/IFN-γ+ in conjoined tumour-bearing mice was related to the participation of CD8+ in conjoined GFP+ mice.
The CD4/CD8 ratio was a measure of whether the immune system reached a steady state. An increased CD4/CD8 ratio during organ transplantation predicts possible rejection [16, 17]. CD4/CD8 in the conjoined group increased gradually after surgery, which was considered to be related to postoperative transplant rejection. CD4/CD8 in the positive control group decreased gradually, which was related to the destruction of immune mechanisms caused by the tumour. However, the CD4/CD8 ratio of the conjoined GFP+ mice was significantly higher than that of the negative control group (p <0.05), which was related to the symbiotic model stimulating the immune system of the GFP+ mice.
CD3 is a T cell marker present on both resting and activated T lymphocytes. Whereas CD4 is immunohistochemically expressed on Th lymphocytes, CD8 is expressed on suppressor-cytotoxic T cells. IFN-γ can promote macrophage phagocytosis and an inflammatory response, directly promote T and B cell differentiation and CTL maturation, and stimulate B cells to secrete antibodies, thereby enhancing the body's immune function [24]. In our study, we found that expression of CD3, CD4, CD8 and IFN-γ was 0.32 ± 0.63, 0.33 ± 0.00 and 0.31 ± 0.91 and 0.28 ± 0.14, respectively, which was significantly higher than that of the positive control group (p <0.05), indicating that the symbiotic model could stimulate the production of CD3, CD4, CD8 and IFN-γ, and thus play a role in regulating the immune system and killing tumour cells. Expression of CD31 and VEGF was 0.19 ± 0.50 and 0.19 ± 0.21, respectively. There was no significant difference compared with the positive control group (p >0.05, see figs12 and 13 and table 2).
CD31 is primarily used to demonstrate the presence of endothelial cell tissue for assessing tumour angiogenesis, which might imply a degree of rapid growth of the tumour. Malignant vascular endothelial cells also usually retain the antigen. CD31 antigen is expressed in the cytoplasm and membrane of malignant melanoma cells. The strong positive expression of CD31 indicates strong adhesion between tumour cells [25].
Vascular endothelial growth factor (VEGF) was a kind of glycoprotein which could selectively promote the formation of vascular endothelial cells in vitro culture fluid of bovine pituitary follicles and was found by Ferrara in 1989 [26]. Vascular endothelial growth factor receptor (VEGFR) promotes endothelial cell proliferation. As well as increasing vascular permeability of endothelial cell migration, it can induce tumour angiogenesis and maintain the continued growth of the tumour. The level of VEGF expression is positively correlated with many factors, such as the size of the primary tumour, angiogenesis and lymphatic metastasis, so it is an important immunohistochemistry indicator of tumour growth [27]. Whereas the positive expression rates of CD31 and VEGF in the conjoined tumour-bearing group were 0.19 ± 0.50 and 0.19 ± 0.21, respectively, the positivity rates of CD31 and VEGF in the positive control group were 0.32 ± 0.35 and 0.29 ± 0.35, respectively. However, there was no significant difference between the two groups. It indicated that the symbiotic model had little effect on CD31 and VEGF.
| Table 2: Immunohistochemistry: expression of cell markers in the tumour-bearing mice. |
| Marker |
Parabiosis tumour-bearing mice day 8
(OD) |
Parabiosis tumour-bearing mice day 14
(OD) |
Positive control day 8
(OD) |
Positive control day 14
(OD) |
|---|---|---|---|---|
| CD3 | 0.27* | 0.36 | 0.22 | 0.17 |
| CD4 | 0.33* | 0.33 | 0.17 | 0.22 |
| CD8 | 0.24* | 0.37 | 0.15 | 0.19 |
| CD31 | 0.15 | 0.22 | 0.29 | 0.34 |
| INF-γ | 0.27* | 0.29 | 0.16 | 0.20 |
| VEGF | 0.17 | 0.20 | 0.26 | 0.31 |
INF-γ = interferon-gamma; VEGF = vascular endothelial growth factor * p <0.05
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Conboy IM , Conboy MJ , Wagers AJ , Girma ER , Weissman IL , Rando TA . Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–4. doi:.https://doi.org/10.1038/nature03260
2 Villeda SA , Luo J , Mosher KI , Zou B , Britschgi M , Bieri G , et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–4. doi:.https://doi.org/10.1038/nature10357
3 Loffredo FS , Steinhauser ML , Jay SM , Gannon J , Pancoast JR , Yalamanchi P , et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153(4):828–39. doi:.https://doi.org/10.1016/j.cell.2013.04.015
4 Castellano JM , Palner M , Li S-B , Freeman GM, Jr , Nguyen A , Shen B , et al. In vivo assessment of behavioral recovery and circulatory exchange in the peritoneal parabiosis model. Sci Rep. 2016;6:29015. doi:.https://doi.org/10.1038/srep29015
5 Mittal D , Gubin MM , Schreiber RD , Smyth MJ . New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr Opin Immunol. 2014;27(27):16–25. doi:.https://doi.org/10.1016/j.coi.2014.01.004
6 Li X , Du W , Liu W , Li X , Li H , Huang SA . Comprehensive flow cytometry phenotype in acute leukemia at diagnosis and at relapse. APMIS. 2010;118(5):353–9. doi:.https://doi.org/10.1111/j.1600-0463.2010.02603.x
7 Zhang K , Deng H , Cagle PT . Utility of immunohistochemistry in the diagnosis of pleuropulmonary and mediastinal cancers: a review and update. Arch Pathol Lab Med. 2014;138(12):1611–28. doi:.https://doi.org/10.5858/arpa.2014-0092-RA
8 Decouvelaere AV . Apport de l’immuno-histochimie dans le diagnostic des sarcomes [Immunohistochemistry in the diagnosis of sarcomas]. Ann Pathol. 2015;35(1):98–106. doi:.https://doi.org/10.1016/j.annpat.2014.11.006
9 Eggel A , Wyss-Coray T . A revival of parabiosis in biomedical research. Swiss Med Wkly. 2014;144:w13914. doi:.https://doi.org/10.4414/smw.2014.13914
10 Lunsford WR , McCAY CM , Lupien PJ , Pope FE , Sperling G . Parabiosis as a method for studying factors which affect aging in rats. Gerontologia. 1963;7(1):1–8. doi:.https://doi.org/10.1159/000211170
11 Deacon RM . Assessing nest building in mice. Nat Protoc. 2006;1(3):1117–9. doi:.https://doi.org/10.1038/nprot.2006.170
12 Kamran P , Sereti KI , Zhao P , Ali SR , Weissman IL , Ardehali R . Parabiosis in mice: a detailed protocol. J Vis Exp. 2013;80:50556.
13 Rando TA , Chang HY . Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell. 2012;148(1-2):46–57. doi:.https://doi.org/10.1016/j.cell.2012.01.003
14 Duyverman AM , Kohno M , Duda DG , Jain RK , Fukumura D . A transient parabiosis skin transplantation model in mice. Nat Protoc. 2012;7(4):763–70. doi:.https://doi.org/10.1038/nprot.2012.032
15 Gaber T , Strehl C , Sawitzki B , Hoff P , Buttgereit F . Cellular energy metabolism in T-lymphocytes. Int Rev Immunol. 2015;34(1):34–49. doi:.https://doi.org/10.3109/08830185.2014.956358
16 Dumitriu IE . The life (and death) of CD4+ CD28(null) T cells in inflammatory diseases. Immunology. 2015;146(2):185–93. doi:.https://doi.org/10.1111/imm.12506
17 Plain KM , Verma ND , Tran GT , Nomura M , Boyd R , Robinson CM , et al. Cytokines affecting CD4(+) T regulatory cells in transplant tolerance. Interleukin-4 does not maintain alloantigen specific CD4(+)CD25(+) Treg. Transpl Immunol. 2013;29(1-4):51–9. doi:.https://doi.org/10.1016/j.trim.2013.10.003
18 Liu B , Zhang H , Li J , Lu C , Chen G , Zhang G , et al. Triptolide downregulates Treg cells and the level of IL-10, TGF-β, and VEGF in melanoma-bearing mice. Planta Med. 2013;79(15):1401–7. doi:.https://doi.org/10.1055/s-0033-1350708
19 Lasfar A , Gogas H , Zloza A , Kaufman HL , Kirkwood JM . IFN-λ cancer immunotherapy: new kid on the block. Immunotherapy. 2016;8(8):877–88. doi:.https://doi.org/10.2217/imt-2015-0021
20 Leiva-Revilla J , Guerra-Castañon F , Olcese-Mori P , Lozada I , Rubio J , Gonzales C , et al. Efecto de la maca roja (Lepidium meyenii) sobre los niveles de IFN-γ en ratas ovariectomizadas [Effect of red maca (Lepidium meyenii) on INF-γ levels in ovariectomized rats]. Rev Peru Med Exp Salud Publica. 2014;31(4):683–8. doi:.https://doi.org/10.17843/rpmesp.2014.314.118
21 Stern C , Kasnitz N , Kocijancic D , Trittel S , Riese P , Guzman CA , et al. Induction of CD4(+) and CD8(+) anti-tumor effector T cell responses by bacteria mediated tumor therapy. Int J Cancer. 2015;137(8):2019–28. doi:.https://doi.org/10.1002/ijc.29567
22 Cui W , Joshi NS , Jiang A , Kaech SM . Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27(15):2177–87. doi:.https://doi.org/10.1016/j.vaccine.2009.01.088
23 Kang S , Xie J , Miao J , Li R , Liao W , Luo R . A knockdown of Maml1 that results in melanoma cell senescence promotes an innate and adaptive immune response. Cancer Immunol Immunother. 2013;62(1):183–90. doi:.https://doi.org/10.1007/s00262-012-1318-1
24 Kiyota T , Takahashi Y , Watcharanurak K , Nishikawa M , Ohara S , Ando M , et al. Enhancement of anticancer effect of interferon-γ gene transfer against interferon-γ-resistant tumor by depletion of tumor-associated macrophages. Mol Pharm. 2014;11(5):1542–9. doi:.https://doi.org/10.1021/mp4007216
25 Li H , Gupta S , Du WW , Yang BB . MicroRNA-17 inhibits tumor growth by stimulating T-cell mediated host immune response. Oncoscience. 2014;1(7):531–9. doi:.https://doi.org/10.18632/oncoscience.69
26 Hsueh C , Lin JD , Wu IC , Chao TC , Yu JS , Liou MJ , et al. Vascular endothelial growth factors and angiopoietins in presentations and prognosis of papillary thyroid carcinoma. J Surg Oncol. 2011;103(5):395–9. doi:.https://doi.org/10.1002/jso.21844
27 Costache MI , Ioana M , Iordache S , Ene D , Costache CA , Săftoiu A . VEGF Expression in Pancreatic Cancer and Other Malignancies: A Review of the Literature. Rom J Intern Med. 2015;53(3):199–208. doi:.https://doi.org/10.1515/rjim-2015-0027
No financial support and no other potential conflict of interest relevant to this article was reported.