Figure 1 Vascular access creation (brachiocephalic arteriovenous fistula) using a punch.
DOI: https://doi.org/10.4414/smw.2018.14668
Functional vascular access, most commonly an arteriovenous fistula (AVF) or an arteriovenous graft (AVG), is essential to deliver adequate haemodialysis therapy to patients. The need for dialysis in patients with chronic kidney disease has been increasing in recent years [1]. Between 1990 and 2000, the global prevalence of maintenance dialysis increased 1.7 times and the incidence more than doubled [2]. Therefore, efficient vascular access creation and maintenance service is essential. With an average life expectancy of patients on dialysis of 5 to 10 years, vascular access longevity is an important issue and unfortunately can be jeopardised by various issues [3]. Non-maturation, occlusion and infection are typical complications occurring during the evolution of the vascular access. This results in frequent surgical revisions and an increased use of central venous catheters (CVCs) as an alternative for haemodialysis access, which in turn is related to higher morbidity and mortality rates and increased costs [4–8]. The primary patency of a vascular access, which represents intervention-free survival, has been reported to be superior for AVFs [9–13], with AVGs and CVCs showing a reduced intervention-free survival. There is conflicting evidence in the literature as to primary AVF creation rates, ranging from 24 to 80% [14], and the percentage of patients on CVC dialysis is reported to be nearly 80% [15].
The optimal surgical management is to create an AVF, and avoid grafts and CVCs for as long as possible. It remains unclear whether this aim is being achieved in current practice. Vascular access guidelines such as the Kidney Diseases Outcomes Quality Initiative (K/DOQI) [16], European Renal Best Practice Guidelines [14] and the Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS) [17] address this issue.
The United States Renal Data System (USRDS) collects data from national and regional renal registries in the USA [1]. However, Switzerland does not have a specific multidisciplinary registry for vascular access and outcome data for vascular access creation is not available, although more than 4500 patients are currently on haemodialysis in this country [18]. This means that we cannot compare our results with other centres and countries or review conformity with guidelines.
The aim of this retrospective study was to assess the quality of vascular access creation in a Swiss tertiary referral centre and to compare it with the current literature and guidelines to define strategies to improve the clinical management process (fig. 1).

Figure 1 Vascular access creation (brachiocephalic arteriovenous fistula) using a punch.
This retrospective analysis was designed as a quality control study of a tertiary referral centre in Switzerland, in accordance with the Helsinki declaration. All consecutive patients >18 years old undergoing primary vascular access creation between January 2013 and December 2014 were included. Other interventions for renal replacement therapy during this time (isolated catheters for peritoneal dialysis, CVCs without simultaneous access creation, secondary vascular access creation and vascular access revisions) were taken into account but not further analysed. Data, including demographics, risk factors, comorbidities, surgical details, outcome parameters and follow-up information, were retrieved from the hospital records of the Department of Cardiovascular Surgery and the Department of Nephrology and Hypertension. We contacted external dialysis units for information on patients followed up at other institutions. Primary vascular access was classified as AVF or AVG, as well as according to its anatomical location. Study data were collected and managed using REDCap research electronic data capture hosted at the institution (REDCap Software, Version 6.9.3, Vanderbilt University). Follow-up data for at least 365 days were collected. Data were collected in a retrospective manner as part of the in-hospital quality control, which is regulated by the federal law for health insurance. Primary endpoints of the study were the primary and the secondary patency after 1 year. Secondary endpoints were reinterventions and CVC placement.
Proportions and 95% confidence intervals (CIs) of primary and secondary endpoints at 1 year after vascular access were calculated to compare the treatment groups using the chi-squared test. The Kaplan-Meier method was used to estimate freedom from events with respect to all endpoints during the entire follow-up time. Hazard ratios and 95% CIs were analysed using Cox regression analysis. The proportional hazard assumption was tested on Schoenfeld residuals. All CIs are two-sided. Statistical analyses were performed using Stata 12 (StataCorp LP, College Station, Tx, USA).
The ESVS definitions of primary and secondary patency were applied [17]. In brief, primary patency is the time between access creation and the first reintervention. Secondary patency is the time between access creation and abandonment after one or more reinterventions.
Between January 2013 and December 2014, there were 365 surgical interventions for renal replacement therapy (fig. 2). Revision surgery represents the largest group (n = 163), followed by CVC placement (n = 78), including 45 exchanges from a non-tunnelled to a tunnelled CVC, 15 isolated tunnelled CVCs and 18 CVC removals. Seventy-four patients with a primary vascular access creation were included in the further analysis: 11 AVG and 63 AVF. Nine patients with a primary access were excluded because of renal transplantation (three), operative abandonment of access (four), no further dialysis required (one), and patient refusal of revision of a failing vascular access (one).
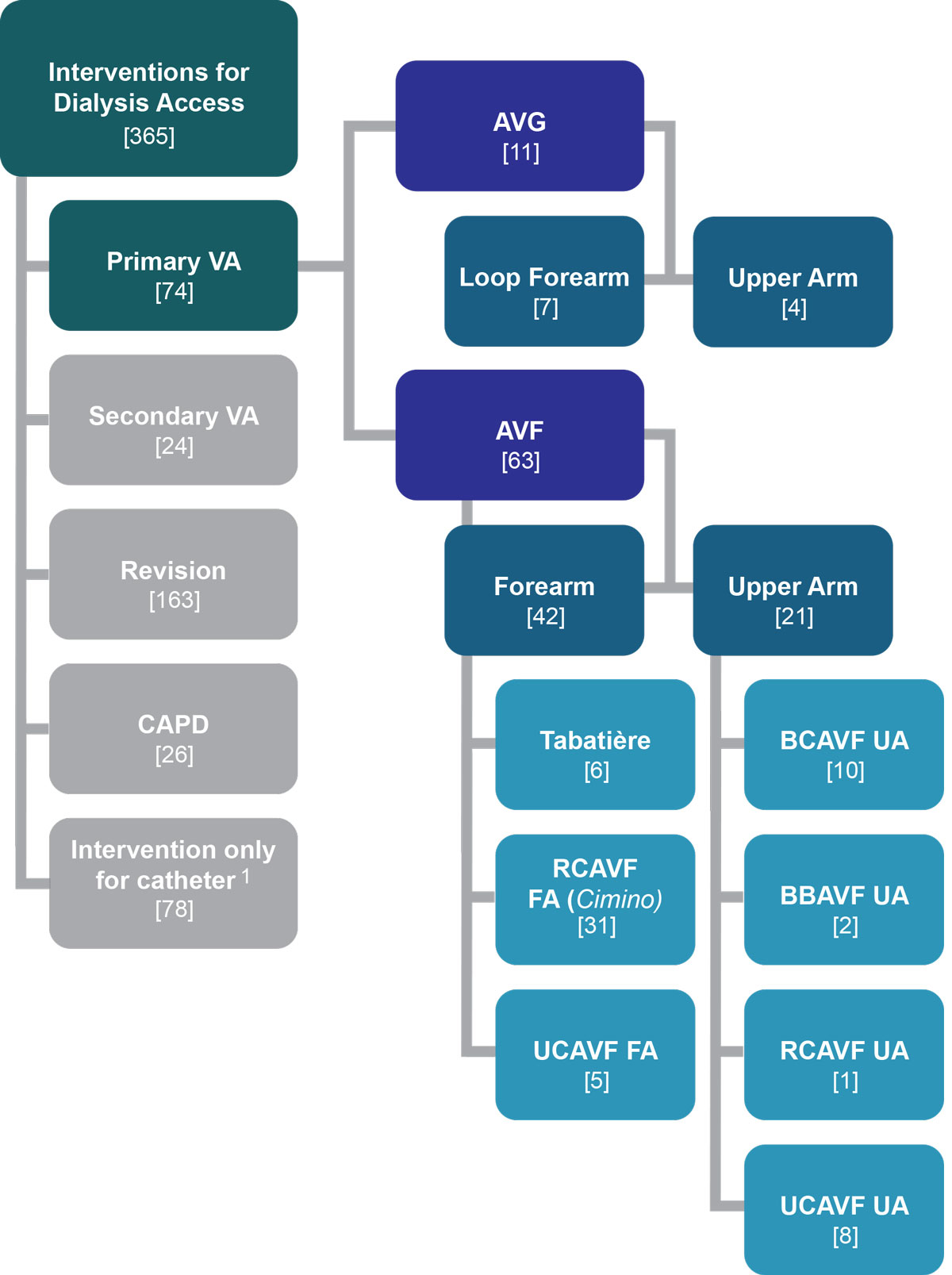
Figure 2 Overview of interventions for renal replacement therapy January 2013 to December 2014.
AVF = arteriovenous fistula; AVG = arteriovenous graft; BBAVF = brachiobasilic AVF, upper arm; BCAVF = brachiocephalic AVF, upper arm; RCAVF =radiocephalic AVF (Cimino), forearm; UCAVF = ulnocephalic AVF forearm and upper arm; VA = vascular access
1 45 changes (2 non-tunnelled), 18 removals, (3 non-tunnelled), 15 new inserted (2 non-tunnelled)
All patients had preoperative venous and arterial ultrasound mapping. The surgeons’ experience was as follows: trainees under supervision (15) and board-certified surgeons (59). Type of anaesthesia used was: local (2), regional (53), and general (19).
Table 1 shows demographic data and comorbidities of patients with a primary vascular access creation. The most common cause of end-stage renal disease was hypertensive nephropathy (36%).
Table 1 Demographics and comorbidities.
|
All patients
(n = 74) |
AVG
(n = 11) |
AVF forearm
(n = 42) |
AVF upper arm
(n = 21) |
|
|---|---|---|---|---|
| General | ||||
| Female, n (%) | 27 (36) | 7 (64) | 12 (29) | 8 (38) |
| Age in years (mean ± SD) | 62.9 ± 15.9 | 62.9 ± 12.5 | 60.9 ± 16.1 | 66.7 ± 16.8 |
| BMI in kg/m2 (mean SD) | 27.8 ± 5.5 | 29.3 ± 6.5 | 28.7 ± 5.4 | 25.1 ± 4.3 |
| Creatinine in μmol/l* (mean ± SD) | 434 ± 254 | 449 ± 348 | 480 ± 265 | 336 ± 126 |
| Estimated creatinine clearance rate† in ml/min/1.73m2 (mean ±SD) | 16.7 ± 7.9 | 18.4 ± 11.7 | 15.9 ± 6.8 | 17.4 ± 7.6 |
| Cause of ESRD‡ | ||||
| Hypertensive nephropathy, n (%) | 27 (36) | 6 (55) | 13 (31) | 8 (38) |
| Diabetic nephropathy, n (%) | 26 (35) | 6 (55) | 11 (26) | 9 (43) |
| Glomerulonephritis, n (%) | 9 (12) | 1 (9) | 5 (12) | 3 (14) |
| Systemic inflammatory disease and vasculitis (e.g., SLE), n (%) | 8 (11) | 0 (0) | 4 (10) | 4 (19) |
| Hereditary nephropathy, n (%) | 7 (9) | 2 (18) | 4 (10) | 1 (5) |
| Others, n (%) | 7 (9) | 0 (0) | 5 (12) | 2 (10) |
| Unknown, n (%) | 7 (9) | 0 (0) | 2 (5) | 5 (24) |
| Tubulointerstitial nephritis, n (%) | 4 (5) | 2 (18) | 2 (5) | 0 (0) |
| Vascular nephropathy, n (%) | 3 (4) | 0 (0) | 2 (5) | 1 (5) |
| Risk factors‡ | ||||
| Hypertension, n (%) | 70 (95) | 11 (100) | 40 (95) | 19 (90) |
| Dyslipidaemia, n (%) | 45 (61) | 7 (64) | 25 (60) | 13 (62) |
| Diabetes mellitus, n (%) | 36 (49) | 7 (64) | 16 (38) | 13 (62) |
| Smoking, n (%) | 26 (35) | 3 (27) | 16 (38) | 7 (33) |
AVF = arteriovenous fistula: AVG = arteriovenous graft; BMI = body mass index; ESRD = end-stage renal disease; SLE = systemic lupus erythematosus * At time of access creation † Cockroft-Gault ‡ Multiple selections possible
Mean follow-up ± standard deviation was 564 ± 294 days. The survival curve is shown in fig. 3. Mortality at 1 year was 11%.
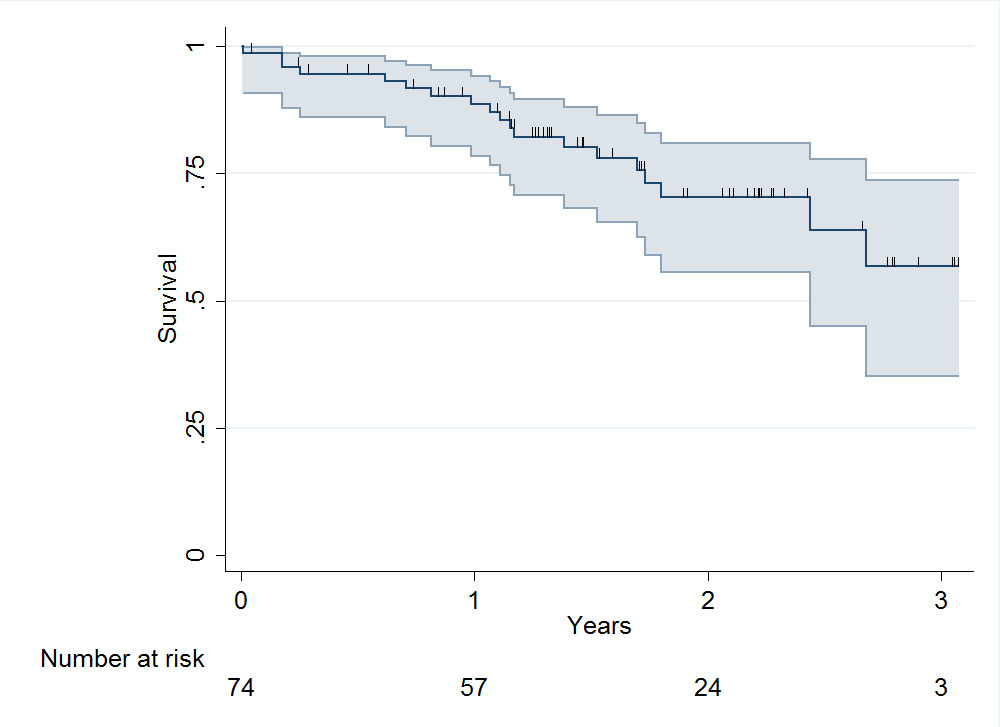
Figure 3 Patient survival after vascular access creation (including 95% confidence intervals): median survival 528 days (interquartile range 371–807).
The intervention-free primary patency rate of the vascular access at 1 year was 46% (95% CI 33–58%) for AVF and 30% (95% CI 7–58%) for AVG (fig. 4). There was no significant difference in primary patency rate between AVG and AVF either in the upper arm (30%, 95% CI 7–58% vs 46%, 95% CI 24–66%, respectively; p = 0.384) or in the forearm (30%, 95% CI 7–58% vs 46%, 95% CI 30–60%, respectively; p = 0.813). A Cox regression analysis showed hazard ratios for primary patency of 0.91 (95% CI 0.41–1.99) for forearm AVF and 0.68 (95% CI 0.28–1.66) for upper arm AVF, when compared with AVG.
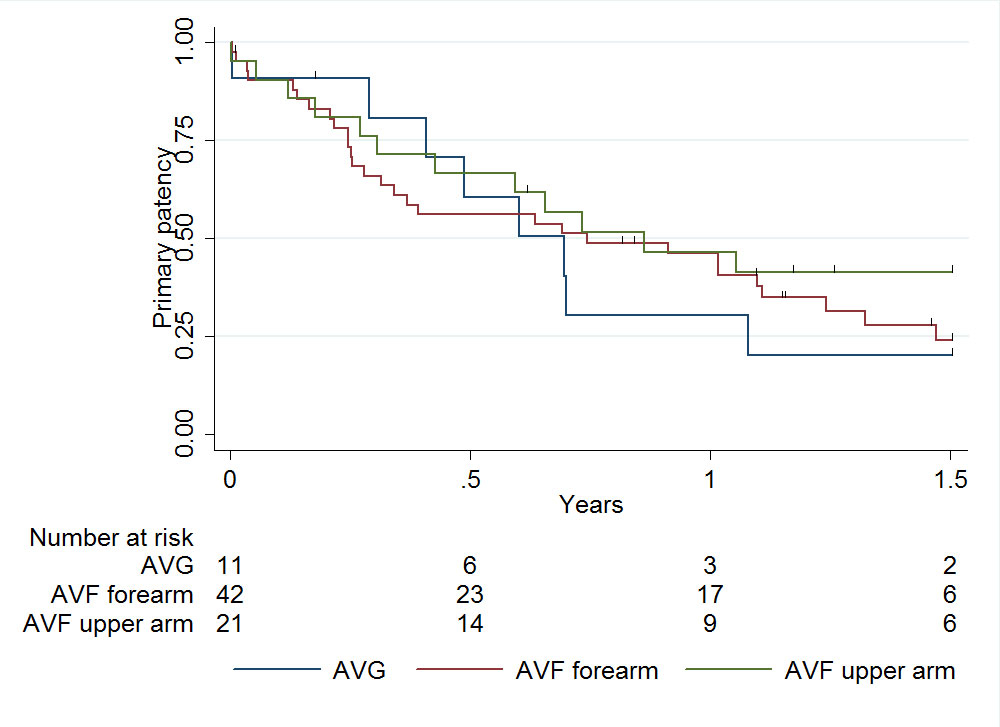
Figure 4 Primary patency of primary vascular access: median primary patency 253.5 days (interquartile range 92–453).
AVF = arteriovenous fistula; AVG = arteriovenous graft
The secondary patency rate of the vascular access at 1 year was 75% (95% CI 63–84%) for AVF and 50% (95% CI 18–75%) for AVG (fig. 5). A trend to a better secondary patency rate at 1 year was seen with AVF in the upper arm (80%, 95% CI 56–92%) as compared with AVF on the forearm (73%, 95 CI 56–84%; p = 0.322). A Cox regression analysis showed hazard ratios for secondary patency of 0.77 (95% CI 0.28–2.12) for forearm AVF and 0.46 (95% CI 0.13–1.6) for upper arm AVF, when compared with AVG.
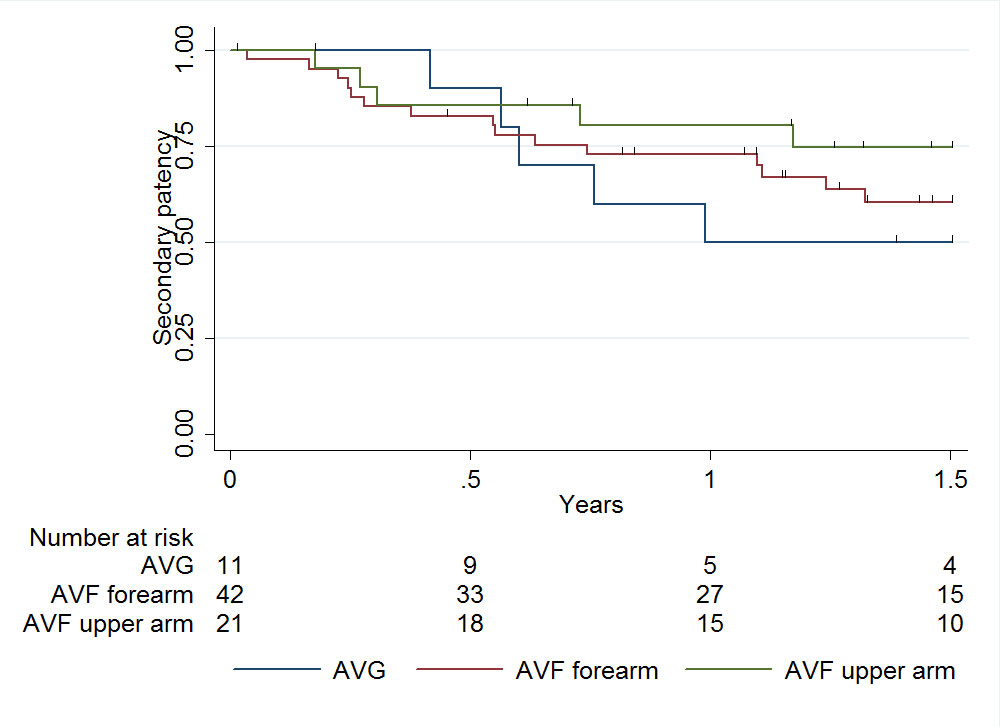
Figure 5 Secondary patency of primary vascular access: median secondary patency 458 days (interquartile range 223–767).
AVF = arteriovenous fistula; AVG = arteriovenous graft
Table 2 shows revisions performed during the follow-up. Twenty-four percent (16/68) of all revisions were performed before the vascular access was used for dialysis. Forty-one patients needed one revision, 19 patients two revisions and 8 patients three revisions.
Table 2 Revisions during the follow-up.
|
All patients
(n = 74) |
AVF forearm
(n = 42) |
AVF upper arm
(n = 21) |
AV graft
(n = 11) |
p-value* | |
|---|---|---|---|---|---|
| No. of revisions per 1000 patient days (95% CI) | 1.7 (1.33–2.15) |
1.51 (1.05–2.10) |
1.46 (0.87–2.31) |
2.87 (1.70–4.54) |
0.755 |
| For bleeding | 0.07 (0.01–0.21) |
0.04 (0.00–0.24) |
0.08 (0.00–0.45) |
0.16 (0.00–0.89) |
0.755 |
| For stenosis | 0.77 (0.52–1.08) |
0.82 (0.49–1.28) |
0.97 (0.50–1.70) |
0.16 (0.00–0.89) |
0.227 |
| For thrombosis | 0.67 (0.45–0.97) |
0.43 (0.21–0.79) |
0.24 (0.05–0.71) |
2.39 (1.34–3.94) |
0.017 |
| For infections | 0.02 (0.00–0.13) |
0 (0.00–0.16) |
0 (0.00–0.30) |
0.16 (0.00–0.89) |
0.632 |
| For other reasons | 0.17 (0.07–0.35) |
0.22 (0.07–0.50) |
0.16 (0.02–0.59) |
0 (0.00–0.59) |
0.559 |
AVF = arteriovenous fistula; AVG = arteriovenous graft; CI = confidence interval * p-value between AVG and the total of AVF (forearm and upper arm combined)
Patients with AVG (72%) underwent more revisions compared with upper arm AVF (57%) and forearm AVF (50%).
Thrombosis was the main reason for AVG revisions and stenosis for AVF revisions. Forearm AVF had more revisions due to thrombosis than upper arm AVF. Infection was observed in only one AVG. Other reasons for reoperations were banding of a high-flow fistula (one), ligation of side branches (two), intervention to correct a lymphocele (one), transposition of cephalic vein (one), and aneurysm ligation (one).
Reasons for abandonment of vascular access (31/74) were the following: spontaneous thrombosis/occlusion (12), renal transplantation (7), and death (4). Other reasons for abandonment were non-maturation (four), stenosis and hand ischaemia (two), stenosis and aneurysm (one) and multiple stenoses (one).
Twenty-seven patients (36%) underwent CVC placement (tunnelled or non-tunnelled) before vascular access creation. Of these, the CVC was removed in 13 (48%) patients during the first postoperative year. Of the 47 patients who did not have a CVC beforehand, 7 (15%) simultaneously received a CVC during vascular access creation and 9 (17%) received a CVC during the first year. Only 31 patients (42%) never received a CVC (fig 6.)
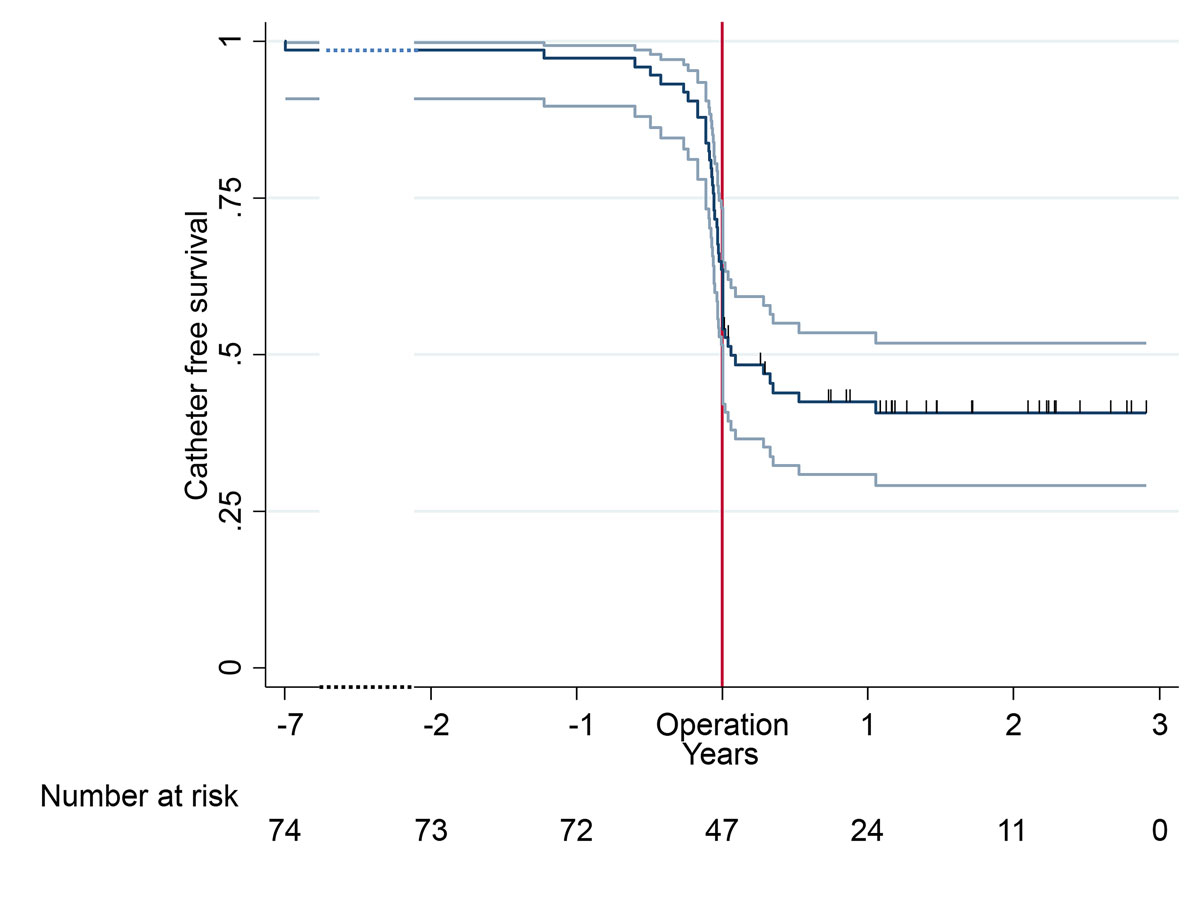
Figure 6 central venous catheter placement in combination with vascular access creation (including 95% confidence intervals).
AVF = arteriovenous fistula; AVG = arteriovenous graft
Thirty-seven (50%) patients had their first dialysis through a CVC. Only 19 (26%) patients had their first dialysis over an AVF and four (5%) over an AVG. At the cut-off of 365 days after surgery, 12 (16%) patients had not undergone dialysis through a catheter or a vascular access (10 AVF / 2 AVG) and 17 (23%) patients were still dialysed via a CVC.
During the follow-up, in 14 patients with an AVF and in 3 patients with an AVG, the primary vascular access was never used for dialysis.
In patients with a primary AVF or AVG, the total amount of time for which a CVC was present was 380 ± 474 days (395 ± 495 days for AVF vs 262 ± 269 for AVG), which includes the pre- and post-vascular access creation period.
During the 2-year observation period, approximately one fifth of all surgical interventions in the field of renal replacement therapy at our tertiary hospital were related to primary vascular access creation. Nearly half of this operative case-load dealt with vascular access revisions and another fifth with interventions for tunnelled CVC. Eighty-five percent of creations of primary native (autologous) vascular access was in accordance with the guidelines and reflects our policy to favour the construction of an AVF over an AVG [16]. In the case of missing forearm veins, a loop graft can be helpful to preserve and remodel the upper arm veins before cubital AVF creation is considered [19–21]. There is an ongoing discussion as to whether for elderly patients with poor veins and a limited life expectancy, the implantation of AVG is a better option than an AVF creation, with the risk of non-maturation and the interim need for a CVC [10].
Patients with end-stage renal disease have a high prevalence of risk factors, related to a high percentage of comorbidities, particularly cardiovascular. Advanced age and poor vessel quality may be another reason why AVF maturation is a problem leading to several reinterventions even before the vascular access is in use.
The mortality from chronic kidney disease increased by 82% between 1990 and 2010 and accounts for a significant proportion of mortality in the world. This risk is dependent on the stage of the chronic kidney disease and is related to coronary heart disease [22]. Not surprisingly, this applies to our cohort with an average age of 63 years and a following drop in the survival curve.
Current literature shows a high percentage of non-maturation [23, 24], related to the fact that older patients and patients with more comorbidities are dialysed. In addition, surgeons try to promote AVF creation, even when the vessel quality seems poor. Maybe a more consistent preoperative ultrasound algorithm, based not only on the absolute arterial inner diameter, but also on the measurement of the arterial wall distensibility, could help to improve the predictability of AVF maturation [25, 26].
We observed a substantial difference in the 1-year results between the primary and the secondary patency. The primary patency rate was below our expectations and below the range of 52 to 83% reported in the literature. A Cox regression analysis did not show a significant difference between AVG and AVF.
On one hand, further investigation is needed to identify patients who are at risk for AVF non-maturation or early access failure. This is important in the context of a “fistula first” approach where surgeons are advised to create a forearm AVF instead of an AVG or upper arm AFV; even if the vessel quality is limited. On the other hand, we need improved judgement of what kind of primary access should be realised first.
Our secondary patency rates for AVF in the forearm or upper arm or for AVG is in accordance with results in the literature; showing a secondary patency for radio-cephalic AVF between 42-83% and for upper arm AVF of 47-88% [17].
This poor outcome with a consecutive need of revisions is a psychological burden for the patient and brings additional costs to the healthcare system.
It is well documented that the use of CVC has a higher morbidity and mortality [27]. Nevertheless, we recorded CVC use in up to 60% of all patients with a primary access, which is consistent with other reports [6].
In a report of from the ERA-EDTA (European Renal Association – European Dialysis and Transplant Association) registry, including 13,044 patients, an increase of CVC use to start haemodialysis from 58% in 2005 to 68% in 2009 was documented. In contrast, there was a reduction from 42 to 32% in the use of AVF as first-line vascular access [28]. In our series, 50% of all patients started their haemodialysis with a CVC. Since 36% of the CVCs were implanted before vascular access creation, this could be due to either late referral or difficulties to correctly predict optimal timing of vascular access creation. Concerning vascular access readiness for dialysis, the timing of vascular access creation and the risk of AVF non-maturation is key. Early referral is recommended: 6 months for AVF and at least 4 to 6 weeks for AVG. The increasing number of patients requiring chronic renal replacement therapy after acute kidney injury will continue to challenge optimal timing of vascular access creation. However, this inevitably can also lead to patients undergoing surgery too early, despite not requiring dialysis, as observed in our series.
This was a retrospective single-centre study with a small number of patients and medium-term follow-up, which limits comparison between the groups and generalisability of the results. The comparison of data of forearm and upper arm vascular access can be crucial when defined by the area of cannulation. The brachial artery in the antecubital fossa can give inflow for a forearm loop graft, as well as for an upper arm cephalic or basilic AVF. Therefore, the definition of the inflow vessel may be a more accurate way to categorise the vascular access than the topography of cannulation.
We assume that the group of patients in our tertiary referral centre have more concomitant diseases than those in smaller peripheral hospitals. For the analysis of intervention-free survival, the bias of different endpoints i.e., revisions, loss of vascular access and death, must be considered. Because of difficulties with documentation of dialysis data, it was assumed that whenever there had been a CVC placement, the patient was undergoing dialysis. Owing to lack of data, acute, acute-on-chronic and chronic kidney failure could not be differentiated in detail.
The primary patency in primary vascular access creation in our centre was lower than expected when compared to the literature, whereas the secondary patency was similar. The percentage of CVCs implanted for dialysis was alarmingly high and this needs to be addressed in the future, especially with regard to the ideal timing of vascular access creation. The vascular access team should implement the evidence of guidelines. There is an initiative starting this year to encourage all Swiss vascular surgeons to provide data on vascular access interventions, including 12-month follow-up, in the national online registry “SwissVasc 2.0”.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Collins AJ , Foley RN , Gilbertson DT , Chen SC . United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int Suppl (2011). 2015;5(1):2–7. doi:.https://doi.org/10.1038/kisup.2015.2
2 Thomas B , Wulf S , Bikbov B , Perico N , Cortinovis M , Courville de Vaccaro K , et al. Maintenance Dialysis throughout the World in Years 1990 and 2010. J Am Soc Nephrol. 2015;26(11):2621–33. doi:.https://doi.org/10.1681/ASN.2014101017
3Foundation NK [Internet]. New York: National Kidney Foundation; c10/02/2017-02/2018 [cited 16.11.2017. NKF KDOQI Guidelines, 2006 Updates, Vascular Access. Available from: http://kidneyfoundation.cachefly.net/professionals/KDOQI/guideline_upHD_PD_VA/va_guide2.htm
4 Torrent DJ , Maness MR , Kachare SD , Zink JN , Haisch CE , Harland RC , et al. Examining Hemodialysis Reliable Outflow catheter performance and cost in hemodialysis access. J Surg Res. 2014;192(1):1–5. doi:.https://doi.org/10.1016/j.jss.2014.03.016
5 Manns B , Tonelli M , Yilmaz S , Lee H , Laupland K , Klarenbach S , et al. Establishment and maintenance of vascular access in incident hemodialysis patients: a prospective cost analysis. J Am Soc Nephrol. 2005;16(1):201–9. doi:.https://doi.org/10.1681/ASN.2004050355
6 Lok CE , Foley R . Vascular access morbidity and mortality: trends of the last decade. Clin J Am Soc Nephrol. 2013;8(7):1213–9. doi:.https://doi.org/10.2215/CJN.01690213
7 Drew DA , Lok CE , Cohen JT , Wagner M , Tangri N , Weiner DE . Vascular access choice in incident hemodialysis patients: a decision analysis. J Am Soc Nephrol. 2015;26(1):183–91. doi:.https://doi.org/10.1681/ASN.2013111236
8 Banerjee S . Dialysis catheters and their common complications: an update. Sci World J. 2009;9:1294–9. doi:.https://doi.org/10.1100/tsw.2009.145
9 Fitzgerald JT , Schanzer A , Chin AI , McVicar JP , Perez RV , Troppmann C . Outcomes of upper arm arteriovenous fistulas for maintenance hemodialysis access. Arch Surg. 2004;139(2):201–8. doi:.https://doi.org/10.1001/archsurg.139.2.201
10 Al-Jaishi AA , Oliver MJ , Thomas SM , Lok CE , Zhang JC , Garg AX , et al. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63(3):464–78. doi:.https://doi.org/10.1053/j.ajkd.2013.08.023
11 Erkut B , Unlü Y , Ceviz M , Becit N , Ateş A , Colak A , et al. Primary arteriovenous fistulas in the forearm for hemodialysis: effect of miscellaneous factors in fistula patency. Ren Fail. 2006;28(4):275–81. doi:.https://doi.org/10.1080/08860220600583617
12 Perera GB , Mueller MP , Kubaska SM , Wilson SE , Lawrence PF , Fujitani RM . Superiority of autogenous arteriovenous hemodialysis access: maintenance of function with fewer secondary interventions. Ann Vasc Surg. 2004;18(1):66–73. doi:.https://doi.org/10.1007/s10016-003-0094-y
13 Nassar GM , Suki D , Rhee E , Khan AJ , Nguyen B , Achkar K . Outcomes of arteriovenous grafts following simultaneous thrombectomy and stent graft placement across the venous anastomosis. Semin Dial. 2014;27(6):639–44. doi:.https://doi.org/10.1111/sdi.12254
14 Tordoir J , Canaud B , Haage P , Konner K , Basci A , Fouque D , et al. EBPG on Vascular Access. Nephrol Dial Transplant. 2007;22(Suppl 2):ii88–117. doi:.https://doi.org/10.1093/ndt/gfm021
15 Collins AJ , Foley RN , Gilbertson DT , Chen SC . The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S5–11. doi:.https://doi.org/10.2215/CJN.05980809
16Foundation NK [Internet]. Clinical Practice Guidelines for Vascular Access, Update 2006 c10/02/2017-02/2018 [cited 16.11.2017] Available from: http://www.kidney.org/professionals/KDOQI/guidelines_commentaries.cfm.
17 Schmidli J , Widmer MK , Basile C , de Donato G , Gallieni M , Gibbons CP , et al.; Esvs Guidelines Committee; ESVS Guidelines Reviewers. Editor’s Choice - Vascular Access: 2018 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55(6):757–818. doi:.https://doi.org/10.1016/j.ejvs.2018.02.001
18 Ambühl PM . Aktuelle Erkenntnisse zur Schweizer Dialysepopulation. Hausarzt Praxis. 2017;12(3):22–6.
19 Chiang N , Hulme KR , Haggart PC , Vasudevan T . Comparison of FLIXENE™ and standard PTFE arteriovenous graft for early haemodialysis. J Vasc Access. 2014;15(2):116–22. doi:.https://doi.org/10.5301/jva.5000213
20 Schuman E . Graft for immediate use as first stage of a native fistula. J Vasc Access. 2009;10(3):203–6. doi:.https://doi.org/10.1177/112972980901000312
21 Lok CE , Thumma JR , McCullough KP , Gillespie BW , Fluck RJ , Marshall MR , et al. Catheter-related infection and septicemia: impact of seasonality and modifiable practices from the DOPPS. Semin Dial. 2014;27(1):72–7. doi:.https://doi.org/10.1111/sdi.12141
22 Murray CJ , Richards MA , Newton JN , Fenton KA , Anderson HR , Atkinson C , et al. UK health performance: findings of the Global Burden of Disease Study 2010. Lancet. 2013;381(9871):997–1020. doi:.https://doi.org/10.1016/S0140-6736(13)60355-4
23 Rooijens PP , Tordoir JH , Stijnen T , Burgmans JP , Smet de AA , Yo TI . Radiocephalic wrist arteriovenous fistula for hemodialysis: meta-analysis indicates a high primary failure rate. Eur J Vasc Endovasc Surg. 2004;28(6):583–9. doi:.https://doi.org/10.1016/j.ejvs.2004.08.014
24 Huijbregts HJ , Bots ML , Wittens CH , Schrama YC , Moll FL , Blankestijn PJ ; CIMINO study group. Hemodialysis arteriovenous fistula patency revisited: results of a prospective, multicenter initiative. Clin J Am Soc Nephrol. 2008;3(3):714–9. doi:.https://doi.org/10.2215/CJN.02950707
25 Malovrh M . Patients with chronic kidney disease: safety aspects in the preoperative management. Contrib Nephrol. 2015;184:13–23. doi:.https://doi.org/10.1159/000365498
26 Tordoir JH , Widmer MK . Patient safety in dialysis access: education and research. Contrib Nephrol. 2015;184:251–63. doi:.https://doi.org/10.1159/000366465
27 Bray BD , Boyd J , Daly C , Donaldson K , Doyle A , Fox JG , et al.; Scottish Renal Registry. Vascular access type and risk of mortality in a national prospective cohort of haemodialysis patients. QJM. 2012;105(11):1097–103. doi:.https://doi.org/10.1093/qjmed/hcs143
28 Tordoir JH , Rooyens P , Dammers R , van der Sande FM , de Haan M , Yo TI . Prospective evaluation of failure modes in autogenous radiocephalic wrist access for haemodialysis. Nephrol Dial Transplant. 2003;18(2):378–83. doi:.https://doi.org/10.1093/ndt/18.2.378
No financial support and no other potential conflict of interest relevant to this article was reported.