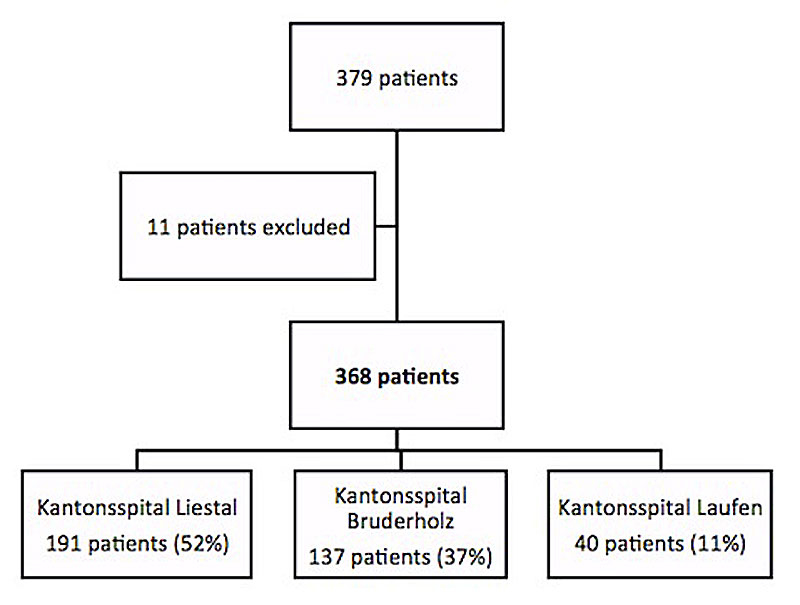
Figure 1 Study population flow chart.
DOI: https://doi.org/10.4414/smw.2018.14662
Hyponatraemia is defined as a plasma sodium concentration less than 135 mmol/l [1] and is the result of a relative excess of total body water in relation to total body sodium content. It is classified into three grades of severity: “mild” is defined as plasma sodium concentration from 125–135 mmol/l, “moderate” as 124–115 mmol/l and “severe” as less than 115 mmol/l.
Hyponatraemia is the most common electrolyte disorder encountered in hospitalised patients [2]. The inpatient prevalence of mild hyponatraemia is estimated to be 30% [3, 4]. Approximately 10% of patients admitted to the Basel University Hospital in 2012 were found to be hyponatraemic on admission [5].
From 20 to 40% of all cases of hyponatraemia are presumed to be due to the syndrome of inappropriate antidiuretic hormone (ADH) secretion (SIADH), but as most of the studies providing these data were retrospective, the number of patients with true SIADH might have been overestimated [6–8]: the retrospective, laboratory-based study design did not allow a reliable diagnosis because the findings of the clinical examination were missing (clinical euvolaemia is mandatory for diagnosis, see Bartter and Schwartz criteria for SIADH [9]).
Based on a prevalence of 3.2 to 6.1 million hyponatraemic patients per year, the direct costs of treating hyponatraemia in the US were estimated to be between 1.6 and 3.6 billion US dollars [10]. No data on the economic importance of treating hyponatraemia in Switzerland have been published. Therefore, the goals of this study were to estimate the costs of hospitalisation due to hyponatraemia in a service population representative of the whole country. In addition, we aimed to assess the quality of diagnosis and treatment, and analysed clinical outcome measures (length of stay, in-hospital mortality, residual symptoms and relapse rate).
This study was conducted at the three acute care hospitals of the canton of Basel-Landschaft, namely the Kantonsspital Bruderholz, Liestal and Laufen (with a total of 934 in-hospital beds).
All patients older than 18 years who were admitted to one of the three hospitals between 1 January 2011 and 31 December 2011, and who had plasma sodium concentrations of less than 135 mmol/l either on admission or developing during the hospital stay, were included (fig. 1). Patients were enrolled when the discharge summary contained either of the two ICD-10 codes E87.1 (hyponatraemia) or E22.2 (SIADH). The volume status (hypovolaemia, euvolaemic SIADH [9] and hypervolaemia) was classified on the basis of the documented clinical analysis of the filling of the jugular veins, orthostasis (fall in systolic blood pressure by >20 mm Hg and/or orthostatic symptoms in the upright position) and the presence/absence of oedema. If available, fractional urinary sodium excretion if equal or lower than 1% was used as a further diagnostic criterion for hypovolaemia.

Figure 1 Study population flow chart.
Patients were considered to have hyponatraemia due to SIADH if they met the Bartter and Schwartz criteria [9]. The essential criteria include decreased serum osmolality (<280 mOsm/kg H2O), urinary osmolality above 100 mOsm/kg H2O and elevated urinary sodium excretion with normal salt intake, clinical euvolaemia, absence of other causes of euvolaemic hypo-osmolality (hypoadrenalism, hypothyreoidism) and no recent use of diuretics [9].
Hyponatraemia was divided into mild, moderate and severe on the basis of the lowest available plasma sodium concentration.
The patients’ history on admission, personal data, aetiological factors, symptoms of hyponatraemia and treatment modalities were obtained from the patient files. The cause of hyponatraemia was defined on the basis of the patients’ volume status, symptoms, aetiological factors and laboratory measurements (see previous section). The physician in charge was a specialist for internal medicine. The multimodal allocation of causes (based on anamnestic, clinical and laboratory findings) reduced possible bias.
The treatment was defined as adequate if it met the following criteria:
As the vaptans were not on the market and urea is typically not used in Switzerland we did not include these drugs in the assessment of appropriateness of treatment. We also checked whether treatment was based on a minimal set of clinical (see classification of volume status in the previous section) or biochemical (measurements of initial plasma electrolytes, blood glucose, renal function, plasma and urine osmolality in euvolaemic patients) diagnostic parameters.
The diagnosis related group (DRG) system in Switzerland, called SwissDRG, is a nationwide reimbursement system of the Swiss mandatory health insurance for the financing of hospital stays. The per-case fixed rate – called “cost weight” – is calculated on the basis of medical (primary and secondary diagnosis), therapeutic (called “procedures”), economic (duration of hospital stay, hospital accommodation) and demographic criteria (age and gender of the hospitalised patient). The resulting cost amount is multiplied by the cost weight (based on case data of selected hospitals [11]) and base rate (differs between hospitals [11], set by the canton).
The reimbursement system of the SwissDRG is annually updated by SwissDRG AG, an organisation consisting of representatives of the cantons, insurers and care providers, and approved by the Federal Council [11]. The updates are based on proposals submitted by the representatives involved with the aim of ensuring fair and cost-covered medical care. The calculations of reimbursement of SwissDRG are based on a list of “Netzwerkspitälern”, which includes hospitals of all over Switzerland [12]. Thus, the reimbursement of SwissDRG is thought to be equal and not based on top performing hospitals. Annual updates allow accurate and cost-covered medical care.
The underlying data for this study came from the year 2011, one year before the introduction of the SwissDRG system. Assuming no major changes in the patient population, we calculated cost weight and flat charges per case using the 2015 version of SwissDRG. The evaluation of the cost of illness was based on the 2011 report of the number of admissions to acute care hospitals in Switzerland [13]. On the basis of published data from the Swiss Federal Statistical Office, the canton of Basel-Landschaft and its hospitals correspond to the average [14, 15] and this allows extrapolation to the rest of Switzerland.
The data were analysed using the statistic program SPSS, Version 23.0 (IBM Corp., Armonk, N.Y., USA) and Excel software packages. Data are means + standard deviation and were tested for significance with the two-tailed t-test. For parameters with uneven distribution, medians were used. Parameters were divided into groups by age, gender and severity of illness and tested for significant correlation. A p-value of <0.05 was considered significant.
Eleven of the initial 379 patients, whose sodium was not measured at least twice or for whom no medical history or record could be found, were excluded. Consequently, 368 patients were included in this study. Table 1 describes the general characteristics of the study population.
Table 1 Patient characteristics.
| Total patient population | 368 (100%) |
|---|---|
| Age median (years) | 75 ± 12 |
| Sex | |
| Female | 229 (62%) |
| Male | 139 (38%) |
| Volume status | |
| Hypervolaemic | 165 (45%) |
| Euvolaemic (SIADH) | 128 (35%) |
| Hypovolaemic | 75 (20%) |
| Body mass index (kg/m2) | 24 ± 5 |
| Severity of hyponatraemia | |
| Mild | 232 (63%) |
| Moderate | 110 (30%) |
| Severe | 26 (7%) |
| Previous hospitalisation due to hyponatraemia | |
| No | 335 (91%) |
| Yes | 33 (9%) |
| Symptoms attributed to hyponatraemia | |
| Neurological (muscle cramps, vertigo, confusion) | 257 (70%) |
| Severe neurological symptoms (e.g. seizures) | 36 (10%) |
| Nausea | 195 (53%) |
| Vomiting | 173 (47%) |
| Pain (e.g., headache) | 162 (44%) |
| Loss of energy, fatigue, weakness, depression | 125 (34%) |
| Anorexia | 110 (30%) |
| None | 0 |
| Most frequent comorbidities (>10%) | |
| Hypertension | 170 (46%) |
| Anemia | 114 (31%) |
| Diabetes mellitus | 103 (28%) |
| Obesity | 101 (27%) |
| Heart failure | 98 (26%) |
| Renal insufficiency | 85 (23%) |
| Cancer, neoplasms | 66 (18%) |
| Breast cancer | 39 (10%) |
| Lung cancer | 15 (4%) |
| Other | 12 (3%) |
| Cirrhosis/hepatic disease | 61 (16%) |
| Alcohol abuse | 41 (11%) |
| Falls within last 2 weeks | 80 (22%) |
| Fracture rate (all fractures) | 39 (10%) |
| Most frequent medication (>10%) | |
| Average number per patient | 6.8 |
| Diuretics | 265 (72%) |
| Thiazides | 93 (25%) |
| Loop diuretics | 90 (24%) |
| Potassium-sparing diuretics | 48 (13%) |
| Angiotensin-converting enzyme inhibitors | 84 (23%) |
| Proton pump inhibitors | 77 (21%) |
| Antidepressants | 66 (18%) |
| Therapy modalities | |
| 0.9% saline infusion | 231 (63%) |
| Water restriction | 90 (24%) |
| Discontinuation or change of current drug therapy | 68 (18%) |
| Hypertonic (3%) saline infusion | 48 (13%) |
| No apparent intervention (spontaneous normalisation) | 28 (8%) |
| Loop diuretics | 25 (7%) |
| Urea | 0 (0%) |
| Vaptans | 0 (0%) |
| Plasma sodium at hospital discharge | |
| Normonatraemic | 188 (51%) |
| Hyponatraemic | 180 (49%) |
| Mild hyponatraemia | 110 (30%) |
| Moderate hyponatraemia | 59 (16%) |
| Severe hyponatraemia | 11 (3%) |
|
Quantitative and qualitative neurological deficits
at hospital discharge |
|
| Overall | 76 (20%) |
| Mild hyponatraemia | 42 (11%) |
| Moderate hyponatraemia | 29 (8%) |
| Severe hyponatraemia | 5 (1%) |
| Average length of hospital stay (days) | |
| Mean (days) | 11 |
| Median (days) | 9 |
| Mortality rate | |
| Overall | 35 (9%) |
| Mild hyponatraemia | 22 (9%) |
| Moderate hyponatraemia | 10 (9%) |
| Severe hyponatraemia | 3 (11%) |
| Causes of Death | |
| Cancer | 17 (49%) |
| Infection (pneumonia, UTI) | 9 (26%) |
| Unknown | 6 (17%) |
| Sepsis | 3 (8%) |
| Relapse rate (within 12 months) | 81 (22%) |
| Poor compliance (start of risk medication, potomania) | 31 (8%) |
| Unknown | 27 (7%) |
| Alcohol abuse | 16 (4%) |
| Cancer, neoplasms | 4 (1%) |
| Refused treatment | 3 (1%) |
SIADH = syndrome of inappropriate antidiuretic hormone secretion; UTI = urinary tract infection Note: Due to rounding not all percentages sum up precisely to 100%.
The distribution of severity, gender and age among the three hospitals was equal. Almost two thirds of patients suffered from mild hyponatraemia, the average age was 75 ± 12 years, the majority (62%) of patients were women. The average body mass index (BMI) was 24 ± 5 kg/m2, hypertension and anaemia (46 and 31%, respectively, of the patient population) represented the most frequent comorbidities. Breast cancer in women and lung cancer in men were the most common neoplasms. Neurological symptoms were the most frequent symptoms attributed to hyponatraemia by the physicians in charge, followed by gastrointestinal symptoms (nausea and/or vomiting). There was no asymptomatic patient. Eighty patients (22%) had fallen within 2 weeks prior to hospitalisation, and 39 of these had fractured at least one bone (just short of a significant correlation, p = 0.055).
Hyponatraemia was often thought to be triggered by multiple factors. One single cause was indentified in 58% (213 patients) of the patients. Hyponatraemia was attributed to two causes in 24% and to at least three different causes in 18% of the patients.
Thiazide diuretics (strong, significant correlation, r = 0.69, p = 0.03) and antidepressants (r = 0.33, p = 0.68, not significant) were the most common drug groups associated with hyponatraemia. There was an unexpectedly high, but statistically not significant, proportion of hyponatraemic patients on proton pump inhibitor therapy (r = 0.61, p = 0.23). Of the 128 patients classified as euvolaemic, only 73, representing 20% of the total hyponatraemic population, were diagnosed as having SIADH. Therefore, a full 55 (or 43% of the euvolaemic patients) were not clearly classified.
Most patients (63%, see table 1) received an initial saline (0.9% NaCl) infusion, irrespective of their volume status. If patients were tested for SIADH (35%), they received therapeutic water restriction as first-line therapy. Later, if there was no improvement of plasma sodium, change of current drug therapy (mainly discontinuation of thiazides) and hypertonic saline infusion were common choices of therapy. Eight percent of patients had a spontaneous normalisation of the plasma sodium level.
The overall in-hospital mortality rate was 9% and was, surprisingly not affected by the severity of hyponatraemia or the volume status on admission. Overall, 180 of the 368 patients were still hyponatraemic at the time of discharge, implying that only 51% of the discharged patients had achieved normonatraemia (fig. 2). Eleven patients were severely hyponatraemic at least 3 days before hospital discharge.
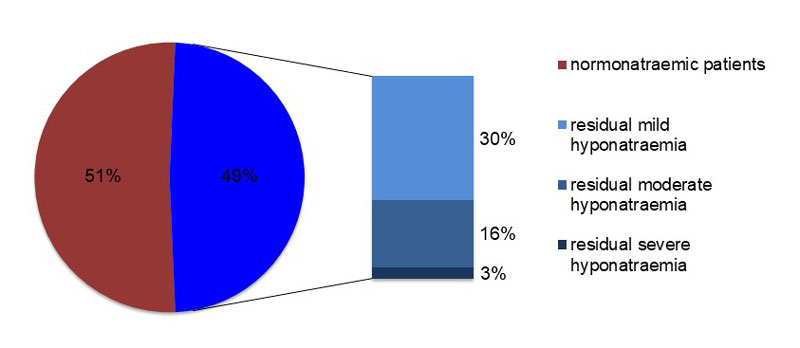
Figure 2 Plasma sodium levels on discharge by severity.
As illustrated by table 1, a total of 76 patients, representing every fifth patient of the study population, had persistent neurological deficits (gait instability, confusion, muscle cramps and dizziness) on discharge from the hospital. Again, most of them were suffering from mild or moderate hyponatraemia.
Eighty-one patients were readmitted because of hyponatraemia within 12 months, the 1-year recurrence rate was thus 22%. There was no difference in relapse rate between hyponatraemia without SIADH and hyponatraemia due to SIADH. Risk factors for relapse were age (r = 0.65, p = 0.03), female sex (r= 0.49, p = 0.12; combined with >75years, r = 0.58, p = 0.049), resumption of risk medication (r = 0.563, p = 0.02). Non-significant correlations were found for overweight (r = 0.32, p = 0.39), persistent alcohol abuse (r = 0.414, p = 0.09) and cancer (r = 0.43, p = 0.11).
Of note, 70 (86%) of the 81 patients who relapsed within 12 months had been discharged from hospital after the first admission while still hyponatraemic (strong and significant correlation, r = -0.51, p = 0.04). The fall rate in the relapse patient population was 36% (29 of 81 patients).
Quality of care and cost of hyponatraemia were analysed and the results are shown separately below.
Different factors with an impact on outcome and prognosis of hyponatraemia were identified. These are further discussed below (see “Discussion” and fig. 4). The absence of or an incomplete initial diagnostic approach in the emergency unit emerged as crucial. In 114 (31%) of the patients, the treatment was not directly based on the physician’s assessment of the patient’s volume status (see “Materials and methods”). This may have had the following effects:
This had not only a considerable effect on the prognosis, but also demonstrably resulted in a prolonged hospital stay and thus in increasing costs (see “Cost analysis”).
The length of in-hospital stay varied from a minimum of 1 day to a maximum of 125 days (see fig. 3). The median length of stay was 9 days. This constitutes at least 2 days more than assumed by the SwissDRG system (6.8 days for hyponatraemia not associated with SIADH). Patients in this study with hyponatraemia due to SIADH stayed on average 1 day less in hospital than hyponatraemic patients of the non-SIADH type. This was exactly in accordance with SwissDRG (8 days in hyponatraemic patients with SIADH).
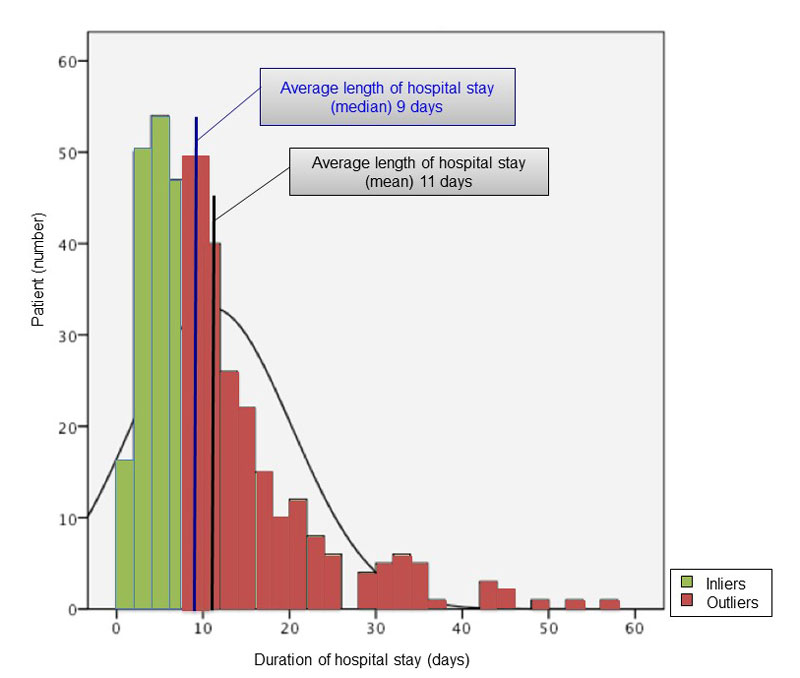
Figure 3 Number of hyponatraemic patients and their duration of in-hospital stay.
Surprisingly, the length of stay for severe hyponatraemia was not longer than for moderate and mild hyponatraemia; in fact a trend to the contrary, albeit not significant, was observed. The time until normalisation of plasma sodium levels was available for a only few patients and could thus not be statistically analysed.
The cost weight (0.789 in non-SIADH hyponatraemia and 1.03 in hyponatraemia due to SIADH) and the flat charge per case (9940 CHF) [16], calculated according to the SwissDRG system 2015, led to a projected deficit of 2.1 million CHF of inpatient treatment of hyponatraemia in the canton of Basel-Landschaft in 2011, due to the overstay of most patients.
In a first analysis, comorbidities were not taken into account in order to estimate the cost of illness for hyponatraemia only. The second analysis incorporated the comorbidities (increase of the patient clinical complexity level resulting in higher cost weight and thus reimbursements), which reduced the deficit of treating hyponatraemia to about 20% (i.e., to 300,000–400,000 CHF). The longer in-hospital stay, i.e., the outlier phenomenon, was primarily responsible for this deficit (the comorbidities of the hyponatraemic patients were included in the analysis of the length of in-hospital stay (overall duration of hospital stay minus comorbidity-[SwissDRG]-based length of hospitalisation). Mild hyponatraemia again contributed most of this deficit owing to the large patient group and long average of hospital stay.
The total incidence rate of hyponatraemia in the hospitalised population of the canton of Basel-Landschaft in 2011 was comparably low at 3.5% (368 hyponatraemic patients out of 10,500 admissions through emergency wards per year [16]). This study used only cases where hyponatraemia was regarded as the SwissDRG-relevant diagnosis. Therefore, the incidence rate was, predictably, lower than in reports using any available laboratory result [17]. Based on the published data of the Swiss population report [13] and under the assumption that the population of the canton of Basel-Landschaft is a representative patient population for Switzerland – number of hospital beds, hospitalisation rate, average age and gender distribution of patients at the time of hospitalisation and comorbidity rate are in the range of Swiss average [13] – the projected annual revenues from hyponatraemia in Switzerland in 2011 were in the order of 287 million CHF with a calculated deficit of 95 million CHF. Hyponatraemia was responsible for approximately 242,800 hospital days per year in Switzerland.
These numbers represent only direct treatment costs during hospitalisation; outpatient treatment and indirect costs (e.g., cost of care due to residual deficits and work loss due to illness) could not be calculated and were thus not included. It is likely that the high proportion of the non-working elderly in the patient population (average age 75 ± 12 years) in this study meant that there was little impact on cost due to work loss. However, because of the cost of the overstay of most patients, high relapse rate and outpatient treatment, the total economic burden would be even higher. Hyponatraemia reimbursment by SwissDRG did not cover its in-hospital treatment costs. For the latter, a maximum in-hospital stay of 5 days for patients with non-SIADH hyponatraemia and 6.5 days for patients with SIADH would be required. However, the actual duration of hospital stay of hyponatraemic patients found in this study was considerably longer (median of 9 days, see table 1).
For the exact calculations, see table 2.
Table 2 Cost of illness of inpatient treatment of hyponatraemia and its resulting deficit.
|
a) Calculation of revenues from hyponatraemia, reimbursed by SwissDRG, in Switzerland in 2011
Incidence of hyponatraemia in Switzerland in the year 2011 (total population 7,954,662) 985,384 total hospitalisations in Switzerland in the year 2011 = 100% 34,488 estimated hospitalisations due to hyponatraemia = 3.5% Projected days of in-hospital treatment due to hyponatraemia ALOS according to SwissDRG in 2011: 6.8 days with non-SIADH hyponatraemia, 8 days with hyponatraemia due to SIADH Non-SIADH hyponatraemia represented 80% of hyponatraemia cases (i.e., 0.8), hyponatraemia due to SIADH 20% (0.2) → (34,488 × 0.8 × 6.8) + (34,488 × 0.2 × 8) = 242,796 days Revenue from hyponatraemia per patient (based on the flat charge per case of canton of Basel-Landschaft in 2011 [ 16 ]) 0.789 non-SIADH hyponatraemia × 9940 CHF = 7842.65 CHF per patient-hospital stay 1.03 hyponatraemia due to SIADH × 9940 CHF = 10,238.20 CHF per patient-hospital stay Total revenue from treating hyponatraemia in Switzerland in 2011 (34,488 × 0.8 × 7842.65) + (34,488 × 0.2 × 10,238.20) = 287,000,858.90 CHF b) Calculation of uncovered costs / deficit in Switzerland in 2011 Cost of one in-hospital treatment day reimbursed by SwissDRG (see above) Non-SIADH hyponatraemia: 7842.65 CHF: 6.8 days = 1153.35 CHF per patient-in-hospital day Hyponatraemia due to SIADH: 10,238.20 CHF: 8 days = 1279.80 CHF per patient-in-hospital day Average cost of an in-hospital treatment day in general 1576 CHF per patient-in-hospital day [18] Calculated daily deficit of in-hospital treatment of hyponatraemia Non-SIADH hyponatraemia: 1576 − 1153.35 = 422.65 CHF Hyponatraemia due to SIADH: 1576 – 1279.80 = 296.20 CHF Calculated total deficit of in-hospital treatment of hyponatraemia Non-SIADH hyponatraemia: 187,614 bed-days × 422.65 CHF = 79,295,057.10 Hyponatraemia due to SIADH: 55,182 bed-days × 296.20 CHF = 16,344,908.40 Total = 95,639,965.50 CHF Consequences, approximately cost-covered (i.e. reimbursed by SwissDRG) duration of hospital stay in Switzerland in 2011 Non-SIADH hyponatraemia: 7842.65 CHF: 1576 CHF = 5 days Hyponatraemia due to SIADH: 10,238.20 CHF: 1,576 CHF = 6.5 days |
ALOS = average length of stay; SIADH = syndrome of inappropriate antidiuretic hormone secretion
The main findings of this study were that female gender (62%), advanced age (average 75 ± 12 years) and the use of thiazides (r = 0.69, p = 0.03) represented the main risk factors with negative prognostic value concerning hyponatraemia and were associated with adverse outcomes in this retrospective analysis of 368 hyponatraemic patients. Hyponatraemia was never asymptomatic and falls occurred in every fifth patient (80 patients, 22%, p = 0.055), of whom half consequently suffered from fractures (39 patients). In one third of the patient population, treatment was not based on the patient’s volume status, which was associated with prolonged hospital stay (median of 9 days), 4 times higher rate of in-hospital mortality (19 vs 5%). The in-hospital mortality rate of patients whose hyponatraemia was not treated at all increased from 9% overall to 37%. However, the decision to withhold treatment was made actively as part of the palliative care concept in 45%, i.e., 34 of the patients. Age (r = 0.65, p = 0.03), female sex (r = 0.49, p = 0.12; in combination with age >75years r = 0.58, p = 0.049) and resumption of risk medication (r = 0.563, p = 0.02) were significantly associated with relapse; nevertheless, persistent hyponatraemia at time of discharge from hospital after the first admission represented an important and direct factor causing a relapse in this study population (r = −0.51, p = 0.04). The fall rate of the patient population suffering from a relapse (81 patients) was 36%. It is conceivable that the high rate of persistent neurological deficits and the inadequate treatment were accountable for the renewed falls.
On an economic basis, the prolonged hospital stay in most patients (median of 9 days, 1 day shorter in hyponatraemia due to SIADH) would translate into a deficit of 95 million CHF in Switzerland. Hyponatraemia was responsible for approximately 242,800 hospital days. The reimbursement by SwissDRG allows a cost-covering maximum in-hospital stay of 5 days for patients with non-SIADH hyponatraemia and 6.5 days for patients with SIADH.
Although hyponatraemia often presented as a symptom of the primary disease or was found by chance in routine laboratory tests in the study, it was significantly associated with worse outcome. In addition to the known risk factors of female gender [4] and old age, [4] hyponatraemia was associated with certain comorbidities (hypertension and anaemia) and the use of thiazides. For further analysis of the impact of the comorbidities on the mortality of the study patient population, patients were subdivided into groups based on the number of their comorbidities. Comorbid patients were defined as suffering from at least two of the following diseases: heart failure (at least New York Heart Association grade III), chronic renal insufficiency (of at least stage 3), chronic obstructive pulmonary disease (at least GOLD stage 3), cirrhosis or chronic cancer. The analysis showed that such patients were mostly (58%) in the moderately hyponatraemic patient group. As the overall mortality of this patient group was equal to that of the mildly hyponatraemic patients (with less comorbidity, 24%), it was concluded that the comorbidities were not solely responsible for death, but that hyponatraemia was at least jointly or partly responsible.
Hospitalisation for hyponatraemia increases mortality at least 5 fold in comparison with the mortality rate of a normonatraemic Swiss general population aged 65 to 79 years [13], and mortality was comparable irrespective of the severity of hyponatraemia [13]. The following factors might explain the lack of correlation between severity and mortality. The clinical features of severe hyponatraemia – headache, lethargy and disturbances of consciousness associated with a sodium concentration below 115 to 120 mmol/l [5] – forces patients to be bed-ridden, resulting in fewer falls and fractures and a lower mortality rate. The comparably low mortality in the severely hyponatraemic patient population in this study might be further explained by the more frequent sodium measurements in patients with at least moderate hyponatraemia. This shows a possible increased awareness of the seriousness of severe hyponatraemia and, in parallel, possible underestimation of the seriousness of milder hyponatraemia. Other studies have confirmed these findings [3, 19]. The latter contention has been demonstrated to be wrong in this study.
Despite the fact that this study evaluated a hospitalised patient population, which is more likely to be clinically symptomatic than outpatients [17, 20, 21], the evaluation showed that there is no such thing as benign hyponatraemia. Furthermore, analysis showed a poor correlation of serum sodium with the severity of illness. Thus an additional, clinically based classification system, as recently recommended in the Clinical practice guideline [22] should be used.
Figure 4 illustrates the factors with the highest impact on outcome and prognosis of hyponatraemia identified in this retrospective analysis. Especially, a comprehensive initial diagnostic approach seems crucial for appropriate classification and thus treatment of hyponatraemia. As this approach was often inadequate or lacking, inappropriate treatment was the consequence (verifiable in 114 of 368 patients). We assume that the lack of sustained normalisation of plasma sodium as evidenced by persistent hyponatraemia at discharge in almost every second patient (this high rate of patients still being hyponatraemic on hospital discharge was confirmed by other studies [23]) was responsible, at least in part, for the high rate of clinically apparent residual deficits at the time of discharge. The report of the Hyponatraemia Registry stated a poor correction rate even with appropriate therapy [23].
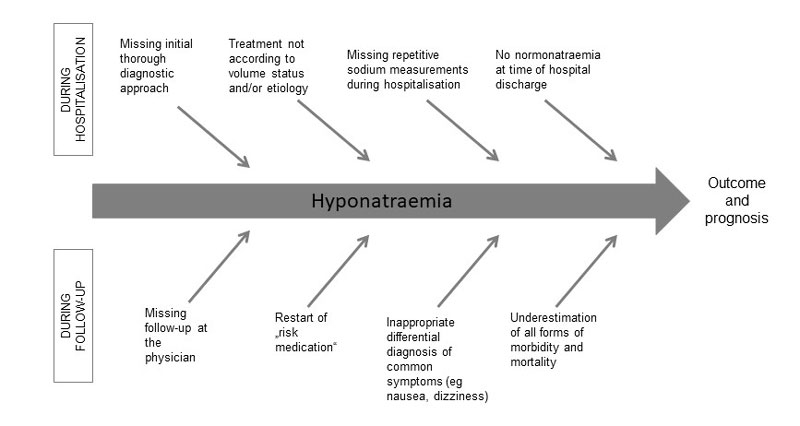
Figure 4 Effects on outcome and prognosis of hyponatraemia.
There was only little information concerning the risk factors for prolonged hyponatraemia combined with excess of hospital stay, which did not allow statistical testing. The fewer sodium measurements during hospital stay in patients with mild hyponatraemia might be associated with a prolonged duration of disease and thus longer hospital stay in this patient group.
Besides these findings, the failure to clearly define outpatient follow-up and the renewed prescription of risk medication were considered responsible for the high rate of relapses and “chronic” hyponatraemia.
Hyponatraemia also constitutes a risk factor for falls and consequently fractures [24, 25]. The latter are not only due to a higher risk of falls, but also directly linked to a hyponatraemia-associated increase in bone fragility [26, 27]. Hyponatraemia has been shown to decrease osteoblast and increase osteoclast acitivities [26, 28]. In addition, it may be relevant, at least for SIADH cases of hyponatraemia, that ADH has been demonstrated to be a negative regulator of bone mass [29]. The resulting reduction of bone density and increased bone fragility result in the unsteadiness of gait, falls and attention deficit of hyponatraemic patients (22% falls within last 2 weeks, 10% fracture rate).
The odds ratio for the development of osteoporosis among mildly hyponatraemic patients was estimated to be significantly higher than among normonatraemic adults by verifying a linear association between the level of serum sodium and mineral density of the femoral neck bone (T-score −2.5 or less at the hip, odds ratio 2.85, 95% confidence interval 1.03–7.86; p <0.01 [26]). These findings were confirmed [28]. Unfortunately, the direct consequences of hyponatraemia on the development of osteoporosis could not be assessed in this study. The many falls – a higher fall rate in the relapse patient population compared to the entire patient population – might have been prevented by proper outpatient management of hyponatraemia, but causality could not be statistically analysed because of the retrospective nature of the analysis.
The inadequate or insufficient treatment of hyponatraemia was associated with a prolonged hospital stay and resulted in higher costs, i.e., a considerable deficit. Overstay of patients suffering from mild hyponatraemia, who were the largest patient group, caused most expense. This is to our knowledge the first study reporting on the cost of illness of hyponatraemia in Switzerland. Findings of US studies align with the conclusion of this analysis [10, 30]. To put the calculated annual cost into context, 650 million CHF are annually spent for the treatment of congestive heart failure [31] and approximately 700 million CHF in the treatment of lung cancer [32]. It is likely that the overall cost of hyponatraemia is actually higher than calculated expenses, since the cost of treatment of severe hyponatraemia in an intensive care unit and outpatient treatment could not be taken into account.
Measurements of initial plasma electrolytes, blood glucose and renal function, as well as plasma and urine osmolality, glucose and urine sodium should be standard in the work-up and form the basis of appropriate treatment of hyponatraemia. In every patient with hyponatraemia, hypoosmolality, and a urine osmolality above 100 mOsm/kg H2O, SIADH should be considered [33].
The therapy should always be according to the patient’s volume status, in order to prevent therapeutic failure and prolonged duration of hospital stay. Spontaneous normalisation of plasma sodium is a rather rare event. Mild hyponatraemia especially has a significant negative prognostic value and needs astute identification of its cause(s) (for prevention of relapses), and, of course, accurate therapy. Laboratory measurements at regular intervals guides successful therapy. Normonatraemia represents the highest aim of therapy and should be reached to lower risk of relapse. If the patient is still hyponatraemic at the time of discharge, the suspected cause should be reevaluated or aggravating medication searched for. Patient, who had diuretic-induced hyponatraemia should, if possible, avoid this medication (i.e., thiazides) for life. The in-hospital stay should be as short as possible, but average length of stay should be not driving force, as it brings a potential risk of discharging patients still hyponatraemic. For more appropiate reimbursement, a further subdivision into the three severities (mild, moderate and severe) would be desirable, as each require different treatment (e.g. intensive medical care in severe hyponataremia) and recovery time.
Hospital standards should aim to reduce the duration of hospital stay and thus be cost effective. Besides prognostic value, hyponatraemia of any degree should always be listed on the discharge report if a therapeutic effort has been made, as it raises the cost weight by increasing the patient clinical complexity level and thus allows n accurate therapy by extending the duration of hospital stay according to SwissDRG. In addition, chronic or repeated hyponatraemia will be recognised.
Medical follow-up visits seem crucial to prevent relapse and development of chronic hyponatraemia, and the discharge report should contain clear and relevant recommendations for outpatient follow-up. Hospital-based follow-up should be considered in those cantons of Switzerland where laboratory measurements of sodium by family physicians are not covered by health insurance.
In conclusion, our study shows that all severities of hyponatraemia can be associated with relevant morbidity and mortality, and that diligent initial diagnostic work up, adherence to in-hospital treatment standards, appropriate organisation of outpatient follow-up and lifelong ban on use of risk medication (i.e., thiazides) are the mainstays to improve prognosis and decrease costs of hyponatraemia in Switzerland.
We thank the staff of the departments of internal medicine of the Kantonsspital Bruderholz, Liestal and Laufen and the Kodierungsstelle Basel-Landschaft for making the archives and data accessible.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Adrogué HJ , Madias NE . Hyponatremia. N Engl J Med. 2000;342(21):1581–9. doi:.https://doi.org/10.1056/NEJM200005253422107
2 Wald R , Jaber BL , Price LL , Upadhyay A , Madias NE . Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med. 2010;170(3):294–302. doi:.https://doi.org/10.1001/archinternmed.2009.513
3 Upadhyay A , Jaber BL , Madias NE . Incidence and prevalence of hyponatremia. Am J Med. 2006;119(7, Suppl 1):S30–5. doi:.https://doi.org/10.1016/j.amjmed.2006.05.005
4 Hawkins RC . Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003;337(1-2):169–72. doi:.https://doi.org/10.1016/j.cccn.2003.08.001
5 Arampatzis S , Frauchiger B , Fiedler GM , Leichtle AB , Buhl D , Schwarz C , et al. Characteristics, symptoms, and outcome of severe dysnatremias present on hospital admission. Am J Med. 2012;125(11):1125.e1–7. doi:.https://doi.org/10.1016/j.amjmed.2012.04.041
6 Sherlock M , O’Sullivan E , Agha A , Behan LA , Rawluk D , Brennan P , et al. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clin Endocrinol (Oxf). 2006;64(3):250–4. doi:.https://doi.org/10.1111/j.1365-2265.2006.02432.x
7 Feldman BJ , Rosenthal SM , Vargas GA , Fenwick RG , Huang EA , Matsuda-Abedini M , et al. Nephrogenic syndrome of inappropriate antidiuresis. N Engl J Med. 2005;352(18):1884–90. doi:.https://doi.org/10.1056/NEJMoa042743
8 Ellison DH , Berl T . Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356(20):2064–72. doi:.https://doi.org/10.1056/NEJMcp066837
9 Bartter FC , Schwartz WB . The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967;42(5):790–806. doi:.https://doi.org/10.1016/0002-9343(67)90096-4
10 Boscoe A , Paramore C , Verbalis JG . Cost of illness of hyponatremia in the United States. Cost Eff Resour Alloc. 2006;4(1):10. doi:.https://doi.org/10.1186/1478-7547-4-10
11Arbeitsgruppe S. Fixed rate per case payments in Swiss hospitals, S. AG, Editor. 2015: Bern, Schweiz.
12Arbeitsgruppe S. Liste der Netzwerkspitaeler Akutsomatik, S. AG, Editor. 2018: Bern, Schweiz.
13Statistik Bf. Die Bevölkerung der Schweiz 2011, B.f. Statistik, Editor. 2011: Neuchâtel.
14Ammann N. Statistik Baselland des Statistischen Amtes Baselland, S.A. Baselland, Editor. 2013, Schul- und Büromaterialverwaltung Baselland.
15Statistik Bf. Regionalporträts 2011: Kantone. Statistischer Atlas der Schweiz, B.f. Statistik, Editor. 2013, Bundesamt für Statistik: Neuchâtel.
16Kantonsspital Baselland HDSS. Qualitätsbericht 2012, K. Baselland, Editor. 2012.
17 Verbalis JG , Goldsmith SR , Greenberg A , Korzelius C , Schrier RW , Sterns RH , et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10, Suppl 1):S1–42. doi:.https://doi.org/10.1016/j.amjmed.2013.07.006
18Statistik Bf. Kosten der stationären Spitalaufenthalte 2011. B.f. Statistik, Editor, 2011: Neuchâtel.
19 Nigro N , Winzeler B , Suter-Widmer I , Schuetz P , Arici B , Bally M , et al. Symptoms and characteristics of individuals with profound hyponatremia: a prospective multicenter observational study. J Am Geriatr Soc. 2015;63(3):470–5. doi:.https://doi.org/10.1111/jgs.13325
20 Adrogué HJ , Madias NE . The challenge of hyponatremia. J Am Soc Nephrol. 2012;23(7):1140–8. doi:.https://doi.org/10.1681/ASN.2012020128
21 Douglas I . Hyponatremia: why it matters, how it presents, how we can manage it. Cleve Clin J Med. 2006;73(Suppl 3):S4–12. doi:.https://doi.org/10.3949/ccjm.73.Suppl_3.S4
22 Spasovski G , Vanholder R , Allolio B , Annane D , Ball S , Bichet D , et al.; Hyponatraemia Guideline Development Group. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol Dial Transplant. 2014;29(Suppl 2):i1–39. doi:.https://doi.org/10.1093/ndt/gfu040
23 Greenberg A , Verbalis JG , Amin AN , Burst VR , Chiodo JA, 3rd , Chiong JR , et al. Current treatment practice and outcomes. Report of the hyponatremia registry. Kidney Int. 2015;88(1):167–77. doi:.https://doi.org/10.1038/ki.2015.4
24 Renneboog B , Musch W , Vandemergel X , Manto MU , Decaux G . Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e1–8. doi:.https://doi.org/10.1016/j.amjmed.2005.09.026
25 Hoorn EJ , Rivadeneira F , van Meurs JB , Ziere G , Stricker BH , Hofman A , et al. Mild hyponatremia as a risk factor for fractures: the Rotterdam Study. J Bone Miner Res. 2011;26(8):1822–8. doi:.https://doi.org/10.1002/jbmr.380
26 Verbalis JG , Barsony J , Sugimura Y , Tian Y , Adams DJ , Carter EA , et al. Hyponatremia-induced osteoporosis. J Bone Miner Res. 2010;25(3):554–63. doi:.https://doi.org/10.1359/jbmr.090827
27 Ayus JC , Moritz ML . Bone disease as a new complication of hyponatremia: moving beyond brain injury. Clin J Am Soc Nephrol. 2010;5(2):167–8. doi:.https://doi.org/10.2215/CJN.09281209
28 Kinsella S , Moran S , Sullivan MO , Molloy MG , Eustace JA . Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol. 2010;5(2):275–80. doi:.https://doi.org/10.2215/CJN.06120809
29 Tamma R , Sun L , Cuscito C , Lu P , Corcelli M , Li J , et al. Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia. Proc Natl Acad Sci USA. 2013;110(46):18644–9. doi:. Corrected in: Proc Natl Acad Sci USA. 2014;111(38):14002. doi:http://dx.doi.org/10.1073/pnas.1415306111 https://doi.org/10.1073/pnas.1318257110
30 Amin A , Deitelzweig S , Christian R , Friend K , Lin J , Belk K , et al. Evaluation of incremental healthcare resource burden and readmission rates associated with hospitalized hyponatremic patients in the US. J Hosp Med. 2012;7(8):634–9. doi:.https://doi.org/10.1002/jhm.1973
31 Szucs TD . Gesundheitsökonomische Aspekte der chronischen Herzinsuffizienz. Schweiz Arzteztg. 2003;84(46):2431–41. doi:.https://doi.org/10.4414/saez.2003.10090
32Gesundheit SWABf. Kosten der nichtübertragbaren Krankheiten in der Schweiz, Schlussbericht, B.f. Gesundheit, Editor. 2014: Neuchâtel.
33 Robertson GL . Physiology of ADH secretion. Kidney Int Suppl. 1987;21:S20–6.
No financial support and no other potential conflict of interest relevant to this article was reported.