The Expanded Risk Score in Rheumatoid Arthritis (ERS-RA): performance of a disease-specific calculator in comparison with the traditional prediction scores in the assessment of the 10-year risk of cardiovascular disease in patients with rheumatoid arthritis
DOI: https://doi.org/10.4414/smw.2018.14656
Fausto
Salaffia, Marina
Carottib, Marco
Di Carloa, Marika
Tardellaa, Valentina
Latoa, Andrea
Becciolinic, Ennio Giulio
Favallic, Andrea
Giovagnonib
aRheumatological Clinic, Ospedale “Carlo Urbani”, Università Politecnica delle Marche, Jesi (Ancona), Italy
bRadiology Department, Ospedali Riuniti, Università Politecnica delle Marche, Ancona, Italy
cRheumatology Department, ASST Gaetano Pini - CTO, Milano, Italy
Summary
AIMS OF THE STUDY
To assess the performance of the Expanded Risk Score in Rheumatoid Arthritis (ERS-RA), a disease-specific cardiovascular disease (CVD) prediction score, in evaluating the 10-year risk, in comparison with other traditional algorithms in patients with rheumatoid arthritis (RA).
METHODS
Consecutive RA patients, aged 40–75 years, without established CVD, were included. We calculated the disease-specific ERS-RA and four traditional CVD prediction scores: the modified Systematic Coronary Risk Evaluation (mSCORE), the Framingham Risk Score using body mass index (FRS BMI), the calculator developed by the American College of Cardiology / American Heart Association in 2013 (ACC/AHA 2013) and the QRISK3. Subjects also underwent ultrasound assessment of the carotid arteries. The presence of a carotid intima-media thickness (CIMT) >0.90 mm or of carotid plaques identified the high-risk patients.
RESULTS
Of the 84 patients evaluated, 33 (39.3%), 16 (19.0%), 24 (28.6%), 25 (29.8%) and 33 (39.3%) subjects were defined as having high CVD risk according to ACC/AHA 2013, mSCORE, FRS BMI, QRISK3 and ERS-RA, respectively. Compared with the ultrasound results, all the areas under the receiver operating characteristic curves (AUC-ROC) showed good discrimination properties (0.848 – FRS BMI, 0.816 – mSCORE, 0.828 – ACC/AHA 2013, 0.844 – QRISK3, 0.869 – ESR-RA). Comparison of the AUC-ROCs did not show that discriminative ability for detecting subclinical atherosclerotic damage was improved with ESR-RA.
CONCLUSIONS
Using a surrogate marker of subclinical atherosclerotic organ damage as indicator of CVD burden, the newly ERS-RA risk score that incorporates specific aspects of RA performs as well as ACC/AHA 2013, mSCORE, FRS BMI and QRISK3 estimators.
Introduction
Rheumatoid arthritis (RA) is a chronic and disabling inflammatory disease with an unpredictable course and wide variations in severity that affects about 0.5% of the Italian population [1].
During recent years, the recognition of an increased risk of cardiovascular disease (CVD) has emerged as a major issue in RA patients [2]. It has been estimated that the CVD burden in RA is comparable to that of diabetes mellitus [3]. This knowledge translates into an increased standardised CVD mortality ratio, which ranges between 1.5 and 1.7 [4, 5]. Compared with the general population, RA patients have a 50% augmented risk of CVD-related death (standardised mortality ratio 1.5) [5]. CVD is the primary cause of death in RA patients, whose median life expectancy is shortened by 6 to 7 years [6].
The major traditional CVD risk scores, derived from the general population, including the Framingham Risk Score (FRS) [7], the Systematic COronary Risk Evaluation (SCORE) [8], the Reynolds Risk Score (RRS) [9], and the algorithm developed by the American College of Cardiology/American Heart Association in 2013 (ACC/AHA 2013) [10], have been tested in patients with RA and all of them seem to perform suboptimally in this poulation, resulting in an underestimation of the CVD risk [11, 12]. Different approaches to solve this issue have been proposed, such as applying a multiplier of 1.5 [13], or adding 10 years to the age of patients with RA [14]. However the validity of these proposals has not been rigorously tested [13].
QRISK3 incorporates more factors than QRISK2 to help physicians to identify those at most risk of heart disease and stroke, such as chronic kidney disease, migraine, corticosteroids use, systemic lupus erythematosus (SLE), atypical antipsychotics, severe mental illness, erectile dysfunction, and a measure of systolic blood pressure variability [14, 15].
The need of effective CVD risk stratification tools specific to RA patients is recognised by the European League Against Rheumatism (EULAR) recommendations, as well as by other experts [13, 16, 17].
Several studies demonstrated an association between CVD risk factors, markers of RA severity and atherosclerosis [18–20].
Recently, the Expanded Risk Score in RA (ERS-RA) calculator was developed and internally validated using data from the Consortium of Rheumatology Researchers of North America (CORRONA) registry in the USA [21]. This risk score incorporates several RA-specific factors such as corticosteroid use, disease duration, disease activity – measured with the Clinical Disease Activity Index (CDAI) – and function – evaluated with the modified Health Assessment Questionnaire (mHAQ).
During recent years the use of measures to assess the subclinical atherosclerosis burden in vivo, as well as of risk prediction scores, has become widespread. The ultrasound examination of both carotid arteries in subjects with inflammatory joint diseases, next to the conventional cardiovascular risk factors, helps to stratify the patients. Through ultrasound, a high frequency of carotid plaques in RA patients is detectable, leading to early initiation of statin use [22]. Moreover, ultrasound allows evaluation of the carotid intima-media thickness (CIMT), which is a safe, noninvasive and cost effective method to detect atherosclerotic disease early [23–25]. CIMT has been reported as representative of subclinical and asymptomatic atherosclerosis, a manifestation of a raised CVD risk [26].
The objective of this study was to assess the performance of the ERS-RA in evaluating the 10-years CVD risk in comparison with the traditional algorithms, the modified SCORE (mSCORE), the FRS using body mass index (FRS BMI), the ACC/AHA 2013, and the QRISK3; the presence of a CIMT over 0.9 mm and/or the presence carotid plaque was considered an indicator of high-risk patients with subclinical atherosclerotic organ damage.
Materials and methods
Study population
Consecutive RA patients, aged from 40 to 65 years and fulfilling the 2010 American College of Rheumatology (ACR) / EULAR classification criteria for RA [27], were recruited from the outpatient clinics of two Italian tertiary rheumatology centres (Rheumatological Clinic, Università Politecnica delle Marche, Jesi, Ancona, Italy and Rheumatology Department, ASST Gaetano Pini - CTO, Milano, Italy) from September 2017 to November 2017. Subjects fulfilled the following inclusion criteria: disease duration ≥5 years; current treatment with ≥1 synthetic or biological disease-modifying anti-rheumatic drug (s/bDMARD) for a period ≥3 months. Patients were excluded if suffering from pre-existing CVD (including ischaemic heart disease, cerebrovascular accident, peripheral arterial disease, heart failure), or if they were already taking prescribed statins. We also excluded patients with diabetes and moderate (estimated glomerular filtration rate [eGFR] 30–59 ml/min/1.73 m2) or severe (eGFR <30 ml/min/1.73 m2) chronic kidney disease, since these conditions represent high or very high CVD risk. Hypertension was defined as a systolic blood pressure ≥140 mm Hg and/or a diastolic blood pressure ≥90 mm Hg, and/or a diagnosis of hypertension by a physician and current treatment according to guidelines for the management of hypertension [28].
All the procedures applied in this research were in accordance to the Helsinki Declaration as revised in 2013. All the patients gave their written informed consent for the anonymous collection of data, and the study was approved by the Ethics Board of the University-Hospital (Comitato Unico Regionale – ASUR Marche).
Laboratory measurements
A standardised set of fasting blood measurements was performed for each patient, in particular total cholesterol, high density lipoprotein (HDL) cholesterol, triglycerides, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), glucose, rheumatoid factor, and anti-citrullinated protein antibodies (ACPA).
Clinical assessments
Clinical assessment included the mHAQ [29], as a function index, and the CDAI for assessing disease activity [30]. In the mHAQ, the number of activities of daily living was reduced from 20 (HAQ) to 8 (mHAQ). The mHAQ total score is between 0.0 and 3.0, in 0.125 increments. Higher scores indicate worse function and greater disability [31].
The CDAI range is from 0 to 76, and it is based on the simple summation of the swollen and tender joint count of 28 joints, with patient and physician global assessments on visual analogue scales (VASs) (0–10 cm). It can essentially be used everywhere and anytime to assess disease activity. Moreover, CDAI cut-off values for remission are more stringent than those of Disease Activity Score-28 joints (DAS-28) [32].
Cardiovascular disease risk prediction scores
CVD risk was calculated with FRS BMI, mSCORE, ACC/AHA 2013, QRISK3, and ERS-RA, via the official web sites. The results of the algorithms that do not include RA among the variables were multiplied by 1.5, in accordance to the EULAR 2015/2016 recommendations [16].
Table 1 summarises the principal features of each index.
Table 1 Characteristics of the cardiovascular risk prediction scores.
| ACC/AHA 2013 |
This algorithm predicts the 10-year risk of heart disease or stroke (cardiovascular morbidity), in patients aged 20 to 79 years. Gender, age, race, diagnosis of diabetes, smoking, treated hypertension, SBP and lipid profile values (TC and HDL-C) are included as variables. |
| ERS-RA |
This tool, developed to assess cardiovascular morbidity, includes four RA-specific factors as variables: disease duration, disease activity, disability and use of corticosteroids. It was developed and internally validated from data in the CORRONA registry, but it has not been externally validated. Laboratory data are not needed. |
| FRS BMI |
This algorithm predicts the 10-year risk of coronary death, myocardial infarction, coronary insufficiency, angina, ischaemic and haemorrhagic stroke, transient ischaemic attack, peripheral artery disease and heart failure (cardiovascular morbidity and mortality). Variables include age, gender, and traditional CV risk factors (presence of diabetes, smoking, and treated hypertension), as well as values of systolic blood pressure and BMI. CV risk can be calculated for individuals aged 30 to 74 years. |
| QRISK3 |
The updated QRISK3 prediction model was developed and validated to quantify absolute risks of cardiovascular disease (cardiovascular morbidity – coronary heart disease, ischaemic stroke, or transient ischaemic attack) in people aged 25 to 84 years. This algorithm includes established risk factors and new risk factors such as an expanded definition of chronic kidney disease, migraine, corticosteroid use, SLE, atypical antipsychotic use, severe mental illness, erectile dysfunction and a measure of blood pressure variability. There is no need to apply a 1.5 multiplication factor according to the EULAR recommendations because RA is included as a variable. |
| SCORE |
This calculator evaluates the 10-year risk of cardiovascular mortality (first fatal atherosclerotic event, (for example, myocardial infarction, stroke, and aortic aneurysm). It is based on age, gender, smoking habits, total cholesterol and SBP. The SCORE model allows calibration of charts for individual countries, which has been done for numerous European countries. At the international level, two sets of charts are provided: one for high-risk and one for low-risk countries. We used the SCORE equation for low-risk countries, since the guidelines consider Italy as a low-risk country. The modified SCORE (mSCORE) refers to the SCORE calculation multiplied by 1.5. |
FRS BMI
The 10-year risk for CVD was calculated using the Framingham equation [33], available as a web-based tool (http://hivpv.org/Home/Tools). The assessed factors include gender, age, systolic blood pressure, antihypertensive treatment, smoking status, type 2 diabetes, total cholesterol and HDL cholesterol or BMI in a simplified model [34]. The score was categorised as follows: <10% low risk; 10–20% moderate risk; ≥20% high risk.
mSCORE
This model evaluates the 10-year risk of the first fatal atherosclerotic event. The SCORE was developed from large European cohort studies. It estimates the risk of dying from CVD (not just coronary artery disease), based on age, gender, smoking habits, total cholesterol and systolic blood pressure [8]. The SCORE model allows calibration of the charts for individual European countries. At the international level, two sets of charts are provided: one for high-risk and one for low-risk countries. We used the SCORE equation for low-risk countries, since the guidelines consider Italy to be a low-risk country. Subjects were considered at low risk if the score was <1%, at moderate risk if score was 1–5%, and at high risk if the score was ≥5%. In this study we used the mSCORE, with the 1.5 multiplication factor for RA patients.
ACC/AHA 2013
This risk score employs the AHA/ACC pooled cohort equation [12, 35, 36], which estimates 10-year cardiovascular risk as low (<5%), intermediate (≥5 to <7.5%), and high (≥7.5%).
QRISK3
The calculator considers RA as a separate CVD risk factor. It calculates the percentage of the risk in the population aged between 24 and 84 years. As well as the variables already included in QRISK2 (age, ethnicity, deprivation, systolic blood pressure, BMI, total/HDL cholesterol ratio, smoking, family history of coronary heart disease in a first degree relative aged less than 60 years, type 1 diabetes, type 2 diabetes, treated hypertension, RA, atrial fibrillation, chronic kidney disease [stage 4 or 5]), QRISK3 takes into account additional factors such as chronic kidney disease (stage 3, 4, or 5), a measure of systolic blood pressure variability (standard deviation of repeated measures), migraine, corticosteroids, SLE, atypical antipsychotics, severe mental illness, human immunodeficiency virus infection / acquired immunodeficiency syndrome) and erectile dysfunction in men.
Pharmacological intervention (statins) is recommended in patients with the threshold of 20% probablitiy of the end event over the following 10 years [14]. It was calculated using QRISK3 -2017 risk calculator https://qrisk.org/three/.
ERS-RA
The ERS-RA was calculated using a publically available Excel macro. For ESR-RA, high risk was defined as a 10-year CVD risk ≥7.5%. As mentioned above, the novel feature of this calculator is the inclusion of RA-specific features in the CVD risk assessment, which contribute to a significantly improved model for the prediction of cardiovascular events [21].
Ultrasound evaluation of the carotid arteries
The ultrasound assessment followed the American Society of Echocardiography (ASE) guidelines [37]. The same, trained sonographer (MC), blinded to the clinical and laboratory data, evaluated the carotid arteries in B-mode, using a 5–12 MHz linear probe (iU22 Philips). Intraobserver reproducibility of readings of mean CIMT was evaluated in 20 patients within one week of the first ultrasound examination. The intraclass correlation coefficient for CIMT was 0.91. Patients were placed in the supine position for ultrasound examination of the common carotid arteries, resting in the supine position for 15 minutes before the assessment.
The CIMT, in accordance with the Mannheim consensus recommendations, was measured on the far wall of the common carotid artery at least 5 mm below its end, which avoids interindividual variability induced by physiological remodeling and reduces gain dependence [38]. Three measurements along a minimum length of 10 mm of a straight arterial segment on both the right and left sides were made. The average of three measurements of the far wall of the artery was recorded for each patient. An upper limit of 0.90 mm was chosen for the present study, based on epidemiological data currently available.
Plaque is defined as a focal structure that encroaches into the arterial lumen by at least 0.5 mm or 50% of the surrounding IMT value or has a thickness of >1.5 mm as measured from the media-adventitia interface to the intima-lumen interface [38]. Plaque presence was evaluated in the bilateral common carotid arteries, internal carotid arteries and carotid artery bulbs. Carotid plaques were counted in each territory and defined as no plaque, unilateral or bilateral plaques. The presence of a CIMT >0.9 mm or of unilateral/bilateral plaques defined the high-risk subjects (US+) with subclinical vascular damage [39].
Statistical analysis
Values were expressed as the mean ± standard deviation (SD) unless indicated otherwise. The univariate analysis to identify variables associated with high-risk patients was investigated using the student’s t-test (parametric data) or the one-way analysis of variance (ANOVA) to compare means of more samples. Qualitative variables were tested using chi-square tests. The ability of the ERS-RA to discriminate between patients with and without subclinical atherosclerosis compared with the other CVD risk indices was determined through area under the receiver operating characteristic curves (AUC-ROCs) analysis.
All statistical analyses were performed using MedCalc 11.3.1.0 version (MedCalc Software, Ostend, Belgium).
Results
Patients
The cross-sectional cohort was composed of 98 eligible patients; 84 were included in the final analysis. Fourteen patients were excluded: 11 because they did not complete the ultrasound assessment and 3 because they were already taking statins. The population’s demographic and clinical characteristics are shown in table 2. Women accounted for the majority of our population (71.4%). Thirty patients (35.7%) were active smokers, and 32 (38.1%) had hypertension. Lipid profile values showed mean total cholesterol of 206.1 ± 44.8 mg/dl, HDL cholesterol 56.7 ± 15.2 mg/dl and total/HDL cholesterol ratio 3.8 ± 1.2.
Table 2 Demographic characteristics of the whole cohort (84 subjects) and of the subgroups of patients according to carotid intima-media thickness (CIMT) stratification and plaque presence (CIMT ≤0.90 mm, n = 43 subjects; >0.90 mm, n = 33 subjects and n = 41 subjects >0.90 mm + carotid plaques).
|
Variable
|
Overall
(n = 84)
|
CIMT ≤0.90 mm
(n = 43)
|
CIMT >0.90 mm without plaques
n = 33
|
CIMT >0.90 mm or plaques
n = 41
|
p-value*
|
|
Mean ± SD
|
Mean ± SD
|
Mean ± SD
|
Mean ± SD
|
| Age (years) |
59.3 ± 10.3 |
58.6 ± 10.6 |
60.5 ± 10.8 |
59.9 ± 11.1 |
n.s. |
| Disease duration of RA (years) |
11.4 ± 6.7 |
10.4 ± 7.4 |
12.4 ± 5.8 |
11.8 ± 6.1 |
n.s. |
| TC (mg/dl) |
206.1 ± 44.8 |
199.8.1 ± 47.3 |
215.5 ± 48.7 |
219.1 ± 50.1 |
0.041 |
| HDL-C (mg/dl) |
56.7 ± 15.2 |
56.5 ± 14.4 |
55.7 ± 15.5 |
56.9 ± 13.9 |
n.s. |
| TC/HDL-C ratio |
3.8 ± 1.2 |
3.7 ± 1.4 |
3.9 ± 1.2 |
3.9 ± 1.7 |
n.s. |
| Systolic blood pressure (mm Hg) |
132.9 ± 14.5 |
126.7 ± 12.6 |
143.8 ± 15.5 |
144.1 ± 16.1 |
0.033 |
| Body mass index (kg/m2) |
24.8 ± 4.3 |
24.2 ± 4.3 |
25.9 ± 3.2 |
26.1 ± 4.1 |
n.s. |
| CDAI |
10.9 ± 6.8 |
9.9 ± 6.2 |
12.5 ± 7.3 |
11.9 ± 7.9 |
n.s. |
| mHAQ |
0.67 ± 0.43 |
0.59 ± 0.41 |
0.80 ± 0.43 |
0.78 ± 0.41 |
n.s. |
| CIMT |
0.806 ± 0.209 |
0.684 ± 0.131 |
0.928 ± 0.141 |
0.933 ± 0.151 |
<0.001 |
| CVD risk scores |
|
|
|
|
|
| FRS BMI |
14.5 ± 14.2 |
8.4 ± 9.3 |
21.1 ± 13.8 |
21.9 ± 12.3 |
<0.001 |
| mSCORE |
3.4 ± 4.2 |
1.9 ± 3.2 |
5.1 ± 4.9 |
5.3 ± 4.3 |
<0.001 |
| ACC/AHA 2013 |
16.6 ± 17.7 |
9.9 ± 11.2 |
23.8 ± 21.9 |
22.9 ± 19.8 |
<0.001 |
| QRISK3 |
17.5 ± 16.1 |
10.8 ± 10.1 |
24.0 ± 17.7 |
23.6 ± 15.5 |
<0.001 |
| ESR-RA |
13.9 ± 16.4 |
7.2 ± 9.0 |
20.9 ± 22.8 |
20.5 ± 17.9 |
<0.001 |
| Education |
n (%) |
n (%)
|
n (%)
|
n (%)
|
|
| Primary school |
18 (21.4) |
8 (18.6) |
8 (24.2) |
11 (26.8) |
n.s. |
| Secondary school |
39 (46.2) |
20 (46.5) |
16 (48.5) |
18 (43.9) |
n.s. |
| High school or university |
27 (32.4) |
15 (34.8) |
9 (27.3) |
12 (29.3) |
n.s. |
| Current smoker |
30 (35.7) |
15 (34.8) |
15 (45.5) |
19 (46.3) |
0.027 |
| Hypertension |
32 (38.1) |
17 (39.5) |
11 (33.3) |
15 (36.5) |
n.s. |
| Hypertension treatment |
29 (34.5) |
15 (34.8) |
13 (30.3) |
14 (34.1) |
n.s. |
| RF positivity |
51 (60.7) |
28 (65.1) |
20 (51.5) |
22 (53.6) |
n.s. |
| ACPA positivity |
48 (57.1) |
27 (62.8) |
18 (54.5) |
23 (56.1) |
n.s. |
| Double seropositivity (ACPA and RF) |
41(48.8) |
22 (51.1) |
16 (48.5) |
|
n.s. |
| Methotrexate treatment |
38 (45.2) |
20 (46.5) |
15 (45.5) |
19 ((46.3) |
n.s. |
| Biological DMARDs |
31 (36.9) |
17 (39.5) |
11 (33.3) |
14 (34.1) |
n.s. |
| Daily use of prednisone |
29 (34.5) |
15 (34.8) |
11 (33.3) |
14 (34.1) |
n.s. |
Patients presented a mean CDAI of 10.9 ± 6.8 and a mean mHAQ of 0.67 ± 0.43. Severe extra-articular manifestations were found in only three patients (3.6%). Rheumatoid factor seropositivity was found in 51 (60.7%), ACPA seropositivity in 48 (57.1%), and both (ACPA and any rheumatoid factor isotype) in 41 (48.8%). A total of 38 (45.2%) subjects were taking methotrexate, with a median dose of 12.5 mg per week. Use of a bDMARD was reported in 31 (36.9%) patients. Daily use of prednisone was documented in 29 subjects (34.5%), with a median dose of 5 mg.
The 10-year cardiovascular disease prediction scores
The mean ± SD values of the 10-year CVD prediction scores were: 14.5 ± 14.2, 95% confidence interval (CI) for the mean 11.3–17.6 for FRS BMI; 3.4 ± 4.2, 95% CI for the mean 2.5–4.3 for mSCORE; 16.6 ± 17.7, 95% CI for the mean 12.7–20.5 for ACC/AHA 2013; 17.5 ± 16.9, 95% CI for the mean 13.7–21.2 for QRISK3; and 13.9 ± 16.4, 95% CI for the mean 10.3–17.6 for ERS-RA. Thirty-three (39.3%), 16 (19.0%), 24 (28.6%), 26 (30.5%) and 33 (39.3%) patients were defined as having high CVD risk according to ACC/AHA 2013, mSCORE, FRS BMI, QRISK3 and ERS-RA, respectively. All the calculators showed a significantly greater risk in male patients.
Carotid artery ultrasound results
The mean CIMT was 0.806 ± 0.209 mm. CIMT values were significantly higher in men than in women (0.944 ± 0.201 vs 0.756 ± 0.181; p = 0.001). Among all patients, 39 (46.4%) had carotid plaques and 33 (39.3%) a CIMT >0.90 mm; 41 (48.8%) patients had either CIMT >0.90 mm or carotid plaques (US+) (table 2).
Discriminating ability of the cardiovascular risk prediction scores
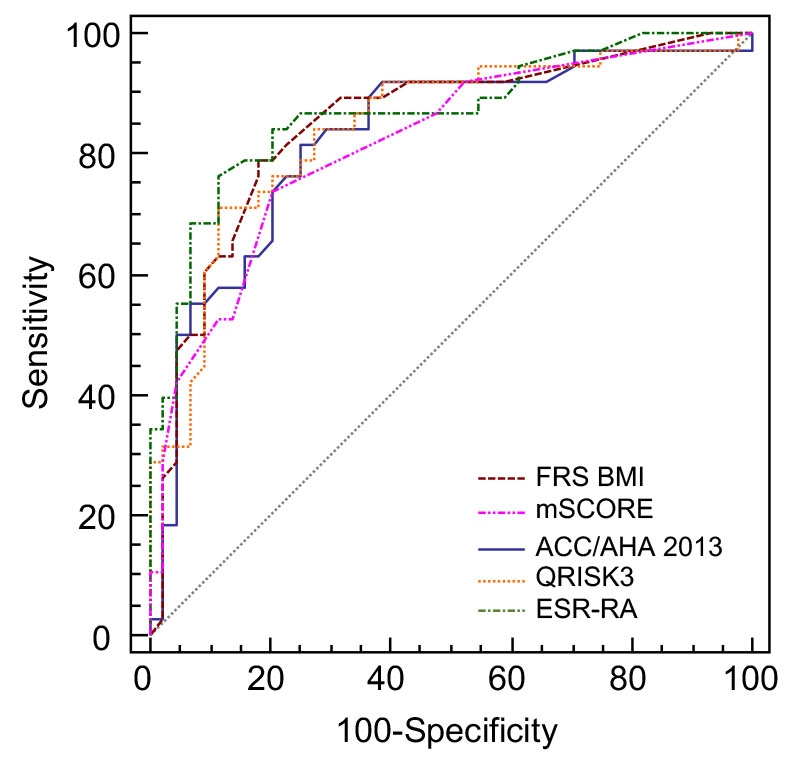
Discriminating ability (to identify the presence of a CIMT >0.9 mm or unilateral/bilateral plaques) of the five risk indices was good, with an AUC-ROC of 0.848 (95% CI 0.729–0.902) for FRS BMI, 0.816 (95% CI 0.715–0.893) for mSCORE, 0.828 (95% CI 0.724–0.902) for ACC/AHA 2013, 0.845 (95% CI 0.749–0.919) for QRISK3 and 0.869 (95% CI 0.776–0.933) for ERS-RA (table 3). The AUC-ROCs of the different CVD risk prediction models are depicted in figure 1. All AUCs showed good discrimination properties, with that of ERS-RA being the best. However, no statisticaly significant difference between the ERS-RA AUC and those of the other four indices was observed (table 4).
Table 3 Area under the receiver operating characteristic curves (standard error and 95% confidence intervals) to distinguish patients in regard to presence of subclinical atherosclerosis as defined by the presence of carotid intima-media thickness over 0,9 mm and/or carotid plaques (US+).
|
Variable
|
AUC
|
SE*
|
95% CI†
|
| FRS BMI |
0.848 |
0.0447 |
0.752 to 0.918 |
| mSCORE |
0.816 |
0.0474 |
0.715 to 0.893 |
| ACC/AHA 2013 |
0.828 |
0.0474 |
0.729 to 0.902 |
| QRISK3 |
0.845 |
0.0439 |
0.749 to 0.919 |
| ERS-RA |
0.869 |
0.0409 |
0.776 to 0.933 |
Table 4 Comparison of the Expanded Risk Score in Rheumatoid Arthritis area under the receiver operating characteristic curve to the areas of the other four cardiovascular risk prediction scores.
|
Variable
|
Difference between areas
|
SD*
|
95% CI
|
Significance level (p-value)
|
| ESR-RA vs ACC/AHA 2013 |
0.0407 |
0.0331 |
−0.0241 to 0.105 |
0.2187 |
| ERS-RA vs FRS BMI |
0.0206 |
0.0347 |
−0.0474 to 0.0887 |
0.5522 |
| ERS-RA vs QRISK3 |
0.0241 |
0.0391 |
−0.0531 to 0.102 |
0.5381 |
| ESR-RA vs mSCORE |
0.0529 |
0.0352 |
−0.0161 to 0.122 |
0.1329 |
Discussion
In the present study, we compared the ability of the ERS-RA, a disease-specific CVD prediction score, with that of four risk algorithms developed for the general population, to predict CVD mortality risk in an Italian RA population. The main finding was a good concurrent and discriminant validity of all the five 10-year CVD risk calculators. Although ERS-RA gave a better AUC-ROC compared with the other four tools, this difference was not statistically significant.
The fact that disease-specific calculators, such as ERS-RA, do not bring significant improvements in CVD risk prediction compared with the traditional instruments has been already highlighted in results derived from a large cohort of patients (1796 subjects) of the Trans-Atlantic Cardiovascular Consortium for RA [42].
CVD risk is an important, but frequently underassessed, topic in RA [43]. The reasons for increased CVD risk in RA patients are complex and are postulated to be related to chronic autoimmune and inflammatory mechanisms, endothelial dysfunction and inadequate management of modifiable risk factors, and potentially to medication including corticosteroids and non-steroidal anti-inflammatory drugs [35, 44, 45].
Several well-known models for CVD mortality risk prediction, which utilise data from multiple risk factors, have been developed and updated in the USA, Japan and Europe in recent decades [7–9, 46–49]. Their values and limitations have been reviewed [50], and it has been demonstrated that algorithms for the general population do not work well in RA patients [11, 12].
Moreover, application of the scores suggested in the international guidelines for cholesterol management in patients with RA can lead to quite discordant results. In a French cohort, eligibility for statin treatment was tested in accordance with the guidelines of the European Society of Cardiology (ESC) (the SCORE), with the 1.5 multiplication factor according to the EULAR, with the Adult Treatment Panel III (ATP-III) (the FRS), and to the ACC/AHA (the Pooled Cohort Equations): statin therapy was recommended in 9.6% of the women and 26.1% of the men according to the SCORE algorithm, whereas according to the ATP-III guidelines statins should be started in 15.5% of the women and 51% of the men [51]. As SCORE and FRS were created for the general population, the EULAR experts recommend multiplying cardiovascular risk in RA patients by a factor of 1.5 [16]. This coefficient has been criticised, since it was derived from the experts’ opinion, with no supporting data, and whereas FRS, SCORE, and RRS seem to undestimate, QRISK2 has been judged to overestimate the CVD risk [11].
Crowson et al., in a study of 525 RA patients aged over 30 years, analysed FRS and RRS, concluding that these tools substantially underestimate cardiovascular risk in RA patients (both genders), mainly in advanced age, in rheumatoid factor positive subjects, and in patients with a persistently high ESR, which is an important marker of inflammatory activity of the disease [12].
Galarza-Delgado et al. in a cohort of Mexican Mestizo RA patients, showed a significant difference between of CVD risk calculators [52]. In the individual comparison, QRISK3 did not show a statistical difference when compared with ERS-RA. In this study, FRS BMI delivered the highest values of predicted CVD risk. Ozen et al. found that the ACC/AHA 2013 algorithm failed to identify the majority (≈55%) of the patients with increased CIMT and/or carotid plaques. However, the ACC/AHA 2013 calculator was better than SCORE and QRISK2 in detecting patients with subclinical atherosclerosis when the high risk thresholds (>7.5%, >5% and >20%, respectively) for all three indices were used [53]. Thus, data coming from the literature revealed differences between the scores, with a trend to underestimation for some prediction tools.
One reason for the underestimate of CVD risk in RA may be the high frequency of asymptomatic atherosclerosis [54–56], which can be visualised by ultrasound of the carotid arteries.
The assessment of CIMT and the presence of carotid plaques with ultrasound has become an efficient technique to measure the presence of subclinical atherosclerosis in RA [57, 58]. Both CIMT and carotid plaques were found to be predictors of cardiovascular events in low and intermediate risk groups of non-rheumatic individuals, and also in RA patients [24, 25, 59]. CIMT is a measure of early atherosclerosis and vascular remodelling. CIMT has been employed as an indicator of atherosclerosis in epidemiological, observational and interventional clinical studies. It has been also applied as a primary endpoint for therapeutic efficacy with various pharmacological therapies and it has been employed as an exposure variable in studies on the prognostic value of predicting coronary artery disease and stroke [60, 61]. Both CIMT >0.90 mm and the presence of carotid plaques are considered expressions of subclinical organ damage, and as factors influencing the CVD prognosis in the general population and in RA patients [24, 25, 28].
In clinical practice the assessment of CIMT is not yet a routine investigation, but the predictive value of this measure has been established in several prospective studies, guidelines and consensus statements [23, 38, 62]. However, the CIMT is not reccomended as a screening tool by the ESC, and the correct integration of additional infomation into the traditional risk models is a sensitive issue [63].
Three potential limitations to our study deserve to be mentioned. First of all, our study was a cross-sectional evaluation, with a small number of patients included, and lacked CVD outcomes. We tried to overcome this limitation with the carotid ultrasound assessment. However, follow-up of these patients is needed. Secondly, the generalisability of our results may be limited because all of our patients were recruited from two Italian centres. An additional limitation is that the “cardiovascular disease-free” criteria might induce a selection bias, especially in patients with longstanding RA.
In conclusion, CVD risk assessment remains inadequate in RA at present. The main finding in our present study was good concurrent and discriminant validity of the five 10-year CVD risk calculators. The ERS-RA does not seem to add significant advantages compared with ACC/AHA 2013, mSCORE, FRS BMI and QRISK3 estimators.
The use of surrogate markers of subclinical atherosclerotic organ damage increases the proportion of correct risk stratification. Up to now, CIMT measured by B-mode ultrasound is the most studied measure and has been validated by official medical agencies [26, 38, 63, 64], but new techniques (i.e., the coronary calcium score) probably will be widely available in the near future [65].
Larger heterogeneous RA cohorts followed longitudinally for clinical CVD endpoints, such as stroke or myocardial infarction, would provide a better assessment of the utility of CVD risk calculators, helping to identify the contribution of RA specific factors to the total predicted burden.
Acknowledgements
We thank all rheumatologists and clinical staff of the Rheumatological Clinic of “Carlo Urbani” Hospital, Jesi, and of the Rheumatology Department, ASST Gaetano Pini - CTO, Milano, Italy for the collaboration in data collection.
Author contributions
FS, AG, AB, and EGF contributed to the study design, protocol, analysis, and data interpretation. MDC, MT, VL, AD, and MC contributed to the data collection and to the manuscript revision. FS and MDC wrote the manuscript. All authors approved the final version of the manuscript.
References
1
Salaffi
F
,
De Angelis
R
,
Grassi
W
; MArche Pain Prevalence; INvestigation Group (MAPPING) study. Prevalence of musculoskeletal conditions in an Italian population sample: results of a regional community-based study. I. The MAPPING study. Clin Exp Rheumatol. 2005;23(6):819–28.
2
Meune
C
,
Touzé
E
,
Trinquart
L
,
Allanore
Y
. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford). 2009;48(10):1309–13. doi:.https://doi.org/10.1093/rheumatology/kep252
3
Ferraccioli
G
,
Gremese
E
. Adiposity, joint and systemic inflammation: the additional risk of having a metabolic syndrome in rheumatoid arthritis. Swiss Med Wkly. 2011;141:w13211.
4
Aviña-Zubieta
JA
,
Choi
HK
,
Sadatsafavi
M
,
Etminan
M
,
Esdaile
JM
,
Lacaille
D
. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59(12):1690–7. doi:.https://doi.org/10.1002/art.24092
5
John
H
,
Kitas
G
. Inflammatory arthritis as a novel risk factor for cardiovascular disease. Eur J Intern Med. 2012;23(7):575–9. doi:.https://doi.org/10.1016/j.ejim.2012.06.016
6
Lassere
MN
,
Rappo
J
,
Portek
IJ
,
Sturgess
A
,
Edmonds
JP
. How many life years are lost in patients with rheumatoid arthritis? Secular cause-specific and all-cause mortality in rheumatoid arthritis, and their predictors in a long-term Australian cohort study. Intern Med J. 2013;43(1):66–72. doi:.https://doi.org/10.1111/j.1445-5994.2012.02727.x
7
D’Agostino
RB, Sr
,
Vasan
RS
,
Pencina
MJ
,
Wolf
PA
,
Cobain
M
,
Massaro
JM
, et al.
General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. doi:.https://doi.org/10.1161/CIRCULATIONAHA.107.699579
8
Conroy
RM
,
Pyörälä
K
,
Fitzgerald
AP
,
Sans
S
,
Menotti
A
,
De Backer
G
, et al.; SCORE project group. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. doi:.https://doi.org/10.1016/S0195-668X(03)00114-3
9
Ridker
PM
,
Buring
JE
,
Rifai
N
,
Cook
NR
. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611–9. doi:.https://doi.org/10.1001/jama.297.6.611
10
Goff
DC, Jr
,
Lloyd-Jones
DM
,
Bennett
G
,
Coady
S
,
D’Agostino
RB, Sr
,
Gibbons
R
, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, 25 Pt B):2935–59. doi:.. Correction in: J Am Coll Cardiol. 2014;63(25):302. doi:https://doi.org/10.1016/j.jacc.2013.11.005
11
Arts
EE
,
Popa
C
,
Den Broeder
AA
,
Semb
AG
,
Toms
T
,
Kitas
GD
, et al.
Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2015;74(4):668–74. doi:.https://doi.org/10.1136/annrheumdis-2013-204024
12
Crowson
CS
,
Matteson
EL
,
Roger
VL
,
Therneau
TM
,
Gabriel
SE
. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110(3):420–4. doi:.https://doi.org/10.1016/j.amjcard.2012.03.044
13
Peters
MJ
,
Symmons
DP
,
McCarey
D
,
Dijkmans
BA
,
Nicola
P
,
Kvien
TK
, et al.
EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69(2):325–31. doi:.https://doi.org/10.1136/ard.2009.113696
14
Hippisley-Cox
J
,
Coupland
C
,
Brindle
P
. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. doi:.https://doi.org/10.1136/bmj.j2099
15
Hippisley-Cox
J
,
Coupland
C
,
Vinogradova
Y
,
Robson
J
,
Minhas
R
,
Sheikh
A
, et al.
Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ. 2008;336(7659):1475–82. doi:.https://doi.org/10.1136/bmj.39609.449676.25
16
Agca
R
,
Heslinga
SC
,
Rollefstad
S
,
Heslinga
M
,
McInnes
IB
,
Peters
MJ
, et al.
EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76(1):17–28. doi:.https://doi.org/10.1136/annrheumdis-2016-209775
17
Martín-Martínez
MA
,
González-Juanatey
C
,
Castañeda
S
,
Llorca
J
,
Ferraz-Amaro
I
,
Fernández-Gutiérrez
B
, et al.
Recommendations for the management of cardiovascular risk in patients with rheumatoid arthritis: scientific evidence and expert opinion. Semin Arthritis Rheum. 2014;44(1):1–8. doi:.https://doi.org/10.1016/j.semarthrit.2014.01.002
18
Roubille
C
,
Richer
V
,
Starnino
T
,
McCourt
C
,
McFarlane
A
,
Fleming
P
, et al.
Evidence-based recommendations for the management of comorbidities in rheumatoid arthritis, psoriasis, and psoriatic arthritis: expert opinion of the Canadian dermatology-rheumatology comorbidity initiative. J Rheumatol. 2015;42(10):1767–80. doi:.https://doi.org/10.3899/jrheum.141112
19
Dessein
PH
,
Norton
GR
,
Woodiwiss
AJ
,
Joffe
BI
,
Wolfe
F
. Influence of nonclassical cardiovascular risk factors on the accuracy of predicting subclinical atherosclerosis in rheumatoid arthritis. J Rheumatol. 2007;34(5):943–51.
20
del Rincón
I
,
Freeman
GL
,
Haas
RW
,
O’Leary
DH
,
Escalante
A
. Relative contribution of cardiovascular risk factors and rheumatoid arthritis clinical manifestations to atherosclerosis. Arthritis Rheum. 2005;52(11):3413–23. doi:.https://doi.org/10.1002/art.21397
21
Solomon
DH
,
Greenberg
J
,
Curtis
JR
,
Liu
M
,
Farkouh
ME
,
Tsao
P
, et al.
Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: a Consortium of Rheumatology Researchers of North America Registry Study. Arthritis Rheumatol. 2015;67(8):1995–2003. doi:.https://doi.org/10.1002/art.39195
22
Rollefstad
S
,
Kvien
TK
,
Holme
I
,
Eirheim
AS
,
Pedersen
TR
,
Semb
AG
. Treatment to lipid targets in patients with inflammatory joint diseases in a preventive cardio-rheuma clinic. Ann Rheum Dis. 2013;72(12):1968–74. doi:.https://doi.org/10.1136/annrheumdis-2012-202789
23
Greenland
P
,
Alpert
JS
,
Beller
GA
,
Benjamin
EJ
,
Budoff
MJ
,
Fayad
ZA
, et al.; American College of Cardiology Foundation; American Heart Association. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):e50–103. doi:.https://doi.org/10.1016/j.jacc.2010.09.001
24
Gonzalez-Juanatey
C
,
Llorca
J
,
Martin
J
,
Gonzalez-Gay
MA
. Carotid intima-media thickness predicts the development of cardiovascular events in patients with rheumatoid arthritis. Semin Arthritis Rheum. 2009;38(5):366–71. doi:.https://doi.org/10.1016/j.semarthrit.2008.01.012
25
Evans
MR
,
Escalante
A
,
Battafarano
DF
,
Freeman
GL
,
O’Leary
DH
,
del Rincón
I
. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63(5):1211–20. doi:.https://doi.org/10.1002/art.30265
26
O’Leary
DH
,
Bots
ML
. Imaging of atherosclerosis: carotid intima-media thickness. Eur Heart J. 2010;31(14):1682–9. doi:.https://doi.org/10.1093/eurheartj/ehq185
27
Aletaha
D
,
Neogi
T
,
Silman
AJ
,
Funovits
J
,
Felson
DT
,
Bingham
CO, 3rd
, et al.
2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580–8. doi:.https://doi.org/10.1136/ard.2010.138461
28
Mancia
G
,
Fagard
R
,
Narkiewicz
K
,
Redón
J
,
Zanchetti
A
,
Böhm
M
, et al.; Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281–357. doi:.https://doi.org/10.1097/01.hjh.0000431740.32696.cc
29
Wolfe
F
. Which HAQ is best? A comparison of the HAQ, MHAQ and RA-HAQ, a difficult 8 item HAQ (DHAQ), and a rescored 20 item HAQ (HAQ20): analyses in 2,491 rheumatoid arthritis patients following leflunomide initiation. J Rheumatol. 2001;28(5):982–9.
30
Aletaha
D
,
Nell
VP
,
Stamm
T
,
Uffmann
M
,
Pflugbeil
S
,
Machold
K
, et al.
Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7(4):R796–806. doi:.https://doi.org/10.1186/ar1740
31
Pincus
T
,
Summey
JA
,
Soraci
SA, Jr
,
Wallston
KA
,
Hummon
NP
. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26(11):1346–53. doi:.https://doi.org/10.1002/art.1780261107
32
Mierau
M
,
Schoels
M
,
Gonda
G
,
Fuchs
J
,
Aletaha
D
,
Smolen
JS
. Assessing remission in clinical practice. Rheumatology (Oxford). 2007;46(6):975–9. doi:.https://doi.org/10.1093/rheumatology/kem007
33
Anderson
KM
,
Odell
PM
,
Wilson
PW
,
Kannel
WB
. Cardiovascular disease risk profiles. Am Heart J. 1991;121(1):293–8. doi:.https://doi.org/10.1016/0002-8703(91)90861-B
34
Pencina
MJ
,
D’Agostino
RB, Sr
,
Larson
MG
,
Massaro
JM
,
Vasan
RS
. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation. 2009;119(24):3078–84. doi:.https://doi.org/10.1161/CIRCULATIONAHA.108.816694
35
Gabriel
SE
,
Crowson
CS
. Risk factors for cardiovascular disease in rheumatoid arthritis. Curr Opin Rheumatol. 2012;24(2):171–6. doi:.https://doi.org/10.1097/BOR.0b013e32834ff2fd
36
Stone
NJ
,
Robinson
JG
,
Lichtenstein
AH
,
Bairey Merz
CN
,
Blum
CB
,
Eckel
RH
, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25, Suppl 2):S1–45. doi:.https://doi.org/10.1161/01.cir.0000437738.63853.7a
37
Stein
JH
,
Korcarz
CE
,
Hurst
RT
,
Lonn
E
,
Kendall
CB
,
Mohler
ER
, et al.; American Society of Echocardiography Carotid Intima-Media Thickness Task Force; Endorsed by the Society for Vascular Medicine. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. J Am Soc Echocardiogr. 2008;21(2):93–111, quiz 189–90. doi:.https://doi.org/10.1016/j.echo.2007.11.011
38
Touboul
PJ
,
Hennerici
MG
,
Meairs
S
,
Adams
H
,
Amarenco
P
,
Bornstein
N
, et al.
Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23(1):75–80. doi:.https://doi.org/10.1159/000097034
39
Corrales
A
,
González-Juanatey
C
,
Peiró
ME
,
Blanco
R
,
Llorca
J
,
González-Gay
MA
. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: results of a population-based study. Ann Rheum Dis. 2014;73(4):722–7. doi:.https://doi.org/10.1136/annrheumdis-2012-203101
40
Hanley
JA
,
McNeil
BJ
. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi:.https://doi.org/10.1148/radiology.143.1.7063747
41
Hanley
JA
,
McNeil
BJ
. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839–43. doi:.https://doi.org/10.1148/radiology.148.3.6878708
42
Crowson
CS
,
Gabriel
SE
,
Semb
AG
,
van Riel
PLCM
,
Karpouzas
G
,
Dessein
PH
, et al.; Trans-Atlantic Cardiovascular Consortium for Rheumatoid Arthritis. Rheumatoid arthritis-specific cardiovascular risk scores are not superior to general risk scores: a validation analysis of patients from seven countries. Rheumatology (Oxford). 2017;56(7):1102–10. doi:.https://doi.org/10.1093/rheumatology/kex038
43
Ikdahl
E
,
Rollefstad
S
,
Olsen
IC
,
Kvien
TK
,
Hansen
IJ
,
Soldal
DM
, et al.
EULAR task force recommendations on annual cardiovascular risk assessment for patients with rheumatoid arthritis: an audit of the success of implementation in a rheumatology outpatient clinic. BioMed Res Int. 2015;2015:515280. doi:.https://doi.org/10.1155/2015/515280
44
Aviña-Zubieta
JA
,
Abrahamowicz
M
,
De Vera
MA
,
Choi
HK
,
Sayre
EC
,
Rahman
MM
, et al.
Immediate and past cumulative effects of oral glucocorticoids on the risk of acute myocardial infarction in rheumatoid arthritis: a population-based study. Rheumatology (Oxford). 2013;52(1):68–75. doi:.https://doi.org/10.1093/rheumatology/kes353
45
Prasad
M
,
Hermann
J
,
Gabriel
SE
,
Weyand
CM
,
Mulvagh
S
,
Mankad
R
, et al.
Cardiorheumatology: cardiac involvement in systemic rheumatic disease. Nat Rev Cardiol. 2015;12(3):168–76. doi:.https://doi.org/10.1038/nrcardio.2014.206
46
Pyörälä
K
,
De Backer
G
,
Graham
I
,
Poole-Wilson
P
,
Wood
D
. Prevention of coronary heart disease in clinical practice. Recommendations of the Task Force of the European Society of Cardiology, European Atherosclerosis Society and European Society of Hypertension. Eur Heart J. 1994;15(10):1300–31.
47
Woodward
M
,
Brindle
P
,
Tunstall-Pedoe
H
; SIGN group on risk estimation. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart. 2007;93(2):172–6. doi:.https://doi.org/10.1136/hrt.2006.108167
48
Hata
J
,
Nagai
A
,
Hirata
M
,
Kamatani
Y
,
Tamakoshi
A
,
Yamagata
Z
, et al.; Biobank Japan Cooperative Hospital Group. Risk prediction models for mortality in patients with cardiovascular disease: The BioBank Japan project. J Epidemiol. 2017;27(3):S71–6. doi:.https://doi.org/10.1016/j.je.2016.10.007
49
Ridker
PM
,
Paynter
NP
,
Rifai
N
,
Gaziano
JM
,
Cook
NR
. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118(22):2243–51, 4p, 2251. doi:.https://doi.org/10.1161/CIRCULATIONAHA.108.814251
50
Cooney
MT
,
Dudina
AL
,
Graham
IM
. Value and limitations of existing scores for the assessment of cardiovascular risk: a review for clinicians. J Am Coll Cardiol. 2009;54(14):1209–27. doi:.https://doi.org/10.1016/j.jacc.2009.07.020
51
Tournadre
A
,
Tatar
Z
,
Pereira
B
,
Chevreau
M
,
Gossec
L
,
Gaudin
P
, et al.
Application of the European Society of Cardiology, Adult Treatment Panel III and American College of Cardiology/American Heart Association guidelines for cardiovascular risk management in a French cohort of rheumatoid arthritis. Int J Cardiol. 2015;183:149–54. doi:.https://doi.org/10.1016/j.ijcard.2015.01.069
52
Galarza-Delgado
DA
,
Azpiri-Lopez
JR
,
Colunga-Pedraza
IJ
,
Cardenas-de la Garza
JA
,
Vera-Pineda
R
,
Serna-Peña
G
, et al.
Assessment of six cardiovascular risk calculators in Mexican mestizo patients with rheumatoid arthritis according to the EULAR 2015/2016 recommendations for cardiovascular risk management. Clin Rheumatol. 2017;36(6):1387–93. doi:.https://doi.org/10.1007/s10067-017-3551-7
53
Ozen
G
,
Sunbul
M
,
Atagunduz
P
,
Direskeneli
H
,
Tigen
K
,
Inanc
N
. The 2013 ACC/AHA 10-year atherosclerotic cardiovascular disease risk index is better than SCORE and QRisk II in rheumatoid arthritis: is it enough?
Rheumatology (Oxford). 2016;55(3):513–22.
54
Roman
MJ
,
Moeller
E
,
Davis
A
,
Paget
SA
,
Crow
MK
,
Lockshin
MD
, et al.
Preclinical carotid atherosclerosis in patients with rheumatoid arthritis. Ann Intern Med. 2006;144(4):249–56. doi:.https://doi.org/10.7326/0003-4819-144-4-200602210-00006
55
Stamatelopoulos
KS
,
Kitas
GD
,
Papamichael
CM
,
Chryssohoou
E
,
Kyrkou
K
,
Georgiopoulos
G
, et al.
Atherosclerosis in rheumatoid arthritis versus diabetes: a comparative study. Arterioscler Thromb Vasc Biol. 2009;29(10):1702–8. doi:.https://doi.org/10.1161/ATVBAHA.109.190108
56
Vandhuick
T
,
Allanore
Y
,
Borderie
D
,
Louvel
JP
,
Fardellone
P
,
Dieudé
P
, et al.
Early phase clinical and biological markers associated with subclinical atherosclerosis measured at 7 years of evolution in an early inflammatory arthritis cohort. Clin Exp Rheumatol. 2016;34(1):58–67.
57
Kerekes
G
,
Soltész
P
,
Nurmohamed
MT
,
Gonzalez-Gay
MA
,
Turiel
M
,
Végh
E
, et al.
Validated methods for assessment of subclinical atherosclerosis in rheumatology. Nat Rev Rheumatol. 2012;8(4):224–34. doi:.https://doi.org/10.1038/nrrheum.2012.16
58
Gonzalez-Gay
MA
,
Gonzalez-Juanatey
C
,
Vazquez-Rodriguez
TR
,
Martin
J
,
Llorca
J
. Endothelial dysfunction, carotid intima-media thickness, and accelerated atherosclerosis in rheumatoid arthritis. Semin Arthritis Rheum. 2008;38(2):67–70. doi:.https://doi.org/10.1016/j.semarthrit.2008.02.001
59
Nambi
V
,
Chambless
L
,
Folsom
AR
,
He
M
,
Hu
Y
,
Mosley
T
, et al.
Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55(15):1600–7. doi:.https://doi.org/10.1016/j.jacc.2009.11.075
60
Salonen
R
,
Salonen
JT
. Determinants of carotid intima-media thickness: a population-based ultrasonography study in eastern Finnish men. J Intern Med. 1991;229(3):225–31. doi:.https://doi.org/10.1111/j.1365-2796.1991.tb00336.x
61
Howard
G
,
Burke
GL
,
Evans
GW
,
Crouse
JR, 3rd
,
Riley
W
,
Arnett
D
, et al.
Relations of intimal-medial thickness among sites within the carotid artery as evaluated by B-mode ultrasound. ARIC Investigators. Atherosclerosis Risk in Communities. Stroke. 1994;25(8):1581–7. doi:.https://doi.org/10.1161/01.STR.25.8.1581
62
de Groot
E
,
van Leuven
SI
,
Duivenvoorden
R
,
Meuwese
MC
,
Akdim
F
,
Bots
ML
, et al.
Measurement of carotid intima-media thickness to assess progression and regression of atherosclerosis. Nat Clin Pract Cardiovasc Med. 2008;5(5):280–8. doi:.https://doi.org/10.1038/ncpcardio1163
63
Kooter
AJ
,
Kostense
PJ
,
Groenewold
J
,
Thijs
A
,
Sattar
N
,
Smulders
YM
. Integrating information from novel risk factors with calculated risks: the critical impact of risk factor prevalence. Circulation. 2011;124(6):741–5. doi:.https://doi.org/10.1161/CIRCULATIONAHA.111.035725
64
Dessein
PH
,
Corrales
A
,
Lopez-Mejias
R
,
Solomon
A
,
Woodiwiss
AJ
,
Llorca
J
, et al.
The Framingham Score and the Systematic Coronary Risk Evaluation at Low Cutoff Values Are Useful Surrogate Markers of High-risk Subclinical Atherosclerosis in Patients with Rheumatoid Arthritis. J Rheumatol. 2016;43(3):486–94. doi:.https://doi.org/10.3899/jrheum.150510
65
Wahlin
B
,
Meedt
T
,
Jonsson
F
,
Henein
MY
,
Wållberg-Jonsson
S
. Coronary Artery Calcification Is Related to Inflammation in Rheumatoid Arthritis: A Long-Term Follow-Up Study. BioMed Res Int. 2016;2016:1261582. doi:.https://doi.org/10.1155/2016/1261582