The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis
DOI: https://doi.org/10.4414/smw.2018.14661
Christoph T.
Bergera, Gregor
Sommerb, Markus
Aschwandenc, Daniel
Staubc, Christof
Rottenburgerb, Thomas
Daikelerd
aTranslational Immunology and Medical Outpatient Clinic, Departments of Biomedicine and Internal Medicine, University Basel and University Hospital Basel,
Switzerland
bDepartment of Radiology and Nuclear Medicine, University Hospital Basel, Switzerland
cDepartment of Angiology, University Hospital Basel, Switzerland
dDepartment of Rheumatology, University Hospital Basel, Switzerland
Summary
Historically, giant cell arteritis (GCA) was considered to be synonymous with temporal arteritis. However, the disease spectrum of GCA extends much further, and includes vasculitis of the aorta and its branches with or without involvement of the temporal arteries. Imaging is crucial for the diagnosis and follow-up of GCA patients. Large vessel GCA (LV-GCA) often presents as an inflammatory syndrome and is only detected by imaging modalities such as: colour duplex sonography (CDS), computed tomography (CT) / CT angiography (CTA), magnetic resonance imaging (MRI) or 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) / CT. Deciding which imaging modality to use in different clinical situations remains a matter of debate. CDS and MRI enable assessment of the temporal arteries with a presumably higher sensitivity than histology. In the context of a typical presentation, CDS can replace a biopsy. In about a third of patients, the temporal arteries are not involved, thus PET/CT, MRI, CT, or CDS of larger arteries is needed to diagnose GCA. The sensitivity of all modalities is affected by glucocorticoid therapy. Therefore, without delaying therapy, imaging should be performed within a few days of treatment initiation. The use of PET/CT for the work-up of inflammatory syndromes in the elderly reveals vasculitis in approximately 20% of examined patients and uncover relevant diagnoses in the majority of remaining patients. The aorta should be routinely assessed in all GCA patients at diagnosis and during follow-up. MRA or CTA are best suited to characterise structural damage of larger arteries. The role of imaging in monitoring GCA disease activity still needs to be further defined.
The disease spectrum of giant cell arteritis
Giant cell arteritis (GCA) is a vasculitis that predominantly affects the medium-sized and larger arteries. It occurs exclusively in people older than 50 years with incidence rates in western countries of around 6.9–32.4/100,000 [1]. With the continuously aging population, GCA is predicted to become a substantial health issue in the coming decades [1]. Typically, the disease manifests with a temporal headache, an inflammatory syndrome, and with the variable presence of polymyalgia and ischaemic symptoms due to vessel occlusion. In recent years, it was further appreciated that disease manifestations could be more variable. Specifically, about a third of large-vessel GCA (LV-GCA) patients have no sonographic evidence of temporal vasculitis [2], and more than half have normal histology of the temporal artery [3]. Moreover, a subset of patients presents clinically with an isolated systemic inflammatory syndrome due to inflammation of large extracranial vessels [3, 4]. Some authors distinguish such LV-GCA from the classical cranial GCA (cGCA). The existence of an LV-GCA subgroup was already proposed almost two decades ago, on the basis of angiographic studies detecting large vessel vasculitis in patients with suspected polymyalgia rheumatica [5]. Indeed, 30 to 80% of patients with biopsy-confirmed GCA have evidence of large-vessel involvement [2, 5–11]. However, many of these studies were not designed to systematically assess the incidence of large-vessel involvement in GCA, and were largely focused on biopsy-proven GCA, thereby introducing a potential selection bias. The disease incidence of “true” GCA and LV-GCA, therefore, remains uncertain [3].
The American College of Rheumatology (ACR) criteria commonly used to classify GCA are strongly focused on temporal vasculitis: both a clinically and histologically pathological temporal artery yield one point out of three needed. The others are age >50 years, an erythrocyte sedimentation rate (ESR) ≥40 mm/h, and new onset headache [12]. Since LV-GCA patients without temporal artery involvement less frequently complain of headaches, the ACR criteria inherently miss patients with extracranial LV-GCA [3]. In extreme cases, LV-GCA presents with inflammation only. This was acknowledged by the diagnosis and classification of vasculitis (DCVAS) study group, which is currently trying to establish, for the first time, diagnostic criteria for primary vasculitides [13]. In fact, patients with imaging-confirmed large-vessel vasculitis that did not meet the ACR criteria have been included in large prospective trials on GCA, namely the GiACTA trial [14]. The larger the predominantly involved vessels are, the more unlikely it is that critical stenosis and ischaemic symptoms will occur. Therefore, LV-GCA patients have jaw claudication less frequently and are at lower risk for the ischaemic complications of GCA – anterior ischaemic optic neuropathy. They do, however, experience more relapses and hence are exposed to higher glucocorticoid doses and need more steroid-sparing agents [3, 5]. Diagnosis in these cases is often delayed because of the nonspecific presentation. Hence, raising awareness of LV-GCA as a frequent cause of inflammation of unknown origin in the elderly is relevant, since ischaemic events still occur and vision loss in elderly patients significantly impacts their independence and quality of life. A careful clinical examination with bilateral blood pressure measurements and auscultation for bruits over the large arteries may point to the diagnosis earlier, but it has low sensitivity for GCA [3]. Currently, cGCA and LV-GCA are considered different spectrums of the same disease; thus, both are treated with systemic glucocorticoids. In cases of relapse, patients are additionally treated with the interleukin (IL)6-receptor blocker tocilizumab (Actemra®). Tocilizumab recently has been proven to be highly effective in preventing relapses and reducing steroid doses to maintain remission [14, 15].
The purpose of this review is to discuss the current state of imaging in GCA and to assign different imaging modalities their respective roles in the diagnosis and follow-up of GCA.
What are the imaging correlates of vasculitis?
An inflamed artery shows vessel wall thickening due to inflammation-related cellular influx, oedema, and deposition of extracellular matrix. Additionally, chronic inflammation results in intimal proliferation and subsequent stenosis of the vessel lumen [16]. The different available imaging modalities probably visualise variable aspects of these processes.
Colour duplex sonography
Colour duplex sonography (CDS) assesses the vascular wall anatomy as well as the lumen and it allows the measurement of blood flow. Vasculitis of the temporal arteries shows as homogenous, typically hypoechogenic (dark), circumferential vessel wall thickening. At the temporal arteries, this finding is referred to as the “halo sign”, because of the halo-like appearance around the coloured lumen in cross-sections (fig. 1A) [17]. We recently established the “compression sign”: when applying transducer-imposed pressure on the temporal arteries, a non-vasculitic artery can be compressed and vanishes on the B-mode image (fig. 1B, C). In contrast, an artery with vasculitis-associated wall thickening is not fully compressible and remains visible under compression (fig. 1D, E). The compression sign relies on B-mode ultrasound only, making it less examiner- and device-dependent. Nonetheless, it still has a sensitivity and specificity comparable to the halo sign [18]. As with temporal arteries, vasculitis of large arteries (e.g., axillary, carotid) also presents as circumferential homogenous hypoechogenic wall thickening (fig. 2A). Stenosis, or even occlusion, can be present. In lower limb arteries an echo-lucent ribbon within the thickened wall can be another sign of vasculitis [7]. In contrast to vasculitis, advanced arteriosclerosis shows more heterogeneous, eccentric, irregular plaques, typically with acoustic shadowing mostly in the carotid and femoral and popliteal arteries (fig. 2B).
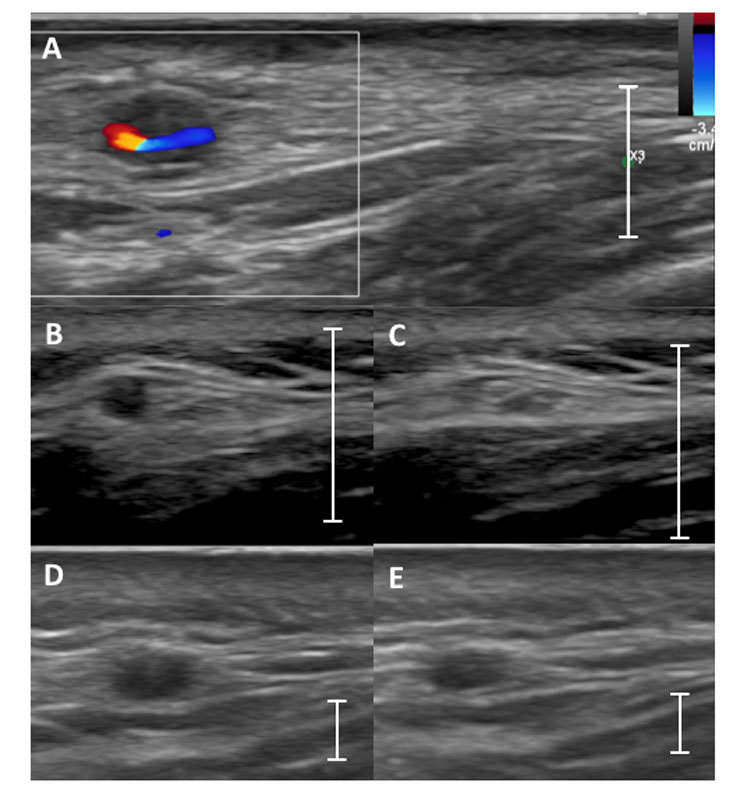
Figure 1
Typical aspect of vasculitis in temporal arteries. (A) Example of the halo sign: a hypoechoic vessel wall around the colour-filled remaining lumen in colour Doppler mode. (B) A noninflamed frontal temporal artery without compression that with (C) a complete lack of visibility of the artery wall upon compression in B-mode = negative compression sign. (D) A temporal artery with vasculitis with a positive compression sign: frontal temporal artery without compression (D) and remaining visibility of the artery upon compression (E) in B-mode is shown. The line indicates the scale of 1 cm.
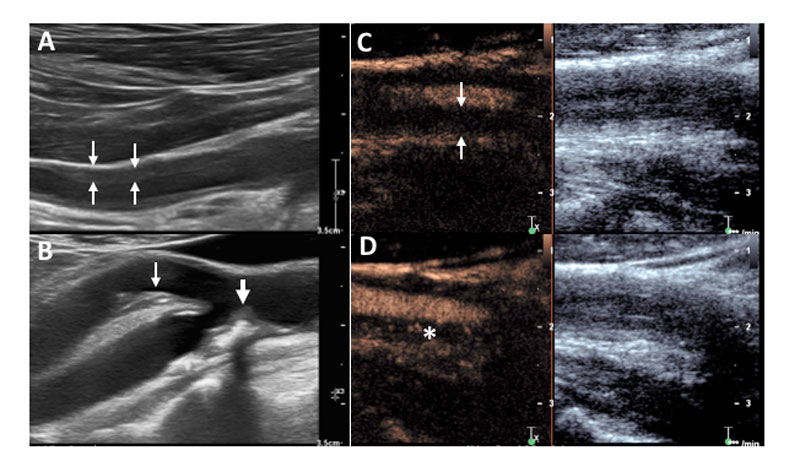
Figure 2
Large vessel findings in colour duplex sonography (CDS) and contrast-enhanced ultrasound. (A) Vasculitis of an axillary artery with homogeneous, typically circumferential, hypoechogenic vascular wall thickening in CDS. (B) Arteriosclerosis in the carotid arteries showing irregularly shaped, eccentric hypo- and hyperechogenic (with acoustic shadowing) plaques in the internal (thick arrow) and external (thin arrow) carotid artery. (C, D) Common carotid artery in another patient with large-vessel vasculitis showing strong hypervascularisation of the vessel wall. (C) Enhanced vessel lumen without contrast bubbles within the thickened vessel wall (arrows) immediately after destroying all bubbles by a so called “flush”. (D) Reappearance of the contrast bubbles within the arterial wall (asterisk) indicates hyperaemia/vascularisation.
Efforts to standardise diagnosis by CDS have recently been proposed. Intima-media thickness cut-off values for vasculitis have been suggested for the temporal and axillary artery. However, this analysis technique has a predictive value similar to that of pure qualitative interpretation [19]. Vasculitis lesions are often segmental. Moreover, there is substantial variation in vessel diameter amongst healthy subjects. We therefore currently refrain from using such measurements, as more studies are needed to validate the findings outside of a study setting.
Contrast-enhanced ultrasound (CEUS) complements and enhances standard CDS, particularly in the setting of inflammatory vascular diseases [20]. Ultrasound contrast agents contain gas-filled microbubbles. After intravenous injection, the microbubbles distribute in the vascular system down to the capillary perfusion level. By using contrast specific ultrasound imaging modalities, their physical properties make them readily detectable in the microcirculation and allow demonstration of hypervascularisation and hyperaemia in the inflamed vessel wall (fig. 2C, D) [21].
Contrast enhanced computed tomography (CT) and CT-angiography
Multislice-detector CT (CT) is a robust and frequently used imaging method for acute (aortic rupture, dissection, inflammation, embolism) and chronic (atherosclerosis, stenosis, aneurysms) vascular pathologies. Endovascular injection of contrast agents renders even small vascular structures visible. CT is the fastest of all cross-sectional imaging methods and can provide coverage from the skull to the upper thigh in less than one minute, depending on the CT machine. Breath-holding, in the order of 10 seconds, and electrocardiographic gating are required to achieve optimal quality for the thoracic aorta and in particular the aortic root [22]. For the diagnosis of vasculitis, CT-angiography (CTA) has to be differentiated from contrast-enhanced CT during parenchymal (venous or portal venous) contrast phases. CTA depicts the vessel lumen using endoluminal contrast agents and a defined temporal acquisition window. Hence, CTA is best for visualising changes in the vascular lumen such as an irregular contour, stenosis, or aneurysms. Parenchymal CT, on the other hand, is performed with a specific delay after a contrast bolus (preferably in the venous phase approximately 50 seconds after contrast injection) to allow passage through tissues. The principal criterion for large vessel vasculitis on parenchymal CT is a concentric thickening of the vessel wall, typically with late contrast enhancement and presence of vascular wall oedema. Cut-offs of ≥2 mm for the aorta and >1 mm for its branches are often used [10]. A case-control study including 64 GCA patients recently reported 98% specificity for an aortic thickness >2.2 mm [23]. Since histological correlation studies are virtually impossible, such cut-offs remain a matter of debate. Optimal diagnostic results can be obtained when CTA and CT in the venous phase are combined [24].
Magnetic resonance imaging (MRI) and MR-angiography
MRI also has an established role in the diagnosis of cardiovascular disease, including the assessment of large and peripheral vessels. Current protocols for MRI of LVV commonly take 30 to 45 minutes and consist of three or four types of sequences [24]:
- The T2-weighted fast-spin echo displays oedema in the vessel wall as a hyperintense rim (fig. 3A). This method is highly sensitive to inflammatory changes, but also prone to artefacts from flow and pulsation, potentially providing a false diagnosis of LVV.
- The T1-weighted imaging with fat suppression (preferably using the Dixon technique) before and after intravenous injection of gadolinium (Gd)-based contrast agents (fig. 3B), simultaneously detects thickening and contrast enhancement of the vessel wall. Modern scanners can run this sequence with full 3D coverage of the chest or abdomen within one breath-hold for each region [25]. A limitation of the two mentioned non-ECG-gated sequences is degraded image quality in the ascending aorta by pulsation artefacts. These artefacts are decreased by
- using an ECG-triggered balanced steady-state free-precession (bSSFP) sequence, initially developed for cardiac MRI. This sequence has little susceptibility to contrast agents but has a higher resolution for morphological changes of the vessel wall.
- Finally, MR-angiography (MRA), like CTA, can identify vasculitic arterial segments by their irregular outline and diameter changes (fig. 3C, D). MRA may be performed either using T1-weighted imaging and Gd-based contrast agents or in a non-contrast-enhanced fashion using ECG and respiratory triggered 3D bSSFP when contrast agent toxicity is an issue.
MRI can also be used to visualise vasculitis of the temporal arteries, especially if a 3 Tesla scanner with higher signal and spatial resolution is available [26].
FDG PET/CT to visualise hypermetabolism in inflamed arteries
Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) is a nuclear medicine technique used to detect increased glucose metabolism anywhere in the body, as it enables whole-body imaging in a single session. Currently, PET is almost exclusively combined with CT (PET/CT) to assess the morphology of the FDG positive area. FDG is a glucose analogue with a positron-emitting radionuclide fluorine-18 introduced into the glucose molecule. FDG shows increased uptake in metabolically active cells, such as brain or heart muscle, but also malignant cells and activated leucocytes [27]. The mechanism of increased FDG uptake in the latter is increased glycolysis and greater activation-induced surface expression of glucose transporters (the Na+-dependent glucose transporters [GLUT]) [28].
Increased FDG uptake in the vessel wall is the hall-mark of vasculitis in PET (figs 3 and 4
). Uptake can be graded visually, by semiquantitative analysis, or using standardised uptake values (SUVs) [29–31]. In general, visual vascular uptake higher than tracer uptake in the liver is considered suspicious for large-vessel vasculitis [30]. Furthermore, a smooth linear or long segmental pattern of FDG uptake in the aorta and its main branches is a characteristic pattern of GCA [31–33]. Differentiation between vasculitis and atherosclerosis remains a challenge with PET/CT [31, 32]. The discrimination is mostly based on qualitative assessments; hence it is strongly dependent on the experience of the reader. Vasculitic vessel wall changes are more homogeneous, linear and without calcification (fig. 4A). In contrast, arteriosclerotic lesions are marked by calcifications, a patchy distribution, or minor vessel wall pathologies at predilection sites of arteriosclerosis. As a result of the high arteriosclerotic load in lower limbs arteries, enhanced tracer uptake in this area has lower specificity than in the aortic branches [34].
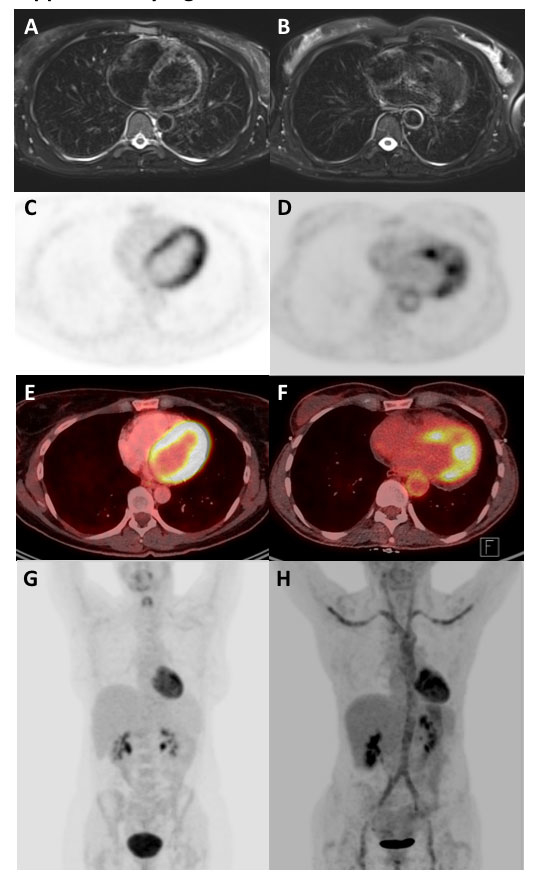
Figure 3
Increased fluorodeoxyglucose (FDG) uptake in the large arteries of a patient with untreated giant cell arteritis. (A) Maximum intensity projection gives an overview including comparison to the liver FDG uptake. Axial positron emission tomography / computed tomography fusion images showing (B) FDG uptake in the vertebral arteries, and (C) FDG uptake in external and internal iliac arteries.
Several semiquantitative methods have been reported. A four-grade scale based on the visual grading of the vascular vs liver uptake was introduced in 2003: grade 0 = no uptake; grade 1 = uptake lower than liver; grade 2 = similar to the liver; and grade 3 = higher than liver [35]. In untreated patients, grades 2–3 are relatively specific for vasculitis, whereas grade 1 (rarely 2) were observed in atherosclerotic vessels, particularly in the thoracic aorta. Alternatively, vessel-to-organ SUV ratios, such as an aorta-to-liver SUVmax ratio, have been used to quantify tracer uptake [36]. Such standardisations allow cut-off ratios for the diagnosis of GCA to be defined and provide robustness as an observer-independent method. We recently evaluated various SUV ratios in different vascular territories and found that a ratio of the SUVmax of the supra-aortic arteries divided by mean liver values enabled the best discrimination between GCA and non-GCA patients [37].
Examples of PET and MRI findings in patients with vasculitis compared to normal findings of healthy controls are shown in supplementary figure S1 in appendix 1.
The role of imaging in diagnosing GCA
In the absence of a disease-specific diagnostic biomarker and given that the invasive temporal artery biopsy is diagnostic in only 50% of patients, imaging is essential for diagnosing GCA [38, 39]. Imaging of the cranial arteries, and specifically the temporal arteries, has to be distinguished from intrathoracic vessel imaging. Moreover, imaging for the diagnosis (presence of an inflamed artery) can be different from imaging to detect vascular complications. This section discusses how the different imaging techniques can be applied to answer specific clinical questions.
Imaging of the temporal arteries
Early and swift diagnosis of GCA is critical to avoid ischaemic complications. The European League against Rheumatism (EULAR) recommends an early imaging test to confirm GCA but it also stresses that treatment should not be delayed for further diagnostic testing [24]. Ischaemic complications are more frequent in patients with temporal arteritis than in those with LV-GCA [40]. Therefore, given its broad availability and the good performance of the “compression” and “halo” signs, diagnosing vasculitis of the temporal arteries is the domain of CDS. CDS has been extensively studied (an overview of all studies is available in [41]). The performance of the halo sign in diagnosing GCA has specificities ranging from 78 to 100% and sensitivities varying from 55 to 100% [17, 42]. In a very recent meta-analysis, pooled sensitivities and specificities for GCA diagnosis using CDS were 77% (95% confidence interval [CI] 62–87%) and 96% (95% CI 85–99%), respectively, relative to the clinical diagnosis [41]. The wide range in sensitivity is largely operator dependent, but it is also affected by patient selection (cGCA or LV-GCA). In cohorts with a high rate of LV-GCA, sensitivity is lower, given that the temporal arteries are less frequently affected in this group. The compression sign is easier to perform, with a sensitivity of 75–79% and a specificity of 100%. Even if performed as a bedside test by a rheumatologist without experience in vascular ultrasound, compression sign specificity and sensitivity are comparable to those of the halo sign [18, 43]. In a prospective study of 381 GCA patients, CDS of temporal arteries was directly compared with histology. CDS had a better sensitivity (54 vs 39%) but a lower specificity (81 vs 100%) [44]. CDS-guided temporal artery biopsy (i.e., marking the site to biopsy) does not increase the diagnostic yield of a biopsy and may not be needed before temporal artery biopsy [45]. Common to all imaging modalities, the halo and compression signs can become negative during glucocorticoid therapy. Diagnostic tests should, therefore, be completed as quickly as possible [24]. EULAR recommends CDS as the first examination to diagnose temporal arteritis [24]. In patients with a higher pre-test probability for GCA, vasculitic CDS findings increase the specificity for diagnosing GCA further (81.8%) [7, 43, 46]. In such cases, the temporal artery biopsy can be omitted [24].
High-resolution MRI can also be used to image the temporal arteries [47, 48]. However, immediate access to MRI is often limited, and if combined with large-vessel MRI to assess the full extent of the disease, two separate examinations are needed. Pooled data on more than 500 patients yielded a 73% sensitivity and 88% specificity of MRI for detecting arteritis in the temporal or occipital arteries, with the clinical diagnosis as the comparator [41]. One advantage of MRI over CDS may be visualisation of the occipital branch of the temporal artery, which is not routinely evaluated with CDS. MRI of the temporal arteries has been included in the recent EULAR recommendations for imaging in GCA as a second-best alternative to CDS to assess the cranial arteries [24].
Imaging for large-vessel involvement
In addition to the temporal arteries, CDS can also access other frequently affected vessels in GCA, namely the carotid, subclavian and axillary arteries, and the vertebral artery. Several studies demonstrated an increased diagnostic yield of CDS when the axillary or the axillary and subclavian arteries were assessed in addition to the temporal arteries [2, 46]. If ultrasound is used, the frequency of subjects with vasculitic findings in large vessels ranges from 29 to 55%, depending on the number of evaluated vascular regions [2, 7, 9]. CDS also allows investigation of the abdominal aorta, pelvic arteries and arteries of the lower extremities, which are affected in 29% of the patients and may rarely be the sole finding in LV-GCA [7].
Aortitis is also frequent in GCA [11, 23, 49], and cases of isolated ascending aortitis might represent a subtype in the spectrum of LV-GCA [50]. The thoracic aorta is inaccessible to CDS. Therefore, contrast-enhanced CT, MRI, or PET/CT are used to assess the aorta.
CT and CTA have a reported sensitivity of 73% and a specificity of 78% for the diagnosis of LV-GCA [51]. Pathologies of the vessel wall or lumen of the larger arteries occur in approximately two thirds of patients with newly diagnosed GCA (67.5%) [6, 10]. These mostly involve the aorta (65%), the brachiocephalic trunk (48%), the subclavian arteries (43%), the carotid arteries (35%) and the femoral arteries (30%) [10]. Dilation of the thoracic aorta is observed in about 15 to 23% of the patients [6, 10, 11]. Patients with aortitis at diagnosis are at risk for developing aortic aneurysms [11].
MRI is often used interchangeably with CT to diagnose large-vessel involvement. The ability to combine the morphological assessment of the vasculature by MRA with tissue characterisation including the detection of oedema (using T2-weighting), contrast agent uptake (using T1-weighting) and fibrosis (using late gadolinium enhancement) is one of the advantages of MRI. However, studies on the diagnostic accuracy of large-vessel MRI are sparse.
FDG-PET/CT assesses all vascular territories in the same examination and allows the exclusion or inclusion of various differential diagnoses of GCA (table 1). Importantly, detection of aortitis on FDG PET/CT is associated with an increased risk of subsequent aortic complications, thus yielding useful outcome-relevant information [52]. Initial findings from a small case series reported vascular hypermetabolism in 29 of 35 patients with GCA [8]. A case-control study on 32 biopsy-proven GCA patients found a sensitivity of 80% and specificity of 79% [10]. These performance marks were slightly lower than those from two recent meta-analyses [53, 54]. The first, which compared 170 patients with GCA or Takayasu arteritis to 230 controls, showed a sensitivity of 83% and specificity of 90% for GCA [53]. The second meta-analysis, which included four studies with GCA, showed a very high pooled sensitivity (90%) and specificity (98%) for FDG-PET [54]. We recently demonstrated that quantitative analysis comparing the FDG uptake ratio (maximal FGD uptake in a vessel region [SUVmax] with the liver SUV) was superior to visual scoring. This was done in a “real-life” cohort of patients with suspected GCA. A cut-off ratio of 1.0 yielded a sensitivity of 71% and a specificity of 91% for vasculitis of the supra-aortic vessels; however, specificity for the aorta and the infra-aortic vessels was lower [37]. FDG uptake is reduced after initiation of glucocorticoid therapy. Thus, a possible decline of the sensitivity of FDG-PET/CT soon after the onset of immunosuppressive treatment must be considered, and examinations should be done as fast as possible [37, 55, 56]. PET/CT can also be used to exclude LV-GCA in patients with clinically isolated polymyalgia rheumatica refractory to standard therapy [29], as up to 20% of refractory polymyalgia rheumatica cases have evidence of vasculitis in PET [57]. Moreover, for findings such as shoulder and iliopectineal bursitis, FDG uptake between spinal spinous processes and around the ischial tuberosities are correlates of polymyalgia rheumatica in PET [29, 57–59].
Table 1 Differential diagnostic challenges in diagnosing giant cell arteritis.
|
CRP
ESR
|
General symptoms*
|
Myalgia
|
Cranial symptoms†
|
Headache
|
| Giant cell arteritis |
+ |
+ |
+ |
+ |
+ |
| Polymyalgia rheumatica |
+ |
+ |
+ |
- |
- |
| Malignancy |
(+) |
+ |
(+) |
- |
(+) |
| Infections |
+ |
+ |
+ |
- |
(+) |
| Autoimmune disease (RA, SLE, Sjögrens…) |
+ |
+ |
+ |
- |
(+) |
| Haematological/myeloma |
-/+ |
+ |
+ |
- |
- |
| Tuberculosis |
+ |
+ |
(+) |
- |
(+) |
CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; RA = rheumatoid arthritis; SLE = systemic lupus erythematosus
* Fatigue, weight loss, fever
† Vision loss, jaw claudication |
FDG-PET/CT as a key diagnostic tool in elderly patients with inflammatory syndromes
FDG-PET/CT plays a particular role in the diagnostic workup of elderly patients with inflammation or fever of unknown origin (IUO or FUO) [60]. The underlying cause of both conditions ranges from malignancy to infectious and autoimmune diseases, and are quite similar in patients with either IUO or FUO [61]. Many of these causes, including LV-GCA, can be identified on FDG-PET/CT, making it a valuable method to screen for the cause of inflammation, if the initial clinical workup was negative [4]. In a prospective PET scan study on elderly IUO patients (>50 years, elevated ESR ≥50 mm/h, nonspecific complaints and a prior protocolled work-up including chest x-ray, abdominal ultrasound and electrophoresis), autoinflammatory disease (43%, 25/58) and specifically large-vessel vasculitis (24%, 14/58) was the most common finding [62]. Similarly, a recent meta-analysis identified abnormal PET findings in one third of FUO patients [63]. In this setting, the most frequent subsequent diagnosis was infection (42%), followed by noninfectious inflammatory disease including large-vessel vasculitis (33%), and malignancy (17%). In a meta-analysis study, pooled sensitivity and specificity of PET/CT in FUO were reported with 98% and 86%, respectively [64].
Thus, after initial clinical assessment, FDG PET/CT is an excellent examination tool to start the diagnostic process by locating suspicious lesions and guiding subsequent focused diagnostic approaches with higher specificity. Although expensive, FDG PET/CT is cost-effective in the work-up of patients with IUO, as the number of diagnostic procedures, as well as the cost of hospitalisation, could be reduced [65]. The negative predictive value of FDG PET/CT is high: focal disease could be correctly identified or excluded in approximately 90% of patients [61]. A negative FDG PET/CT, performed after the initial workup and exclusion of non-focal systemic diseases, therefore justifies a watch-and-wait strategy and obviates the need for further investigations [66].
Challenges in choosing the best modality for large-vessel imaging
The diagnostic accuracy of different imaging techniques used in LV-GCA has been independently investigated in different settings (various patient populations, treatment durations, etc.). Only a few comparative studies of different modalities have been published to date [49, 51, 67, 68] and a benchmark is lacking since histological confirmation, as the “gold standard”, is rarely available [69]. Consequently, the 2018 EULAR recommendations for imaging in large-vessel vasculitis propose CDS, PET/CT, CT or MRI as equivalent possible methods for the detection of GCA-associated changes in extracranial arteries [24]. Thus, personal preference, local expertise and the reimbursement situation often drive the decision of what is used. There are some obvious advantages and disadvantages to specific modalities (table 2). In the EULAR recommendation, CDS is recommended as the first-line method for screening the supra-aortic arteries in patients with suspected predominantly cGCA (fig. 5). In the event of negative or inconclusive findings in the temporal arteries, this can be complemented with axillary ultrasound in the same session. The advantage of CDS is its availability and the absence of radiation and nephrotoxic contrast agents. CDS, however, also has several disadvantages that include: (i) the lack of evaluation of the thoracic aorta and the very proximal part of the supra-aortic arteries, (ii) its operator dependency, and (iii) limited information on inflammatory activity. Therefore, in ambiguous cases, CDS is often complemented with imaging of the large vessels, particularly in patients with arteries that appear normal on CDS (fig. 5).
Table 2 Comparison of the different diagnostic imaging modalities.
|
Modality
|
Typical findings
|
Advantage
|
Disadvantage
|
| CDS |
• Hypoechogenic wall thickening
• Halo sign
• Compression sign |
• Good availability
• High resolution
• Patient can see results
• No radiation
• No nephrotoxic contrast agents
• Low costs
• Dynamic examination (blood flow) |
• Operator-dependent
• No imaging of thoracic aorta and the very proximal part of the supra-aortic arteries
• Limited information on inflammatory activity |
| FDG-PET CT |
• Homogenous 18-FDG uptake in the vessel wall
• Signs of PMR (interspinous uptake, bursitis)
• Activated bone marrow |
• Shows metabolic activity
• Broad detection of differential diagnoses including tumours, occult infection and PMR |
• Availability
• Costs
• Time consuming
• Radiation |
| MRI/MR-A |
• Thickened artery wall
• Contrast uptake
• Ectasia of the aorta, stenosis of aortic branches |
• No radiation
• Combined assessment of inflammation and morphological changes |
• Contraindications (some implants, claustrophobia)
• Time consuming; |
| CT/CT-A |
• Thickened artery wall
• Late contrast uptake
• Ectasia of the aorta, stenosis of aortic branches |
• Fast
• Combined assessment of inflammation and morphological changes
• Good differential diagnosis |
• Radiation
• Nephrotoxic contrast agents |
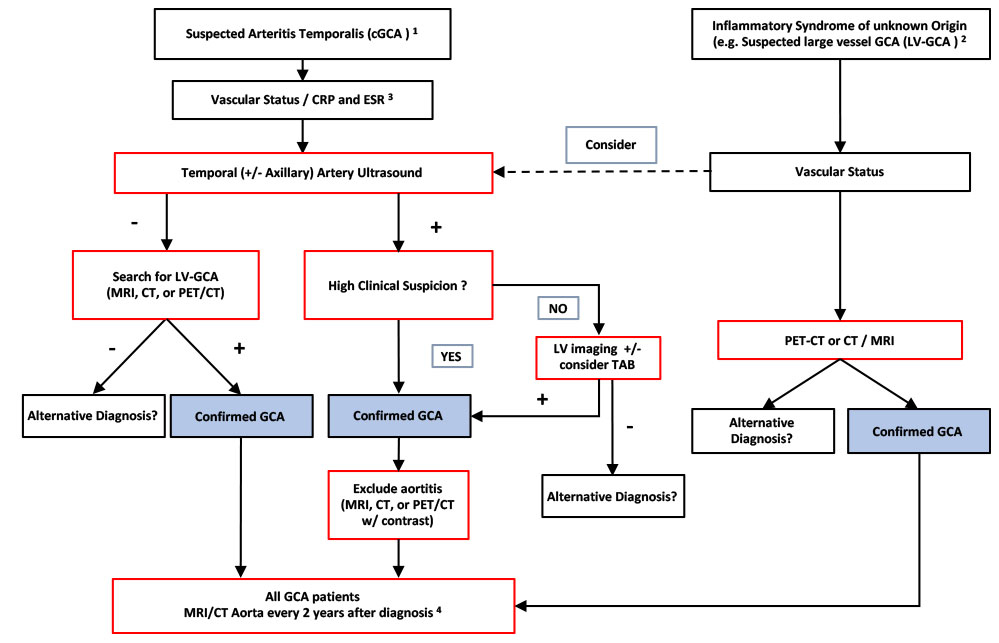
Figure 4
Local imaging strategy in patients with suspected cranial or and large-vessel giant cell arteritis at the University Hospital Basel. As highlighted by the European League against Rheumatism recommendations, temporal artery biopsy (TAB) can be replaced by imaging and the modality of imaging of the large-vessels should be chosen based on local expertise and availability [24]. (1) Cranial giant cell arteritis (cGCA) should be suspected if at least one of the following symptoms is present: new headache, vision loss, jaw claudication, suspicious temporal arteries (hard, painful or pulseless). (2) Suspected large-vessel GCA (LV-GCA) includes patients with fever or inflammation of unknown origin, arm/leg claudication (with inflammation or polymyalgia rheumatica [PMR]), and for the algorithm, absence of the symptoms listed in (1), except headache. (3) Vascular status should include auscultation of the large arteries for bruits; palpation of the peripheral pulses; blood pressure measurements on both arms; and lab tests should include at least C-reactive protein (CRP) / erythrocyte sedimentation rate (ESR). (4) If aortic aneurysm or large vessels stenosis is detected during initial or follow-up screen, follow-up imaging frequency has to be decided on an individual basis.
CT = computed tomography; MRI = magnetic resonance imaging; PET = positron emission tomography
CT and PET/CT further contribute to the diagnostic workup by detecting or excluding essential differential diagnoses, such as malignancy, granulomatous disease and infectious disease. Both have limitations related to radiation exposure and the toxicity of iodine-based contrast agents. This can be relevant if the examinations are frequently performed, as in the context of aneurysm screening and follow-up. In this setting, MRA allows potential vascular complications to be assessed at high resolution without the radiation exposure of CTA.
To date, very few data are available for a head-to-head comparison of different imaging modalities. In a small cohort of 24 patients with suspected GCA, PET/CT outperformed CTA (without contrast-enhanced venous phase) in terms of specificity (100 vs 84.7%) with comparable sensitivity (66.7 vs 73.3%) [51]. A small study in a temporal artery biopsy-positive cohort (15 GCA patients, 9 non-GCA patients) compared PET and CTA [49]. PET was slightly superior to CTA, owing to false positive findings with CTA, resulting in a lower positive predictive value [49]. Others also found comparable performance of PET/CT and CTA, with again a slightly better performance of PET/CT, as it was more sensitive in detecting inflammation in a given segment [70]. In summary, PET/CT slightly outperforms CTA for initial GCA diagnosis, but the two techniques show good concordance.
By contrast, in an observational study of LV-GCA patients, 15/46 had vertebral artery vasculitis as defined by FDG-PET positivity. CDS was performed on all vertebral arteries but showed poor agreement with the PET findings (halo in 5/15 of the PET-positive subjects and 3/31 vertebral artery PET-negative patients) [67]. Comparable discrepancies between different modalities have been reported for MRI vs FDG-PET on 84 patients with large-vessel vasculitis (35 GCA / 30 Takayasu arteritis / 19 controls). The agreement regarding disease extent (i.e. involved vessels) was only 60% between MRA and FDG-PET, with MRA generally showing significantly more extensive disease [68]. Disease activity grading based on imaging (i.e., signs of inflammation) showed similarly poor agreement. Only oedema and wall thickness on MRA correlated well with FDG-uptake in PET [68]. This study also addressed two clinically relevant issues. First, only findings from PET were associated with patient clinical status following comparison of imaging and clinical disease activity. On the other hand, a striking 51% of the patients in clinical remission still had active disease by both MRA and FDG-PET [68]. This study nicely demonstrates the need for systematic imaging studies to define what represents disease activity and what not. Of potential interest is hybrid FDG-PET/MRI to combine the strengths of both methods to improve diagnostic results [71].
The role of imaging during follow-up do determine disease activity
GCA is a relapsing disease, with about 40% of all patients experiencing relapse [72]. Clinical remission is defined as normal C-reactive protein (CRP) and ESR in the absence of clinical symptoms. The high relapse rate suggests subclinical activity during treatment, at least in some patients, which makes clinical assessment challenging. Follow-up examinations to investigate ongoing disease activity in patients without suspected flare are not recommended by the EULAR since their clinical usefulness still needs to be defined [24]. There is, however, an unmet need to identify patients at risk for relapses and those with subclinical activity. This is further emphasised in patients treated with tocilizumab, since CRP, and most likely ESR too, are suppressed independently of clinical activity [73, 74]. Already the earliest reports on tocilizumab in LVV highlighted that a normal CRP and absence of clinical symptoms do not equal remission at the level of the artery [75]. To date, the question of when to stop treatment safely in patients with clinically inactive GCA is still experience-based.
Since PET/CT visualises metabolism, it is plausible that a decreasing or normalised vascular FDG uptake in PET/CT indicates remission. However, despite adequate treatment, a low-grade FDG uptake persists in a relevant number of patients [8, 68, 76]. Whether this represents residual, low-grade vasculitis or noninflammatory metabolic processes in the vessel walls remains to be better defined [77]. Very recently, a large National Institutes of Health (NIH)-funded FDG-PET study reported a link between high PET activity during follow-up and subsequent relapses. This study found that more than half of patients in clinical remission showed signs of active vasculitis on PET [76], supporting findings from a previous, smaller subgroup analysis [68]. The authors used a qualitative summary score to grade activity in PET scans. Patients with a high score were at greater risk for future clinical relapse than those with a low score (55 vs 11% relapse rate) [76].
Little information is available on assessment of disease activity with CT or MRI during follow-up. In a CTA study on 40 GCA patients, a 1-year follow-up examination was performed in 35 subjects. Of those, 68% were found to have continued arterial wall thickening, and contrast enhancement was substantially reduced in the majority of participants (15 of 16 available subjects, 94%) [78]. Oedema and wall thickening observed on MRI can represent active disease, as demonstrated in MRI/PET correlation studies [68]. MRI follow-up data from the Swiss randomised controlled trial of tocilizumab in GCA showed that of 13 patients with initial large vessel involvement, alterations in vessel walls were normalised in only 25% placebo vs 33% tocilizumab patients. Importantly, tocilizumab-treated subjects were all in a sustained remission from GCA for over a year [79]. Similarly, MRI findings in aortitis persist frequently, despite clinical remission and normalisation of PET/CT [80]. These observations warrant further investigation, since the study was small and did not address whether the persistent MRI findings are clinically relevant.
Vasculitic CDS findings rapidly normalise within the temporal arteries but persist in larger vessels in most patients despite clinical remission [7, 44]. In the future, the additional use of CEUS may facilitate monitoring of disease activity in LV-GCA during follow-up. Several case reports and case series suggest that CEUS can detect disease activity in GCA or Takayasu arteritis [21, 74, 81–83]. Germano et al. demonstrated in 31 patients with large-vessel vasculitis that enhancement of the thickened carotid vessel wall correlated with the grade of vascular inflammation on FDG-PET and was more frequent in clinically active disease [21]. However, the clinical relevance of CEUS to assess disease activity at the time of diagnosis or to monitor arterial inflammation during follow-up still needs to be defined.
The role of follow-up imaging to identify complications and structural damage
Large-artery complications are common in GCA. An estimated one quarter to one third of patients develop aortic structural damage detected with a mixed CT (thoracic aorta) and ultrasound (abdominal aorta) assessment [84, 85]. GCA patients are at almost 20 times increased risk for thoracic aortic aneurysm, often occurring years after diagnosis and independent of persistent clinical disease activity [85–88]. Although in many patients, aortic aneurysm or large-vessel stenosis is present at diagnosis, the incidence of newly diagnosed large vessel involvement increases around 5 years after diagnosis [88]. The development of aortic structural damage may occur independently of clinical disease activity [84]. A commonly used, and recommended, follow-up strategy is to start aortic screening 2 years after diagnosis [89]. MRA or CTA are typically used to detect aortic aneurysms or stenosis of aortic branches. However, such strategies primarily assess irreversible structural damage. Detection of aortitis using PET/CT could predict aneurysm formation, which should be further explored to test whether these patients will benefit from more intensive therapy.
Summary and conclusions
The diagnostic algorithm for GCA depends on the clinical presentation, the available imaging methods and the local expertise [24]. We, therefore, recommend establishing immediately accessible specialised fast-track clinics with diagnostic algorithms adapted to the local expertise. This shortens the time to adequate therapy and reduces ischaemic complications [90].
For diagnosing cGCA, the best and most extensive data exist on the use of CDS of the temporal arteries. CDS can easily be extended to the larger vessels, thereby increasing the diagnostic sensitivity. Therefore, CDS is the preferred imaging method in these patients. If CDS is not available, MRI of the temporal arteries is a good alternative. Large vessel imaging should be performed in all GCA patients to assess potential aortitis, aneurysm or subclavian stenosis. In patients with predominantly LV-GCA having, “B-symptoms” only, large vessel imaging is a reasonable first diagnostic step. Figure 5 shows our local diagnostic workup for patients with suspected GCA. This algorithm must be adapted to local availability and expertise. Since CDS misses isolated vasculitis of the thoracic aorta, this is best combined with a cross-sectional imaging modality. Based on current data, none of the other available methods (PET/CT, MRI or CT) can be preferred over another to diagnose LV-GCA. Standardised quantitative, and therefore investigator-independent, image analysis has only been established for PET/CT. Moreover, PET/CT findings link to clinical outcome data: aortitis to an increased risk for future aortic aneurysm and high FDG uptake during follow-up with subsequent relapses. In patients with FUO or IUO, PET/CT allows the exclusion of various alternative diagnoses, making it a very valuable method in this setting.
If the clinical presentation suggests a high pre-test probability (i.e., typical symptoms) and imaging shows vasculitis, temporal artery biopsy is not necessary to confirm the diagnosis. MRA or CTA are best suited for long-term follow-up screening for aortic aneurysms.
The best imaging strategy to diagnose vasculitis of the large extracranial arteries in LV-GCA remains to be defined. The EULAR recommendations do not prefer one method over the others and recommend imaging depending on local settings and expertise [24]. Indeed, only few data are available from comparative studies. The evaluation for the recommendations included, however, only two PET studies [41], as the underlying data were collected before several recent studies in support of PET in diagnosing and monitoring disease activity in GCA were published [37, 52, 68, 76]. Further comparative studies using different imaging modalities in the same patient and at predefined time-points could improve the clinical guidelines. This may contribute to identifying patient subgroups, subsequent risk stratification and adapted treatment strategies. A prerequisite to this is standardisation of quantitative analysis, with cut-off values to determine what is diagnostic for “vasculitis” [10, 19, 30, 37]. Finally, the vessel biology in treated subjects needs to be further studied. The high relapse rate after treatment stop in steroid- and potentially also in tocilizumab-treated subjects suggests that GCA might be still active in asymptomatic patients even in the absence of inflammatory markers tested in peripheral blood. Indeed, subclinical local inflammation can persist despite clinical remission, as highlighted in serial temporal artery biopsies [91]. How and to what extent serial imaging can more accurately assess disease activity and hence can guide treatment decisions remains to be further studied.
Appendix 1 Supplementary figure
Acknowledgments
We thank the clinical trial unit of the University Hospital Basel for their ongoing support of the local GCA cohort, and Dr Glenn Bantug for the editing and critical reading of the manuscript. The patients and caring physicians for their contribution.
References
1
De Smit
E
,
Palmer
AJ
,
Hewitt
AW
. Projected worldwide disease burden from giant cell arteritis by 2050. J Rheumatol. 2015;42(1):119–25. doi:.https://doi.org/10.3899/jrheum.140318
2
Schmidt
WA
,
Seifert
A
,
Gromnica-Ihle
E
,
Krause
A
,
Natusch
A
. Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology (Oxford). 2008;47(1):96–101. doi:.https://doi.org/10.1093/rheumatology/kem322
3
Muratore
F
,
Kermani
TA
,
Crowson
CS
,
Green
AB
,
Salvarani
C
,
Matteson
EL
, et al.
Large-vessel giant cell arteritis: a cohort study. Rheumatology (Oxford). 2015;54(3):463–70. doi:.https://doi.org/10.1093/rheumatology/keu329
4
Muto
G
,
Yamashita
H
,
Takahashi
Y
,
Miyata
Y
,
Morooka
M
,
Minamimoto
R
, et al.
Large vessel vasculitis in elderly patients: early diagnosis and steroid-response evaluation with FDG-PET/CT and contrast-enhanced CT. Rheumatol Int. 2014;34(11):1545–54. doi:.https://doi.org/10.1007/s00296-014-2985-3
5
Brack
A
,
Martinez-Taboada
V
,
Stanson
A
,
Goronzy
JJ
,
Weyand
CM
. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum. 1999;42(2):311–7. doi:.https://doi.org/10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F
6
Agard
C
,
Barrier
JH
,
Dupas
B
,
Ponge
T
,
Mahr
A
,
Fradet
G
, et al.
Aortic involvement in recent-onset giant cell (temporal) arteritis: a case-control prospective study using helical aortic computed tomodensitometric scan. Arthritis Rheum. 2008;59(5):670–6. doi:.https://doi.org/10.1002/art.23577
7
Aschwanden
M
,
Kesten
F
,
Stern
M
,
Thalhammer
C
,
Walker
UA
,
Tyndall
A
, et al.
Vascular involvement in patients with giant cell arteritis determined by duplex sonography of 2x11 arterial regions. Ann Rheum Dis. 2010;69(7):1356–9. doi:.https://doi.org/10.1136/ard.2009.122135
8
Blockmans
D
,
de Ceuninck
L
,
Vanderschueren
S
,
Knockaert
D
,
Mortelmans
L
,
Bobbaers
H
. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum. 2006;55(1):131–7. doi:.https://doi.org/10.1002/art.21699
9
Ghinoi
A
,
Pipitone
N
,
Nicolini
A
,
Boiardi
L
,
Silingardi
M
,
Germanò
G
, et al.
Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology (Oxford). 2012;51(4):730–4. doi:.https://doi.org/10.1093/rheumatology/ker329
10
Prieto-González
S
,
Arguis
P
,
García-Martínez
A
,
Espígol-Frigolé
G
,
Tavera-Bahillo
I
,
Butjosa
M
, et al.
Large vessel involvement in biopsy-proven giant cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis. 2012;71(7):1170–6. doi:.https://doi.org/10.1136/annrheumdis-2011-200865
11
de Boysson
H
,
Daumas
A
,
Vautier
M
,
Parienti
JJ
,
Liozon
E
,
Lambert
M
, et al.
Large-vessel involvement and aortic dilation in giant-cell arteritis. A multicenter study of 549 patients. Autoimmun Rev. 2018;17(4):391–8. doi:.https://doi.org/10.1016/j.autrev.2017.11.029
12
Hunder
GG
,
Bloch
DA
,
Michel
BA
,
Stevens
MB
,
Arend
WP
,
Calabrese
LH
, et al.
The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33(8):1122–8. doi:.https://doi.org/10.1002/art.1780330810
13
Craven
A
,
Robson
J
,
Ponte
C
,
Grayson
PC
,
Suppiah
R
,
Judge
A
, et al.
ACR/EULAR-endorsed study to develop Diagnostic and Classification Criteria for Vasculitis (DCVAS). Clin Exp Nephrol. 2013;17(5):619–21. doi:.https://doi.org/10.1007/s10157-013-0854-0
14
Stone
JH
,
Tuckwell
K
,
Dimonaco
S
,
Klearman
M
,
Aringer
M
,
Blockmans
D
, et al.
Trial of Tocilizumab in Giant-Cell Arteritis. N Engl J Med. 2017;377(4):317–28. doi:.https://doi.org/10.1056/NEJMoa1613849
15
Villiger
PM
,
Adler
S
,
Kuchen
S
,
Wermelinger
F
,
Dan
D
,
Fiege
V
, et al.
Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387(10031):1921–7. doi:.https://doi.org/10.1016/S0140-6736(16)00560-2
16
Weyand
CM
,
Goronzy
JJ
. Immune mechanisms in medium and large-vessel vasculitis. Nat Rev Rheumatol. 2013;9(12):731–40. doi:.https://doi.org/10.1038/nrrheum.2013.161
17
Schmidt
WA
,
Kraft
HE
,
Vorpahl
K
,
Völker
L
,
Gromnica-Ihle
EJ
. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med. 1997;337(19):1336–42. doi:.https://doi.org/10.1056/NEJM199711063371902
18
Aschwanden
M
,
Daikeler
T
,
Kesten
F
,
Baldi
T
,
Benz
D
,
Tyndall
A
, et al.
Temporal artery compression sign--a novel ultrasound finding for the diagnosis of giant cell arteritis. Ultraschall Med. 2013;34(1):47–50.
19
Schäfer
VS
,
Juche
A
,
Ramiro
S
,
Krause
A
,
Schmidt
WA
. Ultrasound cut-off values for intima-media thickness of temporal, facial and axillary arteries in giant cell arteritis. Rheumatology (Oxford). 2017;56(9):1479–83. doi:.https://doi.org/10.1093/rheumatology/kex143
20
Kaspar
M
,
Partovi
S
,
Aschwanden
M
,
Imfeld
S
,
Baldi
T
,
Uthoff
H
, et al.
Assessment of microcirculation by contrast-enhanced ultrasound: a new approach in vascular medicine. Swiss Med Wkly. 2015;145:w14047.
21
Germanò
G
,
Macchioni
P
,
Possemato
N
,
Boiardi
L
,
Nicolini
A
,
Casali
M
, et al.
Contrast-Enhanced Ultrasound of the Carotid Artery in Patients With Large Vessel Vasculitis: Correlation With Positron Emission Tomography Findings. Arthritis Care Res (Hoboken). 2017;69(1):143–9. doi:.https://doi.org/10.1002/acr.22906
22
Fleischmann
D
,
Mitchell
RS
,
Miller
DC
. Acute aortic syndromes: new insights from electrocardiographically gated computed tomography. Semin Thorac Cardiovasc Surg. 2008;20(4):340–7. doi:.https://doi.org/10.1053/j.semtcvs.2008.11.011
23
Berthod
PE
,
Aho-Glélé
S
,
Ornetti
P
,
Chevallier
O
,
Devilliers
H
,
Ricolfi
F
, et al.
CT analysis of the aorta in giant-cell arteritis: a case-control study. Eur Radiol. 2018. [Epub ahead of print] doi:.https://doi.org/10.1007/s00330-018-5311-8
24
Dejaco
C
,
Ramiro
S
,
Duftner
C
,
Besson
FL
,
Bley
TA
,
Blockmans
D
, et al.
EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77(5):636–43. doi:.https://doi.org/10.1136/annrheumdis-2017-212649
25
Michaely
HJ
,
Morelli
JN
,
Budjan
J
,
Riffel
P
,
Nickel
D
,
Kroeker
R
, et al.
CAIPIRINHA-Dixon-TWIST (CDT)-volume-interpolated breath-hold examination (VIBE): a new technique for fast time-resolved dynamic 3-dimensional imaging of the abdomen with high spatial resolution. Invest Radiol. 2013;48(8):590–7. doi:.https://doi.org/10.1097/RLI.0b013e318289a70b
26
Bley
TA
,
Uhl
M
,
Venhoff
N
,
Thoden
J
,
Langer
M
,
Markl
M
. 3-T MRI reveals cranial and thoracic inflammatory changes in giant cell arteritis. Clin Rheumatol. 2007;26(3):448–50. doi:.https://doi.org/10.1007/s10067-005-0160-7
27
Glaudemans
AW
,
Signore
A
. FDG-PET/CT in infections: the imaging method of choice?
Eur J Nucl Med Mol Imaging. 2010;37(10):1986–91. doi:.https://doi.org/10.1007/s00259-010-1587-x
28
Meller
J
,
Sahlmann
CO
,
Scheel
AK
. 18F-FDG PET and PET/CT in fever of unknown origin. J Nucl Med. 2007;48(1):35–45.
29
Slart
RHJA
; Writing group; Reviewer group; Members of EANM Cardiovascular; Members of EANM Infection & Inflammation; Members of Committees, SNMMI Cardiovascular; Members of Council, PET Interest Group; Members of ASNC; EANM Committee Coordinator. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging. 2018;45(7):1250–69. doi:.https://doi.org/10.1007/s00259-018-3973-8
30
Stellingwerff
MD
,
Brouwer
E
,
Lensen
KJ
,
Rutgers
A
,
Arends
S
,
van der Geest
KS
, et al.
Different Scoring Methods of FDG PET/CT in Giant Cell Arteritis: Need for Standardization. Medicine (Baltimore). 2015;94(37):e1542. doi:.https://doi.org/10.1097/MD.0000000000001542
31
Puppo
C
,
Massollo
M
,
Paparo
F
,
Camellino
D
,
Piccardo
A
,
Shoushtari Zadeh Naseri
M
, et al.
Giant cell arteritis: a systematic review of the qualitative and semiquantitative methods to assess vasculitis with 18F-fluorodeoxyglucose positron emission tomography. BioMed Res Int. 2014;2014:574248. doi:.https://doi.org/10.1155/2014/574248
32
Blockmans
D
. The use of (18F)fluoro-deoxyglucose positron emission tomography in the assessment of large vessel vasculitis. Clin Exp Rheumatol. 2003;21(6, Suppl 32):S15–22.
33
Besson
FL
,
Parienti
JJ
,
Bienvenu
B
,
Prior
JO
,
Costo
S
,
Bouvard
G
, et al.
Diagnostic performance of 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011;38(9):1764–72. doi:.https://doi.org/10.1007/s00259-011-1830-0
34
Blockmans
D
,
Bley
T
,
Schmidt
W
. Imaging for large-vessel vasculitis. Curr Opin Rheumatol. 2009;21(1):19–28. doi:.https://doi.org/10.1097/BOR.0b013e32831cec7b
35
Meller
J
,
Strutz
F
,
Siefker
U
,
Scheel
A
,
Sahlmann
CO
,
Lehmann
K
, et al.
Early diagnosis and follow-up of aortitis with [(18)F]FDG PET and MRI. Eur J Nucl Med Mol Imaging. 2003;30(5):730–6. doi:.https://doi.org/10.1007/s00259-003-1144-y
36
Hautzel
H
,
Sander
O
,
Heinzel
A
,
Schneider
M
,
Müller
HW
. Assessment of large-vessel involvement in giant cell arteritis with 18F-FDG PET: introducing an ROC-analysis-based cutoff ratio. J Nucl Med. 2008;49(7):1107–13. doi:.https://doi.org/10.2967/jnumed.108.051920
37
Imfeld
S
,
Rottenburger
C
,
Schegk
E
,
Aschwanden
M
,
Juengling
F
,
Staub
D
, et al.
[18F]FDG positron emission tomography in patients presenting with suspicion of giant cell arteritis-lessons from a vasculitis clinic. Eur Heart J Cardiovasc Imaging. 2018;19(8):933–40. doi:.https://doi.org/10.1093/ehjci/jex259
38
Kistner
A
,
Bigler
MB
,
Glatz
K
,
Egli
SB
,
Baldin
FS
,
Marquardsen
FA
, et al.
Characteristics of autoantibodies targeting 14-3-3 proteins and their association with clinical features in newly diagnosed giant cell arteritis. Rheumatology (Oxford). 2017;56(5):829–34.
39
Monach
PA
. Biomarkers in vasculitis. Curr Opin Rheumatol. 2014;26(1):24–30. doi:.https://doi.org/10.1097/BOR.0000000000000009
40
Schmidt
WA
,
Krause
A
,
Schicke
B
,
Kuchenbecker
J
,
Gromnica-Ihle
E
. Do temporal artery duplex ultrasound findings correlate with ophthalmic complications in giant cell arteritis?
Rheumatology (Oxford). 2009;48(4):383–5. doi:.https://doi.org/10.1093/rheumatology/ken515
41
Duftner
C
,
Dejaco
C
,
Sepriano
A
,
Falzon
L
,
Schmidt
WA
,
Ramiro
S
. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4(1):e000612. doi:.https://doi.org/10.1136/rmdopen-2017-000612
42
Arida
A
,
Kyprianou
M
,
Kanakis
M
,
Sfikakis
PP
. The diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: a second meta-analysis. BMC Musculoskelet Disord. 2010;11(1):44. doi:.https://doi.org/10.1186/1471-2474-11-44
43
Aschwanden
M
,
Imfeld
S
,
Staub
D
,
Baldi
T
,
Walker
UA
,
Berger
CT
, et al.
The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol. 2015;33(2, Suppl 89):S-113–5.
44
Luqmani
R
,
Lee
E
,
Singh
S
,
Gillett
M
,
Schmidt
WA
,
Bradburn
M
, et al.
The Role of Ultrasound Compared to Biopsy of Temporal Arteries in the Diagnosis and Treatment of Giant Cell Arteritis (TABUL): a diagnostic accuracy and cost-effectiveness study. Health Technol Assess. 2016;20(90):1–238. doi:.https://doi.org/10.3310/hta20900
45
Germanò
G
,
Muratore
F
,
Cimino
L
,
Lo Gullo
A
,
Possemato
N
,
Macchioni
P
, et al.
Is colour duplex sonography-guided temporal artery biopsy useful in the diagnosis of giant cell arteritis? A randomized study. Rheumatology (Oxford). 2015;54(3):400–4. doi:.https://doi.org/10.1093/rheumatology/keu241
46
Monti
S
,
Floris
A
,
Ponte
CB
,
Schmidt
WA
,
Diamantopoulos
AP
,
Pereira
C
, et al.
The proposed role of ultrasound in the management of giant cell arteritis in routine clinical practice. Rheumatology (Oxford). 2018;57(1):112–9. doi:.https://doi.org/10.1093/rheumatology/kex341
47
Bley
TA
,
Wieben
O
,
Uhl
M
,
Thiel
J
,
Schmidt
D
,
Langer
M
. High-resolution MRI in giant cell arteritis: imaging of the wall of the superficial temporal artery. AJR Am J Roentgenol. 2005;184(1):283–7. doi:.https://doi.org/10.2214/ajr.184.1.01840283
48
Rhéaume
M
,
Rebello
R
,
Pagnoux
C
,
Carette
S
,
Clements-Baker
M
,
Cohen-Hallaleh
V
, et al.
High-Resolution Magnetic Resonance Imaging of Scalp Arteries for the Diagnosis of Giant Cell Arteritis: Results of a Prospective Cohort Study. Arthritis Rheumatol. 2017;69(1):161–8. doi:.https://doi.org/10.1002/art.39824
49
Hommada
M
,
Mekinian
A
,
Brillet
PY
,
Abad
S
,
Larroche
C
,
Dhôte
R
, et al.
Aortitis in giant cell arteritis: diagnosis with FDG PET/CT and agreement with CT angiography. Autoimmun Rev. 2017;16(11):1131–7. doi:.https://doi.org/10.1016/j.autrev.2017.09.008
50
Cinar
I
,
Wang
H
,
Stone
JR
. Clinically isolated aortitis: pitfalls, progress, and possibilities. Cardiovasc Pathol. 2017;29:23–32. doi:.https://doi.org/10.1016/j.carpath.2017.04.003
51
Lariviere
D
,
Benali
K
,
Coustet
B
,
Pasi
N
,
Hyafil
F
,
Klein
I
, et al.
Positron emission tomography and computed tomography angiography for the diagnosis of giant cell arteritis: A real-life prospective study. Medicine (Baltimore). 2016;95(30):e4146. doi:.https://doi.org/10.1097/MD.0000000000004146
52
de Boysson
H
,
Liozon
E
,
Lambert
M
,
Parienti
JJ
,
Artigues
N
,
Geffray
L
, et al.
18F-fluorodeoxyglucose positron emission tomography and the risk of subsequent aortic complications in giant-cell arteritis: A multicenter cohort of 130 patients. Medicine (Baltimore). 2016;95(26):e3851. doi:.https://doi.org/10.1097/MD.0000000000003851
53
Lee
YH
,
Choi
SJ
,
Ji
JD
,
Song
GG
. Diagnostic accuracy of 18F-FDG PET or PET/CT for large vessel vasculitis. Z Rheumatol. 2016;75(9):924–31. doi:.https://doi.org/10.1007/s00393-015-1674-2
54
Soussan
M
,
Nicolas
P
,
Schramm
C
,
Katsahian
S
,
Pop
G
,
Fain
O
, et al.
Management of large-vessel vasculitis with FDG-PET: a systematic literature review and meta-analysis. Medicine (Baltimore). 2015;94(14):e622. doi:.https://doi.org/10.1097/MD.0000000000000622
55
Fuchs
M
,
Briel
M
,
Daikeler
T
,
Walker
UA
,
Rasch
H
,
Berg
S
, et al.
The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis. Eur J Nucl Med Mol Imaging. 2012;39(2):344–53. doi:.https://doi.org/10.1007/s00259-011-1967-x
56
Nielsen
BD
,
Gormsen
LC
,
Hansen
IT
,
Keller
KK
,
Therkildsen
P
,
Hauge
EM
. Three days of high-dose glucocorticoid treatment attenuates large-vessel 18F-FDG uptake in large-vessel giant cell arteritis but with a limited impact on diagnostic accuracy. Eur J Nucl Med Mol Imaging. 2018;45(7):1119–28. doi:.https://doi.org/10.1007/s00259-018-4021-4
57
Moosig
F
,
Czech
N
,
Mehl
C
,
Henze
E
,
Zeuner
RA
,
Kneba
M
, et al.
Correlation between 18-fluorodeoxyglucose accumulation in large vessels and serological markers of inflammation in polymyalgia rheumatica: a quantitative PET study. Ann Rheum Dis. 2004;63(7):870–3. doi:.https://doi.org/10.1136/ard.2003.011692
58
Cimmino
MA
,
Zampogna
G
,
Parodi
M
. Is FDG-PET useful in the evaluation of steroid-resistant PMR patients?
Rheumatology (Oxford). 2008;47(6):926–7. doi:.https://doi.org/10.1093/rheumatology/ken098
59
Takahashi
H
,
Yamashita
H
,
Kubota
K
,
Miyata
Y
,
Okasaki
M
,
Morooka
M
, et al.
Differences in fluorodeoxyglucose positron emission tomography/computed tomography findings between elderly onset rheumatoid arthritis and polymyalgia rheumatica. Mod Rheumatol. 2015;25(4):546–51. doi:.https://doi.org/10.3109/14397595.2014.978936
60
Calamia
KT
,
Hunder
GG
. Giant cell arteritis (temporal arteritis) presenting as fever of undetermined origin. Arthritis Rheum. 1981;24(11):1414–8. doi:.https://doi.org/10.1002/art.1780241113
61
Balink
H
,
Veeger
NJ
,
Bennink
RJ
,
Slart
RH
,
Holleman
F
,
van Eck-Smit
BL
, et al.
The predictive value of C-reactive protein and erythrocyte sedimentation rate for 18F-FDG PET/CT outcome in patients with fever and inflammation of unknown origin. Nucl Med Commun. 2015;36(6):604–9. doi:.https://doi.org/10.1097/MNM.0000000000000300
62
Lensen
KJ
,
Voskuyl
AE
,
van der Laken
CJ
,
Comans
EF
,
van Schaardenburg
D
,
Arntzenius
AB
, et al.
18F-fluorodeoxyglucose positron emission tomography in elderly patients with an elevated erythrocyte sedimentation rate of unknown origin. PLoS One. 2013;8(3):e58917. doi:.https://doi.org/10.1371/journal.pone.0058917
63
Besson
FL
,
Chaumet-Riffaud
P
,
Playe
M
,
Noel
N
,
Lambotte
O
,
Goujard
C
, et al.
Contribution of (18)F-FDG PET in the diagnostic assessment of fever of unknown origin (FUO): a stratification-based meta-analysis. Eur J Nucl Med Mol Imaging. 2016;43(10):1887–95. doi:.https://doi.org/10.1007/s00259-016-3377-6
64
Dong
MJ
,
Zhao
K
,
Liu
ZF
,
Wang
GL
,
Yang
SY
,
Zhou
GJ
. A meta-analysis of the value of fluorodeoxyglucose-PET/PET-CT in the evaluation of fever of unknown origin. Eur J Radiol. 2011;80(3):834–44. doi:.https://doi.org/10.1016/j.ejrad.2010.11.018
65
Balink
H
,
Tan
SS
,
Veeger
NJ
,
Holleman
F
,
van Eck-Smit
BL
,
Bennink
RJ
, et al.
18F-FDG PET/CT in inflammation of unknown origin: a cost-effectiveness pilot-study. Eur J Nucl Med Mol Imaging. 2015;42(9):1408–13. doi:.https://doi.org/10.1007/s00259-015-3010-0
66
Balink
H
,
Verberne
HJ
,
Bennink
RJ
,
van Eck-Smit
BL
. A Rationale for the Use of F18-FDG PET/CT in Fever and Inflammation of Unknown Origin. Int J Mol Imaging. 2012;2012:165080. doi:.https://doi.org/10.1155/2012/165080
67
Pfadenhauer
K
,
Weinerth
J
,
Hrdina
C
. Vertebral arteries: a target for FDG-PET imaging in giant cell arteritis? Clinical, ultrasonographic and PET study in 46 patients. Nucl Med (Stuttg). 2011;50(1):28–32. doi:.https://doi.org/10.3413/nukmed-0335-10-07
68
Quinn
KA
,
Ahlman
MA
,
Malayeri
AA
,
Marko
J
,
Civelek
AC
,
Rosenblum
JS
, et al.
Comparison of magnetic resonance angiography and 18F-fluorodeoxyglucose positron emission tomography in large-vessel vasculitis. Ann Rheum Dis. 2018;77(8):1165–71.
69
Cimmino
MA
,
Camellino
D
. Large vessel vasculitis: which imaging method?
Swiss Med Wkly. 2017;147:w14405.
70
de Boysson
H
,
Dumont
A
,
Liozon
E
,
Lambert
M
,
Boutemy
J
,
Maigné
G
, et al.
Giant-cell arteritis: concordance study between aortic CT angiography and FDG-PET/CT in detection of large-vessel involvement. Eur J Nucl Med Mol Imaging. 2017;44(13):2274–9. doi:.https://doi.org/10.1007/s00259-017-3774-5
71
Einspieler
I
,
Thürmel
K
,
Pyka
T
,
Eiber
M
,
Wolfram
S
,
Moog
P
, et al.
Imaging large vessel vasculitis with fully integrated PET/MRI: a pilot study. Eur J Nucl Med Mol Imaging. 2015;42(7):1012–24. doi:.https://doi.org/10.1007/s00259-015-3007-8
72
Martinez-Lado
L
,
Calviño-Díaz
C
,
Piñeiro
A
,
Dierssen
T
,
Vazquez-Rodriguez
TR
,
Miranda-Filloy
JA
, et al.
Relapses and recurrences in giant cell arteritis: a population-based study of patients with biopsy-proven disease from northwestern Spain. Medicine (Baltimore). 2011;90(3):186–93. doi:.https://doi.org/10.1097/MD.0b013e31821c4fad
73
Berger
CT
,
Recher
M
,
Daikeler
T
. Interleukin-6 flags infection in tocilizumab-treated giant cell arteritis. Rheumatology (Oxford). 2018;57(1):196–7. doi:.https://doi.org/10.1093/rheumatology/kex336
74
Dikkes
A
,
Aschwanden
M
,
Imfeld
S
,
Glatz
K
,
Messerli
J
,
Staub
D
, et al.
Takayasu arteritis: active or not, that’s the question. Rheumatology (Oxford). 2017;56(10):1818–9. doi:.https://doi.org/10.1093/rheumatology/kex213
75
Unizony
S
,
Arias-Urdaneta
L
,
Miloslavsky
E
,
Arvikar
S
,
Khosroshahi
A
,
Keroack
B
, et al.
Tocilizumab for the treatment of large-vessel vasculitis (giant cell arteritis, Takayasu arteritis) and polymyalgia rheumatica. Arthritis Care Res (Hoboken). 2012;64(11):1720–9. doi:.https://doi.org/10.1002/acr.21750
76
Grayson
PC
,
Alehashemi
S
,
Bagheri
AA
,
Civelek
AC
,
Cupps
TR
,
Kaplan
MJ
, et al.
18 F-Fluorodeoxyglucose-Positron Emission Tomography As an Imaging Biomarker in a Prospective, Longitudinal Cohort of Patients With Large Vessel Vasculitis. Arthritis Rheumatol. 2018;70(3):439–49. doi:.https://doi.org/10.1002/art.40379
77
Clifford
A
,
Burrell
S
,
Hanly
JG
. Positron emission tomography/computed tomography for the diagnosis and assessment of giant cell arteritis: when to consider it and why. J Rheumatol. 2012;39(10):1909–11. doi:.https://doi.org/10.3899/jrheum.120171
78
Prieto-González
S
,
García-Martínez
A
,
Tavera-Bahillo
I
,
Hernández-Rodríguez
J
,
Gutiérrez-Chacoff
J
,
Alba
MA
, et al.
Effect of glucocorticoid treatment on computed tomography angiography detected large-vessel inflammation in giant-cell arteritis. A prospective, longitudinal study. Medicine (Baltimore). 2015;94(5):e486. doi:.https://doi.org/10.1097/MD.0000000000000486
79
Reichenbach
S
,
Adler
S
,
Bonel
H
,
Cullmann
JL
,
Kuchen
S
,
Bütikofer
L
, et al.
Magnetic resonance angiography in giant cell arteritis: results of a randomized controlled trial of tocilizumab in giant cell arteritis. Rheumatology (Oxford). 2018;57(6):982–6. doi:.https://doi.org/10.1093/rheumatology/key015
80
Scheel
AK
,
Meller
J
,
Vosshenrich
R
,
Kohlhoff
E
,
Siefker
U
,
Müller
GA
, et al.
Diagnosis and follow up of aortitis in the elderly. Ann Rheum Dis. 2004;63(11):1507–10. doi:.https://doi.org/10.1136/ard.2003.015651
81
Schinkel
AF
,
van den Oord
SC
,
van der Steen
AF
,
van Laar
JA
,
Sijbrands
EJ
. Utility of contrast-enhanced ultrasound for the assessment of the carotid artery wall in patients with Takayasu or giant cell arteritis. Eur Heart J Cardiovasc Imaging. 2014;15(5):541–6. doi:.https://doi.org/10.1093/ehjci/jet243
82
Herlin
B
,
Baud
JM
,
Chadenat
ML
,
Pico
F
. Contrast-enhanced ultrasonography in Takayasu arteritis: watching and monitoring the arterial inflammation. BMJ Case Rep. 2015;2015:bcr2015211094. doi:.https://doi.org/10.1136/bcr-2015-211094
83
Czihal
M
,
Lottspeich
C
,
Schröttle
A
,
Treitl
KM
,
Treitl
M
,
Leipe
J
, et al.
Relapses in three patients with Takayasu arteritis under tocilizumab treatment detected by contrast enhanced ultrasound. Vasa. 2018;47(2):149–52. doi:.https://doi.org/10.1024/0301-1526/a000679
84
García-Martínez
A
,
Arguis
P
,
Prieto-González
S
,
Espígol-Frigolé
G
,
Alba
MA
,
Butjosa
M
, et al.
Prospective long term follow-up of a cohort of patients with giant cell arteritis screened for aortic structural damage (aneurysm or dilatation). Ann Rheum Dis. 2014;73(10):1826–32. doi:.https://doi.org/10.1136/annrheumdis-2013-203322
85
Nuenninghoff
DM
,
Hunder
GG
,
Christianson
TJ
,
McClelland
RL
,
Matteson
EL
. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum. 2003;48(12):3522–31. doi:.https://doi.org/10.1002/art.11353
86
Gonzalez-Gay
MA
,
Garcia-Porrua
C
,
Piñeiro
A
,
Pego-Reigosa
R
,
Llorca
J
,
Hunder
GG
. Aortic aneurysm and dissection in patients with biopsy-proven giant cell arteritis from northwestern Spain: a population-based study. Medicine (Baltimore). 2004;83(6):335–41. doi:.https://doi.org/10.1097/01.md.0000145366.40805.f8
87
Evans
JM
,
O’Fallon
WM
,
Hunder
GG
. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med. 1995;122(7):502–7. doi:.https://doi.org/10.7326/0003-4819-122-7-199504010-00004
88
Kermani
TA
,
Warrington
KJ
,
Crowson
CS
,
Ytterberg
SR
,
Hunder
GG
,
Gabriel
SE
, et al.
Large-vessel involvement in giant cell arteritis: a population-based cohort study of the incidence-trends and prognosis. Ann Rheum Dis. 2013;72(12):1989–94. doi:.https://doi.org/10.1136/annrheumdis-2012-202408
89
Dasgupta
B
,
Borg
FA
,
Hassan
N
,
Alexander
L
,
Barraclough
K
,
Bourke
B
, et al.; BSR and BHPR Standards, Guidelines and Audit Working Group. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology (Oxford). 2010;49(8):1594–7. doi:.https://doi.org/10.1093/rheumatology/keq039a
90
Diamantopoulos
AP
,
Haugeberg
G
,
Lindland
A
,
Myklebust
G
. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis?
Rheumatology (Oxford). 2016;55(1):66–70. doi:.https://doi.org/10.1093/rheumatology/kev289
91
Maleszewski
JJ
,
Younge
BR
,
Fritzlen
JT
,
Hunder
GG
,
Goronzy
JJ
,
Warrington
KJ
, et al.
Clinical and pathological evolution of giant cell arteritis: a prospective study of follow-up temporal artery biopsies in 40 treated patients. Mod Pathol. 2017;30(6):788–96. doi:.https://doi.org/10.1038/modpathol.2017.10