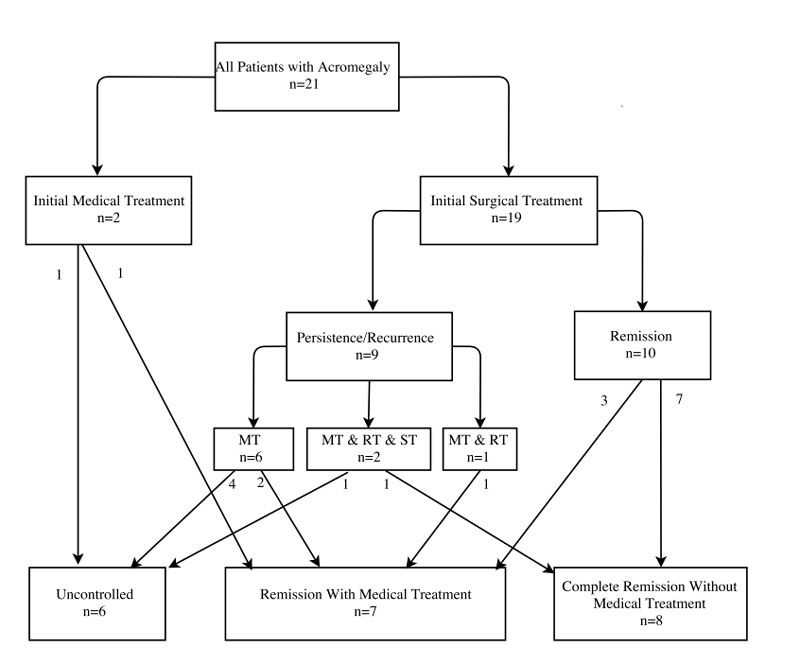
Figure 1 Clinical outcomes of patients according to their initial and follow-up treatments.
MT = medical treatment; ST = surgical treatment; RT = radiotherapy
DOI: https://doi.org/10.4414/smw.2018.14653
Acromegaly is a rare disorder resulting from excess growth hormone secretion after the growth plates have closed [1, 2]. Increased growth hormone secretion stimulates the production of insulin-like growth factor-1 (IGF-1) in the liver leading to organ enlargement and various complications [3, 4]. Initial symptoms typically include enlargement of the hands and feet, and the forehead, jaw and nose [5]. Other symptoms may include joint pain, thicker skin, deepening of the voice, headaches, problems with vision and new onset of diabetes, sleep apnoea, and high blood pressure. Although there are other causes for growth hormone overproduction such as stress or hypoglycaemia, a growth hormone-secreting pituitary tumour is responsible for this disease in a majority (>95%) of cases [3].
Once acromegaly is diagnosed, the treatment goals depend on the extent of the tumour and therapeutic options and include biochemical control (age- and sex-normalised IGF-1 and random growth hormone <1 μg/l or nadir growth hormone <0.4 μg/l) or tumour debulking to prevent damage to the optic nerve with loss of sight [3]. If biochemical control cannot be achieved, stabilisation of tumour volume and alleviation of symptoms remains an important task. Initial treatment of acromegaly is usually surgical with the goal of extirpation of the tumour [4]. Second line treatments include somatostatin analogues, growth hormone receptor antagonists, dopamine agonists and/or radiotherapy, additional surgery and stereotactic radiosurgery (SRS).
So far, there is no standard set of prognostic parameters for patients diagnosed with acromegaly that helps to predict treatment outcomes, and might help to optimise initial treatment decisions and frequencies of follow-up visits. According to the German Acromegaly Register, age at initial presentation and sex turned out to be an independent risk factor [6]. Cavernous sinus invasion is more important than the adenoma size for remission [7, 8]. In the case of high risk for relapse, a more extensive approach may be needed with short-term follow up of the patients, whereas in low risk patients the focus should be on preservation of pituitary function. To address this issue, our aim was to investigate determinants of acromegaly recurrence and predictors of outcomes in patients with acromegaly treated at a tertiary care referral centre in Switzerland within the last 10 years.
This observational study used consecutive patients with acromegaly entered in the Swiss Pituitary Registry (SwissPit). The SwissPit registry was approved by the local ethical committee (EKNZ 2015-375). As an observational registry, patients were informed and had the right to refuse to participate. Up to now, no patient has refused participation.
In brief, all patients suffering from sellar lesions who either underwent surgery at the department of neurosurgery of the Kantonsspital Aarau, a tertiary care hospital in the northern part of Switzerland, or were referred to the department of endocrinology at the same institution between 2006 and 2016 were eligible for inclusion in the study. A total of 21 patients had a final diagnosis of acromegaly and were included in the study. The data collection was based on an electronic data capture system (secuTrial®), a system run on a server maintained by the IT department of the University Hospital Basel, Switzerland. Data collection included detailed baseline data regarding clinical features, radiological features and biochemistry. The therapeutic approach was also recorded, as well as outcomes of patients including need for medical treatments, radiotherapy or repeat surgery.
The diagnosis of acromegaly was confirmed by an endocrinologist based on biochemistry results with increased age- and sex-normalised IGF-1 and no suppression of growth hormone in a glucose suppression test [4]. If there were doubts about the appropriateness of IGF-1 levels (i.e., false elevated IGF-1 levels due to sepsis or preanalytical issues), blood draws were repeated. It was further validated by neuroradiological findings. Radiological findings were graded as suggested by Knosp and colleagues [9] on the basis of the relation of the tumour to the cavernous sinus, with Knosp grade 3 und 4 tumours being defined as invasive and therefore limited to gross total resection. Furthermore we used the classification suggested by Hardy and colleagues [10], which basically describes the suprasellar extension of the tumour.
Our main endpoint was clinical cure, defined as complete remission, remission with need for medical treatment, and uncontrolled disease defined by non-normalisation of IGF-1 and growth hormone levels measured at random or during a 75-g oral glucose tolerance test. In patients with uncontrolled disease, we either did not have any surgical/radiotherapy options with a justifiable risk, or patients refused these treatments, or both. All such cases were discussed between the clinical team, including neurosurgeons and endocrinologists, and the patient, as part of routine care in our institution.
We followed the recommendations of the STROBE statement for reporting observational trials [11]. We used descriptive statistics including mean with standard deviation (SD) for patient’s age and median with interquartile range (IQR) for non-normally distributed values such as duration of symptoms, body mass index (BMI), growth hormone and IGF-1 levels. We further used frequencies for binary data to describe the populations. We investigated the association of initial baseline features and clinical outcome in multivariate logistic regression analyses and report odds ratios (ORs) and 95% confidence intervals (CIs). Tests were two-tailed and carried out at the 5% significance levels. Analyses were performed with Stata 12.1 (Stata Corp., College Station, TX, US).
Between 2006 and 2016, a total of 21 patients with a final diagnosis of acromegaly admitted to our hospital were included in the study, with three different neurosurgeons being in charge of surgical treatment during that time period. Of these patients, 48% (n = 10) were male and 52% (n = 11) were female. The median duration of symptoms until diagnosis was 5 years. The most prevalent symptoms at initial presentation were acral enlargement (n = 17, 81%), coarse facial features (n = 14, 67%), and macroglossia (n = 6, 29%). Cardiovascular comorbidities were common with 14 (67%) patients having arterial hypertension, 3 (14%) patients having coronary artery disease, and 2 (10%) patients having hypertensive cardiopathy. Table 1 displays baseline characteristics of the cohort.
Table 1 Baseline characteristics.
| Total |
Remission without therapy
(n = 8) |
Remission with therapy
(n = 7) |
Uncontrolled disease
(n = 6) |
|
|---|---|---|---|---|
| Mean age at diagnosis in years (SD) | 48.9 (14.9) | 54.9 (6.9) | 44.1 (18.9) | 46.5 (17.1) |
| Gender | 10 male 11 female |
5 male 3 female |
4 male 3 female |
1 male 5 female |
| Smoker | 8 (38%) | 4 (50%) | 2 (29%) | 2 (33%) |
| Median duration of symptoms until diagnosis in years (IQR) | 5 (3–7) | 7 (5–10) | 3 (3) | 2 (1–5) |
| Obesity (BMI ≥30 kg/m2) | 7 (33%) | 4 | 2 | 1 |
| Median BMI in kg/m2 (IQR) | 29.35 (27.7–31.15) | 29.4 (28.4–31.9) | 30.05 (28.8–32.95) | 27.25 (24.5–29.8) |
| Median growth hormone level at diagnosis in µg/l (IQR) | 25.7 (6.34–34.9) | 29 (5.9–29.5) | 24 (4.6–40) | 24.7 (17.55–28.5) |
| Median IGF-1 level at diagnosis in µg/l (IQR) | 440 (101–639) | 106 (73.1– 440) | 308 (86.3–687) | 807.5 (370–994.5) |
| Clinical Features | ||||
| Local tumour effects | ||||
| Visual field defects | 4 (19%) | 2 (25%) | 1 (14%) | 1 (17%) |
| Cranial nerve palsy | 1 (5%) | 0 (0%) | 0(0%) | 1 (17%) |
| Headache | 6 (29%) | 2 (25%) | 0(0%) | 4 (67%) |
| Hypopituitarism | 3 (15%) | 1 (13%) | 1 (14%) | 1 (17%) |
| Fatigue | 4 (19%) | 2 (25%) | 0(0%) | 2 (33%) |
| Skin, joints and visceromegaly | ||||
| Hyperhidrosis | 2 (10%) | 1 (13%) | 0(0%) | 1 (17%) |
| Intestinal polyps | 3 (15%) | 0(0%) | 2 (29%) | 1 (17%) |
| Skin thickening | 1 (5%) | 0(0%) | 1 (14%) | 0 (0%) |
| Macroglossia | 6 (29%) | 1 (13%) | 2 (29%) | 3 (50%) |
| Acral enlargement | 17 (81%) | 7 (88%) | 5 (71%) | 5 (83%) |
| Coarse facial features | 14 (67%) | 6 | 5 (71%) | 3 (50%) |
| Cardiovascular and pulmonary systems | ||||
| Hypertensive cardiopathy | 2 (10%) | 1 (13%) | 1 (14%) | 0 (0%) |
| Arterial hypertension | 14 (67%) | 8 (100%) | 3 (43%) | 3 (50%) |
| Coronary artery disease | 3 (14%) | 1 (13%) | 1 (14%) | 1 (17%) |
| Sleep apnoea (OSAS) | 4 (19%) | 1 (13%) | 1 (14%) | 2 (33%) |
| Endocrine and nervous systems | ||||
| Diabetes mellitus type 2 | 5 (24%) | 3 (38%) | 0 (0%) | 2 (33%) |
| Diabetes insipidus | 1 (5%) | 0(0%) | 0(0%) | 1 (17%) |
| Hypogonadotropism | 2 (10%) | 1 (13%) | 1 (14%) | 0 (0%) |
| Panhypopituitarism | 1 (5%) | 0(0%) | 0(0%) | 1 (17%) |
| Carpal tunnel syndrome | 8 (38%) | 1 (13%) | 3 (43%) | 4 (67%) |
| Paraesthesia of hands | 6 (30%) | 2 (25%) | 2 (29%) | 2 (33%) |
BMI = body mass index; IQR = interquartile range; OSAS = obstructive sleep apnoea syndrome; SD = standard deviation
The patients’ mean initial tumour volume was 5.3 cm3 (σ = 7.5) and the most common Knosp grade was 1 (8, 38%), whereas the most common Hardy grade was 2 (n = 10, 48%). There were 5 microadenomas (24%), 14 macroadenomas (67%) and 2 giant adenomas (10%). Four patients had full or partial compression of the optical nerve. Table 2 shows details about tumour size classification, Knosp grade and Hardy grade in the population.
Table 2 Neuroradiological parameters.
| Total |
Remission without therapy
(n = 8) |
Remission with therapy
(n = 7) |
Uncontrolled
disease (n = 6) |
|
|---|---|---|---|---|
| Knosp grade [9] | 0 (n = 4, 19%) | 2 (25%) | 0 (0%) | 2 (33%) |
| 1 (n = 8, 38%) | 4 (50%) | 2 (29%) | 2 (33%) | |
| 2 (n = 2, 10%) | 0 (0%) | 2 (29%) | 0 (0%) | |
| 3 (n = 4, 19%) | 2 (25%) | 2 (29%) | 0 (0%) | |
| 4 (n = 3, 14%) | 0 (0%) | 1 (14%) | 2 (33%) | |
| Hardy grade [10] | 1 (n = 6, 29%) | 4 (50%) | 1 (14%) | 1 (17%) |
| 2 (n = 10, 48%) | 3 (38%) | 4 (57%) | 3 (50%) | |
| 3 (n = 4, 19%) | 0 (0%) | 2 (29%) | 2 (33%) | |
| 4 (n = 1, 5%) | 1(13%) | 0 (0%) | ||
| Chiasma compression | 4 (19%) | 2 (25%) | 0 (0%) | 2 (33%) |
| Pituitary stalk compression | 10 (48%) | 4 (50%) | 3 (43%) | 3 (50%) |
| Tumour size | ||||
| Microadenoma (<10 mm) | 5 (24%) | 4 (50%) | 0 (0%) | 1 (17%) |
| Macroadenoma (≥10 mm; <40 mm) | 14 (67%) | 4 (50%) | 6 (86%) | 4 (67%) |
| Giant adenoma (≥ 40 mm) | 2 (10%) | 0 (0%) | 1 (14%) | 1 (17%) |
| Mean initial tumour volume in cm2 (SD) | 5.3 (7.5) |
1.3 (2.0) | 7.5 (9.5) | 8.1 (8.2) |
| Postoperative parameters | ||||
| Median postoperative IGF-1 level (5 days after surgery) in µg/l (IQR) | 115 (45.2–507) | 32.4 (25.9–154) | 87 (53.3–136) | 621 (511–625) |
| Median postoperative growth hormone level (5 days after surgery) in µg/l (IQR) | 4.2 (1.7–6.4) | 1.715 (0.9– 2.06) | 4.5 (1.75–5.6) | 6.61 (6.24–11) |
| Hypocorticalism and hypothyroidism | 1 (13%) | 1 (13%) | 0 (0%) | 0 (0%) |
| Mean postoperative tumour volume (at next radiological visit) in cm3 (SD) | 1.1 (2.2) | 0.8 (2.1) | 1.2 (2.5) | 1.4 (2.5) |
| Transient SIADH | 1 (5%) | 1 (13%) | 0 (0%) | 0 (0%) |
IGF-1 = insulin-like growth factor-1; IQR = interquartile range; SD = standard deviation; SIADH = syndrome of inappropriate antidiuretic hormone secretion
Mean follow up time in the cohort was 32 months after initial treatment/surgery. Figure 1 shows clinical outcomes of patients according to their initial and follow-up treatments. All but two patients (n = 19) underwent initial trans-sphenoidal surgery. A total of 15 (79%) patients had microscopic surgery and 4 (21%) endoscopic surgery. Additionally, 14 (74%) patients were operated on with the help of intraoperative magnetic resonance imaging and 4 (21%) also with fluoroscopy. After the initial surgery, in two patients (11%) additional surgery was needed and three (16%) patients received medical treatment followed by conventional external beam radiotherapy.

Figure 1 Clinical outcomes of patients according to their initial and follow-up treatments.
MT = medical treatment; ST = surgical treatment; RT = radiotherapy
Drug therapy as a preoperative treatment to optimise the patients’ condition and/or to reduce tumour volume was used in four (19%) patients. Regarding pituitary function, one patient was diagnosed with hypocorticalism and hypothyroidism after surgery. Another patient with panhypopituitarism before surgery had a complete normalisation of pituitary function after surgery.
Regarding outcomes, 8 patients had complete remission and 13 patients had active disease. More specifically, out of the 19 patients receiving initial surgical treatment, 5 (26%) had uncontrolled disease, 6 (32%) went into remission needing ongoing medical treatment, and 8 (42%) had complete remission with no further medical treatment (one patient had initial persistence but complete remission after use a combination of radiotherapy, surgery and medical therapy) (fig. 1). Patients with no full remission received various medical treatments with all patients having a somatostatin analogue, 23% of patients receiving pegvisomant and 31% of patients having a dopamine agonist (table 3). Multimodal treatment was necessary in most cases.
Table 3 Drug treatment (13/21 = 62%).
| Somatostatin (13) | 13/13 = 100% |
| Octreotide | 11/13 = 85% |
| Lanreotide | 4/13 = 31% |
| Adverse event (diarrhoea; exanthema) | 2/4 = 50% |
| Pasireotide | 3/13 = 23% |
| Pegvisomant (3) | 3/13 = 23% |
| Adverse event (dysaesthesia in lower arms | 1/3 = 33% |
| Dopamine agonist (4) | 4/13 = 31% |
| Cabergoline | 3/13 = 23% |
| Adverse event (alopecia; headaches) | 2/3 = 67% |
| Bromocriptine and quinagolide | 1/13 = 8% |
Finally, we investigated predictors for adverse outcome in the patients with uncontrolled disease and non-full remission. Results of the logistic regression analysis are presented in table 4. A larger adenoma size was a significant predictor of not having full remission (OR 12.0, 95% CI 1.02–141.0; p = 0.048). In addition, higher postoperative IGF-1 levels were predictors of uncontrolled disease (OR 4.53, 95% CI 1.07–19.2; p = 0.040). None of the other parameters showed a significant result in regression analysis.
Table 4 Logistic regression analysis of predictors for adverse outcome in the patients with uncontrolled disease and non-full remission.
| Parameter | Uncontrolled disease | Non-full remission | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR | p-value | |
| Age at diagnosis | 0.984 (0.922–1.05) | 0.63 | 0.950 (0.885 - 1.02) | 0.157 |
| Male gender | 7.5 (0.692–81.2) | 0.097 | 2.67 (0.434–16.4) | 0.29 |
| Duration of Symptoms until diagnosis | 0.606 (0.306–1.20) | 0.152 | 0.478 (0.19–1.21) | 0.118 |
| Macroglossia | 3.99 (0.520–30.8) | 0.183 | 4.38 (0.407–47.0) | 0.223 |
| Initial IGF-1 level | 2.57 (0.657–10.034) | 0.175 | 1.93 (0.628–5.91) | 0.252 |
| Postoperative IGF-1 level | 4.53 (1.07–19.2) | 0.040 | 2.5 (0.807–7.73) | 0.112 |
| Chiasma compression | 3.38 (0.544–21.0) | 0.191 | 0.933 (0.177–4.91) | 0.935 |
| BMI | 0.755 (0.511–1.12) | 0.159 | 0.875 (0.682–1.12) | 0.292 |
| Carpal tunnel syndrome | 2.37 (0.802–6.98) | 0.119 | 2.8 (0.766–10.2) | 0.12 |
| Initial tumour volume | 1.07 (0.944–1.21) | 0.289 | 1.35 (0.905–2.02) | 0.14 |
| Adenoma size | 1.82 (0.16-20.7) | 0.63 | 12 (1.02–141) | 0.048 |
BMI = body mass index; CI = confidence interval; IGF-1 = insulin-growth factor-1; OR = odds ratio
Acromegaly is associated with increased morbidity and a two-fold higher mortality rate, and can result in a severe disease burden with significant distress for the patient [12]. The findings of this registry-based, observational study focusing on predictors of medical outcomes in patients with confirmed acromegaly from a tertiary care centre in Switzerland are threefold.
First, we found an overall relatively high success rate of initial pituitary surgery, with side effects of surgery being rare. A total of 42% of patients had complete remission without need for additional medical treatment. If the tumour did not invade the cavernous sinus (Knosp 0–2), the remission rate after surgery was even higher (67%), which confirms results from other studies [13]. In addition, 33% of patients had remission with medical treatment, but controlled disease.
Second, when studying predictors for outcome, we found initial tumour size to be a predictor for non-full remission, and postoperative IGF-1 levels to be a predictor for uncontrolled disease. Knowing such factors may help to risk stratify patients regarding expected outcomes, which in turn can have an influence on initial and follow-up therapeutic and diagnostic management. It should be mentioned that the reliability of the laboratory analyses depends on the quality in assay performance, of which a certain heterogeneity is well known [7].
Third, despite initial surgery and medical treatment, in 29% of patients the situation was uncontrolled, with patients needing additional surgery and radiotherapy in addition to medical treatment. In accordance with the current guidelines, the next best therapeutic step was decided in the pituitary tumour board (consisting of experts in neurosurgery, endocrinology, radiology, nuclear medicine and oncology). Importantly, in the case of disease persistence, a multimodal approach using drug and radiotherapy within an interdisciplinary care team is mandatory to provide the best possible care for patients.
Our study included a small, but still representative, patient cohort and is in line with previous studies [13, 14]. Specifically, the meta-analysis by Briceno [13], looking at efficacy of trans-sphenoidal surgery in achieving biochemical cure of growth hormone-secreting pituitary adenomas, found an overall remission prevalence of 47.6%, with differences according to type of tumour and sinus invasion. Compared with other registries, our patients had a similar mean age (between 40 and 50 years old) and a similar time lapse from symptom onset to diagnosis of about 5 years. In a population of about 500,000 inhabitants in our area, 21 patients with acromegaly treated over a period of 10 years correspond to a prevalence of 42 patients per million and an incidence of around 4 per year per million. This again is in the range found by studies from other countries. Interestingly, despite the high standard of medical education and treatment in Switzerland, many patients did have typical features of acromegaly and one may argue that patients could have been referred earlier to make the final diagnosis. Owing to the low incidence of acromegaly, general practitioners may be reluctant to measure IGF-1 or to refer patients to a specialised clinic, despite having some suspicion of the diagnosis. It remains an important task to provide ongoing education about this disease to the medical community in order to detect cases early and provide the treatment to patients to prevent secondary complications [14].
The strength of this analysis is the long time period of consecutive patient recruitment and the standardised treatment approach due to local guidelines. As a tertiary care centre, we were able to gather a relatively large number of patients with a rare disease for this analysis. However, several limitations need to be considered. First, the power of our analysis was still low, which negatively impacts on the significance level of predictors in the regression analysis. In addition, the study may have not enough power to show a difference between the surgical approaches. Hence, it is important to persuade more Swiss centres to include their acromegalic patient characteristics into our register. This would allow further analyses with a larger patient collective to confirm the findings and to avoid the bias of the small number of surgeons. On the other hand, inclusion of more data could show additional significant predictors for outcome. Also, pooling data in a meta-analysis would be a possible next step to increase the number of patients and power of the statistical analysis. The study is observational and we do not know if knowledge of prognostic parameters may influence management of patients. Also, we included all patients with acromegaly from our centre based on the medical records, but it is possible that some patients were missed (selection bias). Because over time reference ranges and laboratory tests have changed, there is uncertainty whether all patients included in this registry would fulfill the current diagnostic criteria of acromegaly.
In conclusion, this observational registry study shows a high success rate of initial pituitary surgery in patients with confirmed acromegaly. Initial tumour size and postoperative IGF-1 levels help to risk stratify patients regarding expected outcomes. In case of disease persistence, a multimodal approach using drug and radiotherapy is mandatory.
All authors gave consent for publication
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
We thank all participating patients and caregivers.
The research counsil of the Kantonsspital Aarau provided funding for this study (Forschungsrat Nr. 1410.000.069). No other potential conflict of interest relevant to this article was reported.
1 Buchfelder M , Schlaffer SM . The surgical treatment of acromegaly. Pituitary. 2017;20(1):76–83. doi:.https://doi.org/10.1007/s11102-016-0765-7
2 Lavrentaki A , Paluzzi A , Wass JA , Karavitaki N . Epidemiology of acromegaly: review of population studies. Pituitary. 2017;20(1):4–9. doi:.https://doi.org/10.1007/s11102-016-0754-x
3 Katznelson L , Laws ER, Jr , Melmed S , Molitch ME , Murad MH , Utz A , et al.; Endocrine Society. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933–51. doi:.https://doi.org/10.1210/jc.2014-2700
4 Bonadonna S , Doga M , Gola M , Mazziotti G , Giustina A . Diagnosis and treatment of acromegaly and its complications: consensus guidelines. J Endocrinol Invest. 2005;28(11, Suppl International):43–7.
5 Vilar L , Vilar CF , Lyra R , Lyra R , Naves LA . Acromegaly: clinical features at diagnosis. Pituitary. 2017;20(1):22–32. doi:.https://doi.org/10.1007/s11102-016-0772-8
6 Petersenn S , Buchfelder M , Gerbert B , Franz H , Quabbe HJ , Schulte HM , et al.; Participants of the German Acromegaly Register. Age and sex as predictors of biochemical activity in acromegaly: analysis of 1485 patients from the German Acromegaly Register. Clin Endocrinol (Oxf). 2009;71(3):400–5. doi:.https://doi.org/10.1111/j.1365-2265.2009.03547.x
7 Giustina A , Chanson P , Bronstein MD , Klibanski A , Lamberts S , Casanueva FF , et al.; Acromegaly Consensus Group. A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab. 2010;95(7):3141–8. doi:.https://doi.org/10.1210/jc.2009-2670
8 Almeida JP , Ruiz-Treviño AS , Liang B , Omay SB , Shetty SR , Chen YN , et al. Reoperation for growth hormone-secreting pituitary adenomas: report on an endonasal endoscopic series with a systematic review and meta-analysis of the literature. J Neurosurg. 2017;1:1–13. doi:.https://doi.org/10.3171/2017.2.JNS162673
9 Knosp E , Steiner E , Kitz K , Matula C . Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993;33(4):610–7, discussion 617–8.
10 Hardy J . Transphenoidal microsurgery of the normal and pathological pituitary. Clin Neurosurg. 1969;16(CN_suppl_1):185–217. doi:.https://doi.org/10.1093/neurosurgery/16.CN_suppl_1.185
11 von Elm E , Altman DG , Egger M , Pocock SJ , Gøtzsche PC , Vandenbroucke JP ; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9. doi:.https://doi.org/10.1016/j.ijsu.2014.07.013
12 Ben-Shlomo A , Sheppard MC , Stephens JM , Pulgar S , Melmed S . Clinical, quality of life, and economic value of acromegaly disease control. Pituitary. 2011;14(3):284–94. doi:.https://doi.org/10.1007/s11102-011-0310-7
13 Briceno V , Zaidi HA , Doucette JA , Onomichi KB , Alreshidi A , Mekary RA , et al. Efficacy of transsphenoidal surgery in achieving biochemical cure of growth hormone-secreting pituitary adenomas among patients with cavernous sinus invasion: a systematic review and meta-analysis. Neurol Res. 2017;39(5):387–98. doi:.https://doi.org/10.1080/01616412.2017.1296653
14 Pivonello R , Auriemma RS , Grasso LF , Pivonello C , Simeoli C , Patalano R , et al. Complications of acromegaly: cardiovascular, respiratory and metabolic comorbidities. Pituitary. 2017;20(1):46–62. doi:.https://doi.org/10.1007/s11102-017-0797-7
JK, AS, SB, NM, ND, JF, BM and PS treated the included patients, obtained clinical data or entered the data in the datafile. AS and PS performed the statistical analysis. All authors gave inputs to the final manuscript and agreed to publication.
JK and AS contributed equally to this article.
The research counsil of the Kantonsspital Aarau provided funding for this study (Forschungsrat Nr. 1410.000.069). No other potential conflict of interest relevant to this article was reported.