In-vitro characterisation of a novel antimicrobial agent, TNP-2092, against Helicobacter pylori clinical isolates
DOI: https://doi.org/10.4414/smw.2018.14630
Ben
Wanga, Qiaoyun
Zhaoa, Wenzhu
Yina, Ying
Yuanb, Xiaomei
Wangb, You-hua
Wanga, Hui
Wanga, Wen
Yea, Shuping
Chena, Hai-long
Guoc, Yong
Xiea
aDepartment of Gastroenterology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi province, China
bTenNor Therapeutics (Suzhou) Ltd., Suzhou, Jiangsu province, China
cLeling People's Hospital, Dezhou. Shandong province, China
Summary
BACKGROUND AND OBJECTIVES
TNP-2092 is a novel dual-action lead compound consisting of rifamycin SV and 4H-4-oxo-quinolizine pharmacophores, with a broad spectrum of antibacterial activities. This compound is currently in the early stage of clinical development for Helicobacter pylori infection. The aim of the present study was to determine the antibacterial activity of TNP-2092 against H. pylori isolated from primary patients.
METHODS
A total of 100 H. pylori clinical isolates from primary patients were selected. The minimum inhibitory concentrations (MICs) for clarithromycin, levofloxacin, rifampin and TNP-2092 were determined using an agar dilution method. A time-kill study was performed with different concentrations of TNP-2092 relevant to MIC against H. pylori ATCC strain 43504 for up to 24 hours. The time-kill study with drug concentrations of 0–4 × MIC was also used to determine the antibacterial activity of TNP-2092 against H. pylori under different pH conditions (pH 4–7).
RESULTS
The primary resistance percentages to clarithromycin, levofloxacin, rifampin and TNP-2092 were 13, 18, 1 and 1%, respectively. TNP-2092 killing kinetics were both concentration and time dependent. The effectiveness of TNP-2092 against H. pylori was gradually reduced with a decrease in pH.
CONCLUSIONS
TNP-2092 is highly active against H. pylori and against strains resistant to clarithromycin or levofloxacin. Its antibacterial activity is both concentration- and time-dependent .The antibacterial activity of TNP-2092 appears to be pH-dependent and is more active under neutral pH. TNP-2092 represents a promising new therapy for the treatment of H. pylori infection in primary patients.
Introduction
Helicobacter pylori is one of the most common bacterial pathogens in humans, and is closely associated with a number of prevalent gastrointestinal diseases, such as gastric cancer, chronic gastritis, peptic ulcer diseases, and mucosa-associated lymphoid tissue (MALT) lymphoma [1]. Eradication of H. pylori can prevent the recurrence of peptic ulcers and reduce the risk of developing gastric cancer [2, 3]. Anti-H. pylori therapies consisting of an acid-suppressive drug and/or a bismuth component with two or more antibiotics have been recommended by several consensus reports [3, 4]. However, elevated antimicrobial resistance has limited the efficiency of many once highly successful eradication therapies [5–7]. Therefore, the development of new drugs or drug combinations has been a focus in the field.
TNP-2092 is a novel rifampin-quinolone hybrid antibiotic consisting of covalently conjugated rifamycin SV and 4H-4-oxo-quinolizine pharmacophores; it is currently in the early stage of clinical development for the eradication of H. pylori [8]. TNP-2092 exhibits multitargeting activity against bacterial RNA polymerase as well as DNA gyrase and DNA topoisomerase IV [8–11].A previous study indicated that TNP-2092 might have potential utility in the treatment of persistent Staphylococcus aureus infections [9]. This study sought to assess the in vitro antibacterial activity of TNP-2092 against H. pylori strains isolated from primary patients treated at the First Affiliated Hospital of Nanchang University.
Materials and methods
Patients and bacterial culture
H. pylori isolates were obtained from patients diagnosed with duodenal ulcers who visited the First Affiliated Hospital of Nanchang University from 2013 to 2014 and underwent upper digestive tract endoscopy for the evaluation of dyspepsia. The study was approved by the ethics committee of First Affiliated Hospital of Nanchang University. None of the patients had previous exposure to any eradication therapy for H. pylori infection.
H. pylori was cultured on Columbia agar plates with 5% sheep blood and an H. pylori-selective antibiotic supplement (Oxoid, Basingstoke, UK) containing vancomycin (10 mg/l), cefsulodin (5 mg/l), trimethoprim (5 mg/l) and amphotericin B (5 mg/l). The plates were incubated for up to 2 days at 37°C in microaerophilic conditions (GENbag, Biomerieux). H. pylori was identified from colony and microscopic morphology, as well as positive urease, catalase and oxidase tests.
Susceptibility tests
The minimum inhibitory concentrations (MICs) for clarithromycin (Sigma-Aldrich), rifampin (Sigma-Aldrich), levofloxacin (Sigma-Aldrich) and TNP-2092 (TenNor Therapeutics) were determined using an agar dilution method according to the National Committee for Clinical Laboratory Standards Institute guidelines. H. pylori strains were grown on Mueller-Hinton agar plates. Briefly, H. pylori cells were suspended in physiological saline and adjusted to a turbidity of a McFarland No.2 standard (approximately 107–108 cfu/ml) and inoculated directly onto different concentrations (128–0.0008 µg/ml) of antimicrobial agent-containing agar dilution plates. All plates were incubated as previously described for 3 days. The MIC was determined as the lowest concentration of antimicrobial agent preventing visible growth. For the H. pylori ATCC 43504 strain, the MIC of clarithromycin and levofloxacin should be in the range of 0.016–0.125 µg/ml and 0.016–0.25µg/ml, respectively following the CLSI guidelines. The clarithromycin resistance was defined, according to the EUCAST-approved breakpoint, as ≥0.5 µg/ml. Isolates were defined as resistant to rifampin, TNP-2092, or levofloxacin, when MICs were >1µg/ml [12].
Determination of the killing kinetics of TNP-2092
Time-kill curves were measured by exposing H. pylori ATCC43504 to a range of concentrations (0–64 × MIC) to determine the time required for TNP-2092 to reduce the colony forming units (CFUs) of H. pylori cells. Briefly, bacterial culture at the growth phase was diluted to an OD600 of 0.2 and 20µl of the diluted sample was seeded into 4 ml of Bromfield medium with 10% fetal bovine serum and TNP-2092. After 0, 0.5,1, 3, 5, 7, 9 and 24 hours of incubation in a shaking microaerobic environment, the samples from different concentrations of TNP-2092 were collected, submitted to 10-fold serial dilutions and plated on Karmali’s Campylobacter medium plates. The plates were incubated for 72 hours under 5% CO2, and the CFUs were counted.
Impact of pH changes on the in vitro activity of TNP-2092 against H. pylori
The antibacterial activity of TNP-2092 against H. pylori under different pH conditions was also determined in the time-kill study by applying 0–4 × MIC of the drug against strain 43504 for up to 24 hours at different pH levels. Briefly, bacterial cultures were diluted to an OD600 of 0.2 and 20 µl of the diluted sample was seeded into 4 ml Bromfield medium with 10% fetal bovine serum and TNP-2092 (concentrations 0, 0.25, 0.5 and 1 µg/ml) under different pH conditions (pH 7, 6, 5, 4). After 0, 2, 4, 6, 8, and 24 hours of incubation the samples were collected, diluted and plated on Karmali’s Campylobacter medium plates and incubated for 72 hours under 5% CO2, when CFUs were counted and the log10 value of CFUs was calculated.
Results
MIC and prevalence of antibiotic resistance
The MIC values for clarithromycin, levofloxacin, rifampin and TNP-2092 ranged from 0.008 to16 µg/ml, 0.016 to >128 µg/ml, 0.008 to >128 µg/ml and 0.032 to >128 µg/ml, respectively. The MIC50 of clarithromycin (0.008 µg/ml) was lower than that of levofloxacin (0.25 µg/ml), rifampin (0.125 µg/ml) and TNP-2092 (0.125 µg/ml). However, the MIC90 of TNP-2092 (0.5 µg/ml) and rifampin (0.5 µg/ml) was lower than that of clarithromycin (2 µg/ml) and levofloxacin (8 µg/ml).
Overall resistance percentages among the 100 H. pylori clinical isolates are shown in table 1. The primary resistance percentages to clarithromycin, levofloxacin, rifampin and TNP-2092 were 13% (13/100), 18% (18/100), 1% (1/100) and 1% (1/100), respectively.
Table 1 Resistance percentages and minimum inhibitory concentrations (MICs) of four antibiotics against H. pylori strains.
|
Antibiotic
|
Strains
(n)
|
Range of MIC
(µg/ml)
|
Resistant strains
(%)
|
| Clarithromycin |
100 |
0.008 to 16 |
13 |
| Levofloxacin |
100 |
0.016 to >128 |
18 |
| Rifampin |
100 |
0.008 to >128 |
1 |
| TNP-2092 |
100 |
0.032 to >128 |
1 |
Among the 100 H. pylori isolates, 24 isolates were single-drug resistant to either levofloxacin or clarithromycin (table 1). Double resistance was detected in seven H. pylori isolates (7%) (table 2). All seven of these isolates were resistant to both clarithromycin and levofloxacin. Triple resistance to levofloxacin, rifampin and TNP-2092 was found in one H. pylori isolate (1%) (table 2). No rifampin or TNP-2092 single resistant isolate was detected (0/100).
Table 2 Double and multidrug resistance of H. pylori strains.
|
Antibiotic
|
Resistant strains (%)
|
| Clarithromycin + levofloxacin |
7 |
| Clarithromycin + rifampin |
0 |
| Rifampin + levofloxacin |
0 |
| Clarithromycin + TNP-2092 |
0 |
| levofloxacin+ TNP-2092 |
0 |
| Rifampin + TNP-2092 |
0 |
| Clarithromycin + levofloxacin+ rifampin |
0 |
| levofloxacin+ TNP-2092+ rifampin |
1 |
| Clarithromycin + levofloxacin+ rifampin+ TNP-2092 |
0 |
Killing kinetics of TNP-2092 against H. pylori
The data shown in figure 1 indicate that TNP-2092 is a bactericidal agent. Figure 1 also shows that the slopes of the time-kill curves increased with the increase in drug concentration when the concentration of TNP-2092 was equal to or higher than the MIC (0.25 µg/ml). The higher the concentration of TNP-2092 was, the faster the CFUs were reduced (but not for the concentrations of 2 and 4 µg/ml). In parallel, CFUs also gradually decreased over time. When the concentration of TNP-2092 was lower than that of MIC (0.25 µg/ml), bacterial growth was observed.
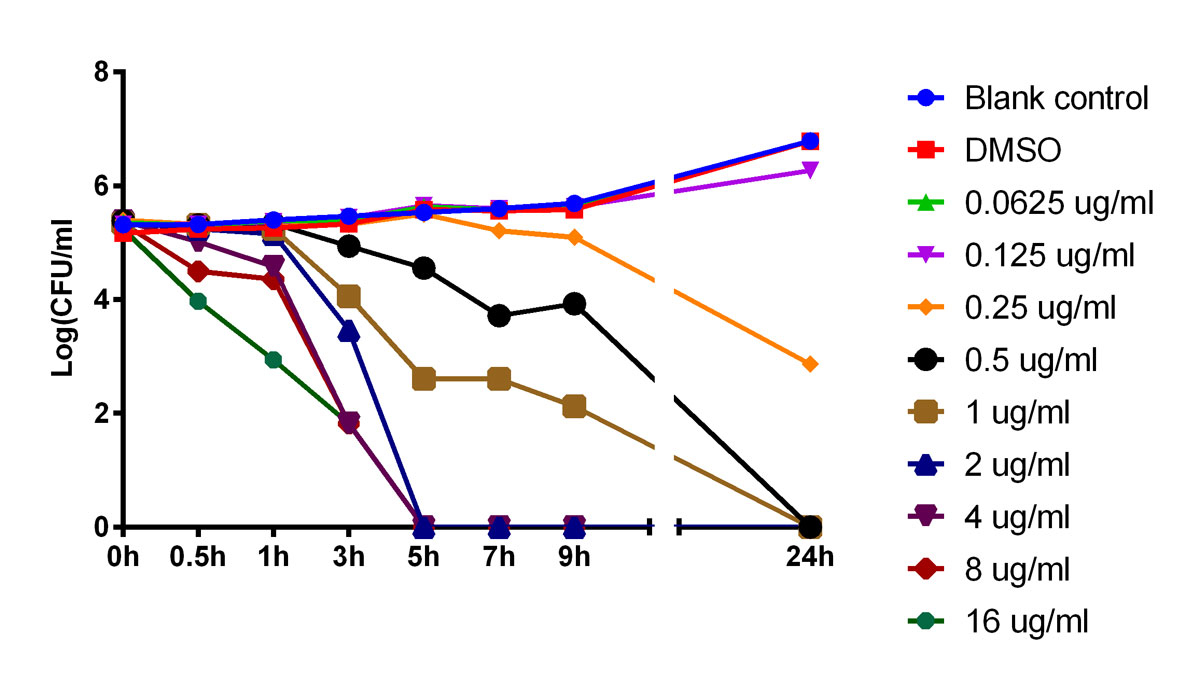
Figure 1 Time-kill kinetics of TNP-2092. The higher the concentration of TNP-2092 was, the faster colony forming units (CFUs) were reduced (but not for concentrations of 2 and 4 µg/ml). In parallel, CFUs were also gradually decreased over time. When the concentrations of TNP was lower than the minimum inhibitory concentration (0.25 µg/ml) bacterial growth was observed.
Determination of the antibacterial activity of TNP-2092 against H. pylori under different pH conditions
As is shown in figure 2, when the concentration of TNP-2092 was 0 µg/ml, H. pylori could not survive at pH 4.0 and not replicate well at pH 5.0. When the concentration of TNP-2092 was 0.25 µg/ml, the antibacterial activity of TNP-2092 against H. pylori seemed to be reduced at pH 5.0 and pH 6.0 compared with activity at pH 7. Similar results were obtained when the concentration of TNP-2092 was 0.5 µg/ml and 1 µg/ml.
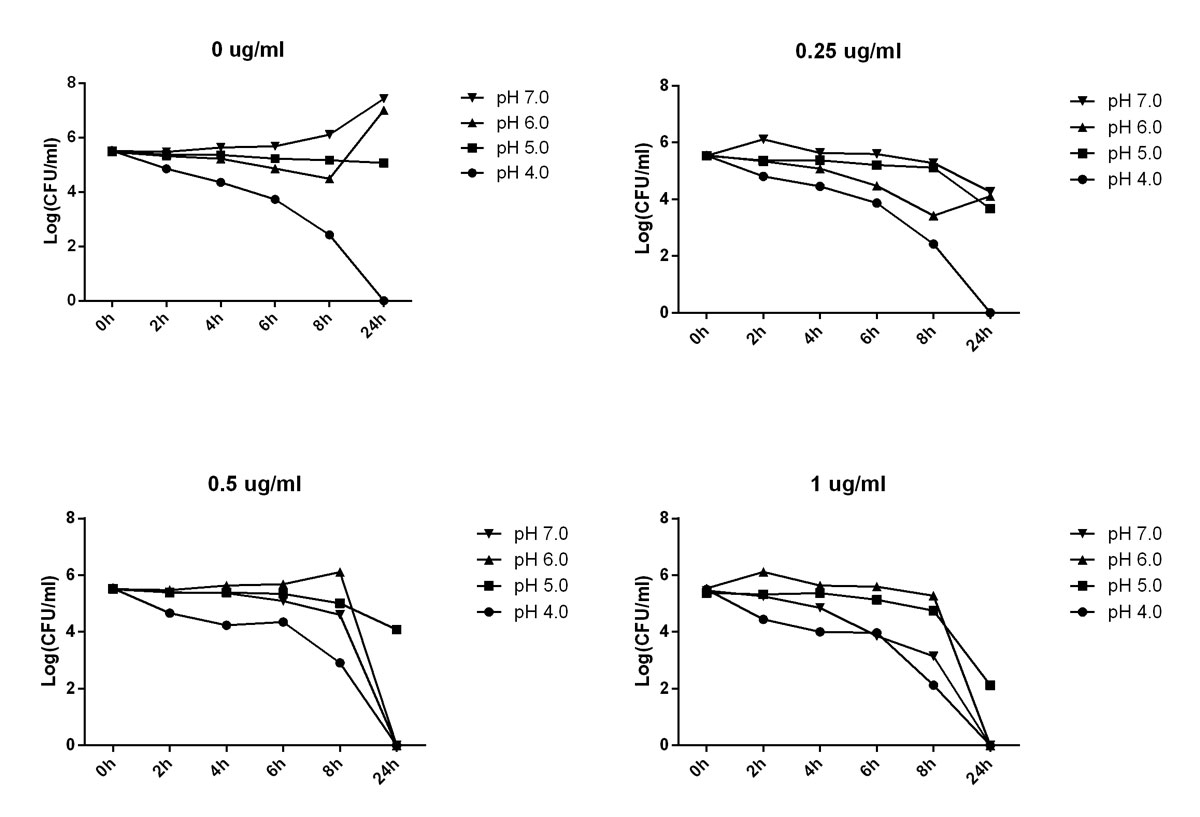
Figure 2 Determination of TNP-2092 in-vitro activity under different pH conditions. When the concentration of TNP-2092 was 0 µg/ml, H. pylori could not survive at pH 4 and could not replicate well at pH 5. When the concentration of TNP-2092 was 0.25 µg/ml, its antibacterial activity against H. pylori seemed to be reduced at pH 5 and 6 compared with pH 7. Similar results were obtained when the concentration of TNP-2092 was 0.5 and 1.0 µg/ml.
Discussion
With the increasing emergence of bacterial resistance to antibiotics, there is an urgent need to develop new regimens and agents for H. pylori infections. TNP-2092, consisting of a rifamycin and a quinolizine pharmacophore, was reported to be highly active against S. aureus and other Gram-positive bacterial species. Since quinolones and rifampin have been used as alternative antibiotics to eradicate H. pylori, it was expected that TNP-2092 may also be active against H. pylori. The results from the present study revealed that TNP-2092 was highly active and bactericidal against H. pylori. Our results also showed that TNP-2092 retained activity against prevalent quinolone-resistant isolates, which indicated that TNP-2092 could be a promising drug when the resistance rate to levofloxacin in the field is high.
Among the 100 H. pylori isolates tested, only one strain was resistant to TNP-2092. The resistance mechanism of this strain is currently under investigation. The dual mechanisms of action of TNP-2092 from both portions of the conjugated pharmacophores appeared to dramatically reduce the resistance rate and were active against the single-drug resistant strains; that is, the compound was active against all 18 quinolone resistant and 13 macrolide resistant isolates.
This study showed that the clarithromycin resistance rate is 13%, which is slightly lower than that reported in some previous studies in China [13, 14]. This discrepancy may have resulted from geographic differences. Clarithromycin belongs to a macrolide family that inhibits protein synthesis at the ribosomal level [15]. Clarithromycin resistance is thought to be closely related to its reduction of the eradication rate as a first-line therapy [16]. Point mutations in the 23 s rRNA gene were described as the main mechanism for clarithromycin resistance in H. pylori [17].
Levofloxacin is a broad spectrum fluoroquinolone inhibiting DNA gyrase, a type II topoisomerase, and topoisomerase IV [18, 19]. Levofloxacin-based therapies have been recommended as a second-line against H. pylori infection by the Maastricht/Florence IV consensus [3]. They showed high efficiency in eradicating H. pylori in many studies [20]. However, the resistance rate of H. pylori to levofloxacin appears to be rising [14, 21, 22] because of its increased use for airway or urinary tract infections [23]. Levofloxacin resistance was linked to the point mutations in the gyrA gene [24]. This present study showed that the levofloxacin resistance rate is 18%, which is consistent with that from a recent multicentre study conducted in China [13]. We also found that 53% of clarithromycin-resistant isolates were also resistant to levofloxacin. As a high resistance rate to levofloxacin may jeopardise the efficacy of clarithromycin, levofloxacin-based therapies may be not good choices as empirical therapies in the clinic. This finding also indicated that levofloxacin should not be administered as a second-line agent to patients in whom clarithromycin-based therapy was not successful, and susceptibility testing is recommended before its administration.
Rifampin is an antibiotic that inhibits bacterial DNA-dependent RNA polymerase, and has been primarily used in the treatment of tuberculosis [25]. In this study, the resistance rate of H. pylori to rifampin only (1%) was much lower than clarithromycin and levofloxacin, which is consistent with other studies conducted in Germany and Japan [26, 27]. Rifampin also retained activity against clarithromycin-and levofloxacin-resistant isolates. Further investigation is needed to explore the mechanism of resistance in this one isolate with resistance to levofloxacin, rifampin and TNP-2092. Rifabutin, a structurally related analogue of rifampin, has been shown to yield acceptable eradication rates as a third-line therapy against H. pylori. However, rifabutin is not available in many areas and is also expensive. Thus, rifampin-based therapies have been considered as a rescue regimen based on its high in-vitro activity against H. pylori.
The pharmacokinetic features of TNP-2092 in figure 1 show that both concentration- and time-dependent activities may serve as references to determine the dosage and the duration of drug administration in animal model studies and human trials.
The data in figure 2 show that the activity of TNP-2092 against H. pylori was reduced at pH 5.0, which might be attributed to several factors such as intrinsic tolerance of the bacterial cells in a nonoptimal growing state, protonation of TNP-2092, which prevents its penetration into bacterial cells and/or the instability of TNP-2092. Further investigation may be needed to address this mechanism. However, the data may suggest coadministration of a proton pump inhibitor to create the optimal local environment for the optimal antibacterial activity of this compound.
Moreover, good compliance with simplicity of drug administration is a vital factor of a successful H. pylori eradication programme [28]. Compliance issues are closely associated with treatment failure in patients with antibiotic-susceptible strains and the development of antibiotic resistance [29]. TNP-2092 may achieve better compliance with its dual-activity feature from both the rifamycin and quinolones pharmacophores, which could decrease the number of drugs administered and simplify the treatment regimen. Moreover, TNP-2092 could make it difficult for H. pylori to develop resistance [8].
Nevertheless, rifampin is an essential antibiotic against Mycobacterium tuberculosis. Furthermore, the wide use of rifampin or rifabutin may increase the resistance rate of M. tuberculosis to this drug class. Thus, rifampin or rifabutin should not be used as a first-line therapy for H. pylori eradication and should be reserved for rescue treatment even though the compounds possess the characteristics of a first-line therapy. However, further in-vitro and in-vivo studies are needed to determine whether it is sufficient to prevent rifamycin resistance in M. tuberculosis.
In conclusion, TNP-2092 is a promising drug candidate for the treatment of diseases associated with H. pylori infection. Clinical studies are needed to further elucidate its efficacy and safety in patients. The antibacterial activity of TNP-2092 appears to be both concentration- and time-dependent, and it is reduced under acidic pH. TNP-2092 has good potential to reduce the development of drug resistance and maintains activity against existing infections caused by drug-resistant H. pylori bacterial cells.
Author contributions
Ben Wang, Qiaoyun Zhao and Wenzhu Yin contributed equally to this work.
References
1
McColl
KE
. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362(17):1597–604. doi:.https://doi.org/10.1056/NEJMcp1001110
2
Gisbert
JP
,
Calvet
X
,
Cosme
A
,
Almela
P
,
Feu
F
,
Bory
F
, et al.; H. pylori Study Group of the Asociación Española de Gastroenterología (Spanish Gastroenterology Association). Long-term follow-up of 1,000 patients cured of Helicobacter pylori infection following an episode of peptic ulcer bleeding. Am J Gastroenterol. 2012;107(8):1197–204. doi:.https://doi.org/10.1038/ajg.2012.132
3
Malfertheiner
P
,
Megraud
F
,
O’Morain
CA
,
Atherton
J
,
Axon
AT
,
Bazzoli
F
, et al.; European Helicobacter Study Group. Management of helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61(5):646–64. doi:.https://doi.org/10.1136/gutjnl-2012-302084
4
Gisbert
JP
,
Calvet
X
,
Gomollón
F
,
Monés
J
; Grupo Conferencia Española de Consenso sobre Helicobacter pylori. Tratamiento erradicador de Helicobacter pylori. Recomendaciones de la II Conferencia Española de Consenso [Eradication treatment of Helicobacter pylori. Recommendations of the II Spanish Consensus Conference]. Med Clin (Barc). 2005;125(8):301–16. Article in Spanish. doi:.https://doi.org/10.1157/13078424
5
Graham
DY
,
Fischbach
L
.
Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59(8):1143–53. doi:.https://doi.org/10.1136/gut.2009.192757
6
Megraud
F
.
Helicobacter pylori and antibiotic resistance. Gut. 2007;56(11):1502. doi:.https://doi.org/10.1136/gut.2007.132514
7
Megraud
F
,
Coenen
S
,
Versporten
A
,
Kist
M
,
Lopez-Brea
M
,
Hirschl
AM
, et al.; Study Group participants.
Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62(1):34–42. doi:.https://doi.org/10.1136/gutjnl-2012-302254
8
Ma
Z
,
Lynch
AS
. Development of a Dual-Acting Antibacterial Agent (TNP-2092) for the Treatment of Persistent Bacterial Infections. J Med Chem. 2016;59(14):6645–57. doi:.https://doi.org/10.1021/acs.jmedchem.6b00485
9
Robertson
GT
,
Bonventre
EJ
,
Doyle
TB
,
Du
Q
,
Duncan
L
,
Morris
TW
, et al.
In vitro evaluation of CBR-2092, a novel rifamycin-quinolone hybrid antibiotic: studies of the mode of action in Staphylococcus aureus
. Antimicrob Agents Chemother. 2008;52(7):2313–23. doi:.https://doi.org/10.1128/AAC.01649-07
10
Robertson
GT
,
Bonventre
EJ
,
Doyle
TB
,
Du
Q
,
Duncan
L
,
Morris
TW
, et al.
In vitro evaluation of CBR-2092, a novel rifamycin-quinolone hybrid antibiotic: microbiology profiling studies with staphylococci and streptococci
. Antimicrob Agents Chemother. 2008;52(7):2324–34. doi:.https://doi.org/10.1128/AAC.01651-07
11
Karpiuk
I
,
Tyski
S
. Looking for the new preparations for antibacterial therapy III. New antimicrobial agents from the quinolones group in clinical trials. Przegl Epidemiol. 2013;67(3):455–60, 557–61.
12European Committee on Antimicrobial Susceptibility Testing. EUCAST clinical breakpoints for Helicobacter pylori. Available from: http://www.eucast.org/clinical_breakpoints/
13
Su
P
,
Li
Y
,
Li
H
,
Zhang
J
,
Lin
L
,
Wang
Q
, et al.
Antibiotic resistance of Helicobacter pylori isolated in the Southeast Coastal Region of China. Helicobacter. 2013;18(4):274–9. doi:.https://doi.org/10.1111/hel.12046
14
Gao
W
,
Cheng
H
,
Hu
F
,
Li
J
,
Wang
L
,
Yang
G
, et al.
The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter. 2010;15(5):460–6. doi:.https://doi.org/10.1111/j.1523-5378.2010.00788.x
15
Hao
H
,
Yuan
Z
,
Shen
Z
,
Han
J
,
Sahin
O
,
Liu
P
, et al.
Mutational and transcriptomic changes involved in the development of macrolide resistance in Campylobacter jejuni
. Antimicrob Agents Chemother. 2013;57(3):1369–78. doi:.https://doi.org/10.1128/AAC.01927-12
16
Molina-Infante
J
,
Perez-Gallardo
B
,
Fernandez-Bermejo
M
,
Hernandez-Alonso
M
,
Vinagre
G
,
Dueñas
C
, et al.
Clinical trial: clarithromycin vs. levofloxacin in first-line triple and sequential regimens for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2010;31(10):1077–84.
17
Sakinc
T
,
Baars
B
,
Wüppenhorst
N
,
Kist
M
,
Huebner
J
,
Opferkuch
W
. Influence of a 23S ribosomal RNA mutation in Helicobacter pylori strains on the in vitro synergistic effect of clarithromycin and amoxicillin. BMC Res Notes. 2012;5(1):603. doi:.https://doi.org/10.1186/1756-0500-5-603
18
Lee
H
,
Hong
SN
,
Min
BH
,
Lee
JH
,
Rhee
PL
,
Lee
YC
, et al.
Comparison of efficacy and safety of levofloxacin-containing versus standard sequential therapy in eradication of Helicobacter pylori infection in Korea. Dig Liver Dis. 2015;47(2):114–8. doi:.https://doi.org/10.1016/j.dld.2014.10.014
19
Drlica
K
,
Zhao
X
. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61(3):377–92.
20
Gisbert
JP
,
Morena
F
. Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther. 2006;23(1):35–44. doi:.https://doi.org/10.1111/j.1365-2036.2006.02737.x
21
Liou
JM
,
Lin
JT
,
Chang
CY
,
Chen
MJ
,
Cheng
TY
,
Lee
YC
, et al.
Levofloxacin-based and clarithromycin-based triple therapies as first-line and second-line treatments for Helicobacter pylori infection: a randomised comparative trial with crossover design. Gut. 2010;59(5):572–8. doi:.https://doi.org/10.1136/gut.2009.198309
22
Federico
A
,
Nardone
G
,
Gravina
AG
,
Iovene
MR
,
Miranda
A
,
Compare
D
, et al.
Efficacy of 5-day levofloxacin-containing concomitant therapy in eradication of Helicobacter pylori infection. Gastroenterology. 2012;143(1):55–61.e1, e13–4. doi:.https://doi.org/10.1053/j.gastro.2012.03.043
23
Karczewska
E
,
Klesiewicz
K
,
Wojtas-Bonior
I
,
Skiba
I
,
Sito
E
,
Czajecki
K
, et al.
Levofloxacin resistance of Helicobacter pylori strains isolated from patients in southern Poland, between 2006-2012. Acta Pol Pharm. 2014;71(3):477–83.
24
Mégraud
F
. Epidemiology and mechanism of antibiotic resistance in Helicobacter pylori
. Gastroenterology. 1998;115(5):1278–82. doi:.https://doi.org/10.1016/S0016-5085(98)70101-5
25
Pinheiro
M
,
Pereira-Leite
C
,
Arêde
M
,
Nunes
C
,
Caio
JM
,
Moiteiro
C
, et al.
Evaluation of the structure-activity relationship of rifabutin and analogs: a drug-membrane study. ChemPhysChem. 2013;14(12):2808–16. doi:.https://doi.org/10.1002/cphc.201300262
26
Fujimura
S
,
Kato
S
,
Kawamura
T
,
Watanabe
A
. In vitro activity of rifampicin against Helicobacter pylori isolated from children and adults. J Antimicrob Chemother. 2002;49(3):541–3. doi:.https://doi.org/10.1093/jac/49.3.541
27
Heep
M
,
Beck
D
,
Bayerdörffer
E
,
Lehn
N
. Rifampin and rifabutin resistance mechanism in Helicobacter pylori
. Antimicrob Agents Chemother. 1999;43(6):1497–9.
28
Chey
WD
,
Wong
BC
; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102(8):1808–25. doi:.https://doi.org/10.1111/j.1572-0241.2007.01393.x
29
O’Connor
JP
,
Taneike
I
,
O’Morain
C
. Improving compliance with helicobacter pylori eradication therapy: when and how?
Therap Adv Gastroenterol. 2009;2(5):273–9. doi:.https://doi.org/10.1177/1756283X09337342

