Baseline characteristics and patterns of care in testicular cancer patients: first data from the Swiss Austrian German Testicular Cancer Cohort Study (SAG TCCS): This article was corrected and republished online on August 29, 2018. Please see Correction ‒ August 29, 2018.
DOI: https://doi.org/10.4414/smw.2018.14640
Christian
Rothermundta, Claudio
Thurneysena, Richard
Cathomasb, Beat
Müllerc, Walter
Mingroned, Anita
Hirschi-Blickenstorfere, Tobias
Wehrhahnf, Christian
Rufg, Sacha I.
Rothschildh, Bettina
Seiferti, Angelika
Terbuchj, Thomas
Grassmuggj, Regina
Woelkyk, Christian
Fankhauserl, Thomas
Kunitm, Natalie
Fischern, Roman
Inaueno, Katrin
Zieglerp, Alan
Haynesp, Peter
Jüniq, Jeanine
Kehla, Silke
Gillessenar
aKlinik für Medizinische Onkologie und Hämatologie, Departement Innere Medizin, Kantonsspital St. Gallen, Switzerland
bKantonsspital Graubünden, Abteilung Onkologie und Hämatologie, Chur, Switzerland
cLuzerner Kantonsspital, Medizinische Onkologie, Luzern, Switzerland
dOnkologiezentrum, Kantonsspital Olten, Switzerland
eOnkozentrum Hirslanden, Klinik Hirslanden, Zürich, Switzerland
fZentrum für Onkologie/Hämatologie, Kantonsspital Aarau AG, T, Switzerland
gUrologischen Abteilung, Bundeswehrzentralkrankenhaus Koblenz, Germany
hDepartement Innere Medizin, Medizinische Onkologie, Universitätsspital Basel, Switzerland
iOnkologie, Kantonsspital Baselland, Liestal, Switzerland
jAbteilung für Onkologie, Universitätsklinik für Innere Medizin, Medizinische Universität Graz, Austria
kMedizinische Onkologie, Kantonsspital Frauenfeld, Switzerland
lDepartment of Urology, University Hospital Zurich, University of Zurich, Switzerland
mUniversitätsklinik Salzburg für Urologie und Andrologie, Landeskrankenhaus, Salzburg, Austria
nMedizinische Onkologie, Kantonsspital Winterthur, Swizterland
oMedizinische Onkologie, Kantonsspital Münsterlingen, Switzerland
pCTU Bern, and Institute of Social and Preventive Medicine (ISPM), University of Bern, Switzerland
qApplied Health Research Centre St. Michaels, Toronto, Ontario, Canada
rUniversity of Bern, Switzerland
This article was corrected and republished online on August 29, 2018. Please see Correction ‒ August 29, 2018.
Summary
BACKGROUND
The majority of germ cell tumour (GCT) patients can be cured by orchiectomy followed by active surveillance or subsequent systemic and/or local treatments. There are various guidelines for a structured follow-up including radiographic and clinical examinations.
OBJECTIVE
The Swiss Austrian German Testicular Cancer Cohort Study (SAG TCCS) prospectively evaluates follow-up, indicator of relapse and late toxicities. This is a descriptive analysis; we present baseline characteristics and treatment strategies for the first 299 patients with primary GCT or relapsed GCT after completion of treatment.
RESULTS
Of the patients included in this study, 192 (64.2%) had seminoma and 107 (35.8%) non-seminoma. Mean age was 41 years (standard deviation [SD] 11.7) for seminoma and 31 (SD 9.3) years for non-seminoma patients. Median tumour size was 3.5 cm (interquartile range 2.5‒5.0 and 2.3‒4.5 in seminoma and non-seminoma, respectively) in both histological groups. Among seminoma patients, 81 (42.2%) had primary tumours >4cm; 154 (80.2%) seminoma patients had stage I, 26 (13.5%) stage II and 12 (6.3%) stage III disease. Fifty-seven (53.3%) non-seminoma tumours were stage I, 29 (27.1%) stage II and 21 (19.6%) stage III. Marker-positive disease was present in 58 (30.2%) seminoma patients and 78 (72.9%) non-seminoma patients. Of 154 stage I seminoma patients, 89 (57.8%) chose active surveillance and 65 (42.2%) adjuvant chemotherapy. Twenty-six (45.6%) stage I non-seminoma patients had high-risk disease; 23 of these were treated with adjuvant chemotherapy and 3 chose active surveillance. Among the 30 (52.6%) low risk stage I patients, all opted for active surveillance. Twelve (46.2%) stage II seminoma patients had radiotherapy, 14 (53.8%) were treated with three to four cycles of chemotherapy. All stage III seminoma patients, and all stage II and III non-seminoma patients were treated with three to four cycles of chemotherapy. Treatment decisions were made at the respective centre. Eleven patients did not receive therapy that conformed with guidelines.
CONCLUSION
It is important to enrol GCT patients in prospective studies in general, but also in follow-up studies to assess baseline characteristics, oncological outcome, and long-term toxicity and to validate the performance of follow-up schedules. This is the first time that the distribution of disease, detailed baseline characteristics and the respective treatment of men with GCT is collected in a prospective manner in German speaking countries (Switzerland, Austria and Germany) and therefore patterns of care have been evaluated. SAG TCCS results will inform on future modifications of surveillance schedules and follow-up procedures.
Trial registration number: NCT02229916 (Clinicaltrials.gov)
Introduction
Germ cell tumours (GCTs) are rare malignancies, but are the most common solid tumours among men between the ages of 15 and 40 years [1]. Throughout the late 20th century, the increase in incidence has been greatest in men of European descent [2]. About 400 men are diagnosed with GCTs in Switzerland every year [3]. Histologically, GCTs comprises pure seminomas in approximately 60% of patients and non-seminomas in 40% [2]. The majority of GCT patients can be cured by orchiectomy and, if necessary, subsequent local or systemic treatments [4]. Active surveillance is a widely accepted strategy in stage I GCT and incorporates close follow-up, with chemotherapy given only to patients subsequently relapsing, thus avoiding treatment in about 50 to 90% of patients [5, 6]. Alternatively, adjuvant chemotherapy can be administered, especially when high-risk features are present, and this minimises the risk of recurrence to below 5% [7–9]. Overall survival for stage I GCT is close to 100% regardless of the management chosen [6]. In stage IIA and selected stage IIB seminoma radiotherapy is an option, whereas all other metastatic GCTs require cisplatin-based chemotherapy [10]. These therapy strategies result in a 10-year overall survival rate of above 95%. However, strict adherence to treatment guidelines is a prerequisite [11, 12].
Given the excellent prognosis, long follow-up after initial treatment is needed, focusing not only on relapse but also on side effects and long-term toxicities including increased risk of secondary malignancies and cardiovascular morbidity, which can occur years after GCT treatment [13]. An interdisciplinary Swiss working group developed evidence-based recommendations for the follow-up of GCT patients that were later adopted by the German Testicular Cancer Study Group [5, 14–16].
Currently, no treatment and outcome data on GCT patients are available for Switzerland. The National Institute for Cancer Epidemiology and Registration (NICER) covers only part of Switzerland and provides statistics only on incidence and mortality. We believe that in GCT patients, more extensive data collection and analysis is needed to study outcome and late toxicities. In contrast to the United Kingdom and Scandinavian countries, in Switzerland, Austria or Germany the care of GCT patients is neither standardised nor centralised. The Swiss Austrian German Testicular Cancer Cohort Study (SAG TCCS) is the first study to prospectively evaluate the initial indicators of relapse in GCT patients after completion of curative treatment, to measure the usefulness of the various follow-up examinations and to collect data on late sequelae following treatment, namely impairment of cardiovascular, renal, pulmonary, gonadal, neuronal and cognitive function, psychosocial disorders, sexual health and socioeconomic aspects.
SAG TCCS is in accordance with recommendations for future research strategies in GCT published 2010 by Travis et al. [13]. These recommendations include: life-long follow-up within the setting of a large prospective cohort study to ascertain risks of emerging toxicities and the evolution of known late sequelae; the development of comprehensive risk prediction models; elucidation of the effect(s) of exposure to platinum; assessment of the overall burden of medical and psychosocial morbidity; and the development of evidence-based guidelines for long-term follow-up and interventions.
Here we report the baseline characteristics and the treatment strategies for the first 299 patients enrolled in this cohort in Switzerland, Austria and Germany between January 2014 and July 2017, while prospective registration is currently ongoing. Therapies were chosen at the discretion of the local investigators based on their respective in-house guidelines. We will report on initial indicators of relapse and on late toxicities in a later manuscript.
Materials and methods
The SAG TCCS of consecutive newly diagnosed and relapsed male GCT patients after completion of curative/salvage treatment is a Swiss, German and Austrian multicentre prospective cohort study. In Switzerland, urologists and medical oncologists in private practice, and secondary and tertiary referral centres were made aware of this cohort study with several mailings. In addition, all participants at the Swiss Group for Clinical Cancer Research Genitourinary Project Group semi-annual meetings were approached. Participation is voluntary.
Male patients of any age can be included after giving written informed consent if they have a histologically proven seminoma or non-seminoma and are within 3 months of treatment completion (surgery of primary tumour, adjuvant chemotherapy, chemotherapy, radiotherapy, salvage therapy, resection of residual disease if applicable). Exclusion criteria are pre-existent malignancy within the past 5 years, with exception of previous GCT, and inability for any reason to comply with the trial investigations of the follow-up schedules. Patients are classified into one of nine follow-up schedules based on initial stage and risk factors, as published by Cathomas et al. [5]
Data collection at study inclusion comprises patient baseline characteristics, tumour assessments, treatment strategies and tumour markers, and thereafter mode of relapse detection and stage of disease at relapse. The database is set to assess late toxicities, including secondary malignancies, gathered prospectively at the standardised follow-up visits and investigations for at least 10 years, or until loss to follow-up or withdrawal of consent. Depending on funding and research interest, follow-up can be extended further while the study is ongoing. Data are entered locally in electronic case report forms and saved and analysed centrally at the Institute of Social and Preventive Medicine (ISPM) in Bern, Switzerland. The data collection is in accordance with the Declaration of Helsinki and ethical approval was obtained.
The primary objective of the SAG TCC study will be to determine the diagnostic performance and the clinical impact of a variety of tests, including conventional radiographs, computed tomography scans, abdominal ultrasound, serum tumour markers including alpha-fetoprotein (AFP), human chorionic gonadotropin (HCG) or lactate dehydrogenase (LDH), and clinical signs and symptoms that aim at early detection of relapse after curative therapy with documented complete remission or partial remission.
Secondary objectives will be: to determine the rate and time-point of relapse, and the rate of intermediate and poor-prognosis disease at relapse; to compare patient characteristics at baseline and at relapse; and to assess late toxicity, i.e., secondary neoplasms, cardiovascular risk factors and disease, treatment sequelae due to organ dysfunction following cancer treatment and, finally, sexual health and socioeconomic wellbeing. These data will be published after a longer follow-up.
We used Fisher’s exact test for binary/categorical variables and Wilcoxon rank-sum tests for continuous variables. This is a descriptive analysis; we present baseline characteristics and treatment strategies for the first 299 patients within SAG TCCS.
Results
A total of 299 patients were included in 14 Swiss, 1 Austrian, and 1 German centre between 10 January 2014 and 1 September 2017. The majority of patients (n = 276) are from Switzerland. The 14 Swiss participating sites are all secondary and tertiary oncology centres; two private urology practices have so far not recruited patients. The German and Austrian sites are tertiary oncology referral centres. Study recruitment status is summarised in figure 1.
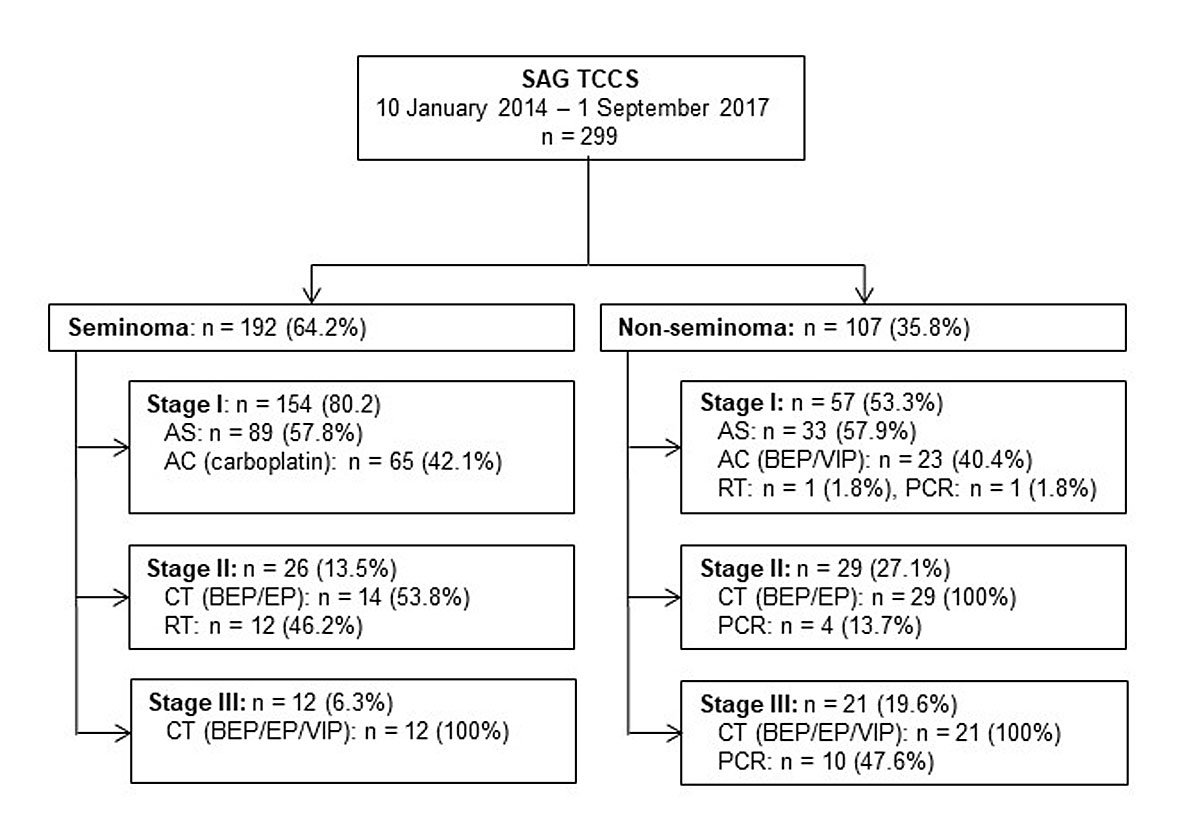
Figure 1 Distribution of GCT patients: AC = adjuvant chemotherapy; AS = active surveillance; BEP = bleomycin, etoposide, cisplatin; CT = curative chemotherapy; EP = etoposide, cisplatin; PCR = postchemotherapy resection; RT = radiotherapy; SAG TCCS = Swiss Austrian German Testicular Cancer Cohort Study; VIP = etoposide, ifosfamide, cisplatin.
Age, histology and tumour size
Mean age at inclusion was 40.9 (standard deviation [SD] 11.7) and 31.1 (SD 9.3) years in seminoma and non-seminoma patients, respectively. Of the 299 patients, 192 (64.2%) had seminoma and 107 (35.8%) non-seminoma.
Median primary tumour diameter was 3.5 cm in seminoma and 3.5 cm in non-seminoma patients, with 81 (42.2%) of the seminoma patients presenting with a primary tumour ≥4 cm. In stage I seminoma, primary tumour size was ≥4 cm in 62 (40.2%) patients. Rete testis invasion (RTI) was found in 89 (46.4%) seminoma patients and 74 (48.1%) patients with seminoma stage I. Lymphovascular invasion (LVI+) was present in 56 (52.3%) non-seminoma patients and 26 (45.6%) non-seminoma patients with stage I.
In SAG TCCS, there is no central pathology review and data are taken from local pathology reports.
Tumour markers and stage of disease
Three serum tumour markers have established roles in the management of men with GCT: alpha-fetoprotein (AFP), human chorionic gonadotropin (HCG) and lactate dehydrogenase (LDH). Any marker elevation constitutes marker-positive disease. Post-orchiectomy levels of tumour markers are used in risk stratification.
In summary, 58 (30.2%) seminoma and 78 (72.9%) non-seminoma patients had marker-positive disease, and 134 (69.8%) seminoma and 29 (27.1%) non-seminoma patients had marker-negative disease. All tumour markers (AFP, HCG and LDH) were available for all patients before start of treatment. In stage I and II seminoma, elevated tumour markers were present in 26.0 and 34.6% of patients, respectively; however, in stage III seminoma, 72.7% of patients had marker-positive disease.
Primary extragonadal GCT (retroperitoneal and mediastinal) was present in eight patients, three with seminoma and five with non-seminoma GCTs.
Twenty-five stage II seminoma and all 29 stage II non-seminoma patients were classified as good prognosis according to the International Germ Cell Cancer Collaborative Group (IGCCCG) classification. One stage II seminoma patient was classified as IGCCCG intermediate prognosis.
In stage III seminoma, 9 (75.0%) patients were classified as IGCCCG [17] good and 3 (25.0%) intermediate prognosis. Of the stage III non-seminoma patients, 10 (47.6%) were classified as IGCCCG good, 6 (28.6%) intermediate and 5 (23.8%) poor prognosis.
Baseline characteristics are summarised in tables 1–3
.
Table 1 Patient characteristics of the SAG TCCS cohort I.
|
Seminoma
|
Non-seminoma
|
p-value
|
|
n
|
%
|
n
|
%
|
|
Total
|
192 |
100.0 |
107 |
100.0 |
|
|
Age at inclusion
|
|
|
|
|
|
| Mean (SD) in years |
40.9 (11.7) |
31.1 (9.3) |
<0.001 |
| <40 years |
96 |
50.0 |
94 |
87.9 |
|
| ≥40 years |
96 |
50.0 |
13 |
12.1 |
<0.001 |
|
Tumour diameter
|
|
|
|
|
|
| Median (IQR) in cm |
3.5 (2.5–5.0) |
3.5 (2.3–4.5) |
0.30 |
| ≥4 cm |
81 |
42.2 |
39 |
36.4 |
|
| <4 cm |
101 |
52.6 |
59 |
55.1 |
|
| Not available |
4 |
2.1 |
5 |
4.7 |
|
|
Clinical stage
|
|
|
|
|
|
| I |
154 |
80.2 |
57 |
53.3 |
<0.001 |
| II |
26 |
13.5 |
29 |
27.1 |
0.47 |
| IIA |
11 |
5.7 |
13 |
12.1 |
|
| IIB |
10 |
5.2 |
14 |
13.1 |
|
| IIC |
5 |
2.6 |
2 |
1.9 |
|
| III |
12 |
6.3 |
21 |
19.6 |
0.18 |
| Extragonadal |
3 |
1.6 |
5 |
4.7 |
|
|
IGCCCG prognosis group
|
|
|
|
|
|
| Good prognosis |
34 |
17.7 |
39 |
36.4 |
|
| Stage II |
25 |
73.5†
|
29 |
74.4†
|
|
| Stage III |
9 |
26.5†
|
10 |
25.6†
|
|
| Intermediate prognosis |
4 |
2.1 |
6 |
5.6 |
|
| Stage II |
1 |
25†
|
|
|
|
| Stage III |
3 |
75†
|
6 |
100†
|
|
| Poor prognosis |
– |
– |
5 |
4.7 |
|
| Stage II |
– |
– |
|
|
|
| Stage III |
– |
– |
5 |
100†
|
|
|
Elevated preoperative tumour markers
|
|
|
|
|
| AFP |
8 |
4.2 |
59 |
55.1 |
<0.001 |
| HCG |
36 |
18.8 |
59 |
55.1 |
<0.001 |
| LDH |
39 |
20.3 |
28 |
26.2 |
0.25 |
| At least one elevated tumour marker |
58 |
30.2 |
78 |
72.9 |
<0.001 |
| Stage I any marker elevation |
40 (of 154) |
26.0*
|
37 (of 57) |
64.9*
|
<0.001 |
| Stage II any marker elevation |
9 (of 26) |
34.6*
|
22 (of 29) |
75.9*
|
0.003 |
| Stage III any marker elevation |
8 (of 11) |
72.7*
|
19 (of 21) |
90.5*
|
0.31 |
|
RTI
|
|
|
|
|
|
| Yes |
89 |
46.4 |
64 |
59.8 |
0.005 |
| No |
86 |
44.8 |
32 |
29.9 |
|
| Not available |
17 |
8.9 |
11 |
10.3 |
|
|
LVI
|
|
|
|
|
|
| Yes |
45 |
23.4 |
56 |
52.3 |
<0.001 |
| No |
134 |
69.8 |
46 |
43.0 |
|
| Not available |
13 |
6.8 |
5 |
4.7 |
|
|
Histological characterisation non-seminoma
|
|
|
|
|
| >50% seminoma |
– |
– |
5 |
4.7 |
|
| >50% embryonal carcinoma |
– |
– |
50 |
46.7 |
|
| >50% yolk sac tumour |
– |
– |
7 |
6.5 |
|
| >50% chorion carcinoma |
– |
– |
3 |
2.8 |
|
| >50% teratoma |
– |
– |
7 |
6.5 |
|
Table 2 Patient characteristics of the SAG TCCS cohort II.
|
Seminoma
|
Non-seminoma
|
p-value
|
|
n
|
%
|
n
|
%
|
|
Total
|
192 |
100.0 |
107 |
100.0 |
|
|
Vital signs
|
|
|
|
|
|
| Mean BMI (SD) in kg/m2
|
25.4 (3.4) |
|
25.5 (4.1) |
|
0.83 |
| Mean BP (SD) in mm Hg |
132/81 (15.7/9.9) |
130/80 (14.0/11.8) |
|
|
Tobacco use
|
|
|
|
|
|
| Current |
49 |
25.5 |
33 |
30.8 |
0.35 |
|
Substance abuse
|
|
|
|
|
|
| Current |
10 |
5.2 |
11 |
10.3 |
0.11 |
|
Education completed
|
|
|
|
|
|
| None |
3 |
1.6 |
3 |
2.8 |
|
| Compulsory education |
15 |
7.8 |
9 |
8.4 |
|
| Apprenticeship |
108 |
56.3 |
61 |
57.0 |
|
| Higher education |
14 |
7.3 |
11 |
10.3 |
|
| Technical college |
29 |
15.1 |
11 |
10.3 |
|
| University/ETH |
23 |
12.0 |
11 |
10.3 |
|
| Not available |
|
|
1 |
0.9 |
|
|
Employment status
|
|
|
|
|
|
| Employed |
176 |
91.7 |
93 |
86.9 |
|
| Employee |
150 |
85.2*
|
88 |
94.6*
|
|
| Self employed |
26 |
14.8*
|
5 |
5.4*
|
|
| Not employed |
16 |
8.3 |
14 |
13.1 |
|
| Unemployed |
4 |
25.0*
|
2 |
14.3*
|
|
| Student |
2 |
12.5*
|
11 |
78.6*
|
|
| Homemaker |
2 |
12.5*
|
|
|
|
| Retired |
4 |
25.0*
|
|
|
|
| Unable to work |
4 |
25.0*
|
1 |
7.1*
|
|
Table 3 Patient characteristics of the SAG TCCS cohort III.
|
Seminoma
|
Non-seminoma
|
p-value
|
|
n
|
%
|
n
|
%
|
|
Total
|
192 |
100.0 |
107 |
100.0 |
|
|
Cryopreservation
|
|
|
|
|
|
| All |
43 |
22.4 |
53 |
49.5 |
<0.001 |
| ≥40 |
5 |
2.6 |
|
|
|
| <40 |
38 |
19.8 |
53 |
49.5 |
0.016 |
|
Contralateral biopsy
|
|
|
|
|
|
| All |
71 |
37.8 |
59 |
55.1 |
0.005 |
| Not available |
4 |
2.1 |
|
|
|
|
Postoperative testosterone
|
|
|
|
|
|
| Normal (9.72–35.17 nmol/l) |
56 |
29.2 |
27 |
25.2 |
|
| Abnormal (<9.72 nmol/l) |
14 |
7.3 |
6 |
5.6 |
|
| Not available |
122 |
63.5 |
74 |
69.2 |
|
| mean (SD value) nmol/l |
5.3 (2.8) |
3.4 (2.8) |
|
Treatment
The majority of stage I seminoma patients (n = 89, 57.8%) chose active surveillance, and of these 42 (47.2%) presented with neither a tumour size ≥4 cm nor RTI. However, 14 (15.7%) patients with a tumour diameter ≥4 cm and RTI opted for active surveillance. Adjuvant treatment with one cycle of carboplatin AUC7 (i.e., the dosage reaching an area under the curve of 7 mg/ml/min) was given to 61 (39.6%) stage I seminoma patients. Three (1.9%) patients were treated with two cycles of carboplatin AUC7 and one patient (0.6%) with carboplatin AUC7 and radiotherapy. The majority of patients who received adjuvant treatment for stage I seminoma presented with either a tumour size ≥4 cm (n = 38, 58.4%), or RTI (n = 42, 64.6%) or both (n = 24, 36.9%). Thirty-nine (51.3%) of the stage I seminoma patients aged over 40 were treated with adjuvant chemotherapy.
Among patients with stage II seminoma, 11 (42.3%) received three cycles of bleomycin, etoposide and cisplatin (BEP) and 3 (11.5%) patients four of cycles of etoposide and cisplatin (EP). Twelve (46.2%) seminoma stage II patients had radiotherapy, 11 in combination with once cycle of carboplatin AUC7 chemotherapy in the Swiss Group for Clinical Cancer Research (SAKK) 01/10 study. One seminoma stage II patient had radiotherapy only.
Radiotherapy was applied in five (45.5%) stage IIA and seven (70.0%) stage IIB seminoma patients.
Five (41.7%) stage III seminoma patients were treated with three cycles BEP, five (41.7%) patients with four cycles of EP, and one (9.1%) patient received two cycles BEP followed by two cycles of etoposide, ifosfamide and cisplatin (VIP) each. One patient was treated in the TIGER study protocol after relapse [18]. One seminoma patient underwent resection of residual disease after chemotherapy completion.
Of the stage I non-seminoma patients, 33 (57.9%) chose active surveillance. All 30 (52.6%) patients without LVI and thus with low-risk disease opted for active surveillance. Twenty-six (45.6%) stage I non-seminoma patients had LVI+ as high-risk feature. Only 3 (11.5%) of these chose active surveillance, whereas 23 (88.5%) were treated with adjuvant chemotherapy consisting of one or two cycles of BEP. One (1.8%) non-seminoma stage IS patient was treated with three cycles of BEP. In one patient with sarcomatoid features on histology, two cycles VIP were given as adjuvant chemotherapy, and one non-seminoma stage I patient (1.8%) was treated with radiotherapy.
Of the stage II non-seminoma patients, 27 (93.1%) were treated with three cycles of BEP, 2 (6.9%) with four cycles of EP and none with radiotherapy. In stage III non-seminoma GCTs, 13 patients (61.9%) were treated with three or four cycles of BEP. Four patients (19.0%) were treated with four cycles of VIP. Two patients (9.5%) received four cycles of EP. One (4.8%) patient was switched to the Group d’Étude des Tumeurs Urogénitales (GETUG) 13 protocol [19] after one cycle of BEP, and one (4.8%) had three cycles of BEP followed by one cycle of EP. Fifteen (14.0%) patients underwent resection of residual disease after completing chemotherapy; most were in stage III.
Summary of treatment is displayed in table 4 for seminomas and table 5 for non-seminoma GCTs.
Table 4 Summary of treatment in seminoma patients.
|
n
|
%
|
|
Total
|
192
|
100.0
|
|
Stage I
|
154
|
80.2
|
| Active surveillance |
89 |
57.8*
|
| Tumour diameter ≥4 cm |
24 |
27.0†
|
| RTI |
32 |
36.0†
|
| Tumour diameter ≥4 cm with RTI |
14 |
15.7†
|
| Tumour diameter <4 cm without RTI |
42 |
47.2†
|
| Age ≥40 years |
37 |
41.6†
|
| Carboplatin AUC7 (1 cycle) |
61 |
39.6*
|
| Tumour diameter ≥4cm |
35 |
57.4†
|
| RTI |
40 |
65.6†
|
| Tumour diameter ≥4 cm with RTI |
23 |
37.7†
|
| Tumour diameter <4 cm without RTI |
9 |
14.8†
|
| Age ≥40 years |
37 |
60.7†
|
| Carboplatin AUC7 (2 cycles, overtreatment) |
3 |
1.9*
|
| Tumour diameter ≥4 cm |
2 |
66.7†
|
| RTI |
2 |
66.7†
|
| Tumour diameter ≥4 cm with RTI |
1 |
33.3†
|
| Age ≥40 years |
1 |
33.3†
|
| Carboplatin AUC7 (1 cycle) and RT (overtreatment) |
1
|
0.6*
|
| Tumour diameter ≥4 cm |
1 |
100.0†
|
| Age ≥40 years |
1 |
100.0†
|
|
Stage II
|
26
|
13.5
|
| Good prognosis |
14 |
53.8*
|
| BEP (3 cycles) |
11 |
78.6‡
|
| EP (4 cycles) |
3 |
21.4‡
|
| Intermediate prognosis |
|
|
| Radiotherapy |
12 |
46.2*
|
| RT only |
1 |
8.3†
|
| RT and carboplatin AUC7 (1 cycle) |
11 |
91.7†
|
| Stage IIA |
5 |
41.7†
|
| Stage IIB |
7 |
58.3†
|
| Stage IIC |
|
|
|
Stage III
|
12
|
6.3
|
| Good prognosis |
9 |
75.0*
|
| BEP (3 cycles) |
4 |
44.4‡
|
| EP (4 cycles) |
4 |
44.4‡
|
| TIGER protocol, ASCT, |
1 |
11.2‡
|
| Intermediate prognosis |
3 |
25.0*
|
| BEP (3 cycles, undertreatment) |
1 |
33.3‡
|
| EP (4 cycles, undertreatment) |
1 |
33.3‡
|
| BEP (4 cycles) |
|
|
| VIP (4 cycles) |
|
|
| BEP (2 cycles), VIP (2 cycles) |
1 |
33.3‡
|
|
Resection
|
1 |
0.5 |
| Stage II |
|
|
| Stage III |
1 |
100.0†
|
Table 5 Summary of treatment in non-seminoma patients.
|
n
|
%
|
|
Total
|
107
|
100.0
|
|
Stage I
|
57
|
53.3
|
| Active surveillance |
33 |
57.9*
|
| LVI+ |
3 |
9.1†
|
| Age ≥40 years |
2 |
12.1†
|
| BEP adjuvant |
22 |
38.6*
|
| LVI+ |
22 |
100.0†
|
| Age ≥40 years |
2 |
3.5†
|
| 1 cycle |
19 |
86.4†
|
| 2 cycles |
2 |
9.1†
|
| 3 cycles (overtreatment) |
1 |
4.5†
|
| EP adjuvant |
|
|
| VIP adjuvant (2 cycles, not standard treatment) |
1 |
1.8*
|
| LVI+ |
1 |
100.0†
|
| Age ≥40 years |
1 |
100.0† |
| Radiotherapy (not standard treatment) |
1 |
1.8*
|
| Age ≥40 years |
1 |
100.0†
|
|
Stage II
|
29
|
27.1
|
| Good prognosis |
29 |
100.0*
|
| BEP (3 cycles) |
27 |
93.1‡
|
| EP (4 cycles) |
2 |
6.9‡
|
| Intermediate and poor prognosis |
0 |
0 |
|
Stage III
|
21
|
19.6
|
| Good prognosis |
10 |
47.6*
|
| BEP (3 cycles) |
8 |
80.0‡
|
| EP (4 cycles) |
2 |
20.0‡
|
| Intermediate and poor prognosis |
11 |
52.4*
|
| BEP (3 cycles, undertreatment) |
1 |
9.1‡
|
| BEP (3 cycles), EP (1 cycle) |
1 |
9.1‡
|
| BEP (4 cycles) |
4 |
36.4‡
|
| VIP (4 cycles) |
4 |
36.4‡
|
| BEP (1 cycle), GETUG-13 Protocol (4 cycles) |
1 |
9.1‡
|
|
Postchemotherapy resection
|
15
|
14.0
|
| Stage I (not standard treatment) |
1 |
1.8*
|
| Stage IIA |
1 |
3.4*
|
| Stage IIB |
3 |
10.3*
|
| Stage IIC |
0 |
0 |
| Stage III |
10 |
47.6*
|
Health-related and socioeconomic patient characteristics
At inclusion, mean body mass index (BMI) was 25.4 (SD 3.4) and 25.5 (SD 4.1) kg/m2, mean blood pressure was 132/81 (SD 15.7/9.9) mm Hg and 130/80 (SD 14.0/11.8) mm Hg in seminoma and non-seminoma patients, respectively.
Testosterone levels after orchiectomy, chemo- or radiotherapy were available for only 70 (36.5%) and 33 (30.8%) of seminoma and non-seminoma patients, respectively. Of these, 14 (7.3%) seminoma and 6 (5.6%) non-seminoma patients had abnormally low testosterone values (<9.72 nmol/l) with a mean value of 5.3 (SD 2.8) and 3.4 (SD 2.8) nmol/l in seminoma and non-seminoma patients, respectively. In seminoma, a total of 43 (22.4%) had semen cryopreservation before treatment. For 53 (49.5%) non-seminoma patients cryopreservation was performed.
Fifteen (7.8%) and 9 (8.4%) of seminoma and non-seminoma patients, respectively, had no or only compulsory education. One hundred and eight (56.3%) and 61 (57.0%), respectively, had finished an apprenticeship, and 14 (7.3%) and 11 (10.3%), respectively, high school. Fifty-two (27.1%) seminoma and 22 (20.6%) non-seminoma patients had a college or university degree. Most patients were employed at inclusion in SAG TCCS: 176 (91.7%) seminoma and 93 (86.9%) non-seminoma patients.
There were 49 (25.5%) current smokers and 10 (5.2%) current substance abusers in the seminoma population at the time of inclusion. Among non-seminoma patients, 33 (30.8%) were current smokers and 11 (10.3%) current substance abusers.
Discussion
We report first data from SAG TCCS. This is the first tri-national cohort study that prospectively collects data from seminoma and non-seminoma patients after completion of GCT treatment and from start of follow-up, thus providing insight into baseline characteristics and patterns of care. Outcome data will be presented at a later time. In contrast, many other reports on GCT baseline characteristics and patterns of care were retrospective in nature.
One major limitation of our cohort is that all recruiting sites are secondary or tertiary referral centres. This causes bias and our data may not reflect routine practise and care for GCT patients in Switzerland, Austria and Germany.
The mean age in our cohort was 40.9 years (SD 11.7) for seminoma and 31.1 years (SD 9.3) for non-seminoma patients and compares well with other series [20, 21]. However, in a retrospective analysis from North Texas, a region with a significant native-born and immigrant Hispanic population, Hispanic patients presented with GCT at a significantly younger age (mean ± SD 29.9 ± 8.9 years ) than non-Hispanic white (NHW) patients (34.0 ± 11.2 years). This approximately 5 year age difference was seen both in the institutional cohort and a query to the National Cancer Database [22]. Interestingly, in the retrospective analysis from Germany, the mean age of patients with GCT increased significantly from 28 years (before 1990) to 36 years (2005–2010). Likewise, age shifts were found in both of the histological subgroups. Mean age rose from 34 to 39 years in seminoma and from 26 to 31 years in non-seminoma patients. The aetiology of the increasing age at diagnosis in a predominantly Caucasian population remains unclear [20].
In our cohort, 192 (64.2%) seminoma patients and 107 (35.8%) non-seminoma patients were enrolled. In accordance with epidemiological studies, the distribution of seminomas and non-seminoma GCTs has been changing over the years, and pure seminoma has become the more common histology. Interestingly, in the stage I seminoma and non-seminoma cohorts presented by Kollmannsberger et al., an equal distribution between seminomas and non-seminoma tumours was seen [6]. The SAG TCCS cohort is similar to the East Anglian GCT group data (58% seminoma patients in the 1996–2002 period) [23] and Danish national data with 60% seminomas and 40% non-seminoma tumours among all stage I GCTs [24].
In past decades there has been a significant reduction in size of the primary tumour from 5 to 4 cm, as reported in the East Anglian GCT group database [23]. The German National Seminoma Registry Study (NSR study) reported a mean tumour size in stage I seminoma of 3.67 ± 2.1 cm. In the cohort from North Texas, testicular tumour size was 4.1 ± 2.8 cm in NHW patients compared with 5.9 ± 4.6 cm in Hispanic patients [22]. Both tumour size ≥4 cm and RTI were present in 38 of 154 (24.6%) stage I seminoma patients in our cohort, compared with 33% in the Spanish Germ-Cell Cancer Group (SGCCG) cohort [25]. In stage I seminoma, tumour size and RTI have been described as risk factors for relapse: presence of both was associated with a 5-year relapse rate of 32% and absence of both with a 5-year relapse rate of 12% [26]. Later studies have not validated both factors, and whereas previously tumour size was used in the form of a cut-off of 4 cm, it is now also postulated to be a continuous variable. However, in a recent publication both tumour diameter >4 cm and RTI were confirmed as independent risk factors for relapse [27]. Furthermore, a systematic literature search and analysis of 19 studies proposed tumour size in stage I seminoma as the most valuable prognostic factor [28].
LVI+ is a risk factor for relapse in non-seminoma stage I. Twenty-six of 57 (45.6%) non-seminoma stage I patients in SAG TCCS were LVI+, whereas in the Swedish and Norwegian Testicular Cancer Group (SWENOTECA) cohort 36% were LVI+ [9].
Fifty-eight (30.2%) seminoma patients presented with marker-positive disease before start of treatment (semicastration or salvage chemotherapy after relapse), mostly as elevated HCG (n = 36, 18.8%). In our cohort, the marker-positive rate for stage I seminoma at diagnosis was a little higher than the rate reported by Aparico et al. (26% in SAG-TCCS vs 15%) [29]. In eight seminoma patients (4.2%), AFP was mildly elevated. Theoretically, elevated AFP is a sign of non-seminomatous histology, but it can also be elevated in non-malignant conditions such as alcohol abuse, hepatitis, cirrhosis, biliary tract obstruction and other conditions such as Fanconi anaemia. Some individuals have familial, hereditary, mildly elevated serum AFP levels in the range of 15 to 30 µg/l [30]. Just recently a report on mildly elevated AFP among patients after treatment completion in GCT, among them 4 seminoma patients, was published. The authors postulated that mildly elevated and stable AFP should be managed with surveillance [31]. For seminoma patients the rate of elevated HCG is reported at 20 to 30% in advanced disease and the rate of increased LDH at 40 to 60% in all stages [32].
A total of 78 (72.9%) non-seminoma patients showed marker-positive disease, which is in line with the experience of the SGCCG [25]. Our data show elevated AFP and HCG with an equal distribution (55.1% each). Any tumour marker was elevated in stage I, II and III in 64.9, 75.9 and 90.5% of patients, respectively. These results are comparable to published data [32].
More seminoma patients were diagnosed in stage I compared with non-seminoma patients (n = 154, 80.2% and n = 57, 53.3%). Seminoma stage I represented >50% of all GCT cases in our study; therefore recommendations for the management of these patients are particularly important since they affect the largest population [20].
As to treatment decisions, 42.2% of stage I seminoma patients received adjuvant chemotherapy in our cohort. In the prospective SWENOTECA cohort of stage I seminomas, 52.3% of the patients opted for adjuvant chemotherapy, and in the NSR study 57.8% of patients did so [21, 27]. Most recurrences on adjuvant chemotherapy occur within 3 years and can effectively be treated with cisplatin-based chemotherapy, with cure rates of 99% [6]. Adjuvant chemotherapy with carboplatin AUC7 yields a 75% relapse risk reduction to about 4% in stage I seminoma without risk adaption, hence significantly fewer men need chemotherapy or radiotherapy for metastatic disease [33]. Recurrence after initial adjuvant treatment with carboplatin displays the same excellent survival probability as de-novo metastatic disease, with a 5-year overall survival of 98% [34]. In a seminal paper, relapse-free rates for patients treated with carboplatin AUC7 were not inferior to adjuvant radiotherapy, but with less short-term toxicity, no significant effect on fertility and almost no long-term toxicity compared with radiation (although long term data are sparse) [35, 36]. Therefore, radiotherapy is no longer standard of care in stage I seminoma and should be reserved for special cases. For stage I seminoma, active surveillance and treatment with adjuvant chemotherapy are options. Shared decision-making with the patient about adjuvant chemotherapy versus active surveillance after orchiectomy should be routine today [37]. It should be taken into consideration that carboplatin offers little risk for short-term toxicity and a significant reduction of relapse risk, but at least an 80% risk of overtreatment. There are insufficient data on long-term toxicity. Relapse, however, necessitates intense salvage chemotherapy with disruption of normal life, impaired quality of life and a substantial risk of long-term toxicity including second malignancies and cardiovascular disease [38]. An adverse prognostic risk factor for GCT-specific mortality is age above 40 years, as salvage therapy cannot be delivered to all such patients [39], thus indicating a potential advantage of adjuvant chemotherapy irrespective of risk factors.
Approximately 56% of high-risk and 86% of low-risk stage I non-seminoma patients are cured after orchiectomy alone [6]. There is a recommendation for active surveillance in low-risk and one cycle of adjuvant BEP in high-risk stage I non-seminoma GCTs [9]. Our data show a majority of stage I non-seminoma patients with high-risk disease (n = 22, 88%) were treated with BEP adjuvant chemotherapy and only 3 patients (12%) preferred active surveillance, whereas all patients with low risk disease received active surveillance. There is no rationale for radiotherapy in non-seminoma stage I tumours and we would not endorse this treatment strategy.
A systematic review and meta-analysis showed that radiotherapy and BEP or EP chemotherapy appear to be equal options in stage IIA and IIB seminoma. However, there is a trend in favour of chemotherapy, with a lower incidence of side-effects and relapse rate, especially in stage IIB [40].
Five patients with stage IIA seminoma (45.5% of all stage IIA) and seven patients with stage IIB seminoma (70% of all stage IIB) were treated with radiotherapy in our cohort.
All patients with advanced disease received poly-chemotherapy and 15 patients underwent resection for residual disease.
Two IGCCCG intermediate-prognosis stage III seminoma patients received either three cycles of BEP or four cycles of EP instead of four cycles of BEP. This is not according to guidelines and represents undertreatment.
One seminoma stage I patient was treated with radiotherapy and carboplatin, one non-seminoma stage I patient had radiotherapy, and another non-seminoma stage I patient received two cycles VIP, all of which are not supported by any data.
We would like to highlight the importance of evidence-based treatment decisions in this highly curative disease.
Prognosis appears to be associated with socioeconomic status. GCT-specific mortality was doubled among US patients diagnosed with seminoma or non-seminoma who were >40 years of age compared with those aged <40 years. Among men with non-seminoma tumours, non-white race and lower socioeconomic status was also associated with increased GCT-specific mortality [39]. In our cohort, 18 (9.4%) and 12 (11.2%) of seminoma and non-seminoma patients, respectively, had no or only compulsory education.
Data on BMI, blood pressure, and testosterone levels serve as a baseline and will be reassessed annually during follow-up.
There are data from a systemic review and meta-analysis on an association of current, chronic, and frequent cannabis use with the development of GCT when compared with never-use of the drug. The strongest association was found for non-seminoma GCTs. In our cohort, twice as many non-seminoma patients as seminoma patients were current substance abusers (10.3 vs 5.2%) [41]. In a recently published cohort study of 49,343 Swedish males born between 1949 and 1951 who underwent extensive medical and psychological assessment at nationwide conscription for the compulsory Swedish military service between 1969 and 1970, no evidence of a significant relation between lifetime “ever” cannabis use and the subsequent development of GCT was found, but “heavy” cannabis use (>50 times in lifetime, as reported at conscription) was associated with a 2.5-fold increased hazard of subsequent GCT. Although the Swedish study and three previous studies [42–44] have shown a relation between frequency and duration of cannabis use and the development of GCT, evidence of a clear dose-response curve – a prerequisite for establishing a persuasive argument for the causal link – is still lacking [45].
GCTs are the only solid tumours that can be cured in the majority of patients with metastatic disease. However, treatment in more advanced disease (intermediate and poor prognosis) is more intensive and cure rates are significantly lower than in the good prognosis group. Active surveillance is a valid strategy in stage I GCT, but early and reliable detection of relapse is essential. It is important to enrol patients in prospective studies to assess not only oncological outcome but also therapy-associated long-term toxicity, and to validate the performance of follow-up schedules and relapse screening modalities stratified by tumour entity, stage and treatment strategy.
This is the first time that the distribution of disease, detailed baseline characteristics and the treatment of men with GCT has been collected in a prospective manner in German speaking countries (Switzerland, Austria and Germany) and therefore patterns of care have been evaluated. Results of SAG TCCS will inform on outcome, future modifications of surveillance schedules and follow-up procedures.
References
1Schweizerischer Krebsbericht. 2015. 14 ed: Bundesamt für Statistik (BFS); Nationales Institut für Krebsepidemiologie und -registrierung (NICER); Schweizer Kinderkrebsregister (SKKR); 2016.
2
Rajpert-De Meyts
E
,
McGlynn
KA
,
Okamoto
K
,
Jewett
MA
,
Bokemeyer
C
. Testicular germ cell tumours. Lancet. 2016;387(10029):1762–74. doi:.https://doi.org/10.1016/S0140-6736(15)00991-5
3http://asrtch/nicer/.
4
Hanna
NH
,
Einhorn
LH
. Testicular cancer--discoveries and updates. N Engl J Med. 2014;371(21):2005–16. doi:.https://doi.org/10.1056/NEJMra1407550
5
Cathomas
R
,
Helbling
D
,
Stenner
F
,
Rothermundt
C
,
Rentsch
C
,
Shahin
O
, et al.
Interdisciplinary evidence-based recommendations for the follow-up of testicular cancer patients: a joint effort. Swiss Med Wkly. 2010;140(25-26):356–69.
6
Kollmannsberger
C
,
Tandstad
T
,
Bedard
PL
,
Cohn-Cedermark
G
,
Chung
PW
,
Jewett
MA
, et al.
Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J Clin Oncol. 2015;33(1):51–7. doi:.https://doi.org/10.1200/JCO.2014.56.2116
7
Oliver
RT
,
Mason
MD
,
Mead
GM
,
von der Maase
H
,
Rustin
GJ
,
Joffe
JK
, et al.; MRC TE19 collaborators and the EORTC 30982 collaborators. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366(9482):293–300. doi:.https://doi.org/10.1016/S0140-6736(05)66984-X
8
Mortensen
MS
,
Bandak
M
,
Kier
MG
,
Lauritsen
J
,
Agerbaek
M
,
Holm
NV
, et al.
Surveillance versus adjuvant radiotherapy for patients with high-risk stage I seminoma. Cancer. 2017;123(7):1212–8. doi:.https://doi.org/10.1002/cncr.30458
9
Tandstad
T
,
Dahl
O
,
Cohn-Cedermark
G
,
Cavallin-Stahl
E
,
Stierner
U
,
Solberg
A
, et al.
Risk-adapted treatment in clinical stage I nonseminomatous germ cell testicular cancer: the SWENOTECA management program. J Clin Oncol. 2009;27(13):2122–8. doi:.. Corrected in: J Clin Oncol. 2010;28(8):1438 https://doi.org/10.1200/JCO.2008.18.8953
10
Glaser
SM
,
Vargo
JA
,
Balasubramani
GK
,
Beriwal
S
. Stage II Testicular Seminoma: Patterns of Care and Survival by Treatment Strategy. Clin Oncol (R Coll Radiol). 2016;28(8):513–21. doi:.https://doi.org/10.1016/j.clon.2016.02.008
11Horner MJRL, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2006. Bethesda, MD: National Cancer Institute; 2009.
12
Verdecchia
A
,
Francisci
S
,
Brenner
H
,
Gatta
G
,
Micheli
A
,
Mangone
L
, et al.; EUROCARE-4 Working Group. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8(9):784–96. doi:.https://doi.org/10.1016/S1470-2045(07)70246-2
13
Travis
LB
,
Beard
C
,
Allan
JM
,
Dahl
AA
,
Feldman
DR
,
Oldenburg
J
, et al.
Testicular cancer survivorship: research strategies and recommendations. J Natl Cancer Inst. 2010;102(15):1114–30. doi:.https://doi.org/10.1093/jnci/djq216
14
Hartmann
M
,
Krege
S
,
Souchon
R
,
De Santis
M
,
Gillessen
S
,
Cathomas
R
; Interdisziplinäre Arbeitsgruppe Hodentumore. Nachsorge von Patienten mit Hodentumoren [Follow-up of testicular germ cell cancer patients: interdisciplinary evidence-based recommendations]. Urologe A. 2011;50(7):830–5. Article in German. doi:.https://doi.org/10.1007/s00120-011-2556-0
15
Cathomas
R
,
Hartmann
M
,
Krege
S
,
Souchon
R
,
Lorch
A
,
Mayer
F
, et al.; interdisziplinäre Arbeitsgruppe Hodentumore. Interdisciplinary evidence-based recommendations for the follow-up of testicular germ cell cancer patients. Onkologie. 2011;34(1-2):59–64.
16
Souchon
R
,
Hartmann
M
,
Krege
S
,
Lorch
A
,
Mayer
F
,
De Santis
M
, et al.
Interdisziplinäre evidenzbasierte Empfehlungen für die Nachbeobachtung von Patienten mit testikulärem Seminom im Frühstadium [Interdisciplinary evidence-based recommendations for the follow-up of early stage seminomatous testicular germ cell cancer patients]. Strahlenther Onkol. 2011;187(3):158–66. doi:.https://doi.org/10.1007/s00066-010-2227-x
17
International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15(2):594–603. doi:.https://doi.org/10.1200/JCO.1997.15.2.594
18
https://clinicaltrials.gov/ct2/show/NCT02375204?term=NCT02375204&rank=1.
19
Fizazi
K
,
Pagliaro
L
,
Laplanche
A
,
Fléchon
A
,
Mardiak
J
,
Geoffrois
L
, et al.
Personalised chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG 13): a phase 3, multicentre, randomised trial. Lancet Oncol. 2014;15(13):1442–50. doi:.https://doi.org/10.1016/S1470-2045(14)70490-5
20
Ruf
CG
,
Isbarn
H
,
Wagner
W
,
Fisch
M
,
Matthies
C
,
Dieckmann
KP
. Changes in epidemiologic features of testicular germ cell cancer: age at diagnosis and relative frequency of seminoma are constantly and significantly increasing. Urol Oncol. 2014;32(1):33.e1–6. doi:.https://doi.org/10.1016/j.urolonc.2012.12.002
21
Dieckmann
KP
,
Dralle-Filiz
I
,
Heinzelbecker
J
,
Matthies
C
,
Bedke
J
,
Ellinger
J
, et al.
Seminoma Clinical Stage 1 - Patterns of Care in Germany. Urol Int. 2016;96(4):390–8. doi:.https://doi.org/10.1159/000443214
22
Woldu
SL
,
Aydin
AM
,
Rao
AV
,
Hutchinson
RC
,
Singla
N
,
Clinton
TN
, et al.
Differences at Presentation and Treatment of Testicular Cancer in Hispanic Men: Institutional and National Hospital-based Analyses. Urology. 2018;112:103–11. doi:.https://doi.org/10.1016/j.urology.2017.08.059
23
Powles
TB
,
Bhardwa
J
,
Shamash
J
,
Mandalia
S
,
Oliver
T
. The changing presentation of germ cell tumours of the testis between 1983 and 2002. BJU Int. 2005;95(9):1197–200. doi:.https://doi.org/10.1111/j.1464-410X.2005.05504.x
24
Daugaard
G
,
Gundgaard
MG
,
Mortensen
MS
,
Agerbæk
M
,
Holm
NV
,
Rørth
M
, et al.
Surveillance for stage I nonseminoma testicular cancer: outcomes and long-term follow-up in a population-based cohort. J Clin Oncol. 2014;32(34):3817–23. doi:.https://doi.org/10.1200/JCO.2013.53.5831
25
Germà-Lluch
JR
,
Garcia del Muro
X
,
Maroto
P
,
Paz-Ares
L
,
Arranz
JA
,
Gumà
J
, et al.; Spanish Germ-Cell Cancer Group (GG). Clinical pattern and therapeutic results achieved in 1490 patients with germ-cell tumours of the testis: the experience of the Spanish Germ-Cell Cancer Group (GG). Eur Urol. 2002;42(6):553–62, discussion 562–3. doi:.https://doi.org/10.1016/S0302-2838(02)00439-6
26
Warde
P
,
Specht
L
,
Horwich
A
,
Oliver
T
,
Panzarella
T
,
Gospodarowicz
M
, et al.
Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol. 2002;20(22):4448–52. doi:.https://doi.org/10.1200/JCO.2002.01.038
27
Tandstad
T
,
Ståhl
O
,
Dahl
O
,
Haugnes
HS
,
Håkansson
U
,
Karlsdottir
Å
, et al.; SWENOTECA. Treatment of stage I seminoma, with one course of adjuvant carboplatin or surveillance, risk-adapted recommendations implementing patient autonomy: a report from the Swedish and Norwegian Testicular Cancer Group (SWENOTECA). Ann Oncol. 2016;27(7):1299–304. doi:.https://doi.org/10.1093/annonc/mdw164
28
Zengerling
F
,
Kunath
F
,
Jensen
K
,
Ruf
C
,
Schmidt
S
,
Spek
A
. Prognostic factors for tumor recurrence in patients with clinical stage I seminoma undergoing surveillance-A systematic review. Urol Oncol. 2017;S1078-1439(17)30331-9. Epub agead of print.
29
Aparicio
J
,
Maroto
P
,
del Muro
XG
,
Gumà
J
,
Sánchez-Muñoz
A
,
Margelí
M
, et al.
Risk-adapted treatment in clinical stage I testicular seminoma: the third Spanish Germ Cell Cancer Group study. J Clin Oncol. 2011;29(35):4677–81. doi:.https://doi.org/10.1200/JCO.2011.36.0503
30
Albany
C
,
Einhorn
L
. Pitfalls in management of patients with germ cell tumors and slight elevation of serum α-fetoprotein. J Clin Oncol. 2014;32(19):2114–5. doi:.https://doi.org/10.1200/JCO.2014.56.0607
31
Wymer
KM
,
Daneshmand
S
,
Pierorazio
PM
,
Pearce
SM
,
Harris
KT
,
Eggener
SE
. Mildly elevated serum alpha-fetoprotein (AFP) among patients with testicular cancer may not be associated with residual cancer or need for treatment. Ann Oncol. 2017;28(4):899–902.
32
Gilligan
TD
,
Seidenfeld
J
,
Basch
EM
,
Einhorn
LH
,
Fancher
T
,
Smith
DC
, et al.; American Society of Clinical Oncology. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28(20):3388–404. doi:.https://doi.org/10.1200/JCO.2009.26.4481
33
Tandstad
T
,
Smaaland
R
,
Solberg
A
,
Bremnes
RM
,
Langberg
CW
,
Laurell
A
, et al.
Management of seminomatous testicular cancer: a binational prospective population-based study from the Swedish norwegian testicular cancer study group. J Clin Oncol. 2011;29(6):719–25. doi:.https://doi.org/10.1200/JCO.2010.30.1044
34
Fischer
S
,
Tandstad
T
,
Wheater
M
,
Porfiri
E
,
Fléchon
A
,
Aparicio
J
, et al.
Outcome of Men With Relapse After Adjuvant Carboplatin for Clinical Stage I Seminoma. J Clin Oncol. 2017;35(2):194–200. doi:.https://doi.org/10.1200/JCO.2016.69.0958
35
Oliver
RT
,
Mason
MD
,
Mead
GM
,
von der Maase
H
,
Rustin
GJ
,
Joffe
JK
, et al.; MRC TE19 collaborators and the EORTC 30982 collaborators. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366(9482):293–300. doi:.https://doi.org/10.1016/S0140-6736(05)66984-X
36
Oliver
RT
,
Mead
GM
,
Rustin
GJ
,
Joffe
JK
,
Aass
N
,
Coleman
R
, et al.
Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol. 2011;29(8):957–62. doi:.https://doi.org/10.1200/JCO.2009.26.4655
37
Gillessen
S
,
De Santis
M
,
Rothermundt
C
. On Nonharming: The Debate Continues in Stage I Testicular Cancer. J Clin Oncol. 2015;33(20):2319–20. doi:.https://doi.org/10.1200/JCO.2015.61.1632
38
Oldenburg
J
,
Aparicio
J
,
Beyer
J
,
Cohn-Cedermark
G
,
Cullen
M
,
Gilligan
T
, et al.; On behalf of: SWENOTECA (Swedish Norwegian Testicular Cancer group), the Italian Germ Cell Cancer Group (IGG), Spanish Germ Cell Cancer Group (SGCCG). Personalizing, not patronizing: the case for patient autonomy by unbiased presentation of management options in stage I testicular cancer. Ann Oncol. 2015;26(5):833–8. doi:.https://doi.org/10.1093/annonc/mdu514
39
Fosså
SD
,
Cvancarova
M
,
Chen
L
,
Allan
AL
,
Oldenburg
J
,
Peterson
DR
, et al.
Adverse prognostic factors for testicular cancer-specific survival: a population-based study of 27,948 patients. J Clin Oncol. 2011;29(8):963–70. doi:.https://doi.org/10.1200/JCO.2010.32.3204
40
Giannatempo
P
,
Greco
T
,
Mariani
L
,
Nicolai
N
,
Tana
S
,
Farè
E
, et al.
Radiotherapy or chemotherapy for clinical stage IIA and IIB seminoma: a systematic review and meta-analysis of patient outcomes. Ann Oncol. 2015;26(4):657–68. doi:.https://doi.org/10.1093/annonc/mdu447
41
Gurney
J
,
Shaw
C
,
Stanley
J
,
Signal
V
,
Sarfati
D
. Cannabis exposure and risk of testicular cancer: a systematic review and meta-analysis. BMC Cancer. 2015;15(1):897. doi:.https://doi.org/10.1186/s12885-015-1905-6
42
Daling
JR
,
Doody
DR
,
Sun
X
,
Trabert
BL
,
Weiss
NS
,
Chen
C
, et al.
Association of marijuana use and the incidence of testicular germ cell tumors. Cancer. 2009;115(6):1215–23. doi:.https://doi.org/10.1002/cncr.24159
43
Trabert
B
,
Sigurdson
AJ
,
Sweeney
AM
,
Strom
SS
,
McGlynn
KA
. Marijuana use and testicular germ cell tumors. Cancer. 2011;117(4):848–53. doi:.https://doi.org/10.1002/cncr.25499
44
Lacson
JC
,
Carroll
JD
,
Tuazon
E
,
Castelao
EJ
,
Bernstein
L
,
Cortessis
VK
. Population-based case-control study of recreational drug use and testis cancer risk confirms an association between marijuana use and nonseminoma risk. Cancer. 2012;118(21):5374–83. doi:.https://doi.org/10.1002/cncr.27554
45
Callaghan
RC
,
Allebeck
P
,
Akre
O
,
McGlynn
KA
,
Sidorchuk
A
. Cannabis Use and Incidence of Testicular Cancer: A 42-Year Follow-up of Swedish Men between 1970 and 2011. Cancer Epidemiol Biomarkers Prev. 2017;26(11):1644–52. doi:.https://doi.org/10.1158/1055-9965.EPI-17-0428
