Ovarian cancer in Switzerland: incidence and treatment according to hospital registry data
DOI: https://doi.org/10.4414/smw.2018.14647
Simon
Wiesera, Marion
Schmidta, André B.
Kindb, Viola A.
Heinzelmann-Schwarzb
aWinterthur Institute of Health Economics, Zurich University of Applied Sciences, Winterthur, Switzerland
bDepartment of Gynaecology and Gynaecological Oncology, University Hospital Basel, University of Basel, Switzerland
Summary
OBJECTIVE
The methods used to diagnose and classify ovarian cancer have changed over the past decade. We used hospital registry data to assess the incidence, treatment durations and hospital costs of ovarian cancer in Switzerland.
METHODS
We carried out a retrospective analysis of a hospital registry covering all inpatient care episodes in Switzerland between 1998 and 2012. Ovarian cancer incidence was assessed by identifying patients with a first ovarian cancer diagnosis as the main reason for hospital stay after an event-free period. We assessed the duration and cost of ovarian cancer treatment sequences as well as the evolution of hospital patient volume over time.
RESULTS
The average age-adjusted incidence rate was 14.6 per 100,000 women per year between 2004 and 2012. This rate is substantially higher (+35.5%) than the corresponding rate published by the National Institute for Cancer Epidemiology and Registration (NICER). Hospital patient volume was low in most cases, with more than 40% of patients treated in hospitals with fewer than 20 cases per year. However, the share of patients treated in hospitals with more than 30 cases per year has increased substantially since 2009.
CONCLUSIONS
We found a substantial difference between the ovarian cancer incidence estimate based on hospital registry data and the corresponding estimate by NICER. The reasons for this substantial difference should be carefully explored. A case-wise comparison could determine whether the difference is due to over- or under-reporting in one of the two registries. The low ovarian cancer patient volume in many hospitals is in conflict with the numbers required for certified specialised cancer centres. The recent increase in patient volume in specialised cancer centres, however, might reflect a growing understanding of the needs and requirements of comprehensive cancer care.
Abbreviations
- CHF
-
Swiss Francs
- CHOP
-
Swiss Classification of Medical Procedures
- ECOG
-
Eastern Cooperative Oncology Group
- EUCAN
-
European Cancer Observatory
- FIGO
-
Fédération Internationale de Gynécologie et d’Obstétrique
- NICER
-
National Institute for Cancer Epidemiology and Registration
- SwissDRG
-
Swiss national prospective payment for inpatient acute care
Introduction
Ovarian cancer is one of the most common cancers in women, and the most aggressive form of cancer of the female reproductive system. The age-adjusted yearly incidence rate was estimated at 13.1 per 100,000 European women in 2012 [1]. Most women are diagnosed at advanced FIGO stages, when the chance of survival for 5 years is less than 30% [2].
Switzerland has one of the most expensive and generous healthcare systems, with universal access and a virtual absence of waiting times [3]. However, Switzerland has an excessive number of hospitals for its population and geography [4]. Among the (in 2012) 174 acute care hospitals (for a population of 8.0 million) [5], many have small case volumes for specialised treatments. The low degree of specialisation of many hospitals treating ovarian cancer patients has been criticised previously. Surgical staging was found to often be incomplete when performed in smaller centres with few patients [6].
The National Institute for Cancer Epidemiology and Registration (NICER) has reported an average age-adjusted incidence rate of 10.8 per 100,000 women in ovarian cancer over the period from 2008 to 2012 [7]. A study from the canton of Vaud based on data collected by the Cancer Registry in the period 1974 to 1988 found an even lower age-adjusted incidence rate of 9.6 [8]. However, this result may underestimate the true ovarian cancer prevalence in Switzerland as the cancer registry data are based on voluntary information on new cases from clinicians. Only the new cancer registration law, which will take effect from 2020, requires all cantons to establish cantonal cancer registries and all healthcare providers to report incident cancer cases to these registries. Little is known about the changes in and the quality of the treatment provided in Swiss hospitals over the past decade. Patient volume per hospital is an additional measure of quality, particularly with respect to expert specialisation, presence of a study nurse, data management and opportunities for a patient to receive the latest drugs via an international trial.
For the first time in Switzerland, the Swiss hospital registry is used in this study to examine the incidence of ovarian cancer as well as its treatment episodes and cost [9]. Surprisingly, this rich and highly representative hospital registry has up to now been rarely used in studies in the field of epidemiology, in health services research, and in health economics [10].
This study assesses the incidence of ovarian cancer in Switzerland between 2004 and 2012 based on a hospital registry covering all inpatient care episodes. It further describes recent trends in the inpatient treatment of ovarian cancer regarding the type and duration of treatment, the type of treating hospital and the yearly hospital patient volume. Finally, it assesses the costs of single inpatient care stays and of the whole inpatient treatment sequence of an ovarian cancer patient.
Materials and methods
Data
Our main data source was the hospital registry provided by the Swiss Federal Statistical Office [9]. The registry contains detailed information on all patients hospitalised since 1998 (socio-demographics, diagnoses, treatments etc.). A unique patient identification number tracks patients over time and across treatment sites. The registry has a virtually complete coverage of all inpatient treatments as participation is mandatory [11]. The coding quality of the registry is high in acute care hospitals, particularly since the introduction of a diagnosis-related group payment system (SwissDRG) in 2012. The hospital registry includes information on the in-hospital mortality but not out-of-hospital mortality, which limits the possibility of assessing the effect of treatment on mortality.
Identification of ovarian cancer patients
Ovarian cancer patients were identified by the ICD-10 code C56 (malignant neoplasm of the ovary) as the main diagnosis in the hospital registry. According to the coding manual, the main diagnosis should correspond to the diagnosis dominating treatment costs [12]. We did not consider cases with C56 as a secondary diagnosis as incident cases. These cases are likely to include patients previously hospitalised with an ovarian cancer main diagnosis.
Incidence calculation
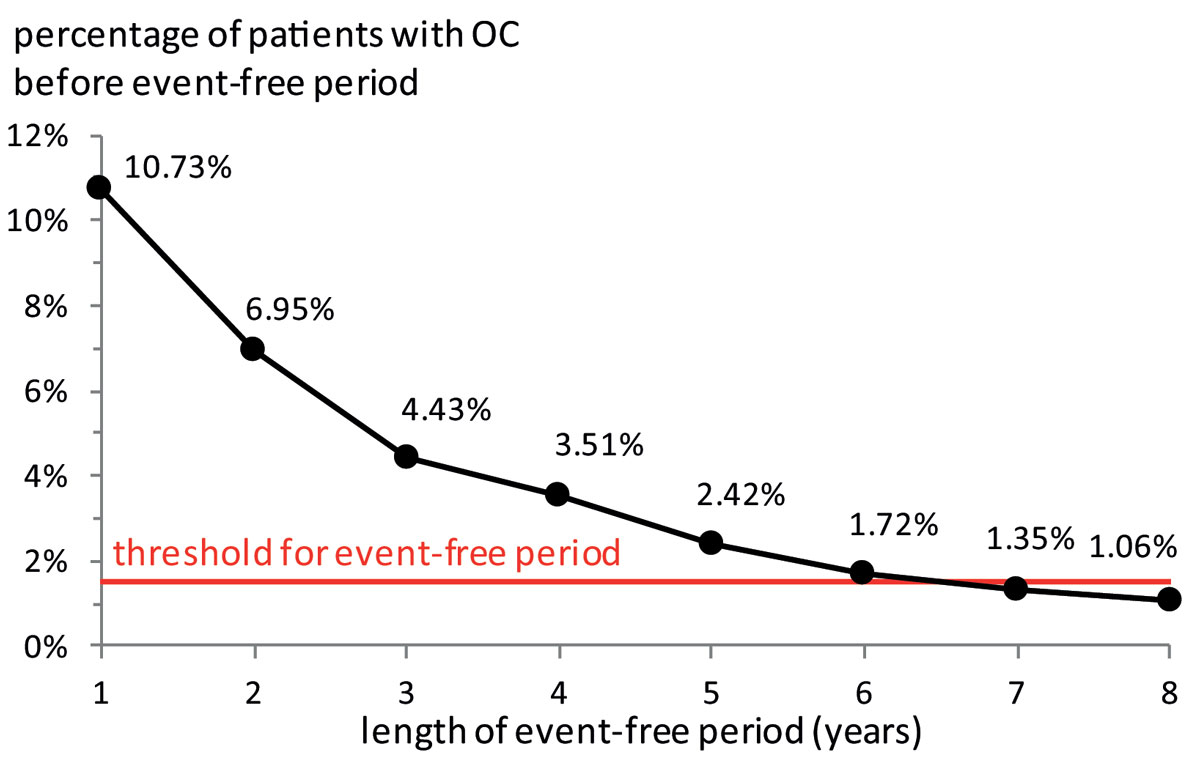
We calculated the yearly age-adjusted ovarian cancer incidence rate per 100,000 women. The number of incident cases was determined by the number of new cases with ovarian cancer as the main diagnosis in a given year. A new case was defined as a patient not diagnosed with ovarian cancer in a preceding event-free period of predetermined duration. The duration of this period was determined by increasing the number of event-free years until the probability of a previous ovarian cancer diagnosis fell below a threshold of 1.5%. We did not choose an even lower threshold because some cases may be the result of data input errors or misclassifications (supplementary fig. S1 in appendix 1).
The age adjustment compensates for demographic changes in the population and allows the international comparison of the incidence rates. We used the direct age adjustment method also applied by NICER [13]. Patients identified as out-of-pocket payers were considered as foreign residents coming to Switzerland for medical treatment. We excluded them from the incidence rate calculation as all Swiss residents are covered by mandatory health insurance.
Our procedure implicitly assumes that all women with ovarian cancer had at least one inpatient care episode with ovarian cancer as main diagnosis. This assumption seems justified in view of the severity of the disease and the standard of care in Switzerland.
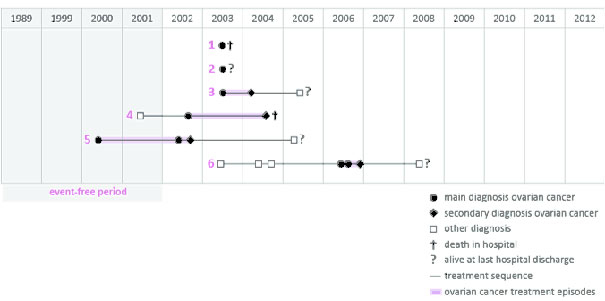
Identification of treatment sequences
We constructed ovarian cancer treatment sequences for all incident patients in order to assess the overall duration and cost of inpatient treatments for each patient. The treatment sequence of a single patient links all her inpatient stays with ovarian cancer as the main or secondary diagnosis. The length of the sequence was calculated as the time from the first to the last of these inpatient stays. The beginning of the treatment sequence was identified by the first inpatient stay with an ovarian cancer main diagnosis. The end of the treatment sequence was unfortunately less clear, except for patients who passed away in the hospital. In order to define the end of the treatment sequence, we therefore introduced a second inpatient-event-free period of three years after the last ovarian cancer stay of the treatment sequence. Women who were alive at the end of the treatment sequence may have been either cured from ovarian cancer, have continued to have treatment in an ambulatory setting or abroad, or have passed away out of hospital. Some patients may seek inpatient treatment after the three-year period. However, the incidence of these cases is likely to be so low that it can be neglected.
Inpatient treatment
In most cases, the beginning of a classical ovarian cancer treatment starts with an operation. We therefore assessed whether patients underwent an ovarian cancer-specific surgical treatment at their first hospitalisation with ovarian cancer as the main diagnosis. The types of treatments provided to patients are coded in the hospital registry with the Swiss Classification of Medical Procedures (CHOP) [14]. The following CHOP-codes were used to identify an ovarian cancer-specific surgical treatment: 65 (surgery of the ovaries), 54 (other surgery in the abdomen) and 68 (other incisions and excisions of the uterus). All treatments other than these CHOP codes were classified as other treatments. A minority of patients with advanced FIGO stage IIIC or IV disease and poor ECOG status are initially treated not by surgery, but by neoadjuvant chemotherapy. A specific coding for such chemotherapy does not exist, so all chemotherapy treatments are identified by the CHOP code 9925XX.
Hospital patient volume
Hospital case volume is an increasingly important indicator of quality of care, as specialised, comprehensive cancer centres have been shown to have a direct impact on the outcome for ovarian cancer patients [6, 15]. Therefore, we calculated the hospital case volume for patients with ovarian cancer as the main diagnosis for each individual hospital and assigned the hospitals to four volume categories: fewer than 10 patients/year, 10–19 patients/year, 20–29 patients/year, 30 or more patients per year. We then assessed whether patient volumes were associated with hospital size and how the share of ovarian cancer cases in individual hospitals changed over time.
Inpatient care costs
Inpatient healthcare costs were calculated using prices for the year 2012, as the new payment system (SwissDRG) was first introduced that year. The average treatment cost of a hospital stay was calculated by multiplying the SwissDRG cost weights with the average cost of an acute care inpatient treatment in 2012. The cost of treatment sequences was calculated by adding the individual costs of the cases identifying the sequence.
Data analysis
The results are presented in terms of means and standard deviations (SDs). Incidence is given as the number of cases per 100,000 per year. T-tests were used to compare differences in mean across hospital types. All analyses were performed with STATA 14.
Results
Incidence of ovarian cancer
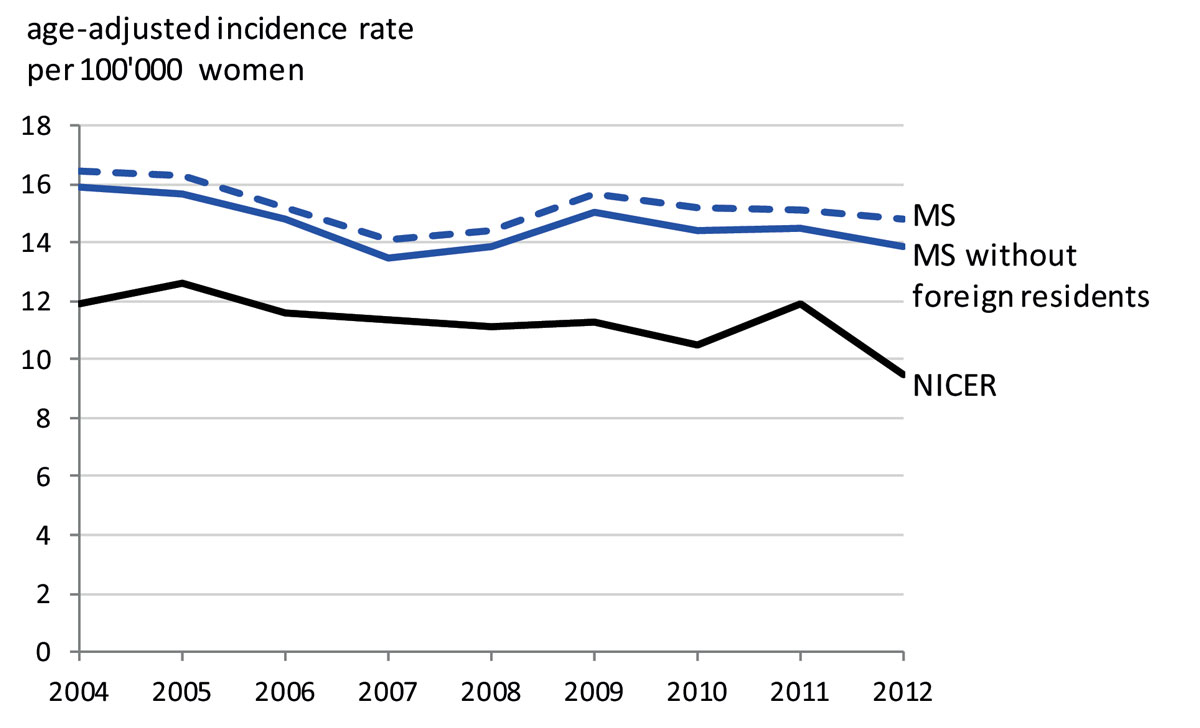
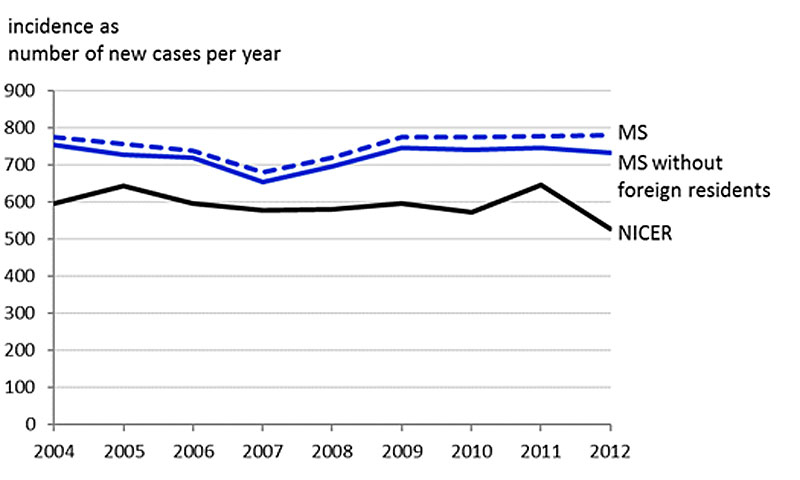
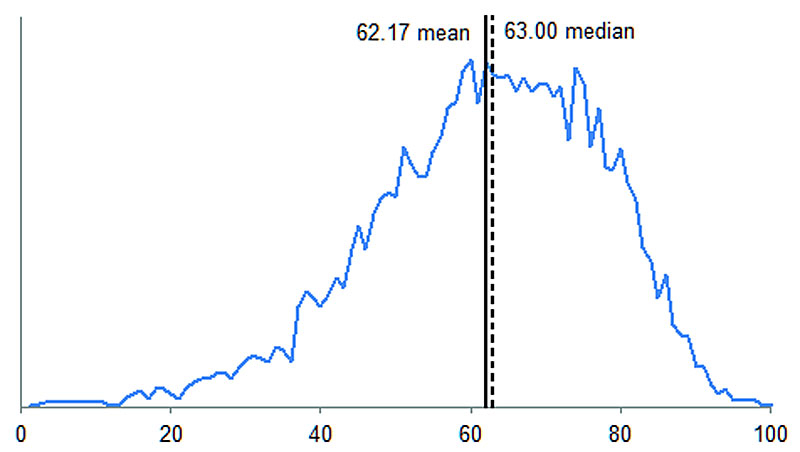
The duration of the event-free period necessary for the incidence calculation was determined at six years based on the data from 2010 to 2012 (fig. 1). The number of incident ovarian cancer cases could thus be assessed for the 2004-2012 period. The yearly average number of incident ovarian cancer cases was 753.1 (SD 31.8), which included an average of 28.2 (SD 7.2) foreign residents (supplementary fig. S2 in appendix 1). The average age-adjusted ovarian cancer incidence rate per 100,000 women (without foreign residents) was 14.6 patients over the 2004-2012 period and decreased to 13.8 in 2012 (fig. 2). The mean age of patients newly diagnosed with ovarian cancer was 62.17 years (SD 15.13) (fig. S3).
Treatment sequences
The average length of an ovarian cancer treatment sequence was 134.0 days (SD 294.2) and consisted of an average number of 2.06 (SD 2.6) hospital stays. The length of a single hospital stay with ovarian cancer as the main diagnosis decreased from 11.2 days (SD 11.2) in 2002 to 10.9 (SD 11.8) days in 2012. We did not find any statistically significant differences in the length of single hospital stays after ovarian cancer surgery between specialised hospitals (more than 30 cases per year) and non-specialised hospitals (fewer than 30 cases per year). Furthermore, we found no statistically significant differences in the length of treatment sequences or in the number of main diagnosis ovarian cancer hospital stays with surgery (table 1).
Table 1 Comparison between specialised and non-specialised hospitals.
|
Specialised hospitals
|
Non-specialised hospitals
|
Difference between hospital types
|
95% confidence interval
|
| Length of overall treatment episode (days: mean, SD) |
106 (242) |
130 (302) |
−23.8 |
−6.99 to 54.49 |
| Length of hospital stay with OC main diagnosis and surgery (mean, SD) |
13 (12) |
12 (10) |
1.1 |
−2.47 to 0.25 |
| Number of hospital stays with OC main diagnosis and surgery (mean, SD) |
4.1 (6) |
4 (4) |
0.1 |
−0.74 to 0.47 |
Hospital case volume
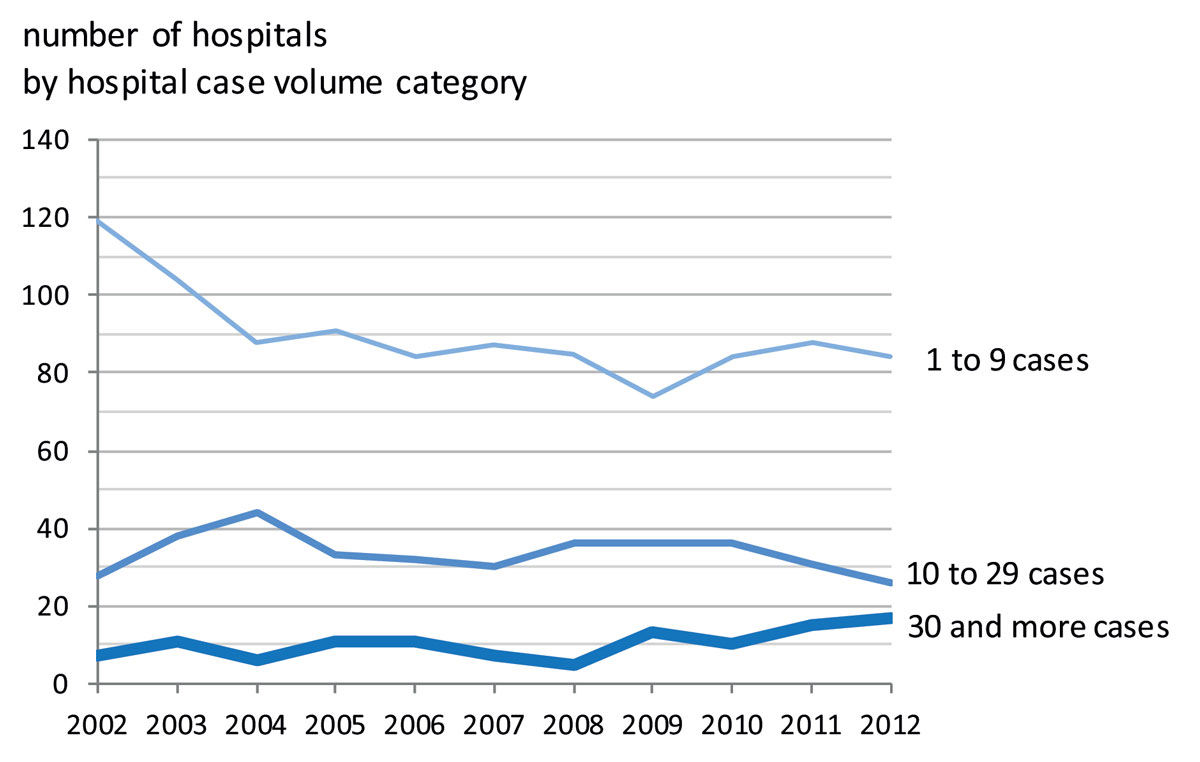
Ovarian cancer hospital patient volume was low in most Swiss hospitals, with over 80 institutions treating fewer than 10 patients per year in 2012. Therefore, up to 2012, most ovarian cancer patients were treated in hospitals with a case volume of less than 30 ovarian cancer patients per year despite the growing evidence that this leads to poorer outcomes (fig. 3).
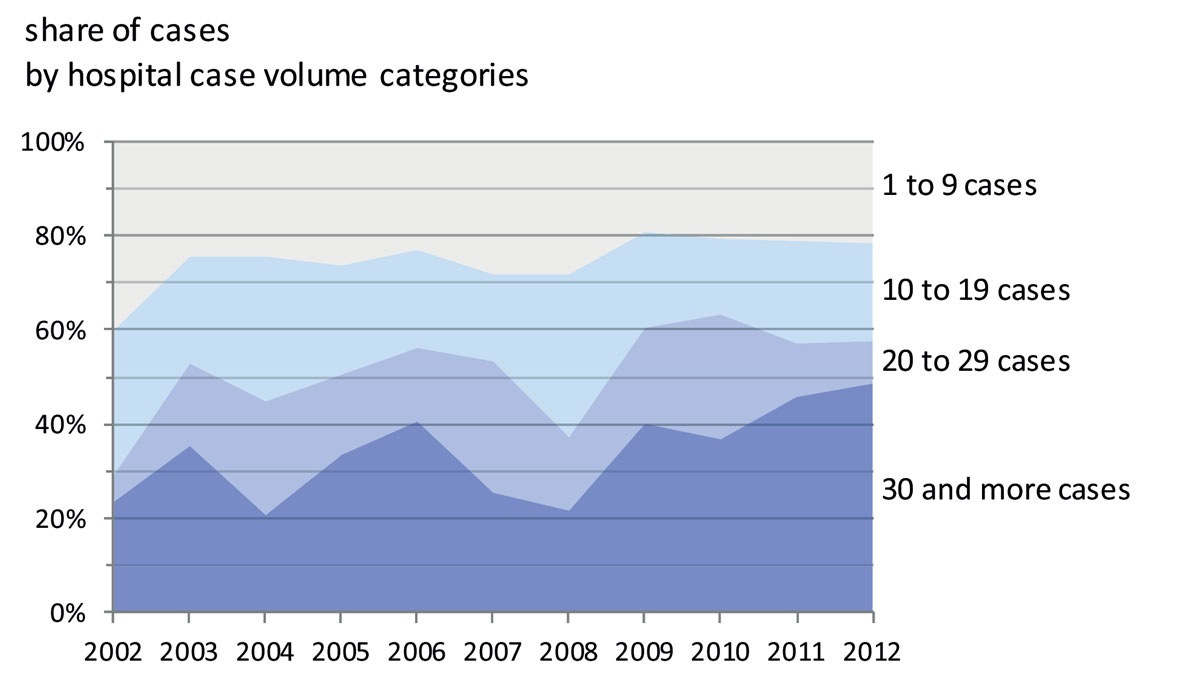
Importantly, the number of specialised centres or comprehensive cancer centres treating 30 or more ovarian cancer patients per year has only increased since 2009. The share of ovarian cancer cases by hospital case volume category was dominated by hospitals with fewer than 30 cases per year (fig. 4). While before 2009 fewer than 20% of surgically treated patients were treated in specialised centres, this proportion has since increased to almost 50% in 2012, probably due to the increasing number of certified cancer centres.
Inpatient care costs
The average cost of a single hospital stay of an ovarian cancer patient was 16,642 CHF (Swiss Francs) in 2012. The cost increased with hospital case volume, from 13,877 CHF in hospitals with 0 to 9 cases per year to 18,346 CHF in hospitals with 30 or more cases per year. The average cost of a hospital stay with a surgical intervention was 22,188 CHF, and the average cost of a stay with chemotherapy was 10,297 CHF. The cost of hospital stays with ovarian cancer as the main diagnosis is slightly higher than the average cost of hospital stays by patients with other cancers requiring surgical interventions: lung cancer (C34.9; CHF 11,249), breast cancer (C50.9; CHF 11,536), prostate cancer (C61; CHF 13,778) and bowel cancer (C18.9; CHF 11,783). The average cost of a complete ovarian cancer inpatient treatment sequence over the period 2004–2009 was CHF 22,246 (calculated at 2012 prices).
Discussion
We find an average age-adjusted ovarian cancer incidence rate of 14.6 cases per 100,000 women per year between 2004 and 2012. This rate is substantially higher (+35.5%) than the corresponding rate of 11.3 per 100,000 women per year reported by NICER for the same period. Both data sources show a slight decrease in ovarian cancer incidence from 2004 to 2012. Furthermore, we find that a considerable proportion of ovarian cancer patients were treated in hospitals with a low hospital case volume. Many patients, therefore, may be being treated in hospitals which are not sufficiently specialised.
Although our estimate of the ovarian cancer incidence rate is substantially higher than the previous estimate for Switzerland, it is within the range of rates reported for other countries. According to EUCAN [16], the ovarian cancer incidence rates in large European countries varied from 16.0 in the UK to 13.3 in Italy, 10.7 in France and 10.2 in Germany in 2012. The highest rate reported in Europe was 18.9 for Latvia, and the lowest was 4.2 for Albania. Our results indicate that part of these differences in incidence rates may be due to differences in the underlying data sources. The reasons for these substantial differences should be carefully explored. A case-wise comparison could, for example, detect whether the difference is due to over- or under-reporting in one of the two registries.
A number of reasons may have led us to under- or overestimate the true ovarian cancer incidence rate. Underestimation may be due to: (1) ovarian cancer cases not diagnosed. (2) Patients diagnosed with ovarian cancer but treated exclusively in an outpatient setting. (3) We excluded patients first diagnosed with ovarian cancer as a secondary diagnosis from the incident cases because this diagnosis is likely to be a consequence of other diseases. However, some of these cases might not have been the consequence of other cancers and should thus have been considered among the incident ovarian cancer cases. Overestimation of incident cases may be primarily due to including cases first diagnosed before the event-free period of six years. However, even extending the event-free period to eight years would only decrease the percentage of patients with ovarian cancer before the event-free period by 1.06% (see fig. 1). Moreover, the assumption of an event-free period of six years appears sensible from a clinical point of view.
The high number of ovarian cancer patients treated in hospitals with a low patient volume casts doubt on the adequacy of the surgical treatments in particular. However, inclusion in clinical system therapy trials with the chance to receive new drugs is also limited. In gynaecological oncology, and particularly in ovarian cancer, treatment regimens are increasingly based not on the organ itself but on its molecular background. In the past two decades, a plethora of molecular methods has delivered a multitude of molecular targets in ovarian cancer. It is a challenge for a subspecialised gynaecological oncologist in a highly specialised cancer centre to be aware of all new technologies, pathways and drugs under development, but this is nearly impossible for a private gynaecologist operating in a small hospital. The standard tumour board involving a pathologist, radiologist, medical oncologist, radiation oncologist and a gynaecologist might still be present in smaller hospitals. In a larger and more comprehensive centre, however, this group is often enriched by a gynaecological oncologist, a geneticist, a gynaecological cancer nurse, study nurses and case management, who aim to incorporate patients into trials of important new drugs with additional benefits, or to improve the recovery phase and lower the morbidity of patients. This might explain the higher costs found in highly specialised cancer centres.
Our study illustrates how the hospital registry can be employed to generate relevant information on the epidemiology and treatment of diseases in Switzerland. This example may be extended to other diseases. However, hospital registry data cannot match cancer registries with regard to diagnostic information on the type of disease or the outcomes of patients. It thus provides complementary information to the cancer registries and could represent a key component of a big data network which monitors diseases, treatments and outcomes.
Conclusion
This analysis identified several issues which may be of concern for the optimal care of ovarian cancer patients in Switzerland and which probably also reflect the situation in other European countries: (1) cancer registry data are not representatively available; (2) half of patients were treated by doctors (specialised or not) in centres with a workload of less than 30 ovarian cancer patients per year.
Appendix 1 Supplementary figures
Acknowledgements
The authors thank the Swiss Federal Office of Statistics for providing the hospital registry data. They also thank the editor and two anonymous reviewers for their comments and suggestions.
Author contributions
SW is the guarantor and drafted the manuscript. All authors contributed to the development of the study design. MS and SW carried out the acquisition and elaboration of the data as well as the statistical analysis. All authors contributed to the interpretation of the results. All authors read, provided feedback and approved the final manuscript.
References
1
Ferlay
J
,
Steliarova-Foucher
E
,
Lortet-Tieulent
J
,
Rosso
S
,
Coebergh
JWW
,
Comber
H
, et al.
Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–403. doi:.https://doi.org/10.1016/j.ejca.2012.12.027
2American Cancer Society. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015.
3
De Pietro
C
,
Camenzind
P
,
Sturny
I
,
Crivelli
L
,
Edwards-Garavoglia
S
,
Spranger
A
, et al.
Switzerland: Health system review. Health Syst Transit. 2015;17(4):1–288, xix.
4OECD. OECD reviews of health systems. Paris: OECD and World Health Organization; 2011.
5Swiss Federal Office of Statistics. Hospital Statistics 2012 (Krankenhausstatistik 2012). Neuchâtel; 2014 https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/erhebungen/ks.html. Accessed 3 June 2017
6
Petignat
P
,
de Weck
D
,
Goffin
F
,
Vlastos
G
,
Obrist
R
,
Luthi
JC
. Long-term survival of patients with apparent early-stage (FIGO I-II) epithelial ovarian cancer: a population-based study. Gynecol Obstet Invest. 2007;63(3):132–6. doi:.https://doi.org/10.1159/000096435
7NICER. Annual incidence rates - National statistics on cancer incidence; 2016 http://www.nicer.org/en/statistics-atlas/cancer-incidence/. Accessed 7 Jan 2016
8
Levi
F
,
Franceschi
S
,
La Vecchia
C
,
Ruzicka
J
,
Gloor
E
,
Randimbison
L
. Epidemiologic pathology of ovarian cancer from the Vaud Cancer Registry, Switzerland. Ann Oncol. 1993;4(4):289–94. doi:.https://doi.org/10.1093/oxfordjournals.annonc.a058484
9Swiss Federal Office of Statistics. Medical Statistic of Hospitals - complete dataset (Medizinische Statistik der Krankenhäuser). Neuchâtel. 2011.
10
Wieser
S
,
Rüthemann
I
,
De Boni
S
,
Eichler
K
,
Pletscher
M
,
Radovanovic
D
, et al.
Cost of acute coronary syndrome in Switzerland in 2008. Swiss Med Wkly. 2012;142:w13655. doi:.https://doi.org/10.4414/smw.2012.13655
11Swiss Federal Office of Statistics. Medizinische Statistik der Krankenhäuser: 2014 – Standardtabellen Neuchâtel; 2015 http://www.bfs.admin.ch/bfs/portal/de/index/news/publikationen.Document.197649.pdf. Accessed 6 Jan 2016
12Swiss Federal Office of Statistics. Kodierungshandbuch - Der offizielle Leitfaden der Kodierrichtlinien in der Schweiz - Version 2012. Neuchâtel: Swiss Federal Office of Statistics; 2012.
13Gordis L. Epidemiology. 5th ed. Philadelphia, PA: Saunders, Elsevier; 2013.
14Swiss Federal Office of Statistics. Schweizerische Operationsklassifikation (CHOP). Swiss Federal Office of Statistics. 2011.
15
Gooiker
GA
,
van Gijn
W
,
Post
PN
,
van de Velde
CJH
,
Tollenaar
RAEM
,
Wouters
MWJM
. A systematic review and meta-analysis of the volume-outcome relationship in the surgical treatment of breast cancer. Are breast cancer patients better of with a high volume provider?
Eur J Surg Oncol. 2010;36(Suppl 1):S27–35. doi:.https://doi.org/10.1016/j.ejso.2010.06.024
16EUCAN. Ovarian Cancer - Estimated incidence, mortality & prevalence, 2012; 2012 http://eco.iarc.fr/eucan/CancerOne.aspx?Cancer=27&Gender=2. Accessed 6 Jan 2016