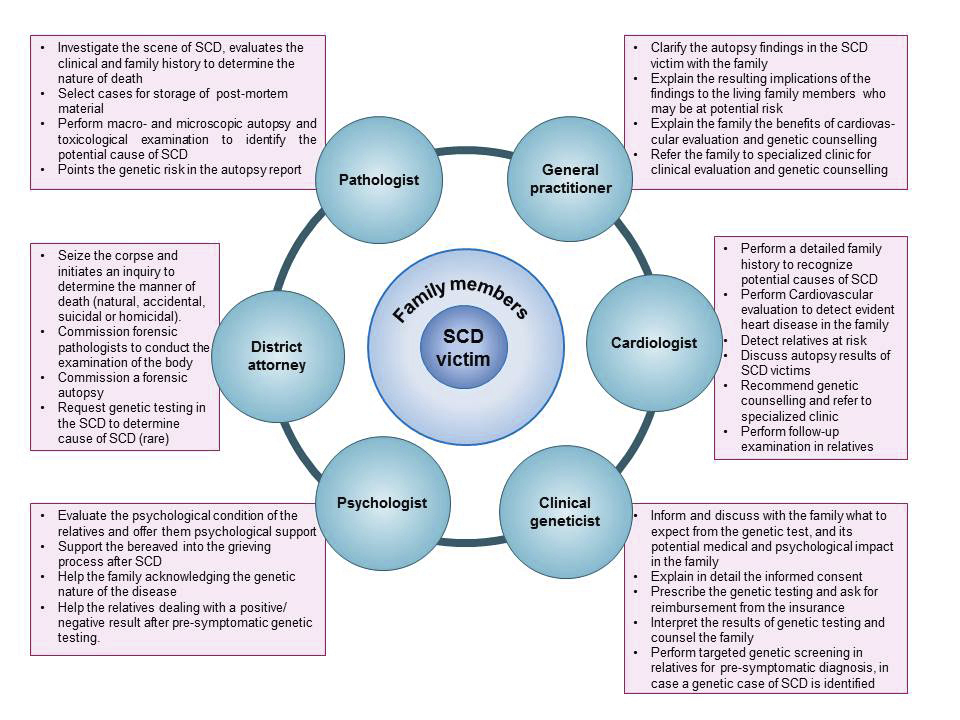
Figure 1 Multidisciplinary work around a sudden cardiac death victim.
DOI: https://doi.org/10.4414/smw.2018.14638
Atherosclerotic coronary artery disease (CAD) is recognised as the most common cause of sudden death in the general population [1]. In a younger population, cardiomyopathies and channelopathies are often involved [2, 3]. In a recent study on sudden cardiac death (SCD) among children and young adults 1 to 35 years of age in Australia and New Zealand, the most common explained causes of SCD were coronary artery disease and inherited cardiomyopathies (24 and 16% of cases, respectively) [4]. Unexplained SCD (40% of cases) was the predominant finding in patients under 31 years of age. The addition of genetic testing to the autopsy investigation substantially increased the rate of identification of a possible cause of SCD among children and young adults, as clinically relevant cardiac gene mutation(s) were identified in 27% of unexplained SCD victims. During follow-up, a clinical diagnosis of an inherited cardiovascular disease was discovered in 13% of the families [4].
Genetic testing can reveal substrates for not only cardiomyopathies and channelopathies [5], but also for aortic diseases and premature atherosclerosis, which may explain some SCD cases [6, 7]. Aortic aneurisms and dissections, mainly involving the vasculature of the aortic root and ascending aorta, may have a genetic origin, particularly in young patients, for whom over 15 genes associated with aortic disease are known today [8–10]. Premature atherosclerosis related to familial hypercholesterolaemia should be suspected in coronary artery disease cases under 55 years in males and under 60 years in females, according to the Consensus Statement of the European Atherosclerosis Society [6]. A possible genetic contribution should also be considered in SCD cases caused by spontaneous coronary artery dissection, mitral valve prolapse and pulmonary embolism [11–14].
In Switzerland, the forensic pathologist usually investigates sudden and unexpected deaths of young patients. Our “Sudden Cardiac Death Working Group” proposed minimum standards for autopsy practice in 2015 [15]; we suggested performing an autopsy in sudden death victims under 40 years of age, collecting and storing post-mortem frozen material for possible genetic testing for a period of ≥5 years, informing the family about the autopsy results and providing, when possible, multidisciplinary cardiogenetic counselling to families of SCD victims [15]. This document was already in line with the updated recommendations proposed recently by the Association for European Cardiovascular Pathology (AECVP) [16]. In the current article, we aim to adapt the guidelines proposed by the ACEVP in 2017 to the Swiss workflow, and to update the recommendations we published in 2015, particularly concerning genetic testing and genetic counselling. An interdisciplinary collaboration involving general practitioners, cardiologists, clinical geneticists, psychologist, molecular biologist and pathologists is proposed, with practical aspects in a Swiss framework [17, 18] (fig. 1).

Figure 1 Multidisciplinary work around a sudden cardiac death victim.
With rising opposition towards autopsies in general, a trend we have noted not only in Switzerland but also in many other countries, one might argue that a simple blood sample or minimally invasive techniques might be sufficient to determine the cause of death. Although valuable information can be obtained with such techniques, they do not replace an autopsy, which provides superior information, leading to a better comprehension of the deceased’s pathologies and/or – likewise importantly – lack of pathologies. A full autopsy is recommended because minimally invasive autopsies with use of modern imaging techniques are less accurate than a standard full autopsy, especially for cardiovascular diseases, and are considered not sufficient to diagnose the cause of SCD [16, 19]. Moreover, not every genetic anomaly or mutation leads to a morphological disorder (incomplete penetrance of genetic disease). Thus, by combining morphological examination techniques (autopsy and histological examination), toxicology and genetic analyses, one reasonably can include or exclude different causes of sudden death. This approach has important implications for the deceased’s family members, who might be at risk for similar diseases and eventually SCD.
According to the European and Swiss recommendations [15, 16], an autopsy should be performed in every SCD of a young adult. The cut-off age is still a subject of discussion. After a recent consensus resulting from the workshop entitled “Ethical, legal and practical aspects of post-mortem genetic analysis for sudden cardiac death in young adults”, organised by the Public and Professional Policy Committee of the European Society of Human Genetics and held in Geneva on November 2016, it is recommended to perform an autopsy in all cases under 40 years of age, it should be considered in victims between 40 and 65 years of age, and can be decided on case-by-case basis in individuals older than 65 years of age. Moreover, cases younger than 40 years who did or did not survive a sudden cardiac arrest have an indication for genetic testing according to the consensus published in 2011 and 2013 [20, 21]. Considering the practical aspects of Swiss forensic practice, we recommended a general cut off age of 45 years old, rather than the previous limit of 40 years old. This is a new proposal and should evidently be applied according to local possibilities; an extended age limit could be also be applied on the basis of the 2017 AECVP recommendations. Even when coronary artery disease or a specific cardiomyopathy is diagnosed in a young case, genetic testing has an important value for diagnosis, prognosis and treatment of family members.
In some cases with a cardiovascular morphological substrate identified at the autopsy, there may be an indication for genetic testing because of the risk that family members could have the disease (table 1).
Table 1 Cardiogenetic studies of family members. Class of recommendations based on autopsy findings of the proband. (Adapted from Basso et al. [16]).
| Finding autopsy | Age limit* | Possible mutated genes | Class of recommendation for referral of first degree family members for clinical/genetic counseling† | Level of evidence‡ |
|---|---|---|---|---|
| Unknown/uncertain cause of death | ≤40 | Mainly ion channel genes | I | C |
| Hypertrophic cardiomyopathy | No limit | Sarcomeric genes and other disease related genes | I | C |
| Arrhythmogenic cardiomyopathy | No limit | Desmosomal genes and other disease related genes | I | C |
| Dilated cardiomyopathy | No limit | Sarcomeric, cytoskeleton genes and other disease related genes | I | C |
| Premature atherosclerosis | Men <40 Women <50 | Familial hypercholesterolaemia genes | IIa | C |
| Thoracic aortic aneurysm / dissection / rupture with medial degeneration | Unknown | Syndromic and non-syndromic aortic aneurysm related genes | I | C |
| Spontaneous coronary artery dissection | No limit | Connective tissue disease related genes | IIa | C |
| Pulmonary embolism | Unknown | Hereditary thrombophilia genes | IIb | C |
| SUDEP | Unknown | Overlap with ion channels related genes | IIa | C |
SUDEP = sudden unexpected death in epilepsy * Age limit refers to age at autopsy of the deceased case. Comment for Swiss practice: we recommend as general practice a cut-off age of 45 years. † I: is recommended; IIa: can be useful; IIb: may be considered; III: is not recommended. ‡ A: data derived from multiple randomised clinical trials or meta-analyses; B: data derived from a single randomised clinical trial or large nonrandomised studies. C: consensus of opinion the experts and/or small studies, retrospective studies, registries.
The genetic risk should be mentioned in the autopsy report because of the growing arguments in favour of post-mortem disclosure to relatives. There is a general need at national and international levels for a well-considered post-mortem disclosure policy that offers an optimal balance between possible benefits and disadvantages. Today, the principles of benefit, the duty to warn, and the potential impact of genetic information for family members, are considered very strong justifications for post-mortem disclosure. These arguments prevail over respect for the deceased’s wishes as expressed in life, and the respect for the deceased’s privacy and confidentiality [22, 23]. In practice, we propose also a short standard letter, which should be sent to the district attorney by the forensic pathologist in order to inform about the storing of post-mortem samples.
Families of a SCD victim find relief when a causal explanation of the event is given [24]. The yield of the genetic test, however, is at best 30% in sudden unexplained death syndrome (SUDS) victims [25] and 10% in sudden infant death syndrome (SIDS) [26, 27]. Since there is a potential unrecognised genetic disorder in the family, it is desirable to offer cardiogenetic counselling to all first-degree relatives of a young (<45 years old) SCD/SUDS or SIDS victim. It should ideally be performed by a multidisciplinary team consisting of a cardiologist, geneticist and psychologist [28], before and after genetic testing [29] (fig. 1). Genetic counselling does not replace the routine clinical cardiac evaluation, expected to be performed in all first-degree relatives of a young SCD or SIDS/SUDS victim. The goal is to offer psychological support, answer all questions and discuss with the relatives the genetic aspects of SCD and the potential implication for their own health. The process of genetic counselling incorporates the interpretation of family and medical histories in search of potential genetic causes. During the counselling, instruction about the inheritance of the suspected genetic disease should be provided. The relatives should be informed about the advantages and disadvantages of the genetic test, including the implications of a positive or a negative test result, mutation detection rate, possibility of unexpected or unclear genetic findings and costs of the test. The genetic counselling session should be non-directive and help the relatives to make informed choices.
It is mandatory to provide informed consent for a genetic test, for which minimum content requirements have been recommended by the Swiss Society of Medical Genetics and are available online (http://sgmg.ch). The patient and the treating physician should sign the document.
After a SCD, relatives display increased anxiety, depression, stress and posttraumatic symptoms with prolonged grief [30, 31]. Some relatives also display guilty feelings or blame others, as it is difficult to make sense of such an event. Often the possible genetic nature of the disease is revealed while relatives are still mourning. As cognitive capacities may be impaired, decision-making about genetic testing may be complicated [32], as relatives also have to deal with the implications of a potential genetic condition affecting their own lives [33]. Genetic knowledge may sometimes be available only months after the death. In this case, the family interpretation of the death meshes with the medical explanation, reshaping family dynamics.
Sharing genetic information with relatives is never trivial and may generate a great deal of anxiety. Disclosing this information may improve, but also worsen, family communication and dynamics [34]. Predictive testing can affect the psychological well-being of parents for a long period of time [35]. Fear of developing symptoms, difficulties in considering prophylaxis (e.g., medical surveillance, beta blockers, internal cardio-defibrillator, exercise restriction, etc.), fear of impairing emotional well-being, especially when it comes to the children, might refrain from genetic testing or communication about it [36]. The inequalities of genetics are also difficult to apprehend (why does one child carry the mutation and not his siblings, for instance?). Paradoxically, being the only one in the family not to carry a pathogenic mutation is sometimes difficult, leading to feelings of exclusion or guilt.
The genetic counselling team should adapt their planed session to the family profile when choosing the setting of the meeting (each member separately or all together) [36, 37]. The information should be adapted to the age and maturity of the persons at risk, especially when talking to children and teenagers [34], but also to the elderly or the disabled. Underage children should be fully part of the genetic counselling process if indicated; their own questions and fears should be addressed.
The presence of a mental health professional in the cardiogenetic team will allow better detection of psychological comorbidity, which may also imply better compliance with and acceptance of medical recommendations [38] (fig. 1).
All first degree relatives of a young SCD victim should be referred to the cardiologist for clinical evaluation, which is usually in the frame of cardiogenetic counselling. The cardiologist will select the necessary examinations on the basis of the personal and family history. Basic evaluation should typically include a resting 12-lead electrocardiogram (ECG), echocardiography, 24-hour ECG, a stress test and if necessary cardiac magnetic resonance imaging (MRI).
Genetic testing should ideally be first performed on samples from the SCD victim. The value of the post-mortem genetic test in a victim of SCD lies in extending genetic screening to the relatives and eventually allowing detection of asymptomatic carriers at risk once the causative mutation has been identified. Establishing a clear genetic diagnosis in victims of SCD allows the initiation of measures to prevent SCD, and guidance with respect to reproductive options in affected families. Implications of a positive genetic test are determined by the association of the identified mutation with a particular disease, and are summarised in the international expert consensus statements [39, 40]. Concerning the diagnostic yield of the genetic test, it should be emphasised that:
There are no specific guidelines on the type or extent of the genetic test to be performed. So far, the number of genes tested and the technique used are selected by each laboratory or suggested by the referral physician. Most of the laboratories nowadays use next-generation sequencing, which allows multiple genes to be screened at the same time. Although there is no evidence that broader panels offer a significantly higher yield in SCD, SUDS or SIDS victims, there is a general tendency to use them.
The autopsy findings and the clinical history can guide the test when a specific condition is diagnosed or suspected. When the death remains unexplained, we recommend performing a genetic examination that includes at least the major genes implicated in channelopathies and cardiomyopathies. These major genes are provided in the guidelines for genetic testing [39]. The addition of minor genes, which usually explain less than 5% of the cases of a particular disease, may slightly increase the yield of the test.
At a clinical level, only pathogenic or probably pathogenic variants, as in the guidelines of the American College of Medical Genetics [41], should be reported. For variants of uncertain clinical significance (also called VUS), it is absolutely essential to discuss during the pre-test genetic counselling whether or not the family members wish to be informed about such findings if detected.
The insurances companies in Switzerland pay for genetic counselling. However, genetic testing in the deceased is not covered, neither by any insurance company nor by the district attorney who orders the forensic autopsy. This represents a real problem in the diagnostic flow of these cases since, according to the international recommendations, the test should be performed first in the deceased person.
For the family members, predictive genetic testing in Switzerland is not reimbursed, unless there is a clear therapeutic implication. Attending physicians should write a request to the insurance provider justifying the test and eventually the insurance will agree to pay.
It is important to recognise that genetic testing has become cheaper and faster, and covers more genes in one test. It is cheaper to perform a genetic test in a SCD victim, than regular medical-cardiovascular check-ups in all first-degree relatives every 3 to 5 years indefinitely. Genetic testing of the deceased needs the consent of the next-of-kin. If no genetic material is available from the deceased person, genetic testing can be performed in selected family members.
Since in 80% of SCD victims older than 45 years old the cause of death is coronary artery disease without a substantial genetic contribution, a genetic test usually has less value as a diagnostic tool in this group [1, 4]. The indication for the genetic test will depend on family history and the evidence of disorders having a genetic origin.
The general practitioner (GP) should recognise a SCD victim in which the cause is unclear and recommend an autopsy, particularly in cases younger than 45 years of age. After a SCD, family members usually turn to their GP first. After the legal section, the GP will receive an autopsy report of the deceased, which discusses possible genetic causes of the SCD; alternatively, questions about hereditary causes of the SCD might come up in the course of the consultation. Thus, the role of the GP in advising, counselling and organising is central to assisting affected family members (see fig. 1).
To help family members to make informed decisions, it is important to explain the findings of the autopsy and the often complex state of affairs. Here, as a person of trust, the GP has the important task of “translating” the autopsy report and explaining the implications of the findings to family members. The right of family members to remain ignorant should be respected as much as their desire for clarification.
The GP should send the autopsy report to the genetic counselling team. The autopsy protocol provides indications of the possibly underlying hereditary disease, as well as a list of the post-mortem material collected, able to be used for genetic testing depending on the situation. However, after the legal section, the permission of the public prosecutor is needed in order to release the autopsy report or autopsy protocol to family members or their physicians. In this respect, the corresponding Institutes of Forensic Medicine may provide organisational assistance to the GP.
The procedures following a SCD involve not only forensic doctors, but also cardiologists, geneticists, general practitioners, pathologists and psychologists, who should provide a comprehensive optimal “cardiogenetic counselling”. There is a need to update and improve the general knowledge concerning the genetic risk of cardiovascular pathologies and the importance of an autopsy and genetic testing, not only among the medical community but also among the juridical and public health authorities.
Family members of SCD victims younger than 45 years old with an unclear cause of death or SCD victims with a clear heritable cause at autopsy at any age, should be directed to a specialised centre for cardiogenetic counselling and genetic testing.
We understand the difficulties faced by different centres in Switzerland in implementing these recommendations, and we invite physicians involved in the management of a SCD victim or family members to contact our working group if assistance is needed.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Chugh SS , Reinier K , Teodorescu C , Evanado A , Kehr E , Al Samara M , et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51(3):213–28. doi:.https://doi.org/10.1016/j.pcad.2008.06.003
2 Semsarian C , Ingles J , Wilde AA . Sudden cardiac death in the young: the molecular autopsy and a practical approach to surviving relatives. Eur Heart J. 2015;36(21):1290–6. doi:.https://doi.org/10.1093/eurheartj/ehv063
3 Lieve KV , Wilde AA . Inherited ion channel diseases: a brief review. Europace. 2015;17(Suppl 2):ii1–6. doi:.https://doi.org/10.1093/europace/euv105
4 Bagnall RD , Weintraub RG , Ingles J , Duflou J , Yeates L , Lam L , et al. A Prospective Study of Sudden Cardiac Death among Children and Young Adults. N Engl J Med. 2016;374(25):2441–52. doi:.https://doi.org/10.1056/NEJMoa1510687
5 Priori SG , Wilde AA , Horie M , Cho Y , Behr ER , Berul C , et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10(12):1932–63. doi:.https://doi.org/10.1016/j.hrthm.2013.05.014
6 Nordestgaard BG , Chapman MJ , Humphries SE , Ginsberg HN , Masana L , Descamps OS , et al.; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34(45):3478–90. doi:.https://doi.org/10.1093/eurheartj/eht273
7 Santos RD , Gidding SS , Hegele RA , Cuchel MA , Barter PJ , Watts GF , et al.; International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 2016;4(10):850–61. doi:.https://doi.org/10.1016/S2213-8587(16)30041-9
8 Halushka MK . Single gene disorders of the aortic wall. Cardiovasc Pathol. 2012;21(4):240–4. doi:.https://doi.org/10.1016/j.carpath.2011.09.004
9 Erbel R , Aboyans V , Boileau C , Bossone E , Bartolomeo RD , Eggebrecht H , et al., The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. Eur Heart J. 2014;35(41):2873–926. doi:.https://doi.org/10.1093/eurheartj/ehu281
10 Ripperger T , Tröger HD , Schmidtke J . The genetic message of a sudden, unexpected death due to thoracic aortic dissection. Forensic Sci Int. 2009;187(1-3):1–5. doi:.https://doi.org/10.1016/j.forsciint.2009.01.020
11 Henkin S , Negrotto SM , Tweet MS , Kirmani S , Deyle DR , Gulati R , et al. Spontaneous coronary artery dissection and its association with heritable connective tissue disorders. Heart. 2016;102(11):876–81. doi:.https://doi.org/10.1136/heartjnl-2015-308645
12 Brandimarti F , Alessandrini F , Pesaresi M , Catalani C , De Angelis L , Galeazzi R , et al. Investigation on genetic thrombophilic factors in FFPE autopsy tissue from subjects who died from pulmonary embolism. Int J Legal Med. 2017;131(2):447–58. doi:.https://doi.org/10.1007/s00414-016-1508-z
13 Konstantinides SV , Torbicki A , Agnelli G , Danchin N , Fitzmaurice D , Galiè N , et al., Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–73. doi:.https://doi.org/10.1093/eurheartj/ehu283
14 Levine RA , Hagége AA , Judge DP , Padala M , Dal-Bianco JP , Aikawa E , et al.; Leducq Mitral Transatlantic Network. Mitral valve disease--morphology and mechanisms. Nat Rev Cardiol. 2015;12(12):689–710. doi:.https://doi.org/10.1038/nrcardio.2015.161
15 Wilhelm M , Bolliger SA , Bartsch C , Fokstuen S , Gräni C , Martos V , et al. Sudden cardiac death in forensic medicine – Swiss recommendations for a multidisciplinary approach. Swiss Med Wkly. 2015;145:w14129. doi:.https://doi.org/10.4414/smw.2015.14129
16 Basso C , Aguilera B , Banner J , Cohle S , d’Amati G , de Gouveia RH , et al.; Association for European Cardiovascular Pathology. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017;471(6):691–705. doi:.https://doi.org/10.1007/s00428-017-2221-0
17 Hofman N , Wilde AA . Role of Genetic Testing in Patients with Ventricular Arrhythmias in Apparently Normal Hearts. Card Electrophysiol Clin. 2016;8(3):515–23. doi:.https://doi.org/10.1016/j.ccep.2016.04.002
18 Michaud K , Fellmann F , Abriel H , Beckmann JS , Mangin P , Elger BS . Molecular autopsy in sudden cardiac death and its implication for families: discussion of the practical, legal and ethical aspects of the multidisciplinary collaboration. Swiss Med Wkly. 2009;139(49-50):712–8.
19 Blokker BM , Wagensveld IM , Weustink AC , Oosterhuis JW , Hunink MG . Non-invasive or minimally invasive autopsy compared to conventional autopsy of suspected natural deaths in adults: a systematic review. Eur Radiol. 2016;26(4):1159–79. doi:.https://doi.org/10.1007/s00330-015-3908-8
20 Ackerman MJ , Priori SG , Willems S , Berul C , Brugada R , Calkins H , et al.; Heart Rhythm Society (HRS); European Heart Rhythm Association (EHRA). HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Europace. 2011;13(8):1077–109. doi:.https://doi.org/10.1093/europace/eur245
21 Priori SG , Wilde AA , Horie M , Cho Y , Behr ER , Berul C , et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85–108. doi:.https://doi.org/10.1016/j.hrthm.2013.07.021
22 Boers SN , van Delden JJ , Knoers NV , Bredenoord AL . Postmortem disclosure of genetic information to family members: active or passive? Trends Mol Med. 2015;21(3):148–53. doi:.https://doi.org/10.1016/j.molmed.2015.01.002
23 Brownsword R , Wale J . The Right to Know and the Right Not to Know Revisited: Part One. Asian Bioeth Rev. 2017;9(1):3–18.
24 Vavolizza RD , Kalia I , Aaron KE , Silverstein LB , Barlevy D , Wasserman D , et al. Disclosing Genetic Information to Family Members About Inherited Cardiac Arrhythmias: An Obligation or a Choice? J Genet Couns. 2015;24(4):608–15. doi:.https://doi.org/10.1007/s10897-014-9783-7
25 Tester DJ , Medeiros-Domingo A , Will ML , Haglund CM , Ackerman MJ . Cardiac channel molecular autopsy: insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing. Mayo Clin Proc. 2012;87(6):524–39. doi:.https://doi.org/10.1016/j.mayocp.2012.02.017
26 Jiménez-Jáimez J , Peinado R , Grima EZ , Segura F , Moriña P , Sánchez Muñoz JJ , et al. Diagnostic Approach to Unexplained Cardiac Arrest (from the FIVI-Gen Study). Am J Cardiol. 2015;116(6):894–9. doi:.https://doi.org/10.1016/j.amjcard.2015.06.030
27 Barefield D , Kumar M , de Tombe PP , Sadayappan S . Contractile dysfunction in a mouse model expressing a heterozygous MYBPC3 mutation associated with hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2014;306(6):H807–15. doi:.https://doi.org/10.1152/ajpheart.00913.2013
28 Mital S , Musunuru K , Garg V , Russell MW , Lanfear DE , Gupta RM , et al.; American Heart Association Council on Functional Genomics and Translational Biology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Stroke Council; Council on Lifestyle and Cardiometabolic Health; and Council on Quality of Care and Outcomes Research. Enhancing Literacy in Cardiovascular Genetics: A Scientific Statement From the American Heart Association. Circ Cardiovasc Genet. 2016;9(5):448–67. doi:.https://doi.org/10.1161/HCG.0000000000000031
29 Dolmatova E , Mahida S , Ellinor PT , Lubitz SA . Genetic etiology and evaluation of sudden cardiac death. Curr Cardiol Rep. 2013;15(8):389. doi:.https://doi.org/10.1007/s11886-013-0389-8
30 Ingles J , James C . Psychosocial care and cardiac genetic counseling following sudden cardiac death in the young. Prog Pediatr Cardiol. 2017;45:31–6. doi:.https://doi.org/10.1016/j.ppedcard.2017.03.001
31 Ingles J , Spinks C , Yeates L , McGeechan K , Kasparian N , Semsarian C . Posttraumatic Stress and Prolonged Grief After the Sudden Cardiac Death of a Young Relative. JAMA Intern Med. 2016;176(3):402–5. doi:.https://doi.org/10.1001/jamainternmed.2015.7808
32 Caleshu C , Kasparian NA , Edwards KS , Yeates L , Semsarian C , Perez M , et al. Interdisciplinary psychosocial care for families with inherited cardiovascular diseases. Trends Cardiovasc Med. 2016;26(7):647–53. doi:.https://doi.org/10.1016/j.tcm.2016.04.010
33 Hidayatallah N , Silverstein LB , Stolerman M , McDonald T , Walsh CA , Paljevic E , et al. Psychological stress associated with cardiogenetic conditions. Per Med. 2014;11(7):631–40. doi:.https://doi.org/10.2217/pme.14.50
34 Vavolizza RD , Kalia I , Aaron KE , Silverstein LB , Barlevy D , Wasserman D , et al. Disclosing Genetic Information to Family Members About Inherited Cardiac Arrhythmias: An Obligation or a Choice? J Genet Couns. 2015;24(4):608–15. doi:.https://doi.org/10.1007/s10897-014-9783-7
35 Hendriks KS , Grosfeld FJ , van Tintelen JP , van Langen IM , Wilde AA , van den Bout J , et al. Can parents adjust to the idea that their child is at risk for a sudden death?: Psychological impact of risk for long QT syndrome. Am J Med Genet A. 2005;138A(2):107–12. doi:.https://doi.org/10.1002/ajmg.a.30861
36 Wiley KA , Demo EM , Walker P , Shuler CO . Exploring the Discussion of Risk of Sudden Cardiac Death. Pediatr Cardiol. 2016;37(2):262–70. doi:.https://doi.org/10.1007/s00246-015-1272-8
37 Erskine KE , Griffith E , Degroat N , Stolerman M , Silverstein LB , Hidayatallah N , et al. An interdisciplinary approach to personalized medicine: case studies from a cardiogenetics clinic. Per Med. 2013;10(1):73–80. doi:.https://doi.org/10.2217/pme.12.108
38 DiMatteo MR , Lepper HS , Croghan TW . Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–7. doi:.https://doi.org/10.1001/archinte.160.14.2101
39 Ackerman MJ , Priori SG , Willems S , Berul C , Brugada R , Calkins H , et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011;8(8):1308–39. doi:.https://doi.org/10.1016/j.hrthm.2011.05.020
40 Priori SG , Blomström-Lundqvist C , Mazzanti A , Blom N , Borggrefe M , Camm J , et al.; ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793–867. doi:.https://doi.org/10.1093/eurheartj/ehv316
41 Richards S , Aziz N , Bale S , Bick D , Das S , Gastier-Foster J , et al.; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–23. doi:.https://doi.org/10.1038/gim.2015.30
No financial support and no other potential conflict of interest relevant to this article was reported.