Adaptation of cost-effectiveness analyses to a single country: the case of bariatric surgery for obesity and overweight
DOI: https://doi.org/10.4414/smw.2018.14626
Zanfina
Ademiab, Yuki
Tomonagac, Joris
van Stiphouta, Dominik
Glinzd, Viktoria L.
Gloyd, Heike
Raatzd, Heiner C.
Bucherd, Matthias
Schwenkglenksac
aInstitute of Pharmaceutical Medicine (ECPM), University of Basel, Switzerland
bSchool of Public Health and Preventive Medicine, Monash University, Melbourne, Australia
cEpidemiology, Biostatistics und Prevention Institute (EBPI), University of Zürich, Switzerland
dBasel Institute for Clinical Epidemiology and Biostatistics (CEB), University Hospital Basel and University of Basel, Switzerland
Summary
OBJECTIVES
The aims of this study were to (a) identify and assess the quality of reporting of published cost-effectiveness studies of bariatric surgery, (b) assess their transferability to Switzerland, and (c) adapt transferable cost-effectiveness results to Switzerland.
METHODS
A systematic literature search was performed in Medline, Embase and other databases. Two reviewers independently undertook screening, extraction, assessment of reporting quality utilising the Consolidated Health Economic Evaluation Reporting Standards, transferability, adaptation of cost data and recalculation of cost-effectiveness results. Cost data were adapted in three steps: correction for different levels of resource utilisation, for different prices of healthcare services and for change in costs over time.
RESULTS
Fifteen studies fulfilled criteria for adaptation of cost data to Switzerland. Four out of fifteen adapted studies with a long time-horizon for patients with a body mass index (BMI) >35kg/m2 indicated bariatric surgery to be a cost-saving (dominant) approach compared with conventional treatment. Other studies for patients with BMI >35kg/m2 showed cost-effective results, with incremental cost-effectiveness ratios (ICERs) below CHF 50,000 per quality adjusted life-year (QALY) gained. Two studies assessed cost-effectiveness for patients with BMI <35kg/m2, and revealed ICERs below 50,000 per QALY gained for bariatric surgery versus conventional treatment. Between-study differences were related to approaches for the modelling effectiveness and costs, time horizon, population, type of intervention and possibly other unidentified reasons. Gastric bypass appeared to be superior to gastric banding, but was more expensive.
CONCLUSIONS
Nearly all studies found bariatric surgery to be a cost saving or cost-effective compared with conventional treatment. The adaptation of existing cost-effectiveness analyses cannot be considered to give accurate ICERs for Switzerland, but may have achieved an approximation of cost-effectiveness levels to be expected for Switzerland. It has made the results of international cost-effectiveness studies reported for different countries and in different currencies more comparable, and may be useful for individual countries in which financing or capacity for economic analyses is scarce.
Introduction
Obesity is a serious health problem associated with an increased risk for comorbidities such as type 2 diabetes, hypertension, obstructive sleep apnoea and musculoskeletal disorders, and for increased mortality [1–3]. Latest global burden of disease estimates showed that the proportion of adults with a body mass index (BMI) of ≥25 kg/m2 in 2013 was 36.9%, with a higher prevalence in women than in men [2]. In addition, obesity with associated health problems has a major economic impact on any healthcare system [4, 5]. For example, in 2008 in the United States, the medical care costs of obesity were $147 billion [5]. In Switzerland, the estimated healthcare costs due to obesity were estimated to be around CHF eight billion in 2011 [6]. Of the Swiss population, 30.8% are overweight (defined as BMI 25.0–29.9 kg/m2) and 10.3% are obese (BMI ≥30 kg/m2) [6, 7]. Conventional treatment for obesity may include nutritional counselling by a nutritionist or physician, behavioural therapy including psychotherapy, diets to reduce caloric intake, physical therapy including physiotherapy and medication, either on their own or in combination with other interventions.
If conventional treatment fails, bariatric (weight-loss) surgery may be considered. Conservative treatment is considered to have failed if, during 2 years of treatment or thereafter, a BMI of <35 kg/m2 cannot be reached and maintained. Swiss statutory health insurance covers certain types of bariatric surgery for obese individuals with a BMI ≥35 kg/m2 independent of existing comorbidities [8].
There is a relatively broad international literature on the cost effectiveness of bariatric surgery, but there are no systematic reviews that examine the transferability of international cost-effectiveness results for Switzerland or another single country. The aims of this study were to (a) identify and assess the quality reporting of published cost-effectiveness studies of bariatric surgery, (b) assess their transferability to Switzerland, and (c) adapt transferable cost-effectiveness results to Switzerland. The underlying notion was that gathering and synthesising international evidence may achieve a certain approximation of cost-effectiveness levels to be expected for a single country, and may be useful for individual countries in which financing or the capacity for economic analyses are scarce.
Methodology
Literature search
A systematic literature search was made in the following electronic databases: Medline, Embase, the Cochrane Library, and the Centre for Review and Dissemination (CRD) databases including the Database of Abstracts of Reviews of Effects (DARE), Cochrane reviews, and Health Technology Assessments (HTA) and the Economic Evaluation Database from the UK National Health Service (NHS EED). Search strings for additional databases were not developed because the above-listed selection of databases has been reported to be both sufficient and very efficient [9]. The economic part of the search string was obtained from the NHS research and development programme, which performed a health technology assessment of “the clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation” [10, 11]. The search strategy included keywords and medical subject heading terms concerning cost, cost-effectiveness and health economic studies. In the Cochrane library we used the option to search for technology assessments and economic evaluations. Search results were restricted by selecting human studies only and removing duplicates. The search was performed on 6 August 2015. Details are provided in appendix 1.
Screening of search results
Screening of the literature comprised three phases. In the first phase, all search results were screened by title. Titles containing relevant keywords such as bariatric surgery, costs, value, cost-effectiveness, cost-utility, quality of life, and burden were considered as potentially relevant. In the second phase, the abstracts of all papers with potentially relevant titles were screened for relevant results – costs, life years gained, quality adjusted life-years (QALYs) or incremental cost-effectiveness ratios (ICERs). Two reviewers independently screened titles and abstracts for eligibility. In the third phase, the full texts of articles with potentially relevant abstracts were assessed for eligibility by two reviewers independently. Any disagreements were resolved by consensus and discussion with a third reviewer when needed.
Eligible full texts were defined as full-scale incremental cost-effectiveness analyses, ideally, but not necessarily, with an endpoint of cost per QALY gained or cost per life-year gained, performed for Switzerland or a jurisdiction with socioeconomic characteristics broadly similar to Switzerland. Studies from north, central and western European countries, the USA, Canada, Australia and New Zealand were considered. Only publications available in English, German, French or Italian were considered.
Extraction of information and quality of reporting
For each study included in the final review, the following data were extracted by two reviewers independently:
- Study population (including country, age and BMI range of the patients)
- Intervention
- Comparator (including “conventional therapy” or “current practice”)
- Setting and perspective of the study
- Cost types included and cost year
- Type of model
- Time horizon and discount rate (adjusting for differences in the timing of costs)
- Approach to sensitivity analysis
- Effectiveness
- Costs ICER
This manuscript was prepared according to the PRISMA flowchart reporting requirements (please refer to fig. 1) [12].

Figure 1 Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) diagram.
Cost utility analysis, cost-effectiveness analysis included: Medline, Embase, Cochrane, CRD (Centre for Reviews and Dissemination). CHEERS = Consolidated Health Economic Evaluation Reporting Standards, ICER = incremental cost effectiveness ratio.
The quality of reporting of the cost-effectiveness studies was assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) [13] 24-item checklist, recommended by the International Society for Pharmacoeconomics and Outcomes Research Health Economic Evaluations Publication Guidelines Task Force. Selected CHEERS criteria were defined as being necessary in order to make a study not originating from Switzerland qualitatively transferable and thus suitable for numerical adaptation of ICER results to Switzerland. It was expected that CHEERS items 4 (population), 7 (intervention/comparator) and 10 (outcome measures) would be met by all studies assessed because studies failing any of these items would not meet the eligibility criteria defined above [13, 14].
Assessment of transferability
A variety of groups worked on criteria for assessing qualitative transferability of studies between jurisdictions, using related multistep procedures [14–16]. For the present study, none of the original models underlying the eligible cost-effectiveness studies was available. Therefore we pursued a modified approach described below and summarised schematically in figure 2.

Figure 2 Steps for study selection and determination of transferability to another country. Studies falling in the lower left box were regarded as being qualitatively transferable.
CHEEERS = Consolidated Health Economic Evaluation Reporting Standards; PICO stands for: P = patient, problem or population; I = intervention; C = comparison, control or comparator; O = outcome.
Essentially, the first step excluded studies that were not full-scale health economic evaluation studies assessing incremental cost-effectiveness, did not meet the PICO criteria, or were performed for countries very different from Switzerland in terms of socioeconomic characteristics. (PICO stands for: P = patient, problem or population; I = intervention; C = comparison, control or comparator; O = outcome.) All remaining studies had to meet CHEERS criteria 4, 7 and 10.
Secondly, studies not meeting CHEERS items 5, 6, 8, 13, 14 and 19 were regarded as not transferable owing to lack of key information. In relation to item 19, the availability of costs and outcomes of interest for both the intervention and the comparator strategies was considered fundamental. Where articles only reported ICERs, the underlying study was considered nontransferable (impossible to adapt the direct healthcare costs) (fig. 2 and appendix 2) [14, 16].
The remaining studies were considered qualitatively transferable, and underwent numerical adaptation of cost-effectiveness results.
The following transferability factors were considered:
- Methodological characteristics (perspective of cost assessment, discount rate, medical cost approach, productivity cost approach)
- Healthcare system characteristics (absolute and relative prices in healthcare, clinical practice variation, differences in resource use, incentives and regulations for health-care providers, technology availability)
- Population characteristics (demography, disease incidence and prevalence, case-mix, life expectancy, health-status preferences, acceptance, compliance, incentives to the patients and productivity and work-loss time)
For the majority of cost-effectiveness studies meeting the general eligibility criteria we did not expect major transferability problems since methodological and population characteristics were expected to be similar to Switzerland. Regarding healthcare system characteristics, we did not expect big differences in availability of technology. Absolute prices in healthcare were adapted numerically (see section “Adaptation of cost-effectiveness”), whereas differences in relative prices were expected to pose certain issues. With respect to clinical practice, bariatric surgery procedures should be well comparable across countries. In contrast, standard care (the comparator strategy) was expected to vary across studies and countries. Therefore, we assessed as far as possible what kind of clinical practice the comparator groups were exposed to.
Adaptation of cost-effectiveness results to Switzerland
The cost data were adapted independently by two reviewers and any disagreements were resolved by a third reviewer, based on an approach previously developed in a study commissioned by the Swiss Federal Office of Public Health, in order to estimate the costs of noncommunicable diseases [17]. The cost data representing direct medical costs to Switzerland were adapted in three steps: correction for different levels of resource utilisation, for different prices of healthcare services, and for change in the level of resource utilisation and prices over time. Subsequently, adapted ICERs were calculated. Although there is no official threshold for cost per QALY gained in Switzerland, a willingness-to-pay threshold of CHF 50,000 per QALY gained were used, in line with published analysis for Swiss setting [18]. The three steps are described below.
Resource utilisation
The types and quantities of healthcare resources used differed between countries. For the same disease, patients in Switzerland often receive more medical treatment than in other countries (i.e., they are treated more intensively for an equivalent diagnosis). Therefore, a quantity correction was based on the Organization for Economic Co-operation and Development (OECD) statistics of healthcare expenses per capita, corrected for purchasing power. A correction for differences in resource utilisation levels (unaffected by price levels) was thus achieved [19]. Correction factors between Switzerland and the countries for which the selected cost-effectiveness analyses were performed are shown in appendix 3.
Prices of healthcare services
The price for the same set of healthcare services or treatments is often different across countries. A price correction was made by applying purchasing power parities provided by the OECD as correction factors representing the proportional costs for identical products in two countries [20]. For more details, please see appendix 3.
Change in costs over time
For eligible cost-effectiveness studies performed in countries other than Switzerland, the two steps described above achieved an approximate adaptation of reported costs. The resulting estimates were valid for the same cost year as in the original study. Corrections for change over time up to 2012 (the latest available), our price year of reference, were based on the yearly growth rates of total Swiss healthcare expenditures [21]. This was expected to be appropriate as, in addition to prices, the healthcare resources used for different strategies of obesity treatment may have changed over time. If the resource used could be assumed to be constant over time, a simple adaptation using inflation rates would be appropriate instead. The implications of this alternative were assessed in a sensitivity analysis. For more details, please see appendix 3.
Example of adaptation of an international CEA
A study by Craig et al. [22] reported that the total costs for gastric bypass in the US in 2001 for a 35-year-old male were US$ 68,000. To adapt the costs for Switzerland, the mentioned costs were multiplied by the ratio Switzerland/US for current expenditure on health, per capita, US$ purchasing power parities, interpreted as the resource utilisation ratio between Switzerland and the US in 2001 (i.e., 0.697) [23], by the purchasing power parity ratio in 2001 (i.e., 1.840) [24], and by the growth in healthcare expenditures in Switzerland between 2001 and 2012 (i.e., 1.492) [25]. This means that the estimated, adapted costs for Switzerland in 2012 would be CHF 131,263 (68,600 × 0.697 × 1.840 × 1.492).
Results
Systematic process of article selection
A total of 76 articles were included in the full-text review, of which 55 were ineligible because of an inappropriate comparator (comparator was not defined), noncomparative design, unsuitable article type (reviews, commentary), or inappropriate outcome measures (fig. 1). The remaining 21 articles fulfilled the inclusion criteria and were assessed using the CHEERS checklist. However, only 15 articles fulfilled criteria for qualitative transferability and were thus suitable for numerical adaptation of ICER results to Switzerland (table 1). There were no cost effectiveness studies from Switzerland.
The six excluded studies (after assessment with CHEERS checklist), Ananthapavan et al. [38], Anselmino et al. [39], Faria et al. [40], Jensen et al. [41], McEwen et al. [42] and Salem et al. [43], were found to be not qualitatively transferable to Switzerland as they did not provide sufficient information on costs (no clear information on cost about intervention and comparator, year of the cost data was missing, discounting information missing, or perspective of analysis was unclear) and effects (missing information on how the quality adjustment life years or life years gained was calculated).
Study and patient characteristics
Of the 15 included studies (table 1), six were from the United States, four from the United Kingdom and one from Australia. The remainder were from continental Europe. All studies were published between 2002 and 2014 and all but two (Craig et al., Borg et al.) [22, 27] reported funding sources. The perspectives of the studies were different. Three studies adopted a healthcare perspective (Keating et al., Mäklin et al., Wang et al.) [32-–36] and nine a payer perspective (Picot et al. 2009, Clegg et al. 2003, Craig et al., Picot et al. 2012, Pollock et al., Ikramuddin et al., Ackroyd et al., Campbell et al., Castilla et al.) [3, 10, 22, 26, 28, 29, 31, 34, 35]. Two were conducted from a societal perspective (Borg et al., Michaud et al.) [27, 33], and in one study the perspective was not defined (i.e., payer, health care or societal perspective) (Hoeger et al.) [30].
Six studies included type 2 diabetes mellitus patients with BMI >35 kg/m2 [3, 26, 30–32, 34, 35]. Hoerger et al. [30] additionally divided the study population into patients with newly diagnosed diabetes and patients with established diabetes. The rest of the studies incorporated patients with BMI >35 kg/m2 and, to a varying degree, obesity-related comorbidities such as type 2 diabetes, hypertension, coronary artery disease, stroke, and other life threatening diseases. Most of the studies assessed patients with BMI ranges above 35 kg/m2 (table 1). Only two studies assessed lower BMI populations. Specifically, Borg et al. [27] assessed costs and effects of bariatric surgery in a population with BMI 30–34 kg/m2 and Keating et al. [32] assessed the cost-effectiveness of laparoscopic adjustable gastric banding (LAGB) in patients with BMI 30–40 kg/m2.
The majority of studies assessed gastric bypass. Eight studies explicitly stated that gastric bypass was performed laparoscopically [3, 10, 26, 28, 29, 33, 34, 36]. In Craig et al. [22] only open gastric bypass was included. Castilla et al. [29] assumed 50% laparoscopic and 50% open gastric bypass. In four studies on gastric bypass it was not clearly stated if the intervention was performed laparoscopically or openly [27, 30, 31, 44]. Nine studies reported the cost-effectiveness of other operations such as adjustable gastric banding (AGB) or LAGB [3, 26, 30–32, 34–36, 44], all compared with conservative treatment or no surgery. Wang et al. [36] reported on laparoscopic roux-en-Y gastric bypass (LRYGB), open roux-en-Y gastric bypass (ORYGB) or LAGB, compared with conservative treatment.
Standard medical management, described as “conventional therapy” or “current practice”, was typically a combination of diet, exercise and behavioural modification, and was the comparator in nine studies [3, 10, 26, 27, 29–32, 44]. Other studies were very specific about current practice, reflecting the population with type 2 diabetes, which included tight glycaemic control, exercise and other behavioural modifications.
Most studies were cost-utility analyses, i.e., cost-effectiveness analyses using QALYs as their benefit measure. The only exception was the study by Michaud et al. [33], which reported life years and costs per life year gained as outcomes of interest. Decision analytic modelling employing Markov elements or life tables was used in all studies. Studies adopted various time horizons, ranging from life time (Craig et al., Borg et al., Keating et al., Wang et al., Campbell et al., Castilla et al., Michaud et al., Hoerger et al.,) [22, 27–30, 32, 33, 36] to 5 years (Ackroyd et al.) [26] (table 1). Ten studies used 3.0% discounting for costs and effects [22, 27–33, 36, 44]. One early study discounted costs by 6.0% and effects by 1.5% [10], and the remaining four studies used 3.5% discounting of costs and effects.
Measurement of cost and data sources
Types of costs included varied, depending on the chosen perspective. The direct medical costs of interventions (gastric bypass, LRYGB, AGB, LAGB) and comparator (conservative therapy, usual care) were reported in all cases. All analyses aimed to include the costs of surgery and related diseases. Only Michaud et al. [33] reported total lifetime medical costs (disease-specific and disease-nonspecific costs). Only two studies included a societal perspective and reported indirect costs [27, 33]. The accuracy in reporting types of costs and the sources of cost data used varied. Details are provided in appendices 4 and 5.
Measurement of clinical effects and data sources
Modelling of clinical effects included body weight loss or BMI reduction, reduced or delayed comorbidities (e.g., type 2 diabetes remission), complications and mortality reduction. Specifics varied across studies and were partially influenced by the study population and time horizon. Almost all studies included short-term effects related to bariatric surgery, except one study [31]. Long-term effects were measured in terms of reduced BMI and reduced mortality [22, 27–29, 32, 33]. Studies also considered surgery-related complications or adverse events in their analyses. However, it was not always clear how surgery-related complications were defined. The most frequent complications listed were reoperation [3, 10, 22, 26, 28, 30–34, 44], infection [3, 10, 22, 26, 32, 34], pulmonary embolism [3, 22, 26] and abdominoplasty [3, 22, 30, 44]. All but three studies included surgical mortality (mortality during 30 days after surgery), except for three studies [26, 33, 34]. Additional information about clinical effects included in the individual studies can be found in appendices 6, 7 and 8.
The sources of effectiveness estimates varied across studies. Some sources were used more frequently than others. For example, the results of the Swedish Obese Subjects (SOS) study [37, 45–47] was used by eleven studies as their main source for effectiveness estimates (appendix 8). Almost all studies combined survival estimates with utilities to generate QALYs as their main summary measures of effectiveness, and one study considered duration of survival only [33].
Adaptation of economic evaluation results to Switzerland
Differences in costs and effects as originally reported by the 15 transferable studies are summarised in appendices 9 and 10. Based on the criteria set by the authors, the results indicate that bariatric surgery is cost-saving or cost-effective.
Adapted results of the 15 transferable studies were quite consistent. Differences in costs and effects between surgery and conventional treatment (usual care) are presented in figure 3 and appendices 11 and 12.
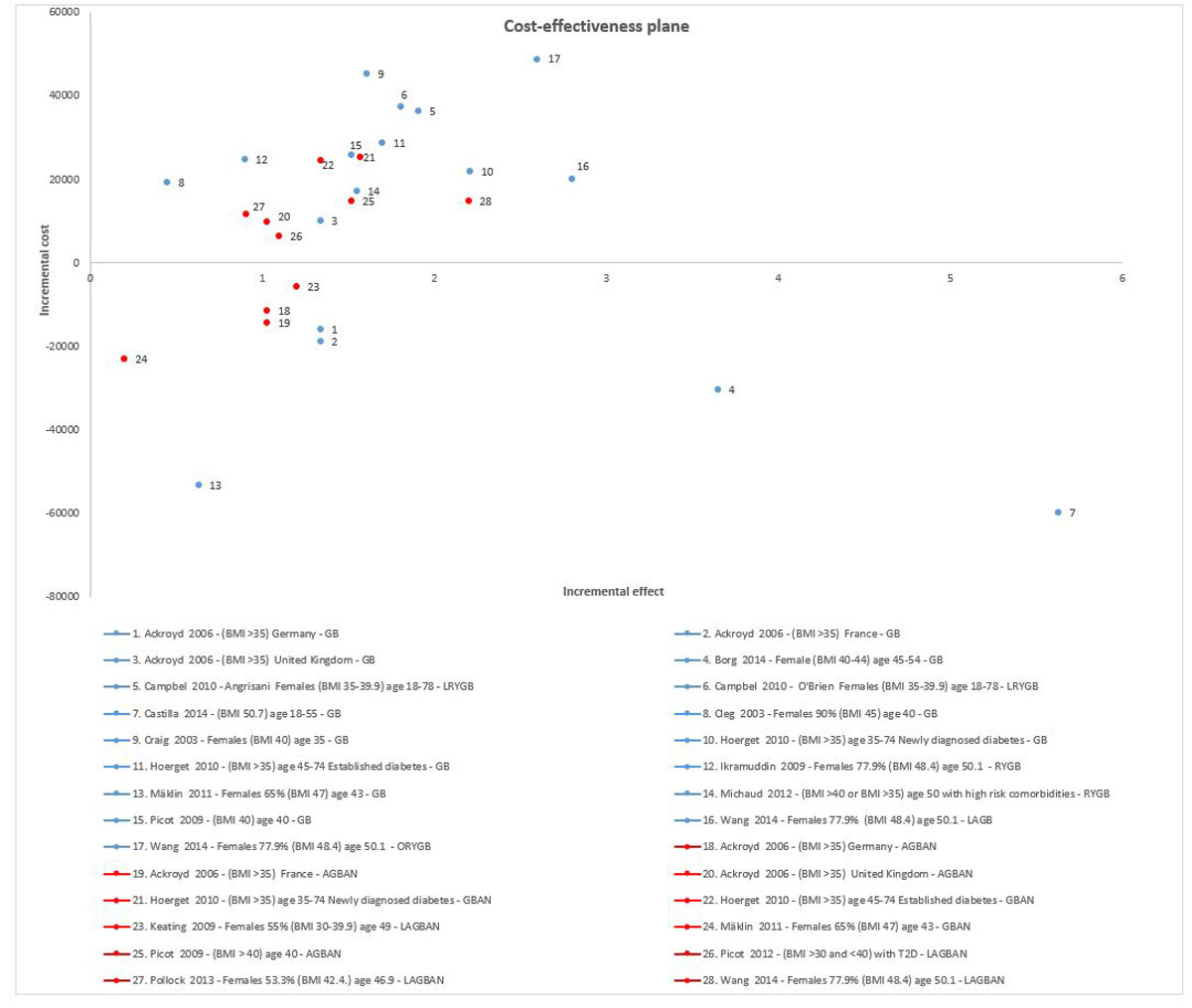
Figure 3 Cost-effectiveness plan, based on costs adapted to Switzerland and original effect estimates.
Blue dots represent the cost-effectiveness studies assessing laparoscopic gastric bypass (or other term used in the studies gastric bypass (GB), laparoscopic roux-en-Y gastric bypass (LRYGB), roux-en-Y gastric bypass (RYGB), versus conservative treatment. Red dots represent cost-effectiveness studies assessing other bariatric surgeries (adjustable gastric banding (AGBAN), gastric banding (GBAN), laparoscopic adjustable gastric banding (LAGBAN) conservative (open) roux-en-Y gastric bypass (ORYGB) versus conservative treatment. In the case of studies providing results for different patient groups, results were selected for presentation based on the longest available time horizon (if there was more than one time horizon available), female gender (when available in the study) and BMI >35 kg/m2. Ackroyd et al. [26] described results based on three different countries, Campbell et al. [28] for two estimates of treatment effects (by Angrisani [48] and O'Brien [49]), Hoerget et al. [30] for two diabetes groups, Wang et al. [36] for two types bypass surgeries and gastric banding, and Picot et al. for two different banding techniques. From the Borg et al. study [27], we selected the results for female patients aged 45–54 years, with BMI 40 kg/m2.
The vast majority of adapted studies of patients with BMI values >35 kg/m2 indicated bariatric surgery compared with conventional treatment or nonsurgical management to be a cost saving (dominant [cost saving and increase in QALYs and life years] or potentially cost-effective (ICERs were generally below CHF 50,000 per QALY gained). More specifically, four studies with long time horizons (10 years to lifetime) showed cost saving results (fig. 3, appendices 11 and 12) [27, 29, 32, 44]. Other studies [3, 10, 22, 28, 30, 31, 34–36] showed cost-effective results, even the study by Ackroyd et al. [26], which utilised a short time horizon (5 years). In the study by Michaud et al. [33], where the effectiveness outcome of interest was life-years gained rather than QALYs gained, the adapted cost-effectiveness ratio was below CHF 9000 per life-year gained.
From a societal perspective, adapted ICERs based on Borg et al. were below CHF 3000 per QALY gained for females aged 45–54 years with BMI 40–44 kg/m2, when gastric bypass was compared with conservative treatment. For males aged 45–54 years with BMI 40–44 kg/m2, gastric bypass was cost-saving and thus dominant [27]. Michaud et al. [33] also reported cost-effectiveness from a societal perspective, for US patients with BMI >40 kg/m2, or with BMI >35 kg/m2 and a high risk of comorbidities. Here, the adapted ICER for gastric bypass compared with conservative treatment was CHF 8158 per life-year gained [33].
For patients in the BMI category <35 kg/m2, the cost-effectiveness of bariatric surgery compared with conventional treatment (usual care) treatment was assessed only by Borg et al. [27] and Picot et al. [34], and both applied a lifetime horizon. In the study by Borg et al. [27], the adapted ICER was below CHF 3000 per QALY gained. In the study by Picot et al. (2012) [34], the adapted ICER was below CHF 50,000 per QALY gained.
From the societal perspective, for patients in the BMI class <35 kg/m2, the cost-effectiveness of bariatric surgery compared with conservative treatment was assessed only by Borg et al. [27], who reported an adapted ICER of CHF 10,458 per QALY gained for females and CHF 12,365 per QALY gained for males.
Procedure-specific differences in terms of benefits and efficiency appear to exist. When LRYGB and LAGB were compared with conservative treatment, LRYGB appeared to be better than gastric banding in terms of clinical benefits (QALYs gained and life-years saved), but also more expensive [26, 30, 36, 44].
Difference between cost-effectiveness studies
In order to understand the observed cost-effectiveness differences between studies, we assessed potential explanatory factors, which are described in appendix 14. Briefly, QALY estimates were a major driver in the difference between cost-effectiveness studies. Also, a partial explanation for the differences between cost-effectiveness studies may be in the cost items taken into consideration, and differences in the modelling techniques of bariatric surgery.
Sensitivity analysis
The aim of the sensitivity analysis was to assess the impact on ICERs adapted for Switzerland when the adjustment of costs over time was based on inflation rates instead of the increase in healthcare costs. This alternative approach to calculating adapted costs and ICERs had no large effect. There were no substantial differences for the studies by Borg et al. [27] and Castilla et al. [29], which were quite recent (cost year 2012). The largest impact on ICERs was seen for the older studies by Clegg et al. (2003) [10] (difference −31.70%) and Craig et al. [22] (difference −28.07%) (appendix 13). For the other 11 studies, the change remained below 20%.
Discussion
The cost-effectiveness of bariatric surgery was systematically reviewed, using data from 15 cost-effectiveness analyses performed in a number of European countries, the US and Australia. The majority of studies assessed cost-effectiveness as cost per QALY gained, with time horizons that ranged from 5 years to lifetime. Results varied mainly owing to: inclusion criteria with regards to age, BMI class, severity of obesity and comorbidities; the assessment and modelling of costs and effects; and the perspective of cost assessment taken.
Nearly all studies assessing patients with BMI >35 kg/m2 indicated bariatric surgery to be cost-saving or cost-effective based on the criteria set by the authors. This result of the present systematic review is consistent with an earlier systematic review published by Padwal et al. in 2011 [50]. The latter suggested that, based on 13 economic evaluations, bariatric surgery compared with conservative treatment appeared attractive, with ICERs ranging from $1000 to $40,000 per QALY gained [50]. However, the authors did not make any definitive conclusions about the cost-effectiveness of alternative surgical procedures.
Similarly, adapted ICER results for patients with a BMI >35 kg/m2 indicated a cost saving (dominant) situation or showed ICERs of CHF 8000 to CHF 44,000 per QALY gained. For patients in the BMI <35 kg/m2 category, the adapted cost-effectiveness of bariatric surgery compared with conventional treatment was below CHF 3000 per QALY gained, from a healthcare perspective (Borg et al. 2014 [27]) or below CHF 50,000 per QALY gained in the study by Pico et al. [34]. Procedure-specific differences in benefits and efficiency appear to exist. However, to date, there is no direct economic evaluation comparing LRYGB with LAGB. Therefore, further information is required to understand the long-term comparative effectiveness and cost-effectiveness of different bariatric procedures.
The adaptation of cost-effectiveness results may have allowed a comparison of results across studies, and an approximation of cost-effectiveness levels to be expected for Switzerland. The majority of studies were from a payer perspective [3, 10, 22, 26, 28, 29, 31, 34, 50] and two were from a societal perspective [27, 33]. It should be noted that adapted ICERs from a payer perspective may reflect a Swiss statutory health insurance perspective (taking into account the direct medical costs of all healthcare services covered by the Swiss statutory health insurance, irrespective of actual payer), rather than an immediate insurer perspective. This is because insurers cover only about 45% of inpatient costs for acute care. The rest is borne by the Swiss cantons.
Limitations of the systematic review
This systematic review had a number of limitations. First, the screening of abstracts was restricted to English, German, French, and Italian language publications, which may have led to excluding evidence published in other languages. Second, the assessment of qualitative transferability to Switzerland was based on a combination and adaptation of published criteria. The estimates emerging from subsequent numerical adaptation of cost results cannot be directly interpreted as “ICERs for Switzerland”, only as an approximation of cost-effectiveness levels to be expected for Switzerland.
Limitations of reviewed cost effectiveness studies
The reviewed cost-effectiveness studies utilised changes in BMI rather than changes in body weight as a percentage change from baseline, and this was due to lack of underlying clinical evidence. Additionally, some studies, again owing to lack of evidence, assumed a re-increase of BMI after a certain time and then a stable difference after the observation period. However, descriptions were not always entirely clear. If the difference in BMI was maintained for longer, this tended to translate into higher net differences in terms of life-years lived and QALYs gained for surgery.
Some studies strongly focused their cost-effectiveness models on the effects on type 2 diabetes. Three of these studies (Ikramuddin et al., Keating et al., Pollock et al.) [31, 32, 35] found relatively small QALY differences; an exception was Hoerger et al. [30]. This might be because the focus on diabetes in these studies led to potentially incomplete coverage of other effects, and thus underestimation of QALY differences.
In addition, it should be noted that in the identified cost-effectiveness studies, the modelling of effectiveness was based on short-term clinical trials and longer-term observational data, such as the Swedish SOS study [47]. This is a frequently seen in health economic evaluations with long time horizons, where an extrapolation of effects observed in clinical trials is needed. Data from observational studies are crucial to understanding the long-term impact of interventions in clinical practice, but are generally affected by higher risks of bias than randomised studies.
Another limitation of the included cost-effectiveness studies was that there were no long-term quality of life data they could have built upon. Bariatric surgery has been shown to improve quality of life in the short-term, and there were cross-sectional data on the correlation between BMI and quality of life. As such, this information was modelled in the available economic evaluations. The authors undertook a number of sensitivity analyses to demonstrate the robustness of the data presented, but not all followed the rule of varying all parameters with potentially relevant, inherent uncertainty.
A final limitation concerns the outcomes considered. It has been well known for many years that diseases such as diabetes, myocardial infarction or stroke are associated with obesity. Not surprisingly, the costs of these diseases are often included in cost assessments of bariatric surgery. However, bariatric surgery may also have a positive or negative impact on the occurrence of additional, potentially costly diseases. For example, the effects of bariatric surgery on bone mineral density have recently been discussed as potentially relevant [51, 52]. Bone mineral loss after bariatric surgery could potentially increase the risk of fractures. This would lead to higher costs and reduced cost-effectiveness.
Conclusions
Nearly all studies found bariatric surgery to be a cost saving or cost-effective, compared with conventional treatment. Differences were due to approaches in the modelling of effectiveness, costs, time horizon, population studied, exact type of intervention studied, and possibly other unidentified reasons. The adaptation process of existing cost-effectiveness-analysis cannot be interpreted as achieving certain ICERs for Switzerland, but may have achieved an approximation of cost-effectiveness levels to be expected for Switzerland. It has certainly made the results of international cost-effectiveness studies, reported for different countries and in different currencies, more comparable, and may be useful for individual countries in which financing or capacity for economic analyses is scarce.
Table 1 Characteristics of the cost-effectiveness studies included.
|
Study
|
Population/country
|
Age at time of intervention
|
Intervention
|
Comparator
|
Perspective/setting
year
|
Time frame
|
Discounting
|
Modelling
|
| Ackroyd 2006 [26] |
Patients with a BMI of >35 kg/m2 and type 2 diabetes in Germany, UK and France |
Not reported |
Laparoscopic gastric bypass and adjustable gastric banding |
Conservative treatment |
Payers perspective
2005 |
5 years |
3.5% |
Decision analytic modelling |
| Borg 2014 [27] |
Females and males in Sweden with age below and above 55 years of age. Stratum age 45–54 years and BMI 40–44 kg/m2 as base case |
Stratum age 45–54 years in the base case |
Gastric bypass (not specified if laparoscopic or open) |
Conservative treatment |
Societal perspective
2012 |
Life |
3% |
A Markov micro-simulation model |
| Campbell 2010 [28] |
Patients with BMI >40 kg/m2 or BMI >35 kg/m2 with comorbid conditions in the US |
18–74 years |
Laparoscopic roux-en-Y gastric bypass and laparoscopic adjustable gastric banding |
No intervention |
Third-party payer
2006 |
Life |
3% |
Markov model |
| Castilla 2014 [29] |
79 patients from Spain with an average BMI of 50.7 kg/m2 (range 36.6 to 76.3 kg/m2) |
18–55 years |
Gastric bypass (50% laparoscopic and 50% open) |
Usual care |
Payer perspective
2012 |
5, 10, 15, 20 years and life |
3% |
Discrete-event simulation model |
| Clegg 2003 [10] |
Hypothetical cohort of 100 patients, 90% females from the UK with an average BMI of 45 kg/m2
|
40 years |
Laparoscopic gastric bypass and laparoscopic adjustable gastric banding |
Nonsurgical management |
NHS / personal social services perspective
1999–2000 |
20 years |
Costs 6% and QALY 1.5% |
Results are based on a systematic review |
| Craig 2002 [22] |
Severely obese >40 kg/m2 patients from the US who were non-smokers without cardiovascular disease |
35–55 years |
Open gastric bypass |
No surgery |
Payer perspective
2001 |
Life |
3% |
Decision model |
| Hoerger 2010 [30] |
Patients from the US with BMI >35 kg/m2 and new or established diabetes |
Not reported |
Gastric bypass and banding surgery (not specified if laparoscopic or open) |
Usual diabetes care |
Unclear
2005 |
Life |
3% |
Markov model |
| Ikramuddin 2009 [31] |
Diabetes patients from the US, 22.1% were males with a mean BMI of 48.4 kg/m2
|
Mean 50.1 years |
Laparoscopic roux-en-Y gastric bypass |
Standard medical treatment |
Third-party payer perspective/2007 |
35 years |
3% |
Markov model |
| Keating 2009 [32] |
Patients with recently diagnosed type 2 diabetes from Australian with a BMI of 30–39.9 kg/m2, 55% female |
Mean 49 years |
Laparoscopic adjustable gastric banding |
Conservative therapy |
Healthcare perspective
2006 |
Life |
3% |
Markov model |
| Mäklin 2011 [25] |
Patients from Finland with a mean BMI of 47 kg/m2 (38-59 kg/m2), 35% male |
Mean 43 years |
Gastric bypass and gastric banding (not specified if laparoscopic or open) |
Ordinary treatment |
Health care perspective
2010 |
10 years |
3% |
Combination of decision tree and Markov model |
Michaud
2012 [33] |
Current eligibility: BMI >40 kg/m2 or BMI >35 kg/m2 with high risk comorbidities (extended eligibility to those with BMI >35 kg/m2 or BMI >30 kg/m2 with qualifying comorbidities) |
50 years |
Laparoscopic roux-en-Y gastric bypass |
Baseline management |
Societal perspective
2010 |
Life |
3% |
Future Elderly Model, microsimulation model of aging and health |
| Picot 2009 [3] |
Patients with a BMI of >40 kg/m2 from the UK |
40 years |
Laparoscopic gastric bypass and adjustable gastric banding |
Nonsurgical comparator |
NHS and personal social services
2007–2008 |
20 years |
3.5% |
Transition model |
| Picot 2012 [34] |
Patients with class I and II obesity (BMI 30–40 kg/m2) with type 2 diabetes or class I obesity (BMI 30–35 kg/m2) in the UK |
Unclear, presumably between 41.8 and 46.6 years |
Laparoscopic adjustable gastric banding |
Nonsurgical interventions |
UK NHS
2009–2010 |
2, 5, and 20 years |
3.5% |
Transition model |
| Pollock 2013 [35] |
Obese patients from the UK with type 2 diabetes, 46.5% were males with a mean BMI of 42.4 kg/m2 (SD 4.5), Duration of diabetes 1 year (SD 0.33) |
46.9 years |
Laparoscopic adjustable gastric banding |
Standard medical management |
National Health Service
2010 |
40 years |
3.5% |
Computer model of diabetes |
| Wang 2014 [36] |
The reference was defined as US female with BMI of 44 kg/m2 (simulation range 18–70 years) |
53 years |
Laparoscopic gastric bypass, conservative (open) roux-en-Y gastric bypass, and laparoscopic adjustable gastric banding |
Nonsurgical intervention |
Health care perspective
2010 |
Life |
3% |
Two-part model using a deterministic approach for the first 5-years post-surgery and separate empirical forecasts for the natural history of BMI |
The appendices 1 to 14 are available in separate file for downloading at https://smw.ch/en/article/doi/smw.2018.14626/
Acknowledgment
We would like to thank the clinicians who participated in the scoping process for their contribution. The authors are solely responsible for the content of the manuscript.
References
1
Dixon
JB
. The effect of obesity on health outcomes. Mol Cell Endocrinol. 2010;316(2):104–8. doi:.https://doi.org/10.1016/j.mce.2009.07.008
2
Ng
M
,
Fleming
T
,
Robinson
M
,
Thomson
B
,
Graetz
N
,
Margono
C
, et al.
Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. doi:.https://doi.org/10.1016/S0140-6736(14)60460-8
3
Picot
J
,
Jones
J
,
Colquitt
JL
,
Gospodarevskaya
E
,
Loveman
E
,
Baxter
L
, et al.
The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess. 2009;13(41):1–190, 215–357, iii–iv. doi:.https://doi.org/10.3310/hta13410
4
Hammond
RA
,
Levine
R
. The economic impact of obesity in the United States. Diabetes Metab Syndr Obes. 2010;3:285–95. doi:.https://doi.org/10.2147/DMSO.S7384
5
Finkelstein
EA
,
Strombotne
KL
. The economics of obesity. Am J Clin Nutr. 2010;91(5):1520S–4S. doi:.https://doi.org/10.3945/ajcn.2010.28701E
6Schneider H, Venetz W. Cost of Obesity in Switzerland in 2012. Report prepared on behalf of the Bundesamt fur Gesundheit, Contact-Number 13.005445. Available at: https://www.bag.admin.ch/dam/bag/fr/dokumente/npp/forschungsberichte/forschungsberichte-e-und-b/cost-of-obesity.pdf.download.pdf/cost-of-obesity.pdf.
7Swiss Society for the Study of Morbid Obesity and Metabolic Disorders (SMOB). Richtlinien zur operativen Behandlung von Übergewicht (Medizinische Richtlinien). In: Medizinische Richtlinien zur operativen Behandlung von Übergewicht. 2013.
8Bundesamt für Gesundheit (BAG). Verordnung des EDI über Leistungen in der obligatorischen Krankenpflegeversicherung, Stand 15. September 2015. Available at: https://www.admin.ch/opc/de/classified-compilation/19950275/201803010000/832.112.31.pdf.
9
Royle
P
,
Waugh
N
. Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system. Health Technol Assess. 2003;7(34):iii
, ix–x, 1–51. doi:.https://doi.org/10.3310/hta7340
10
Clegg
A
,
Colquitt
J
,
Sidhu
M
,
Royle
P
,
Walker
A
. Clinical and cost effectiveness of surgery for morbid obesity: a systematic review and economic evaluation. Int J Obes Relat Metab Disord. 2003;27(10):1167–77. doi:.https://doi.org/10.1038/sj.ijo.0802394
11
Clegg
AJ
,
Colquitt
J
,
Sidhu
MK
,
Royle
P
,
Loveman
E
,
Walker
A
. The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation. Health Technol Assess. 2002;6(12):1–153. doi:.https://doi.org/10.3310/hta6120
12
Liberati
A
,
Altman
DG
,
Tetzlaff
J
,
Mulrow
C
,
Gøtzsche
PC
,
Ioannidis
JP
, et al.
The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1):b2700. doi:.https://doi.org/10.1136/bmj.b2700
13
Husereau
D
,
Drummond
M
,
Petrou
S
,
Carswell
C
,
Moher
D
,
Greenberg
D
, et al.; ISPOR Health Economic Evaluation Publication Guidelines-CHEERS Good Reporting Practices Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)--explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–50. doi:.https://doi.org/10.1016/j.jval.2013.02.002
14
Welte
R
,
Feenstra
T
,
Jager
H
,
Leidl
R
. A decision chart for assessing and improving the transferability of economic evaluation results between countries. Pharmacoeconomics. 2004;22(13):857–76. doi:.https://doi.org/10.2165/00019053-200422130-00004
15
Drummond
M
,
Barbieri
M
,
Cook
J
,
Glick
HA
,
Lis
J
,
Malik
F
, et al.
Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force report. Value Health. 2009;12(4):409–18. doi:.https://doi.org/10.1111/j.1524-4733.2008.00489.x
16
O’Brien
BJ
. A tale of two (or more) cities: geographic transferability of pharmacoeconomic data. Am J Manag Care. 1997;3(Suppl):S33–9.
17Wieser S, Tomonaga Y, Riguzzi M, et al. Die Kosten der nichtübertragbaren Krankheiten in der Schweiz. https://www.bag.admin.ch/dam/bag/de/dokumente/npp/forschungsberichte/forschungsberichte-ncd/kosten-ncd-in-der-schweiz.pdf.download.pdf/Schlussbericht%20COI%20NCDs%20in%20CH%202014%2007%2021.pdf. 2014.
18
Brändle
M
,
Goodall
G
,
Erny-Albrecht
KM
,
Erdmann
E
,
Valentine
WJ
. Cost-effectiveness of pioglitazone in patients with type 2 diabetes and a history of macrovascular disease in a Swiss setting. Swiss Med Wkly. 2009;139(11-12):173–84. doi:https://smw.ch/en/article/doi/smw.2009.12381/.
19OECD. Health Statistics 2015 - Frequently Requested Data - Total health expenditure per capita, US$ PPP. Secondary Health Statistics 2015 - Frequently Requested Data - Total health expenditure per capita, US$ PPP. http://www.oecd.org/els/health-systems/oecd-health-statistics-2014-frequently-requested-data.htm.
20OECD. Health Statistics 2015 - Purchasing Power Parities for GDP and related indicators. Secondary Health Statistics 2015 - Purchasing Power Parities for GDP and related indicators. http://stats.oecd.org/.
21Office SFS. Kosten der Gesundheitswesen seit 1960. Secondary Kosten der Gesundheitswesen seit 1960 2015 http://www.bfs.admin.ch/bfs/portal/de/index/themen/14/01/new/nip_detail.html?gnpID=2014-095.
22
Craig
BM
,
Tseng
DS
. Cost-effectiveness of gastric bypass for severe obesity. Am J Med. 2002;113(6):491–8. doi:.https://doi.org/10.1016/S0002-9343(02)01266-4
23Health Statistics OECD. 2015 - Frequently Requested Data - Total health expenditure per capita, US$ PPP. In.
24Health Statistics OECD. 2015 - Purchasing Power Parities for GDP and related indicators. In.
25Office SFS. Kosten der Gesundheitswesen seit 1960. Secondary Kosten der Gesundheitswesen seit 1960 2015 http://www.bfs.admin.ch/bfs/portal/de/index/themen/14/01/new/nip_detail.html?gnpID=2014-095.
26
Ackroyd
R
,
Mouiel
J
,
Chevallier
JM
,
Daoud
F
. Cost-effectiveness and budget impact of obesity surgery in patients with type-2 diabetes in three European countries. Obes Surg. 2006;16(11):1488–503. doi:.https://doi.org/10.1381/096089206778870067
27
Borg
S
,
Näslund
I
,
Persson
U
,
Ödegaard
K
. Obesity and Surgical Treatment – A Cost-Effectiveness Assessment for Sweden. Nordic Journal of Health Economics.
2014;2(1):257–75. doi:.https://doi.org/10.5617/njhe.207
28
Campbell
J
,
McGarry
LA
,
Shikora
SA
,
Hale
BC
,
Lee
JT
,
Weinstein
MC
. Cost-effectiveness of laparoscopic gastric banding and bypass for morbid obesity. Am J Manag Care. 2010;16(7):e174–87.
29
Castilla
I
,
Mar
J
,
Valcárcel-Nazco
C
,
Arrospide
A
,
Ramos-Goñi
JM
. Cost-utility analysis of gastric bypass for severely obese patients in Spain. Obes Surg. 2014;24(12):2061–8. doi:.https://doi.org/10.1007/s11695-014-1304-0
30
Hoerger
TJ
,
Zhang
P
,
Segel
JE
,
Kahn
HS
,
Barker
LE
,
Couper
S
. Cost-effectiveness of bariatric surgery for severely obese adults with diabetes. Diabetes Care. 2010;33(9):1933–9. doi:.https://doi.org/10.2337/dc10-0554
31
Ikramuddin
S
,
Klingman
D
,
Swan
T
,
Minshall
ME
. Cost-effectiveness of Roux-en-Y gastric bypass in type 2 diabetes patients. Am J Manag Care. 2009;15(9):607–15.
32
Keating
CL
,
Dixon
JB
,
Moodie
ML
,
Peeters
A
,
Playfair
J
,
O’Brien
PE
. Cost-efficacy of surgically induced weight loss for the management of type 2 diabetes: a randomized controlled trial. Diabetes Care. 2009;32(4):580–4. doi:.https://doi.org/10.2337/dc08-1748
33
Michaud
PC
,
Goldman
DP
,
Lakdawalla
DN
,
Zheng
Y
,
Gailey
AH
. The value of medical and pharmaceutical interventions for reducing obesity. J Health Econ. 2012;31(4):630–43. doi:.https://doi.org/10.1016/j.jhealeco.2012.04.006
34
Picot
J
,
Jones
J
,
Colquitt
JL
,
Loveman
E
,
Clegg
AJ
. Weight loss surgery for mild to moderate obesity: a systematic review and economic evaluation. Obes Surg. 2012;22(9):1496–506. doi:.https://doi.org/10.1007/s11695-012-0679-z
35
Pollock
RF
,
Muduma
G
,
Valentine
WJ
. Evaluating the cost-effectiveness of laparoscopic adjustable gastric banding versus standard medical management in obese patients with type 2 diabetes in the UK. Diabetes Obes Metab. 2013;15(2):121–9. doi:.https://doi.org/10.1111/j.1463-1326.2012.01692.x
36
Wang
BC
,
Wong
ES
,
Alfonso-Cristancho
R
,
He
H
,
Flum
DR
,
Arterburn
DE
, et al.
Cost-effectiveness of bariatric surgical procedures for the treatment of severe obesity. Eur J Health Econ. 2014;15(3):253–63. doi:.https://doi.org/10.1007/s10198-013-0472-5
37
Sjöström
L
. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–34. doi:.https://doi.org/10.1111/joim.12012
38
Ananthapavan
J
,
Moodie
M
,
Haby
M
,
Carter
R
. Assessing cost-effectiveness in obesity: laparoscopic adjustable gastric banding for severely obese adolescents. Surg Obes Relat Dis. 2010;6(4):377–85. doi:.https://doi.org/10.1016/j.soard.2010.02.040
39
Anselmino
M
,
Bammer
T
,
Fernández Cebrián
JM
,
Daoud
F
,
Romagnoli
G
,
Torres
A
. Cost-effectiveness and budget impact of obesity surgery in patients with type 2 diabetes in three European countries(II). Obes Surg. 2009;19(11):1542–9. doi:.https://doi.org/10.1007/s11695-009-9946-z
40
Faria
GR
,
Preto
JR
,
Costa-Maia
J
. Gastric bypass is a cost-saving procedure: results from a comprehensive Markov model. Obes Surg. 2013;23(4):460–6. doi:.https://doi.org/10.1007/s11695-012-0816-8
41
Jensen
C
,
Flum
DR
; 2004 ABS Consensus Conference. The costs of nonsurgical and surgical weight loss interventions: is an ounce of prevention really worth a pound of cure?
Surg Obes Relat Dis. 2005;1(3):353–7. doi:.https://doi.org/10.1016/j.soard.2005.03.215
42
McEwen
LN
,
Coelho
RB
,
Baumann
LM
,
Bilik
D
,
Nota-Kirby
B
,
Herman
WH
. The cost, quality of life impact, and cost-utility of bariatric surgery in a managed care population. Obes Surg. 2010;20(7):919–28. doi:.https://doi.org/10.1007/s11695-010-0169-0
43
Salem
L
,
Devlin
A
,
Sullivan
SD
,
Flum
DR
. Cost-effectiveness analysis of laparoscopic gastric bypass, adjustable gastric banding, and nonoperative weight loss interventions. Surg Obes Relat Dis. 2008;4(1):26–32. doi:.https://doi.org/10.1016/j.soard.2007.09.009
44
Mäklin
S
,
Malmivaara
A
,
Linna
M
,
Victorzon
M
,
Koivukangas
V
,
Sintonen
H
. Cost-utility of bariatric surgery for morbid obesity in Finland. Br J Surg. 2011;98(10):1422–9. doi:.https://doi.org/10.1002/bjs.7640
45
Sjöström
CD
,
Peltonen
M
,
Wedel
H
,
Sjöström
L
. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension. 2000;36(1):20–5. doi:.https://doi.org/10.1161/01.HYP.36.1.20
46
Sjöström
L
,
Lindroos
A-K
,
Peltonen
M
,
Torgerson
J
,
Bouchard
C
,
Carlsson
B
, et al.; Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93. doi:.https://doi.org/10.1056/NEJMoa035622
47
Sjöström
L
,
Narbro
K
,
Sjöström
CD
,
Karason
K
,
Larsson
B
,
Wedel
H
, et al.; Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52. doi:.https://doi.org/10.1056/NEJMoa066254
48
Angrisani
L
,
Lorenzo
M
,
Borrelli
V
. Laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass: 5-year results of a prospective randomized trial. Surg Obes Relat Dis. 2007;3(2):127–32, discussion 132–3. doi:.https://doi.org/10.1016/j.soard.2006.12.005
49
O’Brien
PE
,
McPhail
T
,
Chaston
TB
,
Dixon
JB
. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16(8):1032–40. doi:.https://doi.org/10.1381/096089206778026316
50
Padwal
R
,
Klarenbach
S
,
Wiebe
N
,
Hazel
M
,
Birch
D
,
Karmali
S
, et al.
Bariatric surgery: a systematic review of the clinical and economic evidence. J Gen Intern Med. 2011;26(10):1183–94. doi:.https://doi.org/10.1007/s11606-011-1721-x
51
Vilarrasa
N
,
de Gordejuela
AG
,
Gómez-Vaquero
C
,
Pujol
J
,
Elio
I
,
San José
P
, et al.
Effect of bariatric surgery on bone mineral density: comparison of gastric bypass and sleeve gastrectomy. Obes Surg. 2013;23(12):2086–91. doi:.https://doi.org/10.1007/s11695-013-1016-x
52
Carrasco
F
,
Basfi-Fer
K
,
Rojas
P
,
Valencia
A
,
Csendes
A
,
Codoceo
J
, et al.
Changes in bone mineral density after sleeve gastrectomy or gastric bypass: relationships with variations in vitamin D, ghrelin, and adiponectin levels. Obes Surg. 2014;24(6):877–84. doi:.https://doi.org/10.1007/s11695-014-1179-0


