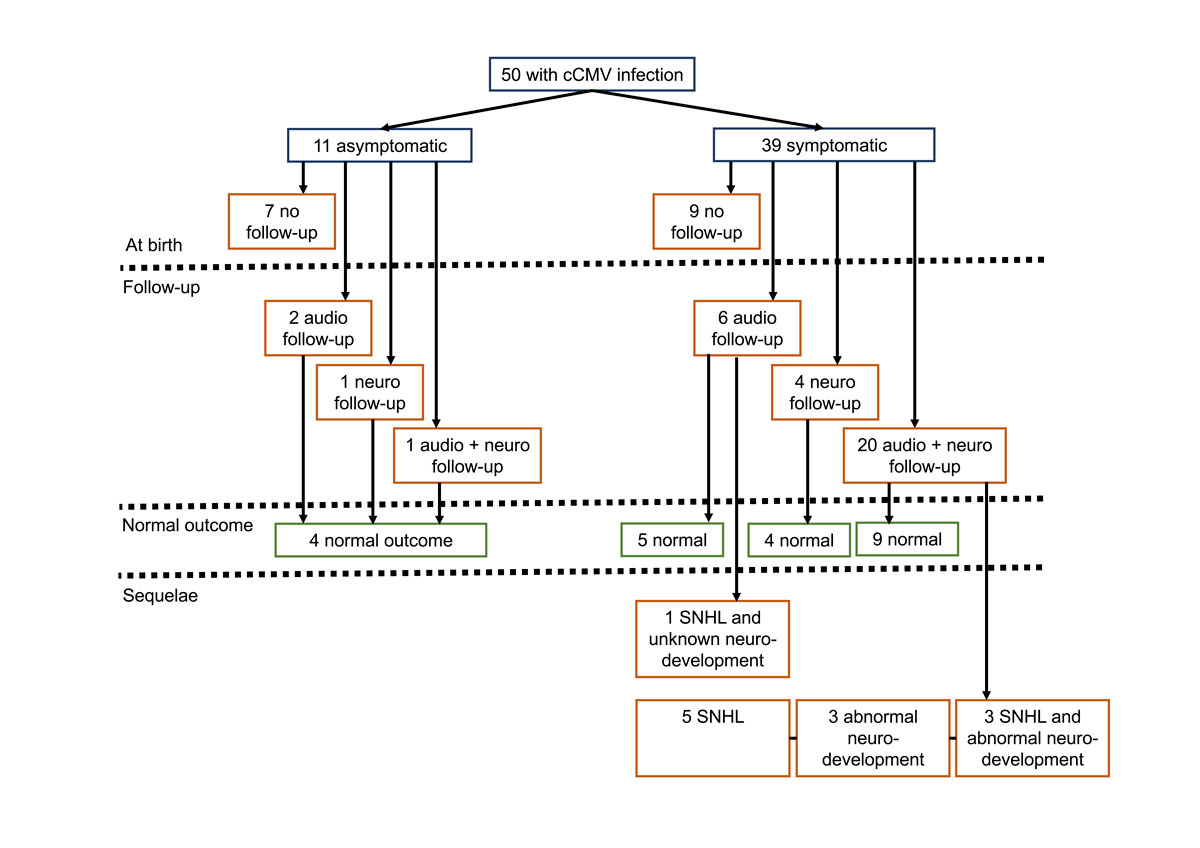
Figure 1 Clinical characteristics at birth and audiological and neurodevelopmental outcomes.
DOI: https://doi.org/10.4414/smw.2018.14627
Congenital cytomegalovirus (cCMV) infections, with a global estimated prevalence of 0.6 to 0.7%, are the leading nongenetic cause of congenital sensorineural hearing loss (SNHL) [1–6]. Cytomegalovirus (CMV) can be transmitted from the mother to the fetus despite pre-existing immunity [1–7], from either reactivation or reinfection [6]. Evidence from the literature suggests that infants born to mothers with primary infection have an equivalent audiological and neurodevelopmental outcome to those born to mothers with a nonprimary infection [4, 5]. Almost all newborns with cCMV infections are asymptomatic at birth. From these asymptomatic neonates, 10 to 15% will develop neurological sequelae [5]. Overall, 17 to 20% of all neonates with symptomatic and asymptomatic cCMV infection will have an abnormal neurological outcome, mainly SNHL, cognitive delay, neuromotor impairment such as cerebral palsy and balance disturbances, seizures or visual impairment [8–10].
Diagnosis of cCMV infection relies on the documentation of a positive CMV polymerase chain-reaction (PCR) in urine or saliva during the first 3 weeks of life [11–13]. Whereas some centres have introduced universal neonatal screening for cCMV [9], others, such as ours, perform targeted neonatal testing [14] in newborns with microcephaly, subependymal pseudocysts or thrombocytopenia [15–17]. Documentation of cCMV disease in newborns is crucial as prompt initiation of oral valganciclovir treatment improves audiological and, possibly, neurodevelopmental outcomes [16, 18, 19]. Universal screening programmes of newborns have not been implemented in part because of a lack of epidemiological data [20]. As a consequence, asymptomatic newborns will be diagnosed late through identification of SNHL [21].
From recent data, we estimate that cCMV infection could affect 500 newborns each year in Switzerland, of whom up to 100 could suffer from lifelong disabilities [9, 22, 23]. We aimed to provide information on neurodevelopmental and audiological outcomes of newborns with cCMV infection diagnosed at the Lausanne University Hospital. In addition, clinical outcomes of infants born to mothers with primary infection were compared with those born to mothers with nonprimary infection.
This was a single-centre retrospective study of neonates born between 1999 and 2014 presenting with cCMV infection and treated at the Lausanne University Hospital, Switzerland. Congenital CMV infection was defined from 1999 to 2004 as a positive CMV viral culture [24], and from 2005 to 2014 by quantitative measures of viral load with molecular assays (detection limit 100–1000 copies/ml [25]) in urine, collected within the first 3 weeks of life [11–13]. Infants born during the study period and diagnosed retrospectively with CMV PCR testing on stored dried blood spots (Guthrie cards) were also included [26].
Indications for neonatal CMV testing included documented maternal seroconversion during pregnancy, abnormal prenatal ultrasounds, documentation of CMV by molecular assays from amniotic fluid (detection limit 100–1000 copies/ml) and postnatal indications such as being small for gestational age (SGA), microcephaly, subependymal pseudocysts and/or thrombocytopenia [15–17, 20, 25]. Screening of pregnant women and newborns relied on physician-based practice and not on predefined screening policies. Maternal seroconversion during pregnancy was defined either as documentation of CMV-specific immunoglobulins in a patient previously known to be seronegative, or a newborn with proven cCMV infection born to a mother without any demonstrable CMV-specific antibodies during pregnancy [1, 5, 27]. A nonprimary maternal infection was defined as positive IgM and IgG serology with an IgG avidity of ≥70% determined within the first 12 weeks of pregnancy. The assay used to measure IgG avidity was an in-house assay based on the Enzygnost CMV IgG assay (Siemens) using an 8 M urea wash, as described elsewhere [28]. The avidity threshold defining remote infection was set using in-house seroconversion panels, and by comparison with the work of Grangeot-Keros et al. [29].
Symptomatic cCMV infection was defined as one or more of the following: SGA (birthweight <10th percentile), petechiae, hepatosplenomegaly, elevated conjugated bilirubinaemia, thrombocytopenia, elevated transaminases or central nervous system involvement [15, 16]. Neurologically symptomatic cCMV disease referred to one or more of the following: microcephaly (head circumference <3rd percentile), abnormal neurological examination, chorioretinitis, SNHL measured with brainstem-evoked response (BSER) testing, an abnormal brain ultrasound or magnetic resonance imaging (MRI) scan [16, 18, 30]. An abnormal brain ultrasound scan was defined by the presence of periventricular calcifications, white matter hyperechogenicities, ventriculomegaly or hydrocephaly. Subependymal pseudocysts and/or lenticulostriated vasculopathy were considered as nonspecific lesions and were thus not included in the criteria for an abnormal brain ultrasound [5, 17, 19, 31]. An abnormal brain MRI referred to white matter abnormalities (particularly in temporal, occipital and parietal white matter), neuronal migration disorders such as schizencephaly, pachygyria, lissencephaly, polymicrogyria or cortical dysplasia, and cerebellar hypoplasia, periventricular calcifications and ventriculomegaly [32–35].
Treatment options for neurologically symptomatic newborns consisted of a 6-week intravenous ganciclovir regimen from 2006 to 2012, 6 weeks of intravenous ganciclovir with 6 to 12 additional months of oral valganciclovir in 2013, or 6 to 12 months oral valganciclovir thereafter [16, 18, 19, 36].
Retrospective chart review and use of the database from the neonatology clinic follow-up unit provided information on relevant baseline characteristics and outcomes. This study was approved by the ethics committee on research involving humans of the canton of Vaud (protocol number 426/14).
BSER was assessed at birth and between 3 and 24 months by an ear-nose-throat (ENT) specialist. Hearing thresholds were defined as follows: 0 to 30 dB for normal hearing, 31 to 45 dB for mild hearing loss, 46 to 70 dB for moderate hearing loss and above 71 dB for severe hearing loss [16, 18]. We used total and best ear analyses as described [18]. Audiological outcomes were adjudicated on the latest BSER.
Neurodevelopmental follow-up was offered to neurologically symptomatic patients from 2001. Infants were examined with the revised Griffiths Mental Development Scales (GMDS) at 6 months of age [37], and the Bayley Scales of Infant and Toddler Development second and third editions (Bayley-II and III) at 12 months and 18 months of age [38]. Older infants were tested with the Kaufman Assessment Battery for Children (K-ABC) first edition [39], or with the Wechsler Preschool and Primary Scale of Intelligence (WPPSI-R) [40]. Neurodevelopmental outcomes were based on the latest scores determined with the GMDS, Bayley-II and III, WPPSI-R, K-ABC, all with an expected mean of 100 and a standard deviation of 15 within the normal population [37–40]. Hence test results were classified as abnormal if one test component was below 85. Sequelae were defined as an abnormal audiological and/or an abnormal neurodevelopmental outcome.
Descriptive statistics were used. Z-scores of the growth parameters weight, height and head circumference of newborns were calculated using the Fenton 2013 Growth Calculator for Preterm Infants [41].
From 1999 to 2014, 38,423 newborns were born at Lausanne University Hospital of whom 2274 were tested for CMV in urine samples. Of these 2274, 48 were found positive by viral culture or PCR. Among the 48 newborns diagnosed with cCMV infection, 24 (50%) were tested for CMV as a result of maternal seroconversion, 21 (44%) because they were SGA, 2 (4%) because of thrombocytopenia and 1 (2%) as a result of abnormal antenatal ultrasounds. Two additional patients (twins) with SNHL (and subependymal pseudocysts on neonatal brain ultrasound) tested positive by PCR on stored dried blood-spots at the age of 3.5 years.
Maternal serology status was available for 46 of 50 (92%) women; 35 of the 46 (76%) had a primary infection. Of the 50 newborns with cCMV infection, 18 (36%) were born prematurely with a median gestational age of 38.6 weeks; 12 (67%) of these were late preterm (34 0/7 to 36 6/7 weeks of gestation) (table 1). Among the 50 newborns with cCMV infection, 39 (78%) were symptomatic, of whom 29 (58%) were neurologically symptomatic and 24 (48%) were SGA. Five (10%) were born with microcephaly and none presented with chorioretinitis. Among the 45 newborns in whom a brain ultrasound was performed, 12 (27%) were abnormal, mostly with periventricular hyperechogenicity (50%). An MRI was done in 20 (40%), of whom 55% presented abnormal findings, all white matter abnormalities. Audiological examination was performed in 40 newborns, of whom 35 (88%) had normal hearing with regards to best ear analysis (supplementary table S1 in appendix 1).
Table 1 Baseline characteristics at birth of all newborns with congenital cytomegalovirus infection.
|
Infected
n = 50 |
||
|---|---|---|
| Maternal infection | Primary | 35/46 (76%) |
| Nonprimary | 11/46 (24%) | |
| Unknown | 4 | |
| Sex | Female | 30/50 (60%) |
| Prematurity <37 weeks | 18/50 (36%) | |
| Median gestational age in weeks | 38.6 (IQR 36–40) | |
| Mean weight z-score | −1.02 (SD 1.06) | |
| Mean height z-score | −1.00 (SD 1.07) | |
| Mean head circumference z-score | −0.57 (SD 1.10) | |
| Anti-viral treatment* | Yes | 13/50 (26%) |
| No | 37/50 (74%) | |
IQR = interquartile range; SD = standard deviation * Intravenous ganciclovir or oral valganciclovir
From the 50 infants with a cCMV infection, audiological assessment (range 3–26 months) performed by the ENT specialist was available for 29 (58%), 27 (93%) of whom had normal hearing in the best ear; among the 58 ears assessed, 81% had normal thresholds. Among the 50 newborns with cCMV infection, neurodevelopment follow-up (range 6–72 months) from 2001 to 2015 was available for 26 (52%) infants born between 2001 and 2014. Of these 26, 21 (81%) were offered follow-up as a result of neurologically symptomatic cCMV infection. Of those 26, 6 (23%) patients presented with abnormal neurodevelopment of whom 3 had SNHL (table S2, appendix 1).
Of the 34 infants for whom audiological and/or neurodevelopmental follow-up was available, 12 (35%) had sequelae. Of these 12, 3 (25%) presented with SNHL and abnormal neurodevelopment, 1 (8%) with SNHL and unknown neurodevelopment, 5 (42%) with isolated SNHL and 3 (25%) with an isolated abnormal neurodevelopmental outcome. All 12 were symptomatic at birth, including 10 (83%) with neurological symptoms. Among the 9 infants with SNHL, 7 (78%) had unilateral, and 2 (22%) bilateral hearing loss (fig. 1 and table 2).

Figure 1 Clinical characteristics at birth and audiological and neurodevelopmental outcomes.
Table 2 Baseline characteristics, audiological and neurodevelopmental outcomes of children with sequelae.
|
Patient
number |
Comorbidities and social difficulties |
Gestational age
(weeks and days) |
Weight z-score | Height z-score |
Head circumference
z-score |
Maternal primary infection | Neurodevelopmental outcome | Hearing loss |
|---|---|---|---|---|---|---|---|---|
| 1 | No | 40 1/7 | 0.04 | −1.13 | −1.29 | Yes | Language delay | Moderate right |
| 2 | No | 37 3/7 | 0.86 | 0.4 | 0.54 | Yes | Language delay | No |
| 3 | No | 39 1/7 | −1.31 | −1.04 | −1.1 | No | Normal | Moderate right |
| 4 | Maternal HIV infection; difficult psychosocial situation | 38 2/7 | −1.57 | −1.1 | −0.07 | No | Global delay | No |
| 5 | Very preterm | 28 3/7 | −2.05 | −3.01 | −3.56 | Unknown | Global delay | Moderate right |
| 6 | Chronic maternal hepatitis B infection | 38 1/7 | −1.87 | −0.3 | −0.47 | No | Unknown | Severe left |
| 7 | Late preterm | 36 0/7 | −0.06 | 0.27 | 0.16 | Yes | Normal | Severe right, mild left |
| 8 | Late preterm | 36 0/7 | −0.29 | −0.55 | −0.17 | Yes | Normal | Severe bilateral |
| 9 | No | 38 0/7 | −2.72 | −2.3 | −2.16 | Yes | Normal | Severe, left |
| 10 | No | 41 5/7 | −2.75 | −2.25 | −2.64 | Yes | Normal | Severe, right |
| 11 | Moderate preterm | 33 0/7 | −2.4 | −2.5 | −1.18 | Unknown | Global delay | Normal |
| 12 | Late preterm | 34 6/7 | −1,54 | −0.33 | −1.21 | No | Motor delay and visual problems | Moderate left |
HIV = human immunodeficiency virus Very preterm = 28 0/7 to 31 6/7, moderate preterm = 32 0/7 to 33 6/7, late preterm = 34 0/7 to 36 6/7 weeks of gestation
Newborns born to mothers with nonprimary infection were more often symptomatic and SGA at birth than those born to mothers with a primary infection. In contrast, petechiae were more frequent in newborns born to mothers with primary infection than in newborns born to mothers with a nonprimary infection. In the two groups, the rates of microcephaly and abnormal audiological outcome at birth were similar (table 3).
Table 3 Clinical characteristics at birth categorised by newborns born to mothers with primary versus nonprimary infection.
|
Maternal primary infection
n = 35 |
Maternal
nonprimary infection n = 11 |
||
|---|---|---|---|
| Symptomatic | 25/35 (71%) | 10/11 (91%) | |
| Neurologically symptomatic | 18/35 (51%) | 8/11 (73%) | |
| SGA | 12/35 (34%) | 9/11 (82%) | |
| Petechiae | 12/35 (34%) | 1/11 (9%) | |
| Thrombocytes Abnormal: <150 G/l |
Abnormal | 9/26 (35%) | 6/11 (55%) |
| Normal | 17/26 (65%) | 5/11 (45%) | |
| Unknown | 9 | 0 | |
| Microcephaly | 3/35 (9%) | 1/11 (9%) | |
| Fundus | Abnormal | 0/31 (0%) | 0/9 (0%) |
| Normal | 26/31 (100%) | 9/9 (100%) | |
| Unknown | 9 | 2 | |
| Brain ultrasound | Abnormal | 7/31 (23%) | 4/10 (40%) |
| Normal | 24/31 (77%) | 6/10 (60%) | |
| Unknown | 4 | 1 | |
| MRI | Abnormal | 5/11 (45%) | 5/8 (63%) |
| Normal | 6/11 (55%) | 3/8 (38%) | |
| Unknown | 24 | 3 | |
| BSER best ear | Normal | 25/28 (89%) | 8/10 (80%) |
| Mild | 0/28 (0%) | 2/10 (20%) | |
| Moderate | 2/28 (7%) | 0/10 (0%) | |
| Severe | 1/28 (4%) | 0/10 (0%) | |
| Unknown | 7 | 1 | |
| BSER total ears | Normal | 45/56 (80%) | 15/20 (75%) |
| Mild | 3/56 (5%) | 2/20 (10%) | |
| Moderate | 5/56 (9%) | 3/20 (15%) | |
| Severe | 3/56 (5%) | 0/20 (0%) | |
| Unknown | 14 | 2 | |
BSER = brain stem evoked response; MRI = magnetic resonance imaging; SGA = small for gestational age
Results of hearing assessments and neurodevelopmental outcomes at 3 months of age and onwards were similar in children born to mothers with a primary infection and those with a nonprimary infection (table 4).
Table 4 Audiological and neurodevelopmental outcome at 3 months of age and onwards, categorised by newborns born to mothers with a primary versus a nonprimary infection.
|
Maternal primary infection
n = 35 |
Maternal nonprimary infection
n = 11 |
||
|---|---|---|---|
| Latest BSER ≥3 months best ear |
Normal | 16/18 (89%) | 9/9 (100%) |
| Mild | 1/18 (6%) | 0/9 (0%) | |
| Moderate | 0/18 (0%) | 0/9 (0%) | |
| Severe | 1/18 (6%) | 0/9 (0%) | |
| Unknown | 17 | 2 | |
| Latest BSER ≥3 months total ears |
Normal | 29/36 (81%) | 15/18 (83%) |
| Mild | 1/36 (3%) | 0/18 (0%) | |
| Moderate | 1/36 (3%) | 2/18 (11%) | |
| Severe | 5/36 (14%) | 1/18 (6%) | |
| Unknown | 34 | 4 | |
| Neurodevelopment score ≥6 months | Abnormal | 2/14 (14%) | 2/8 (25%) |
| Normal | 12/14 (86%) | 6/8 (75%) | |
| Unknown | 21 | 3 | |
BSER = brain stem evoked response
In this first exploratory study to address the burden of cCMV infections in Switzerland, three important observations were made: (1) cCMV infections were detected equally among newborns born to mothers with primary infection and nonprimary infection; (2) most infants who presented with SNHL or abnormal neurodevelopment were neurologically symptomatic at birth; (3) newborns of mothers with a primary infection had similar audiological and neurodevelopmental outcomes as those born to mothers with a nonprimary infection.
We documented a higher proportion of infants born to mothers with a primary infection (76%) versus nonprimary infection, compared with a previous report of countries with a low seroprevalence, like Switzerland (44%) [5]. This finding most likely resulted from the systematic testing for CMV among newborns born to mothers with seroconversion at our centre. We report a lower proportion of symptoms among symptomatic newborns with cCMV infection compared with others studies [15, 16], whereas our proportion of neurological symptoms among symptomatic newborns was similar. These discrepancies could result from a less rigorous assessment of clinical and laboratory outcomes than of neurological outcomes in our setting compared with other studies [15, 16]. Careful clinical and laboratory assessment is warranted in all newborns with cCMV infection, as petechiae and SGA, as well as thrombocytopenia, were identified as significant predictors of subsequent SNHL [42].
Our study reported a similar rate of SNHL or abnormal neurodevelopmental outcomes at 3 months of age and onwards compared with other studies (24% vs 17–20%) [9]. All our children with subsequent SNHL or abnormal neurodevelopmental scores were symptomatic at birth, in contrast to a systematic review [9] that reported that only one third of children with subsequent SNHL or abnormal neurodevelopmental outcomes were symptomatic at birth. As supported by the systematic review [9], neurodevelopmental and audiological follow-up should be offered to all children with cCMV infection identified through universal newborn screening [9, 43].
Confirming findings of prior reports [4, 7], our study indicated that infants born to mothers with a nonprimary infection are at least as often symptomatic at birth as those born to mothers with a primary infection. As documented in other studies [4, 7], we report equivalent rates of abnormal audiological and neurodevelopmental outcomes of children born to mothers with a primary infection compared with those born to mothers with a nonprimary infection.
Our exploratory study represents an important first step in reporting audiological and neurodevelopmental outcomes of newborns with cCMV infection in Switzerland. An important strength relates to the precise documentation of BSER measures, including best ears analysis and total ears analysis, as well as the use of standardised developmental tests [16]. Limitations of our study include the retrospective design, inhomogeneous timeframe of the audiological follow-up and the absence of follow-up of asymptomatic newborns, which may have biased our findings towards more severe outcomes among symptomatic children with longer follow-up. This limitation is inherent to all retrospective studies on cCMV infections conducted before the establishment of recent guidelines on auditory outcomes measures [3], as reflected in a recent European surveillance study [44]. However, at each visit at the neurodevelopmental clinic, hearing was examined by the paediatrician and those with any abnormal findings were systematically referred to the ENT specialist. Therefore, we believe that the wide timeframe of auditory assessment conducted by the ENT specialist minimally affected our findings. Other shortcomings of our study relate to the small sample size inherent to monocentric retrospective studies conducted in a country with a low prevalence of cCMV infection [5]. In addition, the absence of universal screening of newborns may have biased our study towards selecting mothers with a primary infection and mostly symptomatic newborns, who presented mostly with normal BSER and neurodevelopmental scores.
In conclusion, our study supports current findings on the burden and severity of cCMV infections [4, 7, 9] and provides national data on outcomes of newborns with cCMV infection. Combined information on clinical outcomes from the current study and epidemiological data provided by the Swiss Paediatric Surveillance Study (SPSU) will hopefully encourage the establishment of a national registry, which will in turn serve as foundation for future prospective studies.
Table S1 Clinical outcomes at birth of all newborns with congenital cytomegalovirus infection.
|
Infected
n = 50 |
||
|---|---|---|
| Symptomatic | 39/50 (78%) | |
| Neurologically symptomatic | 29/50 (58%) | |
| SGA | 24/50 (48%) | |
| Petechiae | 13/50 (26%) | |
| Thrombocytes Abnormal: <150 G/l |
Abnormal | 17/41 (41%) |
| Normal | 24/41 (59%) | |
| Unknown | 9 | |
| Microcephaly | 5/50 (10%) | |
| Fundus | Abnormal | 0/39 (0%) |
| Normal | 39/39 (100%) | |
| Unknown | 11 | |
| Brain ultrasound | Abnormal | 12/45 (27%) |
| Normal | 33/45 (73%) | |
| Unknown | 5 | |
| MRI | Abnormal | 11/20 (55%) |
| Normal | 9/20 (45%) | |
| Unknown | 30 | |
| BSER best ear | Normal | 35/40 (88%) |
| Mild | 2/40 (5%) | |
| Moderate | 2/40 (5%) | |
| Severe | 1/40 (3%) | |
| Unknown | 10 | |
| BSER total ears | Normal | 64/80 (80%) |
| Mild | 5/80 (6%) | |
| Moderate | 8/80 (10%) | |
| Severe | 3/80 (4%) | |
| Unknown | 20 | |
| BSER = brain stem evoked response; MRI = magnetic resonance imaging; SGA = small for gestational age | ||
Table S2 Audiological and neurodevelopmental outcome at 3 months of age and onwards of all newborns with congenital cytomegalovirus infection.
|
Infected
n = 50 |
||
|---|---|---|
| Latest BSER ≥3 months best ear |
Normal | 27/29 (93%) |
| Mild | 1/29 (3%) | |
| Moderate | 0/29 (0%) | |
| Severe | 1/29 (3%) | |
| Unknown | 21 | |
| Latest BSER ≥3 months total ears |
Normal | 47/58 (81%) |
| Mild | 1/58 (2%) | |
| Moderate | 4/58 (7%) | |
| Severe | 6/58 (10%) | |
| Unknown | 42 | |
| Neurodevelopment score ≥6 months | Abnormal | 6/26 (23%) |
| Normal | 20/26 (77%) | |
| Unknown | 24 | |
| BSER = brain stem evoked response | ||
The authors would like to thank Mrs Karine Perretten the secretary of the Follow-up unit for the great help provided during data collection and Dr René Stricker from the Dianalabs who provided detailed information and references on various diagnostic procedures.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Yamamoto AY , Mussi-Pinhata MM , Isaac M de L , Amaral FR , Carvalheiro CG , Aragon DC , et al. Congenital cytomegalovirus infection as a cause of sensorineural hearing loss in a highly immune population. Pediatr Infect Dis J. 2011;30(12):1043–6. doi:.https://doi.org/10.1097/INF.0b013e31822d9640
2 Schleiss MR . Congenital cytomegalovirus infection: update on management strategies. Curr Treat Options Neurol. 2008;10(3):186–92. doi:.https://doi.org/10.1007/s11940-008-0020-2
3 Rawlinson WD , Boppana SB , Fowler KB , Kimberlin DW , Lazzarotto T , Alain S , et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis. 2017;17(6):e177–88. doi:.https://doi.org/10.1016/S1473-3099(17)30143-3
4 Boppana SB , Fowler KB , Britt WJ , Stagno S , Pass RF . Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics. 1999;104(1):55–60. doi:.https://doi.org/10.1542/peds.104.1.55
5 Manicklal S , Emery VC , Lazzarotto T , Boppana SB , Gupta RK . The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013;26(1):86–102. doi:.https://doi.org/10.1128/CMR.00062-12
6 Fowler KB , Stagno S , Pass RF , Britt WJ , Boll TJ , Alford CA . The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326(10):663–7. doi:.https://doi.org/10.1056/NEJM199203053261003
7 Ross SA , Fowler KB , Ashrith G , Stagno S , Britt WJ , Pass RF , et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr. 2006;148(3):332–6. doi:.https://doi.org/10.1016/j.jpeds.2005.09.003
8 Lombardi G , Garofoli F , Stronati M . Congenital cytomegalovirus infection: treatment, sequelae and follow-up. J Matern Fetal Neonatal Med. 2010;23(sup3, Suppl 3):45–8. doi:.https://doi.org/10.3109/14767058.2010.506753
9 Dollard SC , Grosse SD , Ross DS . New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17(5):355–63. doi:.https://doi.org/10.1002/rmv.544
10 Karltorp E , Löfkvist U , Lewensohn-Fuchs I , Lindström K , Eriksson Westblad M , Teär Fahnehjelm K , et al. Impaired balance and neurodevelopmental disabilities among children with congenital cytomegalovirus infection. Acta Paediatr. 2014;103(11):1165–73. doi:.https://doi.org/10.1111/apa.12745
11 Bale JF , Miner L , Petheram SJ . Congenital Cytomegalovirus Infection. Curr Treat Options Neurol. 2002;4(3):225–30. doi:.https://doi.org/10.1007/s11940-002-0039-8
12 Ross SA , Ahmed A , Palmer AL , Michaels MG , Sánchez PJ , Bernstein DI , et al.; National Institute on Deafness and Other Communication Disorders CHIMES Study. Detection of congenital cytomegalovirus infection by real-time polymerase chain reaction analysis of saliva or urine specimens. J Infect Dis. 2014;210(9):1415–8. doi:.https://doi.org/10.1093/infdis/jiu263
13 de Vries JJC , Vossen ACTM , Kroes ACM , van der Zeijst BAM . Implementing neonatal screening for congenital cytomegalovirus: addressing the deafness of policy makers. Rev Med Virol. 2011;21(1):54–61. doi:.https://doi.org/10.1002/rmv.679
14 Fowler KB , McCollister FP , Sabo DL , Shoup AG , Owen KE , Woodruff JL , et al.; CHIMES Study. A Targeted Approach for Congenital Cytomegalovirus Screening Within Newborn Hearing Screening. Pediatrics. 2017;139(2):e20162128. doi:.https://doi.org/10.1542/peds.2016-2128
15 Boppana SB , Ross SA , Fowler KB . Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57(Suppl 4):S178–81. doi:.https://doi.org/10.1093/cid/cit629
16 Kimberlin DW , Jester PM , Sánchez PJ , Ahmed A , Arav-Boger R , Michaels MG , et al.; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. 2015;372(10):933–43. doi:.https://doi.org/10.1056/NEJMoa1404599
17 Cevey-Macherel M , Forcada Guex M , Bickle Graz M , Truttmann AC . Neurodevelopment outcome of newborns with cerebral subependymal pseudocysts at 18 and 46 months: a prospective study. Arch Dis Child. 2013;98(7):497–502. doi:.https://doi.org/10.1136/archdischild-2012-303223
18 Kimberlin DW , Lin C-Y , Sánchez PJ , Demmler GJ , Dankner W , Shelton M , et al.; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143(1):16–25. doi:.https://doi.org/10.1016/S0022-3476(03)00192-6
19 Amir J , Wolf DG , Levy I . Treatment of symptomatic congenital cytomegalovirus infection with intravenous ganciclovir followed by long-term oral valganciclovir. Eur J Pediatr. 2010;169(9):1061–7. doi:.https://doi.org/10.1007/s00431-010-1176-9
20 Coll O , Benoist G , Ville Y , Weisman LE , Botet F , Anceschi MM , et al., WAPM Perinatal Infections Working Group. Guidelines on CMV congenital infection. J Perinat Med. 2009;37(5):433–45. doi:.https://doi.org/10.1515/JPM.2009.127
21 Fowler KB , Dahle AJ , Boppana SB , Pass RF . Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr. 1999;135(1):60–4. doi:.https://doi.org/10.1016/S0022-3476(99)70328-8
22Bundesamt für Gesundheit - Swiss Paediatric Surveillance Unit (SPSU) [Internet]. [cited 2016 Apr 29]. Available from: http://www.bag.admin.ch/k_m_meldesystem/00737/index.html?lang=de
23 Kenneson A , Cannon MJ . Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–76. doi:.https://doi.org/10.1002/rmv.535
24 Muheim C , Vogel G , Seydoux C , Gillet M , Mosimann F , Von Segesser L , et al. Determinants of protracted cytomegalovirus infection in solid-organ transplant patients. Transplantation. 2002;74(2):226–36. doi:.https://doi.org/10.1097/00007890-200207270-00014
25 Chatellard P , Sahli R , Iten A , von Overbeck J , Meylan PR . Single tube competitive PCR for quantitation of CMV DNA in the blood of HIV+ and solid organ transplant patients. J Virol Methods. 1998;71(2):137–46. doi:.https://doi.org/10.1016/S0166-0934(97)00087-6
26 Boppana SB , Ross SA , Novak Z , Shimamura M , Tolan RW, Jr , Palmer AL , et al.; National Institute on Deafness and Other Communication Disorders CMV and Hearing Multicenter Screening (CHIMES) Study. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303(14):1375–82. doi:.https://doi.org/10.1001/jama.2010.423
27 Benoist G , Leruez-Ville M , Magny JF , Jacquemard F , Salomon LJ , Ville Y . Management of pregnancies with confirmed cytomegalovirus fetal infection. Fetal Diagn Ther. 2013;33(4):203–14. doi:.https://doi.org/10.1159/000342752
28 Bodéus M , Beulné D , Goubau P . Ability of three IgG-avidity assays to exclude recent cytomegalovirus infection. Eur J Clin Microbiol Infect Dis. 2001;20(4):248–52. doi:.https://doi.org/10.1007/s100960100484
29 Grangeot-Keros L , Mayaux MJ , Lebon P , Freymuth F , Eugene G , Stricker R , et al. Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary CMV infection in pregnant women. J Infect Dis. 1997;175(4):944–6. doi:.https://doi.org/10.1086/513996
30 Coats DK , Demmler GJ , Paysse EA , Du LT , Libby C . Ophthalmologic findings in children with congenital cytomegalovirus infection. J AAPOS. 2000;4(2):110–6. doi:.https://doi.org/10.1067/mpa.2000.103870
31 Amir J , Schwarz M , Levy I , Haimi-Cohen Y , Pardo J . Is lenticulostriated vasculopathy a sign of central nervous system insult in infants with congenital CMV infection? Arch Dis Child. 2011;96(9):846–50. doi:.https://doi.org/10.1136/adc.2010.208405
32 Fink KR , Thapa MM , Ishak GE , Pruthi S . Neuroimaging of pediatric central nervous system cytomegalovirus infection. Radiographics. 2010;30(7):1779–96. doi:.https://doi.org/10.1148/rg.307105043
33 Bale JF, Jr . Cytomegalovirus infections. Semin Pediatr Neurol. 2012;19(3):101–6. doi:.https://doi.org/10.1016/j.spen.2012.02.008
34 Boesch C , Issakainen J , Kewitz G , Kikinis R , Martin E , Boltshauser E . Magnetic resonance imaging of the brain in congenital cytomegalovirus infection. Pediatr Radiol. 1989;19(2):91–3. doi:.https://doi.org/10.1007/BF02387893
35 Barkovich AJ , Lindan CE . Congenital cytomegalovirus infection of the brain: imaging analysis and embryologic considerations. AJNR Am J Neuroradiol. 1994;15(4):703–15.
36Kimberlin DW, Jester P, Sanchez PJ, Ahmed A, Sood S, Woods C, et al. Six months versus six weeks of oral valganciclovir for infants with symptomatic congenital cytomegalovirus (CMV) disease with and without central nervous system (CNS) involvement: Results of a Phase III, randomized, double-blind, placebo-controlled, multinational study [abstract LB-1]. In IDWeek 2013, San Francisco, CA, October 2 to 6, 2013; 2013 [cited 2014 Jul 29]. Available from: https://idsa.confex.com/idsa/2013/webprogram/Paper43178.html
37Griffiths R. The Abilities of Babies. Oxford: The Test Agency; 1970.
38Bayley N. Bayley scales of Infant Development. 2nd edition. San Antonio, TX: Harcourt Assessment Company; 1993.
39Kaufman AS, Kaufman NL. Kaufman Assessment Battery for Children. Circle Pines MN: American Guidance Service; 1983.
40Wechsler D. Echelle de Wechsler pour la période préscolaire et primaire. 3ième édition. Paris: Editions du Centre de Psychologie Appliquée; 2002.
41Growth Fenton. 2013 [Internet]. [cited 2016 Apr 30]. Available from: http://www.peditools.org/fenton2013/
42 Rivera LB , Boppana SB , Fowler KB , Britt WJ , Stagno S , Pass RF . Predictors of hearing loss in children with symptomatic congenital cytomegalovirus infection. Pediatrics. 2002;110(4):762–7. doi:.https://doi.org/10.1542/peds.110.4.762
43 Cannon MJ , Griffiths PD , Aston V , Rawlinson WD . Universal newborn screening for congenital CMV infection: what is the evidence of potential benefit? Rev Med Virol. 2014;24(5):291–307. doi:.https://doi.org/10.1002/rmv.1790
44 Gunkel J , Nijman J , Verboon-Maciolek MA , Wolfs T , de Vries LS . International opinions and national surveillance suggest insufficient consensus regarding the recognition and management practices of infants with congenital cytomegalovirus infections. Acta Paediatr. 2017;106(9):1493–8. doi:.https://doi.org/10.1111/apa.13882
No financial support and no other potential conflict of interest relevant to this article was reported.