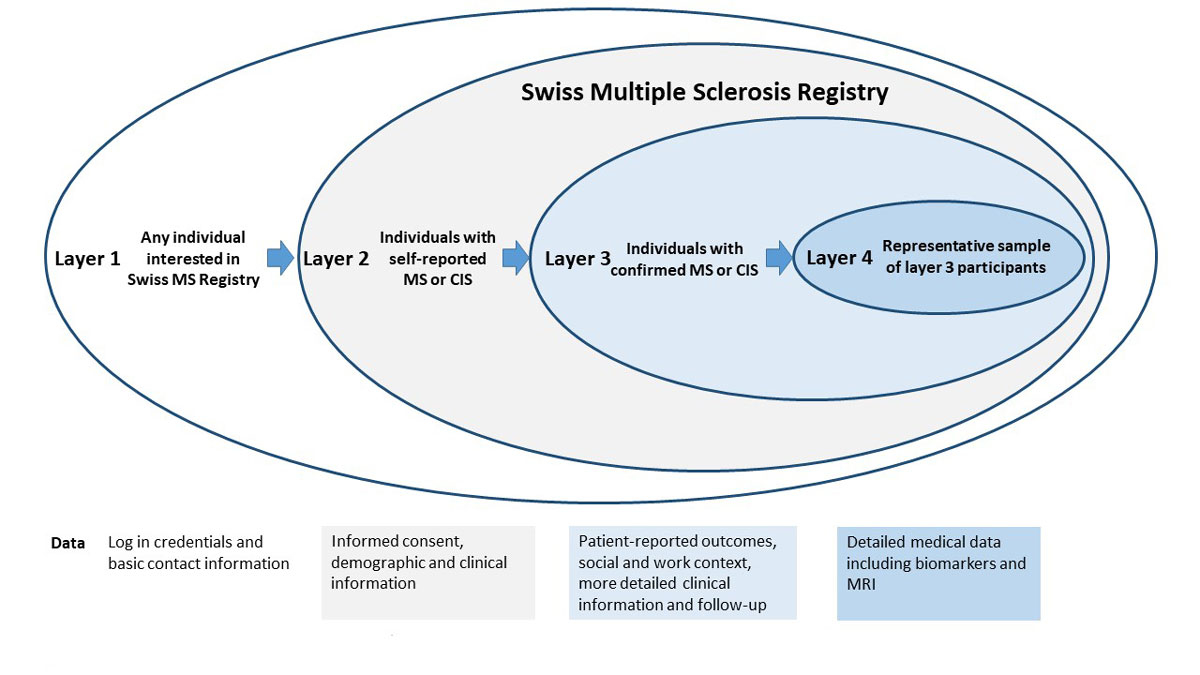
Figure 1 Layered model and type of data collection in the Swiss Multiple Sclerosis Registry.
CIS = clinically isolated syndrome; MRI = magnetic resonance imaging; MS = multiple sclerosis
DOI: https://doi.org/10.4414/smw.2018.14623
Academic or industry scientists who perform studies with limited patient engagement beyond study participation traditionally drive medical research. Although this approach has led to many notable advances, many substantial shortcomings of this framework remain (table 1). First, the knowledge base available for patient and public health decisions is often insufficient, because many issues relevant to patients and caregivers are missing, or because patient-relevant outcomes are not considered [1, 2]. Second, few studies include comparative effectiveness assessments of pharmacological or nonpharmacological interventions, thus hampering medical decision-making [3, 4]. Third, traditional studies often apply restrictive exclusion criteria or rely on recruitment in specialised clinical centres [5, 6]. These methods may improve the chances of finding desired effects, but may endanger the generalisability of the findings and create a lack of diversity in the study population, frequently underrepresenting individuals who choose to receive either care in nonspecialised outpatient settings or no medical care at all. This is particularly problematic for studies that address questions of disease burden such as incidence, prevalence, health-related quality of life and loss of years at full health, as well as questions about the use of healthcare services.
Table 1 Advantages and disadvantages of traditional medical research, and opportunities and risks of novel approaches using societal and technological developments.
| Criterion | Traditional clinical research (e.g., cohort study or clinical trial) | Novel approaches to research using digital tools and citizen or patient engagement (e.g., patientslikeme) |
|---|---|---|
| 1. Legal and ethical framework | (+) Established by law and research community, but often not adaptive to novel trends | (−) Still in development, currently a situation of legal and ethical uncertainty |
| 2. Scientifically rigorous methodology | (+) Extensively developed methodology in terms of study designs and statistical tools | (+) Can partially rely on existing methods (−) Comprehensive methodology still in development, little guidance available |
| 3. Acceptance by medical community, regulatory agencies and policy makers | (+) If guidance on scientifically rigorous methods is followed | (−) Low as yet, mainly due to shortcomings listed above |
| 4. Framework for evidence reporting and interpretation | (+) Well established and widely accepted (e.g., reporting guidelines) | (−) Still in development |
| 5. Citizen science (patient involvement in study design, execution, and analysis) | (−) Often not considered | (+) Core feature; strong involvement considerably enhances participation |
| 6. Includes multiple stakeholder views | (−) Often limited | (+) More flexible, strong focus on citizen and/or patient perspective |
| 7. Patient-relevant study outcomes | (−) Tendency for overreliance on clinical outcomes; patient-reported outcomes not always considered | (+) Core feature (−) Clinical outcomes often not available |
| 8. Creates knowledge base for real-world questions | (−) Not always given, potential overreliance on clinical outcomes and highly selected study populations | (+) If well conducted, delivers more comprehensive evidence, closer to real-life situations |
| 9. Adaptive to novel technological trends | (0) Possible, but sometimes difficult to fit into established frameworks (e.g., online data collection tools) | (+) Core feature; can incorporate behaviour data, social media activities, data from internet of things |
| 10. Clearly defined target, source and study populations | (+) Often good for populations in clinical care (−) Selection may lead to lack of external validity |
(+) Often inclusive recruitment (−) selection process not well understood |
| 11. Risk for measurement and information biases; data quality | (+) If well executed, manageable risk | (+) Self-measurement, interpretation of own data (−) Often patient interpreted; objective measurements sometimes difficult; prone to information biases; difficult to implement data quality procedures |
| 12. Data ownership, oversight, transparent governance | (−) Unclear, patients rarely see their own data; little strategic influence by patients (e.g., regarding study goals) | (+) Core feature if clearly and transparently defined; comprehensive inclusion of patients; study participants see their own data (−) Uncontrolled use of participants’ data (−) Data ownership not regulated |
(+) advantage, (0) neutral, (−) disadvantage, risk
Fortunately, recent developments in society and technology could help to overcome some of the above challenges. For example, patients no longer depend on healthcare professionals for access to their own health data [7], as medical tests and their interpretation become publicly available. Self-tracking devices and big-data analytics provide individualised diagnoses or predictions of disease risks and outcomes. Online platforms (e.g., patientslikeme, CureTogether) and social media [8] invite and enable healthy people and patients to share health data and experiences. These social and technological developments have considerable potential to accelerate medical research and broaden the size and diversity of study populations [9].
However, these novel opportunities for medical research are not without legal, ethical and methodological challenges. Biological or digital data are often amassed on vague ethical and legal bases including unclear information and consent procedures, unspecified purposes, unclear data ownership and undetermined storage durations [10]. Even with informed consent, the purpose of data collection and the sharing rules are often not fully specified at the time of signature [10]. Additionally, the purchase and use of patient-contributed data by third parties can often go unnoticed by participants and the implications of this are unknown. Finally, reliance on self-collected, unstructured patient data raises questions about the internal and external validity of the study results [11]. Self-selection and convenience study samples could impair confounder control and the implementation of measures against other validity threats.
The Swiss Multiple Sclerosis (MS) Registry is designed to leverage the advantages of both traditional medical research and novel societal and technological developments, while mitigating their methodological, ethical and legal disadvantages, and risks. The aim of this article is to assess the first 18 months of participant recruitment in the Swiss MS Registry, which follows a novel approach of digitally facilitated, citizen-science research. Specifically, we aimed at assessing if participant recruitment was indeed accelerated, and whether the study population was more diverse than in research studies following more traditional approaches of participant involvement and recruitment.
The Swiss MS Registry is a prospective, longitudinal, observational study covering the entirety of Switzerland (ClinicalTrials.gov identifier NCT02980640; approved for nationwide conduct as a multicentre study by the Ethics Committee Zurich, study number PB-2016-00894). Based on the notion of well-aligned interests, mutual trust and complementary expertise, the following goals were defined by persons with MS, the Swiss MS Society, researchers and healthcare professionals [1]: to monitor epidemiological trends of MS in Switzerland over time [2]; to estimate the burden on persons with MS and families/proxies; and [3] to establish a flexible network enabling inter- and transdisciplinary, patient-centred research.
We chose a layered model that takes into account the often long diagnostic process of MS and allows for different levels of involvement by participants (fig. 1), similar to the Amyotrophic Lateral Sclerosis Registry in the USA [12]. Layers 1 and 2 do not have any exclusion criteria and are open to persons with MS, persons with a still uncertain diagnoses of MS and to family members or relatives. Information on the Swiss MS Registry can be retrieved via a special section on the Swiss MS Society’s webpage, where additional information on MS in general is also available. This integration creates synergies for both parties by content linkage and increased visitor traffic.

Figure 1 Layered model and type of data collection in the Swiss Multiple Sclerosis Registry.
CIS = clinically isolated syndrome; MRI = magnetic resonance imaging; MS = multiple sclerosis
For data collection, a separate, independent web platform, which is hosted by the University of Zurich, was constructed. In order to gain access to this web platform and layer 1, users have to create an account. In this step, interested persons deposit their contact data (name, address) and provide information about their “role” (patient or relative). During the user account creation, a unique identification number is assigned to that person. The full contact data and unique identification numbers are stored in a secured database, which is kept separate from all other research data and accessible only to defined data managers.
Layer 2 aims at determining whether persons with self-reported confirmed or unconfirmed MS fulfil the eligibility criteria for the Swiss MS Registry, in order to obtain basic information on demography and MS status. The layer 2 questionnaire also includes teaser questions on patient-relevant topics (e.g., alternative medicines, behavioural changes after MS diagnosis) to spark the participant’s interest in additional surveys. Layer 2 provides more time to establish and confirm a diagnosis. This accommodates the often long diagnostic process for MS and avoids excluding people only potentially affected by MS from the Swiss MS Registry right away. Patients wishing to join layer 2 must be at least 18 years of age and have to sign an online informed consent form, which informs them about the type and purpose of data collection and possible risks and benefits of the study, as well as their rights. As part of this agreement, patients also consent to being contacted by members of the Swiss MS Registry data centre staff if needed (e.g., for data clarification, retrieval of diagnosis confirmation, or for invitations to additional substudies). Importantly, participants also have the option to complete the questionnaires on paper, offline. This option was created to enable participation of those without regular use of computers or other devices, or with impairment that makes using the online platform too burdensome.
A confirmed diagnosis of MS or of a Clinical Isolated Syndrome and a signed online informed consent form are prerequisites for entering layer 3. The diagnosis needs to be confirmed in writing by a neurologist, but no restriction regarding type of diagnostic criteria are imposed (e.g., McDonald or Poser criteria). Data collection within this layer is much more comprehensive (see “Measurements” section below). Finally, a subset of layer 3 participants is randomly selected for data ascertainment based on medical records (layer 4). The informed consent for the Swiss MS Registry covers data collection in layers 2 to 4. Participants can consent to layer 4 study modules on an opt-in basis. If provided, the layer 4 informed consent allows study nurses of the Swiss MS Registry to visit the respective clinics and practices and extract specific clinical information from the medical records.
The Swiss MS Registry operates in a spirit of cooperation and mutual trust, reflected in the involvement of all relevant stakeholders and voting rights through their representatives. The scientific assembly and its 20 members form the governance body of the Swiss MS Registry and it takes all strategic decisions, whenever possible by consensus. The members are persons with MS, representatives of the Swiss MS Society, healthcare professionals working in the area of MS, and scientists with particular expertise in clinical or community-based MS research, epidemiology or information technology. The German-, French- and Italian-speaking parts of Switzerland, as well as diverse healthcare settings, are all represented in the scientific assembly. The members of the assembly have regular phone calls and meet biannually in person. All strategic decisions, and approval of regulations, projects and publications are made by this inter- and transdisciplinary group. Professor Jürg Kesselring serves as the first president of the Swiss MS Registry and chairs the scientific assembly meetings.
Each assembly member also contributes to one of three research committees: a Data Committee concerned with the operation of the registry; a Patient- and Population-based Research Committee, and a Laboratory- and Clinic-based Research Committee, both of which oversee and govern the scientific parts of the Registry. The three committees review and approve requests for Swiss MS Registry data for research purposes. Data management and the daily operation of the Swiss MS Registry are led by the Data Centre team at the Epidemiology, Biostatistics and Prevention Institute of the University of Zurich.
A particular challenge in studying MS is its multifaceted manifestation. In order to comprehensively describe this “disease with a thousand faces”, various aspects of life and health need to be covered. The online format of the Swiss MS Registry allows participants to interrupt the completion of questionnaires, which facilitate the comprehensive data collection needed to address the main questions and goals of the Swiss MS Registry. Data collected include sociodemographic and family history data, as well as information on (lifestyle) behaviours and exposures, and living and working conditions. In addition, data on the general cultural, environmental and socioeconomic conditions can be added, depending on where participants live. The breadth of the Swiss MS Registry data collection goes well beyond the clinical context and allows study questions on burden and causes of disease, and prognostic and therapeutic questions, as well as research on health services for persons with MS. Layer 4 provides detailed clinical data from medical records, which are abstracted using standardised forms that are harmonised with data collected in the Swiss MS cohort study.
The Swiss MS Registry closely collaborates with the Swiss MS Cohort Study [13], a clinic-based study at eight major Swiss MS centres. The joint databases offer opportunities for data validation and for innovative projects combining patient-reported Swiss MS Registry data and high quality clinical information and bio-samples from the Swiss MS Cohort Study through harmonised data collection and informed consent for data linkage. Combining data from layer 3 and 4 with data from the Swiss MS cohort allows comprehensive checks and validation of data from both studies and enables continuous improvements in data quality. Furthermore, the provision of regular extracts from their own survey data allows persons with MS to report errors.
Information technology (IT) security and data protection are not only top priorities for the Swiss MS Registry, but were also prime concerns for persons with MS in stakeholder meetings. Furthermore, the flexible study design and the fact that some persons with MS have impaired ability to fill in lengthy online surveys impose high requirements on IT infrastructure. In order to meet these demands, a close collaboration with the Science IT Department of the University of Zurich was established. After careful evaluation of existing IT systems, a panel of IT specialists recommended the development of a custom-built platform with special emphasis on usability and barrier freedom. This platform was developed by a team of specialists on the basis of open-source technologies. IT security features include, amongst others, password protection, TLS-encrypted communication and data transfers, and strict separation of identifiable and unidentifiable data, and were subjected to an external audit by a company specialised in IT security. The research database is hosted in a secure environment on servers of the University of Zurich.
Already at an early planning stage, the strategic goal was declared to be provision of relevant, individualised and understandable feedback to participants, which greatly increases study participation and commitment. The Swiss MS Registry web platform also offers a user interface for data collection through study forms, a diary and a dashboard, which displays ongoing surveys and select displays of user-specific data. Persons with MS and the Swiss MS Society were closely involved in the design and testing of the interface.
To address the first aim of this study – the dynamics of recruitment over the first 18 months of the Swiss MS Registry – we used descriptive statistics to present the number of participants in layers 1 to 4 and plotted the cumulative number of participants against time (June 2016 to November 2017). Our hypothesis was that recruitment would be faster for the Swiss MS Registry than in traditional research studies for several reasons. First, persons with MS themselves wished to have a registry and are therefore very motivated to participate. Second, while the close involvement of and backing by the Swiss MS Society was crucial for gaining trust by key stakeholders, the society also heavily promoted the Swiss MS Registry through its news outlets. Third, access to the study through an online platform within the website of the Swiss MS Society makes participation more convenient and independent of place and time. We compared recruitment against our goal for recruitment for the first year of 400 participants, which was based on prior experience from other observational longitudinal studies in more traditional clinical settings in Switzerland.
The second aim of this study was to assess the diversity of the study population, which we believe has been promoted in healthcare settings, independent recruitment, through the availability of both online and paper questionnaires, and by the layer-based study design, which allows different participation options and takes into account prolonged time to diagnosis. In particular, we hypothesised that the study population of the Swiss MS Registry would be more diverse than those in traditional research studies and better able to capture potentially underrepresented patient groups. To this end, we defined up-front patient groups that tend to be rarely seen in other observational MS studies. These groups were based on the healthcare setting (primary care, neurology clinic or private practice of neurologist) in which participants are looked after the, as well as the place where they live. We compared various characteristics between online participants with those who preferred paper questionnaires. For this analysis, we used descriptive statistics to assess and compare a number of characteristics, such as age, sex, type of MS, use of healthcare services or severity of symptoms for all participants and those who used the online platform or paper form. We chose not to use statistical tests for these comparisons since statistical significance testing is a poor guide to interpretation of important differences between groups. For statistical analysis, we used STATA version 13 (StataCorp LLC, College Station, TX, U.S.A.).
The Swiss MS Registry was launched on 25 June 2016, when the Swiss MS Society organised the first Swiss MS Day. Figure 2 shows the enrolment of participants from the first day, when 160 participants joined, to 30 November 2017. The goal of 400 participants in the first year was reached within 20 days. After this first phase, there was a steady increase with small hikes at 3-month intervals when the Swiss MS Society published articles on the Swiss MS Registry (including enrolment instructions) in its news magazine. Recruitment continued with one or two enrolees per day up to 30 November 2017, when the total number of participants reached 1683. Of these, 1412 completed the layer 2 questionnaire assessment and 915 provided proof of MS diagnosis from their treating physician (mostly neurologists). Of the participants, 80% enrolled using the web-based application whereas 20% preferred the paper version.
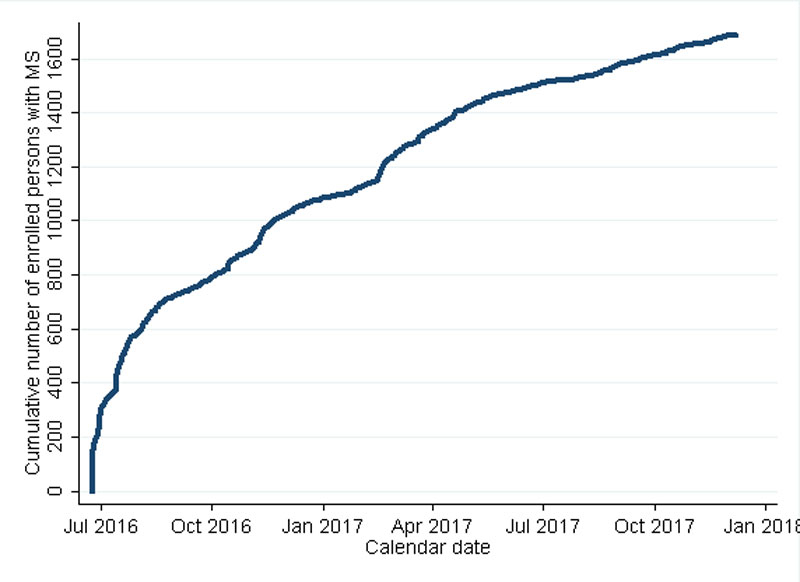
Figure 2 Cumulative enrolment of participants in the Swiss MS Registry.
At the time of analysis, 5% of all entry questionnaires were not complete (in some instances after several reminders). However, in the completed questionnaires, the percentage of missing items for key descriptor variables was low, ranging from, for example, 0.9% on questions about further MS cases among relatives to 5% for the year of first symptom occurrence. Notably, these information gaps are constantly being filled by compilation of data from additional sources (e.g., the diagnosis confirmation), by similar questions in follow-up surveys and through reporting of corrections by participants upon receipt of individualised data feedback.
The analysis of study population diversity was based on the 1387 processed questionnaires of the 1412 participants who had completed the layer 2 assessment. As shown in table 2 (column 2), the median age was 48 years (range 18–84), the median age at onset of symptoms 32 years (range 10–69) and the median number of years since MS diagnosis was 9 years (range 0–57). Although there was at the time an overrepresentation of participants from the German-speaking part of Switzerland, all cantons were well represented, including the French- and Italian speaking regions. Of note, 40 participants (2.9%) had moved to Switzerland relatively recently, since 2006. Moreover, 32 (2.3%) participants lived in a rural mountainous area and 20 (2.0% out of 1041 for whom this information was available) lived in a long-term care facility.
Table 2 Characteristics of study population enrolled in the first 18 months and who completed the layer 2 questionnaire.
| All | Online participation | Paper participation | |
|---|---|---|---|
| N | 1387 | 1154 | 233 |
| Year of birth, median (IQR) | 1969 (1959; 1978) |
1970 (1962; 1979) |
1960 (1951; 1969) |
| Female sex | 1007 (72.6%) | 819 (71.0%) | 188 (80.7%) |
| Region | |||
| German-speaking (incl. Rumantsch) | 1134 (81.8%) | 950 (82.3%) | 182 (78.1%) |
| French-speaking | 205 (14.8%) | 165 (14.3%) | 40 (17.2%) |
| Italian-speaking | 48 (3.5%) | 39 (3.4%) | 11 (4.7%) |
| Nationality | |||
| Swiss | 1262 (90.5%) | 1044 (90.5%) | 218 (93.6%) |
| Other | 125 (9.0%) | 110 (9.5%) | 15 (6.4%) |
| Informed consent module agreements | |||
| Permission for treating physician to confirm diagnosis | 1348 (97.3%) | 1123 (97.3%) | 225 (96.6%) |
| Medical record abstraction | 1293 (93.2%) | 1071 (92.8%) | 222 (95.3%) |
| MS Type | |||
| Clinically isolated syndrome | 59 (4.3%) | 51 (4.4%) | 8 (3.4%) |
| RRMS | 885 (63.8%) | 773 (67.0%) | 112 (48.1%) |
| SPMS | 215 (15.5%) | 160 (13.9%) | 55 (23.6%) |
| PPMS | 149 (10.7%) | 104 (9.0%) | 45 (19.3%) |
| In transition between RRMS and SPMS | 45 (3.2%) | 39 (3.4%) | 6 (2.6%) |
| Missing/unclear | 34 (2.5%) | 27 (2.3%) | 7 (3.0%) |
| Year of MS diagnosis, median (IQR) | 2008 (2000; 2013) |
2008 (2001; 2013) |
2003 (1995; 2010) |
| Relationship with MS Society | |||
| Member | 899 (64.8%) | 714 (61.9%) | 185 (79.4%) |
| News | 224 (16.2%) | 208 (18.0%) | 16 (6.9%) |
| Other (use of SMSS services, consultations, participation in groups) | 145 (10.5%) | 126 (10.0%) | 19 (8.2%) |
| No connection | 98 (7.1%) | 86 (7.5%) | 12 (5.2%) |
| EQ5D domains (best 2 categories) | |||
| Pain/discomfort | 878 (63.3%) | 756 (65.5%) | 122 (52.4%) |
| Mobility | 896 (64.6%) | 782 (67.8%) | 114 (48.9%) |
| Ability to self-care | 1231 (88.8%) | 1041 (90.2%) | 190 (81.6%) |
| Ability to perform daily activities | 885 (63.8%) | 775 (67.2%) | 110 (47.2%) |
| Fear/depression | 1121 (80.8%) | 954 (82.7%) | 167 (71.7%) |
| Received help with filling in questionnaire | 122 (8.8%) | 85 (7.4%) | 37 (15.9%) |
| No DMT in past 6 months | 564 (40.7%) | 454 (39.3%) | 110 (47.2%) |
| MS care received over past 12 months | |||
| From a neurologist | 1061 (76.5%) | 887 (76.9%) | 174 (74.7%) |
| From a general practitioner | 575 (41.5%) | 456 (39.6%) | 119 (51.1%) |
| At a clinic/hospital | 338 (24.4%) | 285 (24.7%) | 53 (22.8%) |
| At a rehabilitation clinic | 150 (10.8%) | 114 (9.9%) | 36 (15.5%) |
| Not in care with a physician / at a clinic | 167 (12.0%) | 136 (11.8%) | 31 (13.3%) |
| DMT = disease-modifying treatment; MS = multiple sclerosis; IQR = interquartile range; PPMS = primary progressive MS; RRMS = elapsing-remitting MS; SPMS = secondary progressive MS | |||
Most participants were cared for by neurologists, but, in contrast to many other studies, many of these 645 participants (46.5%) received care at a private neurology practice. In addition, 12% of the participants did not report any use of healthcare services in the previous 12 months, and 20 (1.4%) participants with relapsing-remitting MS did not receive disease-modifying treatments. Further, 94 (6.8%) participants joined the Swiss MS Registry within 1 year of diagnosis. Combined, these results suggest that the Swiss MS Registry included substantial numbers of participants who would have been unlikely to enrol in a more traditional study in a clinic-based setting.
Table 2 also shows the characteristics of all participants stratified by web-based or paper participation. Those with online participation were around 10 years younger than those favouring the paper forms. Moreover, participants from German-speaking cantons tended to favour online participation, whereas participants from French- and Italian-speaking parts were more likely to favour the paper form. The distribution of types of MS also differed substantially between those participating online (more likely to have relapsing-remitting MS) and those using paper (more likely to have primary or secondary progressive MS). This was further reflected in the burden of MS as measured with EuroQol 5-D, which showed less impairment for those participating online. Two thirds of participants were members of the Swiss MS Society, whereas the remaining one third only received its news or used its services, or were not connected at all with the Swiss MS Society. These results suggested that the Swiss MS Registry study population is more diverse than in studies offering just paper or online participation.
This analysis showed a rapid recruitment of participants into the Swiss MS Registry that vastly exceeded expectations. The study population was diverse and the comparison of online with paper participants showed that using both options increases the diversity of the study population in terms of geographic origin and type and severity of disease. Importantly, data quality appeared to be high, given the high proportion of data completeness. Moreover, preliminary analyses published on the website of the Swiss Multiple Sclerosis Society and cross-comparisons with other Swiss studies [14] further attest to the plausibility of the data and the results.
There are two concurrent studies for which comparisons in terms of the population enrolled and scientific questions are admissible: the Swiss MS Cohort Study and the German MS Registry [13, 15]. In the Swiss MS Cohort Study, persons with MS are a little younger with slightly shorter time since diagnosis, whereas in the German MS Registry participants are of similar age but with reported more years since diagnosis. The clinic-based setting of the Swiss MS Cohort Study and the German MS Registry offer advantages in terms of the clinical, physiological and imaging data that can be collected, whereas the study populations are likely to be less diverse than in the Swiss MS Registry. As a consequence, the Swiss MS Registry complements these other ongoing studies almost ideally. For example, the Swiss MS Cohort Study and the German MS Registry address more clinical questions and questions on biomarkers and imaging for MS, whereas the Swiss MS Registry provides more representative data on disease burden and the everyday life of persons with MS. In addition, since the Swiss MS Registry spans across the healthcare system, questions on diagnostic (from first symptoms to diagnosis) and therapeutic pathways can be addressed.
Comparisons with other large MS studies in terms of diversity of study populations are more challenging. The landscape of MS currently changes rapidly, and there is an increasing awareness of the need to make a rapid diagnosis and of the increasing availability of novel disease-modifying treatments, both of which have a substantial impact on the participants recruited in clinical centres. Thus, a comparison with studies whose recruitment phase was 5 or more years ago is not sensible. Also, the way healthcare is organised differs substantially between countries, which makes interpretation of differences in diagnostic and therapeutic pathways difficult. In addition, some countries, such as Sweden or Denmark, have registries in which persons with a diagnosis of MS are automatically registered either at the time of diagnosis or at the time of treatment initiation [16, 17]. Such enrolment mechanisms are not comparable to those in settings such as Switzerland, where such automatic registration is not foreseen. Finally, online surveys or studies such as the UK MS Register [18] or the North American Research Committee on Multiple Sclerosis [19], the world’s largest MS registry, are conducted online like the Swiss MS Registry but are not primarily longitudinal studies.
The rapid recruitment, the diverse study population and the high data quality demonstrate that the Swiss MS Registry successfully leverages the advantages of traditional medical research and novel approaches using societal and technological developments. Like traditional medical research, this observational study was fully embedded within the legal and ethical framework of medical research, as it obtained approval from ethics committees and was based on a fully developed protocol that described the aims, methods and analytic approaches of the study. Thereby, the study mitigated the risks of novel types of studies or research efforts such as patientslikeme, CureTogether or studies by Google or Facebook that have enormous potential but operate in an, as yet, unclear legal and ethical framework and may be prone to threats to internal and external validity [8–11].
To unleash the full potential of the combination of traditional research and contemporary trends in society and technology, digitally facilitated citizen-science studies should adhere to the highest methodological and ethical standards and guarantee patient rights under the Helsinki Declaration [20]. Community outreach strengthens participation, maximises participation diversity, and can help to manage stakeholder expectations. Active communication is essential for digitally facilitated citizen science. For the Swiss MS Registry, information is spread widely through the network of the Swiss MS Society (e.g., newsletters, website, social media). Additionally, regular presentations to persons with MS and clinicians, including general practitioners and neurologists, raise awareness of the Swiss MS Registry and its goals. Almost 1700 persons with MS had enrolled in the Swiss MS Registry since its launch in June 2016, vastly exceeding expectations. We estimate that the Swiss MS Registry currently represents around 11 to 15% of the entire MS population in Switzerland [21, 22]. These excellent enrolment numbers are indicative of the potential for the combination of traditional research and novel trends.
The combination of patient-reported and clinical data, as well as the close collaboration and possibilities for data exchange with the more clinically oriented Swiss MS Cohort Study, offer possibilities not only for data validation, but also for interesting comparisons of patient- and physician perspectives on symptom severity or adverse drug effects, or for investigations into patient-doctor communication aspects with potentially large implications for healthcare.
Although data collection has been highly standardised so far, the Swiss MS Registry provides a basis for the collection of unstructured data from medical charts, tracking devices or social media. It would have been most straightforward to employ existing online platforms and technologies, but these were not deemed to be safe and flexible enough for our purpose. Therefore, we set up a new and secure online IT infrastructure that offers flexibility to incorporate additional structured but also unstructured data. Although it will take some time to collect large amounts of data and the amount of data may never compare to online platforms such as patientslikeme, our approach mitigates the risks of an unclear legal and ethical framework where not only may persons affected by MS lose trust, but researchers face many uncertainties as well. Another clear advantage is that the source and study populations are well known and characterised and data quality is assured.
The digitally facilitated citizen science approach chosen by the Swiss MS Registry indicates a way to bridge the gap between traditional medical research and novel approaches. The Swiss MS Registry is characterised by transparent governance, operation within clear and established legal and ethical frameworks, active community outreach and communication, and direct involvement of persons with MS in study planning and operation. The rapid recruitment of a diverse study population within the first 18 months since the inception of the Swiss MS Registry and the high quality of the data indicate the potential of this novel concept of digitally enhanced citizen science, which could be useful for many chronic conditions. The Swiss MS Registry may therefore serve as an example for leveraging the advantages of both traditional medical research and novel societal and technological developments, while mitigating their respective disadvantages and risks.
Grant Support: The Swiss MS Registry is funded by the Swiss MS Society with grants to the study coordination centre, located at the Epidemiology, Biostatistics and Prevention Institute, University of Zurich. The Swiss MS Society is a key contributor to the Swiss MS Registry as described in this article, and has contributed as such to the study design of and promotes recruitment in the Swiss MS Registry. Neither the Swiss MS Society nor any other funding body had any role in the decision to publish, or the preparation of the manuscript.
1 Thornton S . Beyond rhetoric: we need a strategy for patient involvement in the health service. BMJ. 2014;348(jun23 18):g4072–4072. doi:.https://doi.org/10.1136/bmj.g4072
2 Hernandez AF , Fleurence RL , Rothman RL . The ADAPTABLE Trial and PCORnet: Shining Light on a New Research Paradigm. Ann Intern Med. 2015;163(8):635–6. doi:.https://doi.org/10.7326/M15-1460
3 Estellat C , Ravaud P . Lack of head-to-head trials and fair control arms: randomized controlled trials of biologic treatment for rheumatoid arthritis. Arch Intern Med. 2012;172(3):237–44. doi:.https://doi.org/10.1001/archinternmed.2011.1209
4 Mills EJ , Ioannidis JPA , Thorlund K , Schünemann HJ , Puhan MA , Guyatt GH . How to use an article reporting a multiple treatment comparison meta-analysis. JAMA. 2012;308(12):1246–53. doi:.https://doi.org/10.1001/2012.jama.11228
5 Van Spall HGC , Toren A , Kiss A , Fowler RA . Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297(11):1233–40. doi:.https://doi.org/10.1001/jama.297.11.1233
6 Boyd CM , Vollenweider D , Puhan MA . Informing evidence-based decision-making for patients with comorbidity: availability of necessary information in clinical trials for chronic diseases. PLoS One. 2012;7(8):e41601. doi:.https://doi.org/10.1371/journal.pone.0041601
7 Frist WH . Connected health and the rise of the patient-consumer. Health Aff (Millwood). 2014;33(2):191–3. doi:.https://doi.org/10.1377/hlthaff.2013.1464
8 Topolovec-Vranic J , Natarajan K . The Use of Social Media in Recruitment for Medical Research Studies: A Scoping Review. J Med Internet Res. 2016;18(11):e286. doi:.https://doi.org/10.2196/jmir.5698
9 Khatri C , Chapman SJ , Glasbey J , Kelly M , Nepogodiev D , Bhangu A , et al.; STARSurg Committee. Social media and internet driven study recruitment: evaluating a new model for promoting collaborator engagement and participation. PLoS One. 2015;10(3):e0118899. doi:.https://doi.org/10.1371/journal.pone.0118899
10 Vayena E , Salathé M , Madoff LC , Brownstein JS . Ethical challenges of big data in public health. PLOS Comput Biol. 2015;11(2):e1003904. doi:.https://doi.org/10.1371/journal.pcbi.1003904
11 Rothman M , Gnanaskathy A , Wicks P , Papadopoulos EJ . Can we use social media to support content validity of patient-reported outcome instruments in medical product development? Value Health. 2015;18(1):1–4. doi:.https://doi.org/10.1016/j.jval.2014.10.001
12 Horton DK , Mehta P , Antao VC . Quantifying a nonnotifiable disease in the United States: the National Amyotrophic Lateral Sclerosis Registry model. JAMA. 2014;312(11):1097–8. doi:.https://doi.org/10.1001/jama.2014.9799
13 Disanto G , Benkert P , Lorscheider J , Mueller S , Vehoff J , Zecca C , et al.; SMSC Scientific Board. The Swiss Multiple Sclerosis Cohort-Study (SMSC): A Prospective Swiss Wide Investigation of Key Phases in Disease Evolution and New Treatment Options. PLoS One. 2016;11(3):e0152347. doi:.https://doi.org/10.1371/journal.pone.0152347
14 Calabrese P , Kobelt G , Berg J , Capsa D , Eriksson J ; European Multiple Sclerosis Platform. New insights into the burden and costs of multiple sclerosis in Europe: Results for Switzerland. Mult Scler. 2017;23(2_suppl):192–203. doi:.https://doi.org/10.1177/1352458517708685
15 Thiel S , Leypoldt F , Röpke L , Wandinger K , Kümpfel T , Aktas O , et al. Neuroimmunological Registries in Germany. Neurol Int Open. 2018;2(1):E25–39. doi:.https://doi.org/10.1055/s-0043-108830
16 Hillert J , Stawiarz L . The Swedish MS registry – clinical support tool and scientific resource. Acta Neurol Scand. 2015;132(199):11–9. doi:.https://doi.org/10.1111/ane.12425
17 Magyari M , Koch-Henriksen N , Sørensen PS . The Danish Multiple Sclerosis Treatment Register. Clin Epidemiol. 2016;8:549–52. doi:.https://doi.org/10.2147/CLEP.S99500
18 UK MS Register. https://ukmsregister.org/Portal/Home
19 North American Research Committee on Multiple Sclerosis (NARCOMS). https://www.narcoms.org/
20 World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. doi:.https://doi.org/10.1001/jama.2013.281053
21 Beer S , Kesselring J . High prevalence of multiple sclerosis in Switzerland. Neuroepidemiology. 1994;13(1-2):14–8. doi:.https://doi.org/10.1159/000110353
22 Blozik E , Rapold R , Eichler K , Reich O . Epidemiology and costs of multiple sclerosis in Switzerland: an analysis of health-care claims data, 2011-2015. Neuropsychiatr Dis Treat. 2017;13:2737–45. doi:.https://doi.org/10.2147/NDT.S143180
Grant Support: The Swiss MS Registry is funded by the Swiss MS Society with grants to the study coordination centre, located at the Epidemiology, Biostatistics and Prevention Institute, University of Zurich. The Swiss MS Society is a key contributor to the Swiss MS Registry as described in this article, and has contributed as such to the study design of and promotes recruitment in the Swiss MS Registry. Neither the Swiss MS Society nor any other funding body had any role in the decision to publish, or the preparation of the manuscript.