The role of molecular imaging in assessing degenerative parkinsonism – an updated review
DOI: https://doi.org/10.4414/smw.2018.14621
Nicolas
Nicastroa, Valentina
Garibottobc, Pierre R.
Burkhardde
aDepartment of Psychiatry, University of Cambridge, United Kingdom
bDivision of Nuclear Medicine and Molecular Imaging, Geneva University Hospitals, Switzerland
cNIMTlab, Faculty of Medicine, University of Geneva, Switzerland
dDivision of Neurology, Geneva University Hospitals, Switzerland
eFaculty of Medicine, University of Geneva, Switzerland
Summary
Diagnosing degenerative forms of parkinsonism still relies on a thorough clinical assessment, which in Parkinson’s disease involves the presence of an asymmetric bradykinesia with rest tremor and/or rigidity that respond substantially to levodopa. Conversely, atypical forms, including multiple system atrophy, progressive supranuclear palsy and corticobasal degeneration, exhibit additional features (cerebellar or pyramidal signs, early postural instability), a poor response to dopamine replacement therapy and a bad prognosis.
Consensus diagnostic criteria have excellent specificity, but lack sensitivity, and a clear diagnosis solely based on clinical evaluation is not always accurate, hence the need for diagnostic biomarkers. Nuclear medicine imaging is definitely one of them, allowing a qualitative and quantitative evaluation of in vivo functional integrity of monoaminergic (e.g., dopaminergic) pathways, brain metabolism and protein deposition and representing a unique window into these complex diseases. It has proved useful for early and accurate diagnosis, and possibly represents a valid biomarker of disease pathogenesis, progression and response to neuroprotective therapies.
This review focuses on the nigrostriatal pathway dysfunctions (demonstrated with presynaptic dopamine positron emission tomography [PET] and single photon emission computed tomography [SPECT] ligands) that confirm a degenerative form of parkinsonism. In addition, 123I-metaiodobenzylguanidine cardiac scintigraphy can unveil postganglionic autonomic failure specifically encountered in Parkinson’s disease. Brain 18F-fluorodeoxyglucose PET may also show a distinct hypometabolism for each degenerative form of parkinsonism. Since a few years ago, the proteins that aggregate in the brain of subjects with neurodegenerative diseases (tau and alpha-synuclein) can be evaluated in vivo by novel radioligands. These developments open new perspectives both as diagnostic tools and to understand the regional topography and burden of protein deposition on motor impairment and cognitive decline. The last part of the review proposes a strategic workup in the practical evaluation of a patient with parkinsonism.
Introduction
Parkinsonism is a neurological syndrome characterised by bradykinesia – slowness and decreased amplitude of movement – associated with resting tremor and muscular rigidity [1], and is related to basal ganglia dysfunction [2]. Degenerative forms of parkinsonism (parkinsonian syndromes) involve the aggregation of toxic protein isoforms in the central and peripheral nervous system leading to progressive neuronal loss. Parkinson’s disease is the most prevalent condition of this subgroup (approx. 80%) [3, 4], whereas multiple system atrophy (MSA), progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) [5–7] are far less common and collectively labelled as atypical parkinsonian syndromes. They all share a variable impairment of nigrostriatal functions and must be distinguished from nondegenerative causes of parkinsonism (e.g., toxic/drug-induced, psychogenic or vascular aetiologies) in which presynaptic nigrostriatal pathways are preserved [8, 9].
Parkinson’s disease is the most frequent neurodegenerative movement disorder in the general population, with an estimated incidence of 21 cases per 100,000 person-years [10] and a 1.5:1 male predominance. It is quite rare before 50 years, and its prevalence increases to over 3% after 80 [11]. Most patients with Parkinson’s disease suffer from a sporadic form, which encompasses complex gene-environment factors (use of pesticides, repeated traumatic brain injury, altered degradation of toxic proteins) [12].
Parkinson’s disease is defined by a progressive and usually asymmetric bradykinesia in association with rest tremor and rigidity [13]. In addition to these cardinal motor features which respond well to dopamine replacement (levodopa, dopamine receptor agonists), Parkinson’s disease is associated with a myriad of non-motor symptoms either preceding the motor phase by several years, such as olfactory loss and rapid eye movement sleep behaviour disorder, or accompanying it (depression, dysexecutive syndrome, orthostatic hypotension). Late-onset complications include dementia and postural instability with subsequent falls. The neuropathological hallmark of Parkinson’s disease is dopamine-containing neurone loss in the substantia nigra pars compacta of the midbrain [14]. In addition, intracellular deposition of misfolded oligomers of alpha-synuclein can be observed in monoaminergic and cholinergic neurones of the brainstem and olfactory bulb (Lewy bodies), with further expansion over cortical areas as the disease progresses. The distinction between Parkinson’s disease with dementia (PDD) and dementia with Lewy bodies (DLB) currently relies on the onset of dementia, which is ≥1 year after motor involvement in PDD and at clinical onset for DLB. In both cases, cognitive impairment is correlated with higher cortical Lewy bodies [15], but in vivo positron emission tomography (PET) and magnetic resonance imaging (MRI) show more severe cortical atrophy, temporoparietal cortical Lewy body deposition and higher concurrent Alzheimer pathology in DLB patients [16–18]. The exact function of alpha-synuclein is not fully understood, but it probably plays a role in synaptic and mitochondrial homeostasis [19].
Atypical parkinsonian syndromes are characterised by a modest or nonexistent response to dopaminergic therapy and a poor prognosis (mean life expectancy 7–10 years from disease onset). Clinical manifestations of each condition reflect the selective neuronal loss in distinct brain regions that differ from those of Parkinson’s disease, where the burden of neuropathology involves the substantia nigra pars compacta and the locus coeruleus [20]. MSA is a synucleinopathy featuring progressive autonomic dysfunction (orthostatic hypotension, urinary incontinence) in combination with variable severity of parkinsonism (in its MSA-parkinsonism or MSA-P form), cerebellar dysfunction (in the MSA-cerebellar or MSA-C subtype) and pyramidal signs [21]. Brain structures more affected in MSA thus include the striatum and pontocerebellar pathways. With an estimated prevalence of 5–7 cases per 100,000 [22], PSP is the second most common degenerative parkinsonian syndrome, its classical form (PSP-Richardson syndrome) presenting with a rather symmetrical motor impairment, oculomotor dysfunction and early postural instability [23]. In PSP, neuropathology is rather extensive and involves the brainstem and frontal cortex. CBD classically features a highly asymmetric parkinsonism with various degree of dystonia, apraxia and pyramidal signs [24], reflecting impairment of cortical frontoparietal areas. PSP and CBD are associated with tau neuropathology, at variance with Parkinson’s disease and MSA, which are related to alpha-synuclein aggregation.
The aim of this review is to summarise the main molecular imaging tools available in clinical practice to help neurologists and general practitioners separate degenerative from nondegenerative forms of parkinsonism, and to increase diagnostic accuracy of Parkinson’s disease and parkinsonian syndromes. After this brief overview of the clinical features of Parkinson’s disease and its atypical variants, we will review the major radioligands used in combination with PET and single photon emission computed tomography (SPECT) – their indications, strength and limitations.
PET and SPECT imaging
PET and SPECT imaging represent invaluable tools for measuring in vivo functional impairment of monoaminergic pathways in neurodegenerative diseases, the more so as structural imaging (brain MRI) usually shows late or unspecific signs [25].
PET requires radioactive compounds labelled with 11C or 18F, which have a short half-life (20–110 minutes) and whose positron-emitting activity can be detected by a dedicated tomograph after intravenous injection to the patient. The radioligand most widely used to measure metabolic activity in the central nervous system is 18F-fluorodeoxyglucose (FDG) PET.
For SPECT, gamma cameras detect low-energy gamma rays emitted by compounds labelled with heavier radioactive isotopes such 123I or 99mTc, which have a longer half-life (6–13 hours). Examples include 123I-FP-CIT SPECT (ioflupane, DaTSCAN®, GE Healthcare), which measures dopamine transporter density on the presynaptic terminals of the nigrostriatal pathway (FP-CIT = fluoropropyl-carbomethoxy-3β-4-iodophenyltropane).
How to detect nigrostriatal degeneration?
The evaluation of presynaptic dopaminergic pathways between the midbrain and the striatum is very useful in distinguishing degenerative conditions such as Parkinson’s disease from nondegenerative forms of parkinsonism and tremor. This can be achieved mainly via three different targets (fig. 1).
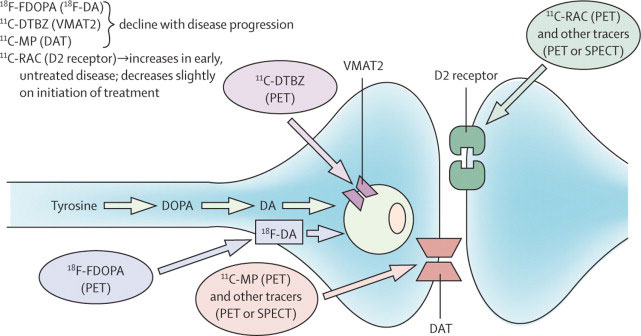
Figure 1 Summary of the dopaminergic pathways within the striatum and the main PET and SPECT targets.
Republished with permission of Elsevier, from “Advances in imaging in Parkinson’s disease”, by A. Jon Stoessl, WR Wayne Martin, Martin J. McKeown, Vesna Sossi, The Lancet Neurology, Vol. 10, permission conveyed through Copyright Clearance Center, Inc.
The enzymatic activity of aromatic amino acid decarboxylase, which converts levodopa into dopamine in nigral cells, can be estimated with 18F-fluorodopa, which acts as an analogue of levodopa. The density of dopamine transporters, which recapture dopamine in the presynaptic compartment, can be measured thanks to 123I-FP-CIT and to specific PET tracers (e.g., 18F-PE2I; N-(3-iodoprop-2E-enyl)-2β-carbomethoxy-3β-(4-methyl-phenyl)nortropane). Finally, 11C- and 18F-dihydrotetrabenazine ligands bind to vesicular monoamine transporter 2. A recent meta-analysis showed a mean uptake decrease of 13 to 77% in Parkinson’s disease subjects compared with healthy controls, vesicular monoamine transporter 2 and the dopamine transporter being the most sensitive targets to assess [26].
Due to the widespread availability of SPECT scans and approval from European (since 2000) and US (since 2011) medical agencies in the evaluation of parkinsonian syndromes, 123I-FP-CIT SPECT has been the most studied molecular imaging technique in degenerative parkinsonian syndromes [27]. There is satisfactory medical evidence that it can distinguish Parkinson’s disease from essential tremor (class I) [28] and drug-induced parkinsonism (class II) [29]. It is indicated for distinguishing degenerative forms of parkinsonism from essential tremor, and DLB from Alzheimer’s disease in doubtful cases. In fact, subjects with essential tremor, drug-induced parkinsonism and psychogenic movement disorders have normal dopamine transporter uptake [30, 31], whereas patients with degenerative conditions exhibit a reduction of striatal dopaminergic binding [32, 33] very early in the disease course, even at the premotor stage. SPECT evaluation has an estimated 97% sensitivity and 100% specificity in distinguishing clinically diagnosed degenerative parkinsonism from essential tremor [28]. Similar accuracy was observed in neuropathologically confirmed cases of DLB versus Alzheimer’s disease [34]. Dopamine uptake impairment is more pronounced in the striatum contralateral to the clinically more affected side. Although a robust distinction between the various degenerative forms of parkinsonism is not possible on the basis of SPECT assessment, a differential pattern has been suggested at the group level. In fact, relative preservation of caudate nucleus compared with putaminal uptake suggests Parkinson’s disease, whereas PSP and MSA-P patients exhibit a substantial alteration of dopamine transporter binding in the whole striatum [35]. For CBD, a wide variation of dopamine uptake has been described [36] and, like MSA-C, a few cases with normal SPECT binding have been reported [37–40]. Differential patterns of dopamine transporter impairment are illustrated in figure 2.
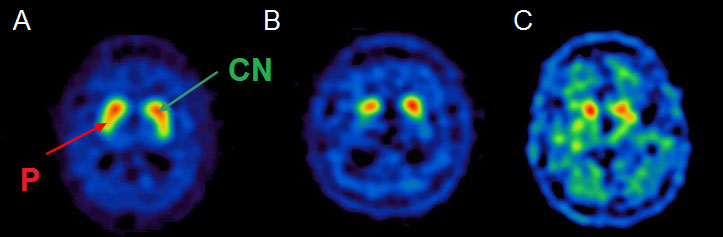
Figure 2 Axial 123I-FP-CIT SPECT (ioflupane, DaTSCAN®) showing a preserved “comma-shaped” uptake in a healthy subject (A) with preserved uptake for both caudate nucleus (CN, green arrow) and putamen (P, red arrow). We can observe typical asymmetric and mostly putaminal uptake reduction in Parkinson’s disease (B), and a severe uptake impairment involving the whole striatum in a patient with PSP (C).
A correlation between striatal SPECT uptake and disease severity is established in Parkinson’s disease with, for example, the Hoehn and Yahr scale, motor Unified Parkinson Disease Rating Scale (UPDRS) scale and bradykinesia, but not with tremor and rigidity which rely on other (nondopaminergic) mechanisms [41, 42]. Similarly, it is debated whether dopamine transporter density represents a valid assessment of nigral cell count in the substantia nigra [43], recent evidence showing that it is mainly correlated with axonal dysfunction but not directly with nigral cell loss [44].
As 123I-FP-CIT does not compete with levodopa for uptake, ongoing dopaminergic treatments do not interfere with SPECT evaluation and therefore do not need to be withdrawn. However, bupropion, fentanyl and amphetamines can decrease DaTSCAN® uptake [45].
A valid alternative to 123I-FP-CIT SPECT is 18F-DOPA PET, which is widely used in neurology clinics for the diagnosis of Parkinson’s disease. However, owing to the up-regulation of aromatic amino acid decarboxylase as a compensatory mechanism in degenerative conditions, the degree of nigrostriatal insult is likely to be underestimated [46, 47].
In addition, 11C- and 18F-labelled dihydrotetrabenazine PET has proven very sensitive in detecting presynaptic dysfunction in Parkinson’s disease [26, 48] by estimating density of vesicular monoamine transporter 2 and, at variance with dopamine transporter and aromatic amino acid decarboxylase ligands, does not seem to be affected by major compensatory changes [46]. Nonetheless, it is not as widely available as dopamine transporter ligands.
The concept of a scan without evidence of dopaminergic deficit reflects preserved presynaptic dopaminergic uptake unexpectedly found in 1 to 15% of patients with suspected Parkinson’s disease. These observations derived from large-scale neuroprotective trials where subjects underwent dopamine transporter imaging as a biomarker of neurodegeneration [49, 50]. As dopamine SPECT is a sensitive method to detect early presynaptic dopamine degeneration, which occurs several years before motor symptoms, it seems unlikely that genuine Parkinson’s disease patients can have normal dopamine transporter function. In fact, current evidence shows that subjects with normal dopamine transporter imaging either have been misdiagnosed (they have a nondegenerative condition mimicking Parkinson’s disease, such as dystonia or essential tremor) or have an abnormal follow-up scan [51–55]. Combining semiquantitative analysis with the standard visual assessment also allows detection of subtle presynaptic changes [33].
Diagnostic differentiation of Parkinson’s disease from other degenerative parkinsonian syndromes
After confirming a presynaptic dopaminergic pathway impairment supporting the diagnosis of a degenerative parkinsonism, molecular imaging tools capable of distinguishing Parkinson’s disease from other degenerative conditions can be useful in selected cases. The clinician can face ambiguous situations, especially early in the disease course when clinical symptoms of Parkinson’s disease and atypical conditions may overlap. Moreover, population-based studies have shown that at least 15% of patients with diagnosed Parkinson’s disease do not fulfill clinical diagnostic criteria and that 20 to 25% with Parkinson’s disease were diagnosed with another condition, such as vascular, atypical degenerative forms of parkinsonism, or essential tremor [56, 57]. Nuclear medicine imaging could therefore be helpful in the differential diagnosis of Parkinson’s disease. Although there is no definite way to separate Parkinson’s disease from atypical conditions, the evaluation of other molecular targets, such as postsynaptic dopaminergic receptors, glucose metabolism or postganglionic adrenergic function [58] can offer precious clues to clinicians.
Brain glucose metabolism can be assessed with an FDG-PET scan [59]. Parkinson’s disease patients usually show normal striatal metabolism, although various degrees of temporoparietal hypometabolism have been described [60]. Atypical parkinsonian syndromes usually show a marked reduction in glucose metabolism. In the case of MSA-P, pronounced hypometabolism can be observed in the striatum, especially in the posterior putamen [59], whereas patients with the cerebellar variant of MSA (MSA-C) usually exhibit reduced metabolism in the pontine and cerebellar regions. In PSP, hypometabolism is commonly described in the caudate nucleus, medial thalamus and midbrain, as well as in frontal areas (anterior cingulate, supplementary motor and premotor areas) [61]. Marked and highly asymmetric frontoparietal and subcortical hypometabolism is commonly seen in patients with CBD [62].
Postsynaptic dopaminergic receptors represent another interesting target (fig. 1). Their density can be measured with both PET and SPECT ligands. The more widely used postsynaptic D2 PET compound is raclopride, which usually shows normal or increased uptake in unmedicated Parkinson’s disease patients [63], but a marked reduction in MSA, PSP and Parkinson’s disease subjects on dopaminergic treatment [64]. 123I-(S)-2-hydroxy-3-iodo-6-methoxy-N-[1-ethyl-2-pyrrodinyl)-methyl]benzamide (IBZM) SPECT shows that Parkinson’s disease patients both with or without dopaminergic medication exhibit normal postsynaptic D2 uptake [65]. Similarly, subjects with CBD usually have preserved post-synaptic binding [66], whereas MSA and PSP harbour a severe uptake reduction [31].
Myocardial SPECT can be used to assess the integrity of postganglionic sympathetic fibres as 123I-metaiodobenzylguanidine (MIBG) is taken up by postganglionic adrenergic neurones. The heart-to-mediastinum ratio (the usual measure used in MIBG SPECT) is decreased in Parkinson’s disease, irrespective of disease severity and duration [67]. Conversely, MIBG SPECT uptake is preserved or only mildly reduced in CBD, PSP and MSA [68, 69] (fig. 3). In fact, although MSA patients suffer from a severe dysautonomia, it is mainly due to presynaptic autonomic dysfunction [70], and the postsynaptic adrenergic fibres are left intact. Sudmeyer et al. showed that combining presynaptic and postsynaptic dopamine imaging with MIBG SPECT resulted in 94% test accuracy in distinguishing Parkinson’s disease from atypical conditions [71]. One caveat about MIBG is that an abnormal scan may be due to other non-neurological conditions, such as coronary disease or diabetes.
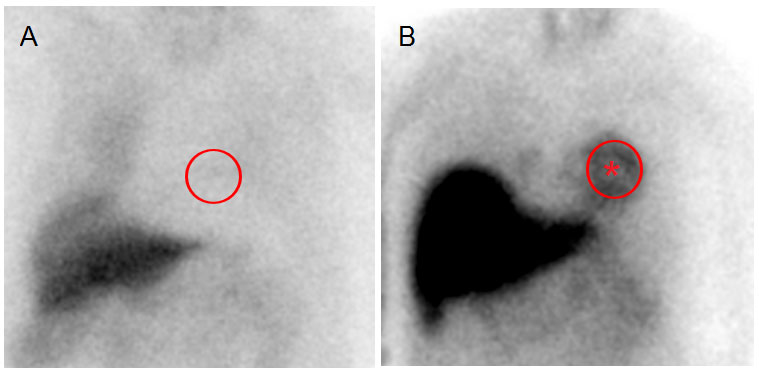
Figure 3 Heart planar 123I-MIBG scintigraphy 4 hours post-injection showing absent (A) heart visualisation (red circle), compatible with Parkinson’s disease. In B, heart visualisation is preserved (red star), as it is in atypical degenerative parkinsonian syndromes.
In summary, after establishing the presence of presynaptic dopaminergic denervation (for example with 123I-FP-CIT SPECT), another PET/SPECT imaging procedure can be performed in selected cases in order to help in the differential diagnosis of degenerative parkinsonism. Based on ligand availability in medical centres and on current literature, cardiac MIBG SPECT has level A evidence that it can separate Parkinson’s disease from atypical conditions [72], whereas postsynaptic dopamine imaging cannot be recommended for routine evaluation [73]. Furthermore, there was insufficient evidence that metabolic PET imaging in 2013 can distinguish Parkinson’s disease from atypical conditions, according to the European Federation of Neurological Societies (EFNS) guidelines, which are currently being revised [72]. Since then, several papers have shown the added value of FDG PET in differentiating parkinsonian syndromes and predicting cognitive impairment in Parkinson’s disease, and FDG-PET can be proposed for the diagnostic work-up on an individual basis [74–76].
Other PET/SPECT ligands of interest
Although not used routinely in clinical evaluation for patients with suspected parkinsonism, several other ligands have been studied and deserve a quick overview.
Human post-mortem studies have shown that microglial activation present in the brain’s resident macrophages is associated with neurodegenerative diseases [77]. Ligands such as PK11195 have the ability to bind to a mitochondrial translocator protein specifically expressed by activated microglia [78]. Consequently, several authors have reported an increased PK11195 PET binding in basal ganglia, pons and frontal cortex in Parkinson’s disease subjects in comparison with healthy controls [79] and a more widespread involvement of cortical areas in Parkinson’s disease patients with dementia [80, 81]. In atypical parkinsonian syndromes, microglial activation was topographically correlated with alpha-synuclein/tau usual deposition, which is in the pallidum and pons for MSA and PSP [79, 82] and frontoparietal areas in CBD [83].
In addition to showing impairment of various monoaminergic pathways, nuclear medicine imaging techniques have now the outstanding ability to estimate specific aggregation of toxic proteins directly involved in parkinsonian syndromes. Regarding tau imaging, several issues must be addressed in the first place. Unlike amyloid, tau aggregation is intracellular, making ligand binding problematic, the more so as several tau isoforms exist, some of which being not pathogenic. In addition, current ligands like 18F-AV1451, 11C-PBB3 and 18F-THK5351, albeit showing substantial tau deposition in tauopathies such as PSP, have been developed to target mainly tau aggregates typical of Alzheimer’s Disease, while their profile of affinity for other tau isoforms is more variable [84–86].
Regarding assessment of alpha-synuclein aggregation, which would be particularly useful for Parkinson’s disease and MSA, research is still ongoing. In fact, it has proved difficult for the current developed ligands to either cross the blood-brain barrier in order to bind to alpha-synuclein or to target selectively its toxic aggregates, due to the interference of other proteins like amyloid [87].
Clinical work-up
The following workflow proposes a clinically-oriented strategy to consider nuclear medicine ancillary tests when facing a patient with suspected parkinsonism (fig. 4).
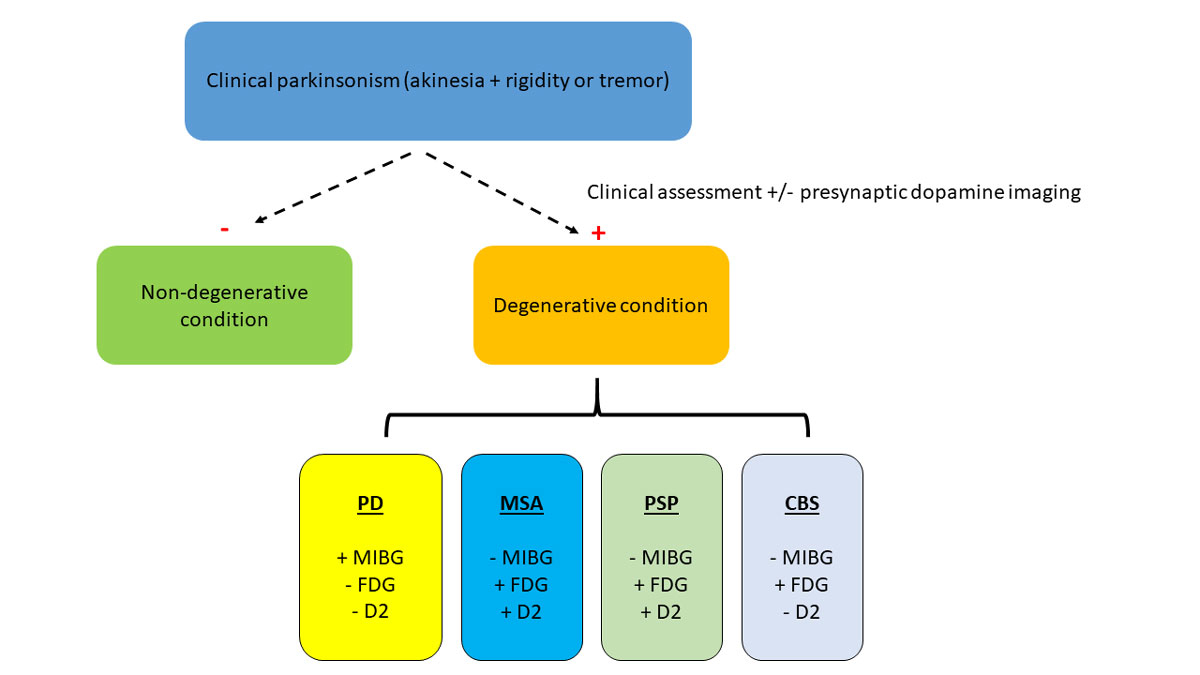
Figure 4 Proposed clinical work-up in the evaluation of a subject with parkinsonism; (-) is normal and (+) pathological.
CBS = corticobasal syndrome; MSA = multiple system atrophy; PD = Parkinson’s disease; PSP = progressive supranuclear palsy
The first step is to confirm parkinsonism, which requires the presence of bradykinesia in association with either rigidity or rest tremor. As a reminder, bradykinesia is defined as the progressive decrease of both speed and amplitude when performing repetitive movements such as opening-closing the hand or foot tapping [88]. Mild and isolated rigidity is frequently encountered in the elderly population and can be due to poor collaboration, difficulty for the patient to relax (paratonia, Gegenhalten) or what is commonly referred to as mild parkinsonian signs considered to be due to various degrees of cerebral small vessel disease [89]. This is not sufficient a criterion to confirm parkinsonism. Postural instability, which was until recently part of the classical tetrad of Parkinson’s disease (with bradykinesia, tremor and rigidity) has been removed as a main criterion because it is not encountered in early Parkinson’s disease, but mainly a sign of advanced Parkinson’s disease or of an atypical parkinsonian syndrome (e.g., PSP).
The next step is to assess whether parkinsonism is secondary to a dopamine-blocking medication (e.g., antipsychotics and various antiemetic drugs such as metoclopramide), vascular, or any strategic lesion damaging the basal ganglia. Therefore, a careful drug history must be performed and a brain MRI with at least T1-, T2- and FLuid Attenuation Inversion Recovery (FLAIR) sequences is usually recommended in the initial evaluation to exclude a secondary cause of parkinsonism [25]. When the clinical picture is not straightforward (unclear medical history, atypical tremor, insidious appearance of parkinsonism in a patient under chronic neuroleptic medication), presynaptic dopaminergic pathway integrity can be assessed with 123I-FP-CIT SPECT imaging. This will invariably show a reduced striatal uptake in degenerative forms of parkinsonism, even early in the disease course [90]. Repeating SPECT imaging cannot be justified by the “scan without evidence of dopaminergic deficit” hypothesis that the patient has a degenerative parkinsonism and the first scan was “too early”. Indeed, there is evidence that SPECT imaging is abnormal even in the pre-motor phase of Parkinson’s disease and in some atypical parkinsonian syndromes, depending on the relative progression of the nigrostriatal vs cortical pathology [91, 92]. On this basis, normal presynaptic dopamine imaging was added as an absolute exclusion criterion in the latest diagnostic recommendations for Parkinson’s disease [13]. In addition, one must be careful with medication interfering with ligand uptake (amphetamines, bupropion) which may require drug withdrawal and washout before rescanning the patient. Abnormal cardiac MIBG SPECT is now considered as a supportive criterion of Parkinson’s disease in the latest diagnostic criteria from the Movement Disorders Society [13].
A degenerative form of parkinsonism is usually associated with a slowly progressive course (over months to years) and shows typical asymmetric rest tremor, with an excellent response to levodopa in the case of Parkinson’s disease. Conversely, a poor response to dopaminergic treatment and the presence of atypical signs suggests MSA (autonomic dysfunction, pyramidal or cerebellar involvement) [93], PSP (early postural instability and prominent trunk rigidity) [23] or CBD (asymmetric parkinsonism in association with dystonia, apraxia and pyramidal signs) [24]. Hereditary neurological conditions with parkinsonism among other features (oculomotor disturbances, dystonia and ataxia) include Wilson’s disease, Huntington disease and spinocerebellar ataxias, which are beyond the scope of the present review. Whenever the distinction between Parkinson’s disease and an atypical parkinsonism proves difficult, additional PET/SPECT can be discussed. In Parkinson’s disease, one will observe decreased MIBG SPECT uptake (due to postganglionic dysautonomia), preserved striatal metabolism on FDG-PET and normal or increased postsynaptic dopaminergic uptake on raclopride PET or IBZM SPECT.
However, when facing an individual patient, neurologists must be aware of a number of practical limitations related to nuclear medicine imaging scans. First, for safety reasons, examinations associated with an exposure to ionising radiation should be prescribed only when clinically relevant information can be obtained, to keep the radiation dose as low as reasonably achievable. Second, these tests are expensive and not always reimbursed by health insurers, which have published limitations usually based on medically questionable but economically logical considerations. Third, nuclear medicine centres are few, sometimes even nonexistent in some regions or countries, making access to them difficult or impossible for the local population. In this instance, deciding which test to perform may be influenced in a restrictive way.
Conclusion
Although the clinical diagnosis of Parkinson’s disease is usually straightforward when showing the cardinal symptoms associated with a clear response to levodopa, this task can be challenging in ambiguous cases and may therefore require well-selected nuclear medicine imaging investigations. The present review shed some light on the most widely used ligands in the evaluation of parkinsonism and emphasised its highly multimodal approach. Functional PET/SPECT imaging has the compelling ability to explore the underlying mechanisms of neurodegenerative diseases in vivo, but must still face several major challenges. These include design of tau and alpha-synuclein compounds with high specificity and confirmation of current observations in larger trials with standardised protocols, in order to improve diagnostic accuracy and tracking treatment response in future therapeutic trials. Therefore, it is of utmost importance for the neurologist and general practitioner to be familiar with the molecular imaging tools available. This would ensure a tailored approach for the diagnostic workup and management of patients with parkinsonism.
References
1
Jankovic
J
. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–76. doi:.https://doi.org/10.1136/jnnp.2007.131045
2
Alexander
GE
,
DeLong
MR
,
Strick
PL
. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9(1):357–81. doi:.https://doi.org/10.1146/annurev.ne.09.030186.002041
3
Bower
JH
,
Maraganore
DM
,
McDonnell
SK
,
Rocca
WA
. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976-1990. Neurology. 1999;52(6):1214–20. doi:.https://doi.org/10.1212/WNL.52.6.1214
4
Nakashita
S
,
Wada-Isoe
K
,
Uemura
Y
,
Tanaka
K
,
Yamamoto
M
,
Yamawaki
M
, et al.
Clinical assessment and prevalence of parkinsonism in Japanese elderly people. Acta Neurol Scand. 2016;133(5):373–9. doi:.https://doi.org/10.1111/ane.12472
5
Stefanova
N
,
Bücke
P
,
Duerr
S
,
Wenning
GK
. Multiple system atrophy: an update. Lancet Neurol. 2009;8(12):1172–8. doi:.https://doi.org/10.1016/S1474-4422(09)70288-1
6
Richardson
JC
,
Steele
J
,
Olszewski
J
. Supranuclear Ophthalmoplegia, Pseudobulbar Palsy, Nuchal Dystonia and Dementia. A Clinical Report on Eight Cases of “Heterogenous System Degeneration”. Trans Am Neurol Assoc. 1963;88:25–9.
7
Wenning
GK
,
Litvan
I
,
Jankovic
J
,
Granata
R
,
Mangone
CA
,
McKee
A
, et al.
Natural history and survival of 14 patients with corticobasal degeneration confirmed at postmortem examination. J Neurol Neurosurg Psychiatry. 1998;64(2):184–9. doi:.https://doi.org/10.1136/jnnp.64.2.184
8
Benamer
TS
,
Patterson
J
,
Grosset
DG
,
Booij
J
,
de Bruin
K
,
van Royen
E
, et al.
Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Mov Disord. 2000;15(3):503–10. doi:.https://doi.org/10.1002/1531-8257(200005)15:3<503::AID-MDS1013>3.0.CO;2-V
9
Lorberboym
M
,
Treves
TA
,
Melamed
E
,
Lampl
Y
,
Hellmann
M
,
Djaldetti
R
. [123I]-FP/CIT SPECT imaging for distinguishing drug-induced parkinsonism from Parkinson’s disease. Mov Disord. 2006;21(4):510–4. doi:.https://doi.org/10.1002/mds.20748
10
Savica
R
,
Grossardt
BR
,
Bower
JH
,
Ahlskog
JE
,
Rocca
WA
. Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol. 2013;70(7):859–66. doi:.https://doi.org/10.1001/jamaneurol.2013.114
11
Pringsheim
T
,
Jette
N
,
Frolkis
A
,
Steeves
TD
. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29(13):1583–90. doi:.https://doi.org/10.1002/mds.25945
12
Ascherio
A
,
Schwarzschild
MA
. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257–72. doi:.https://doi.org/10.1016/S1474-4422(16)30230-7
13
Postuma
RB
,
Berg
D
,
Stern
M
,
Poewe
W
,
Olanow
CW
,
Oertel
W
, et al.
MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591–601. doi:.https://doi.org/10.1002/mds.26424
14
Braak
H
,
Tredici
KD
,
Rüb
U
,
de Vos
RA
,
Jansen Steur
EN
,
Braak
E
. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi:.https://doi.org/10.1016/S0197-4580(02)00065-9
15
Irwin
DJ
,
White
MT
,
Toledo
JB
,
Xie
SX
,
Robinson
JL
,
Van Deerlin
V
, et al.
Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012;72(4):587–98. doi:.https://doi.org/10.1002/ana.23659
16
Garcia-Esparcia
P
,
López-González
I
,
Grau-Rivera
O
,
García-Garrido
MF
,
Konetti
A
,
Llorens
F
, et al.
Dementia with Lewy Bodies: Molecular Pathology in the Frontal Cortex in Typical and Rapidly Progressive Forms. Front Neurol. 2017;8:89. doi:.https://doi.org/10.3389/fneur.2017.00089
17
Jellinger
KA
. Dementia with Lewy bodies and Parkinson’s disease-dementia: current concepts and controversies. J Neural Transm (Vienna). 2018;125(4):615–50.
18
Petrou
M
,
Dwamena
BA
,
Foerster
BR
,
MacEachern
MP
,
Bohnen
NI
,
Müller
ML
, et al.
Amyloid deposition in Parkinson’s disease and cognitive impairment: a systematic review. Mov Disord. 2015;30(7):928–35. doi:.https://doi.org/10.1002/mds.26191
19
Vekrellis
K
,
Xilouri
M
,
Emmanouilidou
E
,
Rideout
HJ
,
Stefanis
L
. Pathological roles of α-synuclein in neurological disorders. Lancet Neurol. 2011;10(11):1015–25. doi:.https://doi.org/10.1016/S1474-4422(11)70213-7
20
Dickson
DW
. Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med. 2012;2(8):a009258. doi:.https://doi.org/10.1101/cshperspect.a009258
21
Fanciulli
A
,
Wenning
GK
. Multiple-system atrophy. N Engl J Med. 2015;372(3):249–63. doi:.https://doi.org/10.1056/NEJMra1311488
22
Coyle-Gilchrist
IT
,
Dick
KM
,
Patterson
K
,
Vázquez Rodríquez
P
,
Wehmann
E
,
Wilcox
A
, et al.
Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736–43. doi:.https://doi.org/10.1212/WNL.0000000000002638
23
Höglinger
GU
,
Respondek
G
,
Stamelou
M
,
Kurz
C
,
Josephs
KA
,
Lang
AE
, et al.; Movement Disorder Society-endorsed PSP Study Group. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord. 2017;32(6):853–64. doi:.https://doi.org/10.1002/mds.26987
24
Armstrong
MJ
,
Litvan
I
,
Lang
AE
,
Bak
TH
,
Bhatia
KP
,
Borroni
B
, et al.
Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496–503. doi:.https://doi.org/10.1212/WNL.0b013e31827f0fd1
25
Heim
B
,
Krismer
F
,
De Marzi
R
,
Seppi
K
. Magnetic resonance imaging for the diagnosis of Parkinson’s disease. J Neural Transm (Vienna). 2017;124(8):915–64. doi:.https://doi.org/10.1007/s00702-017-1717-8
26
Kaasinen
V
,
Vahlberg
T
. Striatal dopamine in Parkinson disease: A meta-analysis of imaging studies. Ann Neurol. 2017;82(6):873–82. doi:.https://doi.org/10.1002/ana.25103
27
Booij
J
,
Tissingh
G
,
Boer
GJ
,
Speelman
JD
,
Stoof
JC
,
Janssen
AG
, et al.
[123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1997;62(2):133–40. doi:.https://doi.org/10.1136/jnnp.62.2.133
28
Benamer
HTS
,
Patterson
J
,
Grosset
DG
,
Booij
J
,
de Bruin
K
,
van Royen
E
, et al.
Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: The [123I]-FP-CIT study group. Mov Disord. 2000;15(3):503–10. doi:.https://doi.org/10.1002/1531-8257(200005)15:3<503::AID-MDS1013>3.0.CO;2-V
29
Tinazzi
M
,
Antonini
A
,
Bovi
T
,
Pasquin
I
,
Steinmayr
M
,
Moretto
G
, et al.
Clinical and [123I]FP-CIT SPET imaging follow-up in patients with drug-induced parkinsonism. J Neurol. 2009;256(6):910–5. doi:.https://doi.org/10.1007/s00415-009-5039-0
30
Brücke
T
,
Asenbaum
S
,
Pirker
W
,
Djamshidian
S
,
Wenger
S
,
Wöber
C
, et al.
Measurement of the dopaminergic degeneration in Parkinson’s disease with [123I] beta-CIT and SPECT. Correlation with clinical findings and comparison with multiple system atrophy and progressive supranuclear palsy. J Neural Transm Suppl. 1997;50:9–24. doi:.https://doi.org/10.1007/978-3-7091-6842-4_2
31
Plotkin
M
,
Amthauer
H
,
Klaffke
S
,
Kühn
A
,
Lüdemann
L
,
Arnold
G
, et al.
Combined 123I-FP-CIT and 123I-IBZM SPECT for the diagnosis of parkinsonian syndromes: study on 72 patients. J Neural Transm (Vienna). 2005;112(5):677–92. doi:.https://doi.org/10.1007/s00702-004-0208-x
32
Im
JH
,
Chung
SJ
,
Kim
JS
,
Lee
MC
. Differential patterns of dopamine transporter loss in the basal ganglia of progressive supranuclear palsy and Parkinson’s disease: analysis with [(123)I]IPT single photon emission computed tomography. J Neurol Sci. 2006;244(1-2):103–9. doi:.https://doi.org/10.1016/j.jns.2006.01.006
33
Nicastro
N
,
Garibotto
V
,
Badoud
S
,
Burkhard
PR
. Scan without evidence of dopaminergic deficit: A 10-year retrospective study. Parkinsonism Relat Disord. 2016;31:53–8. doi:.https://doi.org/10.1016/j.parkreldis.2016.07.002
34
Walker
Z
,
Jaros
E
,
Walker
RW
,
Lee
L
,
Costa
DC
,
Livingston
G
, et al.
Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry. 2007;78(11):1176–81. doi:.https://doi.org/10.1136/jnnp.2006.110122
35
Antonini
A
,
Benti
R
,
De Notaris
R
,
Tesei
S
,
Zecchinelli
A
,
Sacilotto
G
, et al.
123I-Ioflupane/SPECT binding to striatal dopamine transporter (DAT) uptake in patients with Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Neurol Sci. 2003;24(3):149–50. doi:.https://doi.org/10.1007/s10072-003-0103-5
36
Cilia
R
,
Rossi
C
,
Frosini
D
,
Volterrani
D
,
Siri
C
,
Pagni
C
, et al.
Dopamine Transporter SPECT Imaging in Corticobasal Syndrome. PLoS One. 2011;6(5):e18301. doi:.https://doi.org/10.1371/journal.pone.0018301
37
Kaasinen
V
,
Gardberg
M
,
Röyttä
M
,
Seppänen
M
,
Päivärinta
M
. Normal dopamine transporter SPECT in neuropathologically confirmed corticobasal degeneration. J Neurol. 2013;260(5):1410–1. doi:.https://doi.org/10.1007/s00415-013-6886-2
38
O’Sullivan
SS
,
Burn
DJ
,
Holton
JL
,
Lees
AJ
. Normal dopamine transporter single photon-emission CT scan in corticobasal degeneration. Mov Disord. 2008;23(16):2424–6. doi:.https://doi.org/10.1002/mds.22323
39
McKinley
J
,
O’Connell
M
,
Farrell
M
,
Lynch
T
. Normal dopamine transporter imaging does not exclude multiple system atrophy. Parkinsonism Relat Disord. 2014;20(8):933–4. doi:.https://doi.org/10.1016/j.parkreldis.2014.04.022
40
Nicastro
N
,
Garibotto
V
,
Burkhard
PR
. 123I-FP-CIT SPECT Accurately Distinguishes Parkinsonian From Cerebellar Variant of Multiple System Atrophy. Clin Nucl Med. 2018;43(2):e33–6.https://doi.org/10.1097/RLU.0000000000001477
41
Benamer
HT
,
Patterson
J
,
Wyper
DJ
,
Hadley
DM
,
Macphee
GJ
,
Grosset
DG
. Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord. 2000;15(4):692–8. doi:.https://doi.org/10.1002/1531-8257(200007)15:4<692::AID-MDS1014>3.0.CO;2-V
42
Del Sole
A
,
Perini
G
,
Lecchi
M
,
Mariani
C
,
Lucignani
G
,
Clerici
F
. Correlation between 123I-FP-CIT brain SPECT and parkinsonism in dementia with Lewy bodies: caveat for clinical use. Clin Nucl Med. 2015;40(1):32–5. doi:.https://doi.org/10.1097/RLU.0000000000000602
43
Kraemmer
J
,
Kovacs
GG
,
Perju-Dumbrava
L
,
Pirker
S
,
Traub-Weidinger
T
,
Pirker
W
. Correlation of striatal dopamine transporter imaging with post mortem substantia nigra cell counts. Mov Disord. 2014;29(14):1767–73. doi:.https://doi.org/10.1002/mds.25975
44
Saari
L
,
Kivinen
K
,
Gardberg
M
,
Joutsa
J
,
Noponen
T
,
Kaasinen
V
. Dopamine transporter imaging does not predict the number of nigral neurons in Parkinson disease. Neurology. 2017;88(15):1461–7. doi:.https://doi.org/10.1212/WNL.0000000000003810
45
Darcourt
J
,
Booij
J
,
Tatsch
K
,
Varrone
A
,
Vander Borght
T
,
Kapucu
OL
, et al.
EANM procedure guidelines for brain neurotransmission SPECT using (123)I-labelled dopamine transporter ligands, version 2. Eur J Nucl Med Mol Imaging. 2010;37(2):443–50. doi:.https://doi.org/10.1007/s00259-009-1267-x
46
Lee
CS
,
Samii
A
,
Sossi
V
,
Ruth
TJ
,
Schulzer
M
,
Holden
JE
, et al.
In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann Neurol. 2000;47(4):493–503. doi:.https://doi.org/10.1002/1531-8249(200004)47:4<493::AID-ANA13>3.0.CO;2-4
47
Frey
KA
. Can SPET imaging of dopamine uptake sites replace PET imaging in Parkinson’s disease? Against. Eur J Nucl Med Mol Imaging. 2002;29(5):715–7. doi:.https://doi.org/10.1007/s00259-002-0815-4
48
Okamura
N
,
Villemagne
VL
,
Drago
J
,
Pejoska
S
,
Dhamija
RK
,
Mulligan
RS
, et al.
In vivo measurement of vesicular monoamine transporter type 2 density in Parkinson disease with (18)F-AV-133. J Nucl Med. 2010;51(2):223–8. doi:.https://doi.org/10.2967/jnumed.109.070094
49
Fahn
S
,
Oakes
D
,
Shoulson
I
,
Kieburtz
K
,
Rudolph
A
,
Lang
A
, et al., Parkinson Study Group. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351(24):2498–508. doi:.https://doi.org/10.1056/NEJMoa033447
50
Parkinson Study Group. A randomized controlled trial comparing pramipexole with levodopa in early Parkinson’s disease: design and methods of the CALM-PD Study. Clin Neuropharmacol. 2000;23(1):34–44. doi:.https://doi.org/10.1097/00002826-200001000-00007
51
Parkinson Study Group PRECEPT Investigators. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology. 2007;69(15):1480–90. doi:.https://doi.org/10.1212/01.wnl.0000277648.63931.c0
52
Marek
K
,
Seibyl
J
,
Eberly
S
,
Oakes
D
,
Shoulson
I
,
Lang
AE
, et al.; Parkinson Study Group PRECEPT Investigators. Longitudinal follow-up of SWEDD subjects in the PRECEPT Study. Neurology. 2014;82(20):1791–7. doi:.https://doi.org/10.1212/WNL.0000000000000424
53
Erro
R
,
Schneider
SA
,
Stamelou
M
,
Quinn
NP
,
Bhatia
KP
. What do patients with scans without evidence of dopaminergic deficit (SWEDD) have? New evidence and continuing controversies. J Neurol Neurosurg Psychiatry. 2016;87(3):319–23. doi:.https://doi.org/10.1136/jnnp-2014-310256
54
Sixel-Döring
F
,
Liepe
K
,
Mollenhauer
B
,
Trautmann
E
,
Trenkwalder
C
. The role of 123I-FP-CIT-SPECT in the differential diagnosis of Parkinson and tremor syndromes: a critical assessment of 125 cases. J Neurol. 2011;258(12):2147–54. doi:.https://doi.org/10.1007/s00415-011-6076-z
55
Nicastro
N
,
Burkhard
PR
,
Garibotto
V
. Scan without evidence of dopaminergic deficit (SWEDD) in degenerative parkinsonism and dementia with Lewy bodies: A prospective study. J Neurol Sci. 2018;385:17–21. doi:.https://doi.org/10.1016/j.jns.2017.11.039
56
Hughes
AJ
,
Daniel
SE
,
Ben-Shlomo
Y
,
Lees
AJ
. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125(Pt 4):861–70. doi:.https://doi.org/10.1093/brain/awf080
57
Schrag
A
,
Ben-Shlomo
Y
,
Quinn
N
. How valid is the clinical diagnosis of Parkinson’s disease in the community?
J Neurol Neurosurg Psychiatry. 2002;73(5):529–34. doi:.https://doi.org/10.1136/jnnp.73.5.529
58
Meyer
PT
,
Hellwig
S
. Update on SPECT and PET in parkinsonism - part 1: imaging for differential diagnosis. Curr Opin Neurol. 2014;27(4):390–7. doi:.https://doi.org/10.1097/WCO.0000000000000106
59
Eckert
T
,
Barnes
A
,
Dhawan
V
,
Frucht
S
,
Gordon
MF
,
Feigin
AS
, et al.
FDG PET in the differential diagnosis of parkinsonian disorders. Neuroimage. 2005;26(3):912–21. doi:.https://doi.org/10.1016/j.neuroimage.2005.03.012
60
Zhao
P
,
Zhang
B
,
Gao
S
. 18F-FDG PET study on the idiopathic Parkinson’s disease from several parkinsonian-plus syndromes. Parkinsonism Relat Disord. 2012;18(Suppl 1):S60–2. doi:.https://doi.org/10.1016/S1353-8020(11)70020-7
61
Juh
R
,
Kim
J
,
Moon
D
,
Choe
B
,
Suh
T
. Different metabolic patterns analysis of Parkinsonism on the 18F-FDG PET. Eur J Radiol. 2004;51(3):223–33. doi:.https://doi.org/10.1016/S0720-048X(03)00214-6
62
Niethammer
M
,
Tang
CC
,
Feigin
A
,
Allen
PJ
,
Heinen
L
,
Hellwig
S
, et al.
A disease-specific metabolic brain network associated with corticobasal degeneration. Brain. 2014;137(Pt 11):3036–46. doi:.https://doi.org/10.1093/brain/awu256
63
Antonini
A
,
Schwarz
J
,
Oertel
WH
,
Beer
HF
,
Madeja
UD
,
Leenders
KL
. [11C]raclopride and positron emission tomography in previously untreated patients with Parkinson’s disease: Influence of L-dopa and lisuride therapy on striatal dopamine D2-receptors. Neurology. 1994;44(7):1325–9. doi:.https://doi.org/10.1212/WNL.44.7.1325
64
Brooks
DJ
,
Ibanez
V
,
Sawle
GV
,
Playford
ED
,
Quinn
N
,
Mathias
CJ
, et al.
Striatal D2 receptor status in patients with Parkinson’s disease, striatonigral degeneration, and progressive supranuclear palsy, measured with 11C-raclopride and positron emission tomography. Ann Neurol. 1992;31(2):184–92. doi:.https://doi.org/10.1002/ana.410310209
65
Kim
YJ
,
Ichise
M
,
Ballinger
JR
,
Vines
D
,
Erami
SS
,
Tatschida
T
, et al.
Combination of dopamine transporter and D2 receptor SPECT in the diagnostic evaluation of PD, MSA, and PSP. Mov Disord. 2002;17(2):303–12. doi:.https://doi.org/10.1002/mds.10042
66
Klaffke
S
,
Kuhn
AA
,
Plotkin
M
,
Amthauer
H
,
Harnack
D
,
Felix
R
, et al.
Dopamine transporters, D2 receptors, and glucose metabolism in corticobasal degeneration. Mov Disord. 2006;21(10):1724–7. doi:.https://doi.org/10.1002/mds.21004
67
Takatsu
H
,
Nishida
H
,
Matsuo
H
,
Watanabe
S
,
Nagashima
K
,
Wada
H
, et al.
Cardiac sympathetic denervation from the early stage of Parkinson’s disease: clinical and experimental studies with radiolabeled MIBG. J Nucl Med. 2000;41(1):71–7.
68
Raffel
DM
,
Koeppe
RA
,
Little
R
,
Wang
CN
,
Liu
S
,
Junck
L
, et al.
PET measurement of cardiac and nigrostriatal denervation in Parkinsonian syndromes. J Nucl Med. 2006;47(11):1769–77.
69
King
AE
,
Mintz
J
,
Royall
DR
. Meta-analysis of 123I-MIBG cardiac scintigraphy for the diagnosis of Lewy body-related disorders. Mov Disord. 2011;26(7):1218–24. doi:.https://doi.org/10.1002/mds.23659
70
Druschky
A
,
Hilz
MJ
,
Platsch
G
,
Radespiel-Tröger
M
,
Druschky
K
,
Kuwert
T
, et al.
Differentiation of Parkinson’s disease and multiple system atrophy in early disease stages by means of I-123-MIBG-SPECT. J Neurol Sci. 2000;175(1):3–12. doi:.https://doi.org/10.1016/S0022-510X(00)00279-3
71
Südmeyer
M
,
Antke
C
,
Zizek
T
,
Beu
M
,
Nikolaus
S
,
Wojtecki
L
, et al.
Diagnostic accuracy of combined FP-CIT, IBZM, and MIBG scintigraphy in the differential diagnosis of degenerative parkinsonism: a multidimensional statistical approach. J Nucl Med. 2011;52(5):733–40. doi:.https://doi.org/10.2967/jnumed.110.086959
72
Berardelli
A
,
Wenning
GK
,
Antonini
A
,
Berg
D
,
Bloem
BR
,
Bonifati
V
, et al.
EFNS/MDS-ES/ENS recommendations for the diagnosis of Parkinson’s disease. Eur J Neurol. 2013;20(1):16–34. doi:.https://doi.org/10.1111/ene.12022
73
Vlaar
AM
,
de Nijs
T
,
Kessels
AG
,
Vreeling
FW
,
Winogrodzka
A
,
Mess
WH
, et al.
Diagnostic value of 123I-ioflupane and 123I-iodobenzamide SPECT scans in 248 patients with parkinsonian syndromes. Eur Neurol. 2008;59(5):258–66. doi:.https://doi.org/10.1159/000115640
74
Garibotto
V
,
Montandon
ML
,
Viaud
CT
,
Allaoua
M
,
Assal
F
,
Burkhard
PR
, et al.
Regions of interest-based discriminant analysis of DaTSCAN SPECT and FDG-PET for the classification of dementia. Clin Nucl Med. 2013;38(3):e112–7. doi:.https://doi.org/10.1097/RLU.0b013e318279b991
75
Pilotto
A
,
Premi
E
,
Paola Caminiti
S
,
Presotto
L
,
Turrone
R
,
Alberici
A
, et al.
Single-subject SPM FDG-PET patterns predict risk of dementia progression in Parkinson disease. Neurology. 2018;90(12):e1029–37. doi:.https://doi.org/10.1212/WNL.0000000000005161
76
Hellwig
S
,
Amtage
F
,
Kreft
A
,
Buchert
R
,
Winz
OH
,
Vach
W
, et al.
[18F]FDG-PET is superior to [123I]IBZM-SPECT for the differential diagnosis of parkinsonism. Neurology. 2012;79(13):1314–22. doi:.https://doi.org/10.1212/WNL.0b013e31826c1b0a
77
Imamura
K
,
Hishikawa
N
,
Sawada
M
,
Nagatsu
T
,
Yoshida
M
,
Hashizume
Y
. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106(6):518–26. doi:.https://doi.org/10.1007/s00401-003-0766-2
78
Banati
RB
,
Myers
R
,
Kreutzberg
GWPK
. PK (‘peripheral benzodiazepine’)--binding sites in the CNS indicate early and discrete brain lesions: microautoradiographic detection of [3H]PK11195 binding to activated microglia. J Neurocytol. 1997;26(2):77–82. doi:.https://doi.org/10.1023/A:1018567510105
79
Gerhard
A
,
Pavese
N
,
Hotton
G
,
Turkheimer
F
,
Es
M
,
Hammers
A
, et al.
In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21(2):404–12. doi:.https://doi.org/10.1016/j.nbd.2005.08.002
80
Iannaccone
S
,
Cerami
C
,
Alessio
M
,
Garibotto
V
,
Panzacchi
A
,
Olivieri
S
, et al.
In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(1):47–52. doi:. Correction in: Parkinsomism Rel disord. 2013;19(10):921.https://doi.org/10.1016/j.parkreldis.2012.07.002
81
Surendranathan
A
,
Rowe
JB
,
O’Brien
JT
. Neuroinflammation in Lewy body dementia. Parkinsonism Relat Disord. 2015;21(12):1398–406. doi:.https://doi.org/10.1016/j.parkreldis.2015.10.009
82
Gerhard
A
,
Banati
RB
,
Goerres
GB
,
Cagnin
A
,
Myers
R
,
Gunn
RN
, et al.
[11C](R)-PK11195 PET imaging of microglial activation in multiple system atrophy. Neurology. 2003;61(5):686–9. doi:.https://doi.org/10.1212/01.WNL.0000078192.95645.E6
83
Gerhard
A
,
Watts
J
,
Trender-Gerhard
I
,
Turkheimer
F
,
Banati
RB
,
Bhatia
K
, et al.
In vivo imaging of microglial activation with [11C](R)-PK11195 PET in corticobasal degeneration. Mov Disord. 2004;19(10):1221–6. doi:.https://doi.org/10.1002/mds.20162
84
Kepe
V
,
Bordelon
Y
,
Boxer
A
,
Huang
SC
,
Liu
J
,
Thiede
FC
, et al.
PET imaging of neuropathology in tauopathies: progressive supranuclear palsy. J Alzheimers Dis. 2013;36(1):145–53.
85
Marquié
M
,
Normandin
MD
,
Vanderburg
CR
,
Costantino
IM
,
Bien
EA
,
Rycyna
LG
, et al.
Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78(5):787–800. doi:.https://doi.org/10.1002/ana.24517
86
Cho
H
,
Choi
JY
,
Hwang
MS
,
Lee
SH
,
Ryu
YH
,
Lee
MS
, et al.
Subcortical18F-AV-1451 binding patterns in progressive supranuclear palsy. Mov Disord. 2017;32(1):134–40. doi:.https://doi.org/10.1002/mds.26844
87
Fodero-Tavoletti
MT
,
Mulligan
RS
,
Okamura
N
,
Furumoto
S
,
Rowe
CC
,
Kudo
Y
, et al.
In vitro characterisation of BF227 binding to alpha-synuclein/Lewy bodies. Eur J Pharmacol. 2009;617(1-3):54–8. doi:.https://doi.org/10.1016/j.ejphar.2009.06.042
88
Goetz
CG
,
Tilley
BC
,
Shaftman
SR
,
Stebbins
GT
,
Fahn
S
,
Martinez-Martin
P
, et al.; Movement Disorder Society UPDRS Revision Task Force. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–70. doi:.https://doi.org/10.1002/mds.22340
89
Rosso
AL
,
Bohnen
NI
,
Launer
LJ
,
Aizenstein
HJ
,
Yaffe
K
,
Rosano
C
. Vascular and dopaminergic contributors to mild parkinsonian signs in older adults. Neurology. 2018;90(3):e223–9. doi:.https://doi.org/10.1212/WNL.0000000000004842
90
Ba
F
,
Martin
WR
. Dopamine transporter imaging as a diagnostic tool for parkinsonism and related disorders in clinical practice. Parkinsonism Relat Disord. 2015;21(2):87–94. doi:.https://doi.org/10.1016/j.parkreldis.2014.11.007
91
Berendse
HW
,
Booij
J
,
Francot
CM
,
Bergmans
PL
,
Hijman
R
,
Stoof
JC
, et al.
Subclinical dopaminergic dysfunction in asymptomatic Parkinson’s disease patients’ relatives with a decreased sense of smell. Ann Neurol. 2001;50(1):34–41. doi:.https://doi.org/10.1002/ana.1049
92
Sommer
U
,
Hummel
T
,
Cormann
K
,
Mueller
A
,
Frasnelli
J
,
Kropp
J
, et al.
Detection of presymptomatic Parkinson’s disease: combining smell tests, transcranial sonography, and SPECT. Mov Disord. 2004;19(10):1196–202. doi:.https://doi.org/10.1002/mds.20141
93
Gilman
S
,
Wenning
GK
,
Low
PA
,
Brooks
DJ
,
Mathias
CJ
,
Trojanowski
JQ
, et al.
Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–6. doi:.https://doi.org/10.1212/01.wnl.0000324625.00404.15



