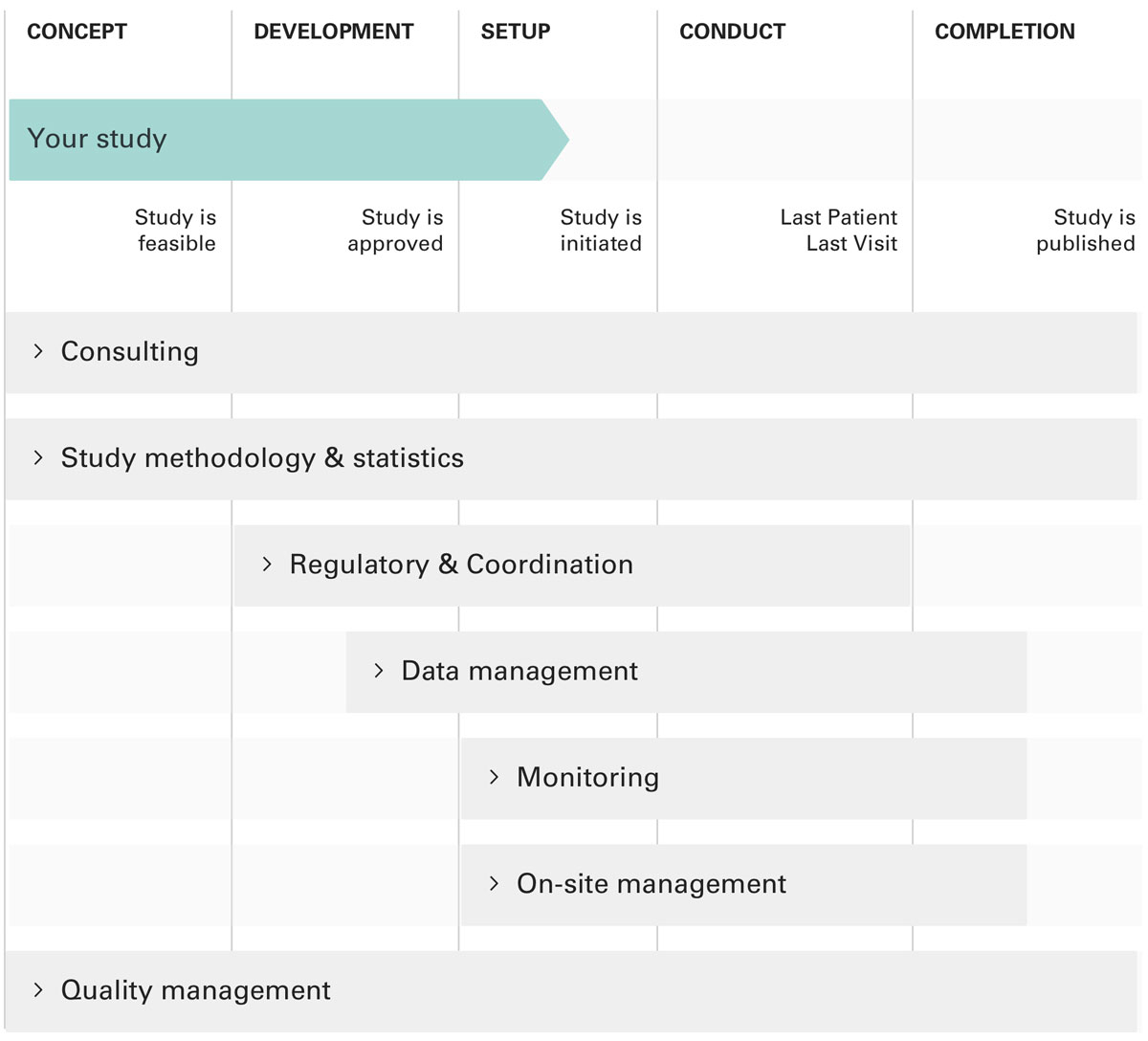
Figure 1 The CTU services apply through all stages of a study.
DOI: https://doi.org/10.4414/smw.2018.14615
As a major driving force of patient-oriented clinical research, academic institutions are ideally placed to lead the movement striving to increase value and reduce waste in clinical research. Academia not only receives large proportions of public funding [1, 2], but it also produces the majority of scientific publications [3]. However, despite significant investment in and improvement of infrastructure, training and methodological support [4, 5], the issues raised by a 2014 series from The Lancet on “Increasing value, reducing waste in biomedical research” [6–11] persist [12–16]. The authors of The Lancet series provided several recommendations on how to improve the system, through (a) setting the right research priorities, (b) ensuring sound research design, conduct, and analysis, (c) lobbying for more efficient research regulation, and (d) ensuring accessibility and reporting of data and research results. However, academic institutions in particular have been slow in taking responsive measures [17]. Potential explanations for this slow progress include a lack of both common academic policies and a definition of what constitutes “good academic research”, across a complex ecosystem of stakeholders and their agendas [17, 18].
Over a decade ago, the number of clinical research projects being performed in Switzerland has been reported to drop [19, 20]. The Swiss Federal Council identified underlying causes for the decline in studies performed in Switzerland, including factors such as the location of trials in countries with facilitated recruitment, ostensibly new markets and the increasing number of multinational studies [20, 21]. The involved stakeholders were concerned with weaknesses in quality, rather than quantity, and so set in motion several constructive initiatives intended to remedy the situation, and to improve the quality of Swiss clinical research [22, 23].
As in other European countries [24], one of these initiatives was to establish clinical trial units (CTUs) as centres of competence at each of the five existing Swiss university hospitals and the St Gallen cantonal hospital, to support the high-quality conduct of academic clinical studies. This 10th anniversary (2007 to 2017) marked a time to reflect on their trajectory and to evaluate whether the CTUs have been successful in improving the value of clinical research conducted in Switzerland.
In this review, we briefly describe the development of the CTUs, providing a portrait of their decade-long efforts to improve the quality of academically initiated clinical studies, evaluate their impact, and provide an outlook on possible future activities of the CTU Network to reduce inefficiencies and improve the value of clinical research in Switzerland.
A decade ago, the Swiss National Science Foundation (SNSF) laid the foundation for establishing CTUs at the five university hospitals of Switzerland (in Basel, Bern, Geneva, Lausanne and Zurich), as well as the St Gallen cantonal hospital, with the aim of improving the quality of Swiss academic clinical research projects. In 2016, the Ente Ospedaliero Cantonale (cantonal hospital network) and its CTU from Ticino, representing the Italian-speaking clinical research community, joined the network as an associate member. Thus combined, this forms the largest national provider of services and education for the academic research force and engages more than 140 employees. The CTUs partner not only with national funders, regulatory bodies and policy makers, but also with international initiatives such as the European Patients’ Academy on Therapeutic Innovation (EUPATI) or the European Clinical Research Infrastructures Network (ECRIN) [22, 25].
The primary aim was to improve the professionalism and quality of research at their respective home institutions, but the CTUs soon realised that for such improvement to be sustainable, the national coordination of these local initiatives would be critical. This led to the establishment in 2009 of the Swiss Clinical Trial Organisation (SCTO) as an umbrella organisation. Its objectives included: positioning Swiss clinical research competitively in the international environment with respect to its innovation and quality; defining and promoting quality standards (via the definition and implementation of internationally recognised quality standards for the conduct of studies); fostering continued education and training coordination; and thereby facilitating national and international multicentre clinical research [26]. Since 2013, the SCTO has been serving as an independent institute housing the relevant expertise, resources and structures to run as a lean, professional and central organisation.
This section outlines the current CTU efforts underway to enhance quality in academic clinical research in Switzerland. With the ground laid, we will then consider new ways forward.
With the common aim of supporting quality throughout all stages of a research project, the CTUs act as multidisciplinary study centres and provide specific services at each study phase (see fig. 1). This includes, for example, asking relevant research questions, using sound methodology, collecting high-quality data, improving recruitment patterns, and transparently disseminating study results. To assure high-quality CTU services, all CTU employees – in addition to being selected on the basis of their expertise in their respective fields – are continually trained according to the newest standards and requirements of regulators, funders and other institutions in the clinical research fields.

Figure 1 The CTU services apply through all stages of a study.
In addition to providing expert support, the CTU Network has strengthened its role as the country’s key academic provider of training in clinical research and, in so doing, is also promoting well-trained, qualified personnel across Switzerland. For example, the CTUs have developed different postgraduate programmes to best serve the different needs of clinical research professionals, such as study nurses and study coordinators, study managers and study physicians, clinical monitors and clinical trial assistants.
The SCTO’s Education Working Group of the CTU Network has compiled a comprehensive catalogue of training and continuous education opportunities in clinical research, thereby supporting the Federal Office of Public Health in building a federal roadmap for developing and nurturing the next generation of clinical researchers. Furthermore, the SCTO Guidelines on Training and Education in Clinical Research in Switzerland gives guidance as to training requirements for different target audiences in clinical research [27].
Besides being actively involved in national activities related to clinical research, the SCTO is recognised by other European countries as a contact point and centre of expertise in Switzerland. Because it participates in ECRIN, the CTU Network benefits from access to research infrastructures across Europe and has provided services to several multinational trials and new calls falling under Horizon 2020. With its public awareness activities, the SCTO continues to be engaged in public dialogue on controversial issues. This includes contributing to the organisation of public seminars, the launch of the national EUPATI platform and fostering contact with research groups and institutions (such as universities and institutes of technology) in order to facilitate a broader reach, “from bench to bedside”.
For the above reasons, it is assumed that the CTUs have indeed contributed substantially to the quality of clinical research in Switzerland. Nonetheless, there is an urgent need for a systematic assessment of their impact. The first independent evaluation of all CTUs, initiated by the SCTO in cooperation with Swissmedic, was completed in 2011. In its final inspection report, Swissmedic rated three out of six CTUs as “well-structured and -organised” [28]. Only a year later, at the end of 2012, the CTUs had completed all required improvement measures and thereby reached an important milestone regarding harmonisation and continuous quality improvement throughout the network.
Quantitative metrics of the number of services rendered by the CTUs have climbed steadily over the past decade. In 2016, the CTU Network provided more than 2600 services to over 270 clinical studies and trained over 3000 course participants [29]. These services are provided in accordance with harmonised quality standards, which were jointly developed by the CTUs and the Swiss Group for Clinical Cancer Research (Schweizerische Arbeitsgemeinschaft für Klinische Krebsforschung). To this end, all CTUs have committed to aligning their management systems in accordance with applicable national and international regulatory requirements. These measures include: the International Council for Harmonisation’s Good Clinical Practice (ICH GCP); internationally acknowledged, process-oriented standards for Quality Management Systems; information security management (compliant with the International Organization for Standardization, ISO 27001); the Guidelines for Good Clinical Data Management Practices (GCDMP); and certain other applicable regulations.
As an additional milestone, the SCTO and the CTUs together developed Guidelines for Good Operational Practice, providing guidance on how to apply GCP in the academic setting [30]. In conjunction with other common guidelines, such as the abovementioned GCDMP, the SCTO’s commitment to education and training, and a set of common Standard Operating Procedures, these documents ensure harmonised quality standards are the basis for implementation at an operational level within each CTU.
In contrast to quantitative measures presented above, the analysis of the network’s qualitative impact has proven much more difficult. In 2015, von Niederhäusern et al. [22] used the number of ethics committee “findings” in study dossiers (indicating potential obstacles or weaknesses) as a first measure, albeit an indirectly correlated one, for the qualitative impact of the CTU Basel on study quality. The study demonstrated that the substantial contribution to the planning and conduct of studies, both academic and industry-sponsored, had successfully decreased the number of findings, which was interpreted as an indirect measure for the increase of research quality.
In June 2017, looking back on their successful history, the CTUs and the SCTO celebrated their ten-year anniversary at a national symposium titled “How to increase the value of academic clinical research in Switzerland” [31]. Several stakeholders were invited to share their thoughts on how they – in collaboration with the system – could improve the value of research. Additionally, the CTU Basel asked stakeholders in a web-based survey to anonymously provide their rating on the Swiss CTUs’ resulting impact on the quality of aspects that were of particular importance to them (i.e., quality of submitted dossiers). The survey participants were asked to rate the CTUs’ impact on these aspects using a visual analogue scale (VAS) ranging from 0 (no impact at all) to 10 (maximum impact). Only two of the participating institutions (ethics committees and Swissmedic) provided more than three individual responses and are therefore displayed in figures 2a and 2b.
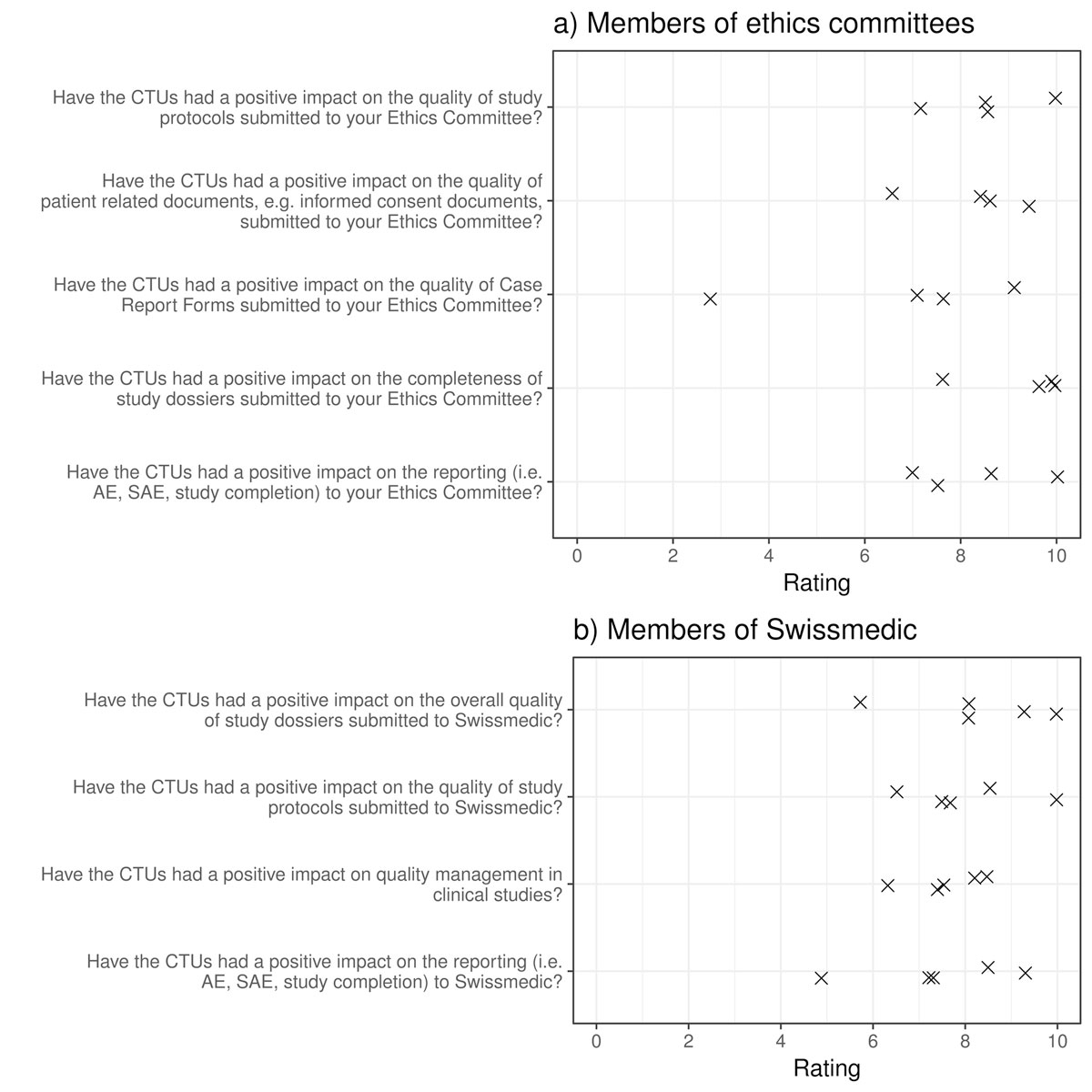
Figure 2 A stakeholder survey on the impact of CTUs on the quality of Swiss-based research: responses from ethics committees and from Swissmedic.
A cross represents one answer of one member of (a) an ethics committee (a) or (b) Swissmedic, respectively, on a visual analogue scale from 0 to 10 (0: no impact at all; 10: maximum impact).
Generally, the CTUs were found to have a positive impact on the quality and completeness of study documents or dossiers and on the reporting to the respective institutions. This improvement is probably a result of an involvement of CTUs in the preparation and submission of study documents, but also of better training of investigators as to the requirements of study protocol and patient information consent.
A range of suggestions were made as to how the CTUs could improve their performance and impact yet further. Both regulatory bodies and researchers recognised the positive impact of CTUs on the quality of submitted study dossiers. Swissmedic and ethics committees offered suggestions on how to further extend the support offered by CTUs (e.g., reaching agreements with other service providers, mandatory checking of study proposals prior to submission, or enforcing good documentation practice). However, researchers noted that cost posed a barrier, especially to young scientists. Suggestions made to overcome the cost issue included allocating CTU start-up packages for promising projects or facilitating access for young researchers. A sample of six of these comments appear in table 1.
Table 1 A selection of suggestions on improving CTUs, from anonymous evaluations.
| Feedback supplied via anonymous evaluation | Source of comment |
|---|---|
| Enforce good documentation practices in the framework of clinical trials (ALCOA principles: Attributable, Legible, Contemporaneous, Original and Accurate). [This includes] how to handle electronic health records, which are not accessible for monitoring purposes … monitoring [is very often] based on printout-outs from health records: [indicating] the importance of “real” certified copies. | A staff member of Swissmedic |
| Try to get agreements with the hospital pharmacies to get reasonable prices for the preparation of investigator medicinal products (IMP) according to Good Manufacturing Practice (GMP) for investigator-initiated trials. These prices are generally very high for small budgets, but they cannot be avoided, because GMP is essential to guarantee the quality and safety of the IMP. | A staff member of Swissmedic |
| All research proposals should go obligatorily through a check/test by the CTUs, prior to their submission to the ethics committees. | A member of an ethics committee |
| It would be great if young research group leaders … could benefit from a start-up “package” of CTU services. I understand that the limiting factor is “Who pays for that?”, but maybe we can work out some solutions. The “winners” of such packages could be selected on academic merit, e.g. number and quality of recent publications. It is great we have CTUs! They are here to stay! | Researcher |
| The CTU should be supportive of research. A distinction must be made between industry- and investigator-driven studies, and the costs of services adapted accordingly. | Researcher |
| We need to facilitate access for young researchers [who are still at the stage of their careers of being] without and industrial [i.e. industry-funded] budget. | Researcher |
Note: These questions were anonymous responses to the question: “Do you have suggestions on how the CTU(s) could improve the impact their services have on the quality of clinical research in the future?”
For the national SCTO symposium held in June 2017, the CTU Network conducted a web-based survey among its customers – the clinical research force at all Swiss university hospitals. The aim of the survey was to evaluate the subjective impact of individual CTU services on the quality of a study in a particular research stage (from concept to dissemination). A total of 155 CTU customers completed the anonymous survey (see table 2 for survey characteristics), which was distributed through SurveyMonkey® and, after completion, descriptively analysed (as total numbers, percentages, means or interquartile ranges, as applicable) by the CTU Basel. The majority of survey participants was made up of sponsor-investigators (35.5%) or investigators (49.7%) who had performed a median number of six clinical studies by then. Half of the survey participants had received services from the CTU Basel, followed by customers of the CTU Zurich (17.4%). Survey participants generally rated the positive influence of CTU services on the quality of their study at 8/10 or higher (see fig. 3). The only service they rated slightly lower than other services was the CTUs’ support with publications, which is indeed not a focus in the CTUs’ portfolio. However, the survey captures feedback only of researchers who have already collaborated with CTUs. The opinions of those who have never used CTU services before are not represented. To further develop and improve the CTU Network, we are aware that it will be important to better understand the reasons for not collaborating with the existing infrastructures.
Table 2 Characteristics of CTU customer survey participants (n = 155) and the studies performed.
| n (%) | |
|---|---|
| All CTU customer survey participants | 155 (100%) |
| Function | |
| Sponsor-investigator | 55 (35.5%) |
| Investigator | 77 (49.7%) |
| Study nurse / Study coordinator | 16 (10.3%) |
| Other | 7 (4.5%) |
| CTU customer at … | |
| CTU Basel | 78 (41.9%) |
| CTU Bern | 24 (12.9%) |
| CTU Geneva | 8 (4.3%) |
| CTU Lausanne | 25 (13.4%) |
| CTU St Gallen | 24 (12.9%) |
| CTC Zurich | 27 (14.5%) |
| Median number of clinical studies performed by survey participants (IQR) | 6 (3, 15) |
| Total number of clinical studies performed | 295 (100%) |
| Type of clinical studies performed | |
| Clinical trials with medical products (HRA, ClinO) | 74 (25.1%) |
| Clinical trials with a medical device (HRA, ClinO) | 31 (10.5%) |
| Other clinical trials (HRA, ClinO) | 80 (27.1%) |
| Clinical trials of transplant products, gene therapy, or clinical trials of genetically modified or pathogenic organisms (HRA, ClinO) | 5 (1.7%) |
| Further use of biological material and health-related personal data for research (HRA, HRO) | 24 (8.1%) |
| Research involving measures for sampling of biological material or collection of health-related personal data from persons (HRA, HRO) | 43 (14.6%) |
| Studies with anonymous data (not under HRA) | 38 (12.9%) |
| Research involving deceased persons, embryos, and foetuses from induced abortions and from spontaneous abortions including stillbirths (HRA, HRO) | 0 (0.0%) |
Only the top three survey answers (ranked by number of respondents) are provided in figure 3, illustrated by the visual analogue scale. Ordinance on Clinical Trials in Human Research (Clinical Trials Ordinance, ClinO): A legal framework to regulate: the requirements for the conduct of clinical trials; the relevant authorisation and notification procedures for clinical trials; the duties and responsibilities of various bodies related to these procedures; and the registration of clinical trials and public access to the registry. Federal Act on Research involving Human Beings (Human Research Act, HRA): A federal Act of 2011 to protect the dignity, privacy and health of human beings involved in research. Ordinance on Human Research with the Exception of Clinical Trials (Human Research Ordinance, HRO): A legal framework to regulate the requirements for the conduct of human research projects (with the exception of clinical trials); and the related authorisation and notification procedures. Interquartile Range (IQR): The interquartile range is defined as the difference between the upper quartile (the highest 25%) and the lower quartile (the lowest 25%) of a data set.
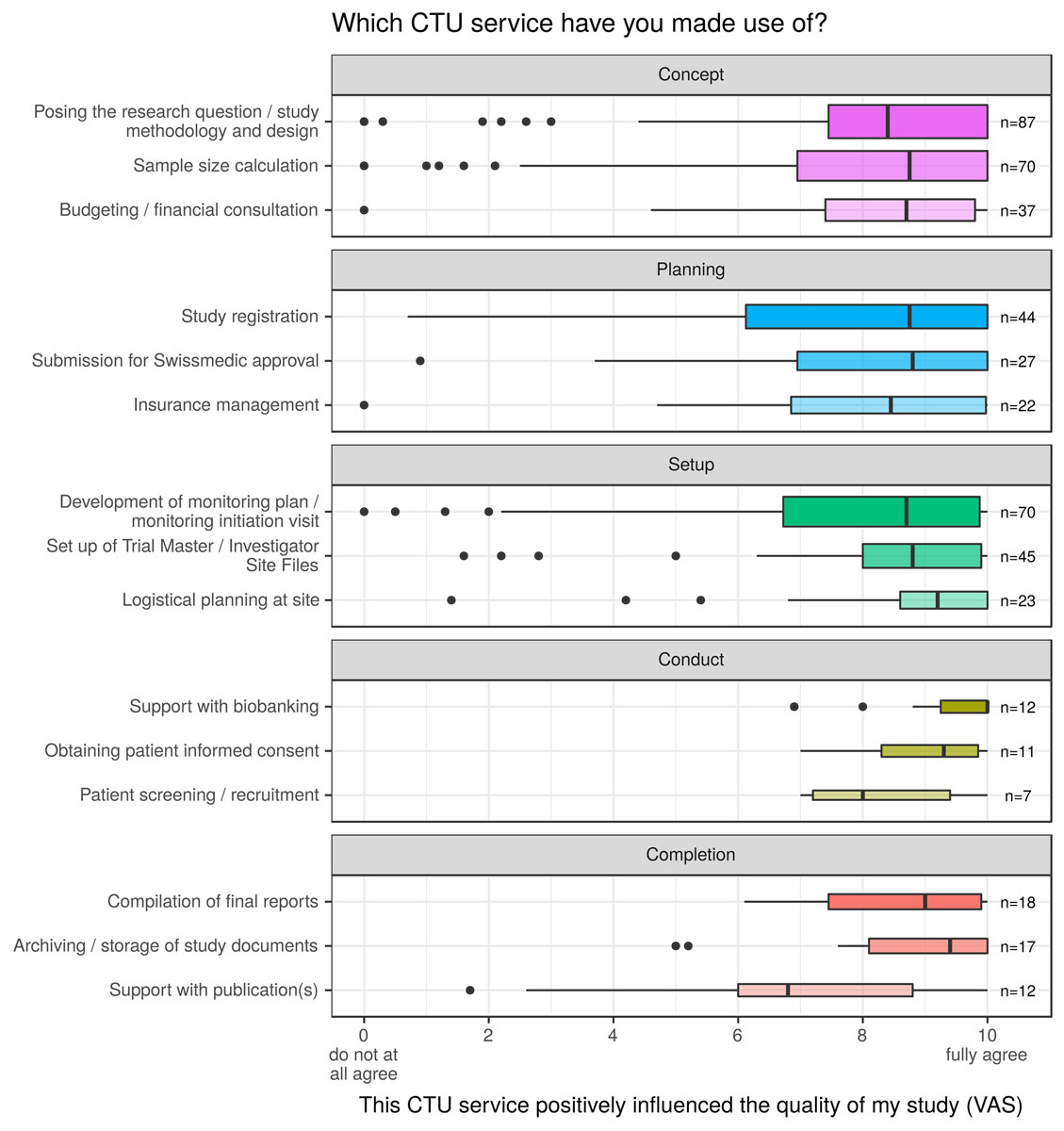
Figure 3 CTU customer survey responses regarding the impact of CTUs on the quality of the study performed (n = 155), by research stage.
The box spans the interquartile range of answers between 0 and 10; the thick vertical line represents the median. Whiskers indicate the most extreme values lying within the box edge and 1.5 times the interquartile range.
Although the feedback of stakeholders who are actively involved with the CTUs has been very positive, praise does not constitute a standardised measure of the actual impact of CTU services on the quality of research. A lack of an existing, common understanding of, and agreement on, the cornerstones that frame good clinical research, and a similar lack of practical guidance on how to enhance its value have been shown to inhibit efforts within the academic system [32].
Based on this, the CTU Basel has created an initiative to INcrease QUality In clinical REsearch (INQUIRE), designed to facilitate adoption of waste-reducing strategies at academic institutions. By applying a systematic survey of quality definitions, concepts, and criteria in the medical literature and on stakeholder websites from 12 countries [32], the CTU Basel, together with affiliated researchers, developed a comprehensive framework for clinical research quality. They conducted four rounds of an online Delphi process among seven stakeholder groups from 16 countries: patient organisations and representatives, academic researchers/initiatives, medical faculties and CTUs, governmental bodies, regulatory agencies, ethics committees, the pharmaceutical industry, and funding agencies. Delphi processes follow a structured communication technique through which experts answer questionnaires and receive an anonymised summary of all the experts' answers after each round, including any reasons provided [33, 34]. Thus, experts are encouraged to revise their earlier answers in light of the replies of other members on their panel. It is believed that during this process, the range of the answers will become narrower and the group will converge towards the “correct” answer.
In order to work in the national context, the INQUIRE framework had also been circulated among relevant Swiss stakeholder representatives. The Delphi process ultimately then achieved consensus on the structure and content of INQUIRE. INQUIRE addresses five study stages that were agreed on by the stakeholders and are similar to the SCTO study phases (concept, planning and feasibility, conduct, analysis and interpretation, reporting and knowledge translation). Its quality dimensions are: (1) protection of participants’ safety and rights, (2) relevance/patient-centredness and involvement, (3) minimisation of bias (internal validity), (4) precision (statistical validity), (5) transparency/access to data, and (6) generalisability (external validity) of study results. All six dimensions should ideally be fulfilled at all stages of a study, from conceptualising a research question to reporting the study’s results. Therefore, for each dimension, specific quality questions were formulated that are applicable to a particular study stage, and that can be used as guidance when assessing the quality of a study.
If all quality dimensions are covered, they will also ideally interact with two quality promoters: infrastructure, and sustainability and education. These promoters include an additional set of factors that may enhance the quality dimensions. For example, having well trained, motivated, and dedicated study staff on site is a prerequisite for high-quality research (infrastructure). Furthermore, high-quality study conduct becomes sustainable through educating, supporting, and mentoring study professionals to continue this practice (sustainability and education). In order to develop practicable tools, both for researchers and the institutions assessing the quality of clinical research, INQUIRE is being implemented at the Department of Clinical Research in Basel, as of November 2017. It will soon also be available for other CTUs, departments, academic institutions, and researchers, as well as other stakeholder groups. Once a harmonised quality assessment standard is applied across Switzerland, it will be possible to evaluate the actual impact of CTUs – and other service providers – on clinical research quality.
After the SCTO symposium, at which the INQUIRE concept was presented, the SCTO and affiliated institutions are now discussing a roadmap on how to implement the framework in academic practice (see fig. 4). Additionally, the SCTO continues to raise awareness on the discussion about research waste and value among its members, researchers, and the public.
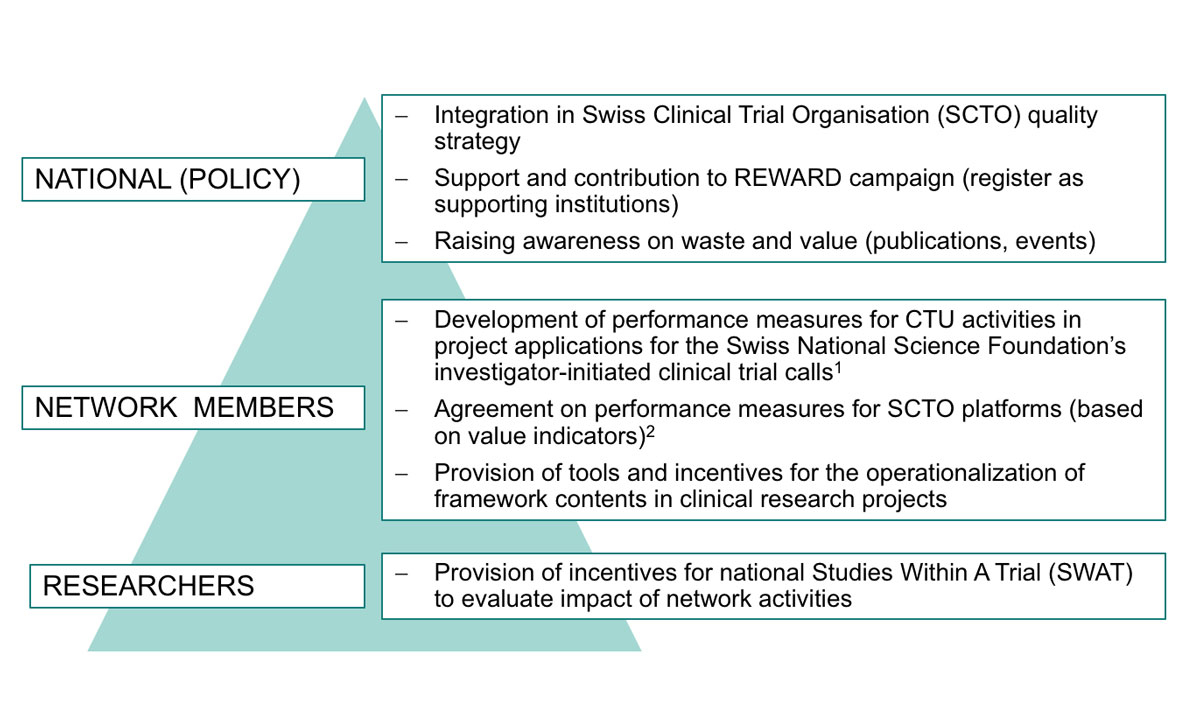
Figure 4 Roadmap on increasing value in Swiss academic clinical research.
1 The CTU Network and SCTO actively support the design, planning, and conduct of investigator-initiated studies that apply for the SNSF’s call for investigator-initiated clinical trials [35]. Integrating performance measures would facilitate a subsequent assessment of the CTU Network’s impact on the success of supported studies.
2 The SCTO Platforms are centres of excellence to provide support on specific areas of expertise, e.g. data management, statistics and methodology, and education and training, and are hosted by different CTUs.
The vast sums of public investment in academic clinical research have significantly influenced how clinical research quality is perceived by all major stakeholder groups. Future efforts should be made to quantify more objectively the impact of the CTU Network on defined value indicators. The consensus-based INQUIRE framework may be used as practical guide to operationalising the concept of value within the CTU Network. Activities to operationalise INQUIRE were underway at the national and local level, as of early 2018. They should ideally result in the development of standardised instruments that allow sources of inefficiency to be addressed and propose valid academic solutions. These instruments should build on and complement already existing tools and be integrated with methodological research projects, challenging their contribution to improve value and reduce waste. In a subsequent step, such an approach could be adapted by other key stakeholders in clinical research, thereby promoting an evidence-based research landscape in Switzerland.
We would like to thank Estelle Jobson from the Swiss Clinical Trial Organisation for editing and proofreading the manuscript.
Belinda von Niederhäusern (PhD) is employed by the Clinical Trial Unit (CTU) Basel. Christiane Pauli-Magnus is the head of the CTU, and the vice-president of the Swiss Clinical Trial Organisation (SCTO). Annette Magnin is the managing director of the SCTO.
1 Dorsey ER , de Roulet J , Thompson JP , Reminick JI , Thai A , White-Stellato Z , et al. Funding of US biomedical research, 2003-2008. JAMA. 2010;303(2):137–43 .https://doi.org/10.1001/jama.2009.1987
2 Moses H, 3rd , Matheson DH , Cairns-Smith S , George BP , Palisch C , Dorsey ER . The anatomy of medical research: US and international comparisons. JAMA. 2015;313(2):174–89 .https://doi.org/10.1001/jama.2014.15939
3 Ioannidis JP , Boyack KW , Klavans R . Estimates of the continuously publishing core in the scientific workforce. PLoS One. 2014;9(7):e101698 .https://doi.org/10.1371/journal.pone.0101698
4 Galsworthy MJ , Hristovski D , Lusa L , Ernst K , Irwin R , Charlesworth K , et al. Academic output of 9 years of EU investment into health research. Lancet. 2012;380(9846):971–2 .https://doi.org/10.1016/S0140-6736(12)61528-1
5 Heinig SJ , Dev A , Bonham AC . The U.S. Public’s Investment in Medical Research: An Evolving Social Contract. Am J Med Sci. 2016;351(1):69–76 .https://doi.org/10.1016/j.amjms.2015.10.016
6 Al-Shahi Salman R , Beller E , Kagan J , Hemminki E , Phillips RS , Savulescu J , et al. Increasing value and reducing waste in biomedical research regulation and management. Lancet. 2014;383(9912):176–85 .https://doi.org/10.1016/S0140-6736(13)62297-7
7 Chalmers I , Bracken MB , Djulbegovic B , Garattini S , Grant J , Gülmezoglu AM , et al. How to increase value and reduce waste when research priorities are set. Lancet. 2014;383(9912):156–65 .https://doi.org/10.1016/S0140-6736(13)62229-1
8 Chan AW , Song F , Vickers A , Jefferson T , Dickersin K , Gøtzsche PC , et al. Increasing value and reducing waste: addressing inaccessible research. Lancet. 2014;383(9913):257–66 .https://doi.org/10.1016/S0140-6736(13)62296-5
9 Glasziou P , Altman DG , Bossuyt P , Boutron I , Clarke M , Julious S , et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet. 2014;383(9913):267–76 .https://doi.org/10.1016/S0140-6736(13)62228-X
10 Ioannidis JP , Greenland S , Hlatky MA , Khoury MJ , Macleod MR , Moher D , et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet. 2014;383(9912):166–75 .https://doi.org/10.1016/S0140-6736(13)62227-8
11 Macleod MR , Michie S , Roberts I , Dirnagl U , Chalmers I , Ioannidis JP , et al. Biomedical research: increasing value, reducing waste. Lancet. 2014;383(9912):101–4 .https://doi.org/10.1016/S0140-6736(13)62329-6
12 Lo B . Serving two masters--conflicts of interest in academic medicine. N Engl J Med. 2010;362(8):669–71 .https://doi.org/10.1056/NEJMp1000213
13 Binder R , Friedli A , Fuentes-Afflick E . The New Academic Environment and Faculty Misconduct. Acad Med. 2016;91(2):175–9 .https://doi.org/10.1097/ACM.0000000000000956
14 Chen R , Desai NR , Ross JS , Zhang W , Chau KH , Wayda B , et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ. 2016;352:i637 .https://doi.org/10.1136/bmj.i637
15 Kasenda B , von Elm E , You J , Blümle A , Tomonaga Y , Saccilotto R , et al. Prevalence, characteristics, and publication of discontinued randomized trials. JAMA. 2014;311(10):1045–51 .https://doi.org/10.1001/jama.2014.1361
16 Meador KJ . Decline of clinical research in academic medical centers. Neurology. 2015;85(13):1171–6 .https://doi.org/10.1212/WNL.0000000000001818
17 Moher D , Glasziou P , Chalmers I , Nasser M , Bossuyt PM , Korevaar DA , et al. Increasing value and reducing waste in biomedical research: who’s listening? Lancet. 2016;387(10027):1573–86 .https://doi.org/10.1016/S0140-6736(15)00307-4
18 von Niederhäusern B , Schandelmaier S , Mi Bonde M , Brunner N , Hemkens LG , Rutquist M , et al. Towards the development of a comprehensive framework: Qualitative systematic survey of definitions of clinical research quality. PLoS One. 2017;12(7):e0180635 .https://doi.org/10.1371/journal.pone.0180635
19Interpharma: Fewer clinical trials 2017 [Internet]. [18 November 2017] Available from: http://www.interpharma.ch/fakten-statistiken/4539-fewer-clinical-trials.
20Akert K, Fey H, Gautier E. Bericht «Medizin Schweiz», Koordination der Forschungsfinanzierung: Standortbestimmung bestehender sowie Förderung der Bildung neuer Kompetenzzentren biomedizinischer Grundlagen-und klinischer Forschung. Bern: 1994.
21Schweizerische Wissenschafts- und Innovationsrat. In der Schweiz hat die klinische Forschung Mühe, mit der hohen Qualität der biomedizinischen Grundlagenforschung Schritt zu halten. 2002. Report 3/2002.
22 von Niederhäusern B , Fabbro T , Pauli-Magnus C . The role of Clinical Trial Units in investigator- and industry-initiated research projects. Swiss Med Wkly. 2015;145:w14161.
23Federal Offfice of Public Health. Master plan of the Federal Council, Boosting Switzerland as a biomedical research and technology centre. 2013.
24 Demotes-Mainard J , Kubiak C . A European perspective--the European clinical research infrastructures network. Ann Oncol. 2011;22(Suppl 7):vii44–9 .https://doi.org/10.1093/annonc/mdr425
25Swiss Clinical Trial Organisation SCTO. Guidelines for Good Operational Practice V2.0. 2013.
26Swiss Clinical Trial Organisation [Internet]. [16November 2017] Available from: https://scto.ch/de/news.html.
27Swiss Clinical Trial Organisation [Internet]. [17 February 2018]. Appendix 4: Training and Education in Clinical Research in Switzerland. Available from: https://scto.ch/de/publications/guidelines.html.
28 Grünig M , Weiss C , Meier-Abt P . Swissmedic durchleuchtet Clinical Trial Units. Schweiz Aerzteztg. 2012;93(03):54–5. doi:.https://doi.org/10.4414/saez.2012.16582
29Publications SCTO. SCTO Short Report 2016 [Internet]. [05 October 2017]. Available from: https://scto.ch/de/publications.html.
30Guidelines for Good Operational Practice V2. 0 2014 [Internet]. [06 February 2016]. Available from: http://www.eoc.ch/dms/site-eoc/documenti/documenti/SCTO20good20operational20practice.pdf.
31Swiss Clinical Trial Organisation [Internet]. Symposium 2017 [17 November 2017]. Available from: https://www.scto.ch/de/event-calendar/symposium/symposium-2017.html.
32 von Niederhäusern B , Schandelmaier S , Mi Bonde M , Brunner N , Hemkens LG , Rutquist M , et al. Towards the development of a comprehensive framework: Qualitative systematic survey of definitions of clinical research quality. PLoS One. 2017;12(7):e0180635. doi:.https://doi.org/10.1371/journal.pone.0180635
33 Sinha IP , Smyth RL , Williamson PR . Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med. 2011;8(1):e1000393 .https://doi.org/10.1371/journal.pmed.1000393
34 Hasson F , Keeney S , McKenna H . Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008–15.
35Programmes SNSF. Investigator initiated clinical trials (IICT) [Internet]. [04 October 2017] Available from: http://www.snf.ch/en/funding/programmes/iict/Pages/default.aspx.
Belinda von Niederhäusern (PhD) is employed by the Clinical Trial Unit (CTU) Basel. Christiane Pauli-Magnus is the head of the CTU, and the vice-president of the Swiss Clinical Trial Organisation (SCTO). Annette Magnin is the managing director of the SCTO.