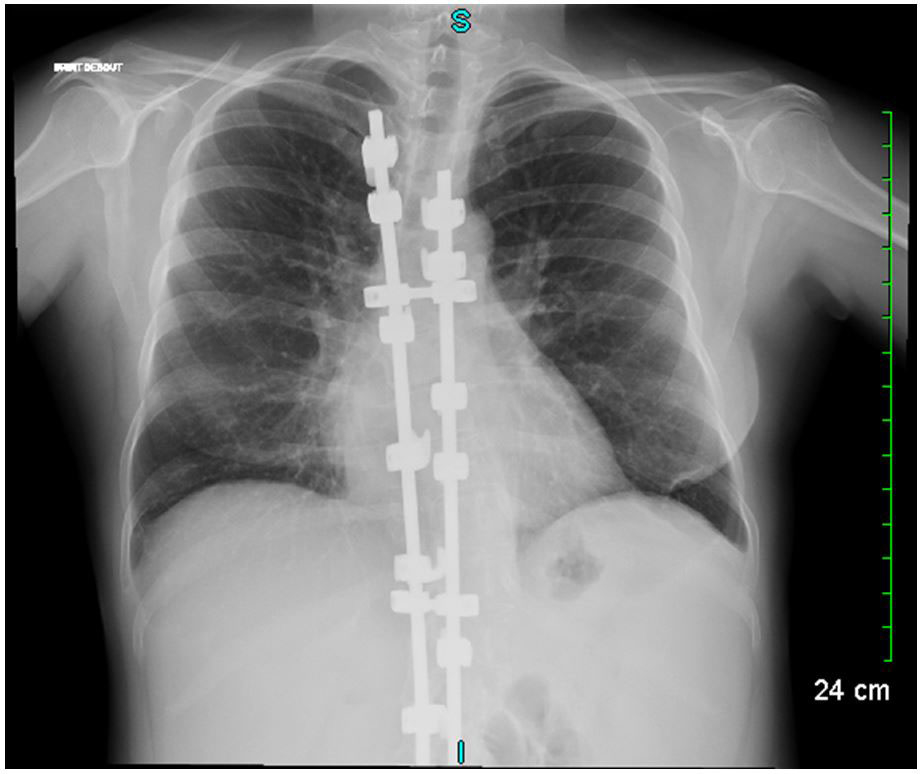
Figure 1 Chest X-ray showing scoliosis with spinal osteosynthesis and images of bronchiectasis.
DOI: https://doi.org/10.4414/smw.2018.14606
Cartilage hair hypoplasia (CHH), is a rare autosomal recessive ribosomopathy related to a mutation in the ribonuclease mitochondrial RNA processing (RMRP) gene, which encodes the mitochondrial portion of the RNA processing endonuclease [1]. Apart from its skeletal and integumentary system manifestations [2], CHH may also present with varied forms and intensities of haematopoietic and/or immune disorders [3–5]. These unfamiliar associations are little-known by the medical community, so the importance of distinguishing CHH from other causes of dwarfism may not be perceived.
Here we report the case of an initially insidious and then chemotherapy-aggravated primary immunodeficiency syndrome in the context of diffuse large B-cell lymphoma. This finally revealed CHH whose clinical presentation, excepting skeletal manifestations, was initially mild. We discuss the causal relation and molecular mechanisms existing between both primary immunodeficiency and lymphoma, those between chemotherapy cytotoxicity and aggravation of immune dysfunction, considering all these components in the background of initially unrecognised CHH.
A 27-year-old female was referred to our department for management of an autoimmune haemolytic anaemia. Recent medical history included a diffuse large B-cell lymphoma at the age of 20 with negative Epstein-Barr virus (EBV) serology. She was considered in remission after four courses of rituximab, adriamycin, cyclophosphamide, vincristine, bleomycin, prednisone and intrathecal methotrexate, and consolidation with 2 infusions of methotrexate followed by successful autologous stem cell transplantation. During and after chemotherapy she had many bacterial infections and two episodes of herpes zoster reactivation. Examination of her childhood medical history found neonatal dwarfism and scoliosis with spinal osteosynthesis (fig. 1A), recurrent acute pulmonary and ear, nose and throat (ENT) infections with otitis media from the ages of 3 to 10 years, requiring placement of tympanostomy tubes at the age of 6 and adenoidectomy. The patient described frequent bronchitis and sinusitis requiring antibiotic courses two or three times a year, complicated by bronchiectasis (fig. 1B).

Figure 1 Chest X-ray showing scoliosis with spinal osteosynthesis and images of bronchiectasis.
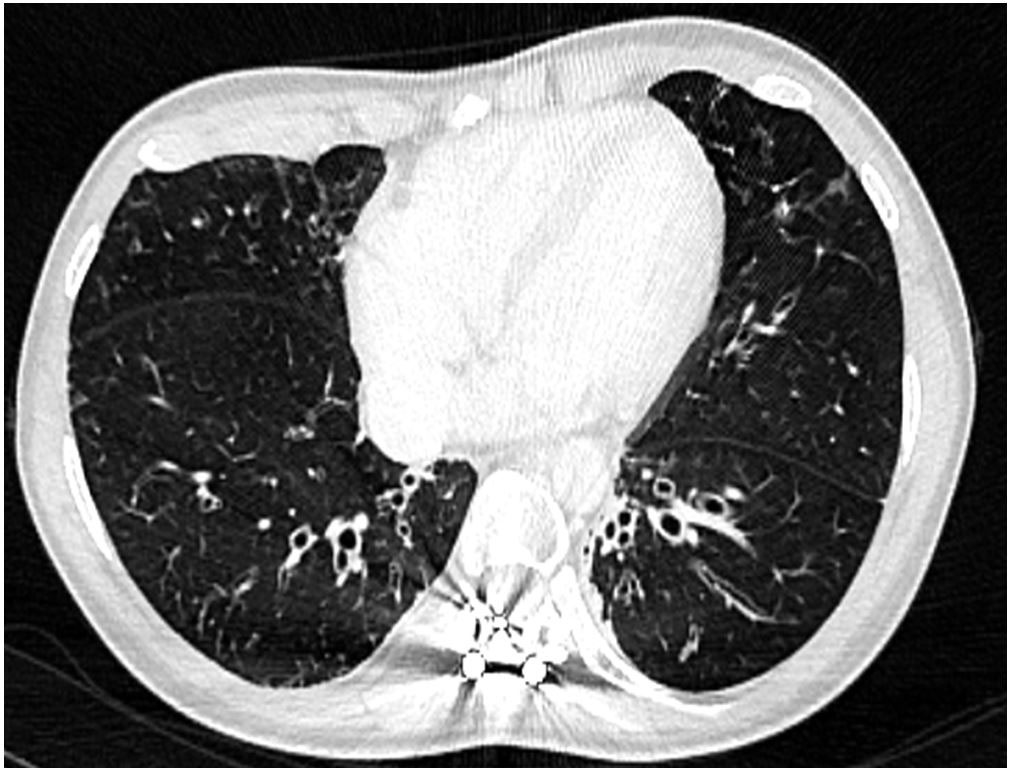
Figure 2 Thoracic computed tomography confirming the X-ray findings.
The clinical examination of the patient was unremarkable, except for short-limb dwarfism (height 130 cm, weight 42 kg), fine thin light hairs and mild hypermobility of large joints. Biological tests matched the criteria for autoimmune haemolytic anaemia, with positive Coombs tests for warm IgG and complement C3d marking, severe hypogammaglobulinaemia (2.0 g/l) and both B and CD4+ T cell lymphopenia. Retrospective measurement of gammaglobulin found that low levels (3.5–4 g/l) were present prior to chemotherapy. Figure 2 shows all previous and recent biological results. A low CD4 count of 209/mm3 was observed beginning at the age of 23 years (3 years after the chemotherapy), with a CD4/CD8 ratio greater than 1 and a normal total lymphocyte count. Monitored lymphocyte counts indicated a shift in the CD4/CD8 ratio to 0.6 and undetectable levels of CD19. Additionally, naïve T cells were undetectable, and the naïve B cell count was increased. On the basis of all the immunological findings, a primary immunodeficiency syndrome and a probable combined immunodeficiency syndrome were determined to have preceded the lymphoproliferative disease, and have been secondarily aggravated by chemotherapy. Finally, from the whole clinical picture, a diagnosis of cartilage hair hypoplasia, which is commonly associated with various immune dysfunction disorders, was made and confirmed with the identification of heterozygote mutation of the RMPR gene (substitutions 214 A>T, 230 C>T). The autoimmune haemolytic anaemia was then effectively treated with steroids as well as supportive intravenous immunoglobulin and oral prophylaxis with sulfamethoxazole-trimethoprim and valaciclovir.
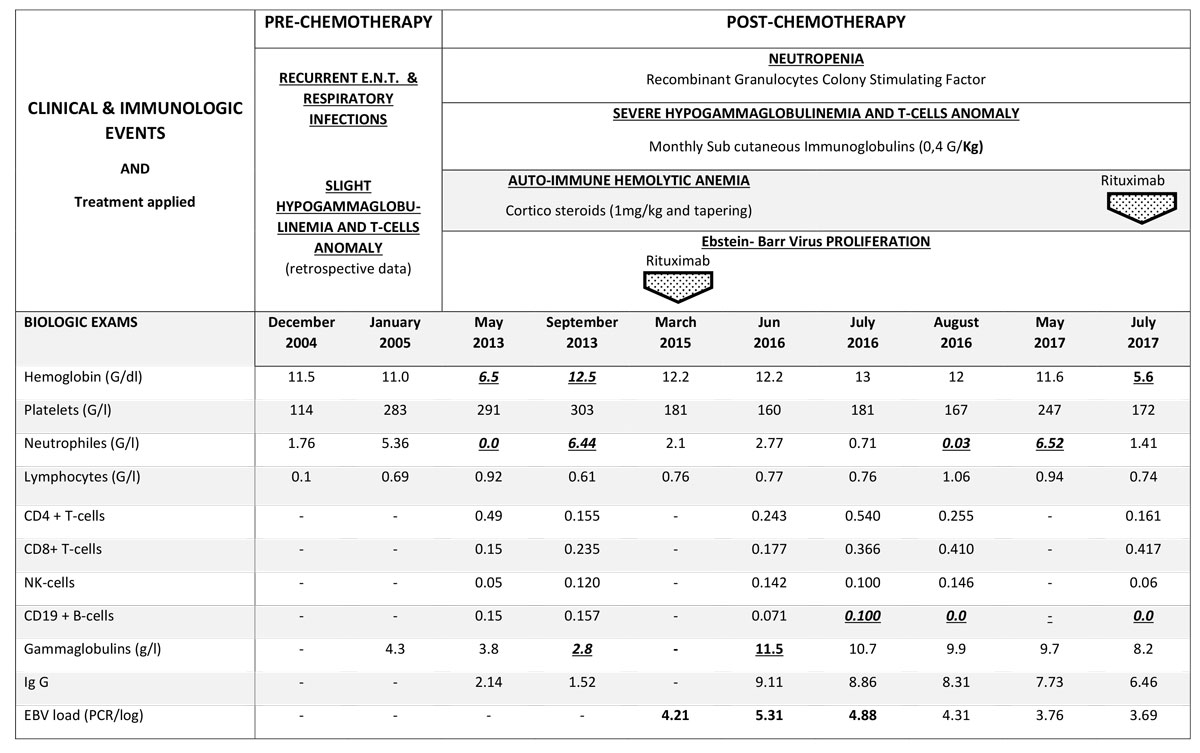
Figure 3 Clinical course and biological test results.
A primary EBV infection was found with a progressively increasing viral load, requiring treatment with anti-CD20 antibodies. She later experienced acute episodes of neutropenia and lymphopenia, without criteria for lymphoproliferation on osteomedullar biopsy, as well as a cutaneous sarcoidosis-like granulomatous disorder without positive tests for infectious causative agents, such as rubeola. Culture of medullar progenitors was not inhibited by the patient’s own serum, and the medullar karyotype remained normal. Ciclosporin treatment resulted in improvement of the granulomatous signs and neutropenia.
This case highlights the importance of screening for primary immunodeficiency syndromes and the value of considering a patient’s whole medical history, including marked skeletal disorders [2], in the context of a diagnosis of lymphoma. Primary immunodeficiency and lymphoproliferative disorders may share a similar pathogenic background [6], which can alter the treatment strategy for a successful therapeutic option, including allogenic bone marrow transplant, in the context of CHH [7].
CHH is pleiotropic in both its physical signs and resulting immune dysfunctions, and is associated with an increased predisposition to malignancies, especially non-Hodgkin’s lymphoma and basal cell carcinoma [6]. In our patient, the initial clinical presentation of immune dysfunction corresponded to that of a slight B-cell immunodeficiency, although retrospective blood analysis found some T-cell anomalies. Secondarily, after chemotherapy, the clinical presentation had features of combined immunodeficiency, with relapsing or persistent and complicated herpesviridae infections, granulomatous disorders [8], autoimmune events [5], some cytopenias with no proven immune dysfunction mechanisms. Immune dysfunction should be considered specifically in relation to the aggravated abnormal myelopoiesis in CHH. Some of the administered cytotoxic drugs could have played a role in the aggravated profile of both immunodeficiency and haematopoiesis in our CHH patient.
CHH is associated with chromosomal instability leading to alterations of the mitotic spindle checkpoint [1, 9], largely due to significantly increased of levels of cyclin B2 mRNA, as well as telomere dysfunction in various types of cells, including T cells [3, 8, 9]. Therefore, in addition to correction of the immunodeficiency [4, 10], reduction in the risk of relapse of autoimmunity [5] and lymphoproliferative disorders [6], mini-allografts or use of C19 CAR T cells allograft procedures that also take into account drug cytotoxicity may be considered first/second-line treatments in haematological manifestations of CHH [7, 11–13]. Early diagnosis of CHH / primary immune deficiency in childhood would have allowed her to receive an allograft, in order to avoid opportunistic infections, autoimmunity and prevent the known risk off lymphoma by correction of the T-cell defect in CHH [3], even though long term outcome after allografts in CHH patients remains unknown.
Finally, paediatric and adult specialists must be made aware of the important immuno-haematological aspects of CHH beyond its skeletal effects, which must be distinguished from other causes of small size. The diagnosis and full investigation must include the molecular characterisation, and management and follow up must be a collaboration with haematologists, immunologists and internists.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Ridanpää M , van Eenennaam H , Pelin K , Chadwick R , Johnson C , Yuan B , et al. Mutations in the RNA component of RNase MRP cause a pleiotropic human disease, cartilage-hair hypoplasia. Cell. 2001;104(2):195–203. doi:.https://doi.org/10.1016/S0092-8674(01)00205-7
2 Riley P, Jr , Weiner DS , Leighley B , Jonah D , Morton DH , Strauss KA , et al. Cartilage hair hypoplasia: characteristics and orthopaedic manifestations. J Child Orthop. 2015;9(2):145–52. doi:.https://doi.org/10.1007/s11832-015-0646-z
3 de la Fuente MA , Recher M , Rider NL , Strauss KA , Morton DH , Adair M , et al. Reduced thymic output, cell cycle abnormalities, and increased apoptosis of T lymphocytes in patients with cartilage-hair hypoplasia. J Allergy Clin Immunol. 2011;128(1):139–46. doi:.https://doi.org/10.1016/j.jaci.2011.03.042
4 Kostjukovits S , Klemetti P , Föhr A , Kajosaari M , Valta H , Taskinen M , et al. High prevalence of bronchiectasis in patients with cartilage-hair hypoplasia. J Allergy Clin Immunol. 2017;139(1):375–8. doi:.https://doi.org/10.1016/j.jaci.2016.07.023
5 Biggs CM , Kostjukovits S , Dobbs K , Laakso S , Klemetti P , Valta H , et al. Diverse Autoantibody Reactivity in Cartilage-Hair Hypoplasia. J Clin Immunol. 2017;37(6):508–10. doi:.https://doi.org/10.1007/s10875-017-0408-4
6 Taskinen M , Ranki A , Pukkala E , Jeskanen L , Kaitila I , Mäkitie O . Extended follow-up of the Finnish cartilage-hair hypoplasia cohort confirms high incidence of non-Hodgkin lymphoma and basal cell carcinoma. Am J Med Genet A. 2008;146A(18):2370–5. doi:.https://doi.org/10.1002/ajmg.a.32478
7 Bordon V , Gennery AR , Slatter MA , Vandecruys E , Laureys G , Veys P , et al.; Inborn Error Working Party of the European Bone Marrow Transplantation (EBMT) group. Clinical and immunologic outcome of patients with cartilage hair hypoplasia after hematopoietic stem cell transplantation. Blood. 2010;116(1):27–35. doi:.https://doi.org/10.1182/blood-2010-01-259168
8 McCann LJ , McPartland J , Barge D , Strain L , Bourn D , Calonje E , et al. Phenotypic variations of cartilage hair hypoplasia: granulomatous skin inflammation and severe T cell immunodeficiency as initial clinical presentation in otherwise well child with short stature. J Clin Immunol. 2014;34(1):42–8. doi:.https://doi.org/10.1007/s10875-013-9962-6
9 Aubert G , Strauss KA , Lansdorp PM , Rider NL . Defects in lymphocyte telomere homeostasis contribute to cellular immune phenotype in patients with cartilage-hair hypoplasia. J Allergy Clin Immunol. 2017;140(4):1120–1129.e1. doi:.https://doi.org/10.1016/j.jaci.2016.11.051
10 Gallimore CI , Lewis D , Taylor C , Cant A , Gennery A , Gray JJ . Chronic excretion of a norovirus in a child with cartilage hair hypoplasia (CHH). J Clin Virol. 2004;30(2):196–204. doi:.https://doi.org/10.1016/j.jcv.2003.10.007
11 Kainulainen L , Lassila O , Ruuskanen O . Cartilage-hair hypoplasia: follow-up of immunodeficiency in two patients. J Clin Immunol. 2014;34(2):256–9. doi:.https://doi.org/10.1007/s10875-013-9981-3
12 Horn J , Schlesier M , Warnatz K , Prasse A , Superti-Furga A , Peter H-H , et al. Fatal adult-onset antibody deficiency syndrome in a patient with cartilage hair hypoplasia. Hum Immunol. 2010;71(9):916–9. doi:.https://doi.org/10.1016/j.humimm.2010.06.002
13 Guggenheim R , Somech R , Grunebaum E , Atkinson A , Roifman CM . Bone marrow transplantation for cartilage-hair-hypoplasia. Bone Marrow Transplant. 2006;38(11):751–6. doi:.https://doi.org/10.1038/sj.bmt.1705520
AN and NMS contributed equally to this work
No financial support and no other potential conflict of interest relevant to this article was reported.