Gamma knife radiosurgery for arteriovenous malformations: general principles and preliminary results in a Swiss cohort
DOI: https://doi.org/10.4414/smw.2018.14602
Matthieu
Rabouda, Constantin
Tuleascaabc, Philippe
Maederad, Luis
Schiappacassee, Maud
Marguetf, Roy Thomas
Danielab, Marc
Levivierab
aUniversity of Lausanne, Faculty of Biology and Medicine, Lausanne, Switzerland
bDepartment of Clinical Neurosciences, Neurosurgery Service and Gamma Knife Centre, Lausanne University Hospital (CHUV), Lausanne, Switzerland
cSwiss Federal Institute of Technology (EPFL), Laboratory of Transmission Signal (LTS-5), Lausanne, Switzerland
dRadiology Department, CHUV, Lausanne, Switzerland
eRadiotherapy Department, CHUV, Lausanne, Switzerland
fInstitute of Radiation Physics, Lausanne, Switzerland
Summary
INTRODUCTION
Arteriovenous malformations (AVMs) are a type of vascular malformation characterised by an abnormal connection between arteries and veins, bypassing the capillary system. This absence of capillaries generates an elevated pressure (hyperdebit), in both the AVM and the venous drainage, increasing the risk of rupture. Management modalities are: observation, microsurgical clipping, endovascular treatment and radiosurgery. The former can be used alone or in the frame of a multidisciplinary approach. We review our single-institution experience with gamma knife radiosurgery (GKR) over a period of 5 years.
MATERIALS AND METHODS
The study was open-label, prospective and nonrandomised. Fifty-seven consecutive patients, benefitting from 64 GKR treatments, were included. All were treated with Leksell Gamma Knife Perfexion (Elekta Instruments, AB, Sweden) between July 2010 and August 2015. All underwent stereotactic multimodal imaging: standard digital subtraction angiography, magnetic resonance imaging and computed tomography angiography. We report obliteration rates, radiation-induced complications and haemorrhages during follow-up course.
RESULTS
The mean age was 46 years (range 13–79 years). The mean follow-up period was 36.4 months (median 38, range 12–75 months). Most common pretherapeutic clinical presentation was haemorrhage (50%). The most common Pollock-Flickinger score was between 1.01 and 1.5 (46%) and Spetzler-Martin grade III (46%). In 39 (60.1%) of cases, GKR was performed as upfront therapeutic option. The mean gross target volume (GTV) was 2.3 ml (median 1.2, range 0.03–11.3 ml). Mean marginal dose was 22.4 Gy (median 24, range 18–24 Gy). The mean prescription isodose volume (PIV) was 2.9 ml (median 1.8, range 0.065–14.6 ml). The overall obliteration rates (all treatments combined) at 12, 24, 36, 48 and 60 months were 4.8, 16.9%, 37.4, 63.6 and 78.4%, respectively. The main predictive factors for complete obliteration were: higher mean marginal dose (23.3 vs 21.0 Gy), lower GTV (mean 1.5 vs 3.5 ml) and absence of previous embolisation (at 60 months 61.8% prior embolisation compared with 82.4% without prior embolisation) (for all p <0.05). Eight (14%) patients experienced complications after GKR. Overall definitive morbidity rate was 3.1%. No patient died from causes related to GKR. However, during the obliteration period, one case of extremely rare fatal haemorrhage occurred.
CONCLUSION
Radiosurgery is a safe and effective treatment modality for intracranial AVMs in selected cases. It can be used as upfront therapy or in the frame of a combined management. Obliteration rates are high, with minimal morbidity. The treatment effect is progressive and subsequent and regular clinical and radiological follow-up is needed to evaluate this effect.
Introduction
Arteriovenous malformations (AVMs) are a type of vascular malformation characterised by abnormal connections between arteries and veins, bypassing the capillary system. The draining veins are dilated because of the arteriovenous shunt. Absence of capillaries generates an elevated pressure (hyperdebit), inside both the AVM and the venous drainage [1]. The high pressure transmitted to the venous system increases the risk of rupture and, in consequence, the risk of haemorrhagic events, as the vascular walls are not adapted to this type of blood flow. The hyperdebit can also create “blood steal phenomenon”, which can in turn generate additional clinical manifestations (epilepsy, other neurological deficits, etc.) [2]. Chronic high blood flow in arterial feeders may induce stenotic or dilated vessel changes with endothelial thickening, abnormal or absence media and elastic lamina, as well as intimal hyperplasia [3]. They are usually solitary, but can also occur as multiple lesions, within the frame of a syndrome, such as Rendu-Osler-Weber, Wyburn-Mason or Wyburn-Mason [4, 5]
The natural history and prevalence of AVMs are not completely understood. The estimated prevalence ranges between 14 and 18 cases per 100,000 [6, 7]. The main location is supratentorial (90%). The feeding artery is usually the middle cerebral artery, followed by the anterior and posterior cerebral arteries. Over time, AVMs may vary in size, increasing, decreasing or even disappearing unexpectedly [8]. The risk of spontaneous brain haemorrhage is 2 to 4% per year [9]. Moreover, ruptured AVMs are responsible for 38% of intracranial haemorrhages in patients aged between 15 and 45 years [10]. Morbidity rates vary, depending on the study, between 4 and 30% [11]. Clinical manifestations include haemorrhage (the most frequent, more than 50% of cases), epilepsy, symptomatic mass effect, ischaemia, neurological deficit or headaches [9, 12, 13].
Management of intracranial AVMs includes observation, microsurgical excision, endovascular treatment and radiosurgery. These treatment modalities can be used alone, or in the frame of multimodal management [9, 14]. Microsurgery directly approaches the AVM, coagulates and clippers the arterial pedicle, followed further by nidus ablation and exclusion of the abnormal drainage vein [15]. Endovascular treatment is based upon injection of embolisation agents (coils, glue, onyx) into the AVM. Radiosurgery is a noninvasive approach, which is being increasingly used and which results in progressive obliteration over a mean period of 2 years. The ideal radiosurgery targets are small to medium size, and for these it represents a safe primary treatment option. The adverse radiation effects are considered low. Five-year obliteration rates vary between 70 and80% [16–19]. A multidisciplinary approach is considered the key for optimal patient management.
The present report evaluates the therapeutic role and the outcomes (both clinical and radiological) of gamma knife radiosurgery (GKR), as primary or combined treatment for intracranial AVMs, during a period of 5 years in a single centre. We report the three main standard outcomes after GKR: obliteration rate, radiation-related complications and postherapeutic GKR haemorrhages.
Materials and methods
Study design
The study was prospective, open-label and nonrandomised. A case report form was created for the first treated case and prospectively filled in for each patient. It included both baseline (pretherapeutic) and follow-up data. Overall information encompassed demographic and dosimetric data, related to the radiosurgical approach.
Patients
All GKR treatments were performed during the period July 2010 and August 2015, in a single centre, Lausanne University Hospital (CHUV). The database was considered closed for new information in October 2017.
Fifty-seven consecutive patients (64 GKR treatments) were included, with variety of AVM stages (see classification below) and locations. The choice of radiosurgery was made after discussion within the interdisciplinary neurovascular board in the CHUV.
Inclusion criteria were: AVM documented by MRI, further and confirmed with digital subtraction arteriography (DSA); patient able to give free and informed consent; cases who benefitted from previous radiosurgery and/or other treatments, regardless of the techniques used (GK or other radiosurgery devices (ex. linear accelerator), microsurgical resection, endovascular approach)
Exclusion criteria were: patients who were not able to give an informed consent and cases lost for follow-up (only one case; taken into consideration for baseline pretherapeutic data only).
Gamma knife radiosurgery procedure
After application of the Leksell Model G stereotactic frame (Elekta Instruments AB, Sweden) under local anaesthesia, all patients underwent stereotactic imaging on the day of GKR. In our Gamma Knife Centre in Lausanne, we always use multimodal imaging for target definition. For AVMs, digital subtraction angiography (DSA), magnetic resonance imaging (MRI) and computed tomography (CT) angiography were used. MRI sequences routinely used Routinely used to better identify the AVM, were time of flight (TOF, 0.6 mm slices) and T1 without and with contrast-enhanced (1 mm thickness). Additional sequences, such as T2 star were employed if necessary to visualise possible previous haemorrhages. CT angiography with bolus contrast injection routinely supplemented the neuroimaging investigation in order to correct for any distortion errors on the MRI and to have complementary information for better targeting (e.g., nidus and complete angioarchitecture) definition (fig. 1).
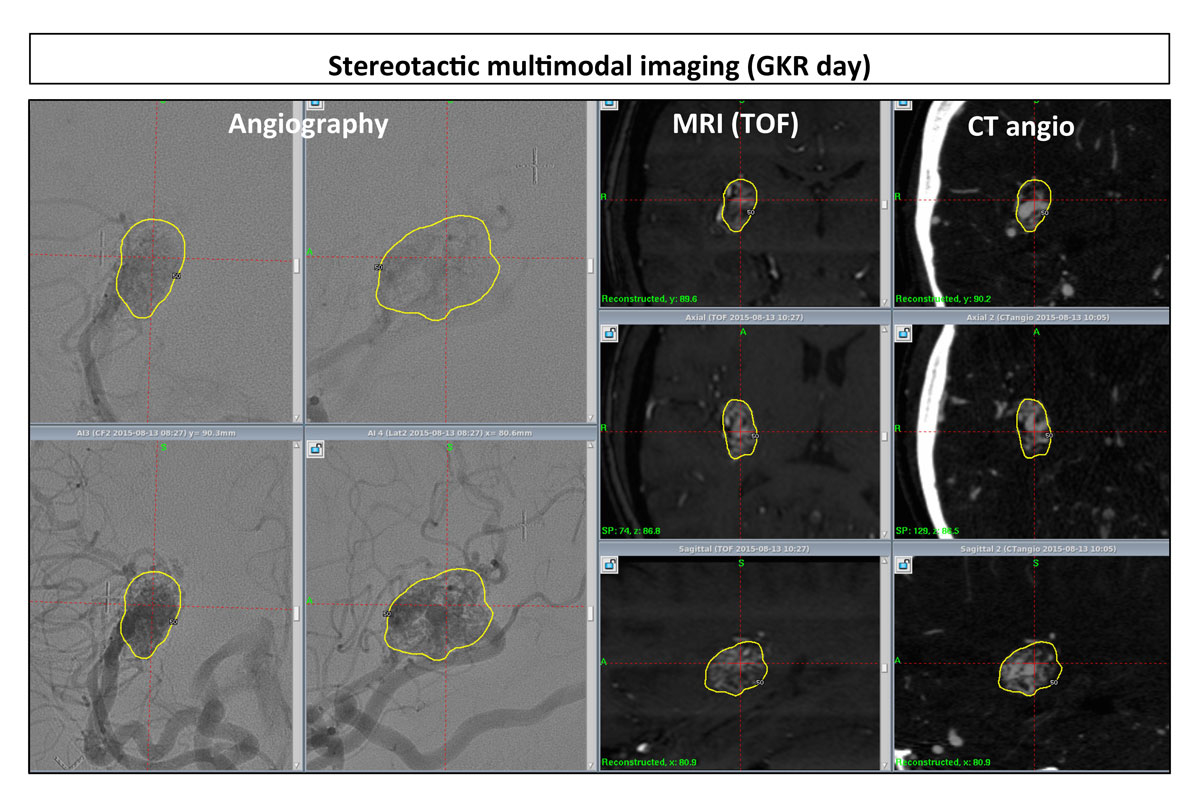
Figure 1 Example of multimodal imaging for gamma knife radiosurgery (GKR) purposes; all the MRI and CT examinations are done after stereotactic frame placement, the day of GKR; from left to right, DSA, MR time-of-flight (TOF) sequence and CT angiography (the former two in coronal-up, axial-middle, and sagittal-bottom reconstruction).
All patients underwent Leksell Gamma Knife Perfexion™ (Elekta Instruments AB, Sweden) by the same operators (ML, CT) within the specified timeframe. Leksell Gamma Plan (LGP version 10.0 and 11.0, Elekta Instruments AB, Sweden) was used for dosimetry planning.
Basic dosimetric data
The mean marginal prescribed dose was 22.4 Gy (median 24, range 18–24 Gy). The delivered dose was established mainly on the basis of the AVM volume and anatomical location. We usually prescribe 24 Gy if possible, based on the published literature within the past three decades, and on the safety and efficacy of radiosurgery in this indication [20].
Follow-up procedures
Data were collected between July 2010 and October 2017. Patients were seen in person at baseline (before therapy) and at 6, 12, 24, 36, 48 and 60 months after GKR, except for cases with clinical and/or radiological complications before these time-points.
Follow-up investigations included a medical consultation and radiological assessment with MRI angiography. If MRI suggested complete obliteration, cerebral DSA was performed. This represents the gold standard, in our setting, for formally confirming complete AVM obliteration. However, in some instances, in both Lausanne and in other Swiss referral hospitals that referred cases for GKR, in rare instances DSA was not done because the patient refused (a total of seven cases had MRI only without DSA for confirmation of complete obliteration).
Reported outcomes
We report the main following three outcomes after GKR: obliteration rate; radiation-induced complications, including new or worsened confirmation clinical symptomatology and/or new radiological findings (cerebral oedema, radionecrosis, cysts, etc.); post-GKR haemorrhages.
Standard classifications for pre- and post-therapeutic evaluations
The most common radiosurgical grading system for brain AVMs is the Pollock-Flickinger score [21]. It is based on three main factors: patient age, AVM volume and AVM location. The score is calculated with the following formula: (0.1 × volume in ml) + (0.02 ×age in years) + (0.5 × location [hemisphere / corpus callosum / cerebellum = 0; basal ganglia / thalamus / brain stem = 1]). The score predicts the success rate, mainly the potential for obliteration without secondary effects after GKR treatment.
The most common surgical grading system is the Spetzler-Martin score. The score takes into account the AVM size (small <3 cm; medium 3–6 cm; large >6 cm; 1, 2, 3 points, respectively), location (eloquent adjacent brain 1 point; non-eloquent 0 points) and the venous drainage pattern (superficial 0 points; deep 1 point). Eloquent brain areas include sensory, motor and visual cortex, language-dedicated areas (Broca and Wernicke areas), hypothalamus, thalamus, internal capsule, cerebral stem, cerebellar peduncles and nodes. The score predicts the surgical success rate and also evaluates other possible modalities of treatment (embolisation and radiosurgery).
Statistical analysis
Stata software (STATA version 11; STATA Corp., Texas, USA) was used for the statistical analysis. Kaplan-Meier analysis was used to evaluate the time-to-event for obliteration and complication rates. Patients’ data were censored at the last follow-up for those who did not have these events. For cases with an event, either obliteration or a complication, the date of the event was recorded and was considered as reference. An acceptable type I error was determined at 0.05 for all statistical tests. Univariate analyses included the two-sample t-test and the log-rank test.
Results
Basic clinical and demographic data
The median age was 46 years (range 13–79 years). The mean follow-up period was 38.2 months (median 38, range 12–75 months). The most common clinical presentation was haemorrhage (50%). The Pollock-Flickinger score was most commonly between 1.01 and 1.5 (46%) and the Spetzler-Martin grade III (46%). In 39 (60.1%) instances, there was no previous treatment and GKR was used as upfront therapy. Other basic demographic data can be found in table 1 and table 2.
Table 1 Demographic and clinical data before gamma knife radiosurgery treatment.
|
Variable
|
n (%)
|
| Sex (n = 64) |
Man |
32 (50.0) |
| Woman |
32 (50.0) |
| Age (years), median (range) |
46 (13–79) |
| Follow-up (months), median (range) |
38 (12–75) |
| Side (n = 64) |
Right |
32 (50.0) |
| Left |
32 (50.0) |
| Clinical presentation (n = 64) |
Haemorrhage |
32 (50.0) |
| Epilepsy |
12 (18.8) |
| Headaches |
6 (9.4) |
| Vertigo |
6 (9.4) |
| Incidental |
6 (9.4) |
| Hemisyndrome |
4 (6.2) |
| Tinnitus |
2 (3.1) |
| Trigeminal neuralgia, behavioural problems, transient neurological deficits, ophthalmological symptoms, aphasia, neck pain |
each with 1 (1.6) |
| Spetzler-Martin grade (n = 63) |
I |
9 (14.3) |
| II |
14 (22.2) |
| III |
29 (46.0) |
| IV |
7 (11) |
| V |
4 (6.3) |
| Modified Pollock-Flickinger score (n = 63) |
<1.0 |
17 (27.0) |
| 1.01–1.5 |
29 (46.0) |
| 1.51–2.0 |
11 (17.5) |
| >2.0 |
6 (9.5) |
| Vascularisation territory (n = 64, more than one possible) |
Right internal carotid |
25 (39.1) |
| Left internal carotid |
16 (25.0) |
| Right vertebrobasilar |
22 (34.4) |
| Left vertebrobasilar |
17 (26.6) |
| Right external carotid |
0 (0.0) |
| Left external carotid |
1 (1.6%) |
| Arterial pedicle (n = 64, more than one possible) |
Anterior cerebral artery |
15 (23.4) |
| Middle cerebral artery |
34 (53.1) |
| Posterior vascularisation |
33 (51.6) |
| Extracranial |
1 (1.6) |
| Previous treatment (n = 64, more than one possible) |
None |
39 (60.1%) |
| Surgery |
1 (1.6) |
| Embolization |
16 (25.0) |
| Radiosurgery: gamma knife |
2 (3.1) |
| Radiosurgery: linear accelerator |
7 (10.9) |
Table 2 Demographic and clinical data after gamma knife treatment: obliterated versus non-obliterated arteriovenous malformations.
|
Variable
|
|
Obliterated
n (%)
|
Non- obliterated
n (%)
|
| Sex |
|
(n total = 39) |
(n total = 25) |
| Man |
16 (41.0) |
16 (64.0) |
| Woman |
23 (59.0) |
9 (36.0) |
| Age (years), median (range) |
|
47 (18–79) |
34 (13–76) |
| Follow-up (months), median (range) |
|
38 (12–60) |
41.5 (12–71) |
| Obliteration time (months), median (range) |
|
35 (8–56) |
– |
| Side |
|
(n total= 39) |
|
| Right |
21 (53.8) |
11 (44.0) |
| Left |
18 (46.2) |
14 (56.0) |
| Spetzler-Martin Grade |
|
(n total = 38) |
(n total = 25) |
| I |
7 (18.4) |
2 (8.0) |
| II |
13 (34.2) |
1 (4.0) |
| III |
17 (44.7) |
12 (48.0) |
| IV |
1 (2.6) |
6 (24.0) |
| V |
0 (0) |
4 (16.0) |
| Modified Pollock-Flickinger score |
|
(n total = 38) |
(n total = 25) |
| <1.0 |
12 (31.6) |
5 (20.0) |
| 1.01–1.5 |
18 (47.4) |
11 (44.0) |
| 1.51–2.0 |
5 (13.2) |
6 (24.0) |
| >2.0 |
3 (7.9) |
3 (12.0) |
| Clinical presentation (more than one possible) |
|
(n = 39) |
(n = 25) |
| Haemorrhage |
16 (41.0) |
16 (64.0) |
| Headaches |
6 (15.4) |
– |
| Incidental |
6 (15.4) |
– |
| Epilepsy |
5 (12.8) |
7 (28.0) |
| Vertigo |
5 (12.8) |
1 (4.0) |
| Tinnitus |
2 (5.1) |
– |
| Behavioural problems |
1 (2.6) |
– |
| Hemisyndrome |
1 (2.6) |
2 (8.0) |
| Trigeminal neuralgia |
1 (2.6) |
– |
| Neck pain |
1 (2.6) |
– |
| Ophthalmological symptoms |
1 (2.6) |
– |
| Aphasia |
1 (2.6) |
– |
| Transient neurological deficits |
– |
1 (4.0) |
| Vascularisation territory (more than one possible) |
|
(n total = 39) |
(n total = 25) |
| Right internal carotid |
14 (35.9) |
11 (44.0) |
| Left internal carotid |
10 (25.6) |
6 (24.0) |
| Right vertebrobasilar |
14 (35.9) |
8 (32.0) |
| Left vertebrobasilar |
8 (20.5) |
9 (36.0) |
| Right external carotid |
0 (0) |
0 (0) |
| Left external carotid |
1 (2.6) |
0 (0) |
| Arterial pedicle (more than one possible) |
|
(n = 39) |
(n = 25) |
| Anterior cerebral artery |
8 (20.5) |
7 (28.0) |
| Middle cerebral artery |
20 (51.3) |
14 (56.0) |
| Posterior vascularisation |
17 (43.6) |
16 (64.0) |
| Extracranial |
1 (2.6) |
0 (0) |
| Previous treatment (more than one possible) |
|
(n total = 39) |
(n total = 25) |
| None |
28 (71.8) |
11 (44.0) |
| Surgery |
6 (15.4) |
0 (0) |
| Embolisation |
1 (2.6) |
10 (40.0) |
| Radiosurgery |
|
|
| Radiosurgery: gamma knife |
0 (0) |
2 (8.0) |
| Radiosurgery: linear accelerator |
5 (12.8) |
2 (8.0) |
Basic dosimetric data
The mean gross target volume was 2.295 ml (median 1.2, range 0.032–11.3 ml). Mean marginal dose was 22.4 Gy (median 24, range 18–24 Gy). The mean prescription isodose volume was 2.984 ml (median 1.77, range 0.065–14.6 Gy). Other basic dosimetric data, including standard radiosurgical indexes, can be found in table 3.
Table 3 Gamma knife radiosurgery dosimetric data (n total = 64).
| Target volume (cm3) |
Mean |
2.295 |
| Median |
1.2 |
| Range |
0.032–11.3 |
| Prescription isodose volume (cm3) |
Mean |
2.984 |
| Median |
1.770 |
| Range |
0.065–14.6 |
| Conformity index |
Mean |
0.979 |
| Median |
0.991 |
| Range |
0.660–1.000 |
| Selectivity |
Mean |
0.677 |
| Median |
0.718 |
| Range |
0.118–0.969 |
| Gradient index |
Mean |
2.718 |
| Median |
2.695 |
| Range |
0.270–4.351 |
| Marginal dose (Gy) |
Mean |
22.406 |
| Median |
24 |
| Range |
18–24 |
Obliteration rates after GKR
For 39 (61%) GKR treatments, there was complete obliteration after a mean period of 32.9 months (median 35, range 8–56 months). The mean follow-up period in the partially obliterated AVM group was higher, at 40.7 ± 8.5 months (range 12–71 months), as compared with complete obliteration 32.9 ± 3.7 months (range 8–56 months). However, the partially obliterated group included more complicated cases, including previous embolisation, larger size with staged-treatments, etc.
The overall obliteration rates at 12, 24, 36, 48 and 60 months were 4.8, 16.9, 37.4, 63.6 and 78.4%, respectively (figs 2 and 3a
). DSA confirmed complete obliteration in 30 (76.9%) out of the 39 cases. Two patients will have DSA evaluation in the next 3 months. The other seven had only MRI angiography confirmation, because they refused DSA evaluation for various reasons.
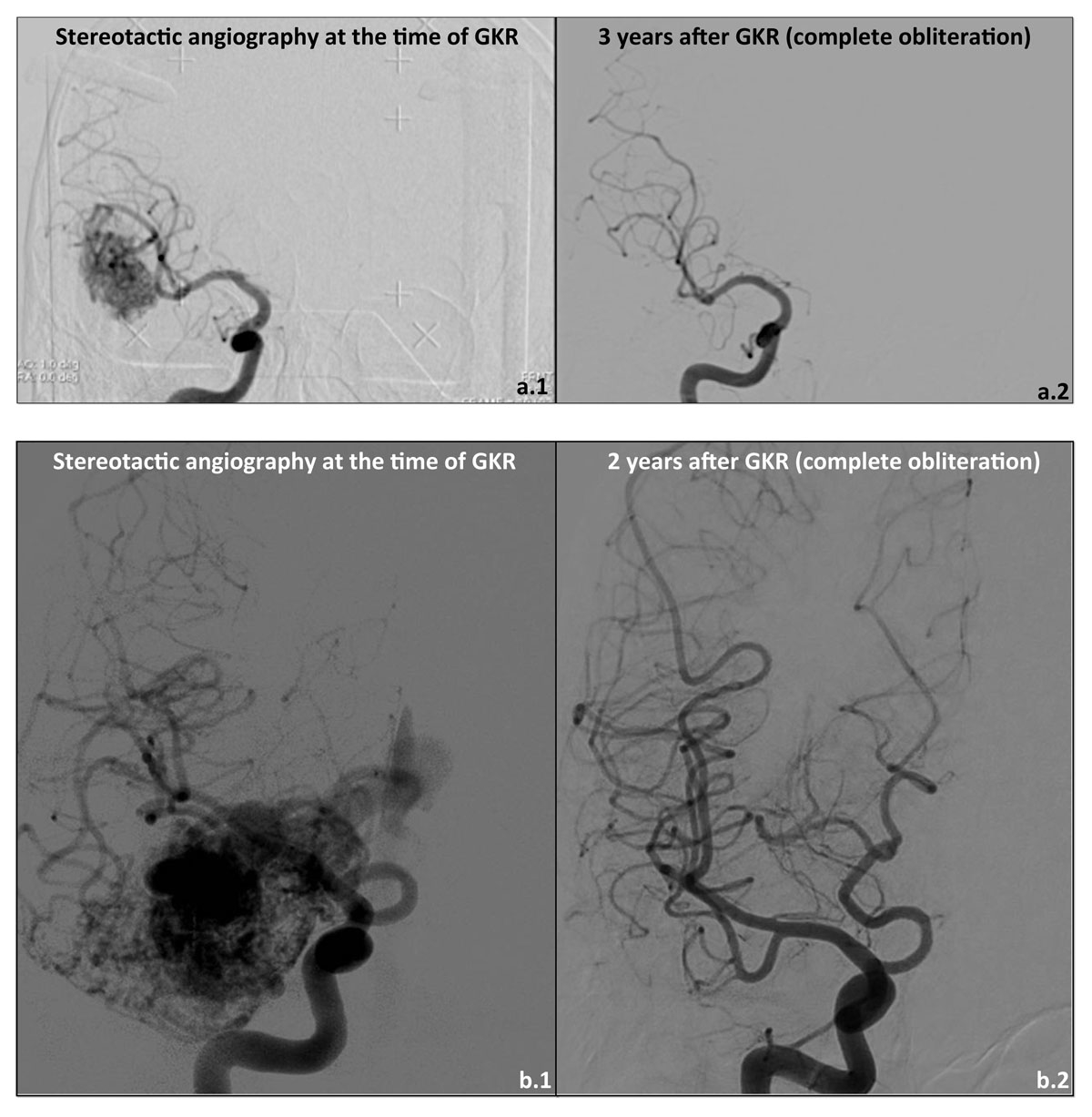
Figure 2 Case illustration 1 (single fraction radiosurgery 24 Gy at the 50% isodose line; images a1 and a2): 50-year-old patient with a right temporal AVM, Spetzler-Martin grade II, fed by temporal branches of the inferior sylvian trunk, with both superficial and deep venous drainage; clinical discovery after initial seizure; (a1) initial DSA, and (a2) DSA 3 years after treatment, showing complete obliteration; clinically, disappearance of seizures.
Case illustration 2 (“staged-volume” radiosurgery, 20 Gy at the 50% isodose line, at 9 months interval; images b1 and b2): 52-year-old patient, presenting with large right temporo-insular AVM, Spetzler-Martin grade III, fed both by the middle and posterior cerebral arteries, with an important afferent component by the anterior choroid artery, with both superficial and deep venous drainage; clinical discovery after initial seizure; (b1) initial DSA, and (b2) DSA 2 years after treatment, showing complete obliteration; clinically, disappearance of seizures.
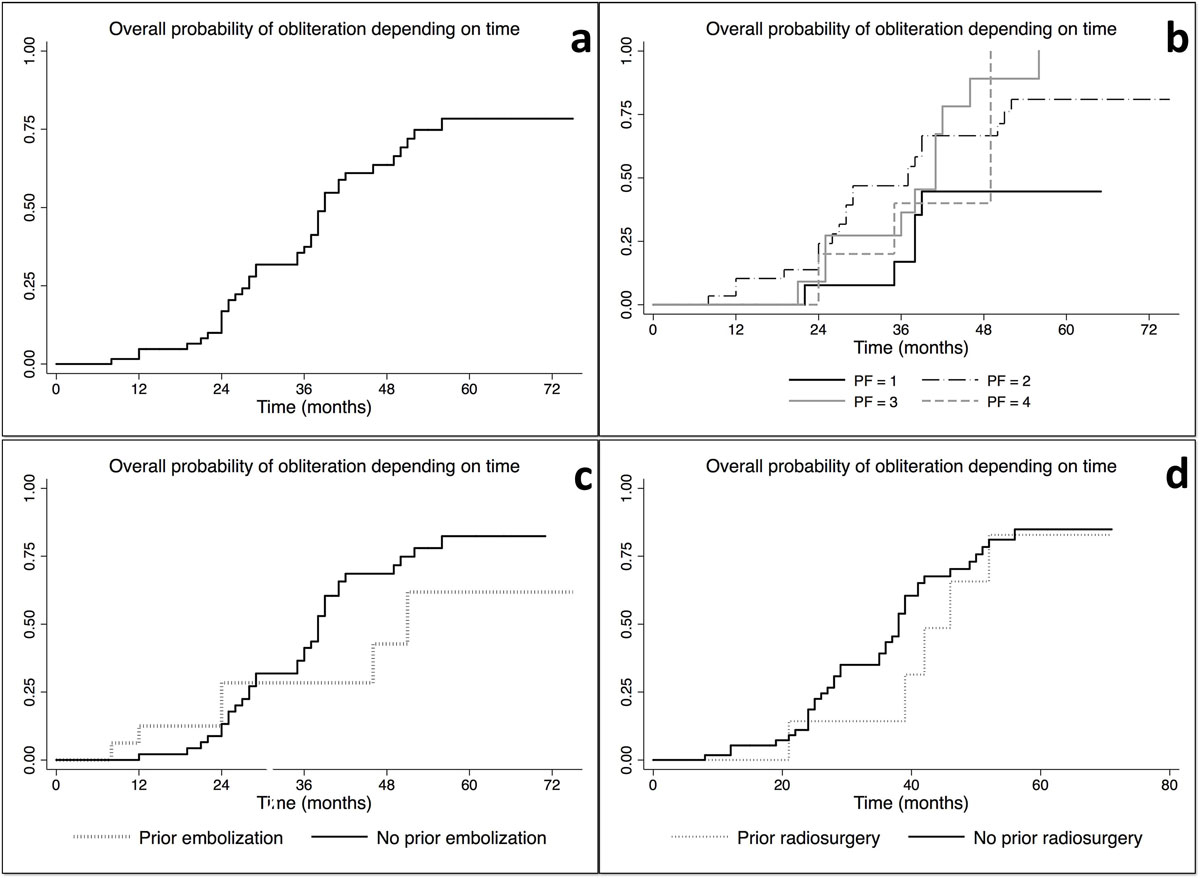
Figure 3 Kaplan-Meier curves showing: (a) the overall probability of obliteration over time; (b) the overall probability of obliteration by Pollock-Flickinger score; (c) the overall probability of obliteration by prior embolisation versus no prior embolisation (p <0.05); (d) the overall probability of obliteration by prior radiosurgery versus no prior radiosurgery (p >0.05).
The overall obliteration rates, by Pollock-Flickinger scores at 12, 24, 36 and 48 months, respectively, (see fig. 3b) were:
- for a score of 1: 0, 7.7, 16.9, 44.6%, staying stable up to 60 months (mean follow-up period 31.2 months, range 12–51 months, mean obliteration time 29.5 months; range 12–51 months);
- for a score of 2: 10.3, 24.1, 54.5 and 71.4%, reaching 80.9% at 60 months (mean follow-up period 37.9 months, range 12–66 months, mean obliteration time 34.7 months, range 21–50 months);
- for a score of 3: 0, 27.3, 45.4 and, 89.1%, reaching 100% at 56 months (mean follow-up period 46.2 months, range 13–75, mean obliteration time 35 months range 26–56 months);
- for a score of 4: 0, 20 and 40%, reaching 100% at 49 months (mean follow-up period 50.4 months, range 35–65 months, mean obliteration time 40.7 months, range 35–52 months).
The main predictive factors for complete obliteration were: higher mean marginal dose (23.3 vs 21.0 Gy), lower gross target volume (1.5 ml, range 0.03–5.55 ml vs 3.5 ml, range 0.06–11.3ml) and no previous embolisation (at 60 months 61.8 vs 82.4%) (p<0.05 for all; fig. 3c).
Previous other radiosurgical treatment was not a statistically significant factor with regard to final obliteration rates (at 60 months, 82.9 compared with 84.9%, p >0.05; fig. 3d). Five of the seven cases (71.4%) were obliterated (mean follow-up time 44 months, range 24–71 months compared with 37.5 months, range 12–75 months).
Clinical and/or radiological complications (including haemorrhage) after GKR
Nine (14%) patients experienced complications after GKR (see table 4). The actuarial complication rate was 8% at 1 year and was 9% at 16 months, remaining stable over time until last follow-up.
Table 4 Complications related to gamma knife radiosurgery treatment (including haemorrhages during the preobliteration period).
|
Type of complication
|
n total = 9/64 (14%)
n (%)
|
| Haemorrhage |
3 (4.7) |
| Ischaemic stroke |
1 (1.6) |
| Ventriculo-peritoneal shunt placement for high intracranial pressure |
1 (1.6) |
| Growth of previous cyst |
1 (1.6) |
| Important perilesional oedema |
1 (1.6) |
| Superior sagittal sinus partial thrombosis |
1 (1.6) |
Three cases (4.7%) had haemorrhage during the follow-up period after GKR. One died, one presented few symptoms and was followed-up with serial MRI (progressive resorption) and one underwent microsurgical resection with a slowly favourable clinical outcome.
The following complications occurred, each in one patient (1.6%): ischaemic stroke; ventriculo-peritoneal shunt placement for high intracranial pressure (12 months after GKR); volumetric growth of a previous asymptomatic cyst; important perilesional oedema; and superior sagittal sinus partial thrombosis. The ischaemic thalamic stroke occurred in a young patient, was clinically symptomatic, and was treated with embolisation, followed by decompression craniectomy and further right partial temporal lobectomy. One case had benefitted previously from a Linear Accelerator (Linac) radiosurgery treatment, 4 years before GKR, with incomplete obliteration. This patient had a transient asymptomatic increase of cysts present after Linac radiosurgery, at 6, 12 and 18 months after GKR; their volume decreased at 24 months, when the AVM was completely obliterated. In one case, there was major perilesional oedema at 6 and 12 months, which disappeared at 24 months, and the patient showed progressive clinical improvement on prolonged corticosteroid therapy. The patient with an asymptomatic partial thrombosis of the superior sagittal sinus was given anticoagulant therapy; 2 years after GKR and 12 months after the start of this therapy, there was complete resolution.
Discussion
Our study analysed the place of GKR, as an upfront or adjuvant treatment, in the upfront and/or multimodal management of brain AVMs. We reported overall obliteration rates at 12, 24, 36, 48 and 60 months of 4.8, 16.9, 37.4, 63.6 and 78.4%, respectively. Furthermore, in the cases without prior embolisation, the 5-year obliteration rate was as high as 82.4%. Eight (14%) patients experienced complications after GKR, most of them transient. Three cases (5.2%) experienced haemorrhage during the follow-up period after GKR, of whom one died. These reported outcomes are in perfect alignment with the current literature.
An important consideration in the decision-making process is the natural history of the AVM. The choice of observation or medical management needs to take into account several factors. Without any treatment, haemorrhage risk is estimated to be approximately 3 to 4% per year; this increases substantially if the venous drainage is exclusively deep, in presence of venous stenosis, or if there is an intranidal aneurysm. Morbidity rate is approximately 2.7% per year and mortality rates at approximately 1%. Overall assessment in the form of the Spetzler-Martin grade indicates surgery for grades I to IIIA, radiosurgery for IIIB to V and combined management for giant AVMs (volume >25 cc). In a recent meta-analysis, which included observational studies, severe complications were observed in 5.1 to 7.4% of patients and median obliteration rates were 13 to 96% [22]. The ARUBA study, designed to answer the question of observation and medical management alone versus medical management plus prophylactic interventional therapy, was stopped because of increased complications rates in the treatment arm compared with the medical management group [23, 24]. Typically, observation and medical management is considered for asymptomatic patients and those without previous haemorrhage.
Treatment strategies may be used alone or in the frame of multimodal therapy. They include microsurgery, endovascular treatment and GKR.
Surgery requires craniotomy and dural opening. Overall mortality rates are approximately 3.3% and morbidity 8.6% (increasing with increase of the Spetzler-Martin grade) [25].
Endovascular treatment has become increasingly used with the advances in this technology, including new microcatheter designs and the development of solid and liquid embolic agents. In a recent meta-analysis, the fatality rate was estimated to be 0.96 per 100 person years and haemorrhage rates 1.7 per 100 person years [22]. Complications leading to permanent neurological deficits or death were seen in 6.6% of cases [22].
AVMs were among the first GKR indications, with a first treatment in Sweden in the early 1970s. This was directed by use of the standard imaging technique, DSA, which already allowed optimal targeting, even before the appearance of MRI in the early 1980s [26]. The physiopathological mechanisms of obliteration after GKR are related to structural changes within the endothelial cells, followed by myofibroblast proliferation, expansion of the extracellular matrix into the intima and further hyaline transformation of the wall of the irradiated vessels. This occurs mainly in two steps, one related to degeneration/proliferation of the vascular wall and the second involving changes within the connective tissue [27, 28].
The crucial factor for successful obliteration with GKR is targeting. This is based upon multimodal imaging, which should include, in our opinion and experience, cranial DSA in addition to the widely used MRI and CT angiography. The definition of the nidus, even in experienced hands, is still sometimes difficult and should be done in collaboration with an experienced neuroradiologist. It is nowadays considered that the foot of the vein should be included in the dosimetry [29]. However, this does not apply to the entire trajectory of the draining vein. In particular, irradiating large parts of the former should be avoided, as this might provoke a collapse of this anatomical structure before the nidus itself has been entirely obliterated, leading to higher blood pressure on the nidus wall and ultimately resulting in accidental haemorrhage. Moreover, in clinical practice, even with use of multimodal imaging (DSA included), draining veins are complicated in their structure, making an accurate delineation and further separation from the target volume difficult [30].
For all Spetzler-Martin grades combined, the overall obliteration rate by surgery is 97%, with a mortality of around 3.3% and a morbidity of 8.6% [31]. Microsurgical treatment completely obliterates high-grade AVMs in 57 to 100% of cases, with non-negligible mortality rates of between 0 and 11% and higher morbidity rates of between 4 and 85% (higher for higher Spetzler-Martin grades III–V) [32]. Combined endovascular and surgical treatment allows higher obliteration rates, of between 83 and 100%, with a complication rate of 15.4% [33, 34].
With embolisation alone, AVM volume reduction is achieved in 79.5% and complete obliteration in 20% of cases, with a complication rate of 12% (range 8–40%) [35]. It is estimated that 40% of AVMs can be treated with embolisation alone, with a morbidity and mortality rate of 1.3% (range 2–7%), depending on the AVM characteristics [36].
With radiosurgery and surgery combined, the overall obliteration rate is 33%. It is nowadays considered that treatment with radiosurgery alone, without prior embolisation, gives a higher probability of obliteration [20]. This is related to several factors, including that combined treatment is used for more complex AVMs, difficult to cure by only one treatment modality. In this context, multimodal management offers a real possibility for cure. Furthermore, there are more radio-induced changes in previously embolised AVMs than in those having had an upfront radiosurgical treatment (43 vs 33%). On the other hand, radiation-induced clinical deficits are lower for the AVMs not previously embolised [20].
In our experience, 70.6% of AVMs with a modified Pollock-Flickinger score equal to or less than 1.0 were obliterated (mean follow-up time 31.2 months, median 27 months, range 12–51 months); obliteration rates were 62.1% for scores between 1.01 and 1.5 (mean follow-up time 34.7 months, median 39 months, range 12–66 months), 45.5% for scores between 1.51 and 2.0 (mean follow-up time 46.2 months, median 47 months, range 13–75 months), and 50% for scores more than 2.01 (mean follow-up time 50.4 months, median 52 months, range 35–65 months). The obliteration rates were higher for low-score AVMs, which was expected and in accordance with previously published data. The mean obliteration time was 29.5 months for low scores, less than or equal to 1.0 (median 28, range 12–51 months). For scores between 1.01 and 1.5, this time increased to 34.7 months (median 38, range 21–50 months) and it further increased for scores between 1.51 and 2.0 at 35 months (median 28, range 26–56 months). Moreover, for scores more than 2.0, the mean obliteration time was even higher, at 40.7 months (median 35, range 35–52 months).
For Spetzler-Martin grades I and II, our obliteration rates were high, at 77.9% (mean follow-up time 33.3, median 34, range 12–56 months) and 92.7%, respectively, (mean follow-up time 36.4, median 39.5, range 12–52 months). The mean obliteration times were quite similar (33.3 vs 36.4 months). For higher Spetzler-Martin grades, obliteration rates decreased to 58.6% for grade III, 14.3% for grade IV and 0.0% for grade V. The mean follow-up periods for these grades were 39.6 months (median 38.5, range 15–75), 45 months (median 44, range 12–67 months) and 39.5 months (median 39.5, range 24–54 months), respectively. The mean obliteration times were 31.1 months for grade III (median 35, range 12–41 months) and 37 months for grade IV (only one case obliterated). No grade V AVM was obliterated.
Our study has several potential limitations. The first is the low number of cases. The second is the relatively short follow-up period, if the mean delay of obliteration after radiosurgery is taken into account; this varies across the studies, but is generally considered to be 2 to 3 years for small to medium size AVMs. The third is the retrospective design.
Radiosurgery is safe and effective as part of a multimodal management of intracranial AVMs, as upfront treatment, or in the frame of combined treatment associated with other modalities (e.g., microsurgical clipping, endovascular). Overall obliteration rates were as high as 80% at 5 years in the present study, in close agreement with what has already been described in the literature. Complications were rare, up to 14% at 5 years, and were transient in the vast majority of cases. The mortality due to radiosurgery itself is 0%. However, during the obliteration period, haemorrhages may still occur until complete AVM cure.
Acknowledgements
Lausanne University Hospital and University of Lausanne
Author contributions
Matthieu Raboud and Constantin Tuleasca contributed equally to the study.
References
1
Solomon
RA
,
Connolly
ES, Jr
. Arteriovenous Malformations of the Brain. N Engl J Med. 2017;377(5):498.
2
Marks
MP
,
Lane
B
,
Steinberg
G
,
Chang
P
. Vascular characteristics of intracerebral arteriovenous malformations in patients with clinical steal. AJNR Am J Neuroradiol. 1991;12(3):489–96.
3
McCormick
WF
,
Nofzinger
JD
. “Cryptic” vascular malformations of the central nervous system. J Neurosurg. 1966;24(5):865–75. doi:.https://doi.org/10.3171/jns.1966.24.5.0865
4
McCormick
WF
. The pathology of vascular (“arteriovenous”) malformations. J Neurosurg. 1966;24(4):807–16. doi:.https://doi.org/10.3171/jns.1966.24.4.0807
5
Jellinger
K
. Vascular malformations of the central nervous system: a morphological overview. Neurosurg Rev. 1986;9(3):177–216. doi:.https://doi.org/10.1007/BF01743136
6
Berman
MF
,
Sciacca
RR
,
Pile-Spellman
J
,
Stapf
C
,
Connolly
ES, Jr
,
Mohr
JP
, et al.
The epidemiology of brain arteriovenous malformations. Neurosurgery. 2000;47(2):389–96, discussion 397. doi:.https://doi.org/10.1097/00006123-200008000-00023
7
Al-Shahi
R
,
Fang
JS
,
Lewis
SC
,
Warlow
CP
. Prevalence of adults with brain arteriovenous malformations: a community based study in Scotland using capture-recapture analysis. J Neurol Neurosurg Psychiatry. 2002;73(5):547–51. doi:.https://doi.org/10.1136/jnnp.73.5.547
8
Minakawa
T
,
Tanaka
R
,
Koike
T
,
Takeuchi
S
,
Sasaki
O
. Angiographic follow-up study of cerebral arteriovenous malformations with reference to their enlargement and regression. Neurosurgery. 1989;24(1):68–74. doi:.https://doi.org/10.1227/00006123-198901000-00011
9
Rubin
BA
,
Brunswick
A
,
Riina
H
,
Kondziolka
D
. Advances in radiosurgery for arteriovenous malformations of the brain. Neurosurgery. 2014;74(Suppl 1):S50–9. doi:.https://doi.org/10.1227/NEU.0000000000000219
10
Hartmann
A
,
Mast
H
,
Mohr
JP
,
Koennecke
HC
,
Osipov
A
,
Pile-Spellman
J
, et al.
Morbidity of intracranial hemorrhage in patients with cerebral arteriovenous malformation. Stroke. 1998;29(5):931–4. doi:.https://doi.org/10.1161/01.STR.29.5.931
11
Heros
RC
,
Tu
YK
. Is surgical therapy needed for unruptured arteriovenous malformations?
Neurology. 1987;37(2):279–86. doi:.https://doi.org/10.1212/WNL.37.2.279
12
Pikus
HJ
,
Beach
ML
,
Harbaugh
RE
. Microsurgical treatment of arteriovenous malformations: analysis and comparison with stereotactic radiosurgery. J Neurosurg. 1998;88(4):641–6. doi:.https://doi.org/10.3171/jns.1998.88.4.0641
13
Schlienger
M
,
Lefkopoulos
D
,
Nataf
F
,
Mammar
H
,
Missir
O
,
Meder
J-F
, et al.
Repeat linear accelerator radiosurgery for cerebral arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2003;56(2):529–36. doi:.https://doi.org/10.1016/S0360-3016(02)04472-3
14
Gruber
A
,
Bavinzski
G
,
Kitz
K
,
Barthelmes
S
,
Mayr
M
,
Knosp
E
. Multimodality Management of Cerebral Arteriovenous Malformations with Special Reference to AVM-Related Hemorrhages During Ongoing Staged Treatment. Acta Neurochir Suppl (Wien). 2016;123:153–8. doi:.https://doi.org/10.1007/978-3-319-29887-0_22
15
Potts
MB
,
Zumofen
DW
,
Raz
E
,
Nelson
PK
,
Riina
HA
. Curing arteriovenous malformations using embolization. Neurosurg Focus. 2014;37(3):E19. doi:.https://doi.org/10.3171/2014.6.FOCUS14228
16
Flickinger
JC
,
Kondziolka
D
,
Maitz
AH
,
Lunsford
LD
. An analysis of the dose-response for arteriovenous malformation radiosurgery and other factors affecting obliteration. Radiother Oncol. 2002;63(3):347–54. doi:.https://doi.org/10.1016/S0167-8140(02)00103-2
17
Lunsford
LD
,
Kondziolka
D
,
Flickinger
JC
,
Bissonette
DJ
,
Jungreis
CA
,
Maitz
AH
, et al.
Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg. 1991;75(4):512–24. doi:.https://doi.org/10.3171/jns.1991.75.4.0512
18
Régis
J
,
Massager
N
,
Lévrier
O
,
Dufour
H
,
Porcheron
D
,
Reyns
N
, et al.
Traitement radiochirurgical gamma-knife des malformations arterio-veineuses du tronc cerebral. Resultats preliminaires [Gamma-knife radiosurgery for brainstem arteriovenous malformations. Preliminary results]. Neurochirurgie. 2001;47(2-3 Pt 2):291–7. [].
19
Massager
N
,
Régis
J
,
Kondziolka
D
,
Njee
T
,
Levivier
M
. Gamma knife radiosurgery for brainstem arteriovenous malformations: preliminary results. J Neurosurg. 2000;93(Suppl 3):102–3.
20
Schwyzer
L
,
Yen
CP
,
Evans
A
,
Zavoian
S
,
Steiner
L
. Long-term results of gamma knife surgery for partially embolized arteriovenous malformations. Neurosurgery. 2012;71(6):1139–47, discussion 1147–8. doi:.https://doi.org/10.1227/NEU.0b013e3182720280
21
Pollock
BE
,
Flickinger
JC
. A proposed radiosurgery-based grading system for arteriovenous malformations. J Neurosurg. 2002;96(1):79–85. doi:.https://doi.org/10.3171/jns.2002.96.1.0079
22
van Beijnum
J
,
van der Worp
HB
,
Buis
DR
,
Al-Shahi Salman
R
,
Kappelle
LJ
,
Rinkel
GJ
, et al.
Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. JAMA. 2011;306(18):2011–9. doi:.https://doi.org/10.1001/jama.2011.1632
23
Mohr
JP
,
Parides
MK
,
Stapf
C
,
Moquete
E
,
Moy
CS
,
Overbey
JR
, et al.; international ARUBA investigators. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383(9917):614–21. doi:.https://doi.org/10.1016/S0140-6736(13)62302-8
24
Mohr
JP
,
Moskowitz
AJ
,
Stapf
C
,
Hartmann
A
,
Lord
K
,
Marshall
SM
, et al.
The ARUBA trial: current status, future hopes. Stroke. 2010;41(8):e537–40. doi:.https://doi.org/10.1161/STROKEAHA.110.580274
25
Pradilla
G
,
Coon
AL
,
Huang
J
,
Tamargo
RJ
. Surgical treatment of cranial arteriovenous malformations and dural arteriovenous fistulas. Neurosurg Clin N Am. 2012;23(1):105–22. doi:.https://doi.org/10.1016/j.nec.2011.10.002
26
Steiner
L
,
Leksell
L
,
Forster
DM
,
Greitz
T
,
Backlund
EO
. Stereotactic radiosurgery in intracranial arterio-venous malformations. Acta Neurochir (Wien). 1974;(Suppl 21):195–209.
27
Schneider
BF
,
Eberhard
DA
,
Steiner
LE
. Histopathology of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 1997;87(3):352–7. doi:.https://doi.org/10.3171/jns.1997.87.3.0352
28
Szeifert
GT
,
Levivier
M
,
Lorenzoni
J
,
Nyáry
I
,
Major
O
,
Kemeny
AA
. Morphological observations in brain arteriovenous malformations after gamma knife radiosurgery. Prog Neurol Surg. 2013;27:119–29. doi:.https://doi.org/10.1159/000341772
29
Nataf
F
,
Schlienger
M
,
Lefkopoulos
D
,
Merienne
L
,
Ghossoub
M
,
Foulquier
JN
, et al.
Radiosurgery of cerebral arteriovenous malformations in children: a series of 57 cases. Int J Radiat Oncol Biol Phys. 2003;57(1):184–95. doi:.https://doi.org/10.1016/S0360-3016(03)00445-0
30
Kawaguchi
O
,
Nyui
Y
,
Kunieda
E
,
Onozuka
S
,
Tsukamoto
N
,
Fukada
J
, et al.
Radiosurgical treatment planning for intracranial AVM based on images generated by principal component analysis--a simulation study. Keio J Med. 2009;58(1):41–9. doi:.https://doi.org/10.2302/kjm.58.41
31
Castel
JP
,
Kantor
G
. Morbidite et mortalite du traitement chirurgical des malformations arterio-veineuses cerebrales. Donnees actuelles et analyse de la litterature recente [Postoperative morbidity and mortality after microsurgical exclusion of cerebral arteriovenous malformations. Current data and analysis of recent literature]. Neurochirurgie. 2001;47(2-3 Pt 2):369–83.
32
Ogilvy
CS
,
Stieg
PE
,
Awad
I
,
Brown
RD, Jr
,
Kondziolka
D
,
Rosenwasser
R
, et al.; Stroke Council, American Stroke Association. Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Circulation. 2001;103(21):2644–57. doi:.https://doi.org/10.1161/01.CIR.103.21.2644
33
Rodríguez-Boto
G
,
Gutiérrez-González
R
,
Gil
A
,
Serna
C
,
López-Ibor
L
. Combined staged therapy of complex arteriovenous malformations: initial experience. Acta Neurol Scand. 2013;127(4):260–7. doi:.https://doi.org/10.1111/j.1600-0404.2012.01706.x
34
Szajner
M
,
Roman
T
,
Markowicz
J
,
Szczerbo-Trojanowska
M
. Onyx(®) in endovascular treatment of cerebral arteriovenous malformations - a review. Pol J Radiol. 2013;78(3):35–41. doi:.https://doi.org/10.12659/PJR.889120
35
Weber
T
,
Maier-Funk
C
,
Ohlhauser
D
,
Hillenbrand
A
,
Cammerer
G
,
Barth
TF
, et al.
Accurate preoperative localization of parathyroid adenomas with C-11 methionine PET/CT. Ann Surg. 2013;257(6):1124–8. doi:.https://doi.org/10.1097/SLA.0b013e318289b345
36
Valavanis
A
,
Yaşargil
MG
. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg. 1998;24:131–214. doi:.https://doi.org/10.1007/978-3-7091-6504-1_4


