How I manage patients with anticoagulation-associated bleeding or urgent surgery
DOI: https://doi.org/10.4414/smw.2018.14598
Thomas C.
Sautera, Balthasar
Eberleb, Walter A.
Wuilleminc, Thomas
Thieled, Anne
Angelillo-Scherrerc, Aristomenis K.
Exadaktylosa, Gabor
Erdoesb, Adam
Cukerf, Michael
Naglere
aDepartment of Emergency Medicine, Inselspital, University Hospital Bern, Switzerland
bDepartment of Anaesthesiology and Pain Therapy, Inselspital, University Hospital Bern, Switzerland
cDivision of Haematology and Central Haematology Laboratory, Luzerner Kantonsspital, Lucerne, Switzerland
dInstitute for Immunology and Transfusion Medicine, Universitätsmedizin Greifswald, Germany
eDepartment of Haematology and Central Haematology Laboratory, Inselspital University Hospital, and Department for BioMedical Research, University of
Bern, Switzerland
fDepartments of Medicine and Pathology & Laboratory Medicine, University of Pennsylvania, Philadelphia, PA, USA
Summary
Antithrombotic treatment puts patients at risk of major bleeding. Fast and adequate response to anticoagulant-associated bleeding may not only stop the bleeding but prevent severe complications. However, practical treatment algorithms to guide physicians in emergency situations are lacking. Important principles that arise from management of bleeding in general are (a) implementation of an in-house algorithm, (b) rapid identification and treatment of the bleeding source, (c) adequate fluid resuscitation, (d) consideration of the application of tranexamic acid and (e) appropriate coagulation testing. We present an algorithm for anticoagulant-associated bleeding and urgent surgery, derived from available data and recommendations, and implemented at our institution. Decisions regarding reversal agents or postponing surgery are based on two questions: the occurrence of a life-threatening bleed or urgent indication for surgery, and the presence of a relevant drug level. Immediate application of reversal agents is suggested if the clinical situation is urgent and laboratory test results are delayed or unavailable. A relevant anticoagulant drug level is required in all other cases. We discuss appropriate laboratory assays for all commonly available anticoagulants, report respective target ranges or expected values, discuss time intervals before surgery, and present critical cut-off values to be used as decision criteria. Specific and unspecific reversal agents for all anticoagulants including the direct oral anticoagulants will be presented. We aim to provide practical guidance for physicians in emergency situations. In addition, we summarise and discuss available experimental and clinical data as well as recommendations provided by scientific societies, authorities and manufacturers.
Introduction
Any anticoagulation treatment is associated with an increased risk of bleeding. Whereas the annual risk of any bleeding is estimated to be 2-4%, the risk of major bleeding increases in some patient populations to as much as 15%/year [1]. Intracranial haemorrhage is the most dangerous adverse event; it is associated with a high risk of death or permanent disability [2]. About 25% of intracranial haemorrhages are linked to oral anticoagulant treatment [3]. The anticoagulant-related bleeding risk is particularly relevant in the case of trauma. Uncontrolled bleeding is the leading cause of death in trauma patients, but it is regarded as potentially preventable [4, 5]. Physicians treating patients with anticoagulation-associated bleeding are confronted with a confusing number of drugs, laboratory tests and reversal agents. Often, clear treatment algorithms are lacking and issues arise in terms of availability of laboratory tests and reversal agents. Moreover, recommendations issued by scientific societies, pharmaceutical companies and regulatory authorities can be vague and sometimes contradictory [6–8].
Management of anticoagulation-associated bleeding has become more challenging as the number of available drugs increases. Several direct oral anticoagulants targeting either factor Xa (rivaroxaban, apixaban, edoxaban) or thrombin (dabigatran etexilate) have entered the market and are increasingly being used [9]. Vitamin K antagonists, unfractionated heparin and low molecular heparin are still widely utilised.
With this review, we aim to provide a practical approach for emergency scenarios. We propose a pragmatic treatment algorithm implemented in our institution (Inselspital, University Hospital, Bern, Switzerland). Our considerations have largely been based on current statements issued by scientific societies, regulatory authorities, companies and experts, which we recognise is a limitation. This is a reflection that high quality evidence in terms of randomised controlled trials or prospective observational research focused on clinical outcomes is scant [10]. Here we collate important concepts of bleeding management in general and regarding anticoagulant-associated bleeding in particular, and discuss all aspects of available reversal agents and laboratory aspects. We also summarise the management in case of urgent surgery. In addition, we provide clear decision criteria in terms of drug levels and time intervals focussing on all common anticoagulants (unfractionated heparin, low molecular weight heparins, vitamin K antagonists, direct oral anticoagulants), but omitting those drugs that are rarely used in Switzerland, such as fondaparinux.
General management of major bleeding
Implementation of algorithms
Several scientific guidelines provide recommendations for the management of major bleeding, such as the current European guideline on management of major bleeding and coagulopathy following trauma, and the European Society of Anaesthesiology guidelines for severe perioperative bleeding [4, 11]. Implementation of in-house algorithms and protocols is regarded as crucial for improving the care of affected patients [12]. Detailed protocols improve communication between multidisciplinary teams in a setting where several diagnostic and therapeutic measures must be taken simultaneously [13]. Indeed, implementation of management protocols have been associated with improved clinical outcomes [14–16].
Rapid identification and treatment of the bleeding source
Identification and treatment of the bleeding source is the critical first step in the management of patients with major bleeding [4, 12]. Care teams must identify bleeding sources as soon as possible by clinical examination and early imaging (ultrasonography, contrast enhanced computed tomography, angiography, or endoscopy). When the bleeding source has been identified, patients should undergo an immediate procedure to control the bleed, which often requires direct transfer to the operating theatre or the angiography or endoscopy suite. This approach is supported by the observation that 80% of trauma deaths occur within the first hour after injury [12]. Indeed, distance from a computed tomography (CT) scanner has been associated with mortality in observational studies [17]. Several additional observational studies also support early bleeding treatment [4, 18, 19].
Fluid resuscitation
In uncontrolled haemorrhage, fluids should be replaced in accordance with the concept of “permissive hypotension” [20, 21]. Although maintenance of an adequate blood pressure is crucial to restore tissue perfusion in haemorrhagic shock, which requires fluid replacement, excessive fluid administration can result in relevant adverse events [22]. A restrictive volume replacement strategy targeting a systolic blood pressure of 80 to 90 mm Hg is therefore suggested, at least in the absence of traumatic brain injury [20, 21]. This approach is supported by clinical data obtained in a small randomised controlled trial [23], and several observational studies and meta-analyses [24–28]. Since colloid use in volume resuscitation has been associated with coagulopathy and kidney injury, a balanced isotonic crystalloid solution is generally recommended [4]. Early administration of red blood cells and fresh frozen plasma (at least in a ratio of 2:1) is another strategy employed to avoid crystalloid overinfusion and to mitigate dilutional coagulopathy, with the goal of achieving a target haemoglobin range of 70 to 90 g/l [4]. Implementation of structured transfusion protocols has been shown to improve survival, to prevent organ failure and to reduce use of blood products and costs [14]. In addition, prevention of hypothermia helps to avoid coagulopathy, acidosis and hypotension associated with a core body temperature less than 35°C.
Tranexamic acid
The anti-fibrinolytic agent tranexamic acid (Cyklokapron®) is a valuable adjunct in the setting of acute bleeding. It has few side effects, is inexpensive and is easily applied. In orthopaedic surgery, tranexamic acid reduced the need for blood transfusions by up to 40% [29]. In trauma patients, the efficacy of early administration of 1 g tranexamic acid was tested in a large scale randomised controlled trial including more than 20,000 patients (CRASH-2) [30]; the overall mortality was reduced by 10%. A small case-control study suggests efficacy also in the context of perioperative venous thromboembolism prophylaxis with a direct oral anticoagulant [31]. Equally important was that none of these studies reported an increase of thromboembolic events, suggesting a good safety profile. Only when tranexamic acid was given in higher dose regimens in the context of cardiac surgery was a small but significantly increased incidence of seizures observed [32]. Based on the available evidence, published reviews suggest that trauma-dose tranexamic acid should also be administered to patients with anticoagulant-associated bleeding [33, 34].
Coagulation testing
Early and repeated determination of coagulation function and platelet counts are recommended in all patients with bleeding events [4]. Conventional coagulation tests are not only useful to detect hereditary diseases (such as haemophilia and von Willebrand’s disease) but also most acquired disorders, namely fibrinogen deficiency, disseminated intravascular coagulation, vitamin K deficiency, liver failure, acute traumatic coagulopathy, and anticoagulant treatment [35]. The Inselspital screening panel for bleeding emergencies contains prothrombin time (INR; Quick %), activated partial thromboplastin time (aPTT), Clauss fibrinogen concentration, thrombin time, and the point-of-care thromboelastometry assay (ROTEM®). ROTEM® or thromboelastography (TEG®) are available in many institutions. These tests can rapidly detect fibrinogen deficiency, thrombocytopenia and (severe) hyperfibrinolysis, and are widely used to guide replacement therapy [11]. An anti-Xa assay for screening of all Xa inhibitors, even though data on its accuracy are lacking, could also be implemented as a screening test. Specific laboratory tests for the monitoring of anticoagulants are discussed further below.
Management of anticoagulant-associated bleeding
General principles
Primary resuscitation of patients with suspected anticoagulant-associated bleeding should follow the same general management strategies outlined above. However, major haemorrhage is but one manifestation of anticoagulant-associated bleeding and is not always in conjunction with trauma. A substantial proportion of patients on anticoagulant treatment suffer a non-traumatic major haemorrhage, such as gastrointestinal bleeding or ruptured abdominal aortic aneurysm; life-threatening bleeding events, such as intracranial haemorrhage or (spontaneous or iatrogenic) spinal haematoma or pericardial tamponade, can occur without hypovolaemia. Moreover, many patients on anticoagulant treatment, though not bleeding actively, may need to be prepared for major emergency surgery.
Thus, there are numerous important differences from non-anticoagulant-associated bleeding, which must be addressed during management. First, specific and nonspecific agents exist that can reverse the anticoagulant effect within minutes and stop active bleeding rapidly [10] or reduce the risk of emergent surgery. Second, patients with anticoagulant-associated bleeding remain at high risk of thromboembolic complications due to their underlying disease (atrial fibrillation, venous thromboembolism, mechanical heart valve), because of the active bleeding event and the associated administration of reversal agents [33]. The risks of bleeding and thrombosis must be monitored and balanced carefully, and the period of reversal must be kept as brief as possible. Third, knowledge of plasma levels of the anticoagulant agent is important for management, with specific laboratory assays required for individual anticoagulant drugs [34]. These issues will be discussed in detail below.
Assessment of severity and urgency
Assessment of the severity of bleeding and urgency of intervention is an important first step in patient management to allocate subsequent treatment [4]. In patients with an anticoagulant-associated bleeding event the question arises whether and when a reversal agent should be applied. Application of reversal agents may stop the bleeding rapidly, but can also increase the risk of thromboembolic events [33]. If the plasma level of the anticoagulant drug is not known, reversal agents should not be used. Although a relevant drug level can be estimated from the time of last administration, this can be uncertain. Laboratory tests to measure anticoagulant drug levels can take time, but they have become increasingly available in the Swiss healthcare setting. Thus, we use – and propose - an algorithm considering both the severity of bleeding as well as assay results (fig. 1). Core aspects of this algorithm were utilised in the pivotal study of idarucizumab, a reversal agent for dabigatran etexilate [36]. This algorithm has been implemented as an in-house guideline for Inselspital University Hospital, Bern, Switzerland.
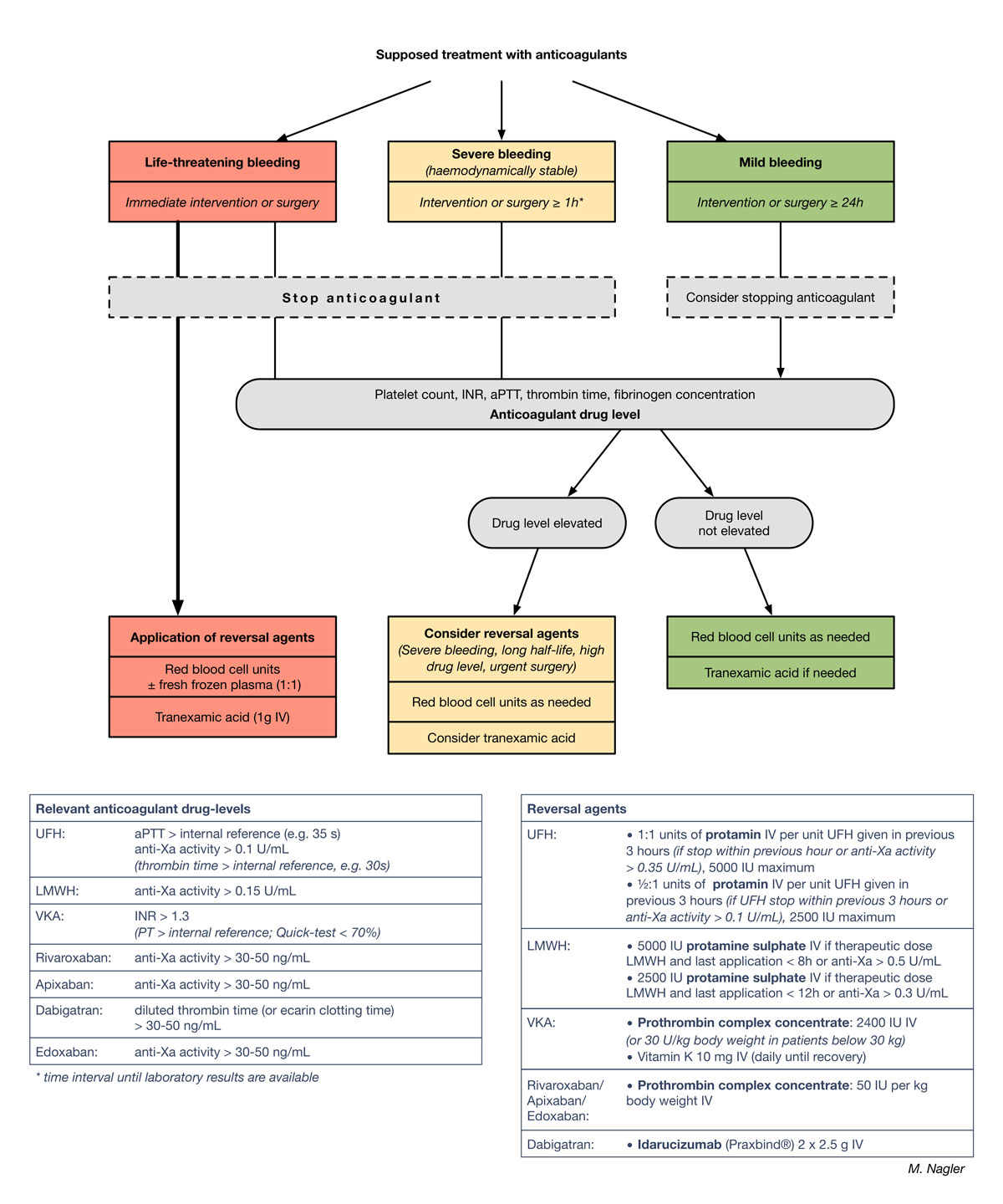
Figure 1 Management of patients with anticoagulant-associated bleeding or urgent surgery. Proposal for a treatment algorithm detailing laboratory tests, cut-off values and administration of reversal agents.
INR = international normalised ratio; aPTT = activated partial thromboplastin time; UFH = unfractionated heparin; LMWH = low molecular weight heparin; VKA = vitamin K antagonists. A larger version of this figure is downloadable from https://smw.ch/en/article/doi/smw.2018.14598.
Proposed treatment algorithm
In patients with supposed or known anticoagulant treatment, assessment of urgency has a high priority (fig. 1). The physician in charge must decide if the bleeding is life threatening and requires immediate action. Identification and treatment of bleeding sources is pivotal. Typical examples include severe haemorrhagic shock or extensive intracranial haemorrhage. In these clinical situations, the risk of bleeding-associated complications is higher than the risk of thromboembolic complications related to reversal agents. In the context of intracranial haemorrhage, several studies have demonstrated markedly improved clinical outcomes if reversal agents are applied rapidly [6]. In our practice, we treat patients suffering from life-threatening bleeding with reversal agents without waiting for laboratory results (fig. 1; left column) [6, 37]. We proceed similarly in patients who require emergency surgery within 1 hour. In contrast, in severely bleeding patients whose haemodynamic and vital functions can be stabilised with treatment according to protocol, we postpone the application of reversal agents until the specific anticoagulant drug level has been determined with the appropriate laboratory test (discussed below) (fig. 1; central “severe bleeding” scenario). For severe bleeding events we use a time cut-off of 1 hour, because all anticoagulant drug levels can be determined and communicated within this timeframe at our institution, but this can be tailored to accommodate laboratory timings at other institutions. Any further patient intake of anticoagulant drugs is of course stopped in both these scenarios. The management of mildly bleeding, haemodynamically stable patients is shown in the right column of figure 1. Patients in this category may require surgery that can be scheduled 24 hours or more in the future. Generally, such patients will not receive reversal agents and anticoagulant intake will be stopped. Treatment decisions, however, for patients with a high thromboembolic risk are assessed on a case-by-case basis (see below).
It is important to note that a panel of screening laboratory tests (blood count, PT, aPTT, fibrinogen and thrombin time) as well as drug-specific assays (discussed below) are required before and after treatment to detect haemostatic disorders and to document treatment efficacy. The drug-level cut-offs for use of reversal agents are shown in figure 1 (left table) and are discussed below.
Anticoagulant drug levels and activity may be estimated by considering the time of last administration. This is, however, fraught with uncertainty as a result of incomplete medication records, the patient’s memory or mental health status and many other pitfalls.
Assessing the thrombotic risk
Patients with anticoagulant-associated bleeding or requiring urgent surgery are at increased risk of thromboembolic complications. This can arise as a result of the underlying disease, the acute event and, potentially, the application of reversal agents. Knowledge of the underlying thromboembolic risk is important for management [38]. Scientific societies have summarised epidemiological data and created risk categories with regard to peri-interventional procedures as reported by the American College of Chest Physicians (ACCP), but these data can also be considered in the case of bleeding as well [38]. Table 1 summarises the data and groups patients into three risk categories. In patients with an intermediate or high thromboembolic risk, anticoagulant treatment should ideally be resumed within 24 hours, because the thromboembolic risk will increase further owing to procedural risks and the effect of reversal.
Table 1 Patient-related risk for thromboembolism according to anticoagulation indication; adapted from [38, 39].
|
Indication
|
Low risk*
|
Intermediate risk†
|
High risk‡
|
| Mechanical heart valve |
Bileaflet mechanical aortic valve without risk factors for stroke |
Bileaflet mechanical aortic valve with risk factors for stroke |
Any mechanical mitral valve
Any caged-ball or tilting-disc valve
Recent stroke/TIA (< 6 months) |
| Atrial fibrillation |
CHA2DS2-VASc score 0 to 3 |
CHA2DS2-VASc score 4 to 7 |
CHA2DS2-VASc score 8 to 9
Recent stroke/TIA (<3 months)
Rheumatic valvular disease |
| Venous thromboembolism |
VTE >1 year |
VTE within <1 year
Recurrent VTE
Active cancer |
Recent VTE (<3 month)
Severe thrombophilia¶
|
Anticoagulants and their reversal agents
Unfractionated heparin
Because of its short half-life, unfractionated heparin is still ubiquitously used in the perioperative setting and in the intensive care unit. Administered intravenously, its biological half-life varies according to the dosage and is typically between 30 minutes and 2.5 hours [8]. The antithrombotic effect is usually gone 4 hours after discontinuation and surgery can be performed safely. Therefore, use of a reversal agent can be avoided in most cases. When reversal is required, protamine (as hydrochloride or sulphate) is employed. It is a small, basic cationic protein made from salmon sperm that binds to the anionic glycosaminoglycan heparin, acting as a stoichiometric reversal agent that can rapidly inactivate unfractionated heparin. One unit (0.01 mg) of protamine will neutralise approximately 1 unit of heparin. Protamine has been used worldwide every day in cardiac surgery for decades, but has well-recognised adverse events such as hypotension, pulmonary vasoconstriction, anaphylactoid reactions and thromboembolic complications [6, 8, 40]. In accordance with guidelines, we recommend the slow application of 1 unit of protamine (diluted in normal saline) per unit unfractionated heparin administered within the previous 3 hours if the heparin was stopped within <1 hour previously or in the case of anti-Xa activity > 0.35 U/ml (with a maximum dose of 5000 units). We propose the application of 0.5 units of protamine per unit unfractionated heparin, if the heparin was stopped within the previous 3 hours or anti-Xa activity is >0.1 U/ml [6, 8]. Protamine should not be given if the ant-Xa level is ≤0.1 U/ml. Protamine dosage can also be monitored using the point-of-care activated clotting time (ACT) test.
Low molecular weight heparin
Low molecular weight heparin consists of short chains of polysaccharides, which have a longer half-life (3–5 hours) than unfractionated heparin, and this permits a once or twice daily subcutaneous application for treatment and prophylaxis of thromboembolism [8]. However, owing to the molecular structure, only about 50% of the anticoagulant effect of low molecular weight heparin can be reversed by protamine, with no other reversal agent currently available. To counteract low molecular weight heparin anticoagulation with protamine, we suggest giving an intravenous dose of 5000 units of protamine slowly (preferably diluted) if a therapeutic dose of low molecular weight heparin was given within the last 8 hours, or if the anti-Xa activity is >0.5 U/ml (fig. 1, right table). A dose of 2500 units is given if a therapeutic dose of low molecular weight heparin were administered more than 8 but ≤12 hours previously or if anti-Xa activity is between 0.3 and 0.5 U/ml. We suggest withholding protamine in all other cases.
Vitamin K antagonists
Vitamin K antagonists block vitamin K epoxide reductase, among other effects, thus inhibiting synthesis of clotting factors II, VII, IX and X [7, 41]. This effect lasts for several days and administration of exogenous agents are required to stop effects of vitamin K antagonists in patients with active bleeding or prior to urgent surgery. Three agents are available to reverse vitamin K antagonist anticoagulant effects.
- Vitamin K itself which provides the substrate for synthesis of the aforementioned clotting factors. Even though repeated doses of vitamin K should be given (in emergencies by slow intravenous injection injection; fig. 1, right table) [6], the earliest physiological effect is 3 hours after administration, rendering this an inadequate treatment for immediate vitamin K antagonist reversal.
- Fresh frozen plasma, which contains all coagulation factors at approximately physiological levels, has been used for vitamin K antagonist reversal for decades as it is inexpensive and widely available. However, treatment with fresh frozen plasma is associated with disadvantages [6], such as a considerable time delay until a correction of anticoagulant effects is observed, a large fluid load, which increases the risk of transfusion-associated circulatory overload, and also, albeit a very small, residual risk of transfusion-related acute lung injury. Thus, recent guidelines recommend vitamin K antagonist reversal with fresh frozen plasma only if the more specific reversal option, prothrombin complex concentrate (see below) is not available [6].
- “Four-factor” prothrombin complex concentrate, which contains vitamin K-dependent coagulation factors (factors II, VII, IX, X; proteins S, C, Z) in variable amounts. Prothrombin complex concentrate is regarded as the vitamin K antagonist reversal agent of choice because of its rapid action, small volume load and low risk of transfusion reactions [6, 7, 42]. Its efficacy has been demonstrated in two randomised controlled trials focusing on patients with major bleeding [43] and urgent surgery [44]. In the setting of intracranial haemorrhage, several observational studies suggest superiority of prothrombin complex concentrate over fresh frozen plasma with regard to time until reversal, haematoma expansion, mortality and functional outcomes [6]. However, prothrombin complex concentrate may increase the risk of thromboembolic complications and not more than 2400 units should be given initially [45] (fig. 1, right table).
Dabigatran etexilate (Pradaxa®)
The main advantage of direct oral anticoagulants is their relatively short half-life, which in the case of dabigatran is 12 to 14 hours [46]. Thus, stopping the drug is sufficient in many cases of bleeding and urgent surgery. Nevertheless, development of a reversal agent was considered an important objective to improve patient care. Idarucizumab, a humanised monoclonal antibody fragment which binds to and inactivates dabigatran, has been developed and tested in a single-arm interventional study (n = 503) [36, 47]. Consecutive infusion of two vials, each containing 2.5 g of idarucizumab, reverses the anticoagulant effect of dabigatran rapidly and completely, both in laboratory testing and clinically. Thus, idarucizumab (Praxbind®) has been licenced in the US (Food and Drug Administration, FDA), in Europe (European Medical Agency, EMA) and Switzerland (Swiss Agency for Therapeutic Products, Swissmedic). In our practice so far, application of idarucizumab has been simple, effective and safe, even in special situations [48]. We suggest administration of idarucizumab as the sole reversal agent in dabigatran-treated patients with severe bleeding or need for urgent surgery (fig. 1). Dialysis might be considered in patients with renal failure if idarucizumab is not available, but this only removes approximately two thirds of dabigatran and typically requires several hours [49].
Oral factor Xa inhibitors: rivaroxaban (Xarelto®), apixaban (Eliquis®) and edoxaban (Lixiana®)
The oral factor Xa inhibitors rivaroxaban, apixaban and edoxaban share important characteristics with dabigatran, in particular the short half-life (5–15 hours) [46]. Thus, stopping these drugs is adequate in many patients. Four-factor prothrombin complex concentrate has been suggested for nonspecific reversal of oral factor Xa inhibitors [10]. The effects have been variable in a number of animal and ex-vivo studies [50–53]. Prothrombin complex concentrate corrected the prothrombin time in healthy volunteers taking rivaroxaban, suggesting potential efficacy [54, 55]. In addition, a number of case reports exist [56–58]. Consistent with existing guidelines [6, 59], we apply prothrombin complex concentrate at a dosage of 50 U per kg body weight in patients with (a) life-threatening bleeding, (b) severe bleeding and relevant drug levels, or (c) need for urgent surgery and relevant drug levels (fig. 1). We generally do not use recombinant factor VIIa because of potential adverse events and a paucity of data.
Future perspectives
Two agents are currently under development for the reversal of factor Xa inhibitors. Andexanet alfa is a recombinant protein mimicking factor Xa but lacks any coagulant activity [60]. Andexanet alfa was effective and well tolerated in animal studies and healthy volunteers [60, 61]. Efficacy and safety have been tested in an open-label single-arm interventional study in elderly patients with acute major bleeding (interim report n = 67) [62]. A partial reversal of rivaroxaban, apixaban or enoxaparin was observed with regard to anti-Xa activity. Clinical efficacy was rated to be good in the majority of patients. However, a high rate of thromboembolic events, as well as deaths, have been observed; this has been suggested to have resulted from the baseline thrombotic risk of the study patients (but it might indeed reflect a certain prothrombotic risk). Andexanet alfa was only moderately effective in reversing low molecular weight heparin in animal experiments [42, 63].
Ciraparantag is another agent developed to reverse the effect of factor Xa inhibitors [64]. It is a positively charged molecule that binds directly to these molecules. It has been tested in a phase I/II study in healthy volunteers [65]. Another study is ongoing in healthy volunteers taking rivaroxaban (NCT03172910). Clinical data obtained in patients are still lacking.
Measurement of anticoagulants
Monitoring of drugs requires established target ranges. The definition of a target range is based on comprehensive data on efficacy and safety outcomes at defined anticoagulant concentrations or activity levels. These criteria are met for vitamin K antagonists only. It is, however, common practice to monitor treatment with unfractionated heparin as well [8]. One of the main benefits of direct oral anticoagulants is that routine laboratory monitoring of anticoagulant activity is not necessary. All direct oral anticoagulants have been studied at fixed doses and no target ranges of drug levels are established. Knowledge of the anticoagulant drug level is, however, important in certain clinical situations [34] and the bleeding patient on anticoagulants is a good example where administration of reversal agents may be lifesaving. Drug concentrations were measured in some of the clinical studies, and an “expected range” for peak and trough levels have been published. For interpretation of test results, it is important to know whether they were measured at peak level (corresponding to the time point tmax after ingestion; table 2) or at the trough level (before the next application). Knowledge of the appropriate laboratory test and expected values is necessary to put results into context. In addition, treating physicians must be aware that large interindividual variabilities exist [66]. Table 3 illustrates appropriate laboratory tests for commonly available anticoagulants and reports target ranges or expected values.
Table 2 Characteristics of commonly used anticoagulants: mechanism of action, pharmacokinetics and elimination.
|
Agent
|
Mechanism of action
|
tmax
|
Half-life
|
Elimination
|
Prolonged half-life
|
Rivaroxaban
(Xarelto®) |
Inhibits factor Xa |
2–4 h |
5–9 h |
66% renal, 28% faecal |
Older patients, renal impairment |
Apixaban
(Eliquis®) |
3–4 h |
8–15 h |
30% renal, 70% faecal |
Older patients, renal impairment |
Edoxaban
(Lixiana®) |
1–2 h |
9–10 h |
50% renal |
Older patients, renal impairment |
Dabigatran
(Pradaxa®) |
Inhibits thrombin |
0.5–2 h |
12–14 h |
Renal |
Older patients, renal impairment |
VKA
(Marcoumar®, Sintrom®) |
Inhibits vitamin K metabolism and reduces Factor II*
|
48–72 h+ |
ca. 160 h+ |
Hepatic metabolism, renal elimination |
Multiple interactions with medications as well as nutritional interactions, impaired hepatic function |
| UFH |
Activates antithrombin |
30–120 min |
30–150 min |
Liver |
Hepatic impairment, high doses |
| LMWH |
Inhibits factor Xa |
2–4 h |
3–5 h |
Mostly renal |
Renal impairment |
Table 3 Laboratory tests, expected drug levels, critical drug levels and required time interval before intervention.
|
Drug
|
Laboratory assay
|
Expected drug levels*
(dosage)
|
Proposed cut-off for surgery
|
Time interval before operations, interventions and spinal anaesthesia
|
Rivaroxaban
(Xarelto®) |
Anti-Xa activity
(PT/INR) |
Peak: 270 ng/m (189–419)†
Trough: 26 ng/ml (6–87)†
(20 mg daily) |
<50 ng/ml for urgent surgery and low bleeding risk
<30 ng/ml for high-risk surgery |
≥24 h if bleeding risk of surgery is low/ intermediate
≥48 h in case of high bleeding risk with surgery, elderly patients, renal failure¶
|
Apixaban
(Eliquis®) |
Anti-Xa activity
(PT/INR) |
Peak: 171 ng/ml (91–321)†
Trough: 103 ng/ml (41–230)†
(5 mg twice daily) |
<50 ng/ml for urgent surgery and low bleeding risk
<30 ng/ml for high-risk surgery |
≥24 h if bleeding risk of surgery is low/intermediate
≥48 h in case of high bleeding risk with surgery, elderly patients, renal failure¶
|
Edoxaban
(Lixiana®) |
Anti-Xa activity
(PT/INR) |
Peak: 170 ng/ml (120–250)‡
Trough: 22 ng/m (10–40)‡
(60 mg daily) |
<50 ng/ml for urgent surgery and low bleeding risk
<30 ng/ml for high-risk surgery |
≥24 h if bleeding risk of surgery is low/intermediate
≥48 h in case of high bleeding risk with surgery, elderly patients, renal failure¶
|
Dabigatran
(Pradaxa®) |
Diluted thrombin time
Ecarin clotting time
(thrombin time) |
Peak: 184 ng/ml (64–443)†
Trough: 90 ng/ml (31–225)†
(150 mg twice daily) |
<50 ng/ml for urgent surgery and low bleeding risk
<30 ng/ml for high-risk surgery |
≥36 h if bleeding risk of surgery is low
≥48 h in the event of high bleeding risk with surgery, elderly patients, renal failure¶
|
VKA
(Marcoumar®, Sintrom®) |
INR |
2.0–3.0 (target range) |
INR <1.3 |
>5 to 7 days |
| UFH |
aPTT
Anti-Xa activity |
0.35–0.7 U/ml (target range) |
aPTT <35 s or
anti-Xa ≤0.1 U/ml |
≥4–6 h (longer in case of renal failure, elderly patients, subcutaneous application) |
| LMWH |
Anti-Xa activity |
0.6–1.0 U/ml (target range twice daily)
0.9–1.6 U/m (target range once daily) |
Anti-Xa ≤0.15 U/ml |
≥12 h (50–100 U/kg body weight)
≥24 h (150–200 U/kg body weight)
≥48 h in the case of high bleeding risk with surgery, elderly patients, renal failure¶
|
Vitamin K antagonists
Prothrombin time (in Switzerland usually expressed in relation to standardised human plasma as a prothrombin ratio [PR] or Quick %) has been used for monitoring of vitamin K antagonists ever since the discovery of “sweet clover disease” [69]. Standardisation of thromboplastins using the international sensitivity index (ISI; based on a WHO standard) has enabled the implementation of the international normalised ratio (INR). Using the INR, the intensity of anticoagulation in individual patients is comparable between individual laboratories using different reagents worldwide. Results of large epidemiological studies have led to a precise definition of the optimal target range, which is 2.0 to 3.0 for most patients and indications [70]. INR values <1.3 or PR >70% Quick are regarded as sufficient for most surgical procedures and in the case of major bleeding.
Unfractionated heparin
It is common practice to monitor treatment with unfractionated heparin though there are major methodological concerns and very few clinical studies support this approach [8, 71]. The aPTT is recommended most often, but it is associated with important shortcomings: a large variability among different reagents and a high sensitivity to other factors that are prevalent in critically ill patients [72–74]. In contrast, chromogenic anti-Xa assays have been developed to determine heparin concentration accurately (though inconsistencies among reagents exist) [75]. Indeed, monitoring with anti-Xa assays was associated with fewer complications than monitoring with aPTT in a small randomised controlled trial [76]. The aPTT target range must be determined by every laboratory in relation to either anti-Xa levels or a protamine/heparin titration curve [8]. In our institution, the aPTT target range is 46–70 sec; the cut-off for surgery and major bleedings is the upper limit of the reference range (35 sec; fig. 1). In terms of anti-Xa activity, the target range is 0.35–0.7 U/ml and a relevant cut-off is 0.1 U/ml (fig. 1) [8]. The thrombin time is also a very sensitive laboratory test to monitor effects of unfractionated heparin, although its response curve is not linear. However, a normal thrombin time essentially excludes the presence of unfractionated heparin.
Low molecular weight heparin
The dosage of low molecular weight heparin is based on body weight, and laboratory monitoring is usually not required [8]. However, knowledge of the plasma concentration is important in the case of major bleeding or urgent surgery. Drug-level adapted treatment is recommended in special situations such as pregnancy, extreme body weight or renal failure [77]. Anti-Xa activity is the most accurate test and is recommended for the measurement of low molecular weight heparin [8]. Target ranges are 0.6–1.0 U/ml (twice daily dosing) and 0.9–1.6 U/ml (once daily dosing) (fig. 1, left table). An appropriate cut-off for most operations is 0.15 U/ml.
Dabigatran etexilate
Thrombin time is a widely available assay and is extremely sensitive to the thrombin inhibitor, dabigatran. Even moderate dabigatran drug levels prolong thrombin time beyond its range of measurement [67]. Thus, a normal thrombin time rules out the presence of dabigatran. Adapted assays have been developed to measure dabigatran drug concentration across the normal therapeutic range [68]. This “diluted thrombin time” provides adequate accuracy, both as an in-house assay and as commercially available test kit (HEMOCLOT®, HYPHEN BioMed, Neuvillesur-Oise, France) [78]. The ecarin clotting time is another assay developed to measure dabigatran activity, but important reproducibility issues remain [68]. Expected dabigatran concentrations at peak and trough are reported in table 3. Cut-off values, which are relevant for considering reversal agents or postponement of surgery, are 50 ng/ml in the case of major bleeding or surgery associated with a low bleeding risk, and 30 ng/ml in the case of life-threatening bleeding or surgery associated with a high bleeding risk (table 3; fig. 1) [37].
Oral factor Xa inhibitors: rivaroxaban, apixaban, and edoxaban
Factor Xa inhibitors affect the PT and PT-based assays, and therefore these assays have been proposed to estimate drug levels [67]. However, most authors do not suggest use of PT-based assays because of limited sensitivity and specificity, and large variation among different reagents [68]. However, the point-of-care coagulometer CoaguChek® XS detects peak rivaroxaban levels with high sensitivity [79].
Anti-Xa assays have been developed for the measurement of oral factor Xa inhibitors [80, 81]. A high accuracy and consistency has been demonstrated, particularly for rivaroxaban plasma concentrations [66]. Expected drug levels at peak (3 hours after intake) and trough (24 hours after intake) with a 20 mg dose are shown in table 3. Scientific societies recommend considering reversal agents or postponement of surgery at an anti-Xa level of 50 ng/ml in the case of major bleeding or urgent surgery, and at 30 ng/ml in the case of life-threatening bleeding or surgery with a high risk of bleeding [37].
Management of anticoagulants in the case of urgent surgery or interventions
The periprocedural risk of patients treated with antithrombotic drugs is determined by four main issues, which must be considered: (1) the thromboembolic risk of the underlying disease which corresponds to the indication for anticoagulation (patient-related risk), (2) the thromboembolic risk associated with the planned surgery or the clinical situation (surgical risk factors), (3) the bleeding risk due to the anticoagulant drugs and the drug level at the time of surgery, and (4) the bleeding risk of the surgery and the potential consequences of a bleeding complication. All issues are discussed in detail elsewhere [38, 39, 82, 83].
Patient-related thromboembolic risk is discussed above and summarised in table 1 [38, 39]. Patients with a high risk should not be left without anticoagulation therapy for a long period of time [38, 39]. The same holds true for patients undergoing a surgical procedure with a high thromboembolic risk (cancer surgery, cardiovascular surgery, extensive abdominal, thoracic or pelvic surgeries, hip fracture, or major trauma) [38, 39]. Nevertheless, a prophylactic dose of anticoagulation treatment should be started about 6 hours postoperatively and a therapeutic dose should be started about 24 hours postoperatively.
The third important aspect is the bleeding risk of the intended surgery or intervention. No interruption of anticoagulation treatment is necessary in procedures with minimal bleeding risk, such as cataract surgery and most dermatology or dental procedures; for colonoscopy, gastrointestinal endoscopy, or arthroscopy decisions should be made on a case by case basis [39]. In contrast, anticoagulation must be interrupted adequately prior to surgeries with a high bleeding risk (intracranial or intraspinal surgery, other major surgery in general).
In the event of emergency surgery and a high bleeding risk (potentially also in cases with an intermediate bleeding risk), use of reversal agents is necessary in virtually all patients treated with a vitamin K antagonist. Specific drugs have been discussed above. Reversal agents are rarely necessary in the case of single anticoagulation treatment with unfractionated heparin. Surgery can usually be conducted 4 hours after stopping unfractionated heparin. Determination of anti-Xa activity can help to adapt the time interval (in the case of high urgency or renal impairment; see table 3). When low molecular weight heparin has been used and paused, most operations can be done after 12 hours (prophylactic dose) or 24 hours (therapeutic dose; table 3). Again, determination of anti-Xa levels can help to adapt the time interval. Protamine can be considered in special situations (table 3) and andexanet or ciraparantag might be options in the future. Manufacturers of direct oral anticoagulants recommend relatively long intervals between administration of the last dose and interventions, up to 72 hours in the case of dabigatran. However, clinical data from large registries suggest that short-term interruption is a safe approach [82]. Considering these data together with manufacturers’ recommendations, we suggest the time intervals mentioned in table 3. Again, determination of the drug level can help to adapt the waiting period in order to reduce or prolong the time to intervention (table 3).
Conclusions
Management of patients with anticoagulant-associated bleeding or urgent surgery is challenging. We present a simple treatment algorithm that is based on the assessment of severity and urgency as well as the measurement of drug levels. In addition, we provide information regarding appropriate drug levels and time intervals to follow in emergency situations. If implemented as a treatment protocol, this algorithm might help emergency and perioperative teams to provide high quality care and to minimise inherent risks of bleeding and thrombosis.
Acknowledgements
We thank Prof. Pierre Fontana, Geneva (Switzerland) for his valuable comments on this manuscript.
References
1
Enriquez
A
,
Lip
GY
,
Baranchuk
A
. Anticoagulation reversal in the era of the non-vitamin K oral anticoagulants. Europace. 2016;18(7):955–64. doi:.https://doi.org/10.1093/europace/euv030
2
Wilson
D
,
Seiffge
DJ
,
Traenka
C
,
Basir
G
,
Purrucker
JC
,
Rizos
T
, et al.; And the CROMIS-2 collaborators. Outcome of intracerebral hemorrhage associated with different oral anticoagulants. Neurology. 2017;88(18):1693–700. doi:.https://doi.org/10.1212/WNL.0000000000003886
3
Schols
AM
,
Schreuder
FH
,
van Raak
EP
,
Schreuder
TH
,
Rooyer
FA
,
van Oostenbrugge
RJ
, et al.
Incidence of oral anticoagulant-associated intracerebral hemorrhage in the Netherlands. Stroke. 2014;45(1):268–70. doi:.https://doi.org/10.1161/STROKEAHA.113.003003
4
Rossaint
R
,
Bouillon
B
,
Cerny
V
,
Coats
TJ
,
Duranteau
J
,
Fernández-Mondéjar
E
, et al.
The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20(1):100. doi:.https://doi.org/10.1186/s13054-016-1265-x
5
Cothren
CC
,
Moore
EE
,
Hedegaard
HB
,
Meng
K
. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World J Surg. 2007;31(7):1507–11. doi:.https://doi.org/10.1007/s00268-007-9087-2
6
Frontera
JA
,
Lewin
JJ, 3rd
,
Rabinstein
AA
,
Aisiku
IP
,
Alexandrov
AW
,
Cook
AM
, et al.
Guideline for Reversal of Antithrombotics in Intracranial Hemorrhage: A Statement for Healthcare Professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit Care. 2016;24(1):6–46. doi:.https://doi.org/10.1007/s12028-015-0222-x
7
Ageno
W
,
Gallus
AS
,
Wittkowsky
A
,
Crowther
M
,
Hylek
EM
,
Palareti
G
. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2, Suppl):e44S–88S. doi:.https://doi.org/10.1378/chest.11-2292
8
Garcia
DA
,
Baglin
TP
,
Weitz
JI
,
Samama
MM
. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2, Suppl):e24S–43S. doi:.. Corrected in: Chest. 2013;144(2):721.https://doi.org/10.1378/chest.11-2291
9
Sauter
TC
,
Amylidi
AL
,
Ricklin
ME
,
Lehmann
B
,
Exadaktylos
AK
. Direct new oral anticoagulants in the emergency department: Experience in everyday clinical practice at a Swiss university hospital. Eur J Intern Med. 2016;29:e13–5. doi:.https://doi.org/10.1016/j.ejim.2015.12.009
10
Siegal
DM
. Managing target-specific oral anticoagulant associated bleeding including an update on pharmacological reversal agents. J Thromb Thrombolysis. 2015;39(3):395–402. doi:.https://doi.org/10.1007/s11239-015-1167-9
11
Kozek-Langenecker
SA
,
Afshari
A
,
Albaladejo
P
,
Santullano
CA
,
De Robertis
E
,
Filipescu
DC
, et al.
Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30(6):270–382. doi:.https://doi.org/10.1097/EJA.0b013e32835f4d5b
12
Rossaint
R
,
Bouillon
B
,
Cerny
V
,
Coats
TJ
,
Duranteau
J
,
Fernández-Mondéjar
E
, et al.; STOP Bleeding Campaign. The STOP the Bleeding Campaign. Crit Care. 2013;17(2):136. doi:.https://doi.org/10.1186/cc12579
13
ATLS Subcommittee; American College of Surgeons’ Committee on Trauma; International ATLS working group. Advanced trauma life support (ATLS®): the ninth edition. J Trauma Acute Care Surg. 2013;74(5):1363–6.
14
Cotton
BA
,
Au
BK
,
Nunez
TC
,
Gunter
OL
,
Robertson
AM
,
Young
PP
. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma. 2009;66(1):41–8, discussion 48–9. doi:.https://doi.org/10.1097/TA.0b013e31819313bb
15
Johnson
JE
,
Mosher
BD
,
Morrison
CA
,
Schneider
PD
,
Stevens
P
,
Kepros
JP
. A disciplined approach to implementation of evidence-based practices decreases ICU and hospital length of stay in traumatically injured patients. J Surg Res. 2010;163(2):327–30. doi:.https://doi.org/10.1016/j.jss.2010.03.074
16
Ruchholtz
S
,
Waydhas
C
,
Lewan
U
,
Piepenbrink
K
,
Stolke
D
,
Debatin
J
, et al.
A multidisciplinary quality management system for the early treatment of severely injured patients: implementation and results in two trauma centers. Intensive Care Med. 2002;28(10):1395–404. doi:.https://doi.org/10.1007/s00134-002-1446-8
17
Huber-Wagner
S
,
Mand
C
,
Ruchholtz
S
,
Kühne
CA
,
Holzapfel
K
,
Kanz
KG
, et al.; TraumaRegister DGU. Effect of the localisation of the CT scanner during trauma resuscitation on survival -- a retrospective, multicentre study. Injury. 2014;45(Suppl 3):S76–82. doi:.https://doi.org/10.1016/j.injury.2014.08.022
18
Martin
M
,
Oh
J
,
Currier
H
,
Tai
N
,
Beekley
A
,
Eckert
M
, et al.
An analysis of in-hospital deaths at a modern combat support hospital. J Trauma. 2009;66(4, Suppl):S51–60, discussion S60–1. doi:.https://doi.org/10.1097/TA.0b013e31819d86ad
19
Smith
W
,
Williams
A
,
Agudelo
J
,
Shannon
M
,
Morgan
S
,
Stahel
P
, et al.
Early predictors of mortality in hemodynamically unstable pelvis fractures. J Orthop Trauma. 2007;21(1):31–7. doi:.https://doi.org/10.1097/BOT.0b013e31802ea951
20
Rixen
D
,
Steinhausen
E
,
Dahmen
J
,
Bouillon
B
. S3-Leitlinie Polytrauma/Schwerverletzten-Behandlung
[S3 guideline on treatment of polytrauma/severe injuries. Initial surgical phase: significance--possibilities--difficulties?].
Unfallchirurg. 2012;115(1):22–9. Article in German. doi:.https://doi.org/10.1007/s00113-011-2104-9
21
Berry
C
,
Ley
EJ
,
Bukur
M
,
Malinoski
D
,
Margulies
DR
,
Mirocha
J
, et al.
Redefining hypotension in traumatic brain injury. Injury. 2012;43(11):1833–7. doi:.https://doi.org/10.1016/j.injury.2011.08.014
22
Haut
ER
,
Kalish
BT
,
Cotton
BA
,
Efron
DT
,
Haider
AH
,
Stevens
KA
, et al.
Prehospital intravenous fluid administration is associated with higher mortality in trauma patients: a National Trauma Data Bank analysis. Ann Surg. 2011;253(2):371–7. doi:.https://doi.org/10.1097/SLA.0b013e318207c24f
23
Morrison
CA
,
Carrick
MM
,
Norman
MA
,
Scott
BG
,
Welsh
FJ
,
Tsai
P
, et al.
Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: preliminary results of a randomized controlled trial. J Trauma. 2011;70(3):652–63. doi:.https://doi.org/10.1097/TA.0b013e31820e77ea
24
Wang
CH
,
Hsieh
WH
,
Chou
HC
,
Huang
YS
,
Shen
JH
,
Yeo
YH
, et al.
Liberal versus restricted fluid resuscitation strategies in trauma patients: a systematic review and meta-analysis of randomized controlled trials and observational studies*. Crit Care Med. 2014;42(4):954–61. doi:.https://doi.org/10.1097/CCM.0000000000000050
25
Kwan
I
,
Bunn
F
,
Roberts
I
; WHO Pre-Hospital Trauma Care Steering Committee. Timing and volume of fluid administration for patients with bleeding. Cochrane Database Syst Rev. 2003;(3):CD002245.
26
Schreiber
MA
,
Meier
EN
,
Tisherman
SA
,
Kerby
JD
,
Newgard
CD
,
Brasel
K
, et al.; ROC Investigators. A controlled resuscitation strategy is feasible and safe in hypotensive trauma patients: results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2015;78(4):687–95, discussion 695–7. doi:.https://doi.org/10.1097/TA.0000000000000600
27
Brown
JB
,
Cohen
MJ
,
Minei
JP
,
Maier
RV
,
West
MA
,
Billiar
TR
, et al.; Inflammation and the Host Response to Injury Investigators. Goal-directed resuscitation in the prehospital setting: a propensity-adjusted analysis. J Trauma Acute Care Surg. 2013;74(5):1207–12, discussion 1212–4.
28
Dick
F
,
Erdoes
G
,
Opfermann
P
,
Eberle
B
,
Schmidli
J
,
von Allmen
RS
. Delayed volume resuscitation during initial management of ruptured abdominal aortic aneurysm. J Vasc Surg. 2013;57(4):943–50. doi:.https://doi.org/10.1016/j.jvs.2012.09.072
29
Henry
DA
,
Carless
PA
,
Moxey
AJ
,
O’Connell
D
,
Stokes
BJ
,
Fergusson
DA
, et al.
Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;(3):CD001886.
30
Shakur
H
,
Roberts
I
,
Bautista
R
,
Caballero
J
,
Coats
T
,
Dewan
Y
, et al., CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. doi:.https://doi.org/10.1016/S0140-6736(10)60835-5
31
Clavé
A
,
Fazilleau
F
,
Dumser
D
,
Lacroix
J
. Efficacy of tranexamic acid on blood loss after primary cementless total hip replacement with rivaroxaban thromboprophylaxis: A case-control study in 70 patients. Orthop Traumatol Surg Res. 2012;98(5):484–90. doi:.https://doi.org/10.1016/j.otsr.2011.12.005
32
Myles
PS
,
Smith
JA
,
Painter
T
. Tranexamic Acid in Patients Undergoing Coronary-Artery Surgery. N Engl J Med. 2017;376(19):1893.
33
Levi
M
. Management of bleeding in patients treated with direct oral anticoagulants. Crit Care. 2016;20(1):249. doi:.https://doi.org/10.1186/s13054-016-1413-3
34
Cuker
A
,
Siegal
D
. Monitoring and reversal of direct oral anticoagulants. Hematology (Am Soc Hematol Educ Program). 2015;2015(1):117–24. doi:.https://doi.org/10.1182/asheducation-2015.1.117
35
Brohi
K
,
Singh
J
,
Heron
M
,
Coats
T
. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–30. doi:.https://doi.org/10.1097/01.TA.0000069184.82147.06
36
Pollack
CV, Jr
,
Reilly
PA
,
van Ryn
J
,
Eikelboom
JW
,
Glund
S
,
Bernstein
RA
, et al.
Idarucizumab for Dabigatran Reversal - Full Cohort Analysis. N Engl J Med. 2017;377(5):431–41. doi:.https://doi.org/10.1056/NEJMoa1707278
37
Levy
JH
,
Ageno
W
,
Chan
NC
,
Crowther
M
,
Verhamme
P
,
Weitz
JI
; Subcommittee on Control of Anticoagulation. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(3):623–7. doi:.https://doi.org/10.1111/jth.13227
38
Douketis
JD
,
Spyropoulos
AC
,
Spencer
FA
,
Mayr
M
,
Jaffer
AK
,
Eckman
MH
, et al.
Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2, Suppl):e326S–50S. doi:.https://doi.org/10.1378/chest.11-2298
39
Spyropoulos
AC
,
Al-Badri
A
,
Sherwood
MW
,
Douketis
JD
. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875–85. doi:.https://doi.org/10.1111/jth.13305
40
Suryanarayan
D
,
Schulman
S
. Potential antidotes for reversal of old and new oral anticoagulants. Thromb Res. 2014;133(Suppl 2):S158–66. doi:.https://doi.org/10.1016/S0049-3848(14)50026-6
41
Nagler
M
,
Angelillo-Scherrer
A
,
Méan
M
,
Limacher
A
,
Abbal
C
,
Righini
M
, et al.
Long-term clinical outcomes of patients with CYP2C9 and VKORC1 variants treated with vitamin K antagonists: a prospective, multicenter cohort study of elderly patients with venous thromboembolism. J Thromb Haemost. 2017;15(11):2165–75. doi:.https://doi.org/10.1111/jth.13810
42
Greinacher
A
,
Thiele
T
,
Selleng
K
. Reversal of anticoagulants: an overview of current developments. Thromb Haemost. 2015;113(5):931–42. doi:.https://doi.org/10.1160/TH14-11-0982
43
Sarode
R
,
Milling
TJ, Jr
,
Refaai
MA
,
Mangione
A
,
Schneider
A
,
Durn
BL
, et al.
Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128(11):1234–43.
44
Goldstein
JN
,
Refaai
MA
,
Milling
TJ, Jr
,
Lewis
B
,
Goldberg-Alberts
R
,
Hug
BA
, et al.
Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015;385(9982):2077–87. doi:.https://doi.org/10.1016/S0140-6736(14)61685-8
45
Sridharan
M
,
Wysokinski
WE
,
Pruthi
R
,
Oyen
L
,
Freeman
WD
,
Rabinstein
AA
, et al.
Periprocedural warfarin reversal with prothrombin complex concentrate. Thromb Res. 2016;139:160–5. doi:.https://doi.org/10.1016/j.thromres.2015.11.024
46
Siegal
DM
,
Garcia
DA
,
Crowther
MA
. How I treat target-specific oral anticoagulant-associated bleeding. Blood. 2014;123(8):1152–8. doi:.https://doi.org/10.1182/blood-2013-09-529784
47
Pollack
CV, Jr
,
Reilly
PA
,
Eikelboom
J
,
Glund
S
,
Verhamme
P
,
Bernstein
RA
, et al.
Idarucizumab for Dabigatran Reversal. N Engl J Med. 2015;373(6):511–20. doi:.https://doi.org/10.1056/NEJMoa1502000
48
Sauter
TC
,
Blum
S
,
Nagler
M
,
Schlittler
FL
,
Ricklin
ME
,
Exadaktylos
AK
. Reversal of Dabigatran Using Idarucizumab in a Septic Patient with Impaired Kidney Function in Real-Life Practice. Case Rep Emerg Med. 2016;2016:1393057. doi:.https://doi.org/10.1155/2016/1393057
49
Ruff
CT
,
Giugliano
RP
,
Antman
EM
. Management of Bleeding With Non-Vitamin K Antagonist Oral Anticoagulants in the Era of Specific Reversal Agents. Circulation. 2016;134(3):248–61. doi:.https://doi.org/10.1161/CIRCULATIONAHA.116.021831
50
Marlu
R
,
Hodaj
E
,
Paris
A
,
Albaladejo
P
,
Crackowski
JL
,
Pernod
G
. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108(2):217–24. doi:.https://doi.org/10.1160/TH12-03-0179
51
Fukuda
T
,
Honda
Y
,
Kamisato
C
,
Morishima
Y
,
Shibano
T
. Reversal of anticoagulant effects of edoxaban, an oral, direct factor Xa inhibitor, with haemostatic agents. Thromb Haemost. 2012;107(2):253–9. doi:.https://doi.org/10.1160/TH11-09-0668
52
Martin
AC
,
Le Bonniec
B
,
Fischer
AM
,
Marchand-Leroux
C
,
Gaussem
P
,
Samama
CM
, et al.
Evaluation of recombinant activated factor VII, prothrombin complex concentrate, and fibrinogen concentrate to reverse apixaban in a rabbit model of bleeding and thrombosis. Int J Cardiol. 2013;168(4):4228–33. doi:.https://doi.org/10.1016/j.ijcard.2013.07.152
53
Perzborn
E
,
Gruber
A
,
Tinel
H
,
Marzec
UM
,
Buetehorn
U
,
Buchmueller
A
, et al.
Reversal of rivaroxaban anticoagulation by haemostatic agents in rats and primates. Thromb Haemost. 2013;110(1):162–72. doi:.https://doi.org/10.1160/TH12-12-0907
54
Eerenberg
ES
,
Kamphuisen
PW
,
Sijpkens
MK
,
Meijers
JC
,
Buller
HR
,
Levi
M
. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573–9. doi:.https://doi.org/10.1161/CIRCULATIONAHA.111.029017
55
Barco
S
,
Whitney Cheung
Y
,
Coppens
M
,
Hutten
BA
,
Meijers
JC
,
Middeldorp
S
. In vivo reversal of the anticoagulant effect of rivaroxaban with four-factor prothrombin complex concentrate. Br J Haematol. 2016;172(2):255–61. doi:.https://doi.org/10.1111/bjh.13821
56
Faust
AC
,
Woodard
S
,
Koehl
JL
,
Mees
W
,
Steinke
D
,
Denetclaw
TH
. Managing Subdural Bleeding Associated With Rivaroxaban: A Series of 3 Cases. J Pharm Pract. 2016;29(3):257–62. doi:.https://doi.org/10.1177/0897190015627116
57
Durie
R
,
Kohute
M
,
Fernandez
C
,
Knight
M
. Prothrombin complex concentrate for the management of severe traumatic bleeding in a patient anticoagulated with apixaban. J Clin Pharm Ther. 2016;41(1):92–3. doi:.https://doi.org/10.1111/jcpt.12339
58
Denetclaw
TH
,
Tam
J
,
Arias
V
,
Kim
R
,
Martin
C
. Case Report: Apixaban-Associated Gluteal Artery Extravasation Reversed With PCC3 Without FFP. J Pharm Pract. 2016;29(4):427–30. doi:.https://doi.org/10.1177/0897190015613231
59
Heidbuchel
H
,
Verhamme
P
,
Alings
M
,
Antz
M
,
Diener
H-C
,
Hacke
W
, et al.
Updated European Heart Rhythm Association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: Executive summary. Eur Heart J. 2017;38(27):2137–49. doi:.https://doi.org/10.1093/eurheartj/ehw058
60
Lu
G
,
Hollenbach
SJ
,
Baker
DC
,
Tan
S
,
Hutchaleelaha
A
,
Curnutte
JT
, et al.
Preclinical safety and efficacy of andexanet alfa in animal models. J Thromb Haemost. 2017;15(9):1747–56. doi:.https://doi.org/10.1111/jth.13768
61
Siegal
DM
,
Curnutte
JT
,
Connolly
SJ
,
Lu
G
,
Conley
PB
,
Wiens
BL
, et al.
Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med. 2015;373(25):2413–24. doi:.https://doi.org/10.1056/NEJMoa1510991
62
Connolly
SJ
,
Milling
TJ, Jr
,
Eikelboom
JW
,
Gibson
CM
,
Curnutte
JT
,
Gold
A
, et al.; ANNEXA-4 Investigators. Andexanet Alfa for Acute Major Bleeding Associated with Factor Xa Inhibitors. N Engl J Med. 2016;375(12):1131–41. doi:.https://doi.org/10.1056/NEJMoa1607887
63
Lu
G
,
DeGuzman
FR
,
Hollenbach
SJ
,
Karbarz
MJ
,
Abe
K
,
Lee
G
, et al.
A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19(4):446–51. doi:.https://doi.org/10.1038/nm.3102
64
Ansell
JE
,
Bakhru
SH
,
Laulicht
BE
,
Steiner
SS
,
Grosso
M
,
Brown
K
, et al.
Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med. 2014;371(22):2141–2. doi:.https://doi.org/10.1056/NEJMc1411800
65
Ansell
JE
,
Bakhru
SH
,
Laulicht
BE
,
Steiner
SS
,
Grosso
MA
,
Brown
K
, et al.
Single-dose ciraparantag safely and completely reverses anticoagulant effects of edoxaban. Thromb Haemost. 2017;117(2):238–45. doi:.https://doi.org/10.1160/TH16-03-0224
66
Studt
JD
,
Alberio
L
,
Angelillo-Scherrer
A
,
Asmis
LM
,
Fontana
P
,
Korte
W
, et al.
Accuracy and consistency of anti-Xa activity measurement for determination of rivaroxaban plasma levels. J Thromb Haemost. 2017;15(8):1576–83. doi:.https://doi.org/10.1111/jth.13747
67
Nagler
M
,
Wuillemin
W
. Labordiagnostik neuer Antikoagulantien - Einfluss auf Hämostaseparameter und Monitoring [Laboratory diagnostic with regard to new anticoagulants - monitoring and influence on coagulation tests]. Ther Umsch. Article in German. 2012;69(11):650–6. doi:.https://doi.org/10.1024/0040-5930/a000343
68
Cuker
A
. Laboratory measurement of the non-vitamin K antagonist oral anticoagulants: selecting the optimal assay based on drug, assay availability, and clinical indication. J Thromb Thrombolysis. 2016;41(2):241–7. doi:.https://doi.org/10.1007/s11239-015-1282-7
69
Gatt
A
,
Chen
D
,
Pruthi
RK
,
Kamath
PS
,
Leise
MD
,
Ashrani
AA
, et al.
From vitamin K antagonists to liver international normalized ratio: a historical journey and critical perspective. Semin Thromb Hemost. 2014;40(8):845–51. doi:.https://doi.org/10.1055/s-0034-1395160
70
Cannegieter
SC
,
Rosendaal
FR
,
Wintzen
AR
,
van der Meer
FJ
,
Vandenbroucke
JP
,
Briët
E
. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333(1):11–7. doi:.https://doi.org/10.1056/NEJM199507063330103
71
Basu
D
,
Gallus
A
,
Hirsh
J
,
Cade
J
. A prospective study of the value of monitoring heparin treatment with the activated partial thromboplastin time. N Engl J Med. 1972;287(7):324–7. doi:.https://doi.org/10.1056/NEJM197208172870703
72
Greaves
M
; Control of Anticoagulation Subcommittee of the Scientific and Standardization Committee of the International Society of Thrombosis and Haemostasis. Limitations of the laboratory monitoring of heparin therapy. Scientific and Standardization Committee Communications: on behalf of the Control of Anticoagulation Subcommittee of the Scientific and Standardization Committee of the International Society of Thrombosis and Haemostasis. Thromb Haemost. 2002;87(1):163–4.
73
Kearon
C
,
Akl
EA
,
Comerota
AJ
,
Prandoni
P
,
Bounameaux
H
,
Goldhaber
SZ
, et al.
Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2, Suppl):e419S–96S. doi:.https://doi.org/10.1378/chest.11-2301
74
Olson
JD
,
Arkin
CF
,
Brandt
JT
,
Cunningham
MT
,
Giles
A
,
Koepke
JA
, et al.
College of American Pathologists Conference XXXI on laboratory monitoring of anticoagulant therapy: laboratory monitoring of unfractionated heparin therapy. Arch Pathol Lab Med. 1998;122(9):782–98.
75
Teien
AN
,
Lie
M
,
Abildgaard
U
. Assay of heparin in plasma using a chromogenic substrate for activated factor X. Thromb Res. 1976;8(3):413–6. doi:.https://doi.org/10.1016/0049-3848(76)90034-7
76
Levine
MN
,
Hirsh
J
,
Gent
M
,
Turpie
AG
,
Cruickshank
M
,
Weitz
J
, et al.
A randomized trial comparing activated thromboplastin time with heparin assay in patients with acute venous thromboembolism requiring large daily doses of heparin. Arch Intern Med. 1994;154(1):49–56. doi:.https://doi.org/10.1001/archinte.1994.00420010073009
77
Nagler
M
,
Angelillo-Scherrer
A
. Thromboembolien und Thrombophilie in der Schwangerschaft. Ther Umsch. 2016;73(7):377–83. doi:.https://doi.org/10.1024/0040-5930/a000807
78
Hawes
EM
,
Deal
AM
,
Funk-Adcock
D
,
Gosselin
R
,
Jeanneret
C
,
Cook
AM
, et al.
Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost. 2013;11(8):1493–502. doi:.https://doi.org/10.1111/jth.12308
79
Fontana
P
,
Alberio
L
,
Angelillo-Scherrer
A
,
Asmis
LM
,
Korte
W
,
Mendez
A
, et al.
Impact of rivaroxaban on point-of-care assays. Thromb Res. 2017;153:65–70. doi:.https://doi.org/10.1016/j.thromres.2017.03.019
80
Asmis
LM
,
Alberio
L
,
Angelillo-Scherrer
A
,
Korte
W
,
Mendez
A
,
Reber
G
, et al.
Rivaroxaban: Quantification by anti-FXa assay and influence on coagulation tests: a study in 9 Swiss laboratories. Thromb Res. 2012;129(4):492–8. doi:.https://doi.org/10.1016/j.thromres.2011.06.031
81
Samama
MM
,
Contant
G
,
Spiro
TE
,
Perzborn
E
,
Guinet
C
,
Gourmelin
Y
, et al.; Rivaroxaban Anti-Factor Xa Chromogenic Assay Field Trial Laboratories. Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost. 2012;107(2):379–87. doi:.https://doi.org/10.1160/TH11-06-0391
82
Beyer-Westendorf
J
,
Gelbricht
V
,
Förster
K
,
Ebertz
F
,
Köhler
C
,
Werth
S
, et al.
Peri-interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur Heart J. 2014;35(28):1888–96. doi:.https://doi.org/10.1093/eurheartj/eht557
83
Nagler
M
,
Erne
P
,
Babst
R
,
Korte
W
,
Wuillemin
WA
. Periinterventionelles Management der Antikoagulation und Antiaggregation. Swiss Med Forum. 2011;11(23-24):407–12. doi:https://doi.emh.ch/10.4414/smf.2011.07539
