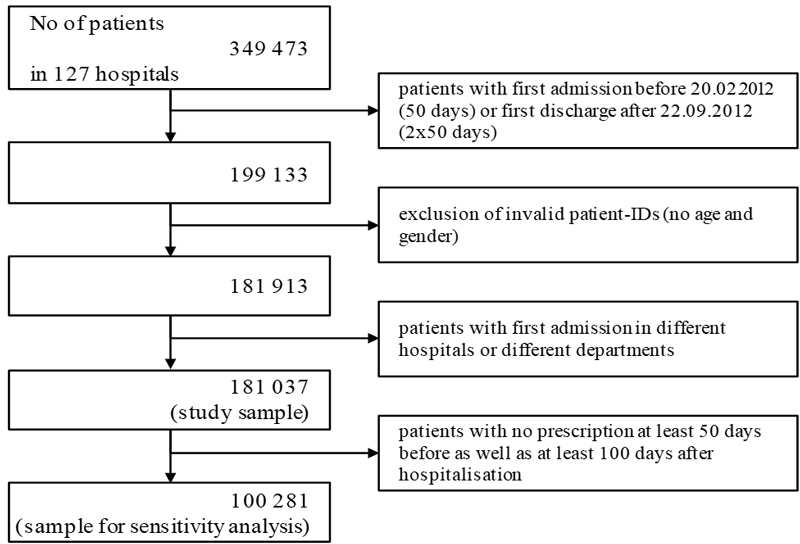
Figure 1 Study inclusion and exclusion.
DOI: https://doi.org/10.4414/smw.2018.14590
Benzodiazepines are often prescribed to treat anxiety and sleep problems even though they can have serious adverse effects, such as craving, withdrawal symptoms upon discontinuation and increased falls, especially in long-term use [1–3] and in elderly patients [4, 5]. When non-benzodiazepine receptor agonists (so-called Z-drugs) were introduced, some practitioners believed that these drugs were superior to benzodiazepines in terms of safety in older adults [6]. However, it is now clear that the spectrum of adverse effects is comparable between Z-drugs and benzodiazepines [7].
An interview study performed in Belgian nursing homes showed that nurses do not avoid using benzodiazepines and view their use as an adequate method for addressing their residents’ sleeping problems [8]. The same may be true for hospitals. For example, in a study performed in a regional hospital in Germany, the majority of nurses used benzodiazepines “often” or “always” in patients on their floor who suffered from insomnia [9]. Hence, it is reasonable to assume that a considerable proportion of patients have their first experiences with sedatives and hypnotics in a hospital setting. Ramesh et al. [10] found that 57% of hospital in-patients who received a benzodiazepine during their hospital stay had not taken benzodiazepines at home prior to hospital admission. Similarly, sedative drugs were initiated in more than one third of patients admitted to an internal ward of a Swiss hospital, most of them naïve to these drugs [11].
The current German guideline on the diagnosis and treatment of insomnia [12], more or less adopted from the Guideline of the European Sleep Research Society [13], recommends the short-term use of benzodiazepines and Z-drugs only if first-line treatment (cognitive behavioural therapy of insomnia and other psychotherapeutic approaches) is ineffective or unavailable. There are also some recommendations on the use of alternatives to benzodiazepines and Z-drugs in the hospital or nursing home setting; these recommendations are, however, rather unsatisfactory. Either the alternatives do not meet the usual quality standards or there is no evidence available. For example, the guideline recommends some sedative antidepressants such as mirtazapine (moderate quality evidence) or antipsychotics such as melperone (low to very low quality evidence). In other words, doctors should know that benzodiazepines and Z-drugs are only second-line options, but may feel let down since alternatives are sometimes time-consuming and do not necessarily meet the standards of evidence-based medicine.
The problems of addiction to and withdrawal from sedatives and hypnotics in patients with insomnia might be, at least in some cases, passed on from the hospital to a primary care setting. Only a few studies [11, 14–16] have explored changes in the use of benzodiazepine or sleep medication as a function of in-hospital use, and only in older patients [14–16] or with small samples in only one hospital [11, 16]. It is therefore difficult to determine the specific role of hospitals in initiating, continuing and discontinuing the use of these drugs. The aim of this study was to ascertain the overall influence of hospitalisation on the prescription use of hypnotics and sedatives in outpatient care. We further considered the roles of different hospital departments and different benzodiazepines and Z-drugs.
The study was part of a larger project on the prescription of hypnotics and sedatives in primary care and during hospitalisation. The ultimate goal of this larger study was to develop, implement and evaluate strategies to reduce the use of hypnotics and sedatives [17]. Whereas most parts of the project are embedded in a local context, we also sought to ascertain the overall influence of hospitalisation on the prescription of hypnotics and sedatives by using a secondary analysis of health insurance data.
The study was approved by the Local Ethics Committee of the University Medical Centre of Göttingen (ref. number 25/2/14).
We used prescription data related to patients who are insured by German Local Health Care Funds [17]. These funds are the largest in Germany and cover approximately 30% of the entire population.
For each patient record, the following data were available:
This was a retrospective follow-up study that compared the use of benzodiazepines and Z-drugs in the primary care sector before and after hospitalisation. The prescriptions for each included patient were analysed for the 50-day period before hospitalisation (t1) and the 100 days after hospitalisation, which were subdivided into two periods of 50 days each (t2 and t3). These periods were chosen because a prescription of benzodiazepines comprises a package of up to 50 units (e.g., tablets).
We included all patients who were hospitalised and spent at least one night at the hospital between 20 February 2012 and 22 September 2012. Thus, we analysed patient prescriptions from 1 January 2012 (50 days before admission = t1) and forward until 31 December 2012 (two periods of 50 days = t2 and t3). We used this raw dataset as the study sample of primary interest.
Because we had no information for cases resulting in death, we could not be sure whether a patient was alive unless they redeemed a prescription. We therefore created a subset of patients who filled at least one prescription at least 50 days before and at least 100 days after hospitalisation and used this sample for sensitivity analyses.
The main outcome of the study was the absolute and relative number of patients with prescriptions of benzodiazepines and Z-drugs before and after hospitalisation.
The drugs under study were benzodiazepines and Z-drugs (ATC codes N05BA, N05CD, and N05CF). We divided the benzodiazepines into either short- or long-acting drugs based on their classification by the European Monitoring Centre for Drugs and Drug Addiction (table 1).
Table 1 Drug classes based on the classifications of the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA).
| Drug class | Drug |
|---|---|
| Short-acting benzodiazepines | Alprazolam |
| Brotizolam | |
| Flunitrazepam* | |
| Lorazepam* | |
| Lormetazepam | |
| Midazolam | |
| Oxazepam | |
| Temazepam | |
| Triazolam | |
| Long-acting benzodiazepines | Bromazepam |
| Chlordiazepoxide | |
| Clobazam | |
| Diazepam | |
| Clorazepate | |
| Flurazepam | |
| Medazepam | |
| Nitrazepam** | |
| Prazepam | |
| Z-drug | Zaleplon |
| Zolpidem | |
| Zopiclone |
* Short/intermediate according to its EMCDDA classification ** Intermediate according its EMCDDA classification
It should be emphasised that we obtained no information regarding drugs that were prescribed or cancelled during hospitalisation. We can infer, with some caution, from the drugs prescribed before and after hospitalisation what drugs might have been initiated, continued or cancelled during hospitalisation.
We classified the different departments at discharge into the following five major categories: internal medicine, surgery, psychiatry, geriatrics and other.
To study the effect of hospitalisation, we modelled the probability of receiving a prescription of hypnotics and/or sedatives in primary care before and after a hospital stay, using a three-factorial design with interactions and covariates. We applied a multivariable logistic regression to account for gender, department and time (before and after admission as specified above) as fixed effects, and age and length of hospital stay as covariates. Time dependence was taken into account by estimating an unstructured covariance matrix within the statistical model. The null hypothesis of interest was that the probability of receiving a prescription did not change over time. The results are reported as odds ratios (ORs) and confidence intervals (CIs) according to a global type-1 error rate of alpha = 0.05. In the case of significant interactions between fixed effects, additional, stratified analyses by these factors were performed, using Bonferroni-adjusted p-values to control the overall type-1 error rate. The type-1 error rate was equally shared among all levels of the stratification factor. We used the closed testing principle [18] when testing for pairwise differences between time points, given the null hypothesis on time was rejected. All statistical analyses were performed in SAS Version 9.4.
Of 349 473 patients who were hospitalised in 2012 (fig. 1), we included only those who had a hospital stay between 20 February 2012 and 22 September 2012. We excluded all patients for whom the data on age and gender were missing and those who did not spend at least one night in hospital. This resulted in a sample of 181 037 patients (the study sample) with a mean age of 64.9 years, 56.5% of whom were women (table 2). These patients had been hospitalised in 127 different hospitals, and most of them had been admitted to an internal medicine (40.8%) or surgery department.

Figure 1 Study inclusion and exclusion.
Table 2 Sample characteristics.
| Patients | Female | Age (yrs.) | Length of hospital stay (days) | |||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | Mean (SD) | Mean (SD) | |
| Overall | 181 037 | (100.0) | 102 297 | (56.5) | 64.9 (20.0) | 7.9 (9.1) |
| Surgery | 60 190 | (33.2) | 30 850 | (51.3) | 63.7 (19.3) | 7.2 (7.9) |
| Geriatrics | 2 268 | (1.3) | 1 658 | (73.1) | 82.8 (8.0) | 16.0 (8.5) |
| Internal medicine | 73 893 | (40.8) | 39 882 | (54.0) | 71.4 (15.7) | 7.6 (7.3) |
| Psychiatry | 7 934 | (4.4) | 3 718 | (46.9) | 49.4 (19.1) | 21.1 (21.1) |
| Other* | 36 752 | (20.3) | 26 189 | (71.3) | 56.1 (23.4) | 6.2 (7.2) |
SD = standard deviation * Other departments include, besides other, gynaecology (with 7% of patients), ENT (3%), neurology (5%), urology (3%)
We then formed a subsample of patients who received at least one prescription during each of the following times: at least 50 days before and at least 100 days after hospitalisation. This resulted in a subset of 100 281 patients (sensitivity sample). As expected, this group was older than the study sample (mean age 70.2 years) and 58.4% were women.
A total of 5648 patients (3.1%) in the study sample (n = 181 037) received one or more of the target drugs before admission (t1), and there was a remarkable difference between men (2.4%) and women (3.7%). The rates at which benzodiazepines and Z-drugs were received were about one percent lower than those in the sensitivity analysis, but the gender pattern was the same (3.2 vs 4.6%).
Within the first 50 days after discharge (t2), the number of patients who were prescribed at least one benzodiazepine or Z-drug increased by 16% from 5648 to 6543 (≈3.6% of all patients). The increase was higher in men (22%) than in women (13%). However, the percentage of patients who received one or more of the target drugs after admission was still higher in women (4.1%) than in men (3.0%).
In the second time window after admission (days 51 to 100, t3), the number of patients returned to, or even fell slightly below, the starting point (5342, ≈3.0%). Accordingly, prescription rates in men (2.5%) and women (3.3%) were close to the initial level before admission.
We observed strong turnover in the patients who no longer received a benzodiazepine or Z-drug or started a new medication after hospitalisation. Of the 5648 patients who received a drug within the 50 days before admission, only 3176 (56.2%) also received a drug in the first or second window or both time-windows after discharge. However, 5961 patients (3.3% of the complete sample) who had no prescription for a benzodiazepine or Z-drug before hospital admission received such a drug in at least one of the two observation windows (i.e., within 100 days) after discharge. In addition, 1049 of these patients (0.6% of the complete sample) received such a drug in both time-windows after discharge.
Figure 2 shows the effect of hospital department on the prescription of benzodiazepines or Z-drugs. Patients discharged from different hospital departments already differed before admission in the relative number of benzodiazepines and Z-drugs received. For example, whereas 4.5% of female patients received a hypnotic or sedative drug before admission to a department of internal medicine, only 3.0% of women did so before admission to a surgical department (fig. 2). However, independently of such differences between hospital departments, we observed an increase in benzodiazepine and Z-drug prescriptions within the first 50 days after discharge (t2) in patients discharged from nearly all departments and again a decrease at t3. For example, 2.4% of patients later admitted to a surgical department received hypnotics or sedatives at t1; this rate was higher (2.9%) within the first 50 days after discharge (t2) and fell to 2.5% at t3. There were three single exceptions to this general trend: women admitted to a psychiatric or geriatric department received fewer hypnotics and sedatives immediately after discharge and men admitted to a geriatric department received even more drugs in the second time-window after discharge (t3) than at t2. These patterns were similar to those observed in the sensitivity analysis (supplementary fig. S1 in appendix 1).
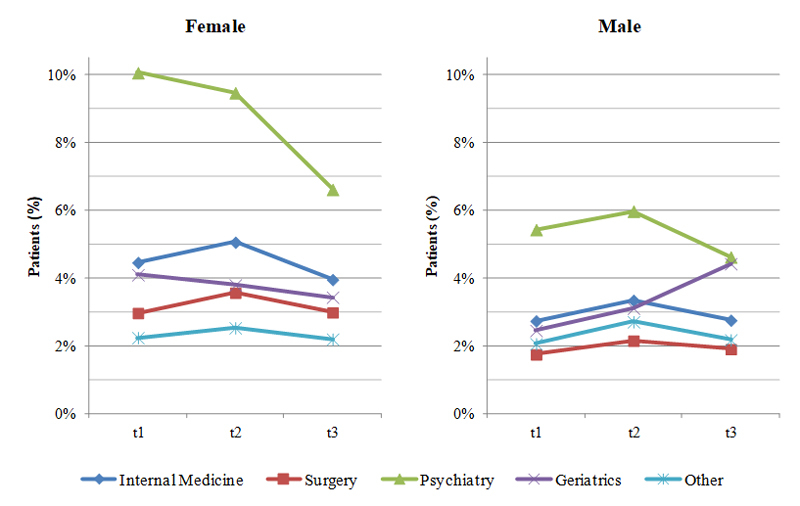
Figure 2 The effect of hospital department on the prescription of benzodiazepines or Z-drugs in males and females (study sample, n = 181 037 patients).
t1 = 50-day period before hospitalisation; t2: = 50-day period after hospitalisation; t3 = days 50 to 100 after hospitalisation
Table 3 shows the results of the multivariable analysis of the hospital effects, i.e., time effects, on the prescription of benzodiazepines and Z-drugs. The hospital effects were first determined for all patients (see the upper section of table 3). Overall, we observed a time effect on the prescription of benzodiazepines and Z-drugs (p = 0.0005). Age and length of hospital stay had an additional effect, both positively correlated with the prescription of these drugs. For gender, more hypnotic or sedative drugs were received by women than men, with an adjusted OR of 1.31 (95% CI 1.20–1.43).
Table 3 Multivariable analysis of hospital effects on the prescription of benzodiazepines and Z-drugs (study sample).
| Parameters | Alpha | p-value | Odds ratio | 95% confidence interval | ||
|---|---|---|---|---|---|---|
| Overall (n = 181 037) | ||||||
| Age | 0.05 | <0.0001 | 1.0145 | 1.0134 | 1.0156 | |
| Hospital stay | <0.0001 | 1.0083 | 1.0065 | 1.0101 | ||
| Gender: female vs male | <0.0001 | 1.3099 | 1.1979 | 1.4324 | ||
| Time | 0.0005 | |||||
| Gender*time | 0.0005 | |||||
| Department | <0.0001 | |||||
| Gender*department | 0.0099 | |||||
| Time*department | <0.0001 | |||||
| Gender*time*department | 0.1328 | |||||
| Internal medicine (n = 73 893) | ||||||
| Age | 0.01** | <0.0001 | 1.0042 | 1.0018 | 1.0067 | |
| Hospital stay | <0.0001 | 1.0105 | 1.0062 | 1.0148 | ||
| Gender: female vs male | <0.0001 | 1.4960 | 1.3778 | 1.6244 | ||
| Time | <0.0001 | |||||
| Gender*time | 0.0090 | |||||
| Male | Time | 0.005** | <0.0001 | |||
| t2 vs t1 | <0.0001 | 1.2282 | 1.1106 | 1.3581 | ||
| t3 vs t1 | 0.7861 | 1.0099 | 0.9120 | 1.1183 | ||
| t3 vs t2 | <0.0001 | 0.8222 | 0.7484 | 0.9033 | ||
| Female | Time | <0.0001 | ||||
| t2 vs t1 | <0.0001 | 1.1426 | 1.0622 | 1.2291 | ||
| t3 vs t1 | <0.0001 | 0.8789 | 0.8149 | 0.9480 | ||
| t3 vs t2 | <0.0001 | 0.7692 | 0.7149 | 0.8276 | ||
| Surgery (n = 60 190) | ||||||
| Age | 0.01** | <0.0001 | 1.0166 | 1.0137 | 1.0195 | |
| Hospital stay | 0.0002 | 1.0091 | 1.0035 | 1.0147 | ||
| Gender: female vs male | <0.0001 | 1.4870 | 1.3299 | 1.6626 | ||
| Time | <0.0001 | |||||
| t2 vs t1 | <0.0001 | 1.2213 | 1.1337 | 1.3158 | ||
| t3 vs t1 | 0.0934 | 1.0496 | 0.9744 | 1.1307 | ||
| t3 vs t2 | <0.0001 | 0.8594 | 0.8003 | 0.9230 | ||
| Gender*time | 0.3988 | |||||
| Geriatrics (n = 2 268) | ||||||
| Age | 0.01** | 0.9215 | 0.9990 | 0.9748 | 1.0239 | |
| Hospital stay | 0.5182 | 0.9942 | 0.9708 | 1.0182 | ||
| Gender: female vs male | 0.4010 | 1.1826 | 0.6957 | 2.0103 | ||
| Time | 0.3336 | |||||
| Gender*time | 0.0284 | |||||
| Psychiatry (n = 7 934) | ||||||
| Age | 0.01** | <0.0001 | 1.0184 | 1.0143 | 1.0224 | |
| Hospital stay | 0.0014 | 1.0052 | 1.0013 | 1.0091 | ||
| Gender: female vs male | <0.0001 | 1.4230 | 1.1760 | 1.7220 | ||
| Time | <0.0001 | |||||
| Gender*time | 0.0276 | |||||
| Male | Time | 0.005** | 0.0004 | |||
| t2 vs t1 | 0.1763 | 1.1080 | 0.8955 | 1.3710 | ||
| t3 vs t1 | 0.0331 | 0.8430 | 0.6732 | 1.0557 | ||
| t3 vs t2 | <0.0001 | 0.7608 | 0.6255 | 0.9254 | ||
| Female | Time | <0.0001 | ||||
| t2 vs t1 | 0.2951 | 0.9341 | 0.7781 | 1.1213 | ||
| t3 vs t1 | <0.0001 | 0.6310 | 0.5142 | 0.7744 | ||
| t3 vs t2 | <0.0001 | 0.6755 | 0.5700 | 0.8005 | ||
| Other (n = 36 752) | ||||||
| Age | 0.01** | <0.0001 | 1.0247 | 1.0217 | 1.0277 | |
| Hospital stay | <0.0001 | 1.0149 | 1.0083 | 1.0214 | ||
| Gender: female vs male | 0.0005 | 1.2323 | 1.0508 | 1.4450 | ||
| Time | <0.0001 | |||||
| t2 vs t1 | <0.0001 | 1.2271 | 1.0984 | 1.3710 | ||
| t3 vs t1 | 0.7707 | 1.0125 | 0.9074 | 1.1297 | ||
| t3 vs t2 | <0.0001 | 0.8250 | 0.7460 | 0.9125 | ||
| Gender*time | 0.1549 | |||||
* Interaction between gender, time and department demands in the stratified analysis ** Stage-wise uniform alpha-splitting based on Bonferroni correction for each department and gender, if necessary
However, the effect of hospitalisation in different hospital departments was not homogenous, as shown in figure 2. Because there were significant interactions between time and department as well as gender and department, we performed stratified analyses by department to identify time effects (see the five lower sections of table 3). In these analyses, we simultaneously controlled for age, gender and length of hospital stay. The results from the study sample were more or less the same as the results in the sensitivity analysis (supplementary table S1 in appendix 1). Because of the larger sample size, more null hypotheses from the study sample could be rejected. In the following, we report the most important results:
Of the patients who received a prescription for a benzodiazepine or Z-drug before admission, 2278 (40.3%) had received a long-acting substance, 2149 (38.0%) a short-acting substance and 1509 (26.7%) had received a Z-drug. Some of these patients (n = 288) received drugs from more than one class, causing the percentages to sum to 105.1%.
The single drug that was most often prescribed before admission was lorazepam, a short-acting substance, which was prescribed for about one quarter of the patients (1439/5648). It was followed by the long-acting substance diazepam (1338 patients, 23.7%) and two Z-drugs, zolpidem (846 patients, 15.0%) and zopiclone (684 patients, 12.1%).
From t1 to t2, the number of patients who received long-term substances fell by 1% from 2278 to 2251, and the number of patients who received short-term substances increased by 35% from 2149 to 2907. In relative numbers, this change represents an increase from 38.0 to 44.4% for short-term substances and a decrease from 40.3 to 34.4% for long-term substances. The number of patients prescribed Z-drugs increased in absolute numbers (from 1509 to 1770) but remained nearly stable in relative numbers (26.7 vs 27.1%). The relationship between these drugs remained constant during t3 (41.9% for short-acting benzodiazepines, 35.5% for long-acting benzodiazepines and 27.9% for Z-drugs).
We observed some remarkable effects for department but no gender effects. For example, of the patients who were prescribed a benzodiazepine or Z-drug and were later admitted to a department of surgery, 34.6% received short-acting substances and 40.8% received long-acting substances at t1. This relationship remained almost unchanged after discharge: 36.7 and 38.4% at t2, and 37.5 and 37.2% at t3, respectively. In contrast, of the patients who were later admitted to a department of internal medicine, 37.2% received short-acting substances and 42.3% long-acting substances at t1. This relationship changed in favour of short-acting substances after discharge: 46.1 and 35.0% at t2, and 42.2 and 36.6% at t3, respectively. Patients admitted to geriatric or psychiatric departments received rather often short-acting substances before admission at t1 (45.8 and 52.4%, respectively) and even more of them received these substances after discharge at t2 (53.7 and 56.1%, respectively). All these results were similar in the sensitivity analysis.
Our data regarding the role of hospitals in initiating and increasing the prescription of hypnotic and sedative drugs showed mixed results. The rate of patients who received these drugs increased by 16% during the 50 days after discharge, representing a considerable number of newly initiated prescriptions. This rate later returned to, or even fell below, the level observed before admission. The relative number of patients who received short-acting substances increased, whereas the number of patients who received long-acting substances fell after discharge.
This study included an unselected sample of German patients who were hospitalised in a large number of different hospitals in 2012. We had access to their prescription data both before and after hospitalisation, but no access to hospital data. We could therefore only indirectly analyse hospital effects using pre-post comparisons.
We had no access to information explaining why prescriptions were continued, cancelled or newly started during hospitalisation, and we therefore could not determine whether these changes represented an active and conscious decision by the hospital doctors or they were initiated by a medical need or for other reasons. Similarly, we could not determine whether primary care doctors followed hospital recommendations, their patients’ wishes or their own medical expertise when they prescribed hypnotic or sedative drugs that were initiated during the hospital stay.
For the final sample, we had to decide whether to include patients who spent at least one night at the hospital during the study period even if they might have died during or shortly after hospitalisation without our knowledge. Alternatively, we could have included only patients who were known to have been followed up for at least two periods of 50 days each after discharge because they received at least one prescription at least 100 days after discharge. To enhance the validity of our results, we chose both variants, with the latter used for a sensitivity analysis.
By defining two time windows after discharge, we could determine the effects of hospitalisation on the prescription of hypnotic or sedative drugs in primary care and, in addition and even more importantly, we could determine whether this effect was sustainable.
A major advantage of this study was its coherent statistical analysis, because use of a multivariable logistic regression model allowed us to go beyond a simple descriptive analysis of the data. Being able to simultaneously evaluate multiple factors in combination with a large sample size enabled us to identify patterns of interaction that would otherwise be missed, while maintaining a type-I error rate.
Hospital admission and discharge are times when a patient’s pharmacological history can be re-evaluated. At these times, drug regimens are often considerably changed [19] or, in the case of specific drugs such as proton-pump inhibitors, significantly increased after discharge [20]. These events are often classified as a negative influence of the hospital [21, 22].
At least four relatively recent studies have investigated the effect of hospitalisation on the subsequent prescription of benzodiazepines or sleep medications. In a Canadian study [14], 3.1% of the patients became new benzodiazepine users after hospital discharge, and more than 1% became chronic users. Similar results were obtained from a study in the same region: benzodiazepine prescriptions without indication were prescribed for the short term after hospitalisation in 3.3% of the patients and for more than 1 year in nearly 1% of the patients [15]. In a survey performed at the Mount Carmel Hospital in Haifa, Israel, patients were asked about changes in their sleep medication that occurred between pre- and post-hospitalisation. The sleep medications evaluated included benzodiazepines, Z-drugs, selective serotonin reuptake inhibitors and antipsychotic drugs with sedating effects. Nearly 8% of prior users of sleep medications discontinued use during their stay in hospital, whereas 14% of those who had not used sleep medications prior to admission initiated use during hospitalisation [16]. A Swiss hospital study on an internal ward showed that sedative medication was initiated for 37% of patients and that, at discharge, the proportion of patients who received a sedative drug prescription had increased by 10% [11].
Our findings during the short time window of the first 50 days after hospital discharge seem to support the results of these previous studies. More than 70% (4054/5648) of those who received a hypnotic and sedative drug after discharge had not received such a drug in the 50 days before admission. In other words, primary care physicians started a new prescription of these drugs in many patients after discharge. However, this does not seem to be a stable trend, because in the following 50 days prescriptions fell back to the level observed before admission.
This overall lack of sustainability is a call to reconsider the role of the hospital as a “turning point” as Zisberg et al. suggested [16]. First, we can confirm this role, as both studies showed that prevalence rates of sleep medication use pre- and post-hospitalisation are fairly similar and, at the same time, the hospital is a significant actor both in initiation and discontinuation of sleep medications. Second, our study identified another important development. Although the increase in prescriptions after discharge is widely recognised [23], the increase in hypnotic and sedative drugs is obviously not a sustainable effect, since 100 days after hospitalisation the prescription rate had returned to its previous level. We therefore conclude that primary care physicians seem to continue hospital medication at first after discharge but stop in the long run as they are indicated for short-term use only.
Moreover, to gain a balanced assessment of the hospital’s role as a “turning point”, we should consider the epidemiological aspects of the use of hypnotics and sedatives in hospitals. Approximately one third or more of all patients, and especially older patients, receive at least one benzodiazepine or a related drug during hospitalisation [23, 24]. If hospitals have a strong knock-on effect on the subsequent use of these drugs in primary care, the increase in the proportion of patients using a benzodiazepine and Z-drug between before admission and shortly after discharge should have been larger than only 0.5% (from 3.1 to 3.6%), as detected in our study.
However, of those patients who visited a hospital without a current prescription for hypnotic or sedative drugs, 0.6% received a prescription in both of the 50-day time windows after discharge that were evaluated in this study. This result is similar to the initiation rates of 1.5% for new chronic benzodiazepine users and 0.7% for patients who continued benzodiazepines after discharge for over 1 year that were observed in the two Toronto studies described above [14, 15].
In general, women receive more antidepressant, hypnotic and sedative drugs [25]. According to the Swedish Prescribed Drug Register, 27% of women but only 18% of men were dispensed at least one hypnotic or sedative drug, and this difference was especially pronounced for benzodiazepines and Z-drugs [26]. We confirmed these differences between men and women, but the primary aim of our study was to ascertain whether a possible effect of hospitalisation on the prescription rate of hypnotics and sedatives is amplified by gender effects. In contrast to the result of the population-based study performed in the Toronto region [14], the results of our study showed that the increase in the prescription of these drugs after discharge is not higher, and even somewhat lower, in female patients than in male patients, especially in internal departments. In this sense, the hospital had, if any, an equalising effect. This effect could also be observed in psychiatric departments.
We also observed department effects in our study. According to previous studies, the prescription/administration of sleep medication is higher in medical than in surgical wards [27–29], with some exceptions [23]. In a cross-sectional study of 800 elderly patients who were admitted to a Swiss teaching hospital [30], no strong differences were found between general medical or geriatric wards. Again, our primary goal was to determine whether the department has an additional influence on the overall effect of hospitalisation. Indeed, we detected big pre-post differences in psychiatric and geriatric departments. Whereas the changes in the geriatric departments were not significant, the decrease in the rate of hypnotics and sedatives after discharge in psychiatric patients, especially in women, was remarkable. Our results are in line with a Scottish study [31] on the influence of hospitalisation on community benzodiazepine prescribing, which showed a reduction of benzodiazepine prescribing in outpatient care after psychiatric hospitalisation. There are two complementary explanations. According to a hospital- and community-based US study [32], benzodiazepines, although indicated for patients with psychiatric diagnoses such as anxiety, are often prescribed for patients with a diagnoses of depression or substance abuse in primary care. According to a literature review [2], patients suffering from severe psychological problems often receive benzodiazepines or other inadequate drugs as a result of long waiting times for psychotherapy. From both explanations we conclude that the decline in benzodiazepines after discharge is due to the initiation of other psychiatric medications or therapies which are substituted for benzodiazepines and Z-drugs.
Longer hospital stays also influenced the effect of hospitalisation. This result is in line with two Canadian studies [14, 15], which also found that longer hospital stays were predictive of the initiation of new benzodiazepine prescriptions or chronic benzodiazepine use after hospitalisation.
Age as another covariate exerted an unclear effect in our study. Whereas the prescription of hypnotics and sedatives slightly increased with age in our study sample as a whole and in patients discharged from a department of internal medicine, we observed an opposite effect in patients discharged from a department of internal medicine in our sensitivity analysis. Mixed results were also found in the two Canadian studies [14, 15]. Currently, we should be cautious when generalising an age effect that has been detected in only a single institution or region.
It is encouraging that lorazepam was the most frequently prescribed drug and that the prescription of short-acting benzodiazepines increased while those of long-acting substances decreased. Lorazepam is less likely to be involved in drug-drug interactions and may be less toxic than its alternatives, such as diazepam or alprazolam [33]. Lists of potentially inappropriate medications classify the prescription of low doses of short- or shorter-acting benzodiazepines or Z-drugs as possible therapeutic alternatives to longer-acting benzodiazepines [4] or explicitly advise against the use of long-acting benzodiazepines and benzodiazepines with long-acting metabolites [34]. Nevertheless, short-acting benzodiazepines are also classified as potentially inappropriate medications in elderly patients, and whether shorter-acting benzodiazepines are actually safer than longer-acting drugs remains controversial [35, 36].
The results of our study regarding the hospital effect on the prescription of hypnotic and sedative drugs in primary care do not seem to be alarming or to indicate a need for action. Indeed, the observed change from short-acting to long-acting substances and the decreases in drug prescriptions after hospital discharge in some groups of patients are reassuring and indicate that hospitals do make corrective measures in some cases. So, no reasons for concern? There are at least two reasons.
Even if primary care physicians seemed to work against an increase in hypnotic and sedative medication in the long run, we should emphasise that the risk of falls and fractures increases after treatment initiation, particularly during the first 1 to 2 weeks of drug exposure. Thus, even a transitory increase in the use of benzodiazepines and Z-drugs can harm the patients and should be checked critically [37].
There was a high turnover of patients who received a benzodiazepine or Z-drug before and after hospitalisation. This finding, in addition to the higher rates of patients, especially elderly patients, receiving hypnotics and sedatives in hospitals, indicates that better communication strategies are needed between hospital doctors, primary care physicians and patients so that patients can be adequately informed about the risks of these drugs.
We identified a small group of patients who require more attention. Approximately 1% of the patients who had not received hypnotic and sedative drugs within the 50 days before hospitalisation received a prescription in both time-windows after hospitalisation. Our study provides valuable data regarding the groups of patients who are most likely to be affected by, and which departments are most likely to contribute to, these developments so that adequate measures can be taken to address them.
The results of our study provide urgently needed data that contribute to a more balanced consideration of the effect of hospitals on the initiation, continuation and discontinuation of hypnotic or sedative drugs. In general, these effects were moderate and only temporary. In some instances, such as the change in favour of short-acting substances, they were even welcome. Moreover, our results suggest that hospitals are not to blame for the fact that hypnotics are prescribed at higher rates to women than to men in primary care, nor for the particularly high prescription rate in the elderly. On the contrary, hospitalisation may be an equilibrating factor for gender inequalities in the use of hypnotic and sedative drugs.
From a clinical perspective, primary care physicians may feel under pressure in the approximately 1% of patients for whom hospitals initiated continuous post-hospitalisation use of these drugs. From a pharmacoepidemiological perspective, it is reassuring that benzodiazepines and related drugs, which are often used in hospitals to combat sleeping problems and other issues, do not seem to be continued in primary care. Whether this finding is the result of a hospital policy that avoids recommending the use of such drugs in the home or a filtering process by primary care physicians should be addressed in future studies.
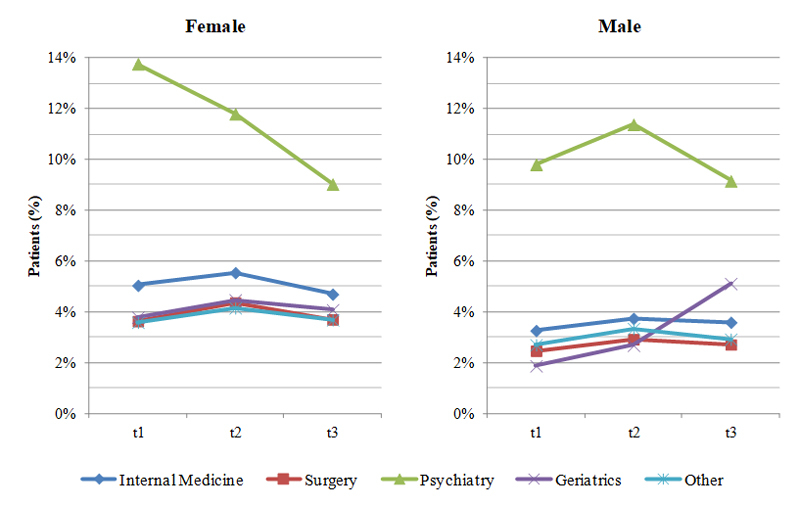
Figure S1 The effect of hospital department on the prescription of benzodiazepines and Z-drugs in males and females (sample for sensitivity analysis, n = 100 281 patients).
t1 = 50-day period before hospitalisation; t2 = 50-day period after hospitalisation; t3 = days 50 to 100 after hospitalisation
Table S1 Multivariable analysis of hospital effects on the prescription of benzodiazepines and Z drugs (sample for sensitivity analysis).
| Parameter | Alpha | p-value | Odds ratio | 95% confidence interval | ||
|---|---|---|---|---|---|---|
| Overall (n = 100 281) | ||||||
| Age | 0.05 | 0.0001 | 1.0030 | 1.0015 | 1.0046 | |
| Hospital stay | <0.0001 | 1.0057 | 1.0032 | 1.0083 | ||
| Gender: female vs male | <0.0001 | 1.3231 | 1.1741 | 1.4911 | ||
| Time | 0.0167 | |||||
| Gender*time | 0.0004 | |||||
| Department | <0.0001 | |||||
| Gender*department | 0.0672 | |||||
| Time*department | <0.0001 | |||||
| Gender*time*department | 0.0339* | |||||
| Internal medicine (n = 45 156) | ||||||
| Age | 0.01** | 0.0093 | 0.9964 | 0.9930 | 0.9999 | |
| Hospital stay | 0.0003 | 1.0094 | 1.0033 | 1.0154 | ||
| Gender: female vs male | <0.0001 | 1.4777 | 1.3365 | 1.6339 | ||
| Time | <0.0001 | |||||
| Gender*time | 0.0007 | |||||
| Male | Time | 0.005** | 0.0022 | |||
| t2 vs t1 | 0.0007 | 1.1513 | 1.0247 | 1.2935 | ||
| t3 vs t1 | 0.0121 | 1.1061 | 0.9880 | 1.2381 | ||
| t3 vs t2 | 0.2885 | 0.9607 | 0.8641 | 1.0681 | ||
| Female | Time | <.0001 | ||||
| t2 vs t1 | 0.0007 | 1.1053 | 1.0171 | 1.2012 | ||
| t3 vs t1 | 0.0094 | 0.9250 | 0.8503 | 1.0063 | ||
| t3 vs t2 | <.0001 | 0.8368 | 0.7700 | 0.9095 | ||
| Surgery (n = 33 057) | ||||||
| Age | 0.01** | 0.0499 | 1.0030 | 0.9990 | 1.0070 | |
| Hospital stay | 0.0303 | 1.0067 | 0.9994 | 1.0140 | ||
| Gender: female vs male | <0.0001 | 1.4386 | 1.2589 | 1.6439 | ||
| Time | <0.0001 | |||||
| t2 vs t1 | <0.0001 | 1.2034 | 1.1038 | 1.3118 | ||
| t3 vs t1 | 0.0424 | 1.0680 | 0.9824 | 1.1612 | ||
| t3 vs t2 | 0.0002 | 0.8876 | 0.8174 | 0.9639 | ||
| Gender*Time | 0.3937 | |||||
| Geriatrics (n = 1 398) | ||||||
| Age | 0.01** | 0.8794 | 0.9981 | 0.9674 | 1.0298 | |
| Hospital stay | 0.1481 | 0.9810 | 0.9473 | 1.0159 | ||
| Gender: female vs male | 0.1482 | 1.4447 | 0.7121 | 2.9308 | ||
| Time | 0.0117 | |||||
| Gender*time | 0.0173 | |||||
| Psychiatry (n = 3 411) | ||||||
| Age | 0.01** | 0.0002 | 1.0080 | 1.0025 | 1.0135 | |
| Hospital stay | 0.4766 | 1.0016 | 0.9960 | 1.0071 | ||
| Gender: female vs male | 0.3773 | 1.0848 | 0.8552 | 1.3759 | ||
| Time | <0.0001 | |||||
| Gender*time | 0.0036 | |||||
| Male | Time | 0.005** | 0.0089 | |||
| t2 vs t1 | ||||||
| t3 vs t1 | ||||||
| t3 vs t2 | ||||||
| Female | Time | <.0001 | ||||
| t2 vs t1 | 0.0210 | 0.8376 | 0.6752 | 1.0391 | ||
| t3 vs t1 | <.0001 | 0.6216 | 0.4904 | 0.7878 | ||
| t3 vs t2 | <.0001 | 0.7421 | 0.6085 | 0.9050 | ||
| Other (n = 17 259) | ||||||
| Age | 0.01** | <0.0001 | 1.0102 | 1.0061 | 1.0143 | |
| Hospital stay | 0.0028 | 1.0131 | 1.0034 | 1.0228 | ||
| Gender: female vs male | <0.0001 | 1.3468 | 1.1136 | 1.6288 | ||
| Time | 0.0009 | |||||
| t2 vs t1 | 0.0004 | 1.1970 | 1.0500 | 1.3646 | ||
| t3 vs t1 | 0.3940 | 1.0428 | 0.9189 | 1.1833 | ||
| t3 vs t2 | 0.0023 | 0.8711 | 0.7752 | 0.9789 | ||
| Gender*time | 0.7814 | |||||
* Interaction between gender, time and department demands in the stratified analysis ** Stage-wise uniform alpha-splitting based on Bonferroni correction for each department and gender, if necessary
We are indebted to AOK Nordost for permission to perform this study.
The study was supported by a research grant from the German Ministry of Health (II A5-2513DSM228).
Markus Harden received personal fees during two internships at Novartis Pharma AG, Basel in 2013 and 2014. The other authors declare that they have no financial relationship with any organisation that might have had an interest in the submitted work. All authors declare that they have no other relationships or activities that could appear to have influenced the submitted work.
1 Olfson M , King M , Schoenbaum M . Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136–42 .https://doi.org/10.1001/jamapsychiatry.2014.1763
2 Janhsen K , Roser P , Hoffmann K . The problems of long-term treatment with benzodiazepines and related substances. Dtsch Arztebl Int. 2015;112(1-2):1–7. doi:.https://doi.org/10.3238/arztebl.2015.0001
3 Lader M . Benzodiazepines revisited--will we ever learn? Addiction. 2011;106(12):2086–109. .https://doi.org/10.1111/j.1360-0443.2011.03563.x
4 Holt S , Schmiedl S , Thürmann PA . Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int. 2010;107(31-32):543–51. .https://doi.org/10.3238/arztebl.2010.0543
5 Pasina L , Djade CD , Tettamanti M , Franchi C , Salerno F , Corrao S , et al.; REPOSI Investigators. Prevalence of potentially inappropriate medications and risk of adverse clinical outcome in a cohort of hospitalized elderly patients: results from the REPOSI Study. J Clin Pharm Ther. 2014;39(5):511–5 .https://doi.org/10.1111/jcpt.12178
6 Antai-Otong D . The art of prescribing. Risks and benefits of non-benzodiazepine receptor agonists in the treatment of acute primary insomnia in older adults. Perspect Psychiatr Care. 2006;42(3):196–200 .https://doi.org/10.1111/j.1744-6163.2006.00070.x
7 Gunja N . In the Zzz zone: the effects of Z-drugs on human performance and driving. J Med Toxicol. 2013;9(2):163–71 .https://doi.org/10.1007/s13181-013-0294-y
8 Anthierens S , Grypdonck M , De Pauw L , Christiaens T . Perceptions of nurses in nursing homes on the usage of benzodiazepines. J Clin Nurs. 2009;18(22):3098–106 .https://doi.org/10.1111/j.1365-2702.2008.02758.x
9 Weiß V , Heinemann S , Himmel W , Nau R , Hummers-Pradier E . Benzodiazepine und Z-Substanzen als Schlaf- und Beruhigungsmittel in einem Krankenhaus [The use of benzodiazepines and Z-drugs for patients with sleeping problems - A survey among hospital doctors and nurses] [in German]. Dtsch Med Wochenschr. 2016;141(13):e121–6. https://www.thieme-connect.de/DOI/DOI?10.1055/s-0042-102618.
10 Ramesh M , Roberts G . Use of night-time benzodiazepines in an elderly inpatient population. J Clin Pharm Ther. 2002;27(2):93–7 .https://doi.org/10.1046/j.1365-2710.2002.00400.x
11 Schumacher L , Dobrinas M , Tagan D , Sautebin A , Blanc AL , Widmer N . Prescription of sedative drugs during hospital stay: a Swiss prospective study. Drugs Real World Outcomes. 2017;4(4):225–34 .https://doi.org/10.1007/s40801-017-0117-6
12 Riemann D , Baum E , Cohrs S , Crönlein T , Hajak G , Hertenstein E , et al. S3-Leitlinie Nicht erholsamer Schlaf/Schlafstörungen [S3 Guidelines on non-restorative sleep/sleep disorders]. [in German]. Somnologie (Berl). 2017;21:2–44. doi:https://doi.org/10.1007/s11818-016-0097-x
13 Riemann D , Baglioni C , Bassetti C , Bjorvatn B , Dolenc Groselj L , Ellis JG , et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700 .https://doi.org/10.1111/jsr.12594
14 Bell CM , Fischer HD , Gill SS , Zagorski B , Sykora K , Wodchis WP , et al. Initiation of benzodiazepines in the elderly after hospitalization. J Gen Intern Med. 2007;22(7):1024–9 .https://doi.org/10.1007/s11606-007-0194-4
15 Scales DC , Fischer HD , Li P , Bierman AS , Fernandes O , Mamdani M , et al. Unintentional continuation of medications intended for acute illness after hospital discharge: a population-based cohort study. J Gen Intern Med. 2016;31(2):196–202 .https://doi.org/10.1007/s11606-015-3501-5
16 Zisberg A , Shadmi E , Sinoff G , Gur-Yaish N , Srulovici E , Shochat T . Hospitalization as a turning point for sleep medication use in older adults: prospective cohort study. Drugs Aging. 2012;29(7):565–76 .https://doi.org/10.1007/BF03262274
17 Heinemann S , Weiß V , Straube K , Nau R , Grimmsmann T , Himmel W , et al. Understanding and reducing the prescription of hypnotics and sedatives at the interface of hospital care and general practice: a protocol for a mixed-methods study. BMJ Open. 2016;6(8):e011908 .https://doi.org/10.1136/bmjopen-2016-011908
18 Marcus R , Peritz E , Gabriel KR . On closed testing procedures with special reference to ordered analysis of variance. Biometrika. 1976;63(3):655–60. doi:.https://doi.org/10.1093/biomet/63.3.655
19 Viktil KK , Blix HS , Eek AK , Davies MN , Moger TA , Reikvam A . How are drug regimen changes during hospitalisation handled after discharge: a cohort study. BMJ Open. 2012;2(6):e001461 .https://doi.org/10.1136/bmjopen-2012-001461
20 Grimmsmann T , Schwabe U , Himmel W . The influence of hospitalisation on drug prescription in primary care--a large-scale follow-up study. Eur J Clin Pharmacol. 2007;63(8):783–90 .https://doi.org/10.1007/s00228-007-0325-1
21 Ahrens D , Chenot JF , Behrens G , Grimmsmann T , Kochen MM . Appropriateness of treatment recommendations for PPI in hospital discharge letters. Eur J Clin Pharmacol. 2010;66(12):1265–71 .https://doi.org/10.1007/s00228-010-0871-9
22 Wermeling M , Himmel W , Behrens G , Ahrens D . Why do GPs continue inappropriate hospital prescriptions of proton pump inhibitors? A qualitative study. Eur J Gen Pract. 2014;20(3):174–80 .https://doi.org/10.3109/13814788.2013.844787
23 Somers A , Robays H , Audenaert K , Van Maele G , Bogaert M , Petrovic M . The use of hypnosedative drugs in a university hospital: has anything changed in 10 years? Eur J Clin Pharmacol. 2011;67(7):723–9 .https://doi.org/10.1007/s00228-010-0983-2
24 Arnold I , Straube K , Himmel W , Heinemann S , Weiss V , Heyden L , et al. High prevalence of prescription of psychotropic drugs for older patients in a general hospital. BMC Pharmacol Toxicol. 2017;18(1):76 .https://doi.org/10.1186/s40360-017-0183-0
25Glaeske G, Gerdau-Heitmann C, Höfel F, Schicktanz C. Gender-specific drug prescription in Germany. Results from prescriptions analyses. In: Regitz-Zagrosek V (ed.) Sex and Gender Differences in Pharmacology (Handbook of Experimental Pharmacology; vol. 214, pp 149–167). https://doi.org/10.1007/978-3-642-30726-3
26 Johnell K , Fastbom J . Gender and use of hypnotics or sedatives in old age: a nationwide register-based study. Int J Clin Pharm. 2011;33(5):788–93 .https://doi.org/10.1007/s11096-011-9536-8
27 Halfens RJ , Lendfers ML , Cox K . Sleep medication in Dutch hospitals. J Adv Nurs. 1991;16(12):1422–7 .https://doi.org/10.1111/j.1365-2648.1991.tb01589.x
28 Nakao M , Takeuchi T , Yano E . Prescription of benzodiazepines and antidepressants to outpatients attending a Japanese university hospital. Int J Clin Pharmacol Ther. 2007;45(1):30–5 .https://doi.org/10.5414/CPP45030
29 Warie H , Petrovic M , Somers A , Mariman A , Robays H , Pevernagie D . The use of hypnosedative drugs in a university hospital setting. Acta Clin Belg. 2003;58(4):225–32 .https://doi.org/10.1179/acb.2003.58.4.003
30 Egger SS , Bachmann A , Hubmann N , Schlienger RG , Krähenbühl S . Prevalence of potentially inappropriate medication use in elderly patients: comparison between general medical and geriatric wards. Drugs Aging. 2006;23(10):823–37 .https://doi.org/10.2165/00002512-200623100-00005
31 Millar HL , Clunie FS , McGilchrist MM , McMahon AD , MacDonald TM . The impact on community benzodiazepine prescribing of hospitalization. J Psychosom Res. 1997;42(1):61–9 .https://doi.org/10.1016/S0022-3999(96)00232-2
32 Kroll DS , Nieva HR , Barsky AJ , Linder JA . Benzodiazepines are prescribed more frequently to patients already at risk for benzodiazepine-related adverse events in primary care. J Gen Intern Med. 2016;31(9):1027–34 .https://doi.org/10.1007/s11606-016-3740-0
33 Nicholson AN . The use of short- and long-acting hypnotics in clinical medicine. Br J Clin Pharmacol. 1981;11(S1, Suppl 1):61S–9S .https://doi.org/10.1111/j.1365-2125.1981.tb01841.x
34 Gallagher P , Ryan C , Byrne S , Kennedy J , O’Mahony D . STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83 .https://doi.org/10.5414/CPP46072
35 de Vries OJ , Peeters G , Elders P , Sonnenberg C , Muller M , Deeg DJ , et al. The elimination half-life of benzodiazepines and fall risk: two prospective observational studies. Age Ageing. 2013;42(6):764–70 .https://doi.org/10.1093/ageing/aft089
36 Chen L , Bell JS , Visvanathan R , Hilmer SN , Emery T , Robson L , et al. The association between benzodiazepine use and sleep quality in residential aged care facilities: a cross-sectional study. BMC Geriatr. 2016;16(1):196 .https://doi.org/10.1186/s12877-016-0363-6
37 Skinner BW , Johnston EV , Saum LM . Benzodiazepine initiation and dose escalation. Ann Pharmacother. 2017;51(4):281–5 .https://doi.org/10.1177/1060028016682530
TG and WH designed and supervised all aspects of the study. TG and TF were responsible for managing and retrieving the data. MH performed the statistical analysis. TG and WH wrote the first draft of the manuscript, and MH critically reviewed and revised the manuscript. All authors read and approved the final submitted version of the manuscript. TG and WH are the principal investigators and guarantors.
The study was supported by a research grant from the German Ministry of Health (II A5-2513DSM228).
Markus Harden received personal fees during two internships at Novartis Pharma AG, Basel in 2013 and 2014. The other authors declare that they have no financial relationship with any organisation that might have had an interest in the submitted work. All authors declare that they have no other relationships or activities that could appear to have influenced the submitted work.